Abstract
Patients with cystic fibrosis (CF) suffer from chronic lung infection and inflammation leading to respiratory failure. Vitamin D deficiency is common in patients with CF, and correction of vitamin D deficiency may improve innate immunity and reduce inflammation in patients with CF. We conducted a double-blinded, placebo-controlled, randomized clinical trial of high-dose vitamin D to assess the impact of vitamin D therapy on antimicrobial peptide concentrations and markers of inflammation. We randomized 30 adults with CF hospitalized with a pulmonary exacerbation to 250 000 IU of cholecalciferol or placebo, and evaluated changes in plasma concentrations of inflammatory markers and the antimicrobial peptide LL-37 at baseline and 12 weeks post intervention. In the vitamin D group, there was a 50.4% reduction in tumor necrosis factor-α (TNF-α) at 12 weeks (P<0.01), and there was a trend for a 64.5% reduction in interleukin-6 (IL-6) (P = 0.09). There were no significant changes in IL-1β, IL-8, IL-10, IL-18BP and NGAL (neutrophil gelatinase-associated lipocalin). We conclude that a large bolus dose of vitamin D is associated with reductions in two inflammatory cytokines, IL-6 and TNF-α. This study supports the concept that vitamin D may help regulate inflammation in CF, and that further research is needed to elucidate the potential mechanisms involved and the impact on clinical outcomes.
Keywords: cystic fibrosis, vitamin D, inflammation, tumor necrosis factor-α, interleukin-6
INTRODUCTION
Cystic fibrosis (CF) is the most common life-shortening, inherited disease among Caucasians in the United States. Chronic pulmonary infection and inflammation lead to respiratory failure, the most common cause of morbidity and mortality in CF.1 Pulmonary inflammation is associated with poor outcomes, and antiinflammatory therapies have had limited extended use owing to adverse side effects.2
Vitamin D insufficiency has been estimated to affect up to 90% of the adults with CF.3 Vitamin D has been shown to suppress in vitro and in vivo the production of proinflammatory cytokines, such as interleukin-6 (IL-6), IL-8 and tumor necrosis factor-α (TNF-α), as well as increase the production of the antimicrobial peptide (AMP), LL-37, from CF respiratory epithelial cells.4–6
Increased production of LL-37 and decreased production of inflammatory messengers may reduce pulmonary complications in CF and improve clinical outcomes. The purpose of this communication is to describe the impact of a vitamin D repletion strategy on markers of inflammation and LL-37 in adults with CF during pulmonary exacerbation.
MATERIALS AND METHODS
Study design
As described previously, adult CF patients of the Emory University CF Center hospitalized for treatment of a pulmonary exacerbation were eligible. After consent was obtained, subjects were randomized to either 250 000 IU cholecalciferol or placebo. Blood samples were obtained at baseline, 1 week and 12 weeks.7
Analytical methods
Serum IL-1β, IL-10, IL-18-binding protein (IL-18BP) and plasma IL-6, IL-8 and TNF-α were assessed by DuoSet ELISA (R&D Systems, Minneapolis, MN, USA), and plasma LL-37 by ELISA (Hycult Biotech, Uden, The Netherlands). All cytokine measurements were recorded in duplicate. Pancreatic insufficiency was assessed by the requirement for physician-prescribed pancreatic enzymes.
Statistical analysis
All statistical analyses were completed using SAS 9.3 (SAS Institute, Cary, NC, USA). This was a secondary analysis; the original study was designed to provide 90% power to detect a 10 ng/ml difference in serum 25(OH)D between the groups at a significance level of α = 0.05. Variables were assessed for normality, and the following variables were transformed because of their right-skewed distributions before inclusion in statistical analyses: IL-1β, IL-6, IL-10 and LL-37. Paired t-tests were applied to assess within-group changes in mean serum/plasma concentrations from baseline. Mixed-effects linear regression models with a random intercept to account for within-subject clustering of data were used to evaluate the difference in mean serum/plasma concentrations of the vitamin D and placebo group at each time point, based on repeated measurements of IL-1β, IL-6, IL-8, IL-10, IL-18BP, TNF-α, NGAL (neutrophil gelatinase-associated lipocalin) and LL-37. The following confounders were assessed: age, BMI, sex, race, CFRD (cystic fibrosis-related diabetes), pancreatic insufficiency, lung function measured as baseline forced expiratory volume in one second, % of predicted, and vitamin D intake.
The confounders found to significantly impact the random intercept model were forced expiratory volume in one second, % of predicted, CF-related diabetes, pancreatic insufficiency and age. The variables whose mean values were significantly different between treatment groups at α<0.10, were adjusted for these confounders.
RESULTS
Baseline demographics have been reported elsewhere.7 There were no statistically significant differences in the demographics of the two groups. There were also no significant differences between the groups in the qualitative microbiological analysis of sputum specimens at the time of hospitalization or the types of antibiotic therapy prescribed during hospitalization.7 As previously reported, in the vitamin D group, serum concentrations of 25(OH)D increased at 1 week and 12 weeks, that is, 27.5±13 and 6.2±11 ng/ml, respectively (P<0.001 and 0.06); there was no significant change in the placebo group.7
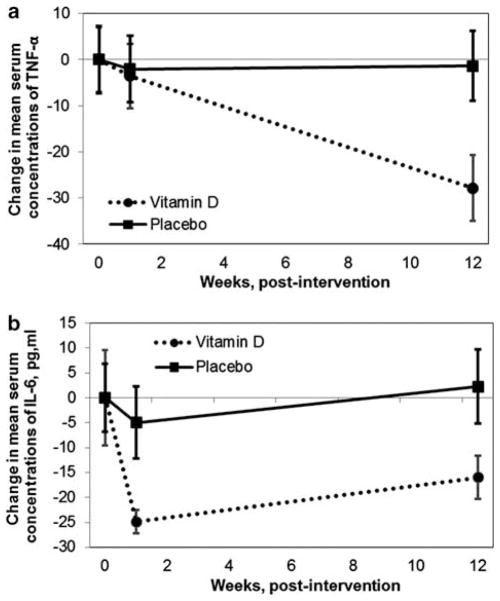
At 12 weeks, mean serum TNF-α concentrations in the vitamin D group were significantly less than those in the placebo group (Table 1). Compared with baseline, TNF-α decreased 3.54±8.3 and 27.62±8.4 pg/ml at 1 week and 12 weeks, respectively (P = 0.60, 0.0002); when adjusted for confounders, the change in TNF-α remained statistically significant. In the placebo group, there was no significant change in TNF-α from baseline at 1 week or 12 weeks (Figure 1).
Table 1.
Comparison of mean serum and plasma concentrations of inflammatory markers
| Time | Vitamin D3a | Placeboa | P-valueb | P-valuec |
|---|---|---|---|---|
| IL-1βd, pg/ml | ||||
| Baseline | 1.95 (1.32) | 2.44 (1.17) | 0.48 | |
| 1 week | 1.27 (0.74) | 2.76 (1.08) | 0.18 | |
| 12 weeks | 2.47 (1.73) | 4.74 (3.69) | 0.15 | |
| IL-6d, pg/ml | ||||
| Baseline | 38.63 (9.60) | 34.38 (6.76) | 0.85 | 0.50 |
| 1 week | 13.72** (2.35) | 29.41 (7.20) | 0.09 | 0.09 |
| 12 weeks | 22.65 (4.34) | 36.62 (7.46) | 0.21 | 0.11 |
| IL-8, pg/ml | ||||
| Baseline | 34.64 (1.07) | 41.40 (2.79) | 0.03 | 0.05 |
| 1 week | 34.51 (0.84) | 40.24 (1.96) | 0.07 | 0.13 |
| 12 weeks | 39.59* (1.88) | 48.54** (4.28) | 0.003 | 0.01 |
| IL-10d, pg/ml | ||||
| Baseline | 42.03 (21.17) | 28.54 (5.56) | 0.99 | |
| 1 week | 30.01 (11.22) | 29.62 (5.80) | 0.73 | |
| 12 weeks | 49.92 (28.89) | 37.24* (11.22) | 0.40 | |
| IL-18-1BP, pg/ml | ||||
| Baseline | 2.64 (0.16) | 2.77 (0.18) | 0.57 | |
| 1 week | 2.65 (0.14) | 2.80 (0.18) | 0.57 | |
| 12 weeks | 2.50 (0.12) | 2.92 (0.20) | 0.11 | |
| TNF-α | ||||
| Baseline | 55.67 (8.18) | 69.52 (7.50) | 0.18 | 0.41 |
| 1 week | 52.13 (5.62) | 67.48 (7.92) | 0.13 | 0.34 |
| 12 weeks | 27.62** (5.82) | 68.54 (8.61) | 0.0003 | 0.0049 |
| NGAL | ||||
| Baseline | 79.65 (1.71) | 78.56 (2.18) | 0.77 | |
| 1 week | 76.11 (2.84) | 76.85 (1.81) | 0.88 | |
| 12 weeks | 71.64* (3.13) | 74.95 (4.01) | 0.39 | |
| LL-37d | ||||
| Baseline | 73.10 (7.40) | 192.60 (46.50) | 0.003 | |
| 1 week | 128.10 (33.80) | 174.60 (28.40) | 0.17 | |
| 12 weeks | 142.60 (45.90) | 135.30 (28.80) | 0.67 | |
Abbreviations: IL, interleukin; BP, binding protein; TNF-α, tumor necrosis factor-α; NGAL, neutrophil gelatinase-associated lipocalin. Mean concentrations at baseline, 1 week and 12 weeks in CF adults hospitalized with a pulmonary exacerbation randomized to either a single oral 250 000 IU dose of cholecalciferol or placebo.
Within-group comparison, P-value <0.05, compared with baseline.
Within-group comparison, P-value <0.01, compared with baseline.
Unadjusted mean from mixed model (s.e.m).
Unadjusted P-value comparing means from mixed model.
Adjusted for forced expiratory volume in one second, % of predicted, CFRD, pancreatic insufficiency and age.
Variable was log-transformed before inclusion in model.
Figure 1.
Mean change in plasma concentrations of TNF-α (tumor necrosis factor-α) and IL-6 (interleukin-6) at baseline, 1 week and 12 weeks in CF (cystic fibrosis) adults randomized to 250 000 IU cholecalciferol or placebo. (a) Mean change in TNF-α plasma concentrations. In the vitamin D group, TNF-α decreased 3.56 and 27.83 pg/ml at 1 week and 12 weeks (P = 0.6, 0.0002); TNF-α remained unchanged in the placebo. (SEM bars). (b) Mean change in plasma concentrations of IL-6. In the vitamin D group, IL-6 decreased 12.39 and 5.16 pg/ml at 1 week and 12 weeks (P = 0.004, 0.35); IL-6 remained unchanged in the placebo (SEM bars).
At 1 week, there was a trend for decreased plasma IL-6 concentrations in the vitamin D group compared with the placebo group (Table 1). Compared with baseline, mean plasma IL-6 concentrations decreased significantly in the vitamin D group: 24.91±8.4 pg/ml (P = 0.004) at 1 week and 18.84±11.8 pg/ml (P = 0.4) at 12 weeks; there was no significant change from baseline in the placebo group at 1 week or 12 weeks (Figure 1).
Comparison of the mean concentrations of markers of inflammation and LL-37 are summarized in Table 1. In IL-1β, IL-10, IL18BP, NGAL and LL-37, there were no significant differences in the mean values between groups at any time point. Both groups exhibited a significant increase in IL-8 concentrations from baseline to 12 weeks. Therefore, the change in IL-8 concentration (baseline to 12-weeks) was compared between the groups. This change did not differ significantly between the groups; the increase in IL-8 concentration in the placebo group was 17.2% and in the vitamin D group was 14.3% (P = 0.2)
DISCUSSION
Inflammation is an important contributor to CF disease progression, and anti-inflammatory therapies may improve clinical outcomes.2 In CF, increased inflammation has been associated with reduced lung function.1 The hormonal form of vitamin D, 1,25-dihydroxyvitamin D has been shown to increase the production of the AMP, LL-37 from cultured CF respiratory epithelial cells and reduce concentrations of inflammatory markers.4,6 Anti-inflammatory therapies have been shown to improve lung function and slow disease progression in CF. However, anti-inflammatories such as corticosteroids have undesirable side-effect profiles that vitamin D does not have.2 Therefore, vitamin D supplementation is an attractive method that may reduce the bacterial burden and inflammation in the CF lung and improve lung function. Given the high prevalence of insufficiency, vitamin D should be evaluated for its potential to increase LL-37 and reduce inflammation.3 This is the first evaluation of multiple markers of inflammation, and the AMP LL-37 in response to a marked increase in 25(OH)D produced by high-dose vitamin D supplementation during a CF exacerbation. The data we present indicate that vitamin D supplementation may reduce systemic concentrations of IL-6 and TNF-α.
Vitamin D may reduce transcription of inflammatory cytokines through modulation of nuclear factor κB and mitogen-activated protein kinase pathways.2,8 Serum 25(OH)D concentrations have been negatively correlated with the proinflammatory marker, IgG, and positively correlated with lung function in cross-sectional studies of CF subjects.9,10 Pretreatment of isolated CF epithelial cells with the hormonal form of vitamin D 1,25(OH)2D, decreases the secretion of IL-8 and IL-6 in response to antigen stimulation.4 1,25(OH)2D has also been shown to increase the production of LL-37, the AMP and hCAP-18 (human cathelicidin antimicrobial protein) mRNA from which it is synthesized.4,6 In this randomized, controlled trial, we have found that increasing vitamin D status in CF subjects may also reduce concentrations of proinflammatory markers.
This study was conducted as a secondary analysis. The objective of the parent study was to evaluate the impact of this single, oral bolus of cholecalciferol on vitamin D status and clinical outcomes.7 The objective of this study was to evaluate serum concentrations of LL-37 and markers of inflammation in response to vitamin D supplementation in adults with CF hospitalized for a pulmonary exacerbation. As a secondary analysis, this study had several limitations; the sample size of this pilot study may not have been powered to evaluate smaller changes in these inflammatory markers. Further, we were unable to completely evaluate other factors that may have impacted the inflammatory outcomes. In addition, our evaluation of changes in systemic markers of inflammation and LL-37 concentrations are only surrogates for possible changes in the respiratory tract.
Vitamin D has the properties of an ideal anti-inflammatory therapy, as it may suppress concentrations of proinflammatory cytokines while supporting innate immune functions, such as AMP synthesis. This pilot study provides baseline data for the design of future studies to assess the impact of vitamin D on pulmonary and systemic inflammation in CF.
Acknowledgments
This work was supported in part by the following grants: PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, grant numbers TL1 RR025010 and K23AR054334, and the CF Foundation.
Footnotes
CONFLICT OF INTEREST
Dr Tangpricha received an unrestricted research grant from BTR, Group (a vitamin D supplement company). The remaining authors declare no conflict of interest.
References
- 1.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 2.Jacquot J, Tabary O, Le Rouzic P, Clement A. Airway epithelial cell inflammatory signalling in cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1703–1715. doi: 10.1016/j.biocel.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Hall WB, Sparks AA, Aris RM. Vitamin D deficiency in cystic fibrosis. Int J Endocrinol. 2010;2010:218691. doi: 10.1155/2010/218691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNally P, Coughlan C, Bergsson G, Doyle M, Taggart C, Adorini L, et al. Vitamin D receptor agonists inhibit pro-inflammatory cytokine production from the respiratory epithelium in cystic fibrosis. J Cyst Fibros. 2011;10:428–434. doi: 10.1016/j.jcf.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D-3. J Cyst Fibros. 2007;6:403–410. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossmann RE, Tangpricha V, Zughaier SM, Liu S. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: a randomized, controlled trial. Dermato-Endocrinology. 2012;4:1–8. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, et al. Treatment and Prevention of Vitamin D Insufficiency in Cystic Fibrosis Patients: Comparative Efficacy of Ergocalciferol, Cholecalciferol, and UV Light. J Clin Endocrinol Metab. 2009;94:2037–2043. doi: 10.1210/jc.2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincikova T, Nilsson K, Moen IE, Karpati F, Fluge G, Hollsing A, et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr. 2011;65:102–109. doi: 10.1038/ejcn.2010.194. [DOI] [PubMed] [Google Scholar]