Abstract
Objective
To describe the use and feasibility of cold saline to decrease body temperature in pediatric neurocritical care.
Design
Retrospective chart review.
Setting
Pediatric tertiary care university hospital.
Patients
Children between 1 week and 17 yrs of age admitted to the pediatric intensive care unit with acute brain injury and having received intravenous cold saline between June-August 2009.
Intervention(s)
None.
Measurements and Main Results
Eighteen subjects accounted for 20 infusions with mean infusion volume 18 ± 10 cc/kg. Eight subjects had traumatic brain injury (TBI), 2 had intracranial hemorrhage, 6 had cardiac arrest, and one each had ischemic stroke and status epilepticus. The mean age was 9.5 ± 4.8 yrs. Temperature decreased from 38.7 ± 1.1°C to 37.7 ± 1.2°C and 37.0 ± 2.0 to 35.3 ± 1.6°C one h after infusion for fever (n=14, p<.05) or hypothermia (HT) induction (n=6, p=.05), respectively. Cold saline was not bolused, rather infused over 10–15 minutes. Mean arterial blood pressure and oxygenation parameters (PaO2/FiO2 ratio, mean airway pressure) were unchanged, but heart rate decreased in HT subjects (121 ± 4 vs. 109 ± 12; p<.05). Serum sodium concentration and International normalized ratio were significantly increased after cold saline infusion. There were no differences between pre- and post-infusion serum glucose and hematocrit, nor cerebral perfusion pressure or intracranial pressure in TBI patients.
Conclusions
Cold saline was an effective method of reducing temperature in children with acute brain injury. This approach can be considered to treat fever or to induce HT. Prospective study comparing safety and efficacy versus other cooling measures should be considered.
Keywords: Brain injury, Hypothermia, Fever, Pediatric, Cold saline
INTRODUCTION
Fever is common after acute brain injury causing increased oxygen consumption, release of excitatory neurotransmitters, injury to the blood brain barrier, increased neuronal cell death and unfavorable neurological outcome in a variety of experimental models of brain injury 1,2, 3.
Fever in adults with cardiac arrest and stroke, children with traumatic brain injury (TBI), and neonates with birth asphyxia is independently associated with worse outcome versus patients who are normothermic 4–6. In children, fever is common in the first 24 hours after resuscitation from cardiac arrest 7, 8.
Current American Heart Association guidelines recommend avoidance of fever and the consideration of therapeutic hypothermia (HT) for children remaining comatose after cardiac arrest but there are no recommendations for how to monitor or manipulate temperature 9, 10. Avoidance of fever is also recommended in the pediatric TBI guidelines but again without treatment recommendations if fever occurs 11.
Therapeutic HT (target temperature 32–34°C) is used clinically and is being tested as a neuroprotectant in randomized, clinical trials after cardiac arrest and TBI in our pediatric ICU 12. Neuroprotection is best achieved by rapid achievement of target temperature 13, 14. Previous experience has shown that induction of HT can be slow when using surface cooling in children with brain injury 15. Optimized surface cooling devices or catheter-based cooling systems are not available for children, and intravenous cold saline is an attractive alternative method as studies using intravenous cold saline in adults have a good safety profile and efficacy 16–18.
We describe our center’s experience with the use of intravenous cold saline for temperature control in pediatric neurocritical care. We hypothesized that intravenous cold saline would be a relatively safe and effective method to decrease temperature promptly in children with acute brain injury.
MATERIALS AND METHODS
Design and Setting
This study was approved by the University of Pittsburgh institutional review board. We performed a retrospective chart review of subjects admitted to the pediatric ICU at the Children’s Hospital of Pittsburgh between June 1 and August 31, 2009.
Inclusion and Exclusion Criteria
We studied children between 1 week and 17 years of age admitted with the following diagnoses: TBI, cardiac arrest, stroke (hemorrhagic and ischemic), status epilepticus, and central nervous system (CNS) infection. Subjects were identified by searching ICU pharmacy records and a neurocritical care service database for use of cold saline. Two subjects received cold saline on two separate occasions in the same admission, and both interventions were recorded.
Data Collection
Medical record review was used for data collection. Demographical data including age, weight, and sex were recorded. Physiological data (temperature, heart rate, and mean arterial blood pressure, central venous pressure, weight) were recorded one hour pre and post cold saline infusion along with details of cold saline infusion (volume infused, method of infusion). Laboratory (arterial blood gas, electrolytes, blood counts, coagulation studies) and ventilator settings pre and post cold saline infusion were recorded as close to the time of infusion as possible. Hourly rectal or esophageal temperatures were measured on most patients, but method of temperature measurement was not uniform across subjects.
Fever and HT induction
Subject temperatures >38°C provoke a search for an etiology and an effort to actively reduce the temperature. Treatment of fever in children with acute brain injury in our ICU includes one or a combination of the following methods: cooling blanket (Cincinnati Sub-Zero Plastipad ®) positioned under the patient and controlled by an automated cooling system (Gaymar Medi-Therm III ®) set to the target temperature, ice packets, bath and fan, lowering of the room and ventilator humidifier thermostat, acetaminophen, and intravenous cold saline. Cold saline is typically infused over 10–20 minutes. Analgesics, sedatives, and neuromuscular blockade medications were used at the discretion of the attending physician. One liter saline bags are stored in two areas of our ICU in Pyxis® MedStation® system at 4°C and their use is monitored by the hospital pharmacy. The 5 subjects who received iced saline for induction of therapeutic HT after cardiac arrest were cooled as part of their post-resuscitation clinical care. Up to 40% of children have temperatures at or below target temperature (32–34°C) post-resuscitation, which has been taken into consideration in our protocol which has been published elsewhere 8, 12.
Outcome Measures
The primary outcomes were logistics related to the application of cold saline and changes in physiological and laboratory parameters pre and post cold saline infusion.
Data analysis
Data were analyzed for differences pre- and post-cold saline infusion using paired t-test. All p-values were two-sided. Missing data were not imputed. Data are presented as mean ± standard deviation (SD). Data analysis was performed using Stata software, version 10 (College Station, Texas).
RESULTS
In the 3 month study period, 18 children received cold saline for a total of 20 infusions. Cold saline was used most frequently in children with TBI or cardiac arrest (Table 1). All cardiac arrests occurred outside the hospital. The youngest child was 18 months of age and the smallest child was 12 kg in body weight.
Table 1.
Subject information.
| n=18 subjects | |
|---|---|
|
| |
| Age, yrs (mean ± SD) | 9.5 ± 4.8 |
|
| |
| Gender, male/female | 7/11 |
|
| |
| Weight, kg | 33.9 ± 21.2 |
|
| |
| Etiology of brain injury | |
| TBI | 8 |
| Cardiac arrest | 6 |
| Hemorrhagic stroke | 2 |
| Ischemic stroke | 1 |
| Status epilepticus | 1 |
TBI, traumatic brain injury
Details of cold saline infusion
Cold saline infusions were prescribed for fever reduction (n=15) and induction of therapeutic HT (n=5). Mean infusion volume was 18.2 ± 10.1 cc/kg cold saline per dose (16.4 ± 10.6 cc/kg for fever group and 23.7 ± 6.8 cc/kg for HT induction, p=n.s.). Peripheral or central venous catheters were used (Table 2). Adjunctive cooling measures such as cooling blanket and ice packs were simultaneously applied in 75% of the subjects. One child placed on ECMO received cold saline infusion after initially being warmed from a presenting temperature of 28°C to 34.6°C. The ECMO circuit’s return blood temperature read 33°C at the time.
Table 2.
Cold saline infusion details.
| n=20 infusions | |
|---|---|
|
| |
| Indication, n | |
| Fever | 15 |
| Induced hypothermia | 5 |
|
| |
| Infusion volume, cc/kg | 18.2 ± 10.1 |
|
| |
| Infusion site, n | |
| CVC femoral | 10 |
| CVC jugular | 3 |
| Peripheral | 4 |
| Unspecified | 3 |
|
| |
| Adjunct cooling measures, n | |
|
| |
| Cooling blanket | 13 |
| Ice packs | 3 |
| ECMO circuit | 1 |
|
| |
| Neuromuscular blockade, n | 15 |
CVC, central venous catheter; ECMO, extracorporeal membrane oxygenation
Physiological variables
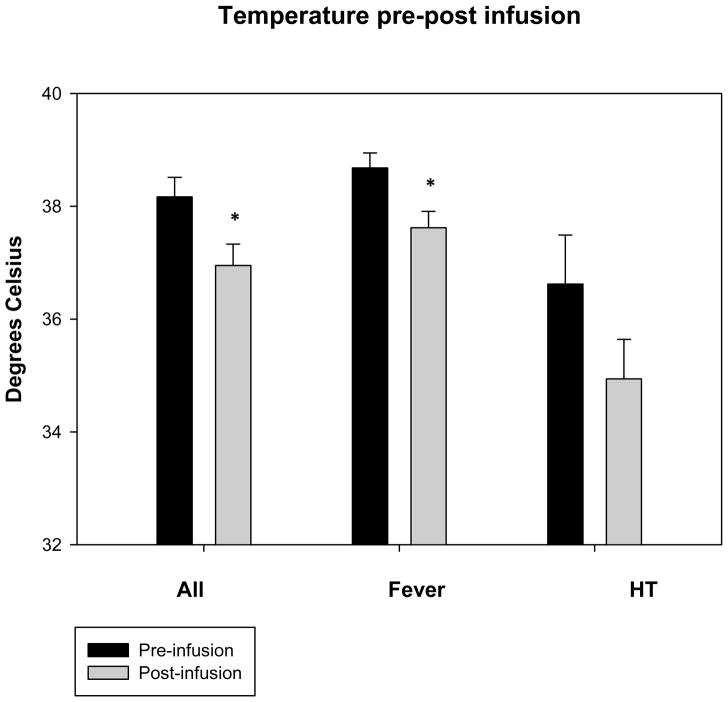
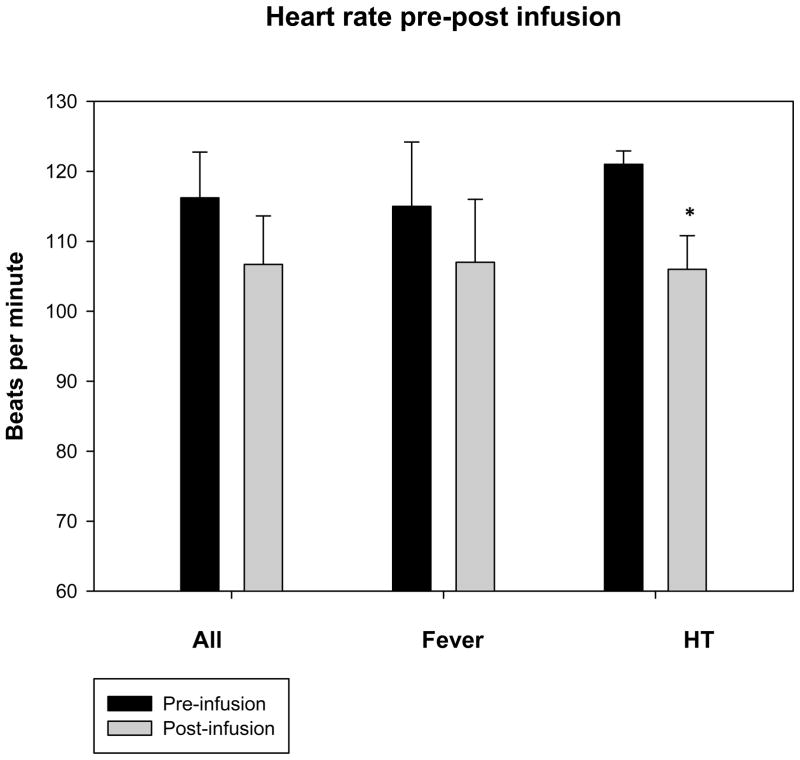
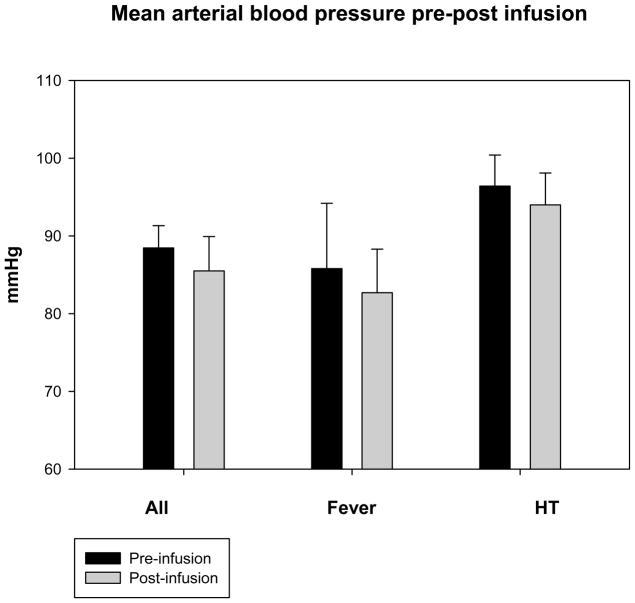
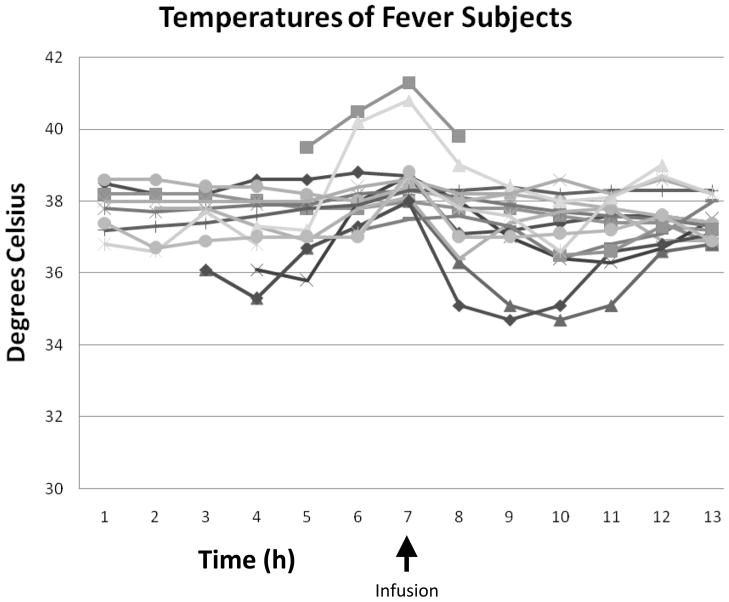
Mean temperatures 1 hour post-infusion were decreased versus baseline overall from 38.2 ± 1.6°C to 36.9 ± 1.6°C, p < .05) (Table 3). Mean temperatures in the fever group decreased from 38.7 ± 1.0°C to 37.6 ± 1.1°C (p < .05) and in the HT group from 36.6 ± 1.9°C to 34.9 ± 1.6°C (p = .1)(Figures 1a, b, and c) (Figures 2a and b). Cold saline infusion resulted in 8/15 children with fever and 2/5 children with HT having temperature reductions to < 38°C and < 34°C, respectively, after completion of the infusion. Median time from ICU admission to target temperature for the HT group was 8 hours (range 0–12 hours). Heart rate was significantly decreased post-infusion among children undergoing HT but not children with fever (115 ± 34 bpm to 107 ± 36 bpm, p=.2 for fever and 121 ± 4 bpm to 106 ± 11 bpm, p < .05 for HT), and mean arterial blood pressures were unaffected (86 ± 13 mmHg to 83 ± 22 mmHg for fever and 96 ± 9 mmHg to 94 ± 9 mmHg for HT). Central venous pressure was unchanged after cold saline infusion (9.8 ± 4.0 mmHg to 11.3 ± 6.8 mmHg). In children with TBI and invasive monitoring, intracranial pressure (ICP) (15 ± 7 mmHg to 13 ± 7 mmHg) and cerebral perfusion pressure (CPP) (73 ± 20 mmHg to 72 ± 18 mmHg) were unaffected by cold saline infusion. There was no difference in PaO2 (269 ± 164 mmHg to 271 ± 159 mmHg), FiO2 (.73 ± .24 to .70 ± .24), or mean airway pressure (12.4 ± 5.2 mmHg to 12.8 ± 5.2 mmHg) pre- and post-infusion.
Table 3.
Physiological effects 1 hour pre and post iced saline infusion.
| N | 1 hour Pre-infusion (Mean ± SD) | 1 hour Post-infusion (Mean ± SD) | p-value | |
|---|---|---|---|---|
|
| ||||
| Temperature, °C | ||||
| Total | 20 | 38.2 ± 1.6 | 37.0 ± 1.7 | < .05 |
| Fever | 15 | 38.7 ± 1.0 | 37.6 ± 1.1 | < .05 |
| Induced hypothermia | 5 | 36.6 ± 1.9 | 34.9 ± 1.6 | .11 |
|
| ||||
| Heart rate, bpm | ||||
| Total | 20 | 116 ± 29 | 107 ± 31 | .06 |
| Fever | 15 | 115 ± 34 | 107 ± 36 | .23 |
| Induced hypothermia | 5 | 121 ± 4 | 106 ± 11 | < .05 |
|
| ||||
| Mean arterial blood pressure, mmHg | ||||
| Total | 20 | 88.5 ± 12.8 | 85.5 ± 19.8 | .33 |
| Fever | 15 | 85.8 ± 13.1 | 82.7 ± 21.7 | .40 |
| Induced hypothermia | 5 | 96.4 ± 8.9 | 94.0 ± 9.3 | .67 |
|
| ||||
| Central venous pressure, mmHg | 13 | 9.8 ± 4.0 | 11.3 ± 6.8 | .42 |
|
| ||||
| Intracranial pressure, mmHg | 10 | 15 ± 7 | 13 ± 7 | .28 |
|
| ||||
| Cerebral perfusion pressure, mmHg | 10 | 73 ± 20 | 72 ± 18 | .44 |
|
| ||||
| PaO2, mmHg | 16 | 269 ± 164 | 271 ± 159 | .94 |
|
| ||||
| Fraction of inspired O2 | 19 | .73 ± .24 | .70 ± .24 | .56 |
|
| ||||
| PO2/FiO2 ratio | 16 | 361 ± 149 | 363 ± 144 | .91 |
|
| ||||
| Mean airway pressure, mmHg | 17 | 12.4 ± 5.2 | 12.8 ± 5.2 | .34 |
|
| ||||
| Sodium, mmol/L | 20 | 140 ± 5 | 142 ± 6 | < .05 |
|
| ||||
| Glucose, mg/dl | 20 | 154 ± 46 | 158 ± 75 | .63 |
|
| ||||
| Hemoglobin, g/dl | 20 | 11.2 ± 1.7 | 10.6 ± 1.9 | .25 |
|
| ||||
| Hematocrit, % | 20 | 32.7 ± 5.4 | 30.9 ± 5.7 | .25 |
|
| ||||
| Platelet (x 109/L) | 20 | 232 ± 93 | 208 ± 109 | .18 |
|
| ||||
| International normalized ratio | 15 | 1.3 ± 0.3 | 1.4 ± 0.4 | < .05 |
|
| ||||
| Partial thromboelastin time, sec | 15 | 36.9 ± 24 | 37.2 ± 9.5 | .96 |
Figure 1.
Figures 1a, b, and c. Temperature, heart rate, and mean arterial blood pressure 1 hour pre and post-cold saline infusion.
*p < .05 post-infusion versus pre-infusion
Error bars are reported as standard error
*p < .05 post-infusion versus pre-infusion
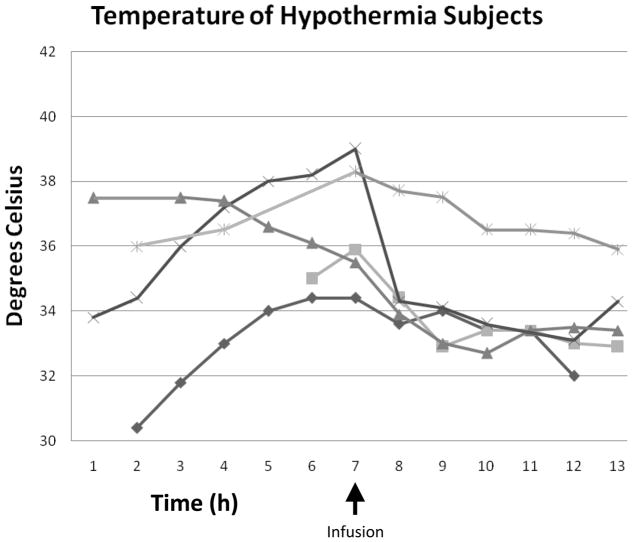
Figure 2.
Figures 2a and b. Expanded temperature curves for subjects with hypothermia and fever.
There was no difference in the rate of temperature decrease over one hour between the group that received adjunctive cooling measures and the group that did not (1.28°C versus 0.95°C, p=.6). All but 5 subjects received neuromuscular blockade during the infusion. There was no difference in the rate of temperature decrease between subjects weighing less than 20 kg (n=8) versus subjects weighing more than 20 kg (n=12) (0.86°C versus 1.45°C, p=.3).
Laboratory values
Serum sodium (140 ± 5 mmol/L to 142 ± 6 mmol/L) and International normalized ratio (INR) (1.3 ± 0.3 to 1.4 ± 0.4 were increased post-infusion (p < .05). Glucose (154 ± 46 mg/dl to 158 ± 75 mg/dl), hemoglobin (11.2 ± 1.7 g/dl to 10.6 ± 1.9 g/dl), hematocrit (32.7 ± 5.4 % to 30.9 ± 5.7 %), and platelet (232 ± 93 × 109/L to 208 ± 109 × 109/L) measurements were unaffected by cold saline infusion.
DISCUSSION
We found that a mean infusion of 18 cc/kg cold saline decreased core temperature by ~1.0°C in children with acute brain injury who were treated for fever and ~1.7°C in children treated to induce therapeutic HT. Cold saline infusion in this study represents a tool to promptly treat fever and initiate induction of HT, but we did not study maintenance of target temperature. During induction of HT, it may be that additional infusions or other methods are needed to continue cooling if the reduction was still above the target temperature. Cold saline was not bolused, rather infused over 10–15 minutes.
The detrimental effects of fever on the outcome of brain injured patients and the importance of achieving therapeutic HT quickly for maximum effect are well documented 4–6, 13. The need for improved methods for the safe and rapid reduction and maintenance of patient temperatures has led to the investigation of new devices such as advanced surface cooling systems, intravascular catheters, and even nasal cooling approaches, but these are similarly unavailable in younger infants and children.
Surface cooling with ice packs and cooling blankets can be slow to decrease temperature in children and adults 15, 19, 20 but may be more effective in infants due to their large surface area to volume ratio 21. Cold intravenous fluid can be readily available and is relatively cheap. Similar to our findings, prospective pilot studies using ~20–30 ml/kg cold intravenous fluids in adults surviving cardiac arrest decreased temperatures by 1.4–1.8°C over 30–60 minutes. All subjects were given sedation and neuromuscular blockade to prevent agitation and shivering, and fluid composition was lactated Ringer’s solution or normal saline 16, 18, 22. Recently a randomized, controlled trial comparing standard methods of cooling with or without intravenous cold saline in children less than 22 months of age with congenital heart disease for treatment of junctional ectopic tachycardia showed that 10–20 cc/kg cold normal saline decreased temperature by 4.2°C per hour 23. One child was slowly warmed after overcooling beyond the 32–34°C target temperature. Subjects received continuous infusions of analgesics and sedatives and it was not described as to how many were given neuromuscular blocking agents. Additionally, a case report described success using cold saline infusion (30 cc/kg) to induce therapeutic HT in a 12 year old child post–resuscitation from cardiac arrest after the combination of a cooling blanket with sedation and neuromuscular blockade failed 24. The median time from ICU admission to target temperature in our five patients was relatively long overall due to one child in whom therapeutic HT was initiated later upon decline in mental status.
Decreases in heart rate and bradycardia (variously defined) are well known consequences of decreased temperature. We found that while fever and HT groups had decreased heart rates after cold saline infusion, only the latter achieved statistical significance. No subjects required clinical interventions for bradycardia. There were no differences in other hemodynamic and oxygenation parameters in our subjects but other studies have found changes after infusion including increased central venous pressure, increased blood pressure, decreased PO2 and PO2/FiO2 ratios, and clinical evidence of pulmonary edema 16, 18, 22.
Other complications such as changes in sodium homeostasis or alterations in coagulation are important considerations when instituting an iced saline infusion. In our series, we found short term alterations in serum sodium and INR which did not have clear evidence of clinical consequences. Normal saline is our standard fluid for volume expansion because it is isotonic and is less likely to decrease serum sodium concentration than other fluids, which might be detrimental to patients with brain injury who are already at risk of brain edema and sodium dysregulation. Other groups have found derangements in coagulation, electrolyte concentrations, and decreased in platelet and hematocrit count after cold fluid administration. We did not record other boluses or fluid infusions the subjects received during the cold saline infusion period.
Neuromuscular blockade and sedation were used were used during 15/20 cold saline infusions to facilitate effective temperature reduction though prevention of shivering. We and other groups have successfully used peripheral and central venous intravenous catheters to deliver the cold saline. Catheters in large caliber peripheral veins (i.e., antecubital) are often placed for initial resuscitation and have less tubing length, which lessens the chance of the fluid temperature increasing prior to reaching the vasculature of the patient.
Study limitations
The purpose of this observational study was to describe our experience using cold saline infusion to decrease temperatures in children with acute brain injury and help care providers in anticipating and preventing adverse events when using this approach. Data collection was limited by available documentation. Rectal temperature probes are routinely used in our ICU, largely because they are simple to use. However, they may less accurately measure core temperature than other methods 25–30. Laboratory values were measured at room temperature regardless of subject temperature, which may have an affected the results.
CONCLUSIONS
Cold saline was an effective method of promptly decreasing temperature in children with acute brain injury who have fever or are undergoing induction of therapeutic HT. Prospective study of the optimal fluid volume, composition, and concurrent adjunctive measures to best achieve temperature targets are needed.
Acknowledgments
1K23NS065132-01; 5K12HD047349-02; NS052478; The Laerdal Foundation.
Special thank you to Christine Modery, R.Ph, Lead Pharmacist, Children’s Hospital of Pittsburgh of UPMC for assistance in data collection and Marci Provins for assistance with the submission of this manuscript.
References
- 1.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008 Jun 7;371(9628):1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 2.Hickey RW, Kochanek PM, Ferimer H, Alexander HL, Garman RH, Graham SH. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Crit Care Med. 2003 Feb;31(2):531–535. doi: 10.1097/01.CCM.0000050323.84293.11. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke. 1996 Dec;27(12):2274–2280. doi: 10.1161/01.str.27.12.2274. discussion 2281. [DOI] [PubMed] [Google Scholar]
- 4.Laptook A, Tyson J, Shankaran S, et al. Elevated temperature after hypoxic-ischemic encephalopathy: risk factor for adverse outcomes. Pediatrics. 2008 Sep;122(3):491–499. doi: 10.1542/peds.2007-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer DM, Funk SE, Reaven NL, Ouzounelli M, Uman GC. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008 Nov;39(11):3029–3035. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 6.Natale JE, Joseph JG, Helfaer MA, Shaffner DH. Early hyperthermia after traumatic brain injury in children: risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000 Jul;28(7):2608–2615. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- 7.Hickey RW, Kochanek PM, Ferimer H, Graham SH, Safar P. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000 Jul;106(1 Pt 1):118–122. doi: 10.1542/peds.106.1.118. [DOI] [PubMed] [Google Scholar]
- 8.Fink EL, Clark RSB, Kochanek PM, Watson RS. Mild therapeutic hypothermia in a cohort of patients surviving cardiac arrest. Pediatr Crit Care Med. 2010;11(1):66–74. doi: 10.1097/PCC.0b013e3181c58237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association AH. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric advanced life support. Pediatrics. 2006 May;117(5):e1005–1028. doi: 10.1542/peds.2006-0346. [DOI] [PubMed] [Google Scholar]
- 10.Neumar RW, Nolan JP, Adrie C, et al. Post-Cardiac Arrest Syndrome. Epidemiology, Pathophysiology, Treatment, and Prognostication A Consensus Statement From the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008 Oct 23; doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 11.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 14. The role of temperature control following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003 Jul;4(3 Suppl):S53–55. [PubMed] [Google Scholar]
- 12.Fink EL, Kochanek PM, Clark RS, Bell MJ. How I Cool Children in Neurocritical Care. Neurocrit Care. 2010 Feb 10; doi: 10.1007/s12028-010-9334-5. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993 Sep;21(9):1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Busto R, Dietrich WD, Globus MY, Ginsberg MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. 1989 Jul 3;101(3):299–304. doi: 10.1016/0304-3940(89)90549-1. [DOI] [PubMed] [Google Scholar]
- 15.Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005 Apr;56(4):740–754. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740–754. [DOI] [PubMed] [Google Scholar]
- 16.Kim F, Olsufka M, Carlbom D, et al. Pilot study of rapid infusion of 2 L of 4 degrees C normal saline for induction of mild hypothermia in hospitalized, comatose survivors of out-of-hospital cardiac arrest. Circulation. 2005 Aug 2;112(5):715–719. doi: 10.1161/CIRCULATIONAHA.105.544528. [DOI] [PubMed] [Google Scholar]
- 17.Kollmar R, Schellinger PD, Steigleder T, Kohrmann M, Schwab S. Ice-cold saline for the induction of mild hypothermia in patients with acute ischemic stroke: a pilot study. Stroke. 2009 May;40(5):1907–1909. doi: 10.1161/STROKEAHA.108.530410. [DOI] [PubMed] [Google Scholar]
- 18.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56(1):9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 19.Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008 Jun 5;358(23):2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 20.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002 Feb 21;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 21.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 22.Kliegel A, Losert H, Sterz F, et al. Cold simple intravenous infusions preceding special endovascular cooling for faster induction of mild hypothermia after cardiac arrest--a feasibility study. Resuscitation. 2005 Mar;64(3):347–351. doi: 10.1016/j.resuscitation.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Kelly BP, Gajarski RJ, Ohye RG, Charpie JR. Intravenous induction of therapeutic hypothermia in the management of junctional ectopic tachycardia: a pilot study. Pediatr Cardiol. Jan;31(1):11–17. doi: 10.1007/s00246-009-9526-y. [DOI] [PubMed] [Google Scholar]
- 24.Kim YM, Jeong JH, Kyong YY, et al. Use of cold intravenous fluid to induce hypothermia in a comatose child after cardiac arrest due to a lightning strike. Resuscitation. 2008 Nov;79(2):336–338. doi: 10.1016/j.resuscitation.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Maxton FJ, Justin L, Gillies D. Estimating core temperature in infants and children after cardiac surgery: a comparison of six methods. J Adv Nurs. 2004 Jan;45(2):214–222. doi: 10.1046/j.1365-2648.2003.02883.x. [DOI] [PubMed] [Google Scholar]
- 26.Robinson JL, Seal RF, Spady DW, Joffres MR. Comparison of esophageal, rectal, axillary, bladder, tympanic, and pulmonary artery temperatures in children. J Pediatr. 1998 Oct;133(4):553–556. doi: 10.1016/s0022-3476(98)70067-8. [DOI] [PubMed] [Google Scholar]
- 27.Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperature exceeds systemic temperature in head-injured patients. Crit Care Med. 1998 Mar;26(3):562–567. doi: 10.1097/00003246-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 28.Henker RA, Brown SD, Marion DW. Comparison of brain temperature with bladder and rectal temperatures in adults with severe head injury. Neurosurgery. 1998 May;42(5):1071–1075. doi: 10.1097/00006123-199805000-00071. [DOI] [PubMed] [Google Scholar]
- 29.Stone JG, Young WL, Smith CR, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995 Feb;82(2):344–351. doi: 10.1097/00000542-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Crowder CM, Tempelhoff R, Theard MA, Cheng MA, Todorov A, Dacey RG., Jr Jugular bulb temperature: comparison with brain surface and core temperatures in neurosurgical patients during mild hypothermia. J Neurosurg. 1996 Jul;85(1):98–103. doi: 10.3171/jns.1996.85.1.0098. [DOI] [PubMed] [Google Scholar]