Abstract
Over the past decade there has been increasing research and clinical interest in the role of exercise therapy/rehabilitation as an adjunct therapy to improve symptom control and management following a cancer diagnosis. More recently, the field of ‘exercise – oncology’ has broadened in scope to investigate whether the benefits extend beyond symptom control to modulate cancer-specific outcomes (i.e., cancer progression and metastasis). Here we review the extant epidemiological evidence examining the association between exercise behavior, functional capacity/exercise capacity, and cancer-specific recurrence and mortality as well as all-cause mortality individuals following a cancer diagnosis. We also evaluate evidence from clinical studies investigating the effects of structured exercise on blood-based biomarkers associated with cancer progression/metastasis as well findings from preclinical investigations examining the effects and molecular mechanisms of exercise in mouse models of cancer. Current gaps in knowledge are also discussed.
Keywords: Exercise, Physical activity, Mouse models, Cancer, Carcinogenesis, Mechanisms
1. Introduction
The health benefits of regular exercise have been recognized for centuries. Indeed, structured exercise training is established as the cornerstone of primary and secondary disease prevention in multiple clinical settings. In contrast, it has not been until the past decade or so that exercise has gained acceptance as a potential adjunct therapy following a cancer diagnosis (Jones and Demark-Wahnefried, 2006; Jones et al., 2010a). To date, approximately, 80 studies have been conducted investigating the effects of structured exercise training in patients following a diagnosis of cancer. Meta-analyses and systematic reviews report that structured exercise training is a safe and well-tolerated therapeutic strategy associated with significant improvements in a broad range of cancer-related toxicities including fatigue, exercise capacity, and physical quality of life (Jones et al., 2011; McNeely et al., 2006; Speck et al., 2010). Based on the extant literature, several national and international organizations have published exercise guidelines for cancer patients both during and following the completion of adjuvant therapy (Hayes et al., 2009; Schmitz et al., 2010).
While the importance of exercise therapy to control and/or mitigate the adverse consequences of cancer therapy are undisputed, there is growing interest in determining whether the benefits extend beyond symptom control to modulate cancer-specific outcomes (i.e., cancer progression and metastasis). Elucidation of the effects and underlying mechanisms will be critical to inform hypothesis-driven clinical trials and ensure the optimal safety and efficacy of exercise in cancer control. Accordingly, over the past several years, exercise–oncology researchers from a wide variety of disciplines have started to investigate the association between exercise behavior, objective measures of exercise capacity/functional capacity, and prognosis following a cancer diagnosis as well as the cellular and molecular mechanisms underlying these associations. The purpose of this paper is to review: (1) the extant epidemiological evidence examining the association between exercise behavior, functional capacity/exercise capacity, and cancer-specific recurrence and mortality as well as all-cause mortality, and (2) studies elucidating the host and cellular mechanisms underlying the exercise–prognosis relationship. In terms of the latter, we evaluate evidence from clinical studies investigating the effects of structured exercise training (‘structured’ exercise is defined as studies testing a specific plan of physical activity designed to improve exercise capacity as opposed to studies designed to increase physical activity) on changes in blood-based biomarkers proposed to mediate the association between exercise behavior/exercise capacity and cancer-specific prognosis.
Further, we also review the evidence from preclinical investigations examining the effects of exercise on tumor progression and metastasis in mouse models of cancer.
2. Search strategy
A comprehensive literature review using PubMed, MEDLINE, Sport Discus, and Cochrane Controlled Trials Register (1966 through January, 2012) was conducted using the following MESH terms and text words: exercise, cardiorespiratory fitness, exercise capacity, cardiopulmonary fitness, functional capacity, oncology, and cancer. Relevant reference lists were also hand-searched. Studies in pediatric patients (<18 years) and adult patients with hematological malignancies were excluded.
3. Relationship between exercise and prognosis: epidemiological evidence
As summarized in Table 1 and 20 epidemiological studies to date have examined the association between self-reported exercise behavior and prognosis following a diagnosis of cancer. (Bertram et al., 2011; Borugian et al., 2004; Chen et al., 2011; Dal Maso et al., 2008; Holick et al., 2008; Holmes et al., 2005; Irwin et al., 2011, 2008b; Jones et al., 2011; Kenfield et al., 2011; Meyerhardt et al., 2006a, 2009a,b, 2006b; Moorman et al., 2011; Morikawa et al., 2011; Pierce et al., 2007; Richman et al., 2011; Ruden et al., 2011; Sternfeld et al., 2009). In brief, the majority of studies were conducted in women with early breast cancer (50%), with fewer conducted in patients with gastrointestinal malignancies (25%) and prostate cancer (10%). One study each was performed in patients with ovarian, non-small cell lung cancer, and primary malignant glioma. Overall, 15 studies (75%) found a significant inverse relationship between exercise and prognosis (cancer-specific or all-cause mortality) with a range of risk reduction between 15% to 67% and 18% to 67% for cancer-specific mortality and all-cause mortality, respectively. A dose–response was reported in 11 (55%); five studies found no significant association between exercise and cancer-specific mortality. Finally, the dose of exercise required for mortality reduction was not uniform either within or between cancer populations ranging from ≥9 metabolic equivalent time (MET)-hours per week of exercise (MET-h wk−1) of moderate intensity exercise (equivalent to approximately 180 min wk−1 of moderate intensity exercise) to ≥27 MET-h wk−1 (equivalent to ≥500 min wk−1 of moderate intensity exercise).
Table 1.
Association between self-reported exercise and cancer-specific mortality and all-cause mortality.
| Tumor type (study) | N | Cohort/setting | Cancer-specific mortality
|
All-cause mortality
|
||||
|---|---|---|---|---|---|---|---|---|
| Risk reduction | Physical activity dose | Dose response | Risk reduction | Physical activity dose | Dose response | |||
| Breast adenocarcinoma (Borugian et al., 2004) | 603 | Breast cancer patients after surgery before the start of adjuvant treatment | 1.0 (multi-variate adjusted relative risk). Exercise was not associated with breast cancer mortality | >1 time/wk | No | n/a | n/a | n/a |
| (Holmes et al., 2005) | 2987 | Stage I–III breast cancer; Nurses’ Health Study | 0.5 (multi-variate adjusted relative risk) | 9–14.9 MET-h/wk | No | 0.56 (multi-variate adjusted relative risk) | 15–23.9 MET-h/wk (p < 0.05) | No |
| (Pierce et al., 2007) | 1490 | Stage I–IIIa breast cancer; Women’s Healthy Eating and Living Study | 0.58 | ≥1320–6420 MET-min/wk | Yes | n/a | n/a | n/a |
| (Holick et al., 2008) | 4482 | Invasive breast cancer free of recurrence >2 years since diagnosis | 0.51 (multi-variate adjusted relative risk) | ≥21 MET-h/wk (p < 0.05) | Yes | 0.44 (multi-variate adjusted relative risk) | ≥21 MET-h/wk (p < 0.05) | Yes |
| (Irwin et al., 2008) | 933 | Breast cancer survivors; Health, Eating, Activity and Lifestyle Study | 0.65 (multi-variate adjusted relative risk) | ≥9 MET-h/wk | Yes | 0.33 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | Yes |
| (Dal Maso et al., 2008) | 1453 | Invasive breast cancer survivors | 0.85 (multi-variate adjusted relative risk) | ≥2 h/wk | n/a | 0.82 (multi-variate adjusted relative risk) | ≥2 h/wk NS | n/a |
| (Sternfeld et al., 2009) | 1970 | Stage I–IIIa breast cancer; Life After Cancer Epidemiology | 0.69 (multi-variate adjusted relative risk) | 3 to <6 h/wk moderate activity | No | 0.66 (multi-variate adjusted relative risk) | 3 to <6 h/wk moderate activity (p < 0.05) | No |
| (Bertram et al., 2011) | 2361 | Stage I–III breast cancer survivors within 4 years, no current chemotherapy; WHEL study | n/a | n/a | n/a | 0.47 (multi-variate adjusted relative risk) | ≥24.7 MET-h/wk NS | Yes |
| (Chen et al., 2011) | 4826 | Stage I–III breast cancer, 6 mo after diagnosis; Shanghai Breast Cancer Survival Study | 0.60 (multi-variate adjusted relative risk) | ≥2 times/wk (p < 0.05) | Yes | 0.70 (multi-variate adjusted relative risk) | ≥2 times/wk (p < 0.05) | Yes |
| (Irwin et al., 2011) | 4643 | Invasive breast cancer survivors; Women’s Health Initiative | 0.60 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | Yes | 0.58 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | Yes |
| Colon/colorecta adenocarcinoma (Meyerhardt et al., 2006) | 832 | Patients with stage III colon cancer | 0.51 (multi-variate adjusted relative risk), cancer recurrence or death from any cause (disease-free survival) | ≥18 MET-h/wk (p < 0.05) | No | 0.37 (multi-variate adjusted relative risk) | ≥27 MET-h/wk (p < 0.05) | Yes |
| (Meyerhardt et al., 2006) | 573 | Women with stage I–III colorectal cancer; Nurses’ Health Study | 0.39 (multi-variate adjusted relative risk) | ≥18 MET-h/wk (p < 0.05) | Yes | 0.43 (multi-variate adjusted relative risk) | ≥18 MET-h/wk (p < 0.05) | Yes |
| (Meyerhardt et al. 2009) | 484 | Men and women with colon cancer; Nurses’ Health Study and Health Professional Follow-up Study | 0.64 (multi-variate adjusted relative risk) | ≥18 MET-h/wk (p < 0.05) | n/a | 0.60 (multi-variate adjusted relative risk) | ≥18 MET-h/wk (p < 0.05) | n/a |
| (Meyerhardt et al. 2009) | 668 | Men with stage I–III colorectal cancer; Nurses’ Health Study | 0.47 (multi-variate adjusted relative risk) | ≥27 MET-h/wk (p < 0.05) | No | 0.59 (multi-variate adjusted relative risk) | ≥27 MET-h/wk (p < 0.05) | No |
| (Morikawa et al., 2011) | 995 | Men and women with stage I–IV colorectal cancer; Nurses’ Health Study and Health Professionals Follow-up Study | In patients with negative CTNNB1 status and stages I–III cancer: 0.33 (multi-variate adjusted relative risk). No difference in survival for CTNNB1 + patients | ≥18 MET-h/wk (p = 0.05) | Yes | n/a | n/a | n/a |
| Prostate adenocarcinoma (Kenfield et al., 2011) | 2705 | Men with prostate cancer from Health Professionals Follow-up Study | 0.65 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | Yes | 0.67 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | Yes |
| (Richman et al., 2011) | 1455 | Men with prostate cancer from CaPSURE Study | 0.43 (for progression) | Walking ≥3 h/wk at ≥3 mph (p < 0.05) | No | n/a | n/a | n/a |
| Lung adenocarcinoma (Jones et al., 2012b,2011) | 118 | Stage IIIb and IV NSCLC | n/a | n/a | n/a | 0.67 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | n/a |
| Ovarian adenocarcinoma (Moorman et al., 2011) | 638 | Invasive disease; NC Ovarian Cancer Study | n/a | n/a | n/a | 0.69 (multi-variate adjusted relative risk) | ≥2 h/wk (p < 0.05) | No |
| Primary glioma (Ruden et al., 2011) | 243 | WHO grade III/IV recurrent glioma | n/a | n/a | n/a | 0.64 (multi-variate adjusted relative risk) | ≥9 MET-h/wk (p < 0.05) | Yes |
Abbreviations: MET, metabolic equivalent task; NSCLC, non-small cell lung cancer; WHO, World Health Organization; NS, not-significant.
Of importance, the association between exercise and cancer-specific mortality is not uniform and appears to vary according to volume of physical activity and even cancer type. For example, the first reported study in women with early breast cancer found that that women reporting ≥9 MET-h wk−1 (equivalent to walking briskly for 30 min, 5 days/week), had a 6% reduction in unadjusted absolute mortality risk at 10 years compared with women who reported less than 3 MET-h wk−1 (equivalent to walking 2–2.9 mph for 1 h) (Holmes et al., 2005). In contrast, Holick et al. that found to ≥21 MET-h wk−1 (brisk walking for 75 min, 5 d wk−1) was required for significant reductions in cancer specific and all-cause mortality in women with invasive breast cancer.
The majority of studies in women with early breast cancer have examined the association between exercise levels in the period following the completion of primary adjuvant therapy (i.e., surgery, radiotherapy, chemotherapy) and prognosis. Of interest, Borugian et al. found that self-reported exercise before adjuvant therapy was not associated with breast cancer mortality at 10 years. The reasons for the discrepant findings are not known but may relate to significant reductions in exercise behavior during primary adjuvant therapy (Irwin et al., 2003) or the lack of additional protective effect of exercise in combination with adjuvant therapy. To this end, exercise may be more effective as a ‘secondary’ adjuvant therapy. In other words, from a disease recurrence perspective, exercise may be more effective as a chronic maintenance adjunct therapy following the completion of primary adjuvant therapy akin to long-term endocrine therapy, although evidence to support this notion is currently lacking. The lack of uniformity of the association between exercise and prognosis following a breast cancer diagnosis also appears to translate to other solid malignancies. Specifically, in a series of studies by Meyerhardt and colleagues, they found that ≥18 MET-h wk−1 (equivalent to walking briskly for 60 min, 5 days/week) was associated with 36% to 40% reductions in colorectal-specific mortality and all-cause mortality in one study whereas ≥27 MET-h wk−1 (equivalent to walking briskly for 90 min, 5 days/week) was required to confer the equivalent reduction in colorectal-specific mortality and all-cause mortality in 668 patients with colorectal cancer.
The lack of consistent effects of exercise on cancer-specific outcomes may be partially explained by differences in tumor biology (phenotype) or expression of certain molecular markers. For example, Holmes et al. (2005) found that ≥9 MET-h wk−1 was associated with a relative risk reduction in mortality of only 9% in women with estrogen receptor (ER) negative tumors relative to a mortality reduction of 50% in women with ER positive tumors. This finding was corroborated by Irwin et al. (2008) In a detailed analysis of tumor samples obtained at the time of surgical resection, Meyerhardt et al. (2009c) found that patients with tumors exhibiting loss of p27, a protein that regulates cell cycle, the hazard ratio (HR) for colon cancer-specific mortality was 1.40 (95% CI, 0.41–4.72) for those reporting ≥18 MET-h wk−1 compared with <18 MET-h wk−1. The corresponding HR for patients with tumors exhibiting expression of p27, was 0.33 (95% CI, 0.12–0.85) for those reporting ≥18 MET-h wk−1 compared with <18 MET-h wk−1. In subsequent analyses Morikawa et al. found that patients reporting ≥18 MET-h wk−1 whose tumors did not express CTNNB1 (β-catenin), a key mediator of the WNT signaling pathway that plays an important role in colorectal carcinogenesis, had an adjusted HR for colorectal cancer-specific survival of 0.33 (95% CI: 0.13–0.81) compared with patients reporting <18 MET-h wk−1 (Morikawa et al., 2011). Conversely, there was no significant relationship between exercise and prognosis in patients with tumors that were positive for nuclear CTNNB1 (adjusted HR: 1.07; 95% CI: 0.50–2.30). Although more studies are clearly required, the putative evidence provides promising preliminary evidence that regular exercise may be protective in select types (i.e., histological or molecular sub-types) of solid malignancies.
4. Relationship between fitness and prognosis: epidemiological evidence
The vast majority of studies to date in the field of exercise–oncology have examined the relationship between self-reported exercise behavior (via surveys) and prognosis in cancer patients. While there are several advantages to self-report methodology, particularly in large observational epidemiological studies, these tools are subjective, lack sensitivity and have poor reliability and validity. In contrast, exercise tolerance tests provide an objective measure of exercise capacity or functional capacity. There are several methods available that provide an objective determination of exercise capacity. For a more detailed review of exercise testing methods in clinical oncology, the reader is referred to a prior review by our group (Jones et al., 2008).
The strong, independent prognostic importance of exercise capacity and functional capacity in numerous clinical populations is well-established (Warburton et al., 2006). However, a paucity of studies have examined the prognostic importance of these measures in patients with cancer. As summarized in Table 2, five studies to date have examined this question. In the first study, our group examined the prognostic value of peak oxygen consumption (VO2peak), the gold standard assessment of exercise capacity. VO2-peak was a strong independent predictor of death in 398 surgical candidates with NSCLC after adjustment for KPS, age, gender, and pulmonary function (Jones et al., 2010c). In further work, we found that VO2peak was also a strong predictor of all-cause mortality in 52 women with metastatic breast cancer. Again, VO2peak added incremental value to the prediction of mortality beyond traditional markers of prognosis in this population (Jones et al.).
Table 2.
Association between objective measures of cardiorespiratory fitness, functional capacity and all-cause mortality.
| Tumor type (study) | N | Cohort/setting | Objective measure | Prognostic findings |
|---|---|---|---|---|
| Non-small cell lung cancer (Kasymjanova et al., 2009) | 64 | Newly diagnosed patients with stage IIIA, IIIB, or IV NSCLC with a life expectancy of at least 4 months | 6MWT conducted three times: at time of enrollment, pre-chemotherapy, and after two cycles of chemotherapy | Patients with an initial 6 MWT ≥400 m had significantly longer survival than patients with initial 6 MWT < 400 m (multivariate adjusted HR: 0.44; 95% CI: 0.23–0.83; p = 0.001) |
| (Jones et al., 2010a,b,c) | 398 | Patients with suspected stage I–IIIA NSCLC who were candidates for primary surgery with an estimated life expectancy of at least 2 years; CALGB protocol 9238 Study | CPET on cycle ergometer to determine VO2 peak measured once before surgery | Patients achieving VO2 peak > 1.29 L/min had significantly better survival than patients with a VO2 peak < 0.96 L/min (multivariate adjusted HR: 0.56; 95% CI: 0.39–0.80; p = 0.0037) |
| (Jones et al., 2010a,b,c) | 118 | Patients with histologically confirmed stage IIIB, IV or recurrent metastatic NSCLC | 6MWT assessed one time | Patients achieving 6MWT ≥ 450 m had significantly better survival than patients with 6MWT <358.5 m (multivariate adjusted HR: 0.48; 95% CI: 0.24–0.93; p = 0.025). Every 50 m improvement in distance was associated with a 13% reduction in risk of death |
| Glioma (Ruden et al., 2011) | 243 | Patients with histologically confirmed WHO grades 3 and 4 malignant glioma who had received or were receiving salvage therapy | 6MWT assessed one time | 6MWT was not an independent prognostic factor. There was no significant difference in all-cause mortality between patients achieving 6MWT > 489 m and 6MWT <390 m (multivariate adjusted HR: 0.97; 95% CI: 0.63–1.48) |
| Breast adenocarcinoma (Jones et al., in press- a,2012a) | 52 | Women with histologically confirmed IV breast cancer receiving cytotoxic chemotherapy | CPET on cycle ergometer to determine VO2 peak measured once | CPET performance was not predictive of overall survival. Patients achieving VO2peak ≥ 15.4 mL/kg-min did not have significantly different survival rates from those with a VO2 peak ≤ 15.4 mL/kg-min (multivariate adjusted HR: 0.59; 95% CI: 0.29– 1.19; p = 0.141) |
Abbreviations: 6MWT, 6 min walk test; CPET, cardiopulmonary exercise testing; NSCLC, non-small cell lung cancer; WHO, World Health Organization.
Three studies have also investigated the prognostic importance of measures of functional capacity, specifically 6-min walk distance (6MWD), in patients with cancer. Kasymjanova et al. investigated the association between 6MWD and survival in 64 patients with inoperable NSCLC (Kasymjanova et al., 2009). Relative to <400 m, a 6MWD ≥ 400 m was associated with a 56% reduction in the risk of death after adjustment for important covariates. In two subsequent studies by our group, we found that 6MWD was a strong, independent predictor of prognosis that provided incremental mortality risk prediction beyond traditional risk factors in patients with metastatic NSCLC, but was not associated with survival in patients with primary malignant recurrent glioma (Jones et al., 2011; Ruden et al., 2011). Of interest, in our metastatic NSCLC study, 6MWD remained an independent predictor of mortality after adjustment for self-reported exercise behavior, suggesting that an objective measure of exercise exposure (e.g., functional capacity) may provide a more sensitive prediction of mortality in patients with advanced cancer.
5. Mechanisms underlying the exercise-prognosis relationship
While the emerging literature base suggests that both self-reported exercise behavior and objective measures of exercise capacity/functional capacity are associated with survival in select cancer populations, the underlying biological mechanisms remain to be elucidated. Postulated mechanisms underlying the potential effects of exercise and/or fitness on cancer progression include modulation of metabolic (e.g., markers of glucose–insulin homeostasis) and sex-steroid (e.g., estrogens) hormone levels, improvements in immune surveillance, and reduced systemic inflammation and oxidative damage (McTiernan, 2008). However, to date, there is a paucity of correlative or direct evidence on these postulated host pathways in the clinical setting, or whether modulation of these pathways modulates the hallmarks of cancer in preclinical (animal) studies. Of course, there is a significant genetic contributor to exercise capacity therefore it is currently not clear how genetic predictors of exercise capacity relate to those contributing to cancer progression/metastasis or how the contribution of lifestyle factors (to exercise capacity) influence the fitness–prognosis relationship beyond genetic contributions. These factors need to be considered when elucidating the mechanisms by which exercise and/or fitness may influence tumorgenesis. In the following sections, we review the available evidence from clinical studies investigating the effects of structured exercise on changes in blood-based biomarkers as well as data from preclinical investigations investigating tumor progression and metastasis in mouse models of cancer. Of note, unless otherwise stated, we assume that all blood-based measurements were performed in the resting state (and not immediately following an acute exercise bout).
5.1. Clinical data
As summarized in Table 3 and 24 studies to date have investigated the effects of structured exercise training on changes in circulating concentrations of host-related factors in persons with cancer (Allgayer et al., 2004, 2008a; Evans et al., 2009; Fairey et al., 2003, 2005a,b; Galvao et al., 2010; George et al., 2010; Irwin et al., 2006, 2007, 2005, 2009; Janelsins et al., 2011; Jones et al., 2012b; Ligibel et al., 2008; Na et al., 2000; Payne et al., 2008; Pierce et al., 2009; Schmitz et al., 2005; Segal et al., 2003, 2009; Tosti et al., 2011; Yuasa et al., 2009; Zeng et al., 2011).
Table 3.
Effects of exercise training on blood-based biomarkers from clinical studies.
| Tumor type (study) | N | Design/cohort/setting | Biological mechanisms |
|---|---|---|---|
| Breast adenocarcinoma (Fairey et al., 2003) | 53 | RCT. Postmenopausal breast cancer patients randomized to a supervised 15-week aerobic training intervention or sedentary control | Exercise was associated with decreases in IGF-1 (10.9%, p = 0.045) and IGF-1:IGFBP-3 M ratio (18.2%, p = 0.017) and increase in IGFBP-3 (p = 0.021). No significant effect on fasting insulin, glucose, or insulin resistance |
| (Irwin et al., 2005) | 710 | Observational. Stage 0–IIIA breast cancer survivors; Health, Eating, Activity, and Lifestyle Study | Exercise was associated with significantly lower C-peptide (p = 0.001) and leptin levels (p = 0.001) and higher IGF-1 (p = 0.0037). There was a trend toward increased IGFBP-3 levels (p = 0.055) |
| (Fairey et al., 2005a,b) | 53 | RCT of exercise training in postmenopausal survivors of stage I–IIIB breast cancer who had completed surgery, RT, and/or chemo with or without tamoxifen or arimidex; Rehabilitation Exercise for Health after Breast Cancer Trial | Exercise was associated with a trend toward decreased C-reactive protein levels (p = 0.066) |
| (Fairey et al., 2005a,b) | 53 | RCT of exercise training in postmenopausal survivors of stage I–IIIB breast cancer after surgery, RT, and/or chemo with or without tamoxifen or arimidex; Rehabilitation Exercise for Health after Breast Cancer Trial | Exercise was associated with increases in natural killer cell cytotoxic activity (p = 0.035) and unstimulated mononuclear cell function (p = 0.007). No significant differences were observed in blood mononuclear cell production of pro-inflammatory (IL-1, TNF-α, IL-6) and anti-inflammatory cytokines (IL-4, IL-10, transforming growth factor-1) |
| (Schmitz et al., 2005) | 85 | RCT. Posttreated breast cancer patients 4–36 months after adjuvant therapy. | Six months of weight training significantly decreased IGF-II (p = 0.02) and IGFBP-3 levels (p = 0.03). There were no significant changes in glucose, insulin, IGF-I, IGFBP-1, and IGFBP-2 |
| (Irwin et al., 2006) | 474 | Observational. Stage 0–IIIA breast cancer survivors; Health, Eating, Activity, and Lifestyle Study | Exercise was associated with a significant decrease in mammographic dense area (p = 0.046) and percent density (p = 0.026) in postmenopausal women with BMI ≥ 30 kg/m2. Exercise was associated with a significant increase in percent density in premenopausal women with BMI < 30 kg/m2 (p = 0.037) |
| (Irwin et al., 2007) | 522 | Observational. Stage 0–IIIA breast cancer survivors; Health, Eating, Activity, and Lifestyle Study | Exercise was associated with a significant decrease in mammographic dense area in postmenopausal women with BMI ≥ 30 kg/m2 (p = 0.036). Exercise was associated with an increase in dense area in women with BMI < 25 kg/m2, but this difference was not statistically significant |
| (Payne et al., 2008) | 20 | RCT. Postmenopausal breast cancer patients receiving hormonal therapy | No significant differences were observed between groups with respect to cortisol or IL-6 levels. Serotonin levels (p = 0.009) were significantly affected by exercise |
| (Ligibel et al., 2008) | 101 | RCT. Sedentary, overweight early stage breast cancer patients to home-based aerobic and resistance training program or sedentary control for 16 weeks | Exercise was associated with a 28% reduction in fasting insulin (p = 0.03) and a nonsignificant improvement in insulin sensitivity (p = 0.09) |
| (Pierce et al., 2009) | 741 | Observational. Stage 0–IIIA breast cancer survivors; Health, Eating, Activity, and Lifestyle Study | Exercise was associated with significantly lower concentrations of C-reactive protein (p = 0.005) and non-significant decreases in serum amyloid A concentrations (p = 0.06) |
| (Evans et al., 2009) | 14 | Pre-post. Posttreated breast cancer patients within 6 months of completion of all major cancer therapy and healthy controls matched for age and physical activity level; Get REAL & HEEL Breast Cancer Program | Breast cancer patients had significantly lower post-exercise blood lactate levels following high-intensity exercise (70% of VO2max, p < 0.0005). There was no significant difference between patients and sedentary controls at low (40%) and moderate (60%) intensity |
| (Irwin et al., 2009) | 75 | RCT. Sedentary postmenopausal breast cancer survivors diagnosed 1–10 years prior with stage 0–IIIA breast cancer after adjuvant treatment at least 6 months before enrollment | Exercise was associated with a decrease in insulin (p = 0.089), IGF-I (p = 0.026), and IGFBP-3 (p = 0.0006) levels |
| (George et al., 2010) | 746 | Observational. Stage 0–IIIA breast cancer survivors; Health, Eating, Activity, and Lifestyle Study | Physical activity offset the negative effects of poor diet quality on C-reactive protein levels (p = 0.03) in breast cancer survivors |
| (Tosti et al., 2011) | 14 | Pre-post. Stage I–III invasive breast cancer within 6 months of completion of all major cancer treatments and no evidence of metastatic disease, matched to healthy controls | Patients with breast cancer had significantly lower blood lactate response to exercise (p ≤ 0.05) across a variety of intensities. There was a trend toward more elevated glucose responses in women with cancer before and after exercise (p < 0.08) |
| (Janelsins et al., 2011) | 21 | RCT. Sedentary patients with stage 0–IIIb breast cancer after primary treatment 1–30 months prior to enrollment | Insulin levels did not rise in exercising patients compared to an increase in sedentary controls that received psychosocial therapy (significance not reported). IGF-1 decreased more in exercising patients than controls and IGFBP-3 increased in the exercising group (compared with a decrease in the control group), but neither of these changes was significant |
| (Zheng et al., 2011) | 75 | Pre-post/observational. Physically inactive postmenopausal women diagnosed with stage 0–IIIA breast cancer after completion of adjuvant treatment at least 6 months prior to enrollment. | Exercise was associated with a significant reduction in methylation of the L3MBTL1 tumor suppressor gene (p = 2.9 × 10−5) |
| Colon/colorectal adenocarcinoma (Allgayer et al., 2004) | 23 | RCT. Stage II or III colorectal cancer patients at least 4 weeks after completion of primary therapy (surgery and radiation and/or chemotherapy) | Short-term (2 weeks) of moderate intensity exercise was associated with decreased IL-1 receptor agonist response to LPS (p < 0.05) and a more pro-inflammatory state (decreased LPS antagonist/IL-1β, TNF-α, and IL-6 cytokine ratio; p < 0.05 for IL-6, all others non-significant) |
| (Allgayer et al., 2008) | 48 | RCT. Colorectal cancer patients after completion of primary therapy (surgery and radiation and/or chemotherapy) | Short-term (2 weeks) of moderate intensity exercise significantly decreased urinary 8-oxo-dG excretion levels (p = 0.02). High intensity exercise resulted in a non-significant increase in 8-oxo-dG levels (p = 0.18) |
| Prostate adenocarcinoma (Segal et al., 2003) | 155 | RCT. Men with a confirmed prostate cancer diagnosis scheduled to receive at least 3 months of androgen deprivation therapy after enrollment | Exercise did not significantly affect testosterone or PSA levels |
| (Segal et al., 2009) | 121 | RCT. Men with a confirmed prostate cancer diagnosis scheduled to receive radiation therapy with or without androgen deprivation therapy after enrollment | Neither resistance nor aerobic exercise significantly affected hemoglobin, testosterone, or PSA levels |
| (Galvao et al., 2010) | 57 | RCT. Men with confirmed prostate cancer at least 2 months of androgen deprivation therapy and were expected to undergo at least 6 more months of ADT with no evidence of disease | Exercise was associated with a significant decrease in C-reactive protein (p = 0.008). No significant differences were observed in testosterone, PSA, cholesterol, triglyceride, insulin, glucose, or homocysteine levels |
| Gastric adenocarcinoma (Na et al., 2000) | 35 | RCT. Stomach cancer patients after surgery | Exercise was associated with a significant sequential change in function in vitro of natural killer cells isolated from stomach cancer patients (p < 0.05) |
| (Yuasa et al., 2009) | 106 | Observational. Patients with primary gastric carcinoma | There was an association between physical activity and decreased methylation of CACNA2D3 (commonly methylated, poor prognostic factor in gastric cancer) (p = 0.03) |
| Lung adenocarcinoma (Jones et al., 2011,2012b) | 16 | Pre-post. Stage I–IIIB non-small cell lung cancer | Exercise was associated with an increase in urinary F2-isoprostanes (markers of oxidative stress) (p = 0.08) |
Abbreviations: RCT, randomized controlled trial; 8-oxo-dG-8-Oxo-2′-deoxyguanosine; BMI, body mass index; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein-3; RT, radiation therapy; LPS, lipopolysaccharide; PSA, prostate-specific antigen.
The most widely investigated pathway is the effect of exercise on circulating metabolic factors, especially the insulin–glucose axis. Of the nine studies that evaluated these factors, most suggest that exercise is associated with changes in insulin-like growth factor-1 (IGF-1) concentration, insulin-like growth factor binding protein-3 (IGFBP-3) concentration, and IGF-1:IGFBP-3 M ratio, but there were no significant changes in insulin and glucose levels. Fairey et al. were the first to report that supervised exercise training following traditional exercise prescription guidelines (i.e., moderate intensity aerobic training at 60–75% of baseline VO2peak, 3×/wk, 30–45 min/session for 15 weeks) was associated with significant alterations in IGF-1 concentrations (−7.4 ng/mL), IGFBP-3 concentrations (+180.5 ng/mL), and IGF-1:IGFBP-3 M ratio (−0.006) in 53 early-stage postmenopausal breast cancer patients compared to a sedentary control group (Fairey et al., 2003). There were no differences in fasting measures of glucose, insulin, or insulin resistance. Similar results were observed in larger studies of both aerobic and resistance exercise in breast cancer patients (Irwin et al., 2005; Janelsins et al., 2011; Schmitz et al., 2005). Ligibel et al. randomly assigned 101 overweight, sedentary early stage breast cancer patients to a 16-week home aerobic and resistance exercise program or sedentary control (Ligibel et al., 2008). Interestingly, in this population, patients experienced a borderline significant decrease in fasting insulin (28%, p = 0.07), a result that was corroborated by Irwin and colleagues (Irwin et al., 2009). Beyond breast cancer, Galvão et al. investigated the effects of a combined aerobic and resistance exercise training intervention metabolic factors in 57 prostate cancer patients undergoing androgen deprivation therapy. No significant differences were observed in any metabolic outcomes.
Exercise-induced effects on immune surveillance and inflammatory pathways have received some attention. Nine studies evaluated immunity and inflammation, but the results were quite variable. Na et al. reported that physical activity was associated with significant changes in in vitro function of natural killer cells isolated from gastric cancer patients (Na et al., 2000). Fairey et al. observed similar results as a result of 15-weeks of aerobic training in early stage breast cancer patients (Fairey et al., 2003). In this study, the authors also used ELISA to examine mononuclear cell cytokine production, but they did not see significant differences in either pro-inflammatory (e.g., IL-1, TNF-α, IL-6) or anti-inflammatory cytokines (e.g., IL-4, IL-10, transforming growth factor-1). A 2008 study by Payne et al. also reported no significant effects of exercise on serum IL-6 and cortisol levels in 20 postmenopausal breast cancer patients receiving hormonal therapy (Payne et al., 2008). In contrast, Allgayer et al. conducted a study of 23 stage II–III colorectal cancer patients after they completed primary therapy and found that short-term moderate intensity exercise was associated with decreases in IL-1 receptor agonist response to stimulation with LPS in heparinized whole blood and a more pro-inflammatory state, which the authors defined as decreased ratios of IL-1ra/IL-6 and IL-1ra/IL-1β (Allgayer et al., 2004). One common measure of systemic inflammation is C-reactive protein (CRP), an acute-phase biomarker that has been widely shown to rise in the blood in accordance with systemic inflammation. Four studies, three in breast cancer and one in prostate cancer, have shown that exercise is associated with decreases in CRP levels (Fairey et al., 2005b; Galvao et al., 2010; George et al., 2010; Pierce et al., 2009).
Fewer studies have examined the effects of exercise training on markers of oxidative balance/status. Allgayer et al. found that short-term moderate intensity exercise significantly reduced urinary excretion of 8-oxo-dG, a marker of oxidative damage to DNA, in colorectal cancer patients after the completion of primary adjuvant therapy (Allgayer et al., 2008). In contrast, Jones et al. found that moderate to high-intensity cycle ergometry was associated with significant increases in F2-isoprostanes, eicosanoid markers of oxidative stress, in 16 patients with stage I–IIIB NSCLC (Jones et al., 2012b). Finally and somewhat surprisingly, a limited number of studies have examined the effects of exercise on circulating concentrations on sex-hormone factors –factors that are clearly of most relevance breast and prostate cancer. In terms of the latter, three studies to date have examined the effects of exercise on levels of testosterone in men with both early and locally-advanced prostate cancer; all studies to date have reported no change in testosterone or prostate-specific antigen between men randomized to exercise compared with sedentary control (Galvao et al., 2010; Segal et al., 2003, 2009).
Based on the current evidence, there are no clear conclusions regarding the efficacy of exercise training to modulate the postulated host-related mechanisms underlying the exercise–prognosis relationship. However, the postulated pathways appear reasonable targets since there is considerable evidence in healthy populations that exercise training can modulate circulating levels of metabolic hormones (Umpierre et al., 2011), immune/inflammatory factors (Walsh et al., 2011), and sex-steroid hormones (Friedenreich et al., 2010; McTiernan et al., 2004). Modulating effects of exercise on pro-oxidative and anti-oxidative products is less conclusive at present. Clearly, the pathways postulated to underpin the exercise–cancer prognosis relationship encompasses essentially all host-related factors in humans. As a consequence, there are literally hundreds of potential candidate biomarkers that could, in theory, modulate exercise efficacy. To tackle this issue, the integration of high-throughput discovery approaches in preclinical and/or clinical investigations will be critical to identify a parsimonious panel of exercise-responsive factors – the mechanistic action of which can then be further interrogated using an integrated approach involving in vivo and in vitro experiments. Following the identification of the major players within and across the various host-related pathways, the clinical importance of these factors can be examined in existing epidemiological datasets.
Whether exercise training can directly alter tumor biology in cancer patients has not been investigated. However, Yuasa et al. found an inverse association between self-reported exercise levels and methylation of CACNA2D3 in tumor tissue from 106 patients with gastric cancer (Yuasa et al., 2009). Similarly, Zeng et al. found a significant association between self-reported exercise levels and decreased methylation of the L3MBTL1 tumor suppressor gene in 75 postmenopausal women with stage 0–IIIA breast cancer (Zeng et al., 2011).
5.2. Preclinical data
The utilization of animal models is a critical scientific method to dissect the effects and underlying host-related and molecular mechanisms of exercise on tumor biology to complement clinical investigations, to facilitate a translational approach to optimize the safety and efficacy of exercise in the oncology setting. Surprisingly, while numerous research groups have utilized mouse models to examine the effects of exercise on the initiation and incidence of several different cancer types (i.e., exercise exposure followed by tumor initiation), far fewer have adopted this experimental system to investigate the effects of exercise on progression and metastasis (i.e., initiation of exercise following tumor establishment, which mimics the clinical scenario of using exercise as an adjunct cancer therapy). As summarized in Table 4, we found a total of 14 studies investigating the effects of endurance exercise on a variety of different tumor types and endpoints, in several different model systems (Baracos, 1989; Cohen et al., 1991; Foley et al., 2004; Hoffman et al., 1962; Hoffman-Goetz et al., 1994b; Japel et al., 1992; Jones et al., 2005, 2010b; MacNeil and Hoffman-Goetz, 1993; Roebuck et al., 1990; Saez Mdel et al., 2007; Uhlenbruck and Order, 1991; Zheng et al., 2011; Zielinski et al., 2004). In summary, 11 (79%) studies reported primary tumor growth as an endpoint; of these, four found that exercise resulted in inhibition of primary tumor growth (Baracos, 1989; Cohen et al., 1991; Hoffman et al., 1962; Zheng et al., 2011). For example, Hoffman et al. implanted Walker 256 sarcoma cells subcutaneously into Wistar rats that were subsequently randomly allocated to voluntary wheel running in combination with daily swimming for 21 days relative to a sedentary control group. Tumor weight was significantly lower in the exercise group (percent of control: −97%) (Hoffman et al., 1962).
Table 4.
Effects of exercise on tumor progression and metastasis in mouse models.
| Tumor type (study) | Rodent model | Animal strain | Method of tumor initiation | Effect of exercise on primary tumor growth (% of control) | Description of primary tumor growth | Description of mechanistic findings
|
||
|---|---|---|---|---|---|---|---|---|
| Intratumoral | Systemic | |||||||
| Breast adenocarcinoma (Cohen et al., 1991) | Rat | F344 | Tail vein injection of 37.5 mg/kg NMUb | Inhibition (NE) | Exercise significantly delayed tumor appearance and increased tumor latency. Tumor incidence was significantly lower in exercised animals compared with controls. There was no significant difference in tumor burden or mean tumor volume | Not evaluated | Exercise did not significantly affect prolactin levels | |
| (Hoffman-Goetz et al., 1994a,b) | Mouse | BALB/c | Lateral tail vein injection of 1 × 104 MMT 66 cells | Not evaluated | This study evaluated pulmonary metastases from a tail vein injection of tumor cells. Exercise beginning after tumor injection did not significantly affect multiplicity of lung metastases | Not evaluated | Mice that began running on a voluntary wheel only after tumor injection had significantly higher LAK cell activity than those that had access to a voluntary running wheel before and after tumor injection | |
| (Jones et al., 2005) | Mouse | Athymic nu/nu | Subcutaneous injection of 5 × 106 MDA-MB-231 cells | No change (NE) | There was no significant difference in tumor growth delay | Not evaluated | Not evaluated | |
| (Saez Mdel et al., 2007) | Rat | Sprague– Dawley | Gastric intubation of DMBA.c 5 mg weekly for 4 w. | Augmentation (+200%) | The exercise group had a significantly higher tumor growth rate. There were no significant differences in survival time or tumor multiplicity | Not evaluated | Not evaluated | |
| (Jones et al., 2010a,b,c) | Mouse | Athymic | Orthotopic (dorsal mammary fat pad) injection of 1 × 106 MDA-MB-231 cells | No change (+21%) | There was no significant difference in survival time based on tumor growth to 1500 mm3 | The exercised group had significantly more perfused vessels. HIF-1 protein levels were significantly higher in the viable tumor the exercised group. There were no significant differences in the levels of CD31, VEGF, ATP, PGC-1α, or AMPK | Not evaluated | |
| Sarcoma (Hoffman et al., 1962) | Rat | Wistar | Subcutaneous injection of 2 cc of Walker 256 cell suspension (concentration not specified) | Inhibition (− 97%) | Tumor weight was significantly lower in the exercise group | Not evaluated | Not evaluated | |
| (Uhlenbruck and Order, 1991) | Mouse | BALB/c | Subcutaneous injection of 2.5 × 104 L−1 cells | Inconclusive (−44% to +86%) | The group that ran 200 m daily had significantly lower tumor weights, but the other running distances did not significantly affect tumor weight | Not evaluated | Not evaluated | |
| (Foley et al., 2004) | Rat | F344 | Subcutaneous injection of 1 × 107 C10 cells | No change (− 31%) | There was no significant difference in tumor weight as a result of exercise | Not evaluated | Exercise significantly increased insulin-stimulated glucose transport. No significant differences in blood glucose levels or lipid peroxidation in skeletal muscle based on exercise treatment | |
| Hepatoma (Baracos, 1989) | Rat | Sprague– Dawley | Subcutaneous injection of 20 μL Morris hepatoma 777 finely chopped tumors | Inhibition (− 25%) | Tumor weight was significantly lower in the exercise groups. No significant differences in tumor weight between groups exercised for different durations | Not evaluated | Not evaluated | |
| Pancreatic adenocarcinoma (Roebuck et al., 1990) | Rat | F344, Lewis | F344 rats received 3 doses of 30 mg/kg azaserine 4–5 d apart. Lewis rats received one dose of 30 mg/kg azaserinea | Inconclusive (−1% to −11%) | F344 rats that were exercised had significantly fewer foci and smaller volume percentage of foci, but there was no difference in focal diameter. No significant differences in Lewis rats | No significant difference in the amount of DNA synthesis based on treatment condition | Not evaluated | |
| Transformed salivary gland cells (Japel et al., 1992) | Mouse | NMRI | Subcutaneous injection of 1.5 × 106 S-180 cells | Not evaluated | Not evaluated | Not evaluated | Moderate intensity physical exercise begun after injection of tumor cells did not significantly change macrophage phagocytosis | |
| Transformed fibroblasts (MacNeil and Hoffman-Goetz, 1993) | Mouse | C3H/He | Lateral tail vein injection of 3 × 105 CIRAS1 cells | Not evaluated | This study evaluated pulmonary metastases from a tail vein injection of tumor cells. Exercise beginning after tumor injection did not alter lung tumor density relative to sedentary controls | Not evaluated | Not evaluated | |
| Neoplastic lymphoid cells (Zielinski et al., 2004) | Mouse | BALB/c | Subcutaneous injection of 2 × 107 EL-4 cells | Inconclusive (+5%) | Exercise significantly delayed tumor appearance. There was no significant difference in peak tumor volume. Non-syngeneic tumors were rejected by the immune system of exercised mice | No significant difference in the fluid content of tumors. Significant reduction in vessel density in exercised animals on days 6, 8, 10, and 14 compared to controls | Not evaluated | |
| Prostate adenocarcinoma (Zheng et al., 2011) | Mouse | SCID | Subcutaneous injection of 2.5 × 106 LNCaP cells. Tumors grew for 4–6 wk then animals were castrated to mimic androgen deprivation | Inhibition | Voluntary wheel running moderately inhibited androgen-independent tumor growth | Not evaluated | Not evaluated | |
Azaserine is a glutamine analog carcinogen used to induce pancreatic cancer in preclinical models.
N-Nitroso-N-methylurea (NMU) is an alkylating agent used to induce breast cancer.
7,12-Dimethylbenzanthracene (DMBA) is an aromatic hydrocarbon used to induce breast cancer.
In contrast, three studies reported mixed results, meaning that tumor growth was inhibited in some exercise conditions and not in others (Roebuck et al., 1990; Uhlenbruck and Order, 1991; Zielinski et al., 2004).
Roebuck et al. examined the effects of up to 18 weeks of voluntary wheel running in a pancreatic adenocarcinoma model induced by injection of azaserine (Roebuck et al., 1990). They found that F344 rats that exercised had significantly fewer tumors and smaller volume percentage, but Lewis rats subjected to the same treatment did not exhibit significant differences between groups. In a different model, Uhlenbruck and Order injected L-1 sarcoma cells subcutaneously into BALB/c mice that were forced to run 200 m, 400 m, or 800 m daily on a treadmill (Uhlenbruck and Order, 1991). Tumor weight was significantly lower in the group that ran 200 m daily compared with sedentary animals, but there were no significant differences in tumor weight for the other distances. Additionally, Zielinski et al. injected EL-4 neoplastic lymphoid cells subcutaneously in BALB/c mice and found that forced treadmill running delayed tumor appearance, but there was no significant difference between groups with respect to maximum tumor volume (Roebuck et al., 1990; Zielinski et al., 2004).
Finally, in three studies tumor growth was comparable between exercise and sedentary control groups (Foley et al., 2004; Jones et al., 2005, 2010b). Foley et al. found no effect of 17 days of voluntary wheel running following subcutaneous injection of C10 sarcoma cells in F344 rats (Foley et al., 2004). Jones et al. observed similar results in two independent experiments in a model of human breast cancer. In the first, MDA-MB-231 breast cancer cells were subcutaneously into athymic nude mice and then randomized to 8 weeks of treadmill running or sedentary control (Jones et al., 2005). In the second experiment, MDA-MB-231 breast cancer cells were orthotopically implanted into the dorsal mammary fat pad and then animals were randomized to 41–48 days of voluntary wheel running or sedentary control (Jones et al., 2010b). In both experiments, exercise did not significantly inhibit or augment tumor growth. It is important to note that in contrast to the aforementioned studies, one has reported augmentation of primary tumor growth with exercise training. In this experiment, Sprague–Dawley rats were administered 7,12-dimethylbenz[α]anthracene to induce breast adenocarcinoma, and following tumor establishment, all animals were randomized to forced swimming (30 min/day for 38–65 days) or sedentary control (Saez Mdel et al., 2007). Tumor growth rate increased 200% compared to control, although there were no significant differences in overall survival time.
The vast majority of cancer deaths result from metastasis, as such studies investigating the effects of exercise on metastatic dissemination and progression are of clear relevance. Only three studies to date have examined this question. In the first study, MacNeil and Hoffman-Goetz injected CIRAS1 transformed fibroblasts into the lateral tail vein of C3H/He mice and then randomized animals to voluntary wheel running or sedentary controls for three weeks (MacNeil and Hoffman-Goetz, 1993). Hoffman-Goetz et al. followed up on that work by injecting MMT 66 breast cancer cells intravenously into BALB/c mice and then randomizing animals to three weeks of treadmill running, voluntary wheel running, or sedentary control (MacNeil and Hoffman-Goetz, 1993). Exercise following tumor cell injection did not impact the development of lung metastases in either of these experiments. However, it is important to note that both of these experiments involved intravenous injection of cancer cells into otherwise healthy recipients, a model that fails to evaluate the ability of tumor cells to break through the tissue’s basement membrane and invade into the capillary.
In an effort to address the limitations of prior research, our group examined the effects voluntary wheel exercise, compared with sedentary control, in male mice orthotopically implanted (into the prostate) with murine prostate cancer cells (TRAMP C-1). Results indicated that primary tumor growth rate was comparable between groups but expression of prometastatic genes was significantly modulated in exercising animals with a shift towards reduced metastasis (Jones et al., in press-a,2012a). Paradoxically, exercise was associated with significant increases in tumor vascularization, as measured by magnetic resonance blood perfusion imaging, while multiplex ELISAs revealed distinct reductions in plasma concentrations of interleukin-6 and CXCL1 in the exercise group. Based on the present data, we conclude that the effects of exercise on tumor progression and metastasis are equivocal. Reasons for the variable results to date likely pertain to differences in study methods, tumor cell line or carcinogen, location of tumor growth, and differences in the volume and type of exercise.
Researchers have also started to investigate the effects of exercise on host-related circulating factors as well as changes in the tumor phenotype that may mediate the exercise–tumor progression relationship. Specifically, three studies (21%) evaluated changes in intratumoral markers of neoplastic phenotype (Table 4). For example, in a model of azaserine-induced pancreatic cancer, Roebuck et al. reported that voluntary wheel running did not significantly affect the rate of DNA synthesis assessed by 3H-thymidine incorporation (Roebuck et al., 1990). The other two experiments assessed markers of tumor angiogenesis. Zielinski et al. found that treadmill running decreased intratumoral blood vessel density in BALB/c mice subcutaneously with EL-4 neoplastic lymphoid cells (Zielinski et al., 2004). More recently, we found that voluntary wheel running increased hypoxia-inducible factor-1 protein levels and number of perfused blood vessels in athymic mice bearing human breast cancer xenografts, although there were no differences in protein levels of 5′ adenosine monophosphate-activated protein kinase, which regulates cellular energy homeostasis, or the angiogenic markers CD31 (a marker of endothelial cells) and vascular endothelial growth factor (Jones et al., 2010b). Two (14%) studies have examined changes in circulating concentrations of various growth factors with exercise, with both finding no changes in systemic glucose or prolactin levels (Cohen et al., 1991; Foley et al., 2004). Additionally, two studies investigated immune effects of exercise. Hoffman-Goetz et al. reported that lymphokine-activated killer cell activity was higher in exercising animals, whereas Japel et al. did not observe a difference in macrophage phagocytosis (Hoffman-Goetz et al., 1994a; Japel et al., 1992). Overall, the effects of exercise on tumor growth and metastasis are inconclusive at present due to the low number and considerable heterogeneity between studies.
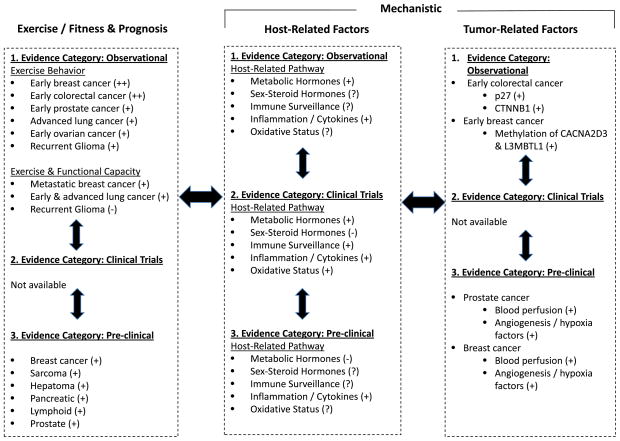
The existing literature base suggests that exercise may modulate select circulating concentrations of several different growth factors across multiple host pathways in both mouse and clinical studies. In conjunction, data from animal studies suggesting that exercise may impact tumor progression. Together, these data create a novel hypothesis that changes in these circulating factors in the host may, in turn, influence release of secondary factors from other organ sites such as the bone marrow, skeletal muscle, or liver with the end result of altering ligand availability in the primary tumor microenvironment and/or distant ectopic sites to influence tumorigenesis and metastasis, respectively. In other words, exercise may be a critical strategy to modulate the host–tumor interaction (Goodwin, 2008). The known effects and mechanisms of exercise on tumor progression adopting a bi-directional translational research approach is presented in Fig. 1.
Fig 1.
Evidence-based representation of the known effects and mechanisms of exercise on tumor progression adopting a bi-directional translational research or scientific discovery (T0) paradigm. Exercise/fitness and prognosis, evidence supporting association between self-reported exercise behavior, objective measures of exercise capacity or functional capacity, and cancer prognosis; Host-Related Factors, postulated systemic (host-related) pathways mediating the association between exercise behavior and exercise/functional capacity and cancer prognosis; Tumor-Related Factors, intratumoral factors shown to mediate the association between exercise and prognosis or factors shown to be modulated in response to exercise. +++, strong evidence; ++, moderate evidence; +, weak evidence; —, null; ?, unknown at present.
Investigations that adopt a scientific discovery (also known as T0 or ‘bench-to-bedside’) translational approach are now required to elucidate to further understand the role and mechanisms of exercise to modulate host milieu–tumor–distant organ cross-talk. Such research will dramatically increase understanding of the mechanistic properties and application of exercise in the oncology setting.
6. Gaps in knowledge
Our recommendations for future research in this field are shown in Table 5.
Table 5.
Future directions for exercise–oncology research on cancer progression.
Epidemiological studies
|
Clinical biomarker intervention studies
|
Preclinical studies
|
Potential translational (cross-cutting/transdisciplinary) studies
|
7. Conclusion
There is growing recognition and acceptance of the beneficial role of exercise training/rehabilitation following a cancer diagnosis to prevent and/or mitigate disease and/or treatment-related toxicities to optimize symptom control and recovery. The results from an increasing number of epidemiological studies, however, suggest a previously unexpected role of exercise – as a therapeutic strategy to potentially delay cancer recurrence and mortality. It is important to stress that the evidence base is emergent with a small number of studies in comparison with the scientific literature base investigating the exercise–cancer prevention/incidence relationship. In addition, the strong association between exercise and prognosis following a cancer diagnosis are yet to be confirmed in randomized trials, although at least one trial is currently ongoing. For example, the Colon Health and Life-Long Exercise Change (CHALLENGE) trial is a phase III trial investigating the effects of regular exercise on recurrence and cancer-specific mortality in 962 colorectal cancer patients (Courneya et al., 2008). In conjunction with epidemiological studies and ongoing RCTs, a more thorough and structured understanding of the systemic and molecular mechanisms underlying the purported effects of exercise on tumor progression is critical. Such an understanding can be effectively achieved through the development of carefully designed correlative science (as part of forthcoming and/or ongoing randomized trials) or biomarker-driven studies together with a broad base of basic and clinical investigations encompassing a scientific discovery (T0 or ‘bench-to-bedside’) translational approach. This work will inform the design of adequately powered, mechanistically driven phase II–III trials to ensure that the appropriate exercise dose is prescribed to the right patients, at the right time, to facilitate the shift towards personalized, medicine to optimize therapeutic outcomes.
Acknowledgments
L.W.J. is supported by NIH CA143254, CA142566, CA138634, CA133895, CA125458 and funds from George and Susan Beischer Foundation. M.W.D. is supported by NIH CA40355. A.S.B. is supported by DOD BC093532.
References
- Allgayer H, Nicolaus S, Schreiber S. Decreased interleukin-1 receptor antagonist response following moderate exercise in patients with colorectal carcinoma after primary treatment. Cancer Detect Prev. 2004;28:208–213. doi: 10.1016/j.cdp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Allgayer H, Owen RW, Nair J, Spiegelhalder B, Streit J, Reichel C, Bartsch H. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography–electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol. 2008;43:971–978. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- Baracos VE. Exercise inhibits progressive growth of the Morris hepatoma 7777 in male and female rats. Can J Physiol Pharmacol. 1989;67:864–870. doi: 10.1139/y89-135. [DOI] [PubMed] [Google Scholar]
- Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W, Flatt SW, Newman VA, Rock CL, Thomson CA, Pierce JP. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22:427–435. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, Gallagher RP, Hislop TG. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:1163–1172. [PubMed] [Google Scholar]
- Chen X, Lu W, Zheng W, Gu K, Matthews CE, Chen Z, Zheng Y, Shu XO. Exercise after diagnosis of breast cancer in association with survival. Cancer Prev Res (Phila) 2011;4:1409–1418. doi: 10.1158/1940-6207.CAPR-10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LA, Choi K, Backlund JY, Harris R, Wang CX. Modulation of N-nitrosomethylurea induced mammary tumorigenesis by dietary fat and voluntary exercise. In Vivo. 1991;5:333–344. [PubMed] [Google Scholar]
- Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, Au HJ, Brundage MD, Tu D, Dhillon H, Meyer RM. The Colon Health and LifeLong Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol. 2008;15:279–285. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, Falcini F, Franceschi S. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–2194. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- Evans ES, Battaglini CL, Groff DG, Hackney AC. Aerobic exercise intensity in breast cancer patients: a preliminary investigation. Integr Cancer Ther. 2009;8:139–147. doi: 10.1177/1534735409335506. [DOI] [PubMed] [Google Scholar]
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–727. [PubMed] [Google Scholar]
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol. 2005a;98:1534–1540. doi: 10.1152/japplphysiol.00566.2004. [DOI] [PubMed] [Google Scholar]
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Martin BS, Mackey JR. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005b;19:381–388. doi: 10.1016/j.bbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Foley JM, Stark KD, Zajchowski S, Meckling KA. Fatty acids and exercise affect glucose transport but not tumour growth in F-344 rats. Can J Appl Physiol. 2004;29:604–622. doi: 10.1139/h04-039. [DOI] [PubMed] [Google Scholar]
- Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, Terry T, Boyd NF, Yaffe MJ, Irwin ML, Jones CA, Yasui Y, Campbell KL, McNeely ML, Karvinen KH, Wang Q, Courneya KS. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- George SM, Neuhouser ML, Mayne ST, Irwin ML, Albanes D, Gail MH, Alfano CM, Bernstein L, McTiernan A, Reedy J, Smith AW, Ulrich CM, Ballard-Barbash R. Postdiagnosis diet quality is inversely related to a biomarker of inflammation among breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:2220–2228. doi: 10.1158/1055-9965.EPI-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ. Host-related factors in breast cancer: an underappreciated piece of the puzzle? J Clin Oncol. 2008;26:3299–3300. doi: 10.1200/JCO.2007.15.4526. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12:428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Hoffman SA, Paschkis KE, Debias DA, Cantarow A, Williams TL. The influence of exercise on the growth of transplanted rat tumors. Cancer Res. 1962;22:597–599. [PubMed] [Google Scholar]
- Hoffman-Goetz L, Arumugam Y, Sweeny L. Lymphokine activated killer cell activity following voluntary physical activity in mice. J Sports Med Phys Fitness. 1994a;34:83–90. [PubMed] [Google Scholar]
- Hoffman-Goetz L, May KM, Arumugam Y. Exercise training and mouse mammary tumour metastasis. Anticancer Res. 1994b;14:2627–2631. [PubMed] [Google Scholar]
- Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Aiello EJ, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, Ballard-Barbash R. Pre-diagnosis physical activity and mammographic density in breast cancer survivors. Breast Cancer Res Tr. 2006;95:171–178. doi: 10.1007/s10549-005-9063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Aiello EJ, McTiernan A, Bernstein L, Gilliland FD, Baumgartner RN, Baumgartner KB, Ballard-Barbash R. Physical activity, body mass index, and mammographic density in postmenopausal breast cancer survivors. J Clin Oncol. 2007;25:1061–1066. doi: 10.1200/JCO.2006.07.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, Wactawski-Wende J, Craft L, Lane D, Martin LW, Chlebowski R. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res (Phila) 2011;4:522–529. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, Dipietro L, Mayne ST, Yu H. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Davis PG, Wideman L, Katula JA, Sprod LK, Peppone LJ, Palesh OG, Heckler CE, Williams JP, Morrow GR, Mustian KM. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11:161–170. doi: 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japel M, Lotzerich H, Appell HJ. Physical exercise may improve macrophage phagocytic activity of tumor bearing mice. In Vivo. 1992;6:215–218. [PubMed] [Google Scholar]
- Jones LW, Antonelli J, Masko EM, Lascola CD, Dewhirst MW, Dyck JRB, Nagendran J, Flores CT, Betof AS, Young ME, Nelson ER, Pollak M, Barry WT, Broadwater G, Freedland SJ. Exercise modulation of the host–tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. doi: 10.1152/japplphysiol.01575.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, Douglas PS, Haykowsky M. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012a doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7:1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Courneya KS, Chiu BK, Baracos VE, Hanson J, Johnson L, Mackey JR. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clin Cancer Res. 2005;11:6695–6698. doi: 10.1158/1078-0432.CCR-05-0844. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, Lane AT, West M, Eves ND, Gradison M, Coan A, Herndon JE, Abernethy AP. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012b;76:248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Peppercom J, Scott JM, Battaglini C. Exercise therapy in the management of solid tumors. Curr Treat Options Oncol. 2010a;11:45–58. doi: 10.1007/s11864-010-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, Potter MQ, Moon EJ, Schroeder T, Herndon JE, 2nd, Dewhirst MW. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010b;108:343–348. doi: 10.1152/japplphysiol.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Watson D, Herndon JE, 2nd, Eves ND, Haithcock BE, Loewen G, Kohman L. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010c;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, Lajeunesse L, Sharma R, Small D. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:602–607. doi: 10.1097/JTO.0b013e31819e77e8. [DOI] [PubMed] [Google Scholar]
- Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29:726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, Adloff K, Keshaviah A, Winer EP. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- MacNeil B, Hoffman-Goetz L. Exercise training and tumour metastasis in mice. influence of time of exercise onset. Anticancer Res. 1993;13:2085–2088. [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, Potter JD, Schwartz RS. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64:2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006a;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009a;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006b;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Ogino S, Kirkner GJ, Chan AT, Wolpin B, Ng K, Nosho K, Shima K, Giovannucci EL, Loda M, Fuchs CS. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009b;15:5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Ogino S, Kirkner GJ, Chan AT, Wolpin B, Ng K, Nosho K, Shima K, Giovannucci EL, Loda M, Fuchs CS. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009c;15:5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman PG, Jones LW, Akushevich L, Schildkraut JM. Recreational physical activity and ovarian cancer risk and survival. Ann Epidemiol. 2011;21:178–187. doi: 10.1016/j.annepidem.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa T, Kuchiba A, Yamauchi M, Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS, Ogino S. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305:1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na YM, Kim MY, Kim YK, Ha YR, Yoon DS. Exercise therapy effect on natural killer cell cytotoxic activity in stomach cancer patients after curative surgery. Arch Phys Med Rehabil. 2000;81:777–779. doi: 10.1016/s0003-9993(00)90110-2. [DOI] [PubMed] [Google Scholar]
- Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35:635–642. doi: 10.1188/08.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, Gilliland FD, Baumgartner KB, Sorensen B, McTiernan A, Ulrich CM. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Tr. 2009;114:155–167. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, Al-Delaimy WK, Thomson CA, Kealey S, Hajek R, Parker BA, Newman VA, Caan B, Rock CL. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck BD, McCaffrey J, Baumgartner KJ. Protective effects of voluntary exercise during the postinitiation phase of pancreatic carcinogenesis in the rat. Cancer Res. 1990;50:6811–6816. [PubMed] [Google Scholar]
- Ruden E, Reardon DA, Coan AD, Herndon JE, 2nd, Hornsby WE, West M, Fels DR, Desjardins A, Vredenburgh JJ, Waner E, Friedman AH, Friedman HS, Peters KB, Jones LW. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011;29:2918–2923. doi: 10.1200/JCO.2011.34.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Saez MC, Barriga C, Garcia JJ, Rodriguez AB, Ortega E. Exercise-induced stress enhances mammary tumor growth in rats: beneficial effect of the hormone melatonin. Mol Cell Biochem. 2007;294:19–24. doi: 10.1007/s11010-005-9067-5. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14:1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, Venner PM, Quinney HA, Jones LW, D’Angelo ME, Wells GA. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol. 2003;21:1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- Segal RJ, Reid RD, Courneya KS, Sigal RJ, Kenny GP, Prud’Homme DG, Malone SC, Wells GA, Scott CG, Slovinec D’Angelo ME. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27:344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Weltzien E, Quesenberry CP, Jr, Castillo AL, Kwan M, Slattery ML, Caan BJ. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti KP, Hackney AC, Battaglini CL, Evans ES, Groff D. Exercise in patients with breast cancer and healthy controls: energy substrate oxidation and blood lactate responses. Integr Cancer Ther. 2011;10:6–15. doi: 10.1177/1534735410387600. [DOI] [PubMed] [Google Scholar]
- Uhlenbruck G, Order U. Can endurance sports stimulate immune mechanisms against cancer and metastasis? Int J Sports Med. 1991;12 (Suppl 1):S63–68. doi: 10.1055/s-2007-1024753. [DOI] [PubMed] [Google Scholar]
- Umpierre D, Ribeiro PA, Kramer CK, Leitao CB, Zucatti AT, Azevedo MJ, Gross JL, Ribeiro JP, Schaan BD. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: immune function and exercise. Exerc immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa Y, Nagasaki H, Akiyama Y, Hashimoto Y, Takizawa T, Kojima K, Kawano T, Sugihara K, Imai K, Nakachi K. DNA methylation status is inversely correlated with green tea intake and physical activity in gastric cancer patients. International journal of cancer. J Int Cancer. 2009;124:2677–2682. doi: 10.1002/ijc.24231. [DOI] [PubMed] [Google Scholar]
- Zeng H, Irwin ML, Lu L, Risch H, Mayne S, Mu L, Deng Q, Scarampi L, Mitidieri M, Katsaros D, Yu H. Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res Tr. 2011 doi: 10.1007/s10549-011-1716-7. [DOI] [PubMed] [Google Scholar]
- Zheng X, Cui XX, Gao Z, Zhao Y, Shi Y, Huang MT, Liu Y, Wagner GC, Lin Y, Shih WJ, Rao CV, Yang CS, Conney AH. Inhibitory effect of dietary atorvastatin and celecoxib together with voluntary running wheel exercise on the progression of androgen-dependent LNCaP prostate tumors to androgen independence. Exp Ther Med. 2011;2:221–228. doi: 10.3892/etm.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 2004;96:2249–2256. doi: 10.1152/japplphysiol.01210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]