Abstract
Aim
To evaluate the relationship of diet to incident diabetes among non-Black and Black participants in the Adventist Health Study-2.
Methods and Results
Participants were 15,200 men and 26,187 women (17.3% Blacks) across the U.S. and Canada who were free of diabetes and who provided demographic, anthropometric, lifestyle and dietary data. Participants were grouped as vegan, lacto ovo vegetarian, pesco vegetarian, semi-vegetarian or non-vegetarian (reference group). A follow-up questionnaire after two years elicited information on the development of diabetes. Cases of diabetes developed in 0.54% of vegans, 1.08% of lacto ovo vegetarians, 1.29% of pesco vegetarians, 0.92% of semi-vegetarians and 2.12% of non-vegetarians. Blacks had an increased risk compared to non-Blacks (odds ratio [OR] 1.364; 95% confidence interval [CI], 1.093–1.702). In multiple logistic regression analysis controlling for age, gender, education, income, television watching, physical activity, sleep, alcohol use, smoking and BMI, vegans (OR 0.381; 95% CI 0.236–0.617), lacto ovo vegetarians (OR 0.618; 95% CI 0.503–0.760) and semi-vegetarians (OR 0.486, 95% CI 0.312–0.755) had a lower risk of diabetes than non-vegetarians. In non-Blacks vegan, lacto ovo and semi-vegetarian diets were protective against diabetes (OR 0.429, 95% CI 0.249–0.740; OR 0.684, 95% CI 0.542–0.862; OR 0.501, 95% CI 0.303–0.827); among Blacks vegan and lacto ovo vegetarian diets were protective (OR 0.304, 95% CI 0.110–0.842; OR 0.472, 95% CI 0.270–0.825). These associations were strengthened when BMI was removed from the analyses.
Conclusion
Vegetarian diets (vegan, lacto ovo, semi-) were associated with a substantial and independent reduction in diabetes incidence. In Blacks the dimension of the protection associated with vegetarian diets was as great as the excess risk associated with Black ethnicity.
Keywords: Vegetarianism, Black, African American, Diabetes, Ethnicity, Diet
Introduction
Beyond the effect of diet on body weight, dietary patterns or specific foods may be important determinants of risk of type 2 diabetes. The prudent pattern characterized by high intakes of fruit, salads and cooked vegetables, fish, poultry, and whole grains seems to protect against type 2 diabetes when compared with typical Western patterns, characterized by high intakes of red and processed meats, sweets, desserts, soft drinks, fatty foods and refined grains [1–6]. Protective diets are generally characterized by high intakes of plant-based foods including legumes and soy as suggested in prospective observational studies [7–9].
Observational evidence supporting an association between vegetarianism and a reduction in the incidence of diabetes was first observed by Snowdon and Phillips in 1985 [10]. Taken together with studies done in non-vegetarian populations [11,12], compelling evidence suggests that meat intake is a dietary risk factor for diabetes. In a recent systematic review and meta-analysis consumption of red meat (relative risk [RR] 1.21; 95% confidence interval [CI] 1.07–1.38), and processed meat (RR 1.41; 95% CI 1.25–1.60) was associated with an increased risk of type 2 diabetes [13]. Meat subgroups such as hamburger, bacon, and hotdogs were specifically associated with an increased risk [13]. Suggested mechanisms include components of meat such as saturated fat, heme iron, as well as nitrites and nitrates found in processed meats.
The vegetarian diet is characterized both by avoidance of meat and a portfolio of natural substances of potential benefit in preventing type 2 diabetes. Although avoidance of red meat is common to all forms of vegetarianism, diets differ in regard to the inclusion of dairy products and eggs (lacto ovo vegetarian), fish (pesco vegetarian) or avoidance of all animal products entirely (vegan). A recent study done in Seventh day Adventists (Adventists), a religious group that promotes vegetarianism and eschews certain meats and shellfish, found that all types of vegetarianism were associated with lower prevalence of type 2 diabetes, including pesco vegetarian and semi-vegetarian diets though vegan and lacto ovo diets seemed to give the greatest protection [14].
The effects of the specific types of vegetarianism on incident diabetes have not been studied previously. In addition, studies have not represented ethnic and racial minorities well and the association between dietary patterns and diabetes has not been verified in these populations [15]. As an example, the Black/African American population carries an increased risk of diabetes, and could potentially benefit from a vegetarian diet [16]. Thus, in the present study, we examined the incidence of diabetes in relation to diet using data from the Adventist Health Study-2 (AHS-2). Furthermore we investigated whether a potential relationship was found in Black participants, a substantial subgroup within the cohort.
Methods
AHS-2 began in 2002 as a prospective study conducted among Adventist church members in the USA and Canada [17]. The main purpose of the study is to investigate the role of foods in regard to various forms of cancer. Participants were eligible if they were proficient in English and were aged 30 years or older. All instruments and procedures were approved by the Loma Linda University Institutional Review Board in June 2001 and approval was renewed annually thereafter.
Race and ethnicity were divided into Black (African American, West Indian/Caribbean, African or other Black) and non-Black (White non-Hispanic, Hispanic, Middle Eastern, Asian, Native Hawaiian/other Pacific Islander or American Indian). Participants were recruited through their churches using a somewhat different approach for Blacks than for non-Blacks [18,19]. Recruitment was church-by-church and staged by geographic region. Local church pastors selected study coordinators/recruiters (church recruiters) from their congregation. Each church had a suggested enrollment goal, which was largely based on active membership [17]. The church recruiters distributed questionnaires to the congregation. Once enrolled, each participant received a previously validated questionnaire with the informed consent materials. Follow-up post cards tracked members who did not respond within 4 weeks. Black participants received $10 for returning a completed questionnaire and the pastors and church recruiters received a financial incentive of $200-$1000 pro-rated on church size and the percentage of local goal achieved [17].
The questionnaire was piloted among Black and non-Black respondents for readability, comprehension, cultural sensitivity and relevance. The questionnaire was divided into sections on disease and medication history, frequency of consumption of various foods, physical activity, and other lifestyle practices. Questionnaires were returned by mail and edited for missing data and stray marks. Educational level was categorized to high school or less, some college and college or higher based on eight options. Income was categorized into earnings of ≤$10,000, $11,000—30,000, $31,000—50,000 and ≥$51,000.
Assessment of lifestyle exposures
As described previously [14], the food frequency portion of the questionnaire covered 130 hard coded foods or food groups that are commonly consumed and space for about 50 write-ins and assessed the past year. Previous validation of the questionnaire pertained to nutrients including vitamin, antioxidant and fatty acid intakes [20,21]. Vegetarian status was categorized by defining vegans as participants that reported consuming no animal products (red meat, poultry, fish, eggs, milk and dairy products <1 time/ month), lacto ovo vegetarians consumed dairy products and/or eggs ≥1 time/month but no fish or meat (red meat, poultry and fish <1 time/month); pesco vegetarians consumed fish ≥1 time/month and dairy products and/or eggs but no red meat or poultry (red meat and poultry <1 time/month); semi-vegetarians consumed dairy products and/or eggs and (red meat and poultry ≥1 time/month and <1 time/week); while non-vegetarians consumed animal products (red meat, poultry, fish, eggs, milk and dairy products >1 time/week). Alcohol was defined as consumption of any amount or none during the past 12 months.
Physical activity questions were previously validated in non-Black and Black participants, respectively [22,23]. We asked about sweat producing physical activity and categorized responses as never or <1 time/week, 1-2 times/week and 3 times or more/week. Participants reported average number of hours of sleep and hours per day of TV watching. Responses were divided into three categories (≤6, 7, and ≥8 h of sleep and <1, 1-2 and ≥3 h of TV watching).
Disease ascertainment
The Bi-Annual Hospitalization History follow-up questionnaire was administered two years after the baseline questionnaire returns starting from August 2004 to December 2007. Participants were asked to respond to the question, “During the last two years, have you developed the following conditions for the first time?” and one of the options was diabetes (type 1 or 2 was not specified).
We randomly selected a list of 99 participants reporting diabetes for verification of the self-reported development of diabetes. A telephone interview was attempted with all these participants. Of this total, 4 participants were deceased, and 16 could not be contacted by telephone or in a few cases by email, despite several attempts. Of 79 participants remaining, all except two confirmed that they had been told by their physician that they had “high blood sugar” or diagnosis of diabetes. One of the respondents who denied that he had developed diabetes reported a strong family history of diabetes and a spouse with diabetes.
Final population and statistical analyses
Among 97,586 participants, there were 66,188 respondents with dietary and anthropometric information at baseline and 71,679 respondents to the Bi-Annual Hospitalization History follow-up questionnaire two years after inclusion. When these respondents were combined, there were 53,536 respondents. Of these 4396 prevalent cases of type 1 and 2 diabetes were excluded, leaving a sample of 49,140. Further, respondents with missing study variables (TV watching, alcohol, smoking, physical activity, income, education, sleep hours, vegetarian status) were excluded leaving 41,387 participants. Among this total, 616 reported being diagnosed with diabetes by December 2007 giving a proportion of ∼1.5%. Participants that were included were slightly younger (57.9 [SD 13.5] years versus 58.8 [SD 15.0]), less likely to be female (63.3% versus 65.7%), less likely to be Black (17.3% versus 33.8%) and had attained a higher educational level (44.4% college graduates versus 31.8%) than excluded subjects.
All data were entered into SAS/STAT database and analyzed using SAS 9.2. Using chi-square, the number of new cases of diabetes in regard to category of vegetarian diet (vegan, lacto ovo, pesco or semi-vegetarian) or non-vegetarian diet was compared. Confounders associated with both diet and diabetes including age, gender, ethnicity (Black versus non-Black), education, income, television watching, hours of sleep, alcohol consumption, smoking and physical activity were assessed. Logistic regression was used to predict the incidence of diabetes while controlling for confounding variables in the entire population and repeated separately in Black (n = 7171) and non-Black (n = 34,216) participants. P-values <0.05 were considered statistically significant.
Results
Table 1 shows the distribution of participants in regard to report of diabetes and dietary and non-dietary variables. Participants who reported new diabetes were older, more likely to be Black, had a higher body mass index (BMI), reported a lower educational level and income, watched more TV, were less physically active, obtained less sleep, were less likely to be “never smokers” and were less likely to follow vegetarian-type diets than participants not reporting diabetes.
Table 1.
Distribution of participants, incident cases of diabetes and nondietary variables.
| Diabetes reported | Not reported | P-value | |
|---|---|---|---|
| N | 616 | 40,771 | |
| Age, years, mean (SD) | 62.3 (12.8) | 57.9 (13.5) | <0.0001 |
| Female, % | 62.3 | 63.3 | 0.63 |
| Black, % | 24.7 | 17.2 | <0.0001 |
| BMI, kg/m2, mean (SD) | 31.2 (6.9) | 26.6 (5.4) | <0.0001 |
| Education, % | <0.0001 | ||
| High school or less | 22.9 | 16.7 | |
| Some college | 43.2 | 38.8 | |
| College or higher | 33.9 | 44.5 | |
| Income, % | <0.0001 | ||
| ≤$10,000 | 22.7 | 19.0 | |
| $11,000–30,000 | 41.4 | 36.6 | |
| $31,000–50,000 | 22.2 | 23.5 | |
| ≥$51,000 | 13.6 | 20.9 | |
| TV watching, % | <0.0001 | ||
| None to < 1 h/day | 15.3 | 27.9 | |
| 1–2 h/day | 47.9 | 48.0 | |
| ≥3 h/day | 36.9 | 24.1 | |
| Physical activity, %, frequency/week | <0.0001 | ||
| <1 time/week | 43.7 | 33.9 | |
| 1–2 times/week | 18.7 | 20.2 | |
| ≥3 times/week | 37.7 | 45.9 | |
| Sleep, % | |||
| ≤6 h/night | 38.3 | 28.9 | |
| 7 h/night | 31.2 | 38.4 | |
| ≥8 h/night | 30.5 | 32.7 | |
| Alcohol, % last 12 months | 0.89 | ||
| Never | 59.4 | 59.7 | |
| Ever | 40.6 | 40.3 | |
| Cigarette Smoking, % | 0.001 | ||
| Never | 77.1 | 82.2 | |
| Ever | 22.9 | 17.8 | |
| Vegetarian status, % | <0.0001 | ||
| Vegan | 3.1 | 8.7 | |
| Lacto ovo vegetarian | 24.7 | 34.2 | |
| Pesco vegetarian | 7.6 | 8.8 | |
| Semi-vegetarian | 3.6 | 5.8 | |
| Non-vegetarian | 61.0 | 42.5 |
The incidence of diabetes increased incrementally among vegans, lacto ovo vegetarians, pesco vegetarians, semi-vegetarians and non-vegetarians (Table 2). All sociodemographic variables, BMI and other lifestyle variables differed according to dietary pattern with non-vegetarians reporting the lowest mean age, highest proportion with a low educational level, highest proportion engaged in TV watching for ≥3 h/day, and highest proportion with ever use of alcohol, and ever smokers (Table 2). Non-vegetarians were also least likely to report higher amounts of physical activity. More Black participants were found in the non- or pesco vegetarian group than in the other dietary groups.
Table 2.
Incidence of diabetes and distribution of non-dietary variables according to diet.
| Vegan | Lacto-ovo vegetarian | Pesco Vegetarian | Semi-Vegetarian | Non-vegetarian | P-Value | |
|---|---|---|---|---|---|---|
| N | 3545 | 14,099 | 3644 | 2404 | 17,695 | <0.0001 |
| Diabetes, % | 0.54 | 1.08 | 1.29 | 0.92 | 2.12 | <0.0001 |
| Age, years, mean (SD) | 59.6 (13.5) | 59.5 (13.9) | 58.7 (13.7) | 58.4 (13.6) | 56.1 (13.0) | <0.0001 |
| Female, % | 59.5 | 62.8 | 65.3 | 65.7 | 63.7 | <0.0001 |
| Black, % | 15.5 | 8.7 | 27.9 | 11.4 | 23.2 | <0.0001 |
| BMI, kg/m2, mean (SD) | 23.8 (4.4) | 25.6 (4.8) | 25.9 (4.8) | 26.8 (5.2) | 28.2 (5.9) | <0.0001 |
| Education, % | <0.0001 | |||||
| High school or less | 16.4 | 12.9 | 15.5 | 18.4 | 20.0 | |
| Some college | 37.8 | 34.4 | 37.8 | 38.9 | 42.8 | |
| College or higher | 45.8 | 52.7 | 46.7 | 42.6 | 37.2 | |
| Income, % | <0.0001 | |||||
| ≤$10,000 | 26.5 | 19.8 | 17.4 | 19.5 | 17.4 | |
| $11,000–30,000 | 39.5 | 36.2 | 34.4 | 37.5 | 36.7 | |
| $31,000–50,000 | 19.4 | 24.4 | 24.2 | 23.0 | 23.5 | |
| ≥$51,000 | 14.6 | 19.6 | 24.0 | 20.0 | 22.4 | |
| TV watching, % | <0.0001 | |||||
| None to < 1 h/day | 49.7 | 35.5 | 27.6 | 25.6 | 17.4 | |
| 1–2 h/day | 37.7 | 46.9 | 50.1 | 49.7 | 50.2 | |
| ≥3 h/day | 12.6 | 17.6 | 22.3 | 24.7 | 32.4 | |
| Sleep, % | <0.0001 | |||||
| ≤6 h/day | 24.3 | 23.8 | 32.7 | 27.3 | 33.7 | |
| 7 h/day | 39.6 | 40.4 | 37.7 | 38.8 | 36.3 | |
| ≥8 h/day | 36.2 | 35.8 | 29.6 | 33.9 | 30.0 | |
| Physical activity, % Frequency/week | <0.0001 | |||||
| Never or <1 time/week | 29.8 | 32.5 | 31.0 | 35.9 | 36.6 | |
| 1–2 times/week | 17.4 | 19.8 | 19.4 | 20.1 | 21.1 | |
| 3 or more times/week | 52.8 | 47.7 | 49.6 | 44.0 | 42.3 | |
| Alcohol, last 12 months, % | <0.0001 | |||||
| No | 66.7 | 74.8 | 61.9 | 61.0 | 45.6 | |
| Yes | 33.3 | 25.2 | 38.1 | 39.0 | 54.4 | |
| Cigarette smoking, % | <0.0001 | |||||
| Never | 84.3 | 89.3 | 84.5 | 82.0 | 75.5 | |
| Ever | 15.7 | 10.7 | 15.5 | 18.1 | 24.5 |
In multiple logistic regression analysis adjusted for age, the odds ratio (OR) of incident diabetes for vegans was 0.228 (95% CI 0.140, 0.372), lacto ovo vegetarians, 0.461 (95% CI, 0.373, 0.569), pesco vegetarians, 0.597 (95% CI 0.433, 0.823), and semi-vegetarians, 0.380 (95% CI 0.236, 0.613) compared to the non-vegetarian reference group. With adjustment for age and BMI, these point estimates were somewhat weakened but remained statistically significant with the exception of the pesco vegetarian group. For vegans the OR was 0.383 (95% CI 0.233, 0.629), for lacto ovo vegetarians, 0.635 (95% CI, 0.511, 0.789), for pesco vegetarians 0.791 (0.572, 1.095) and for semi-vegetarians 0.447 (95% CI 0.277, 0.722). Adjustment for lifestyle factors in addition had very minor effects on the estimates compared to the age and BMI adjusted results (data not shown).
In the final model, adjustment was made for age, BMI, lifestyle, and sociodemographic factors including gender, ethnicity, income and education (Table 3). Black ethnicity was associated with an increased incidence of diabetes (OR 1.364, 95% CI 1.093–1.702) as was age, male gender, and BMI, while a higher income and more sleep were associated with a lower incidence. Vegan (OR 0.381, 95% CI 0.236–0.617), lacto ovo (OR 0.618, 95% CI 0.503–0.760), and semi-vegetarian (OR 0.486, 95% CI 0.312–0.755) diets were associated with a lower incidence of diabetes, while pesco vegetarian diets were not associated with lower incident diabetes (Table 3).
Table 3.
Multiple logistic regression analysis of the relation between diet and diabetes in the total population (n = 41,387).
| Odds ratio | 95% CI | |
|---|---|---|
| Age in 1-year increments | 1.031 | 1.024–1.038 |
| BMI per 1 kg/m2 | 1.109 | 1.096–1.122 |
| Black vs. non-Black | 1.364 | 1.093–1.702 |
| Female vs. male | 0.730 | 0.608–0.876 |
| Education | ||
| Some college vs high school or less | 1.014 | 0.819–1.256 |
| College or higher vs high school or less | 0.943 | 0.740–1.202 |
| Income | ||
| $11,000–$30,000 vs <$10,000 | 0.864 | 0.696–1.073 |
| $31,000–$50,000 vs <$10,000 | 0.868 | 0.670–1.125 |
| ≥$51,000 vs <$10,000 | 0.628 | 0.461–0.854 |
| Television watching | ||
| 1–2 h/day vs <1 h/day | 1.228 | 0.967–1.559 |
| ≥3 h/day vs <1 h/day | 1.253 | 0.967–1.623 |
| Sleep | ||
| 7 h/night vs ≤6 h/night | 0.778 | 0.638–0.948 |
| ≥8 h/night vs ≤6 h/night | 0.738 | 0.607–0.897 |
| Alcohol use | ||
| Ever vs. never | 0.840 | 0.687–1.026 |
| Physical activity, frequency/week | ||
| 1–2×/week vs <1 ×/week or never | 0.989 | 0.788–1.242 |
| >3×/week vs <1 ×/week or never | 0.950 | 0.789–1.143 |
| Smoking | ||
| Ever vs never | 1.019 | 0.809–1.283 |
| Dietary status | ||
| Vegan vs non-vegetarian | 0.381 | 0.236–0.617 |
| Lacto-ovo vegetarian vs non-vegetarian | 0.618 | 0.503–0.760 |
| Pesco-vegetarian vs non-vegetarian | 0.790 | 0.575–1.086 |
| Semi-vegetarian vs non-vegetarian | 0.486 | 0.312–0.755 |
The test of interaction between dietary group and ethnicity was not statistically significant (p = 0.9). In non-Black participants findings were similar to the total population (Table 4). Vegan, lacto ovo and semi-vegetarian diets were protective against diabetes (OR 0.429, 95% CI 0.249–0.740; OR 0.684, 95% CI 0.542–0.862; OR 0.501, 95% CI 0.303–0.827). Among Black participants, female gender was not protective (Table 5) while age and BMI were risk factors as expected. Higher income and increased exercise as well as vegan and lacto ovo vegetarian diets were protective against diabetes (vegan diet, OR 0.304, 95% CI 0.110–0.842; lacto ovo vegetarian diet, OR 0.472, 95% CI 0.270–0.825) (Table 5).
Table 4.
Multiple logistic regression analysis of the relation between vegetarian diet, and diabetes in non-Blacks (n = 34,215).
| Odds ratio | 95% CI | |
|---|---|---|
| Age | 1.033 | 1.025–1.041 |
| BMI | 1.119 | 1.104–1.134 |
| Female vs. male | 0.681 | 0.554–0.841 |
| Education | ||
| Some college vs high school or less | 1.145 | 0.890–1.472 |
| College or higher vs high school or less | 1.121 | 0.847–1.484 |
| Income | ||
| $11,000–$30,000 vs <$10,000 | 0.740 | 0.578–0.947 |
| $31,000–$50,000 vs <$10,000 | 0.886 | 0.663–1.184 |
| ≥$51,000 vs <$10,000 | 0.531 | 0.370–0.763 |
| Television watching | ||
| 1–2 h/day vs <1 h/day | 1.314 | 1.000–1.728 |
| ≥3 h/day vs <1 h/day | 1.412 | 1.046–1.905 |
| Sleep | ||
| 7 h/night vs ≤6 h/night | 0.767 | 0.610–0.964 |
| ≥8 h/night vs ≤6 h/night | 0.719 | 0.571–0.905 |
| Alcohol use | ||
| Ever vs never | 0.898 | 0.710–1.134 |
| Physical activity, frequency/week | ||
| 1–2×/week vs <1×/week or never | 0.958 | 0.730–1.257 |
| >3×/week vs <1×/week or never | 1.053 | 0.852–1.303 |
| Smoking | ||
| Ever vs never | 0.940 | 0.717–1.232 |
| Dietary status | ||
| Vegan vs non-vegetarian | 0.429 | 0.249–0.740 |
| Lacto-ovo vegetarian vs non-vegetarian | 0.684 | 0.542–0.862 |
| Pesco-vegetarian vs non-vegetarian | 0.868 | 0.593–1.270 |
| Semi-vegetarian vs non-vegetarian | 0.501 | 0.303–0.827 |
Table 5.
Multiple logistic regression analysis of the relation between vegetarian diet and diabetes in Blacks (n = 7172).
| Odds ratio | 95% CI | |
|---|---|---|
| Age | 1.034 | 1.020–1.048 |
| BMI | 1.072 | 1.0461.098 |
| Female vs. male | 0.872 | 0.595–1.279 |
| Education | ||
| Some college vs high school or less | 0.722 | 0.477–1.094 |
| College or higher vs high school or less | 0.541 | 0.328–0.895 |
| Income | ||
| $11,000–$30,000 vs <$10,000 | 1.346 | 0.833–2.176 |
| $31,000–$50,000 vs <$10,000 | 0.787 | 0.432–1.434 |
| ≥$51,000 vs <$10,000 | 1.054 | 0.561–1.981 |
| Television watching | ||
| 1–2 h/day vs <1 h/day | 0.904 | 0.552–1.482 |
| ≥3 h/day vs <1 h/day | 0.761 | 0.454–1.274 |
| Sleep | ||
| 7 h/night vs ≤6 h/night | 1.073 | 0.695–1.658 |
| ≥8 h/night vs ≤6 h/night | 0.967 | 0.656–1.424 |
| Alcohol use | ||
| Ever vs never | 0.760 | 0.513–1.126 |
| Physical activity, frequency/week | ||
| 1–2×/week vs <1×/week or never | 1.015 | 0.667–1.544 |
| >3×/week vs <1×/week or never | 0.653 | 0.443–0.967 |
| Smoking | ||
| Ever vs never | 1.214 | 0.776–1.898 |
| Dietary status | ||
| Vegan vs non-vegetarian | 0.304 | 0.110–0.842 |
| Lacto ovo vegetarian vs non-vegetarian | 0.472 | 0.270–0.825 |
| Pesco vegetarian vs non-vegetarian | 0.618 | 0.352–1.086 |
| Semi-vegetarian vs non-vegetarian | 0.469 | 0.153–1.438 |
Discussion
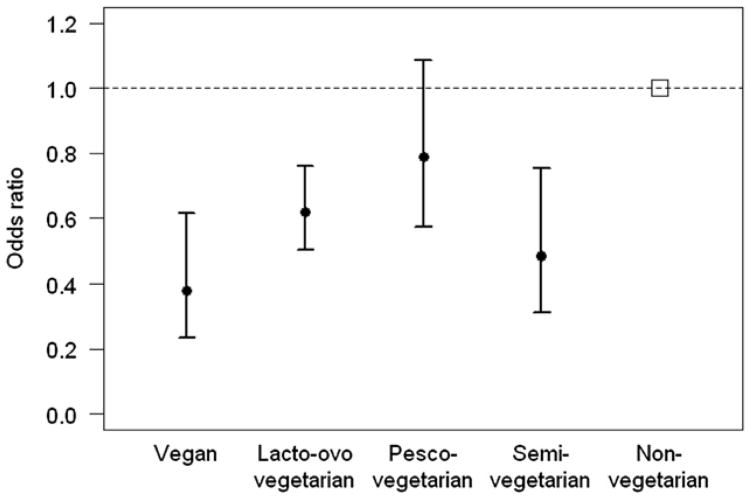
The main finding of this study was that vegan, lacto ovo vegetarian and semi-vegetarian diets were associated a substantial reduction in risk of diabetes compared to non-vegetarian diets, after adjusting for BMI and sociodemographic and lifestyle factors. Among non-Black participants, vegan, lacto ovo and semi-vegetarian diets were associated with a decreased risk of diabetes, while among Black participants, only vegan and lacto ovo vegetarian diets were associated with a decreased risk of diabetes. The increased risk of diabetes in the Black subgroup was in the order of one third with the upper confidence limit at about 70%, while the protection afforded by vegan diets in this subgroup was ∼70% and by lacto ovo vegetarian diets ∼50% suggesting that a vegetarian-type diet may be a way to counteract the increased diabetes risk for Blacks (Fig. 1).
Figure 1.
Odds ratios with 95% confidence intervals for incident diabetes by dietary group adjusted for age, BMI, ethnicity, gender, educational level, income, TV watching, sleep, alcohol, physical activity and cigarette smoking.
These results are congruent with our earlier prevalence study of determinants of type 2 diabetes [14]. In this study, there appeared to be an incremental protection as dietary pattern moved from non-vegetarian to semi-vegetarian to pesco vegetarian to lacto ovo vegetarian to vegan [14]. In the current study the vegan diet appeared to afford the greatest protection against the development of diabetes, however, confidence limits of the vegan and lacto ovo and semi-vegetarian diets largely overlapped, indicating no significant differences between the dietary patterns. Furthermore, the results for vegans should be interpreted with care, as there were only a very small number of vegans who developed diabetes.
A vegetarian diet that included fish was not associated with a significant risk reduction, though the point estimate was suggestive of an effect. Whether the advantage of the prudent diet in protecting against diabetes extends specifically to fish is still open for investigation. Ecologic studies suggest significantly reduced prevalence of type 2 diabetes among high consumers of fish and seafood in countries with a high prevalence [24]. In contrast, at least two prospective studies have shown an increase in risk associated with long-chain omega-3 fatty acids or total fish intake [25,26]. In the European Prospective Investigation of Cancer (EPIC)-Norfolk study fish intake (other than fried fish) was associated with decreased risk of incident type 2 diabetes, while shellfish was associated with an increased risk [27]. Adventists eschew shellfish, thus the lack of a significant protection of the pesco vegetarian diet is not likely due to consumption of shellfish.
Various explanations may be offered for the conflicting results of fish and seafood studies. One may be the method of the preparation of fish. In one study, fried or steamed fish showed opposite effects on the risk of diabetes [5]. Fish contains eicosapentanoic (EPA) and docosahexaenoic (DHA) acids, which may increase insulin sensitivity. When fish is fried, EPA and DHA are lowered impairing the metabolic advantages of fish [25]. We did not address method of preparation in this study. Another explanation may be elevated plasma selenium levels associated with fish intake, which have been associated with risk of diabetes. More research is needed to understand mechanisms by which selenium is implicated in diabetes risk [26,27].
Part of the protection associated with vegetarian diets is due to the lower BMI of vegetarians compared to non-vegetarians, though the notion that meat intake is associated with weight gain has been called into question recently [28]. When the logistic regression analyses were run without control for BMI associations between vegetarian diets and diabetes were strengthened, indicating that BMI accounts for some of the observed protection associated with vegetarian diets. However, associations remained strong after considering BMI. Furthermore, plausible mechanisms have been proposed to explain the protection associated with vegetarian diets. Fruits and vegetables may contribute to a decreased incidence of type 2 diabetes through their low energy density, low glycemic load, and high fiber and macronutrient content. Other features of the vegetarian diet are whole grains and legumes. These foods have been shown to improve glycemic control, slow the rate of carbohydrate absorption and the risk of diabetes [29,7].
Our study provides data on other determinants of development of diabetes in Black compared to non-Black samples. While the effects of age and BMI on risk were similar in both groups, female gender among Blacks was not protective, in contrast to findings in non-Black females. Higher education but not income was protective in Blacks while the opposite was observed in non-Blacks. A previous study indicated that education was less effective as a control for socioeconomic differences between African Americans and Whites, however, most of the Blacks in the study were poorly educated [30]. Some of the differences between Blacks and non-Blacks may be due to differences in study power in the two groups.
Study strengths and limitations
The strengths of the study are that the data were collected prospectively in a well-designed, established cohort study. Much effort has gone into validating the dietary and physical activity questionnaires [20–23]. The prospective design minimized recall bias. Study results were strengthened due to measurement of several well known confounders. After accounting for potential confounding, the associations remained strong.
A number of study limitations must be considered when interpreting these findings. Because screening for blood glucose level was not feasible in this large cohort, under-diagnosis of diabetes is likely, especially in the Black subgroup [31]. This means that cases may be more severe in Blacks than in non-Blacks. The sample of Blacks was substantially smaller than that of non-Blacks. The data are self-reported, however, we attempted to assess the consistency of self-reports of diabetes. We confirmed the consistency in 97.4% of those who reported diabetes, in line with a previous study of self-reported diabetes in the same population, where 96% of those who reported diabetes were confirmed to have diabetes [14].
Some selection bias is present due to non-response to the follow-up questionnaire. The enrollment questionnaire was lengthy and may have discouraged completion of the survey. Dietary measurement error is inevitable and may result in misclassification. Measurement errors may be introduced by under or over reporting of foods eaten. Random error in measuring intake and dietary change attenuate the associations. The vegetarian diet was positively associated with some lifestyle related factors and inversely associated with others. It is possible that some residual confounding has remained after adjustment because of inaccurate measurements.
Finally it may be argued that the population in this study is not representative of the general population due to their lack of smoking, low alcohol consumption and general health consciousness. On the other hand, the choice of population with a very low level of confounders like smoking, which is associated with the development of diabetes [32], and strongly associated with dietary practices, may allow true associations between diet and health to appear.
Perspective
In this prospective study, vegetarian diets were associated with a substantial and independent lower incidence of diabetes non-Black and Black participants, indicating the potential of these diets to stem the current diabetes epidemic.
Acknowledgments
This work was funded in part by the National Institutes of Health grant number 1R01CA94594 and by the School of Public Health, Loma Linda University.
References
- 1.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164:2235–40. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 2.Van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2004;136:201–9. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 3.Montonen J, Knekt P, Härkänen T, Järvinen R, Heliövaara M, Aromaa A, et al. Dietary patterns and the incidence of type 2 diabetes. Am J Epidemiol. 2005;161:219–27. doi: 10.1093/aje/kwi039. [DOI] [PubMed] [Google Scholar]
- 4.Heidemann C, Hoffmann K, Spranger J, Klipstein-Grobusch K, Mölig M, Pfeiffer AFH, et al. A dietary pattern protective against type 2 diabetes in the European Prospective investigation into Cancer and Nutrition (EPIC)-Potsdam Study cohort. Diabetologia. 2005;48:1126–34. doi: 10.1007/s00125-005-1743-1. [DOI] [PubMed] [Google Scholar]
- 5.Hodge AM, English DR, O'Dea K, Giles GG. Dietary patterns and diabetes incidence in the Melbourne Collaborative cohort study. Am J Epidemiol. 2007;165:603–10. doi: 10.1093/aje/kwk061. [DOI] [PubMed] [Google Scholar]
- 6.Brunner EJ, Mosdøl A, Witte DR, Martikainen P, Stafford M, Shipley MJ, et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–21. doi: 10.1093/ajcn/87.5.1414. [DOI] [PubMed] [Google Scholar]
- 7.de Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RB. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med. 2007;4:1385–94. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villegas R, Shu XO, Gao Y, Yang G, Elasy T, Li H, et al. Vegetable but not fruit consumption reduces the risk of type 2 diabetes in Chinese women. J Nutr. 2008;138:574–80. doi: 10.1093/jn/138.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, et al. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr. 2008;87:162–7. doi: 10.1093/ajcn/87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snowdon DA, Phillips RL. Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health. 1985;75:507–12. doi: 10.2105/ajph.75.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Manson JE, Buring JE, Liu SL. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women. Diabetes Care. 2004;27:2108–15. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 12.Schulze MB, Manson JE, Willett WC, Hu FB. Processed meat intake and incidence of type 2 diabetes in younger and middle-aged women. Diabetologia. 2003;46:1465–73. doi: 10.1007/s00125-003-1220-7. [DOI] [PubMed] [Google Scholar]
- 13.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia. 2009;52:2277–87. doi: 10.1007/s00125-009-1481-x. [DOI] [PubMed] [Google Scholar]
- 14.Tonstad S, Butler T, Yan R, Fraser G. Type of vegetarian diet, body weight and prevalence of type 2 diabetes. Diabetes Care. 2009;32:791–6. doi: 10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinlin F, Binyou W, Terry C. A new approach to the study of diet and risk of type 2 diabetes. J Postgrad Med. 2007;53:139–43. doi: 10.4103/0022-3859.32219. [DOI] [PubMed] [Google Scholar]
- 16.Brancati FL, Whelton PK, Kuller LH, Klag MJ. Diabetes mellitus, race, and socioeconomic status. A population-based study. Ann Epidemiol. 1996;6:67–73. doi: 10.1016/1047-2797(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 17.Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, et al. Cohort profile: the Adventist health Study-2 (AHS-2) Int J Epidemiol. 2008;37:260–5. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery S, Herring P, Yancey A, Beeson L, Butler T, Knutsen S, et al. Comparing self-reported disease outcomes, diet and lifestyles in a national cohort of black and white Seventh-day Adventists. Prev Chronic Dis [serial online] 2007 Jul; Available from, http://www.cdc.gov/ped/issues/2007/jul/06_0103.htm. [PMC free article] [PubMed]
- 19.Herring P, Montgomery S, Yancey AK, Williams D, Fraser G. Understanding the challenges in recruiting blacks to a longitudinal cohort study: the Adventist Health Study. Ethn Dis. 2004;14:423–30. [PubMed] [Google Scholar]
- 20.Knutsen S, Fraser G, Lindsted K, Beeson W, Shavlik D. Validation of assessment of nutrient intake. Comparing biological measurements of vitamin C, folate, alpha-tocopherol and carotene with 24-hour dietary recall information in non-Hispanic blacks and whites. Ann Epidemiol. 2001;11:406–16. doi: 10.1016/s1047-2797(01)00224-1. [DOI] [PubMed] [Google Scholar]
- 21.Knutsen S, Fraser G, Beeson W, Lindsted K, Shavlik D. Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24-hour recall and food frequency questionnaire in Black and White Adventists: the Adventist Health Study. Ann Epidemiol. 2003;13:119–27. doi: 10.1016/s1047-2797(02)00260-0. [DOI] [PubMed] [Google Scholar]
- 22.Singh PN, Tonstad S, Abbey DE, Fraser GE. Validity of selected physical activity questions in white Seventh-day Adventists and non-Adventists. Med Sci Sports Exer. 1996;28:1026–37. doi: 10.1097/00005768-199608000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Singh PN, Fraser GE, Knutsen SF, Lindsted DS, Bennett H. Validity of a physical activity questionnaire among African-American Seventh-day Adventists. Med Sci Sports Exer. 2000;33:468–75. doi: 10.1097/00005768-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Nkondjok A, Receveur O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab. 2003;6:635–42. doi: 10.1016/s1262-3636(07)70080-0. [DOI] [PubMed] [Google Scholar]
- 25.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr. 2009;90:613–20. doi: 10.3945/ajcn.2008.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Woudenbergh GJ, van Ballegooijen AJ, Kuijsten A, Sijbrands EJ, van Rooij FJ, Geleijnse JM, et al. Eating fish and risk of type 2 diabetes: a population-based, prospective follow-up study. Diabetes Care. 2009;32:2021–6. doi: 10.2337/dc09-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel PS, Bingham SA, Sharp SJ, Wareham NJ, Luben RN, Forouhi NG, et al. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes. Diabetes Care. 2009;32:1857–63. doi: 10.2337/dc09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astrup A, Clifton P, Layman DK, Mattes RD, Westerterp-Plantenga MS. Meat intake's influence on body fatness cannot be assessed without measurement of body fat. Am J Clin Nutr. 2010;92:1274–6. doi: 10.3945/ajcn.110.000661. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins DJA, Kendall CWC, Marchie A, Jenkins AL, Augustin LSA, Ludwig DS, et al. Type 2 diabetes and the vegetarian diet. Am J Clin Nutr. 2003;78(Suppl):610S–6S. doi: 10.1093/ajcn/78.3.610S. [DOI] [PubMed] [Google Scholar]
- 30.Robbins JM, Vaccarino V, Zhang H, Kasl S. Excess type 2 diabetes in African-American women and men aged 40–74 and socioeconomic status: evidence from the third national health and nutrition examination survey. J Epidemiol Comm Health. 2000;54:839–45. doi: 10.1136/jech.54.11.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fryar C, Hirsh R, Eberhardt MS, Yoon S, Wright JD. National Health and Nutrition Examination Surveys, 1999-2006. Center for Disease Control and Prevention/National Center for Health Statistics; 2010. Hypertension, high serum total cholesterol, and diabetes: Racial and ethnic prevalence differences in U.S. adults, 1999–2006. [Google Scholar]
- 32.Yeh HC, Duncan BB, Schmidt MI, Want NY, Brancati FL. Smoking, smoking cessation and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–7. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]