Abstract
Hydrogen sulfide (H2S) has been recently found to act as a potent priming agent. This study explored the hypothesis that hydroponic pretreatment of strawberry (Fragaria × ananassa cv. Camarosa) roots with a H2S donor, sodium hydrosulfide (NaHS; 100 μM for 48h), could induce long-lasting priming effects and tolerance to subsequent exposure to 100mM NaCI or 10% (w/v) PEG-6000 for 7 d. Hydrogen sulfide pretreatment of roots resulted in increased leaf chlorophyll fluorescence, stomatal conductance and leaf relative water content as well as lower lipid peroxidation levels in comparison with plants directly subjected to salt and non-ionic osmotic stress, thus suggesting a systemic mitigating effect of H2S pretreatment to cellular damage derived from abiotic stress factors. In addition, root pretreatment with NaHS resulted in the minimization of oxidative and nitrosative stress in strawberry plants, manifested via lower levels of synthesis of NO and H2O2 in leaves and the maintenance of high ascorbate and glutathione redox states, following subsequent salt and non-ionic osmotic stresses. Quantitative real-time RT-PCR gene expression analysis of key antioxidant (cAPX, CAT, MnSOD, GR), ascorbate and glutathione biosynthesis (GCS, GDH, GS), transcription factor (DREB), and salt overly sensitive (SOS) pathway (SOS2-like, SOS3-like, SOS4) genes suggests that H2S plays a pivotal role in the coordinated regulation of multiple transcriptional pathways. The ameliorative effects of H2S were more pronounced in strawberry plants subjected to both stress conditions immediately after NaHS root pretreatment, rather than in plants subjected to stress conditions 3 d after root pretreatment. Overall, H2S-pretreated plants managed to overcome the deleterious effects of salt and non-ionic osmotic stress by controlling oxidative and nitrosative cellular damage through increased performance of antioxidant mechanisms and the coordinated regulation of the SOS pathway, thus proposing a novel role for H2S in plant priming, and in particular in a fruit crop such as strawberry.
Key words: Ascorbic acid, glutathione, hydrogen sulfide, nitrosative stress, oxidative stress, polyethylene glycol, priming, redox signalling, salinity, salt overly sensitive, sodium hydrosulfide.
Introduction
Salt and non-ionic osmotic stresses pose major limitations to plant growth and productivity in arid and semi-arid regions of the world, resulting in considerable yield losses (Krasensky and Jonak, 2012). Both stresses exert their malicious effects mainly by disrupting the ionic and osmotic equilibrium of the cell and by the accumulation of reactive chemicals, imposing an additional oxidative (Gill and Tuteja, 2010) and nitrosative stress (Valderrama et al., 2007). It is well known that stress signals are first perceived by receptors at the membrane level and then transmitted via different signalling pathways to the cell in order to activate adaptive responses (Zhu, 2002; Mahajan and Tuteja, 2005). As a result, gene products involved directly or indirectly in cellular protection, such as compatible organic solutes, plant growth regulators, antioxidants, detoxification enzymes, and transcription factors, accumulate in cells (Bartel and Sunkar, 2005; Parida and Das, 2005; Mazzucotelli et al., 2008; Munns and Tester, 2008).
Hydrogen sulfide (H2S) is a colourless, highly soluble, flammable gas that has been known for many years due to its toxic effect. Intriguingly, H2S has only been identified as an important biological player in plants within the last decade (reviewed in Filippou et al., 2012). It is now touted as the third major endogenous gasotransmitter, besides nitric oxide (NO) and carbon monoxide (CO) (Wang, 2002; Olson, 2009; Tan et al., 2010). This gas is endogenously generated during the metabolism of l-cysteine by the enzymes cystathionine β-synthase and cystathionine γ-lyase and exerts significant effect in cellular physiology and pathology at physiologically relevant concentrations (Hughes et al., 2009; Mancardi et al., 2009). Recent evidence revealed the central role of H2S as a stimulatory or inhibitory compound in inflammatory, nervous, cardiovascular, gastrointestinal, and endocrine systems (Mancardi et al., 2009; Nicholson and Calvert, 2010), mainly by regulating intracellular signalling molecules, activating KATP channels and modulating endothelial Ca2+ concentration (Bauer et al., 2010). As a result, H2S-releasing drugs are currently being tested in order to evaluate their pharmacological properties (Sparatore et al., 2009; Kodela et al., 2012).
Despite long knowing that plants synthesize and release H2S (Wilson et al., 1978), relatively few studies focused in H2S biology in plant systems. Only recently, several types of specific cysteine desulphydrases have been identified and functionally characterized, confirming that cysteine isoforms are the main substrates for endogenous H2S production in plants (Bloem et al., 2004; Rausch and Wachter, 2005; Riemenschneider et al., 2005). Although at present there is no direct evidence that H2S acts as an endogenous regulator or a signal molecule in plants, emission of H2S from plants exposed to SO2 injury (Hallgren and Fredriksson, 1982), the induction of l-cysteine desulphhydrase upon pathogen attack (Bloem et al., 2004), and its involvement in guard cell signalling (García-Mata and Lamattina, 2010) all suggest that this is likely the case. Increasing interest is shown in studies focusing on abiotic stress acclimation in plants supplied with exogenous H2S donor, including Cu2+, Al3+, and Cr6+ tolerance (Zhang et al., 2008, 2010a, b ), osmotic stress tolerance (Zhang et al., 2009a), boron toxicity alleviation (Wang et al., 2010), drought (Zhang et al., 2010c), and heat tolerance (Li et al., 2012a). Furthermore, H2S was found to promote root organogenesis in sweet potato, willow, and soybean (Zhang et al., 2009b). Rausch and Wachter (2005) reviewed sulphur metabolism as a versatile platform for launching defence operations and revisited the hypothesis of ‘sulphur-induced resistance’, which may play an important role in the defence potential of plants.
However, no evidence has yet been presented demonstrating whether H2S priming could induce a systemic activation of the plant’s defence mechanism, ultimately resulting in tolerance to moderate and/or severe stress conditions. The present study hypothesized that transient pre-exposure of roots to H2S may induce tolerance to subsequent salt and non-ionic osmotic stress in hydroponically grown strawberry plants, examining its ameliorative effect in plants subjected to both stress conditions immediately after NaHS root pretreatment, as well as 3 d after root pretreatment. As far as is known, this is the first study dealing with the employment of H2S for the protection of a fruit crop from abiotic stress factors. Towards this objective, a combined physiological, biochemical, and molecular approach was employed, aiming to assess several key components of stress tolerance mechanisms in the leaves of strawberry plants exposed to NaCl or polyethylene glycol (PEG)-6000, including cellular damage indicators, physiological parameters, reactive sulphur, oxygen and nitrogen species content, and ascorbate and glutathione redox states, as well as expression analysis of key defence-related genes.
Materials and methods
Plant growth and stress treatments
Seventy-two 6-month-old strawberry plants (Fragaria × ananassa cv. Camarosa) grown in peat in the greenhouse were transferred to constantly aerated distilled water in 15-l pots for 7 d in a growing room with 16/8 light/dark cycle (250 μmol m–2 s–1), 23/20 °C and 65% relative humidity. Subsequently, the plants were transferred in half-strength Hoagland nutrient solution for an additional 7 d until the initiation of the experiment. Initially, roots of 18 plants were incubated in deionized water containing 100 μM sodium hydrosulfide (NaHS, H2S donor) for 48h (changed every 12h) and then transferred to half-strength Hoagland nutrient solution for an additional period of 3 d serving as an acclimation period. Three days after the initiation of the incubation, another set of 27 plants were incubated with 100 μM NaHS as described with no acclimation period. As a result, all strawberry plants were transferred simultaneously (day 0) to nutrient solution with or without either 100mM NaCl or 10% (w/v) PEG-6000 for 7 d. Overall, strawberry plants were subjected to eight treatments, as detailed in the legend to Fig. 1 and described schematically in Supplementary Fig. S1 (available at JXB online). The experimental set up was largely based on the recent study of Tanou and coworkers (2012a). Each treatment was independently run in triplicate, and each replicate consisted of three individual plants. Fully expanded leaves were sampled immediately after addition of NaCl and PEG-6000 (0 d) and after 7 d of stress exposure. Leaves were flash-frozen in liquid nitrogen and stored at –80 °C.
Fig. 1.
Phenotypic effects of H2S donor NaHS (100 μΜ) on strawberry plants exposed to 100mM NaCl or 10% (w/v) PEG-6000, for 7 d, with respective controls. (A) Control, pretreated with H2O, no acclimation, not stressed. (B) H2S, pretreated with H2S, no acclimation, not stressed. (C) NaCl, pretreated with H2O, no acclimation, 100mM NaCl stressed. (D) H2S(0)→NaCl, pretreated with H2S, no acclimation, 100mM NaCl stressed). (E) H2S(3)→NaCl, pretreated with H2S, 3 d acclimation, 100mM NaCl stressed. (F) PEG, pretreated with H2O, no acclimation, 10% (w/v) PEG-6000 stressed. (G) H2S(0)→PEG, pretreated with H2S, no acclimation, 10% (w/v) PEG-6000 stressed. (H) H2S(3)→PEG, pretreated with H2S, 3 d acclimation, 10% (w/v) PEG-6000 stressed. Red arrows indicate wilted, necrotic leaves.
Leaf water potential and relative water content
For the estimation of leaf water potential (MPa), leaf segments were obtained using a cork borer and placed at a WP4-T Dewpoint Potential Meter (Decagon Devices). Measurements were performed at 25 °C. Leaf relative water content (LRWC) was calculated according to Yamasaki and Dillemburg (1999). Fully expanded leaves were removed from the stem and weighted to obtain fresh mass (FM). In order to determine the turgid mass (TM), leaves were floated on distilled water for 3h inside a closed Petri dish. At the end of the incubation period, leaves were placed in a preheated oven at 80 °C for 48h to obtain dry mass (DM) and LRWC was calculated using the following equation:
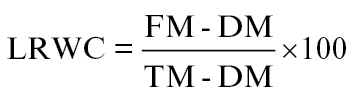 |
Physiological parameters
Stomatal conductance was measured using a ΔΤ-Porometer AP4 (Delta-T Devices, Cambridge, UK). Maximum Fv/Fm photochemical quantum yields of PSII were measured with the OptiSci OS-30p Chlorophyll Fluorometer (Opti-Sciences). Leaves were incubated in dark for 1h prior to measurements.
Lipid peroxidation
The level of lipid peroxidation, as an indicator of cellular damage, was measured in terms of malondialdehyde (MDA) content according to Heath and Packer (1968). Leaf samples (~0.1g) were homogenized in 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 15,000 g for 10min at 4 °C. The supernatant (0.5ml) was mixed with 1.5ml of 20% (w/v) TCA containing 0.5% (w/v) 2-thiobarbituric acid (TBA). The mixtures were heated at 95 °C for 30min and then quickly cooled in an ice bath. The mixtures were centrifuged at 10,000 g for 5min at 4 °C and their absorbance was measured at 532nm. The value of non-specific absorption at 600nm was subtracted from the 532nm reading. The MDA content was calculated using the Lambert-Beer law, with extinction coefficient of 155mM–1 cm–1 and expressed as nmol MDA per g freshweight.
Hydrogen sulfide, hydrogen peroxide, and nitric oxide quantification
Hydrogen sulfide quantification was performed as described by Nashef et al. (1977). Briefly, strawberry leaf tissue was ground into fine powder with a mortar and pestle under liquid nitrogen and ~0.3g of frozen tissue were homogenized in 1ml of 100mM potassium phosphate buffer (pH 7) containing 10mM EDTA. The homogenate was centrifuged at 15,000 g for 15min at 4 °C and 100 μl of the supernatant was used for the quantification of H2S, in an assay mixture containing also 1880 μl extraction buffer and 20 μl of 20mM 5,5’-dithiobis(2-nitrobenzoic acid), in a total volume of 2ml. The assay mixture was incubated at room temperature for 2min and the absorbance was read at 412nm. Hydrogen sulfide was quantified based on a standard curve of known concentrations of NaHS.
Leaf hydrogen peroxide content was assayed as described by Loreto and Velikova (2001). Frozen leaf material (~0.1g) was homogenized on ice with 0.1% (w/v) TCA. The homogenate was centrifuged at 15,000 g for 15min at 4 °C and 0.5ml of the supernatant was added to 0.5ml of 10mM potassium phosphate buffer (pH 7.0) and 1ml of 1M KI. The absorbance of assay mixture was read at 390nm and the content of H2O2 was calculated based on a standard curve of known concentrations of H2O2.
Nitric oxide content was determined according to Zhou et al. (2005). Briefly, frozen leaf material (~0.1g) was homogenized in 50mM cool acetic acid (pH 3.6) containing 4% zinc acetate and centrifuged at 10,000 g for 15min at 4 °C. The supernatant was collected and the pellet was washed with 0.5ml extraction buffer and centrifuged again. The two supernatants were combined and 0.1g charcoal was added. The mixture was agitated and centrifuged at 15,000 g for 15min at 4 °C. To 1ml of clear supernatant, 1ml Griess reagent was added and the mixture was incubated at room temperature for 30min. The absorbance of the mixture was read at 540nm and NO content was calculated by comparison to a standard curve of NaNO2.
ASC and GSH contents and redox states
Reduced ascorbate (ASC) and oxidized ascorbate (dehydroascorbate; DHA) were measured according to Foyer et al. (1983). Dehydroascorbate content was estimated as the difference between total ascorbate and ASC, while the redox state of ascorbate was expressed as the percentage of ASC to total ascorbate: (ASC/(ASC+DHA)) × 100.
The levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured according to Griffith (1980). The amount of reduced glutathione was estimated as the difference between total glutathione and GSSG. The glutathione redox state was expressed as the percentage of GSH to total glutathione: (GSH/(GSH+GSSG)) × 100.
RNA isolation, cDNA synthesis, and gene expression analysis
Total RNA from strawberry leaves was isolated following the protocol described by Mortaji et al. (2008), with slight modifications. These concerned the addition of one instead of three volumes of absolute ethanol along with 0.1 volumes of 3M NaOAC for RNA precipitation. For first-strand cDNA synthesis, 1 μg total RNA was reversed transcribed using the Primescript 1st Strand Synthesis kit (Takara Bio, Japan), according to manufacturer’s instructions. Quantitative real-time RT-PCR (qRT-PCR) was performed in an IQ5 real-time PCR cycler (Bio-Rad, USA) in a final volume of 10 μl, containing 4 μl 10-fold diluted first-strand cDNA, 0.5 μl each gene-specific primer (10 pM) and 5 μl KAPA SYBR FAST qPCR supermix (Takara Bio). Reaction conditions were 95 °C for 3min, followed by 40 cycles of 95 °C for 30 s, 56 °C for 45 s, and 72 °C for 45 s. The amplification cycle was followed by a melting curve run, with 61 cycles with 0.5 °C increments between 65 and 95 °C. The housekeeping reference gene used was 18S (Ta 46 °C) (Bustamante et al., 2006). The list of gene-specific primers used is presented in Supplementary Table S1.
Statistical analysis
Statistical analysis was carried out using the software package SPSS version 21.0 (SPSS, Chicago, USA) and the comparison of averages of each treatment was based on the analysis of variance (one-way ANOVA) according to Duncan’s multiple range test at a significance level of 5% (P ≤ 0.05). The statistical analysis of qRT-PCR results (pairwise fixed reallocation randomization test) was performed using the REST software, according to Pfaffl et al. (2002).
Results
Phenotypic observations
Fig. 1 presents the effect of NaHS root pretreatment on both salinity and non-ionic osmotic stress tolerance of strawberry plants grown in the presence of 100mM NaCl or 10% (w/v) PEG-6000, for 7 d. Strawberry plants in both positive controls (100mM NaCl and 10% (w/v) PEG-6000) exhibited intense symptoms of foliar injury, evident as wilting, leaf desiccation, and chlorotic/necrotic lesions on leaf margins (Fig. 1C, F). On the contrary, NaHS pretreatment prior to stress imposition exhibited obvious mitigating effects. Plants sustained their turgor at control levels, while wilting and necrotic lesions had limited extent if any (Fig. 1D, E, G). Only NaHS-pretreated plants stressed with PEG-6000 after 3 d of acclimation (H2S(3)→PEG) showed less pronounced mitigating effects compared with positive controls (NaCl- and PEG-stressed plants) amongst all priming treatment scenarios (Fig. 1H).
Leaf H2S content
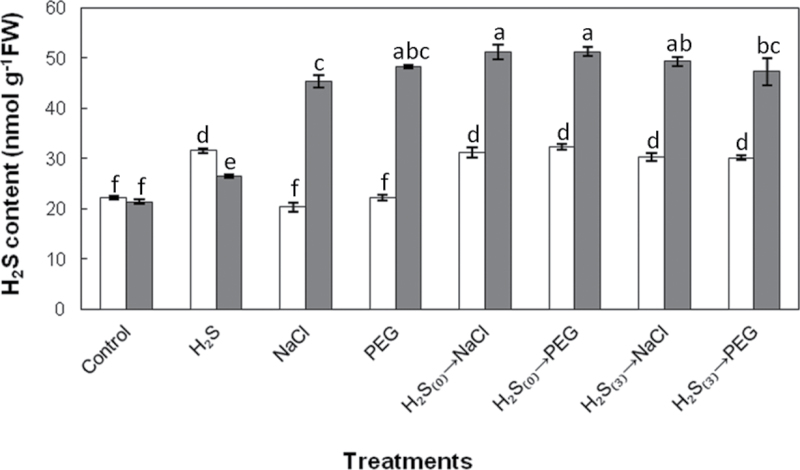
Exogenous root application of NaHS resulted in significantly elevated H2S concentration in leaves compared with control plants up to 7 d after NaHS application. Prolonged exposure to salinity and non-ionic osmotic stress greatly enhanced H2S concentration in leaves, while NaHS-pretreated plants (with no acclimation) subsequently exposed for 7 d to both stress factors were found to accumulate significantly higher amounts of H2S in their leaves compared with NaCl-stressed plants without NaHS pretreatment. The increase in leaf H2S content was less pronounced after 7 d of continuous exposure to salt and non-ionic osmotic stress in plants subjected to 3 d of acclimation after NaHS pretreatment (Fig. 2).
Fig. 2.
Effect of 100 μΜ NaHS root pretreatment on leaf H2S content at the initiation (day 0, white bars) and 7 d (grey bars) after stress imposition. Treatment acronyms are as described in the legend to Fig. 1. Data are means ± SE of three replications. Bars with different letters are significantly different (P < 0.05). FW, freshweight.
Effects on leaf hydration status
In order to evaluate leaf hydration status, LRWC and leaf water potential were monitored. As shown in Supplementary Fig. S2B, salt and PEG-6000 stress lead to a significant decrease in LRWC, while NaHS pretreatment prior to stress exposure substantially mitigated LRWC reduction in all stressed plants. As expected, progressive water deficit considerably lowered leaf water potential (more than 10-fold in osmotic stress conditions). On the other hand, plants pretreated with NaHS prior to stress exposure retained their leaf turgidity, as indicated by the slight modulation of leaf water potential compared with controls (less than 2-fold decline), with the exception of a 3-fold decline in NaHS-pretreated plants subjected to PEG-6000 stress after 3 d of acclimation (Supplementary Fig. S2A).
Physiological parameters
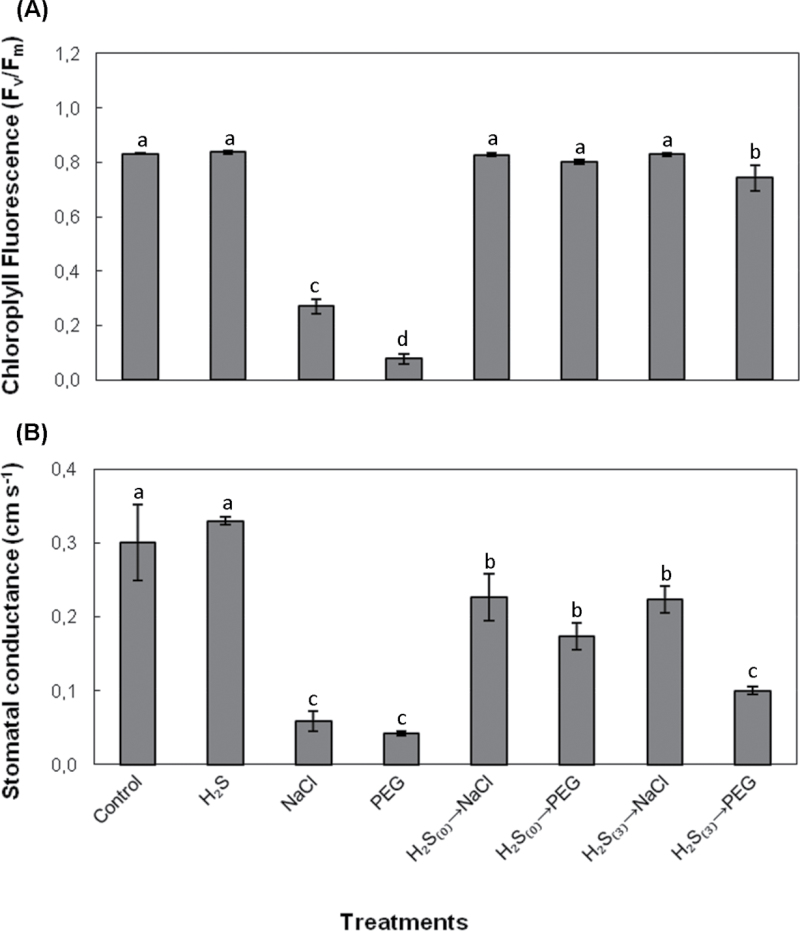
Maximum quantum yield of PSII was monitored in terms of Fv/Fm ratio. Clear negative effects of salt and non-ionic osmotic stress on chlorophyll fluorescence were registered as dramatic decreases of Fv/Fm ratio in both cases. Pretreatment with NaHS and subsequent exposure to stress conditions (100mM NaCl or 10% (w/v) PEG-6000), as well as pretreatment and exposure after 3 d of acclimation, both enabled plants to preserve PSII maximum efficiency at levels similar to control (Fig. 3A). Stomatal conductance decreased significantly after 7 d exposure to salt and non-ionic osmotic stress. This drop in conductivity was significantly reversed in NaHS-pretreated plants subsequently subjected to NaCl and PEG stress, with the sole exception of PEG-stressed plants previously pretreated with NaHS (3 d of acclimation) (Fig. 3B).
Fig. 3.
Effect of 100 μΜ NaHS on chlorophyll fluorescence (A) and stomatal conductance (B) in leaves of strawberry plants after 7 d of exposure to 100mM NaCl or 10% (w/v) PEG-6000. Treatment acronyms are as described in the legend to Fig. 1. Data are means ± SE of three replications. Bars with different letters are significantly different (P < 0.05).
Cellular damage effects
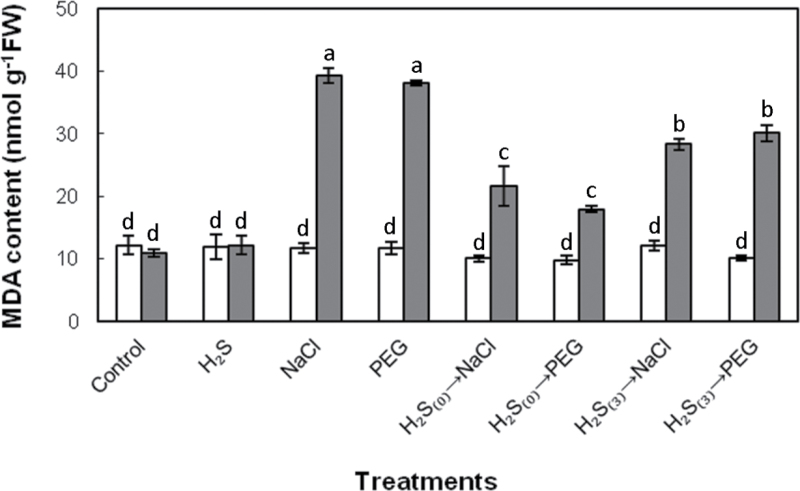
Strawberry plants grown in the presence of 100mM NaCl or 10% (w/v) PEG-6000 for 7 d, exhibited significantly higher (c.3-fold) level of lipid peroxidation, indicative of cell membrane damage. Intriguingly, NaHS root pretreatment prior to NaCl/PEG stress exposure managed to sustain membrane integrity, as illustrated by significantly lower MDA content in comparison with stressed plants not pretreated with NaHS (Fig. 4). Mitigation of cell membrane damage was less pronounced in plants pretreated with NaHS and exposed to stress after 3 d of acclimation compared with NaHS-pretreated plants with no acclimation prior to stress imposition (Fig. 4). Hydrogen sulfide treatment by itself, caused no significant modulation of MDA content compared with control samples, at the dose applied.
Fig. 4.
Lipid peroxidation, measured as leaf malondialdehyde (MDA) content, as affected by H2S donor NaHS (100 μΜ), at the initiation of stress imposition (day 0, white bars) and 7 d (grey bars) after treatment with 100mM NaCl or 10% (w/v) PEG-6000. Treatment acronyms are as described in the legend to Fig. 1. Data are means ± SE of three replications. Bars with different letters are significantly different (P < 0.05). FW, freshweight.
Reactive oxygen and nitrogen species content
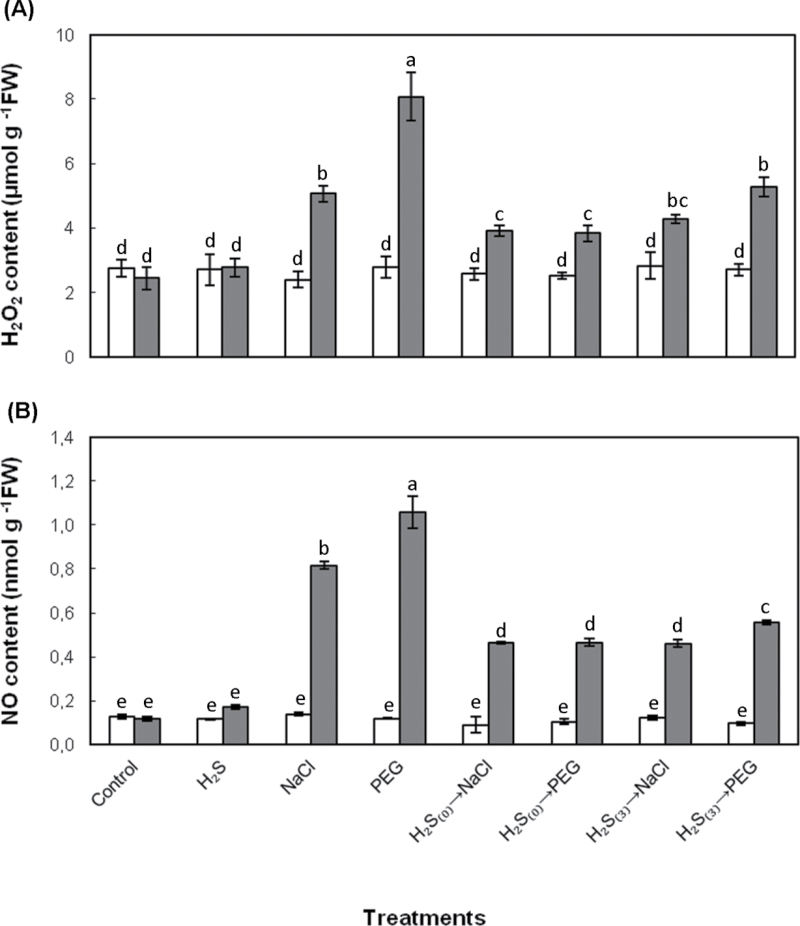
In order to determine the NaCl- and PEG-induced oxidative and nitrosative stress, the contents of H2O2 and NO, representing the major reactive oxygen species (ROS) and reactive nitrogen species (RNS), respectively, were estimated. A similar trend in the levels of both reactive molecules was recorded, in response to NaHS root pretreatment and exposure to salt and non-ionic osmotic stress conditions. As shown in Fig. 5, both H2O2 and NO content increased considerably in stressed plants. Salt exposure resulted in significant increase in H2O2 and NO leaf content, respectively, while PEG-6000 exposure resulted in even higher increase of both reactive species. Both NaHS pretreatments (with or without acclimation) resulted in a decline in stress-induced accumulation of both reactive species. In turn, H2O2 content increase was much less pronounced in NaHS-pretreated plants subsequently exposed to salt and PEG-6000 stress (0.4-fold and 0.6-fold, respectively), while the H2O2 increase quantified in plants exposed to salt and non-ionic osmotic stress after 3 d of acclimation was 0.8-fold and 1.0-fold, respectively. A similar trend was registered for NO content respectively, since the NO content quantified in pretreated plants imposed to both stresses immediately after NaHS root pretreatment was 3.9-fold, while the increase of NO in plants stressed 3 d after NaHS root pretreatment was 3.9- and 4.7-fold in salt and PEG-6000 stressed strawberry plants, respectively. It is noteworthy that H2O2 and NO content in leaves was similar among treatments on day 0, revealing the non-oxidative and nitrosative effects of NaHS pretreatment in plants, at the dose applied (Fig. 5).
Fig. 5.
Effect of 100 μΜ NaHS on H2O2 (A) and NO (B) leaf content in strawberry plants at the initiation of stress imposition (Day 0; white columns) and 7 d (grey bars) after treatment with 100mM NaCl or 10% (w/v) PEG-6000. Treatment acronyms are as described in the legend to Fig. 1. Data are means ± SE of three replications. Bars with different letters are significantly different (P < 0.05). FW, freshweight.
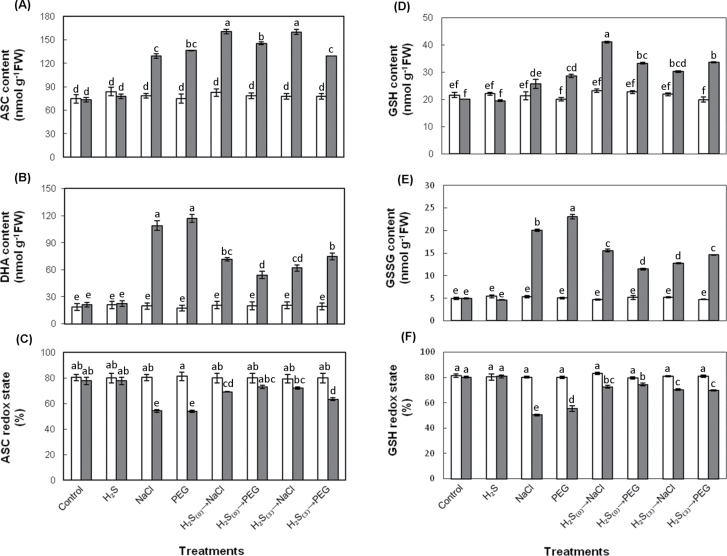
ASC and GSH contents and redox states
The bioavailable ascorbate and glutathione antioxidant pool in response to NaHS pretreatment and subsequent exposure to salt and non-ionic osmotic stress is presented in Fig. 6. Both NaCl- and PEG-6000-treated plants exhibited a marked increase in ascorbate pool compared with controls (Fig. 6A, B), with the contribution of reduced to total ASC being ~54%. Reduced ASC increased by ~1.8-fold, while DHA increased by 5.3-fold in both NaCl- and PEG-stressed plants, compared with controls. NaHS pretreatment prior to stress exposure resulted in more pronounced increase in ASC content and a marked decrease in DHA content in comparison with stressed plants (Fig. 6A, B). Interestingly, reduced ascorbate appeared to contribute to the total ascorbate pool to a much higher degree in plants pretreated with NaHS prior to subsequent NaCl or PEG stress exposure in comparison with NaCl- and PEG-treated plants, as indicated by the higher redox state values (Fig. 6C). In turn, a similar trend in the glutathione antioxidant pool and redox state was recorded. More precisely, NaHS pretreatment prior to subsequent stress exposure (0 and 3 d acclimation) resulted in overall higher levels of GSH and lower levels of GSSG compared with NaCl- and PEG-stressed plants not pretreated with NaHS (Fig. 6D, E). Furthermore, the disturbances in glutathione redox state appeared to be less pronounced in plants pretreated with NaHS prior to stress exposure than non-pretreated stressed plants, compared with control (Fig. 6F). In addition, ascorbate and glutathione content measurements verified the non-oxidative effects of H2S, since both reduced and oxidized forms of ascorbate and glutathione in NaHS-treated plants were kept at the same levels as controls. Finally, exposure to stress conditions, either directly or 3 d after NaHS root pretreatment, appeared to have similar effects on the ASC and GSH antioxidant pools, with an exception being recorded in the samples subjected to 3 d of acclimation prior to non-ionic osmotic stress (H2S(3)→PEG), which appeared to be in a more oxidized state (lower ASC and GSH redox state; Fig. 6C, F).
Fig. 6.
Effects of 100 μΜ NaHS on ascorbate and glutathione pool and redox state at the initiation of stress imposition (day 0, white bars) and 7 d after stress treatments (grey bars): (A) reduced ascorbate, ASC, (B) oxidized ascorbate, DHA, (C) ascorbate redox state, (D) reduced glutathione, GSH, (E) oxidized glutathione, GSSG, and (F) glutathione redox state. Treatment acronyms are as described in the legend to Fig. 1. Data are means ± SE of three replications. Bars with different letters are significantly different (P < 0.05). FW, freshweight.
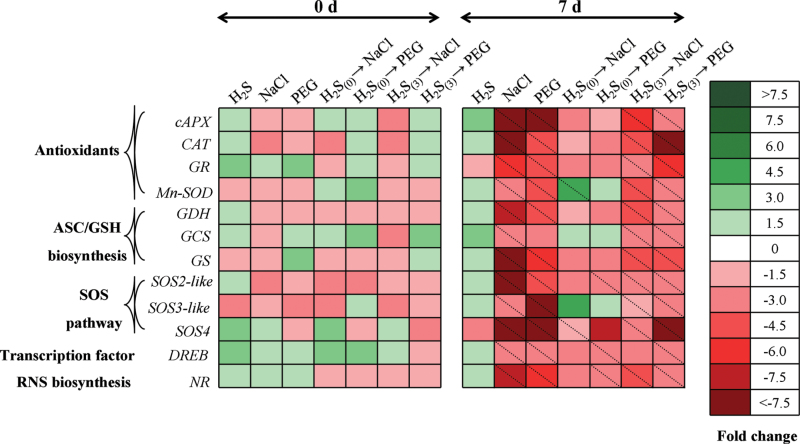
Gene expression analysis
Sodium hydrosulfide pretreatment (followed by subsequent stress imposition) exerted an adverse effect on the mRNA expression levels of all examined genes in comparison with non-pretreated stressed samples, suggesting that H2S plays a key role in the coordinated regulation of multiple transcriptional pathways. These included key antioxidant (cytosolic ascorbate peroxidase, cAPX; catalase, CAT; manganese superoxide dismutase, MnSOD; glutathione reductase, GR), ascorbate and glutathione biosynthesis (glutamylcysteine synthetase, GCS; l-galactose dehydrogenase, GDH; glutathione synthetase, GS), RNS biosynthesis (nitrate reductase, NR), transcription factor (dehydration-responsive element binding factor, DREB), and salt overly sensitive (SOS) pathway (SOS2-like, SOS3-like, SOS4) genes.
The main trends observed were the overall low levels of regulation (up to 1.5-fold up- or downregulation) of most gene-treatment combinations at time point 0 d (when imposition of stress factors commenced) in comparison with control samples (Fig. 7), while all values showed no significant difference to controls. However, examination of results after 7 d of stress imposition revealed a significant suppression of all genes following NaCl and PEG-6000 application (with the only exception of GCS expression in PEG-stressed samples), which was greatly ameliorated when plants were pretreated with NaHS without an acclimation period prior to stress imposition, lowering the suppression levels and showing no significant difference to respective control samples (Fig. 7). Intriguingly, the protective effect of NaHS root pretreatment 3 d before NaCl or PEG-6000 stress imposition was not so pronounced, as most genes maintained significantly suppressed expression levels compared with controls. Nonetheless, relative expression levels of plants pretreated with NaHS 3 d before NaCl or PEG-6000 stress imposition showed lower suppression compared with NaCl- and PEG-stressed samples (Supplementary Table S2), while SOS pathway elements showed no significant difference in H2S(0)→NaCl samples compared with controls, highlighting the importance of the SOS pathway under salinity conditions and the potential importance of its regulation by H2S. It is noteworthy that NaHS-treated (and not subsequently stressed) samples demonstrated induced expression levels of most genes examined (Fig. 7), supporting the protective, non-toxic function of the concentration applied.
Fig. 7.
Heat map showing temporal expression pattern in selected genes associated with enzymatic antioxidants, RNS biosynthesis, redox homeostasis, and SOS pathway in leaves of strawberry plants under non-stress and NaCl/PEG stress conditions. Following root pretreatment with 100 μΜ NaHS 3 d before stress imposition or until application of the stress factor, plants were grown with or without 100mM NaCl or 10% (w/v) PEG-6000 for 7 d as described schematically in Supplementary Fig. S1. Tissues were sampled immediately after H2S pretreatment (day 0) and 7 d after pretreatment. Relative mRNA abundance was evaluated by quantitative real-time RT-PCR using three biological repeats. Upregulation is indicated in green; downregulation is indicated in red; diagonal dotted lines represent statistically significant differences compared with control samples (P < 0.05). A scale of colour intensity is presented as a legend. Actual relative expression data, obtained from three independent replicates, are shown in Supplementary Table S2.
Discussion
Salinity and drought are the most important among abiotic stresses, contributing greatly to the shaping of plant evolution. Therefore, compounds that may result in mitigating various stresses’ detrimental effects should be of prime importance from both the theoretical and applied point of view (Uchida et al., 2002). Several bioactive compounds, such as H2O2, NO, abscisic acid, and polyamines have been shown to induce tolerance to various abiotic stresses (Arasimowicz and Floryszak-Wieczorek, 2007; Filippou et al., 2012; Tanou et al., 2012a, b). The present study provides novel evidence that root pretreatment with the H2S donor NaHS promoted both salt and non-ionic osmotic stress tolerance in strawberry plants, an important fruit crop of high nutritional value and antioxidant capacity (Wang et al., 1996). Furthermore, this study provides evidence that the role of NaHS in alleviating NaCl and PEG-6000 stress could be attributed to H2S, as the levels of endogenous H2S increased following NaHS pretreatment and subsequent stress imposition, in accordance with similar findings by Zhang et al. (2010b). Importantly, Zhang et al. (2009a, b) reported that only H2S, and not other Na+- or sulphur-containing compounds released from NaHS, have a protective role during abiotic stresses.
Hydrogen sulfide pretreatment for 48h prior to NaCl or PEG-6000 exposure decreased the detrimental effects of these abiotic stress factors, while no visible toxicity symptoms were observed. Physiological and hydration status indices demonstrated that plants pretreated with NaHS prior to stress exposure sustained their leaf turgor and membrane integrity in comparison with plants directly exposed to stress conditions. This observation is supported by the higher LWRC and leaf water potential recorded in stressed plants pretreated with NaHS compared with those directly exposed to NaCl or PEG-6000. Furthermore, the conservation of electron transport rate and photochemical efficiency of PSII in plants pretreated with NaHS prior to stress imposition further support this statement. In agreement with the observations of García-Mata and Lamattina (2010) and Lisjak et al. (2010), who reported that H2S is involved in guard cell signalling via an abscisic acid-dependent signalling network, this study indicated that H2S-pretreated and subsequently stressed plants sustained their stomatal conductance to substantially higher levels than stressed plants without pretreatment. Zhang et al. (2009a, 2010c) showed that NaHS-sprayed sweet potato and soybean plants exposed to osmotic stress restricted lipid peroxidation as evidenced by lower MDA content. The present study offers supporting evidence that salt and non-ionic osmotic stress-induced cellular damage is mitigated by NaHS pretreatment, as revealed by lower MDA content.
An increase in ROS levels can provoke partial or severe oxidation of cellular components inducing redox status changes, so continuous control of ROS and therefore of their metabolism is imperative under stress conditions (Jubany-Mari et al., 2010). Recent studies showed that RNS are also overproduced under stress conditions, indicating that nitrosative stress could participate as a significant component in the mechanism of damage produced by abiotic stress conditions in plant cells (Corpas et al., 2007; Valderrama et al., 2007; Filippou et al., 2011). Although reactive oxygen and nitrogen species are toxic in high concentrations, they also function as signalling molecules regulating many biological processes, including responses to abiotic stresses (Neill et al., 2002; Arasimowicz and Floryszak-Wieczorek, 2007; Xu et al., 2010; Molassiotis and Fotopoulos, 2011). Quantification of H2O2 and NO content verified the role of H2S in alleviating NaCl- or PEG-6000-induced oxidative and nitrosative damage, showing that NaHS pretreatment managed to sustain both H2O2 and NO content in much lower concentrations in comparison with stressed strawberry plants. Hydrogen sulfide was shown to regulate ROS and RNS content in a coordinated manner, being in accordance with several reports suggesting a dynamic cross-talk between these reactive forms (Filippou et al., 2011; Molassiotis and Fotopoulos, 2011; Tanou et al., 2012a). Furthermore, these results are in agreement with the findings of Zhang et al. (2010b, c), who also showed that H2O2 was maintained in lower levels in NaHS-pretreated and subsequently stressed plants, while Lisjak et al. (2010) reported reduced NO accumulation in Arabidopsis plants treated with NaHS. No differences in H2O2 and NO content were registered among treatments on day 0 (including samples that underwent NaHS pretreatment for 48h prior to stress imposition at day 0), revealing the non-oxidative or nitrosative effects of NaHS at the dose applied. Interestingly, highest NO content was observed in NaCl- and PEG-stressed plants, correlating with lowest NR expression levels, with nitrate reductase being recognized as a key enzyme involved in the generation of NO in plants (Yamasaki and Sakihama, 2000). Such a negative correlation could be attributed to feedback inhibition of NR, in accordance with findings by Rosales et al. (2011), possibly due to NO toxicity (Shapiro, 2005).
Enzymatic antioxidants such as superoxide dismutases, catalases, and ascorbate peroxidases are in the first line of antioxidant enzymes and have been well studied in numerous plants (Gill and Tuteja, 2010; Singh et al., 2010). Superoxide dismutase catalyses the disproportion of superoxide anion into H2O2, while CAT and APX scavenge H2O2 and convert it into water (Kuk et al., 2003). The higher MDA and H2O2 leaf content observed in stressed plants, indicative of an oxidative burst, could be partly attributed to the downregulation of cAPX and CAT. Accordingly, the induction of MnSOD and the preservation of cAPX and CAT relative expression to levels similar to controls in plants stressed immediately after NaHS root pretreatment could justify the lower concentrations of MDA and H2O2 observed in the leaves of primed plants. The transcript levels observed are in line with previous antioxidant enzymatic activity observations, where plants pretreated with NaHS showed a similar trend retaining higher SOD, CAT, and APX activity compared with cadmium- (Li et al., 2012b) and drought-stressed plants (Zhang et al., 2010c), as well as salt-stressed germinating seeds (Wang et al., 2012). It is interesting to note that expression of all studied genes was downregulated in salt- and PEG-stressed samples 7 d after stress imposition. This could be the result of a general ‘shutdown’ of defence pathways following prolonged stress imposition in a naturally osmotic stress-sensitive crop such as strawberry, in accordance with similar findings by Tanou et al. (2012a) who demonstrated general suppression in defence gene expression levels in roots of hydroponically grown sour orange seedlings subjected to salt stress for 8 d.
Ascorbate and glutathione are major non-enzymatic antioxidant molecules in plants, with significant contribution to the plant antioxidant machinery and tolerance to abiotic stresses (Gill and Tuteja, 2010; Potters et al., 2010). High ascorbate and glutathione redox ratios are necessary to achieve optimal metabolism and promote tolerance to abiotic stress (Foyer and Noctor, 2005; Fotopoulos et al., 2010), while low ascorbate redox ratios result in increased sensitivity to oxidizing agents (Fotopoulos et al., 2006). In the present study, the ability of NaHS-pretreated plants (both applied until exposure to stress or undergoing a 3 d of acclimation after NaHS pretreatment until stress imposition) to cope with subsequent salt and non-ionic osmotic stress correlated with their ability to maintain increased ascorbate and glutathione redox states compared with stressed plants. This was achieved by increasing reduced ascorbate and glutathione content, in accordance with findings by Shan et al. (2011, 2012), who also observed similar induction in ASC and GSH content in wheat plants pretreated with NaHS and subsequently subjected to water and copper stress, respectively. It is noteworthy that NaHS pretreatment of stressed plants resulted in significantly lower GSSG content, possibly via an induction in GR activity as that recorded by Shan et al. (2011) in water-stressed plants, since GR is involved in GSH regeneration from GSSG (Gill and Tuteja, 2010). Such an induction is in line with the relative lower levels of suppression in GR mRNA expression in NaHS-pretreated and stressed plants compared with positive control plants.
Further evidence on the regulatory role of H2S in ascorbate and glutathione redox homeostasis can be given by the expression of key ASC and GSH biosynthetic genes. The expression pattern of GDH (ASC biosynthesis) as well as GCS and GS (GSH biosynthesis) in NaHS-pretreated and subsequently stressed plants supports the increased levels in ASC and GSH content in comparison with NaCl- and PEG-stressed plants, in line with analogous increases in GCS activity in NaHS-pretreated water-stressed wheat plants (Shan et al., 2011) and increases in l-galactono-1,4-lactone dehydrogenase activity in NaHS-pretreated copper-stressed plants (Shan et al., 2012). Thiol redox modification in particular is of great importance and represents a major element of the modus operandi of H2S as a priming agent, as cysteine acts as a common source for H2S emission in plants (Riemenschneider et al., 2005), as well as glutathione biosynthesis (Gill and Tuteja, 2010).
The regulation of ions within the cell cytosol by both plasma membrane and endomembrane transporters is an indispensable component of growth and adaptation (Cheng et al., 2004). The SOS pathway, identified through isolation and study of Arabidopsis mutants, is essential for maintaining ion homeostasis in the cytoplasm and for salt stress tolerance (Zhu, 2002; Mahajan and Tuteja, 2005). SOS3 encodes a Ca2+-binding protein that activates SOS2 serine/threonine protein kinase in a Ca2+-dependent manner (Liu and Zhu, 1998; Halfter et al., 2000; Zhu, 2000). This SOS3–SOS2 protein kinase complex directly phosphorylates SOS1, a NA+/H+ antiporter, resulting in an efflux of excess Na+ ions or its vacuolar sequestration, leading to ion homeostasis and salt tolerance (Qiu et al., 2002; Mahajan and Tuteja, 2005). Furthermore, Shi et al. (2002) identified an additional component of the pathway denoted SOS4, which represents a pyridoxal kinase that is involved in the biosynthesis of pyridoxal-5-phosphate, an active form of vitamin B6. In addition, several reports have established the existence of two other families of calcium sensors (calcineurin B-like proteins) and protein kinases (CBL-interacting protein kinases) which show similar activity to SOS3 and SOS2 components, respectively, and which are often referred to as SOS-like (Gong et al., 2004; Du et al., 2011). The present work provides novel evidence demonstrating that NaHS root pretreatment can induce salt and non-ionic osmotic stress tolerance through the modulation of the SOS pathway. More precisely, H2S pretreatment managed to sustain putative SOS2-like, SOS3-like, and SOS4 relative expression in levels similar to controls in plants directly exposed to stress conditions following NaHS pretreatment. Interestingly, significant suppression of the SOS pathway was observed in plants pretreated with H2S and stressed after 3 d of acclimation, but to a much lesser extent in comparison with stressed plants that were not subjected to NaHS pretreatment. The induction in relative gene expression observed for the SOS pathway elements, following NaHS pretreatment, highlights the potential importance of H2S in the regulation of K+ sequestration/uptake; SOS4 mutants are known to retain less K+ under NaCl stress compared with wild-type plants (Shi et al., 2002), while the protein kinase CIPK23 and calcium sensor CBL1 are identified as the essential K+ uptake regulators in Arabidopsis and also function in response to low K+ stress (Xu et al., 2006), with the two putative SOS2-like and SOS3-like homologues identified in this study showing highest sequence similarity to AtCIPK23 and AtCBL1, respectively (data not shown).
Another key element involved in abiotic stress signalling pathways is the DREB transcription factor, which binds to drought-responsive cis-acting elements and regulates the expression of several genes related in responses to abiotic stress (Agarwal et al., 2006). Recent reports demonstrated that DREB expression manipulation in transgenic plants can confer tolerance to various abiotic stresses (Ban et al., 2011; Cui et al., 2011; Tang et al., 2011). In this study, DREB expression analysis revealed that another mechanism of NaHS-induced tolerance to salt and non-ionic osmotic stress could be via the mitigation of DREB downregulation, in accordance with recent findings demonstrating that DREB2A and DREB2B were induced in Arabidopsis plants fumigated with NaHS (Jin et al., 2011).
The contribution of sulphur compounds and sulphur fertilization in the protection of plants against pests and diseases has resulted in a hypothesis for plant sulphur-induced resistance against biotic stresses being postulated (Bloem et al., 2004; Klikocka et al., 2005). Sulphur-containing defence compounds, such as elemental sulphur, H2S, glutathione, phytochelatins, various secondary metabolites, and sulphur-rich proteins, are crucial for the survival of plants under biotic and abiotic stress (Rausch and Wachter, 2005). In the current study, novel evidence is presented showing that H2S induces significant, systemic tolerance in both NaCl- and PEG-stressed strawberry plants. The tolerance observed in plants pretreated with NaHS and subjected to salt and non-ionic osmotic stress 3 d later, albeit lower compared with simultaneously NaHS-pretreated and stressed plants, suggests that H2S priming is capable of inducing long-lasting systemic tolerance, possibly via regulation of several transduction pathways. This energy-consuming coordinated orchestration of several independent pathways is most likely feasible through increased photosynthetic capacity in NaHS-treated plants (Chen et al., 2011). Furthermore, recent evidence by Hou et al. (2013) and Liu et al. (2012) further demonstrated the potential cross-talk between H2S and other signalling molecules such as ethylene and NO, thus supporting the link observed in the present study between H2S and NO/H2O2 biosynthesis. This study sheds some light on the mechanisms of H2S-induced tolerance and H2S plant signalling to salt and non-ionic osmotic stress. The employment of comprehensive systems biology approaches towards the complete elucidation of H2S signalling in abiotic stress, including the potential application of synthetic inhibitors of H2S biosynthesis (e.g. Hou et al., 2013), stands as a challenging future perspective.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Schematic depiction of experimental treatments.
Supplementary Fig. S2. Effects of H2S donor NaHS (100 μΜ) on leaf water potential and leaf relative water content in strawberry plants exposed either to 100mM NaCl or 10% PEG-6000 for 7 d.
Supplementary Table S1. Oligonucleotides used as primers for qRT-PCR.
Supplementary Table S2. Effects of H2S donor NaHS on the relative mRNA expression of enzymatic antioxidants and proteins involved in RNS biosynthesis, redox homeostasis, SOS pathway, and transcription regulation, in leaves of strawberry plants under non-stress, salt, and PEG stress conditions.
Acknowledgements
The authors would like to thank DR Panagiota Filippou, Ms Anna Mlynarczyk, and Ms Chrystalla Antoniou for excellent technical assistance. This work was supported by the Cyprus University of Technology (EX032 to VF).
References
- Agarwal PK, Agarwal P, Reddy MK, Sopory SK. 2006. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Reports 25, 1263–1274. [DOI] [PubMed] [Google Scholar]
- Arasimowicz M, Floryszak-Wieczorek J. 2007. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Science 172, 876–887. [Google Scholar]
- Ban Q, Liu G, Wang Y. 2011. A DREB gene from Limonium bicolor mediates molecular and physiological responses to copper stress in transgenic tobacco. Journal of Plant Physiology 168, 449–458. [DOI] [PubMed] [Google Scholar]
- Bartel D, Sunkar R. 2005. Drought and salt tolerance in plants. Plant Science 24, 23–58. [Google Scholar]
- Bauer CC, Boyle JP, Porter KE, Peers C. 2010. Modulation of Ca2+ signalling in human vascular endothelial cells by hydrogen sulfide. Atherosclerosis 209, 374–380. [DOI] [PubMed] [Google Scholar]
- Bloem E, Riemenschneider A, Volker J, Jutta P, Schmidt A, Salac I, Haneklaus S, Schnug E. 2004. Sulphur supply and infection with Pyrenopeziza brassicae influence l -cysteine desulphydrase activity in Brassica napus L. Journal of Experimental Botany 55, 2305–2312. [DOI] [PubMed] [Google Scholar]
- Bustamante CA, Rosli HG, Añón MC, Civello PM, Martinez GA. 2006. β-Xylosidase in strawberry fruit: isolation of a full-length gene and analysis of its expression and enzymatic activity in cultivars with contrasting firmness. Plant Science 171, 497–504. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu F-H, Wang W-H, Zheng C-J, Lin G-H, Dong X-J, He J-X, Pei Z-M, Zheng H-L. 2011. Hydrogen sulfide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. Journal of Experimental Botany 62, 4481–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N-H, Pittman JK, Zhu J-K, Hirschi KD. 2004. The protein kinase SOS2 activates the Arabidopsis H+/Ca2 + antiporter CAX1 to integrate calcium transport and salt tolerance. Journal of Biological Chemistry 279, 2922–2926. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, del Río LA, Barroso JB. 2007. Need of biomarkers of nitrosative stress in plants. Trends in Plant Science 12, 436–438. [DOI] [PubMed] [Google Scholar]
- Cui M, Zhang W, Zhang Q, Xu Z, Zhu Z, Duan F, Wu R. 2011. Induced over-expression of the transcription factor OsDREB2A improves drought tolerance in rice. Plant Physiology and Biochemistry 49, 1384–1391. [DOI] [PubMed] [Google Scholar]
- Du W, Lin H, Chen S, Wu Y, Zhang J, Fuglsang AT, Palmgren MG, Wu W, Guo Y. 2011. Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis . Plant Physiolοgy 156, 2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippou P, Antoniou C, Fotopoulos V. 2011. Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signaling and Behavior 6, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippou P, Tanou G, Molassiotis A, Fotopoulos V. 2012. Plant acclimation to environmental stress using priming agents. In: Tuteja N, Gill SS, eds, Plant acclimation to environmental stress. USA: Springer Science & Business Media; 1–28. [Google Scholar]
- Fotopoulos V, Sanmartin M, Kanellis AK. 2006. Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. Journal of Experimental Botany 57, 3933–3943. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Ziogas V, Tanou G, Molassiotis A. 2010. Involvement of AsA/DHA and GSH/GSSG ratios in gene and protein expression and in the activation of defence mechanisms under abiotic stress conditions. In: Anjum NA, Chan M-T, Umar S, eds, Ascorbate-glutathione pathway and stress tolerance in plants. Netherlands: Springer; 265–302. [Google Scholar]
- Foyer C, Rowell J, Walker D. 1983. Measurement of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157, 239–244. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell 17, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L. 2010. Hydrogen sulfide, a novel gasotransmitter involved in guard cell signalling. New Phytologist 188, 977–984. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48, 909–930. [DOI] [PubMed] [Google Scholar]
- Gong D, Guo Y, Schumaker KS, Zhu JK. 2004. The SOS3 family of calcium sensors and SOS2 family of protein kinases in Arabidopsis . Plant Physiology 134, 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. 1980. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry 106, 207–212. [DOI] [PubMed] [Google Scholar]
- Halfter U, Ishitani M, Zhu J-K. 2000. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proceedings of the National Academy of Sciences, USA 97, 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren J-E, Fredriksson S-A. 1982. Emission of hydrogen sulfide from sulfur dioxide-fumigated pine trees. Plant Physiology 70, 456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R, Packer L. 1968. Photooxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics 125, 189–198. [DOI] [PubMed] [Google Scholar]
- Hou Z, Wang L, Liu J, Hou L, Liu X. 2013. Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana . Journal of Integrative Plant Biology (in press; doi: 10.1111/jipb.12004 ) [DOI] [PubMed] [Google Scholar]
- Hughes MN, Centelles MN, Moore KP. 2009. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radical Biology and Medicine 47, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y. 2011. Hydrogen sulfide improves drought resistance in Arabidopsis thaliana . Biochemical and Biophysical Research Communications 414, 481–486. [DOI] [PubMed] [Google Scholar]
- Jubany-Mari T, Munné-Bosch S, Alegre L. 2010. Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiology and Biochemistry 48, 351–358. [DOI] [PubMed] [Google Scholar]
- Klikocka H, Haneklaus S, Bloem E, Schnug E. 2005. Influence of sulfur fertilization on infection of potato tubers with Rhizoctonia solani and Streptomyces scabies . Journal of Plant Nutrition 28, 819–833. [Google Scholar]
- Kodela R, Chattopadhyay M, Kashfi K. 2012. NOSH-aspirin: a novel nitric oxide-hydrogen sulfide-releasing hybrid: a new class of anti-inflammatory pharmaceuticals. ACS Medicinal Chemistry Letters 3, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasensky J, Jonak C. 2012. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany 63, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuk YI, Shin JS, Burgos NR, Hwang TE, Han O, Cho BH, Jung S, Guh JO. 2003. Antioxidative enzymes offer protection from chilling damage in rice plants. Crop Science 43, 2109–2117. [Google Scholar]
- Li L, Wang Y, Shen W. 2012b. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. BioMetals 25, 617–631. [DOI] [PubMed] [Google Scholar]
- Li Z-G, Gong M, Xie H, Yang L, Li J. 2012a. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Science 185–186 185–189. [DOI] [PubMed] [Google Scholar]
- Lisjak M, Srivastava N, Teklic T, Civale L, Lewandowski K, Wilson I, Wood ME, Whiteman M, Hancock JT. 2010. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiology and Biochemistry 48, 931–935. [DOI] [PubMed] [Google Scholar]
- Liu J, Hou Z, Liu G, Hou L, Liu X. 2012. Hydrogen sulfide may function downstream of nitric oxide in ethylene-induced stomatal closure in Vicia faba L. Journal of Integrative Agriculture 11, 1644–1653. [Google Scholar]
- Liu J, Zhu J-K. 1998. A calcium sensor homolog required for plant salt tolerance. Science 280, 1943–1945. [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V. 2001. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiology 127, 1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. 2005. Cold, salinity and drought stresses: an overview. Archives of Biochemistry and Biophysics 444, 139–158. [DOI] [PubMed] [Google Scholar]
- Mancardi D, Penna C, Merlino A, Del, Soldato P, Wink DA, Pagliaro P. 2009. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochimica et Biophysica Acta Bioenergetics 1787, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucotelli E, Mastrangelo AM, Crosatti C, Guerra D, Stanca AM, Cattivelli L. 2008. Abiotic stress response in plants: when post-transcriptional and post-translational regulations control transcription. Plant Science 174, 420–431. [Google Scholar]
- Molassiotis A, Fotopoulos V. 2011. Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signaling and Behaviour 6, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaji Z, Tavakkol, Afshari R, Alizadeh H, Yazdi, Samadi B. 2008. RNA isolation and expression from different dormant and after-ripened wheat (Triticum aestivum) seed tissues rich in polysaccharides and proteins. Asian Journal of Plant Science 7, 201–206. [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59, 651–681. [DOI] [PubMed] [Google Scholar]
- Nashef AS, Osuga DT, Feeney RE. 1977. Determination of hydrogen sulfide with 5,5β’-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Analytical Biochemistry 79, 394–405. [DOI] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J. 2002. Hydrogen peroxide signalling. Current Opinion in Plant Biology 5, 388–395. [DOI] [PubMed] [Google Scholar]
- Nicholson CK, Calvert JW. 2010. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacological Research 62, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KR. 2009. Is hydrogen sulfide a circulating ‘gasotransmitter’ in vertebrate blood? Biochimica et Biophysica Acta Bioenergetics 1787, 856–863. [DOI] [PubMed] [Google Scholar]
- Parida AK, Das AB. 2005. Salt tolerance and salinity effects on plants: a review. Ecotoxicology and Environmental Safety 60, 324–349. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research 30, e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Horemans N, Jansen MAK. 2010. The cellular redox state in plant stress biology – a charging concept. Plant Physiology and Biochemistry 48, 292–300. [DOI] [PubMed] [Google Scholar]
- Qiu Q-S, Guo Y, Dietrich MA, Schumaker KS, Zhu J-K. 2002. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proceedings of the National Academy of Sciences, USA 99, 8436–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch T, Wachter A. 2005. Sulfur metabolism: a versatile platform for launching defence operations. Trends in Plant Science 10, 503–509. [DOI] [PubMed] [Google Scholar]
- Riemenschneider A, Wegele R, Schmidt A, Papenbrock J. 2005. Isolation and characterization of a d-cysteine desulfhydrase protein from Arabidopsis thaliana . FEBS Journal 272, 1291–1304. [DOI] [PubMed] [Google Scholar]
- Rosales EP, Iannone MF, Groppa MD, Benavides MP. 2011. Nitric oxide inhibits nitrate reductase activity in wheat leaves. Plant Physiology and Biochemistry 49, 124–130. [DOI] [PubMed] [Google Scholar]
- Shan C, Dai H, Sun Y. 2012. Hydrogen sulfide protects wheat seedlings against copper stress by regulating the ascorbate and glutathione metabolism in leaves. Australian Journal of Crop Science 6, 248–254. [Google Scholar]
- Shan C-J, Zhang S-L, Li D-F, Zhao Y-Z, Tian X-L, Zhao X-L, Wu Y-X, Wei X-Y, Liu R-Q. 2011. Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiologiae Plantarum 33, 2533–2540. [Google Scholar]
- Shapiro AD. 2005. Nitric oxide signaling in plants. Vitamins and Hormones 72, 339–398. [DOI] [PubMed] [Google Scholar]
- Shi H, Xiong L, Stevenson B, Lu T, Zhu JK. 2002. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. The Plant Cell 14, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BK, Sharma SR, Singh B. 2010. Antioxidant enzymes in cabbage: variability and inheritance of superoxide dismutase, peroxidase and catalase. Scientia Horticulturae 124, 9–13. [Google Scholar]
- Sparatore A, Perrino E, Tazzari V, Giustarini D, Rossi R, Rossoni G, Erdman K, Schröder H, Soldato PD. 2009. Pharmacological profile of a novel H2S-releasing aspirin. Free Radical Biology and Medicine 46, 586–592. [DOI] [PubMed] [Google Scholar]
- Tan BH, Wong PTH, Bian J-S. 2010. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochemistry International 56, 3–10. [DOI] [PubMed] [Google Scholar]
- Tang M, Liu X, Deng H, Shen S. 2011. Over-expression of JcDREB, a putative AP2/EREBP domain-containing transcription factor gene in woody biodiesel plant Jatropha curcas, enhances salt and freezing tolerance in transgenic Arabidopsis thaliana . Plant Science 181, 623–631. [DOI] [PubMed] [Google Scholar]
- Tanou G, Filippou P, Belghazi M, Job D, Diamantidis G, Fotopoulos V, Molassiotis A. 2012a. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. The Plant Journal 72, 585–599. [DOI] [PubMed] [Google Scholar]
- Tanou G, Fotopoulos V, Molassiotis A. 2012b. Priming against environmental challenges and proteomics in plants: update and agricultural perspectives. Frontiers in Plant Science 3, 216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T. 2002. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Science 163, 515–523. [Google Scholar]
- Valderrama R, Corpas FJ, Carreras A, Fernández-Ocaña A, Chaki M, Luque F, Gómez-Rodríguez MV, Colmenero-Varea P, del Río LA, Barroso JB. 2007. Nitrosative stress in plants. FEBS Letters 581, 453–461. [DOI] [PubMed] [Google Scholar]
- Wang B-L, Shi L, Li Y-X, Zhang W-H. 2010. Boron toxicity is alleviated by hydrogen sulfide in cucumber (Cucumis sativus L.) seedlings. Planta 231, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao G, Prior RL. 1996. Total antioxidant capacity of fruits. Journal of Agricultural and Food Chemistry 44, 701–705. [Google Scholar]
- Wang R. 2002. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB Journal 16, 1792–1798. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Cui W, Xu S, Shen W, Wang R. 2012. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant and Soil 351, 107–119. [Google Scholar]
- Wilson LG, Bressan RA, Filner P. 1978. Light-dependent emission of hydrogen sulfide from plants. Plant Physiology 61, 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li H-D, Chen L-Q, Wang Y, Liu L-L, He L, Wu W-H. 2006. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis . Cell 125, 1347–1360. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sun X, Jin J, Zhou H. 2010. Protective effect of nitric oxide on light-induced oxidative damage in leaves of tall fescue. Journal of Plant Physiology 167, 512–518. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y. 2000. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Letters 468, 89–92. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Dillemburg LR. 1999. Measurements of leaf relative water content in Araucaria angustifolia . Revista Brasileira de Fisiologia Vegetal 11, 69–75. [Google Scholar]
- Zhang H, Hu L, Li P, Hu K, Jiang C, Luo J. 2010a. Hydrogen sulfide alleviated chromium toxicity in wheat. Biologia Plantarum 54, 743–747. [Google Scholar]
- Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP. 2008. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. Journal of Integrative Plant Biology 50, 1518–1529. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jiao H, Jiang C-X, Wang S-H, Wei Z-J, Luo J-P, Jones R. 2010c. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiologiae Plantarum 32, 849–857. [Google Scholar]
- Zhang H, Tan ZQ, Hu LY, Wang SH, Luo JP, Jones RL. 2010b. Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. Journal of Integrative Plant Biology 52, 556–567. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tang J, Liu X-P, Wang Y, Yu W, Peng W-Y, Fang F, Ma D-F, Wei Z-J, Hu L-Y. 2009b. Hydrogen sulfide promotes root organogenesis in Ipomoea batatas, Salix matsudana and Glycine max . Journal of Integrative Plant Biology 51, 1086–1094. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ye Y-K, Wang S-H, Luo J-P, Tang J, Ma D-F. 2009a. Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regulation 58, 243–250. [Google Scholar]
- Zhou B, Guo Z, Xing J, Huang B. 2005. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis . Journal of Experimental Botany 56, 3223–3228. [DOI] [PubMed] [Google Scholar]
- Zhu J-K. 2000. Genetic analysis of plant salt tolerance using Arabidopsis . Plant Physiology 124, 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K. 2002. Salt and drought stress signal transduction in plants. Annual Review of Plant Biology 53, 247–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.