Abstract
Aluminium (Al)-activated citrate secretion plays an important role in Al resistance in a number of plant species, such as rice bean (Vigna umbellata). This study further characterized the regulation of VuMATE1, an aluminium-activated citrate transporter. Al stress induced VuMATE1 expression, followed by the secretion of citrate. Citrate secretion was specific to Al stress, whereas VuMATE1 expression was not, which could be explained by a combined regulation of VuMATE1 expression and Al-specific activation of VuMATE1 protein. Pre-treatment with a protein translation inhibitor suppressed VuMATE1 expression, indicating that de novo biosynthesis of proteins is required for gene expression. Furthermore, post-treatment with a protein translation inhibitor inhibited citrate secretion, indicating that post-transcriptional regulation of VuMATE1 is critical for citrate secretion. Protein kinase and phosphatase inhibitor studies showed that reversible phosphorylation was important not only for transcriptional regulation of VuMATE1 expression but also for post-translational regulation of VuMATE1 protein activity. These results suggest that citrate secretion is dependent on both transcriptional and post-transcriptional regulation of VuMATE1. Additionally, VuMATE1 promoter–β-glucuronidase fusion lines revealed that VuMATE1 expression was restricted to the root apex and was entirely Al induced, indicating the presence of cis-acting elements regulating root tip-specific and Al-inducible gene expression, which will be an important resource for genetic improvement of plant Al resistance.
Key words: aluminium toxicity, cis-acting element, promoter, reversible phosphorylation, signalling transduction, transcription factor.
Introduction
Aluminium (Al)-inducible organic acid (OA) secretion from plant roots is an important mechanism controlling the degree of resistance to Al toxicity in many plant species (Ryan et al., 2001; Kochian et al., 2004; Delhaize et al., 2012). These plant species can be divided into two groups based on the timing of secretion (Ma et al., 2001). In the pattern I type, roots secrete OAs immediately upon suffering Al stress; wheat (Triticum aestivum) and buckwheat (Fygopyrum esculentum Moench) are examples of this pattern. In plants of the pattern II type, an induction period after the onset of Al stress is required before the roots secrete OAs; Cassia tora and sorghum (Sorghum bicolor) fit this pattern. The major difference between the two patterns lies in whether or not gene induction is required for OA secretion. The malate transporter gene ALUMINIUM-ACTIVATED MALATE TRANSPORTER 1 (ALMT1) was first isolated in wheat (Sasaki et al., 2004), and homologous genes have been isolated in Arabidopsis (Hoekenga et al., 2006) and oilseed rape (Brassica napus) (Ligaba et al., 2006). Genes involved in Al-induced citrate secretion, which are members of the multidrug and toxic compound extrusion (MATE) family, have also been isolated in barley (Hordeum vulgare) (Furukawa et al., 2007), sorghum (Magalhaes et al., 2007), Arabidopsis (Liu et al., 2009), maize (Zea mays) (Maron et al., 2010), wheat (Ryan et al., 2009), rye (Secale cereale) (Yokosho et al., 2010), rice (Oryza sativa) (Yokosho et al., 2011) and rice bean (Vigna umbellata) (Yang et al., 2011). However, the mechanisms regulating the expression of these genes are not clear.
Protein phosphorylation has been implicated in Al-inducible OA secretion. For example, protein kinase inhibitors have been demonstrated to block Al-activated malate secretion from wheat roots (Osawa and Matsumoto, 2001) and citrate secretion from soybean (Glycine max) roots (Shen et al., 2004). As ALMT1 is expressed constitutively in wheat roots (Sasaki et al., 2004), it is reasonable to deduce that these inhibitors may have been acting on the protein rather than on the pathways regulating expression. Ligaba et al. (2009) generated a series of mutations targeting possible phosphorylation sites on TaALMT1, and expression of some of these mutant proteins in Xenopus oocytes resulted in altered current events either in the absence or presence of Al. However, Kobayashi et al. (2007) demonstrated that malate secretion from Arabidopsis roots under Al stress requires protein phosphorylation, which acts on AtALMT1 at both transcriptional and post-translational levels. These results indicated that protein phosphorylation could be important for OA secretion from plant roots with either the pattern I or pattern II response.
In Arabidopsis, both malate and citrate are secreted from roots in response to Al stress (Hoekenga et al., 2003). Expression of both AtMATE1 and AtALMT1 requires SENSITIVE TO PROTON RHIZOTOXICITY 1 (STOP1), a putative Cys2His2-type zinc-finger transcription factor involved in low pH resistance and Al tolerance in Arabidopsis (Iuchi et al., 2007; Liu et al., 2009). Yamaji et al. (2009) identified the rice transcription factor ALUMINIUM RESISTANCE TRANSCRIPTION FACTOR 1 (ART1), which regulates 31 genes implicated in both internal and external detoxification of Al at different cellular levels. Among these, the expression of OsFRDL4, which mediates Al-activated citrate secretion from rice roots, requires ART1 (Yokosho et al., 2011). Both STOP1 in Arabidopsis and ART1 in rice are expressed constitutively, indicating that Al may trigger the post-translational regulation of both transcription factors to affect downstream gene expression.
Rice bean is an Al-resistant species and is well adapted to grow on acid soils by means of secretion of citrate from root apices (Yang et al., 2006). However, citrate secretion upon Al exposure is delayed by several hours, suggesting a pattern II response for this species. Recently, we isolated a gene responsible for Al-inducible citrate secretion from rice bean roots, VuMATE1 (Yang et al., 2011). In the absence of Al stress, VuMATE1 is not expressed (Yang et al., 2011).
This study sought to clarify the interplay between VuMATE1 expression and citrate secretion in rice bean to elucidate the underlying regulatory mechanisms. The results demonstrated that, although citrate secretion was specific to Al stress, VuMATE1 expression was not, and it was found that citrate secretion comprised a complex regulation pathway involving both de novo protein biosynthesis and reversible protein phosphorylation. Furthermore, we characterized the 5’ flanking sequence of the VuMATE1 gene, which contains cis-acting elements regulating both root tip-specific and Al-inducible gene expression.
Materials and methods
Plant materials and culture conditions
Seeds of rice bean (V. umbellata) were soaked in deionized water overnight, and germinated at 26 ºC in the dark. After germination, the seeds were transferred to a net floating on a 0.5mM CaCl2 solution (pH 4.5). The solution was renewed daily. On d 3, seedlings were transferred to a 1.2 l plastic pot (eight seedlings per pot) containing aerated nutrient solution. The nutrient solution contained the following macro- and micronutrients (μM): CaSO4 (200), CaCl2 (200), MgSO4 (100), KNO3 (400), NH4NO3 (300), NaH2PO4 (5), H3BO3 (3), MnCl2 (0.5), ZnSO4 (0.4), CuSO4 (0.2), Fe-EDTA (10), and (NH4)6Mo7O24 (1). The solution was adjusted to pH 4.5 with HCl and renewed daily. The plants, which were grown for an additional 3–5 d in an environmentally controlled growth room with a 14h/26 ºC day (light intensity of 300 μmol photons m–2 s–1) and a 10h/22 ºC night regime, were used for the following experiments.
Treatments
Root apices (apical 10mm) were excised with a razor blade, and ten root apices were incubated at 26 ºC in a 5ml centrifuge tube containing 3ml of 0.5mM CaCl2 solution (pH 4.5; Ca solution). Root apices were rinsed with the Ca solution three times (10min each) to diminish ion leakage derived from cutting damage. During all incubation periods, root apices were shaken at 80rpm. For the time-course study, root apices were exposed to Ca solution containing either 25 μM AlCl3 (Al solution) or 25 μM LaCl3 every 3h,and the old solutions and roots were collected at each time point. The protein translation inhibitor cycloheximide (CHX) and the transcription inhibitor actinomycin D were dissolved in deionized water and applied at a final concentration of 20 and 10 μM, respectively. Protein kinase and protein phosphatase inhibitors K252a and cyclosporin A were applied at a final concentration of 5 μM. They were first dissolved in a small amount of dimethyl sulfoxide and then added to treatment solutions. The effects of various inhibitors on gene expression and citrate secretion were determined in two ways. The first set of experiments was carried out by incubating the root apices in inhibitors for 1h, rinsing the roots with fresh Ca solution, and then transferring them to either Ca solution or Al solution for various periods. The second set of experiments was carried out by incubating the root apices in Al solution for 6h, rinsing the roots with fresh Ca solution, and then treating them with various inhibitors for 3h.
Citrate measurement
Citrate concentrations were determined according to the enzymatic method described by Zhao et al. (2003). Briefly, to 1ml of treatment solution, 120 μLl 1M Tris/HCl buffer (pH 7.8) and 15 μl 10mM NADH were added. After incubation at 25 ºC for 40min, 2 μl enzyme mixture (containing 1.25U lactate dehydrogenase and 0.5U malate dehydrogenase) was added and the reaction mixture was incubated for a further 40min. The change in A 340 was monitored after the reaction was initiated with citrate lyase (0.5U). Standard measurements were performed using the same procedure.
RNA extraction and quantitative real-time RT-PCR
Plant material was ground in liquid nitrogen and total RNA was extracted using an RNAplant_plus kit (Tiangen, Shanghai, China). First-strand cDNAs were synthesized from 1 μg total RNA using a PrimeScriptTM RT-PCR Kit (Takara Bio, Japan), and diluted to 50–100ng μl–1. Quantitative real-time PCR was performed on a LightCycler480 machine (Roche Diagnostics, Basel, Switzerland) using a SYBR PremixEx Taq kit (Takara Bio). The primer pairs used have been described previously (Yang et al., 2011). Three biological replicate RNA/cDNA samples were generated, and each cDNA sample was tested with three technical replicates, from which the relative expression was calculated against that of the internal control 18S rRNA gene using the formula 2–ΔΔCp.
Construction of genomic libraries and isolation of the VuMATE1 promoter
The 1.55kb VuMATE1 promoter was obtained according to the user manual of the GenomeWalkerTM Universal Kit (Clontech, CA, USA). In brief, four genome walker libraries were constructed by digesting separate aliquots of DNA with four different restriction enzymes (DraI, EcoRV, PvuII, and StuI), followed by ligation to a genome walker adaptor. Nested PCR was performed using the outer/inner adaptor primer provided by the kit and two VuMATE1 gene-specific primers (5’-TTGGTGCTGAAAGATGACTGGAGAAGTGAT-3’ and 5’-GATCTTCTACATTGGGTGCCTAACT-3’). After the nested PCR, amplified fragments were cloned into the pMD18-T vector (Takara Bio). The sequences that extended upstream of the cDNA clones were isolated as the 5’-upstream regions of the gene.
Construction of the VuMATE1–β-glucuronidase (GUS) chimaeric gene and histochemical staining of VuMATE1–GUS plants
The 1.55kb VuMATE1 promoter was amplified from genomic DNA (primers MATE1GUSfor 5’-GGTACCTAAGGCTCGCA CAAGCTTCA-3’ and MATE1GUSrev 5’-CCATGGTAATGAATT TTGATGAACTC-3’) and cloned into the pMD18-T vector (Takara Bio). The correct nucleotide sequence was verified and subcloned into the multicloning site between KpnI and NcoI of pCAMBIA1301. The generated VuMATE1p::GUS construct was introduced into the Agrobacterium tumefaciens strain EHA105, and A. tumefaciens-mediated transformation of Arabidopsis plants (Col-0) was accomplished according to the floral dip protocol (Clough and Bent, 1998). T1 seeds were surface sterilized and plated on one-half strength MS medium supplemented with hygromycin. The resistant plants were transferred to soil and allowed to set seed (T2). Transgenic lines that displayed a 3:1 ratio for hygromycin resistance in the T3 generation were selected for further analysis. All experiments were performed using plants corresponding to the T4 generation. GUS staining was performed according to the method of Jefferson et al. (1987) after exposure to Al (1.0 μM activity) at pH 5.0. Seedlings were observed and photographed with a Nikon AZ100 microscope.
Results
Time course of Al-induced VuMATE1 expression and citrate secretion
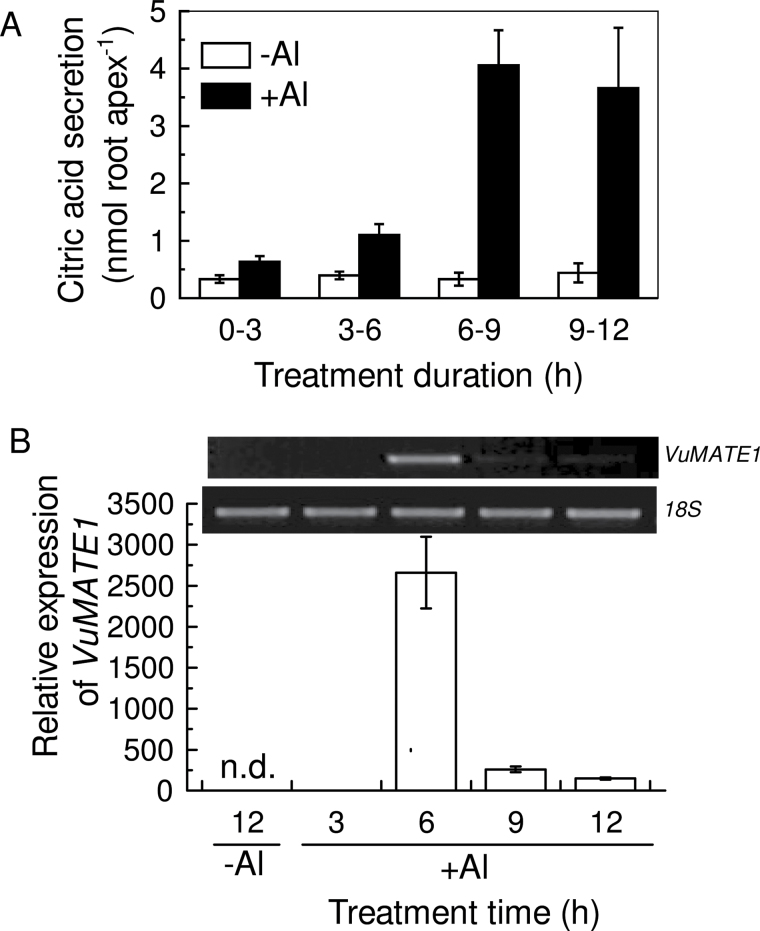
There is a lag phase for secretion of citrate from the intact roots of rice bean in response to Al treatment (Yang et al., 2006), which is typical of pattern II OA release. To establish whether gene induction was required for this pattern II response, we performed a time-course analysis of citrate secretion and VuMATE1 expression in the root apex. Citrate secretion rates were equivalent in both Al-treated and control plants at the first 3h time point (Fig. 1A). Citrate secretion rates remained unchanged for the duration of the treatment in the absence of Al, but increased with Al treatment, reaching a steady state after 6–9h of treatment. In parallel, no signals could be detected for VuMATE1 mRNA in the absence of Al treatment, and only very low levels of expression were observed within 3h of Al treatment. However, the transcript abundance of VuMATE1 greatly increased after 6h of Al treatment and then decreased to relatively low levels thereafter (Fig. 1B). Our previous study demonstrated that VuMATE1 expression remained constant after reaching a maximum by 6h of Al exposure (Yang et al., 2011). This discrepancy might arise from the different experimental conditions used, i.e. whole roots in the previous study and excised root apices in the present study. From the above results, it was clear that, in addition to the lag between the onset of Al stress and VuMATE1 expression, there was a further 3h lag between gene induction and citrate secretion.
Fig. 1.
Citrate release and VuMATE1 expression time series. (A) Induction of citrate secretion from root apices of rice bean by Al treatment. Excised root apices (10mm in length) were placed in 0.5mM CaCl2 solution containing 0 or 25 μM Al. Data are means ± standard deviation (SD) of three independent experiments (n=3 for each experiment). (B) Time course of VuMATE1 expression in the roots of rice bean in response to Al treatment. Expression levels were normalized to that of the 18S rRNA gene and then to the expression of VuMATE1 in root apices at 3h of Al treatment, which was arbitrarily fixed at 1. VuMATE1 expression in root apices treated with 0.5mM CaCl2 solution for 12h was included. Results are shown as mean expression ±SD of three independent experiments. n.d. indicates that no signal was detected. The inset shows semi-quantitative RT-PCR of VuMATE1 expression.
Effect of La on VuMATE1 expression and citrate secretion
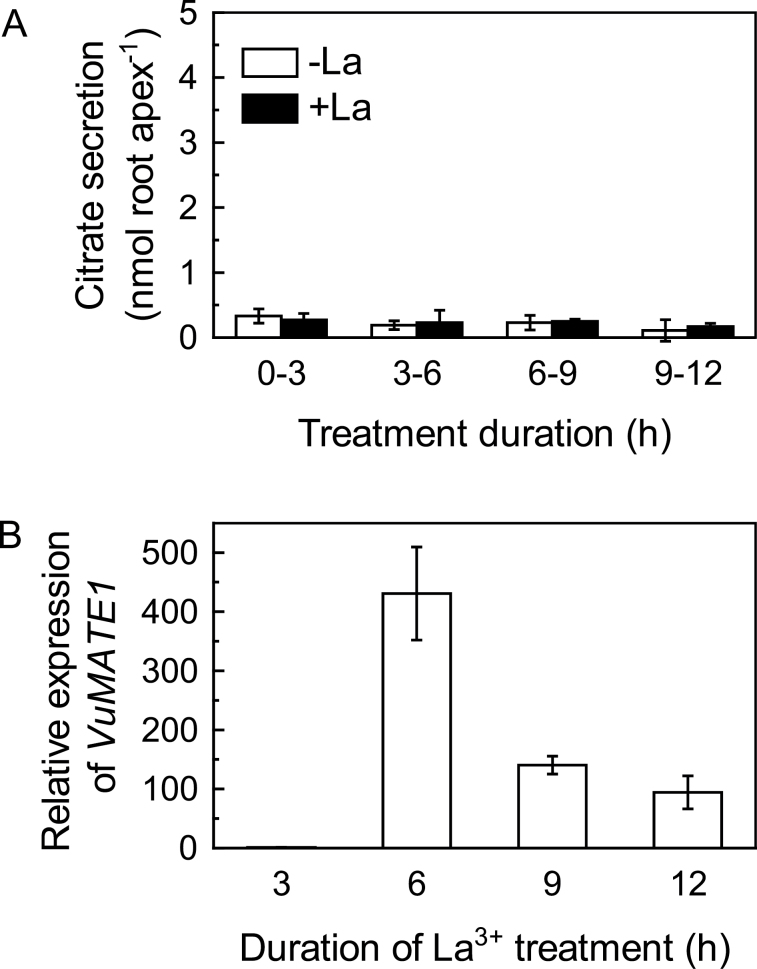
Several lanthanides such as La3+ and Yb3+ have ionic properties similar to those of Al3+ in an aqueous solution. In the Al-resistant wheat genotype ET8, these lanthanides can induce malate secretion (Kataoka et al., 2002). However, whether TaALMT1 induction is involved in lanthanide-induced malate secretion from wheat roots is not clear. In our previous study, we demonstrated that rice bean roots did not respond to La stress in terms of citrate secretion, indicating that citrate secretion is specific to Al stress in rice bean (Yang et al., 2011). To determine whether VuMATE1 expression was specific to Al treatment as well, we exposed root apices to La stress treatment. Citrate secretion rates were equivalent in both La-stressed roots and controls during the entire 12h of treatment (Fig. 2A). However, VuMATE1 expression was induced by La stress and the timing of expression was similar to that under Al stress, although the expression level was lower in La-stressed roots than in Al-stressed roots (Figs 1B and 2B).
Fig. 2.
Time course of citrate release and VuMATE1 expression in the roots of rice bean in response to LaCl3 treatment. (A) Induction of citrate secretion from root apices of rice bean by La treatment. Excised root apices (10mm in length) were placed in 0.5mM CaCl2 solution containing 0 or 25 μM La. Data are means ±SD of three independent experiments (n=3 for each experiment). (B) Time course of VuMATE1 expression in the roots of rice bean in response to La treatment. Expression levels were normalized to that of the 18S rRNA gene and then to VuMATE1 expression in the root apices at 3h of La treatment, which was arbitrarily fixed at 1. Results are shown as mean expression ±SD of three independent experiments.
De novo protein biosynthesis is involved in Al-induced gene expression and citrate secretion
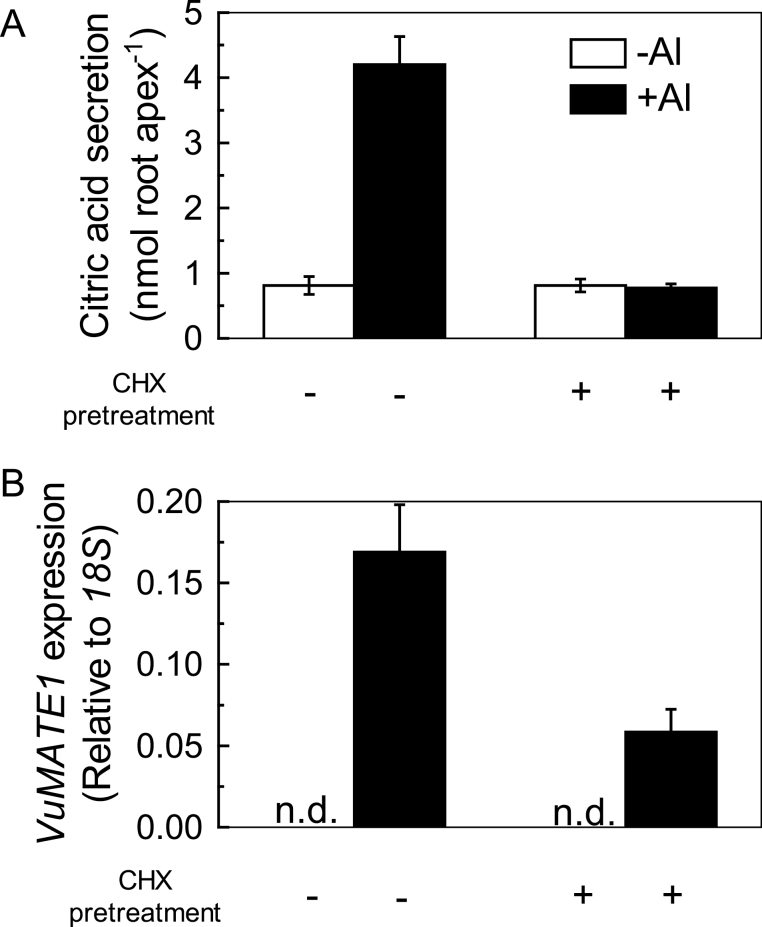
We demonstrated previously that application of CHX, a broad translation inhibitor, effectively inhibited Al-triggered citrate secretion from the intact roots of rice bean (Yang et al., 2006). To examine whether de novo biosynthesis of proteins was required for VuMATE1 induction, CHX was applied in two sets of experiments. First, excised root apices were treated with or without CHX for 1h and then moved to Ca solution with or without Al. CHX pre-treatment completely blocked Al-inducible citrate secretion during the following 9h of Al exposure. CHX pre-treatment also resulted in inhibition of VuMATE1 expression by 65% (Fig. 3). The time course analysis of VuMATE1 expression with CHX pre-treatment showed that the transcript abundance was very low in the first 6h of Al exposure (data not shown), indicating that the VuMATE1 expression detected at the 9h time point was probably due to diminishing CHX action. In the absence of Al stress, VuMATE1 expression was not detected, irrespective of treatment with CHX or not. These results implied that de novo protein biosynthesis is required for VuMATE1 expression, and rule out the possible involvement of a repressor protein suppressing VuMATE1 transcription in the absence of Al.
Fig. 3.
Effect of protein-synthesis inhibitor (CHX, 20 μM) pre-treatment on the activation of citrate secretion from rice bean roots by Al treatment. Root apices pre-incubated for 1h in 0.5mM CaCl2 solution with or without CHX were transferred to solutions with or without Al for 9h. (A) Root exudates were collected for measurement of citrate. Results are shown as means ±SD of three independent experiments (n=3 for each experiment). (B) Root apices were used to analyse gene expression. Expression of VuMATE1 was analysed by quantitative RT-PCR, using the 18S rRNA gene as a reference. Results are shown as mean expression ±SD of three independent experiments. n.d. indicates that no signal was detected.
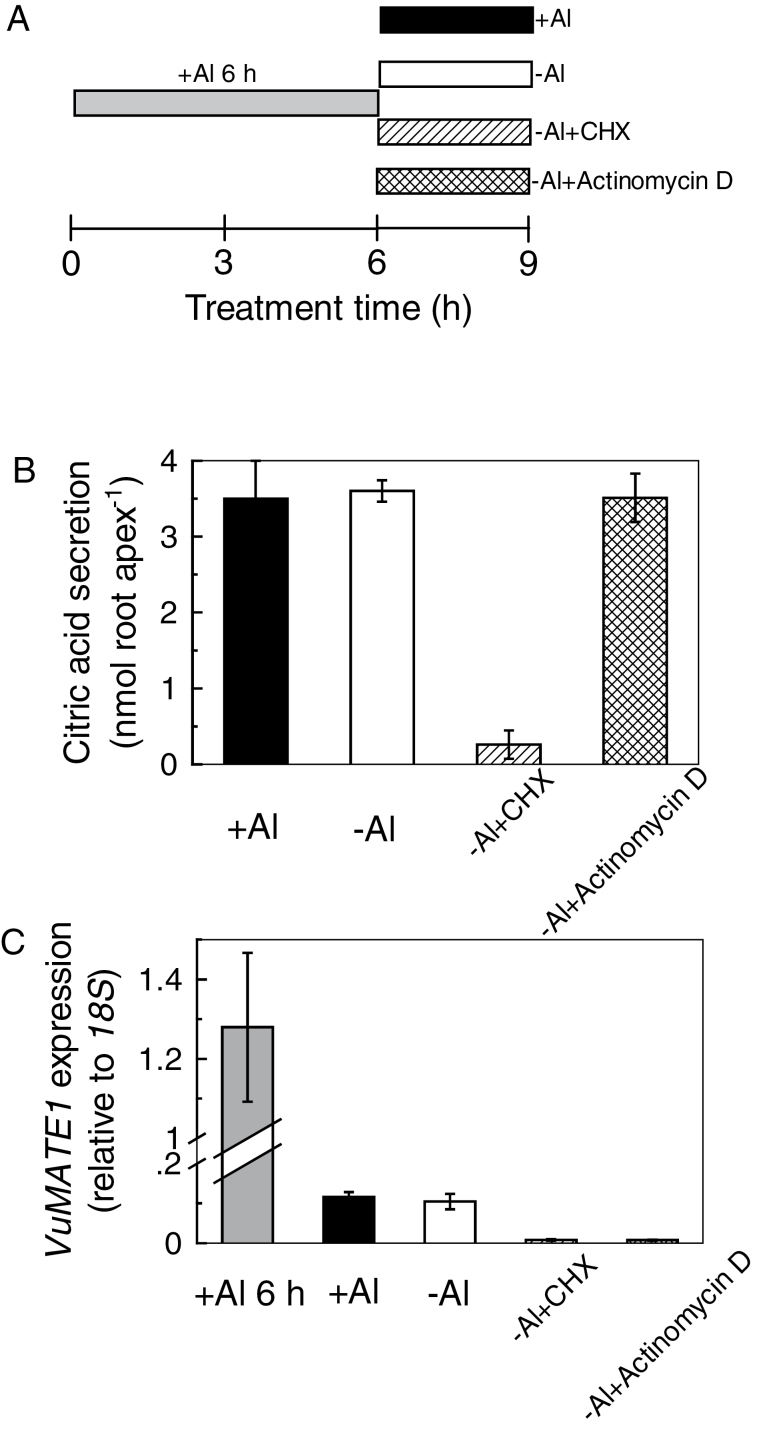
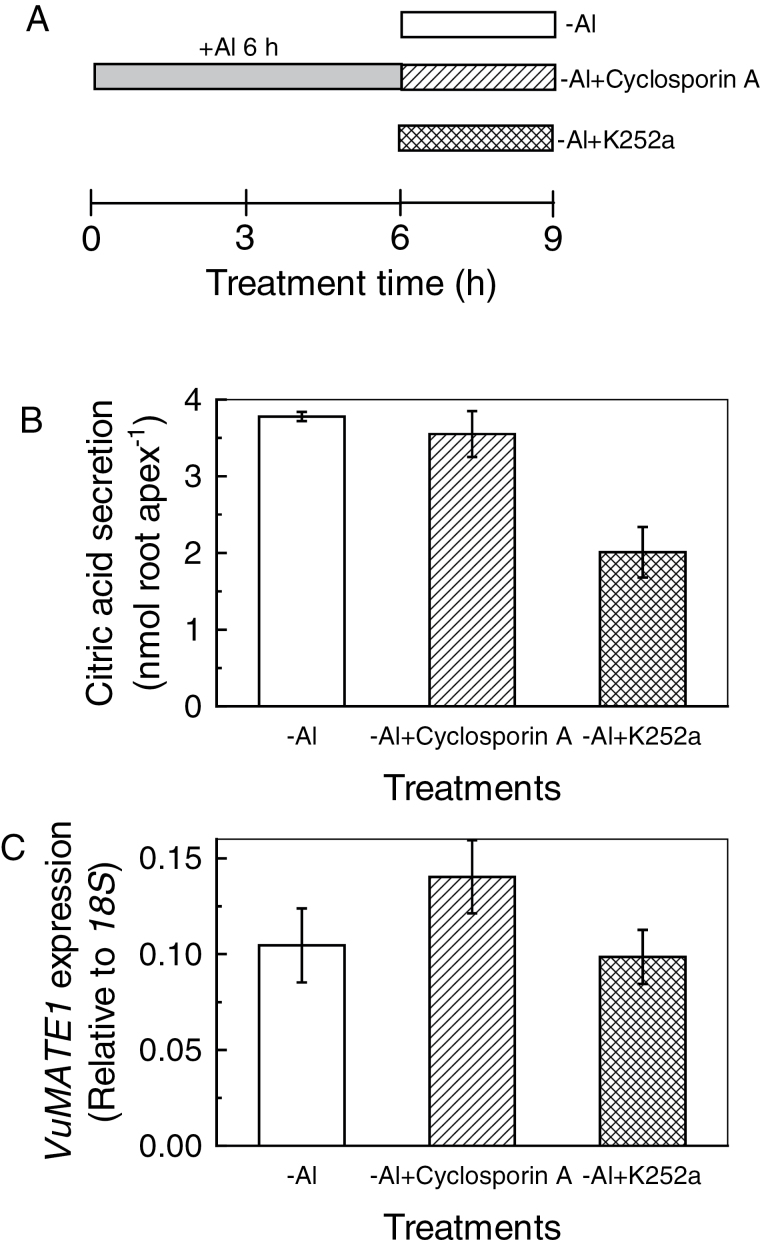
Secondly, root apices were treated with Al for 6h and then moved to Al-containing solution (as a control) or Al-free solution with or without CHX for 3h (CHX post-treatment; Fig. 4A). In comparison with continued Al stress, withdrawal of Al had no effect on citrate efflux during the following 3h of treatment (Fig. 4B), indicating either that Al is not required for citrate efflux after gene induction or that the residual Al was sufficient to maintain secretion. However, when CHX was included in the medium, secretion of citrate was almost completely blocked (Fig. 4B). In parallel, there were no significant differences in VuMATE1 transcript abundance between continuous Al stress and withdrawal of Al, and the expression under both conditions was significantly lower than that after 6h of Al stress (Fig. 4C). The VuMATE1 mRNA decreased to a very low level with CHX post-treatment (Fig. 4C). These results indicated that the translation of VuMATE1 into protein after gene induction is the critical step for citrate secretion. To rule out the contribution of VuAMTE1 transcription to citrate efflux in this experiment, we used the transcription inhibitor actinomycin D. Application of actinomycin D to pre-activated root apices completely inhibited VuMATE1 expression but had no effect on citrate secretion (Fig. 4).
Fig. 4.
Effect of a translation inhibitor (CHX, 20 μM) or a transcription inhibitor (actinomycin D) on pre-activated citrate secretion from rice bean roots. (A) Summary of treatment conditions. Root apices were pre-incubated in Al (25 μM) for 6h (shaded bar), and transferred to Al solution (+Al; filled bar) or to Ca solution with CHX (–Al+CHX; striped bar), actinomycin D (–Al+actinomycin D; hatched bar), or neither (–Al; open bar) for 3h. (B) Root exudates were collected for citrate measurement. Results are shown as means ±SD of three independent experiments (n=3 for each experiment). (C) Root apices were used to analyse gene expression. For comparison, VuMATE1 expression in pre-activated roots (Al 6h) was included. Expression of VuMATE1 was analysed by quantitative RT-PCR, using the 18S rRNA gene as a reference. Results are shown as mean expression ±SD of three independent experiments.
Protein phosphorylation is involved in Al-induced gene expression and citrate secretion
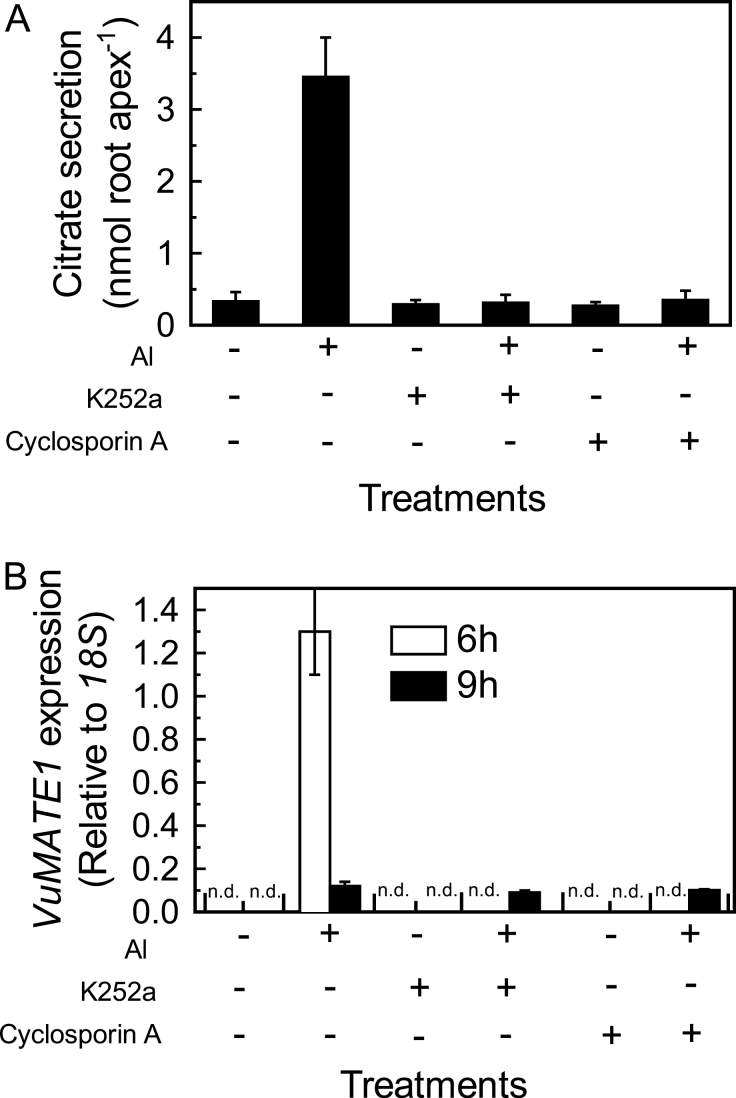
Protein phosphorylation has been implicated in Al-induced malate and citrate secretion from plant roots (Osawa and Matsumoto, 2001; Shen et al., 2004; Ligaba et al., 2009). We examined whether reversible protein phosphorylation affected Al-inducible citrate secretion in rice bean roots by perturbing protein kinase and phosphatase function using the protein kinase inhibitor K252a and the protein phosphatase inhibitor cyclosporin A. These inhibitors were applied in two sets of experiments. First, inhibitors were used to pre-treat root apices before Al exposure. Pre-treatment with either K252a or cyclosporin A completely blocked Al-induced citrate secretion from root apices during the following 9h (Fig. 5A). There are various levels at which reversible protein phosphorylation could regulate Al-inducible citrate secretion: acting on VuMATE1 protein to modulate transport activity, influencing VuMATE1 expression, or both. After 6h of Al exposure, VuMATE1 expression was not detected in root apices pre-treated by the inhibitors, and after 9h of Al exposure, it was only beginning to be expressed (Fig. 5B), indicating that protein phosphorylation is required for transcriptional regulation of VuMATE1 expression.
Fig. 5.
Effect of protein kinase inhibitor or protein phosphatase inhibitor pre-treatment on the activation of citrate secretion from rice bean roots. Root apices pre-incubated for 1h in 0.5mM CaCl2 solution containing 5 μM protein kinase inhibitor K252a, 5 μM protein phosphatase inhibitor cyclosporin A, or neither were transferred to solutions with or without Al for 9h. (A) Root exudates were collected for measurement of citrate. Results are shown as means ±SD of three independent experiments (n=3 for each experiment). (B) Root apices were used to analyse gene expression. Expression of VuMATE1 was analysed by quantitative RT-PCR, using the 18S rRNA gene as a reference. Results are shown as mean expression ±SD of three independent experiments. n.d. indicates that no signal was detected.
Secondly, these inhibitors were used to treat root apices already stressed by Al in order to examine the effect of these inhibitors on transcribed VuMATE1. Root apices were treated with Al for 6h and then transferred to Ca solution with or without the inhibitors (Fig. 6A). In comparison with Ca solution, application of cyclosporin A had no effect on citrate secretion, whilst K252a decreased citrate secretion by 47% (Fig. 6B). In parallel, these inhibitors had no effect on VuMATE1 expression (Fig. 6C). The results indicated that a K252a-sensitive protein kinase is involved in post-transcriptional regulation of VuMATE1 activity.
Fig. 6.
Effect of protein kinase inhibitor (K252a, 5 μM) or protein phosphatase inhibitor (cyclosporin A, 5 μM) on pre-activated citrate secretion from rice bean roots. (A) Treatment conditions. Root apices were pre-incubated in Al (25 μM) for 6h, and transferred to Ca solution with cyclosporin A (–Al+cyclosporin A; striped bar), K252a (–Al+K252a; hatched bar) or neither (–Al; open bar) for 3h. (B) Root exudates were collected for citrate measurement. Results are shown as means ±SD of three independent experiments (n=3 for each experiment). (C) Root apices were used to analyse gene expression. Expression of Vu-MATE1 was analysed by quantitative RT-PCR, using the 18S rRNA gene as a reference. Results are shown as mean expression ±SD of three independent experiments.
Tissue-level localization of VuMATE1 expression via promoter–GUS fusions
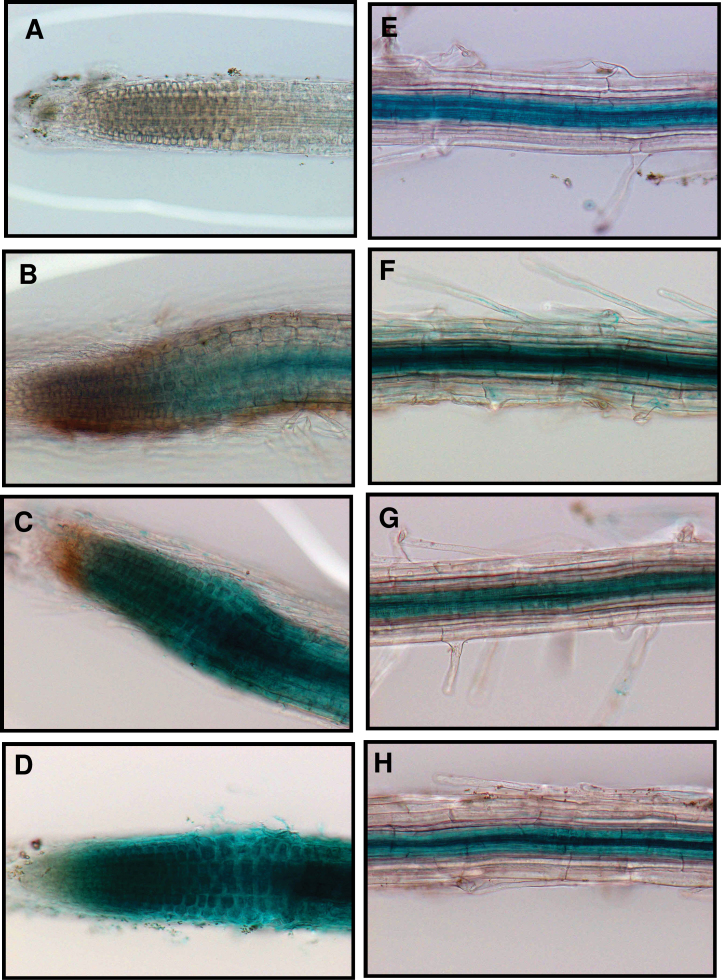
To analyse further the expression pattern and location of VuMATE1, a 1.55kb DNA sequence upstream of the start codon was isolated. This promoter fragment was fused to a GUS reporter gene and transformed into wild-type Arabidopsis by Agrobacterium-mediated transformation. In the absence of Al stress, GUS staining was not observed in the root apex (Fig. 7A). After a 3h exposure of the roots to Al, GUS staining appeared from the central cylinder, and extended to the entire root apex after prolonged exposure (Fig. 7B–D). In the mature root, the vascular cylinder was the site of GUS activity independent of Al stress, whilst the cortical and epidermis cells did not show expression (Fig. 7E–H).
Fig. 7.
VuMATE1p::GUS expression in transgenic plants. Ten-d-old seedlings were treated with modified one-fifth-strength Hoagland solution (pH 5.0) containing 7 μM Al3+ (1 μM activity) for 0 (A, E), 3 (B, F), 6 (C, G), or 9 (D, H) h. (A)–(D) show the root apex, and (E)–(H) show the mature zone.
Discussion
Al-induced citrate secretion is a major Al tolerance mechanism in both dicots and monocots, mediated by members of the MATE family (Delhaize et al., 2012). VuMATE1 is responsible for Al-activated citrate secretion in rice bean roots (Yang et al., 2011). We have shown here that the function of VuMATE1 under Al stress is subject to both transcriptional and post-transcriptional regulation, and that VuMATE1 expression is necessary but not sufficient for citrate secretion in rice bean.
Citrate secretion was more tightly regulated by VuMATE1 protein activity than by the pattern of gene expression. First, whereas La treatment enhanced accumulation of VuMATE1 mRNA, it did not increase the rate of citrate secretion from the root apex (Fig. 2). Secondly, VuMATE1 expression was maximal after 6h of Al exposure, but citrate secretion rates took until 9h of Al exposure to reach their maximum (Fig. 1). This inconsistency suggests either that it takes about 3h to translate VuMATE1 mRNA into protein or that regulation of the transport activity of VuMATE1 depends on some other activating factors. Thirdly, actinomycin D was able to completely inhibit VuMATE1 expression but had no effect on citrate secretion once the root apex had been pre-activated by Al stress (Fig. 4). Finally, the protein kinase inhibitor K252a could block citrate secretion without reducing VuMATE1 expression levels (Fig. 6).
VuMATE1 is upregulated by Al stress, suggesting the involvement of upstream sensing and/or signalling components. Recent studies on rice and Arabidopsis have identified ART1 (Yamaji et al., 2009) and STOP1 (Liu et al., 2009) as the core components regulating MATE and ALMT genes involved in OA secretion. It is reasonable to infer that a functional homologue of STOP1 or ART1 also exists in rice bean. As neither STOP1 in Arabidopsis nor ART1 in rice expression is induced by Al, the signal transduction pathway probably involves post-transcriptional and post-translational regulation of both regulators. It can be assumed that STOP1 and ART1 proteins accumulate upon Al stress and activate gene transcription, or that protein abundance is not affected but instead the proteins are activated directly by the Al signal. In rice bean, pre-treatment with CHX for 1h resulted in inhibition of VuMATE1 transcription (Fig. 3), implying that regulator(s) involved in VuMATE1 expression need to be synthesized de novo.
We have demonstrated that reversible protein phosphorylation is necessary for VuMATE1 expression and citrate secretion. Reversible protein phosphorylation is also implicated in Al-induced malate secretion from the wheat root apex and citrate secretion from soybean roots (Osawa and Matsumoto, 2001; Shen et al., 2004). However, the question arises as to whether protein kinases and phosphatases act upstream or downstream of transporters to regulate OA secretion. Pre-treatment with the protein kinase inhibitor K252a or protein phosphatase inhibitor cyclosporin A blocked citrate secretion, which closely followed the blocking of gene expression (Fig. 5). This indicated that both protein kinases and phosphatases are required to activate regulators acting upstream of VuMATE1. This is consistent with similar experiments in Arabidopsis, where both a protein kinase inhibitor (staurosporine) and a phosphatase inhibitor (calyculin A) reduce Al-induced malate secretion, probably due to a reduction in AtALMT1 expression (Kobayashi et al., 2007). On the other hand, we further demonstrated that protein kinases are also involved in post-transcriptional regulation of VuMATE1 activity. When Al was withdrawn from the solution bathing the root apex, citrate secretion rates remained unchanged (Figs 4B and 6B). Cyclosporin A application did not interfere with secretion rates; however, K252a treatment reduced citrate secretion rates by 47% (Fig. 6B). Because the non-inhibitory or inhibitory effects of cyclosporin A or K252a, respectively, on citrate secretion were not related to the pattern of gene expression (Fig. 6C), it is reasonable to speculate that K252a-sensitive protein kinases are involved in activating or maintaining the activity of the VuMATE1 transporter.
We demonstrated previously that the expression of VuMATE1 is entirely Al-induced and restricted to the root apex (Yang et al., 2011). In the present study, the reporter gene (GUS), when driven by the 5’ flanking sequence of the VuMATE1 gene, exhibited temporal and spatial expression patterns similar to those observed by RT-PCR analysis of gene expression (Fig. 7; Yang et al., 2011). However, we also observed constitutive expression of reporter activity in the vascular cylinder of the mature root region. Fujii et al. (2012) reported that barley HvAACT1 is typically expressed in the pericycle cells of the root mature zone, functioning as a citrate transporter required for Fe nutrition. However, in Al-tolerant barley cultivars, a 1kb insertion upstream of the HvAACT1 coding region changes the location of expression to the root tip, thereby protecting the root tip from Al toxicity (Fujii et al., 2012). Whether VuMATE1 plays two physiological roles in both detoxifying Al toxicity and regulating Fe translocation has yet to be investigated. The root tip is the major site for Al toxicity (Ryan et al., 1993). Only when the expression of Al tolerance genes is confined to this critical area is detoxification capacity maximized. For instance, although a moderate amount of citrate is secreted from Arabidopsis roots under Al stress, the role of AtMATE1 in Al resistance is diminished, as its expression is localized to the mature root regions (Liu et al., 2012). The restricted spatial distribution of Al-induced expression of VuMATE1 and GUS to the root apex suggested that cis-acting elements involved in root tip-specific expression are present in the 5’ flanking sequence of the VuMATE1 gene.
In addition to the presence of root tip-specific elements, there are Al-inducible sequences within the promoter of VuMATE1. To date, MATE genes involved in Al-mediated citrate secretion have been isolated from at least nine plant species (Delhaize et al., 2012). However, unlike VuMATE1, which is not transcribed in the root apex in the absence of Al (Fig. 7A), other MATE genes exhibit varying degrees of constitutive expression, e.g. HvAACT1 expression in barley is not affected by Al and SbMATE expression in sorghum is further increased by Al stress (Furukawa et al., 2007; Magalhaes et al., 2007). Although the transgenic approach has been proven to be a useful strategy for genetic improvement of plant performance in acidic soils, most of the transgenes are driven by constitutive promoters. For example, overexpression of VuMATE1 under the control of the CaMV35S promoter in tomato results in citrate secretion in the absence of Al (Yang et al., 2011). Considering that the efflux of OA anions constitutes a significant carbon cost to plants and may affect plant growth, and that transcription of genes consumes additional energy, limiting the expression of Al tolerance genes to only when Al toxicity occurs constitutes an efficient resource-saving strategy. Thus, dissection of cis-acting elements that regulate Al-inducible gene expression will be useful for genetic improvement of Al resistance through promoter reconstruction. However, the cis-acting sequences regulating root tip-specific gene expression and responsible for Al-inducible gene expression need further characterization.
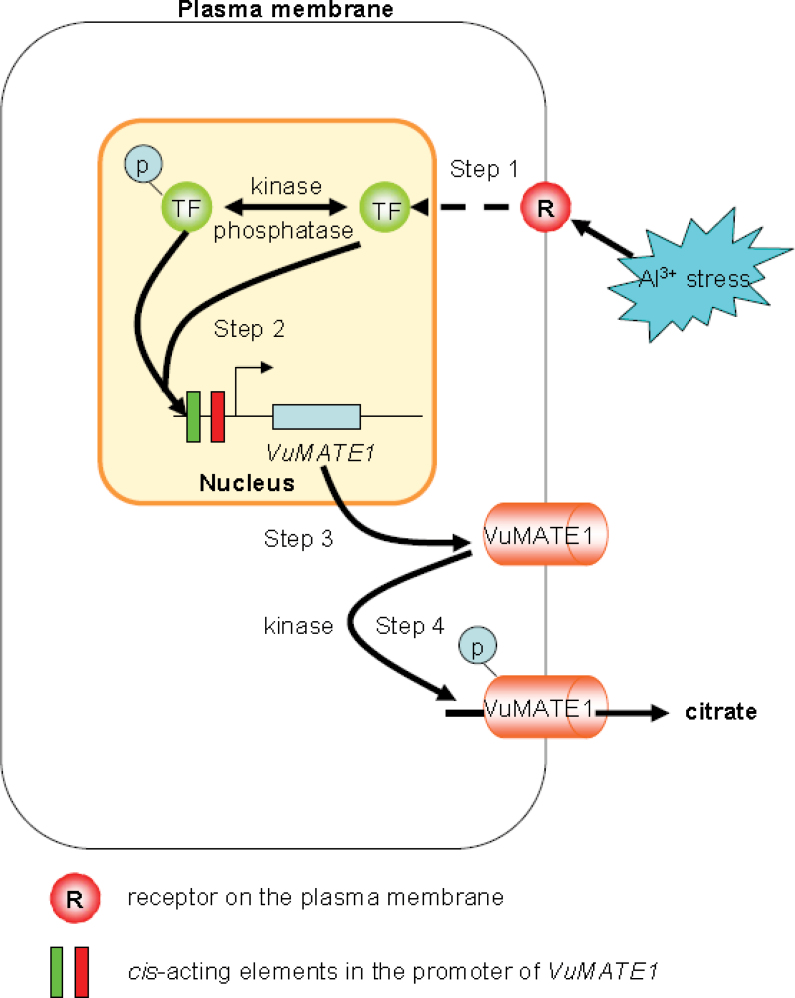
In summary, we have demonstrated that the regulation of citrate secretion in response to Al stress operates on multiple levels. As illustrated in Fig. 8, at least four steps are involved in the process. In step 1, transcription factor(s) or upstream control elements are synthesized de novo after sensing of the Al stress signal. In step 2, reversible protein phosphorylation is directly or indirectly involved in the interaction of transcription factors with the promoter of VuMATE1 to initiate the transcription of VuMATE1. As the accumulation of VuMATE1 mRNA and the appearance of GUS activity driven by the promoter of VuMATE1 began after 3h of Al exposure (Figs 1 and 7), it takes about 3h to complete the first two steps. In step 3, the full induction of VuMATE1 mRNA requires about 3h; at this time point, a portion of the mRNA has been translated into functional protein, as citrate secretion has begun. In step 4, translation of the majority of VuMATE1 mRNA into protein and translational modification are required for maximal citrate secretion, and this step may take about 3h, as the maximum secretion of citrate occurred after 9h of exposure to Al stress (Fig. 1). We also demonstrated that the promoter of VuMATE1 contains cis-acting elements regulating both root tip-specific and Al-inducible gene expression, which provides an important resource for generation of crop varieties more suitable for growth on acidic soils.
Fig. 8.
Hypothetical model for Al-induced citrate secretion from roots of a typical pattern II-type rice bean plant. At least four steps are involved in the process. Step 1: Al3+ interacts with a receptor (R) on the plasma membrane to initiate a signal transduction pathway, which triggers the de novo biosynthesis of an unidentified transcription factor (TF). Step 2: reversible protein phosphorylation is required for the TF to interact with sequences in the promoter of VuMATE1 and induce its expression. Step 3: translation of Vu-MATE1 mRNA into protein. Step 4: a K252a-sensitive protein kinase is involved in the phosphorylation of VuMATE1 for activation of VuMATE1 to transport citrate out of the cell through the plasma membrane. (This figure is available in colour at JXB online.)
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (31071849 and 31222049), the Zhejiang Education Department (20070170), and the Changjiang Scholarship and Innovative Research Team (IRT1185).
Glossary
Abbreviations:
- Al
aluminium
- CHX
cycloheximide
- GUS
β-glucuronidase
- MATE
multidrug and toxic compound extrusion
- OA
organic acid
- SD
standard deviation.
References
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ma JF, Ryan PR. 2012. Transcriptional regulation of aluminium tolerance genes. Trends in Plant Science 17, 341–348. [DOI] [PubMed] [Google Scholar]
- Fujii M, Yokosho K, Yamaji N, Saisho D, Yanane M, Takahashi H, Sato K, Nakazono M, Ma JF. 2012. Acquisition of aluminium tolerance by modification of a single gene in barley. Nature Communications 3, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF. 2007. An aluminum-activated citrate transporter in barley. Plant & Cell Physiology 48, 1081–1091. [DOI] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Piñeros MA, et al. 2006. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proceedings of the National Academy of Sciences, U S A 103, 9738–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekenga OA, Vision TJ, Shaff JE, Monforte AJ, Lee GP, Howell SH, Kochian LV. 2003. Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta×Columbia) by quantitative trait locus mapping: a physiologically simple but genetically complex trait. Plant Physiology 132, 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M. 2007. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and co-regulates a key gene in aluminum tolerance. Proceedings of the National Academy of Sciences, U S A 104, 9900–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Benan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Stekelenburg A, Nakanishi TM, Delhaize E, Ryan PR. 2002. Several lanthanides activate malate efflux from roots of aluminium-tolerant wheat. Plant Cell & Environment 25, 453–460. [Google Scholar]
- Kobayashi Y, Hoekenga OA, Itoh H, Nakashima M, Saito S, Shaff JE, Maron LG, Piñeros MA, Kochian LV, Koyama H. 2007. Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiology 145, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorus efficiency. Annual Review of Plant Biology 55, 459–493. [DOI] [PubMed] [Google Scholar]
- Ligaba A, Katsuhara M, Ryan PR, Shibasaka M, Matsumoto H. 2006. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiology 142, 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligaba A, Kochian LV, Piñeros M. 2009. Phosphorylation at S384 regulates the activity of the TaALMT1 malate transporter that underlies aluminum resistance in wheat. The Plant Journal 60, 411–423. [DOI] [PubMed] [Google Scholar]
- Liu J, Luo X, Shaff J, Liang C, Jia X, Li Z, Magalhaes J, Kochian LV. 2012. A promoter-swap strategy between the AtALMT and AtMATE genes increased Arabidopsis aluminum resistance and improved carbon-use efficiency for aluminum resistance. The Plant Journal 71, 327–337. [DOI] [PubMed] [Google Scholar]
- Liu J, Magalhaes JV, Shaff JE, Kochian LV. 2009. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. The Plant Journal 57, 389–399. [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. 2001. Aluminum tolerance in plants and the complexing role of organic acids. Trends in Plant Science 6, 273–278. [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimaraes CT, et al. 2007. A member of the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genetics 39, 1156–1161. [DOI] [PubMed] [Google Scholar]
- Maron LG, Piñeros MA, Guimaraes CT, Magalhaes JV, Pleiman JK, Mao CZ, Shaff J, Belicuas SNJ, Kochian LV. 2010. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. The Plant Journal 61, 728–740. [DOI] [PubMed] [Google Scholar]
- Osawa H, Matsumoto H. 2001. Possible involvement of protein phosporylation in aluminum-responsive malate efflux from wheat roots apex. Plant Physiology 126, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. 2001. Function and mechanism of organic anion exudation from plant roots. Annual Review of Plant Physiology and Plant Molecular Biology 52, 527–560. [DOI] [PubMed] [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. 1993. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany 44, 437–446. [Google Scholar]
- Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E. 2009. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiology 149, 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. 2004. A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal 37, 645–653. [DOI] [PubMed] [Google Scholar]
- Shen H, Ligaba A, Yamaguchi M, Osawa H, Shibata K, Yan XL, Matsumoto H. 2004. Effect of K-252a and abscisic acid on the efflux of citrate from soybean roots. Journal of Experimental Botany 55, 663–671. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Huang CF, Ngao S, Yano M, Sato Y, Nagamura Y, Ma JF. 2009. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. The Plant Cell 21, 3339–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Zhang L, Li YY, You JF, Wu P, Zheng SJ. 2006. Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Annals of Botany 97, 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Yang JL, Zhou Y, Piñeros MA, Kochian LV, Li GX, Zheng SJ. 2011. A de novo synthesis citrate transporter, Vigna umbellata multidrug and toxic compound extrusion, implicates in Al-activated citrate efflux in rice bean (Vigna umbellata) root apex. Plant Cell & Environment 34, 2138–2148. [DOI] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ma JF. 2010. Isolation and characterization of two MATE genes in rye. Functional Plant Biology 37, 296–303. [Google Scholar]
- Yokosho K, Yamaji N, Ma JF. 2011. An Al-inducible MATE gene is involved in external detoxification of Al in rice. The Plant Journal 68, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ma JF, Sato K, Takeda K. 2003. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 217, 794–800. [DOI] [PubMed] [Google Scholar]