Abstract
Identification of Protein Tyrosine Phosphatase (PTP) substrates is critical in understanding cellular role in normal cells as well as cancer cells. We have previously shown that reduction of PTPL1 protein levels in Ewings sarcoma (ES) inhibit cell growth and tumorigenesis. Therefore, we sought to identify novel PTPL1 substrates that may be important for tumorigenesis. In this current work, we demonstrated that mouse embryonic fibroblasts without PTPL1 catalytic activity fail to form foci when transfected with oncogenes. We proved that catalytic activity of PTPL1 is important for ES cell growth. Using a substrate-trapping mutant of PTPL1 we identified putative PTPL1 substrates by mass-spectrometry. One of these putative substrates was characterized as Valosin Containing Protein (VCP/p97). Using multiple biochemical assays we validated VCP as a novel substrate of PTPL1. We also provide evidence that tyrosine phosphorylation of VCP might be important for its midbody localization during cytokinesis. In conclusion, our work identifies VCP as a new substrate for PTPL1, which may be important in cellular transformation. Our investigation link an oncogenic transcription factor EWS-FLI1, with a key transcriptional target protein tyrosine phosphatase PTPL1, and its substrate VCP. Given our observation that PTPL1 catalytic activity is important for cell transformation, our results may also suggest that VCP regulation by PTPL1 might be important for tumorigenesis.
Keywords: ES, PTPL1, VCP, Phosphorylation
Introduction
Elucidating key signalling pathways in oncogenesis has revealed biologically vulnerable proteins, which can serve as valuable therapeutic targets. Ewings sarcoma (ES) are characterized by a pathognomonic translocation which fuses the N-terminal of the gene EWS with the C-terminal of an ets-family gene [1]. The protein product of this translocation is an oncogenic transcription factor called EWS-FLI1. Genes modulated by EWSFLI1 have a profound influence on ES tumorigenesis. Our previous work showed that protein tyrosine phosphatase L1 (PTPL1) is a direct transcriptional target of EWS-FLI1 and critical for the maintenance of the oncogenic phenotype [2].
PTPL1 (a.k.a PTPN13, FAP-1, PTP-BAS, PTP1E) is a 270 kDa non-receptor type protein tyrosine phosphatase with published opposing roles in tumorigenesis [3]. Few putative PTPL1 substrates have been identified and additional substrates may clarify its cell-specific functions. These substrates include IRS-1 [4], IκBα [5], HER2 [6], STAT4 [7] and Src [8]. Even though no cell cycle dependent substrates of PTPL1 have been identified, there is evidence that PTPL1 is localized to the centrosomes and to the midbody during the cell cycle [9]. Interestingly, in breast cancer PTPL1 acts as a tumor suppressor through IRS-1 dephosphorylation [4] and PTPL1 expression is a prognostic factor for favorable outcome in these cancers [10]. Since PTPL1 likely has different functions in diverse cellular contexts, a more complete knowledge of the enzymatic substrates and protein scaffold partners of PTPL1 are necessary to resolve ambiguity.
A multifunctional chaperon Valosin Containing Protein (VCP) has many diverse roles in the cell [11,12]. A central role of VCP is to link ubiquitinated substrates with either the ubiquitin conjugation or deconjugation machineries in endoplasmic reticulum-associated degradation (ERAD) [13] and ubiquitin-proteosome pathways [14] thereby influencing protein fate in the cell [15]. VCP in the context of ERAD and ubiquitin-proteosome pathways is being widely studied. On the other hand, VCP’s role in cell cycle has been first proposed in Saccharomyces cerevisiae in 1998 [16], however until recently, has not been investigated further. Studies show that VCP is involved in the terminal stages of cell division by participating in the re-emergence of the nuclear envelope in Xenopus egg extracts by removing Aurora B [17]. In addition VCP has been shown to antagonize Aurora B in HeLa cells for proper chromosome segregation [18]. Such emerging studies suggest a critical role for VCP in cell cycle.
To enhance our understanding of the role of PTPL1 in ES tumorigenesis we sought to identify novel PTPL1 substrates. In this study, we generated a substrate-trapping mutant of PTPL1 and used it in a screen for novel substrates. Our screen identified VCP as a candidate substrate of PTPL1 and further biochemical studies validated VCP as a novel PTPL1 substrate. Further on we provide critical evidence suggesting a role for VCP in late stage mitosis, specifically during cytokinesis. In addition, a key finding in our study demonstrates the importance of PTPL1 catalytic activity in oncogenic transformation.
Methods
Cell lines
MEFs were isolated from PTP-BLΔPTP/ΔPTP or wild type mice according to standard procedures [19]. TC32 ES cells were maintained in RPMI (Invitrogen) with 10% FBS and 1% HEPES (Invitrogen). HEK293 cells and MEFs (PTP-BLΔPTP/ΔPTP and PTP-BLWT/WT) were maintained in DMEM (Invitrogen) with 10% FBS (Quality Biologicals). COLO-357, COLO-PL and COLO-SL were described before [20] and were a kind gift from Dr John Jessup.
Antibodies
Actin-HRP, pY99, PTPL1 and GST antibodies were purchased from Santa Cruz Biotechnology. VCP antibodies were purchased from Abcam. 4G10 phosphotyrosine antibody was purchased from Upstate. Flag-M5 antibody was purchased from Sigma.
Plasmids
Full-length Flag-tagged PTPL1 and GFP-VCP plasmids were generous gifts from Dr. Jan Saras and Dr Len Neckers, respectively. Flag-tagged PTPL1 was cloned into pCDNA4/TO (Invitrogen). ΔPTP-PTPL1 was generated by removing the PTP domain of PTPL1 using restriction digest. The GST-PTP constructs were created by cloning the cDNA part encoding the PTPL1 phosphatase domain into pET42 vector (Stratagene). All of the cloning strategies were designed using pDRAW32 DNA Analysis Software (http://www.acaclone.com). Primers for site-directed mutagen esis were designed with PrimerX (http://bioinformatics.org/primerx) and all the reactions were performed by QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer’s protocol. Site-directed mutagenesis primer sequences were 5- ACTGCCTGGCCAGCCCATGATACACCTTC for the PTPL1-DA mutation and 5- CAATGACGATGACCTGTTCGGCGG TACCACCATGG for VCP-YF mutation. The fidelity of all constructs was confirmed via sequencing.
Soft-agar assays
Soft-agar assays were performed in 12 well plates using 0.4% SeaPlaque Agar (Cambrex Bioscience) in PBS. Colonies were stained with 200–300 µl per well of 50 mg/ml MTT (Sigma) for 3 h at 37 °C and imaged with Kodak 2D imaging system.
GST fusion protein purification
BL21 CodonPlus Competent cells (Stratagene) were transformed with pET42-GST-PTP plasmids. An aliquot was induced with 1 mM IPTG for 4 h at 37 °C, lysed and recombinant proteins were affinity purified using GSH-Agarose beads.
In vitro substrate-trapping
For small scale experiments, log phase TC32 cells were treated with 300 µM pervanadate for 1 h before being lysed with NP40 lysis buffer (50 mM Tris pH 7.5, 5 mM EDTA, 150 mM NaCl, 20 mM NaF, 1% NP40, 10 mM Iodoacetic acid, 1 mM PMSF, 2 µg/ml Aprotinin and 2 µg/ml Leupeptin). The lysate was treated with 10 mM DTT for 10 min on ice prior to pull-down. Two milligrams of protein was mixed with 10 µl of affinity purified GST-PTP recombinant protein on GSH-Agarose beads. Protein complexes were eluted from the beads with 2X sample buffer, boiled and then subject to immunoblotting. Scaled up experiments were done with 17 mg of protein and 100 µl of recombinant protein. Bands were excised from Coomassie blue stained SDS-PAGE. Tryptic peptides were extracted and analyzed with MALDI-TOF-TOF spectrometer (4800 Proteomics Analyzer).
In vivo substrate-trapping
TC32 cells were transiently transfected with full-length Flag-tagged PTPL1 constructs using electroporation. After 24 h, cells were lysed and 2 mg of protein was mixed with 30 µl of Flag- Agarose beads (Sigma). Samples were subjected to immunoblotting. The immunoblots were developed using the LAS-3000 Imaging System (Fujifilm Life Science) and band intensities were quantified using the built in software.
Immunofluorescence assays
TC32 cells were plated on collagen-treated coverslips and next day fixed with 4% paraformaldehyde for 15 min at room temperature. Blocking was performed with 1% Blocker BSA (Pierce), 0.05% Triton-X100 in 10% normal goat serum for 30 min at room temperature. VCP specific antibody (mouse) was used at 1/2000 dilution and Aurora-B specific antibody (rabbit) was used at 1/1000. Coverslips were mounted on glass slides with mounting media containing DAPI (Invitrogen). HEK293 cells were plated on poly-lysine treated 35 mm FluoroDish (World Precision Instruments). Cells were transfected with GFP-tagged VCP expression plasmids at 50% confluence with Fugene-6 (Roche). Thirty hours post-transfection, cells were fixed with paraformaldehyde for 15 min at room temperature. Images were taken on an Olympus Fluoview FV300 Laser Confocal Microscope (Olympus) using a 60X/1.4 N.A. objective lens.
Results
PTPL1 is pro-tumorigenic in mouse embryonic fibroblasts
We hypothesized that PTPL1 catalytic activity had a role in transformation. In order to investigate the requirement of PTPL1 catalytic activity in transformation of MEFs, we transiently transfected PTP-BLWT/WT or PTP-BLΔPTP/ΔPTP MEFs with known oncogenes. We observed that in both PTP-BLWT/WT or PTP-BLΔPTP/ΔPTP MEFs, neither oncogenic RasV12 or small-T nor S37A-β-Catenin alone caused foci formation (Fig. 1). Oncogenic RasV12 together with either small-T or S37A-β-Catenin resulted in foci formation only in PTP-BLWT/WT MEFs but not in PTP-BLΔPTP/ΔPTP MEFs. This supported a requirement that PTPL1 catalytic activity is critical in the transformation of mouse embryonic fibroblasts.
Fig. 1.
PTPL1 is required for oncogenic transformation. PTP-BLWT/WT or PTP-BLΔPTP/ΔPTP MEFs were transiently transfected with control, single-oncogene, or double-oncogenes. Cells were plated in 100 cm2 dishes, selected with 600 µg/ml G418 for two weeks, and foci were stained with crystal violet.
PTPL1 phosphatase activity is necessary for ES cell proliferation
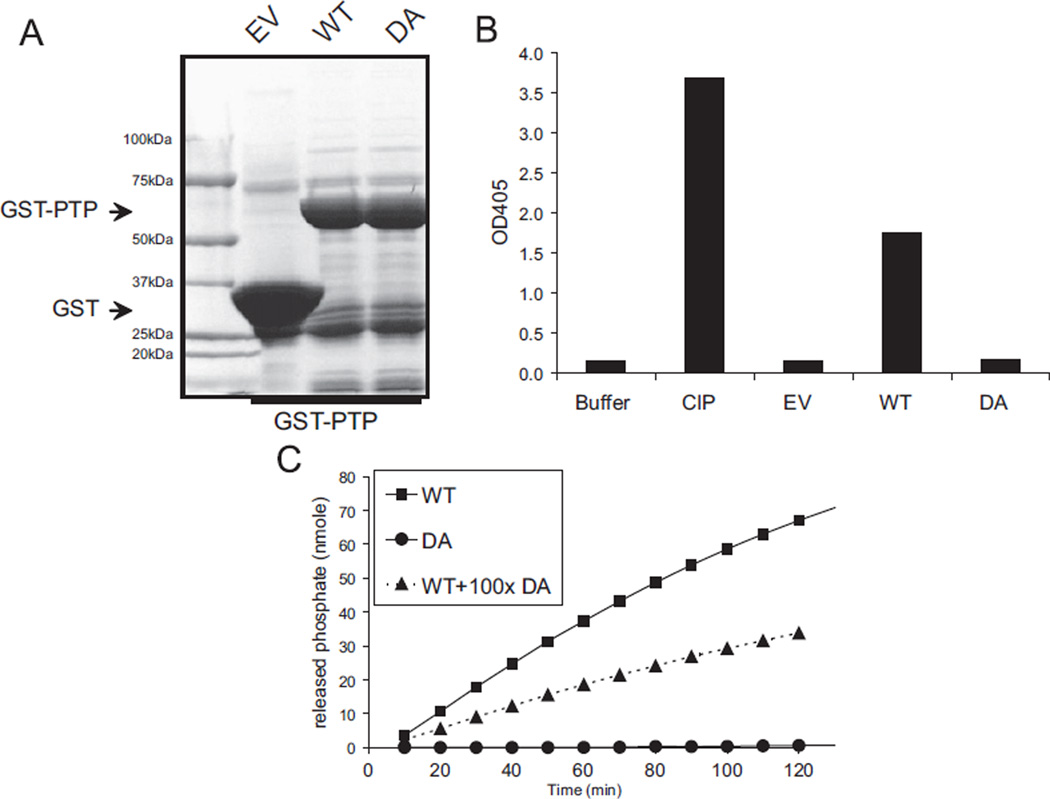
In order to determine the role of PTPL1 phosphatase activity in ES cells, we developed an Asp to Ala mutant of PTPL1 (PTPL1-DA) that lacked phosphatase activity [21,22]. Initially we tested this mutation on a GST-tagged PTPL1 PTP domain (GST-PTP), which we purified from bacterial extracts (Fig. 2A). We performed in vitro phosphatase activity to measure the inorganic phosphate accumulation due to phosphatase activity of purified proteins. As expected, the DA substrate-trapping mutation eliminated catalytic activity of GST-PTP fragment of PTPL1 (Fig. 2B). In addition, we also confirmed that the DA mutant PTP was able to compete for the synthetic substrate p-nitrophenyl phosphate (Fig. 2C).
Fig. 2.
PTPL1-DA lacks phosphatase activity and competes with wild-type protein. (A) Bacteria were induced with IPTG and GST-PTP recombinant proteins were affinity purified. Twenty microliters of purified protein was resolved on SDS-PAGE and coomassie stained. (B) The wild-type (WT) alone (■), wild-type mixed with 100 fold-excess substrate-trapping mutant (DA) (●), and DA mutant alone (▲) recombinant proteins were mixed and assayed over time for 2 h using the colorimetric phosphatase substrate pNPP. (C) In the competition assay PTP-WT protein is mixed with molar excess of PTP-DA. The rate of the reactions where calculated from the slope of the best line-fit as 0.563, 0.280, and 0.006 nmole/min for PTP-WT alone, PTP-WT together with PTP-DA and PTP-DA alone, respectively.
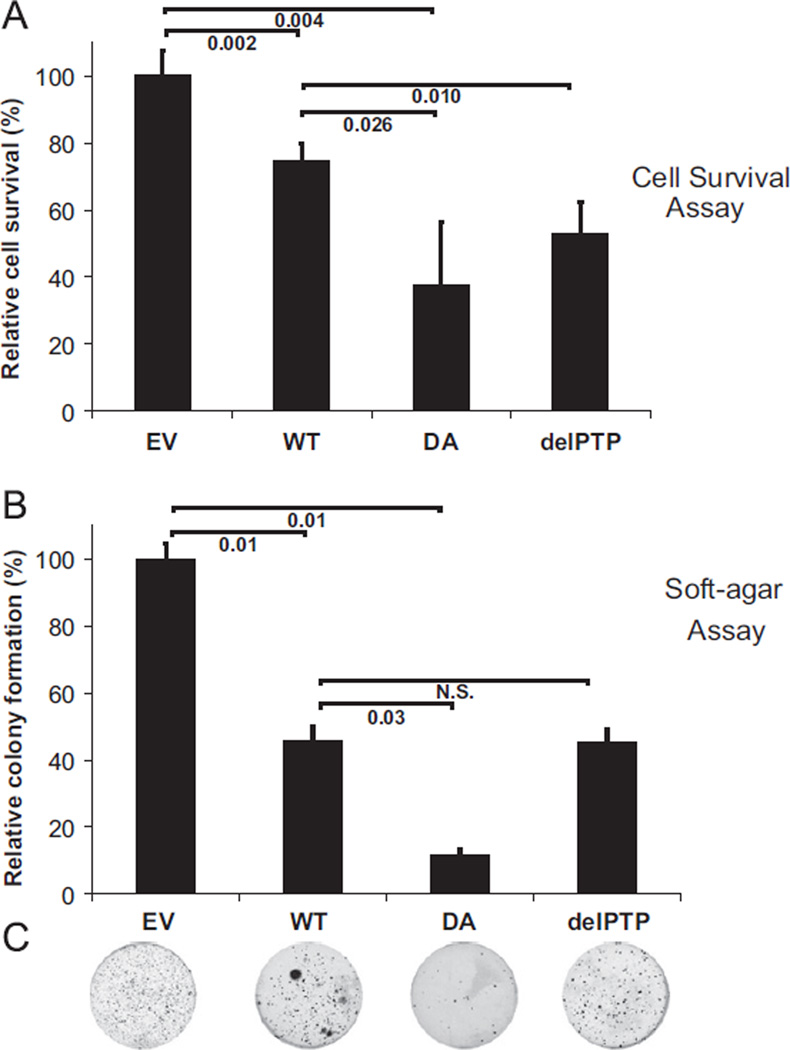
We tested the effect of the substrate-trapping mutant PTPL1 on ES cell growth by transfecting Ewing Sarcoma cells with wild-type PTPL1 (PTPL1-WT) or substrate-trapping mutant PTPL1 (PTPL1-DA) or a PTP domain deleted PTPL1 (PTPL1-delPTP) or an empty vector control (EV). Transiently transfected ES cells were subsequently grown either in liquid-adherent or semi-solid non-adherent culture. Even-though wild-type PTPL1 transfected cells showed 25% of reduction in cell survival, PTPL1-DA showed a 60% reduction in cell survival at 24 h (Fig. 3A). In concordance, the anchorage-independent growth assay revealed a ten-fold reduction in colony counts in PTPL1-DA transfected cells when compared to empty vector transfected cells, which only contained endogenous PTPL1 (Fig. 3B). Exogenous expression of full-length PTPL1 in Ewing Sarcoma cells decreased anchorage independent colony formation (Fig. 3C). The importance of PTPL1 substrates is supported by the PTPL1-DA phenotype, in contrast to exogenous delPTP or full-length PTPL1 showing similar growth in both liquid-adherent or semi-solid non-adherent culture.
Fig. 3.
PTPL1-DA inhibits ES cell proliferation. (A) TC32 cells were transfected with wild-type (WT) or DA substrate-trapping mutant (DA) PTPL1 expression vectors, or with control empty vector (EV). Cells were plated on 96-well plates in triplicate. Twenty-four hours after transfection viable cells were quantified by MTT. Bar graph represents cell survival reported as mean ± s.d. relative to empty vector transfected control cells. Numbers under brackets represent p-values calculated using Student’s t-test. (B) TC32 cells were transiently transfected with PTPL1 expression vectors wild-type (WT), DA substrate-trapping mutant (DA), catalytic PTP-domain removed from PTPL1 (delPTP) or control empty vector (EV). Cells were seeded in duplicate in soft-agar after transfection. After 3 weeks visible colonies were stained with MTT. Bar graph represents the colony counts reported as mean ± s.d. relative to empty vector transfected control cells. Numbers under brackets represent p-values calculated using Student’s t-test. Representative images from plates carrying colonies of cells transfected with each construct is shown below the bar graph.
Substrate-trapping PTPL1 shows enhanced affinity for phosphoproteins
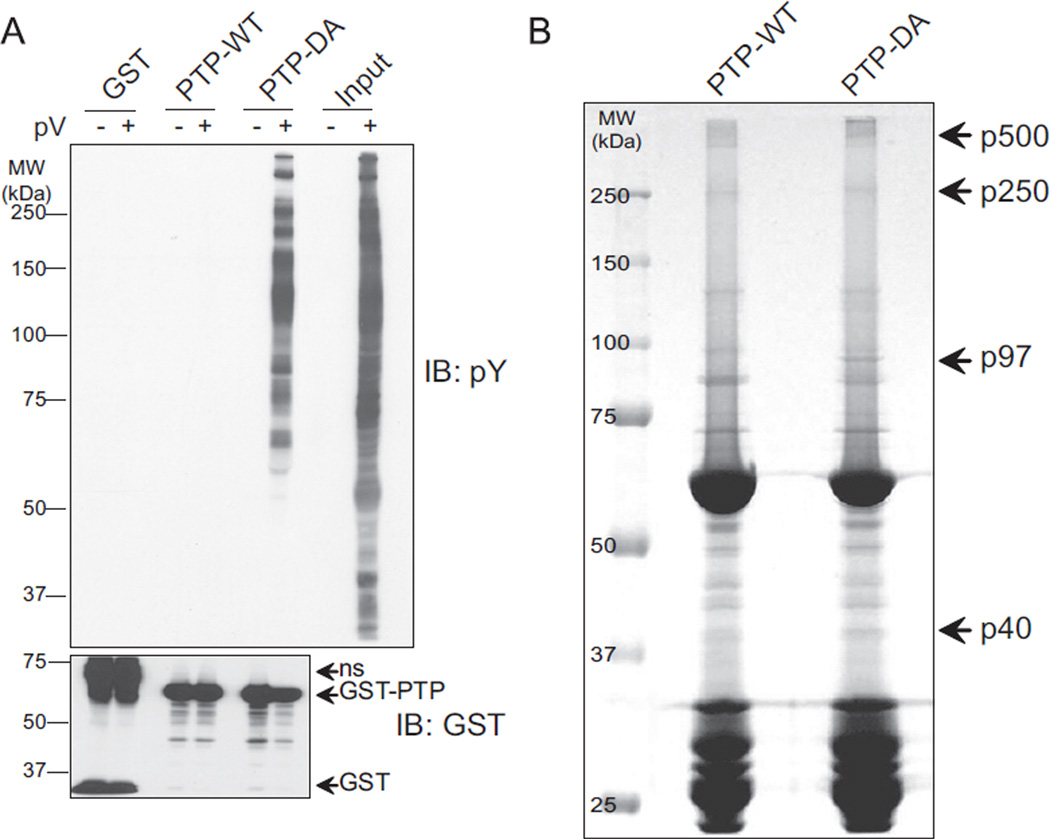
ES cells may appear to require a regulated amount of PTPL1 for cell survival and transformation. The 90% decrease in colony formation in substrate trapping mutant (DA) transfected cells directed us to determine PTPL1 substrates, which might be involved in tumorigenesis. In order to identify substrates, we used an in vitro affinity assay with recombinant GST fusion proteins for both wild type (GST-PTP-WT) and substrate-trapping mutant PTPL1 phosphatase domain (GST-PTP-DA). Lysates from TC32 cells were pre-treated with pervanadate, and compared to untreated controls, to identify proteins that bound to GST-PTP-WT, GST-PTPDA, or GST-alone. Phosphotyrosine immunoblotting demonstrated that PTP-DA was able to form stable complexes with tyrosine phosphorylated proteins whereas neither PTP-WT nor GST-alone bound phosphoproteins (Fig. 4A). Reprobing the membrane with GST antibodies confirmed equal recombinant protein loading (Fig. 4A, lower panel). In order to identify potential substrate proteins, we scaled up the affinity-purification using 17 mg of total protein (Fig. 4B). Those protein bands that appeared to be differentially present or more abundantly bound to PTP-DA (arrow heads) were isolated and analyzed by mass-spectrometry. This experiment provided a list of proteins that were identified by mass-spectrometry in the four most prominent protein bands (Table 1).
Fig. 4.
PTPL1-DA effectively binds phosphorylated proteins. (A) TC32 cell were pretreated with 300 µM pervanadate (pV) for 30 min or left untreated. Equal amounts of lysates were mixed with GST, PTP-WT or PTP-DA recombinant protein-coupled agarose-beads. Associating proteins were eluted and subjected to immunoblotting using phosphotyrosine specific antibodies. The blot was reprobed using GST antibodies. ns: non-specific reactivity. (B). Seventeen milligrams TC32 cell lysate was mixed with beads coupled to GST-PTP wild-type or (PTP-WT) the Asp to Ala substrate trapping mutant (PTP-DA) recombinant proteins. Bound proteins were eluted, separated by SDS-PAGE and stained with Coomassie blue. The arrow shows the band that was cut out and analyzed by mass-spectrometry for protein identification.
Table 1.
Results of mass-spectrometric analysis of proteins isolated by PTPL1 substrate-trapping mutant.
| Band | Protein | MW | PI | Count | Score | C.I. % |
|---|---|---|---|---|---|---|
| p500 | BCOR | 192,069 | 6.06 | 17 | 64.90 | 99.61 |
| LYRIC | 63,799 | 9.33 | 8 | 54.80 | 96.01 | |
| FBLN4 | 195,690 | 6.42 | 13 | 53.30 | 94.37 | |
| RBBP1 | 49,358 | 4.79 | 7 | 50.30 | 88.76 | |
| C6orf60 | 119,254 | 5.52 | 13 | 50.00 | 87.96 | |
| FATH | 505,963 | 4.84 | 19 | 48.80 | 84.13 | |
| M3K9 | 44,947 | 6.92 | 8 | 48.80 | 84.13 | |
| PCLO | 566,309 | 6.12 | 19 | 48.20 | 81.77 | |
| MP2K3 | 39,293 | 7.05 | 9 | 47.40 | 78.09 | |
| AP4B1 | 83,208 | 5.59 | 12 | 47.10 | 76.52 | |
| FAT2 | 479,093 | 5.01 | 20 | 46.50 | 73.04 | |
| HEX | 52,027 | 4.72 | 7 | 45.50 | 66.06 | |
| S3TC1 | 146,836 | 5.85 | 12 | 45.30 | 64.46 | |
| IFIT1 | 55,351 | 6.73 | 9 | 44.40 | 56.28 | |
| MCF2L | 123,906 | 6.16 | 13 | 44.20 | 54.22 | |
| C2orf4 | 33,711 | 6.67 | 7 | 43.90 | 50.94 | |
| C14orf104 | 36,920 | 4.54 | 6 | 42.60 | 33.82 | |
| TF2AA | 41,488 | 4.4 | 5 | 42.60 | 33.82 | |
| MAP2 | 199,417 | 4.83 | 13 | 42.10 | 25.75 | |
| KIAA0256 | 121,700 | 5.76 | 12 | 42.00 | 24.02 | |
| NRG2 | 91,622 | 9.51 | 11 | 41.80 | 20.44 | |
| HNRPQ | 69,590 | 8.68 | 8 | 41.00 | 4.35 | |
| ZN169 | 67,046 | 9.34 | 10 | 41.00 | 4.35 | |
| p250 | MAP1B | 270,454 | 4.73 | 20 | 84.10 | 100.00 |
| C14orf94 | 42,373 | 5.51 | 9 | 62.30 | 99.29 | |
| WISP1 | 40,304 | 6.84 | 10 | 61.80 | 99.20 | |
| CLEC5 | 21,508 | 9.04 | 6 | 48.40 | 82.59 | |
| ASXL1 | 165,329 | 5.85 | 13 | 48.00 | 80.91 | |
| ZNF35 | 58,134 | 8.52 | 10 | 47.60 | 79.07 | |
| BAZ1B | 170,796 | 8.7 | 12 | 47.30 | 77.58 | |
| SPTB2 | 274,461 | 5.41 | 15 | 46.40 | 72.41 | |
| ANR28 | 116,455 | 5.74 | 10 | 44.50 | 57.27 | |
| TCP10 | 45,502 | 8.22 | 6 | 43.00 | 39.65 | |
| NTAN1 | 34,525 | 5.83 | 6 | 42.80 | 36.80 | |
| SFXN3 | 35,481 | 9.26 | 6 | 42.60 | 33.82 | |
| ZBED1 | 78,106 | 5.79 | 10 | 42.10 | 25.75 | |
| GOGB1 | 375,848 | 4.95 | 16 | 41.40 | 12.76 | |
| BCAT1 | 42,925 | 5.17 | 7 | 41.10 | 6.52 | |
| FLOT1 | 47,326 | 7.08 | 8 | 41.10 | 6.52 | |
| HTF9C | 68,682 | 8.21 | 9 | 40.90 | 2.12 | |
| p97 | VCP | 89,135 | 5.14 | 18 | 106.00 | 100.00 |
| SFR12 | 59,345 | 10.39 | 4 | 54.90 | 96.10 | |
| ZBT12 | 49,116 | 7.26 | 9 | 53.30 | 94.37 | |
| MACF1 | 620,034 | 5.27 | 30 | 46.50 | 73.04 | |
| STS1769 | 39,768 | 4.65 | 8 | 41.80 | 20.44 | |
| KIAA0182 | 130,244 | 6.91 | 8 | 41.70 | 18.59 | |
| p40 | ENPP7 | 51,445 | 6.39 | 9 | 57.40 | 97.81 |
| RAB7L | 23,140 | 6.73 | 5 | 46.30 | 71.77 | |
| HSST2 | 100,811 | 8.81 | 9 | 45.30 | 64.46 | |
| TRPA1 | 127,296 | 6.52 | 12 | 43.30 | 43.68 | |
| C14orf94 | 42,373 | 5.51 | 8 | 42.40 | 30.71 | |
| TSP4 | 105,802 | 4.44 | 11 | 41.70 | 18.59 |
Band: Approximate MW excised from the gel, Protein: Protein name, MW: Estimated molecular weight of the protein, P.I: Isoelectric point, Count: the number of peptides detected, Score: MASCOT score assigned with the identification, C.I. confidence interval (ion scores of greater than 95% are considered most reliable for condifent protein identification).
Four protein bands (p500, p250, p97 and p40) as labeled in Fig. 4, were subjected to mass-spectrometry. The table lists all the protein identified and is in concordance with the expected molecular weight. The highest scoring proteins are VCP and MAP1B.
97 kDa protein identified as Valosin Containing Protein
In three rounds of affinity purification, the most reproducible protein band was found at a molecular weight of 97 kDa (Supplemental Fig. 1A). Mass-spectrometry analyses of duplicate samples from two independent experiments showed an average of 15 and 30 peptides, respectively, which identified this 97 kDa protein as Valosin Containing Protein (VCP). Peptide coverage for VCP was 25% and 46% in the two replicate mass-spectrometry experiments (Supplemental Fig. 1B). These results identify the 97 kDa protein as VCP, but as such do not demonstrate that VCP is a PTPL1 substrate. Mass-spectrometry analysis yielded additional proteins as possible substrates, but their identity could not be assessed as robustly as for VCP.
VCP is a novel PTPL1 substrate
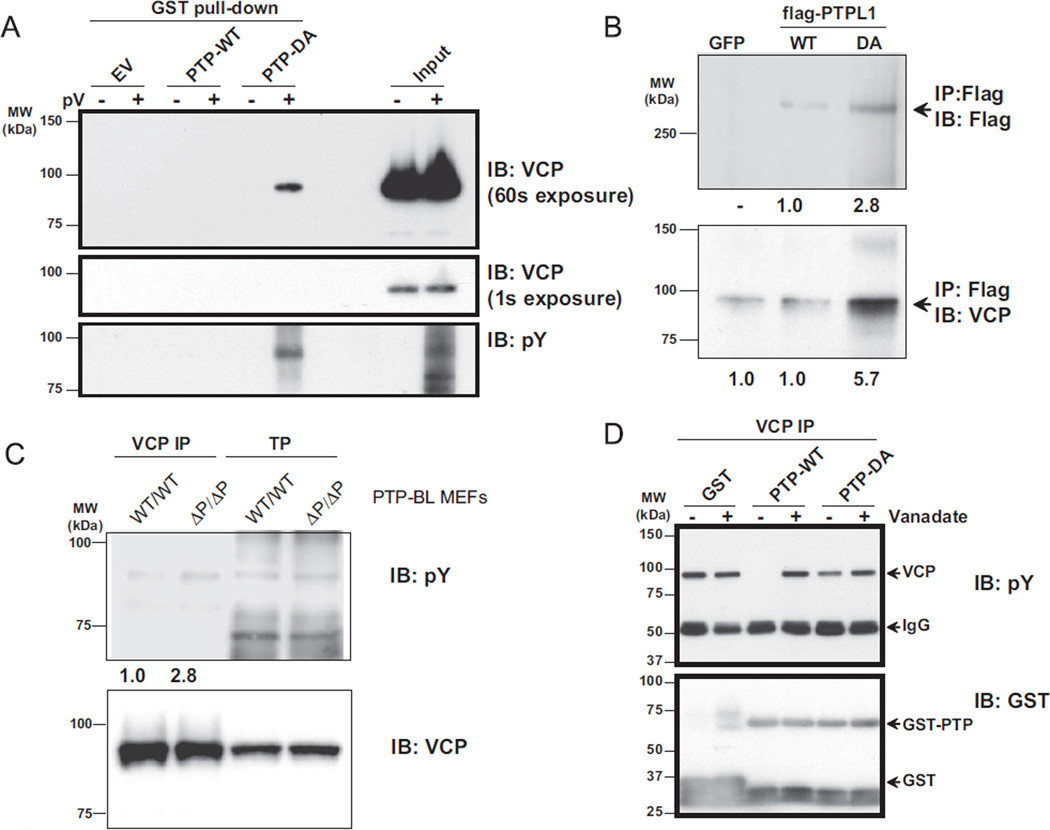
Definitive validation of VCP as a PTPL1 substrate was confirmed according to established criteria [22]. First, we showed that VCP can affinity purify with PTP-DA but not with PTP-WT or with GST alone. The affinity purification was only successful after cells were pretreated with pervanadate (Fig. 5A, top panel) suggesting that PTP-DA could only bind tyrosine phosphorylated VCP. Pervanadate treatment did not affect VCP protein stability as demonstrated by the equal levels of VCP in the input (Fig. 5A, middle panel). Reprobing with phosphotyrosine antibodies confirmed VCP tyrosine phosphorylation (Fig. 5A, lower panel). In order to further demonstrate that VCP is a PTPL1 substrate, we performed additional studies in HEK cells. In a second approach, TC32 cells were transfected with either full-length Flag-PTPL1-WT or Flag-PTPL1-DA. Protein complexes from total cell lysates were immunoprecipitated using Flag-specific antibodies. Results demonstrated that endogenous VCP co-immunoprecipitates with PTPL1-DA but not with PTPL1-WT, which showed similar levels compared to the background in control cells transfected with GFP (Fig. 5B). Densitometric analysis of the immunoblot revealed a 5.7 fold enrichment of VCP in PTPL1-DA over PTPL1-WT immunoprecipitation (Fig. 5B lower panel). In order to further validate VCP as a PTPL1 substrate, we evaluated mouse embryonic fibroblasts (MEF) derived from PTP-BLΔPTP/ΔPTP animals, which lack the catalytic PTP domain of the mouse ortholog of PTPL1, PTP-BL [23]. VCP was immunoprecipitated from these MEF cells grown under regular growth conditions and immunoblotted for antibodies against phospho-tyrosine. In PTP-BLΔPTP/ΔPTP MEFs we measured a 2.8-fold increase in VCP tyrosine phosphorylation when compared to PTP-BLWT/WT MEFs (Fig. 5C upper panel). Lastly, we investigated whether PTPL1 can dephosphorylate VCP in vitro. TC32 cells were pervanadate treated to enrich for phosphotyrosine-VCP, followed by VCP- specific immunoprecipitation. The immunoprecipitates were mixed with GST-PTP wild-type or Asp to Ala mutant constructs in the presence or absence of ortho-vanadate. The reactions were then resolved on SDS-PAGE and immunoblotted for phosphotyrosine or GST. The results from this experiment demonstrated that phospho-VCP was completely dephosphorylated by PTP-WT but not with PTPDA recombinant protein, and the dephosphorylation by PTP-WT was inhibited by ortho-vanadate showing a protein tyrosine phosphatase specific action (Fig. 5D, upper panel). Equal recombinant protein loading was confirmed with immunoblotting against GST specific antibody (Fig. 5D, lower panel). These four separate validations provide further proof that VCP is a PTPL1 substrate.
Fig. 5.
Validation of VCP as a PTPL1 substrate. (A) Five microliters of GST pull-downs (similar to Fig. 2A) were separated by SDS-PAGE and immunoblotted using VCP specific antibodies. Top panel is a 60 s exposure while the middle panel is a 1 s exposure of the VCP blot. Same blot was reprobed for phosphotyrosine antibodies (bottom panel). (B) TC32 were cells transiently transfected with either GFP or full-length Flag-tagged PTPL1-WT or full-length Flag-tagged PTPL1-DA expression vectors using electroporation and lysed after 24 h. Protein complexes were immunoprecipitated with Flag-M2-Agarose beads, and immunoblotted using Flag-M5 (top panel) or VCP (bottom panel) antibodies. Numbers below the figure represent relative band density in arbitrary units determined by densitometry as fold over PTPL1-WT for the Flag-M5 immunoblot or GFP for the VCP immunoblot. (C) VCP immunoprecipitated from PTP-BLWT/WT or PTP-BLΔPTP/ΔPTP MEFs maintained under regular growth conditions was immunoblotted with phosphotyrosine and VCP specific antibodies. Numbers below the figure represent relative band density in arbitrary units determined by densitometry as fold over PTP-BLWT/WT. (D) VCP was immunoprecipitated from pervanadate treated TC32 cells. Immunoprecipitates were aliquot and mixed with GST, PTP-WT or PTP-DA recombinant proteins for 1 h at 37 °C in the presence or absence of ortho-vanadate. Reactions were run on SDS-PAGE and immunoblotted with phosphotyrosine antibodies (top panel) or GST (bottom panel) antibodies.
There is one tyrosine residue in VCP that is phosphorylated in ES cells
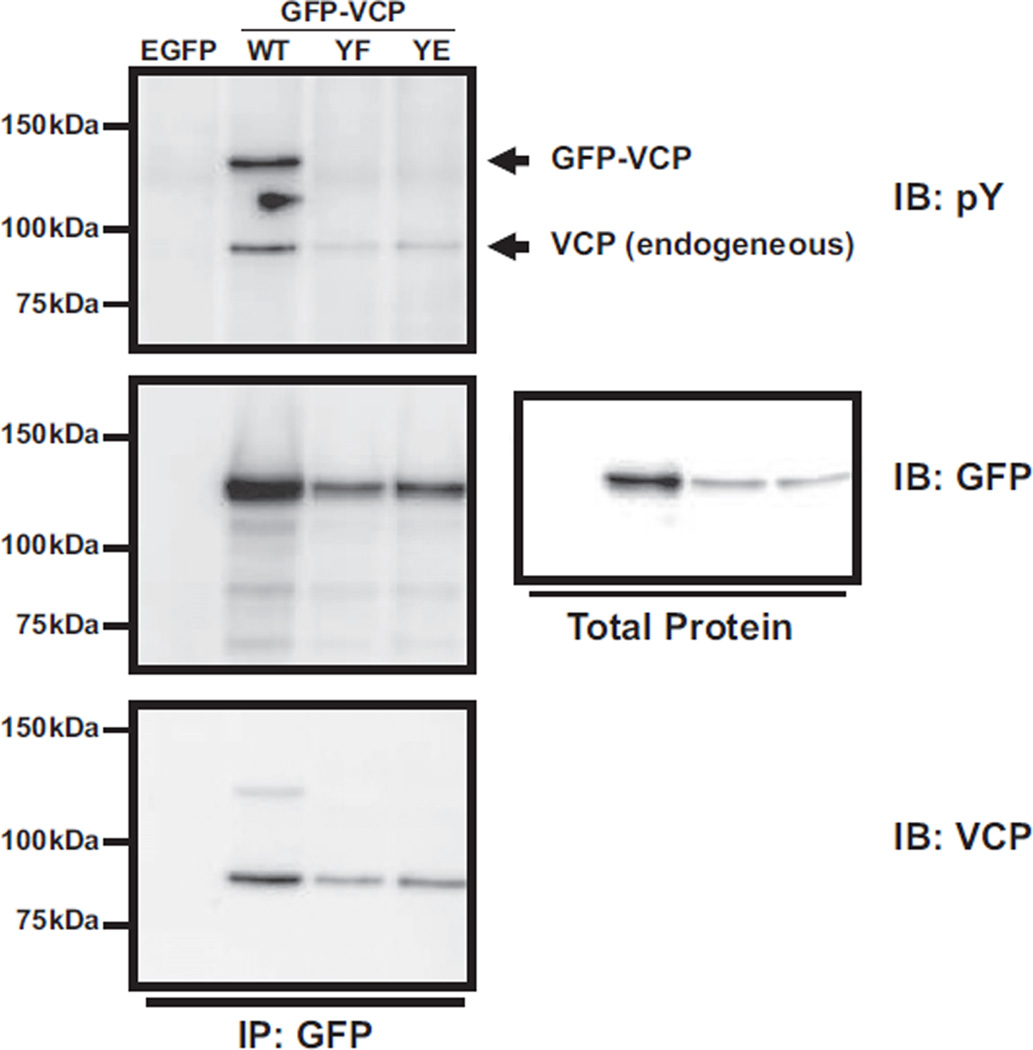
VCP tyrosine residue, Y805, has been shown as the major site for tyrosine phosphorylation [24]. In order to determine if other VCP tyrosines residues are phosphorylated in ES cells, we transiently transfected TC32 cells with wild type VCP or various VCP Y805 mutants, Y805E and Y805F, all fused to GFP. No phosphorylation was seen with a phospho-tyrosine immunoprecipitation for either of these single amino acid substitutions (Fig. 6, upper panel). Therefore, Y805 is the sole residue phosphorylated in ES cells. Immunoblotting with GFP of precipitated proteins also showed that the tagged-VCP protein can interact with endogenous VCP as expected since VCP forms a hexamer in the cell (Fig. 6 middle and lower panel) [25].
Fig. 6.
VCP Y805 is the sole tyrosine phosphorylated in ES cells. TC32 cells were transiently transfected with either GFP expressing control vector, or various VCP expression vectors (GFP-VCP-WT, GFP-VCP-YF, or GFP-VCP-YE) using electroporation. Cells were treated with 300 µM pervanadate for 30 min, lysed and subsequently subjected to immunoprecipitation using antibodies against GFP. The top panel is the immunoblot with antibodies against pY and the bottom blot is for GFP. A non-specific band (n.s.) was marked with the small arrow.
VCP tyrosine phosphorylation is induced with stimulation
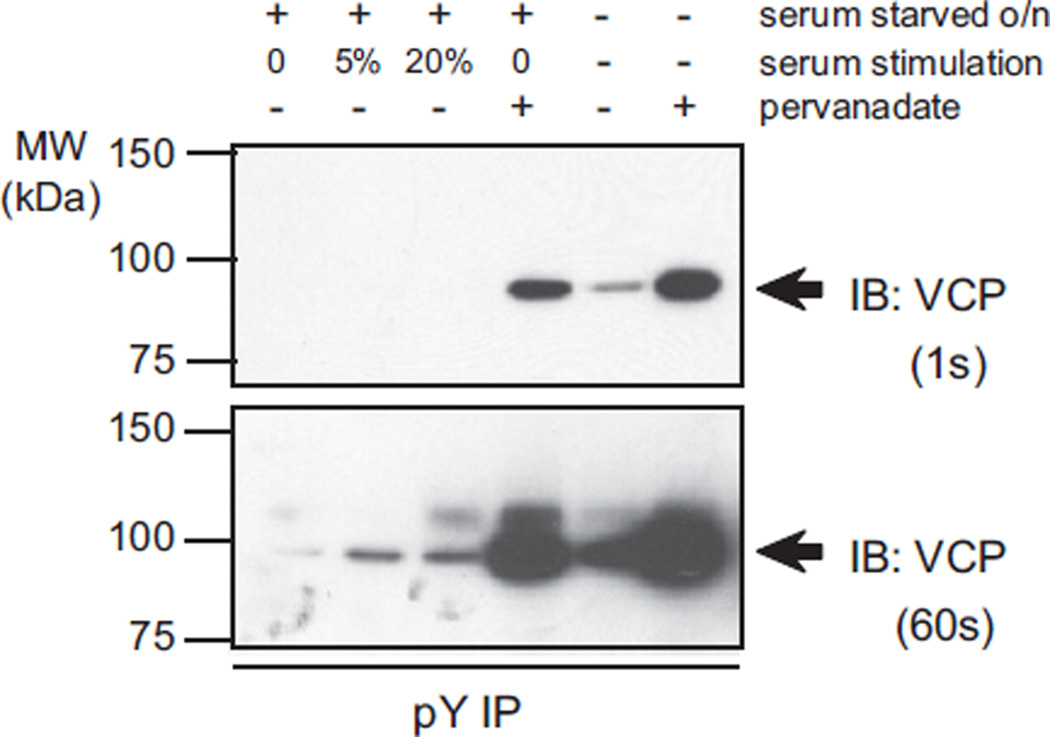
The role of VCP tyrosine phosphorylation in mammalian cells remains cryptic. In yeast, tyrosine phosphorylation of VCP was suggested to be a cell cycle specific event [16]. We therefore evaluated whether VCP tyrosine phosphorylation would be altered upon serum starvation and subsequent stimulation. Overnight serum starved cells displayed very low levels of phosphotyrosine-VCP while serum stimulation of cells increased phosphorylation (Fig. 7). Cells that were grown in regular growth media had higher basal levels of phosphorylation (Fig. 7). Pervanadate treatment of either serum starved cells or cells grown in regular media resulted in robust VCP phosphorylation, (Fig. 7). These results suggested that VCP tyrosine phosphorylation is a physiological event triggered by serum stimulation, suggesting cell growth dependent tyrosine phosphorylation of VCP thus supporting a regulatory role for PTPL1.
Fig. 7.
Phosphorylation of VCP varies with the serum stimulation. TC32 cells were either serum starved or grown in complete medium overnight. Serum starved cells were treated as indicated (NT: no treatment, S: serum, pV: 300 µM pervanadate). Total protein was subject to immunoprecipitation with phosphotyrosine specific antibodies, and immunoblotted using VCP antibodies. A short (1 s, top panel) and a longer (60 s, bottom panel) exposure of the same immunoblot are provided.
Tyrosine 805 may be required for VCP localization to the midbody
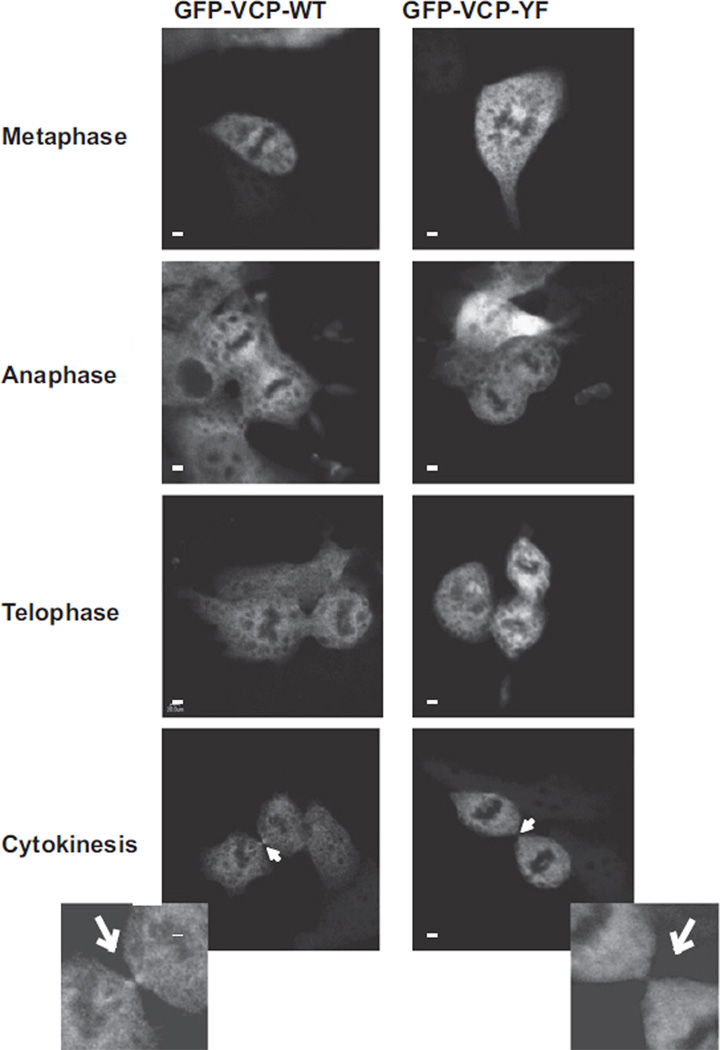
In view of our findings that there is serum-induced tyrosine phosphorylation of VCP (Fig. 7) and the separate observation by others that PTPL1 enriches in the midbody [9] we sought for localization of VCP. In TC32 cells, VCP displayed diffuse staining in both non-dividing cells and dividing cells (Fig. 8). However, in dividing cells at the stage of cytokinesis, enhanced staining of exogenously expressed GFP-VCP was observed at structures that resemble the midbody (Fig. 8). Multiple attempts to show co-enrichment of PTPL1 and VCP at the midbody complex were unsuccessful, which may be due to difficulties in over-expressing PTPL1 variants (data not shown) and the ability of the antibodies to identify the native protein. However, expression studies with VCP phospho-mutants provided evidence for the role of VCP tyrosine phosphorylation. When we transiently transfected HEK293 cells with GFP fused wild type VCP or a Y805F mutant construct, VCP localization in cell throughout cell cycle did not differ regardless of the Y805 residue (Supplemental Fig. 3). However, we did observe reduced VCP localization at the midbody during cytokinesis when Y805 is mutated (Fig. 8 lowest panel). In cells expressing GFP-VCP-Y805F only 20% of the cells at cytokinesis (n = 10) displayed GFP fluorescence at the midbody while 70% of wild type transfected cells at cytokinesis (n = 13) displayed GFP-positivity at the midbody. We conclude that tyrosine 805 is important for midbody localization of VCP.
Fig. 8.
VCP is localized to the midbody. HEK293 cells were transiently transfected with GFP-VCP wild-type (WT) or GFP-VCP-Y805F mutant using Fugene-6. Thirty-six hours post-transfection cells were fixed and imaged. Pictures show representatives of cells at cytokinesis from both VCP-WT and VCP-Y805F transfected HEK293 cells, respectively. Cells at cytokinesis were counted for each WT (n = 13) and Y805F (n = 10) and scored for the presence of GFP-VCP at the midbody. The insets are digitally enlarged images of the midbody, marked by the white arrowhead. The scale bars in each panel represent 20 µm.
Discussion
Phosphatases are no longer considered housekeeping enzymes and understanding their regulatory potential in cell biology requires identifying specific substrates. Identification of PTP substrates can be performed using techniques such as substrate-trapping and strict criteria for the validation of bona fide PTP substrates have been defined [22]. We previously identified PTPL1 as a critical transcriptional target of the oncogene EWS-FLI1 [2]. In order to understand why an oncogenic transcription factor would activate transcription of PTPL1, we first assessed that PTPL1 phosphatase activity influences ES cell survival and continued with a search for the PTPL1 substrates involved. Our affinity purification provided a list of potential PTPL1 substrates of which we validate VCP as a substrate using established criteria [22].
We observed a series of proteins that were identified by the PTPL1-DA substrate-trapping mutant. Multiple Coomassie-stained protein bands with apparent molecular weights of 500, 250, 97, and 40 kDa, were reproducibly observed. The 97 kDa band was identified by mass spectroscopy as VCP and rigorously validated as a substrate in the current study. VCP was identified as a substrate for PTPH1 [26], which resembles PTPL1 in protein domain build up since both phosphatases contain FERM and PDZ domains that are involved in membrane targeting and protein-protein interactions, respectively, suggesting that PTPL1 and PTPH1 may have overlapping substrates. We were unable to unequivocally demonstrate a functional link between PTPL1 and VCP in expression studies, which may reflect their respective impact on cell growth and the need for reliable inducible expression systems to perform such studies. One of the other potential PTPL1 substrates, the 250 kDa protein, could be identified with high confidence (Table 1) as microtubule-associated protein 1 B (MAP1B). MAP1B also carries a terminal tyrosine that is critical for its function [27]. However, phosphorylation at this site has not been reported yet, leaving the possibility that MAP1B is a functional substrate of PTPL1 to be explored in future work.
Evidence shows that PTPL1 can either support tumor growth or function as a tumor suppressor in different experimental models [3,28]. In support of PTPL1 as a tumor growth enhancing protein as in shown in anchorage independent growth of MEF cells, many solid tumors of different tissue of origin display higher staining for PTPL1 compared to benign tissue [29]. ES [2] and the pancreatic cancer cell lines we looked at, PTPL1 levels are higher in metastases (Supplemental Fig. 2). Both wild-type PTPL1 and delPTP over-expression in ES cells resulted in a decrease in growth. This contrasts the need for full-length PTPL1 that is required for oncogenic transformation. Together, these data suggests that the overexpression of PTPL1 behaves differently than its absence in difference cell types (ES cells and fibroblasts) and that these differences can account for discrepancies in the literature about the role of PTPL1 in oncogenesis. In addition, changes in PTPL1 intracellular levels likely alter the balance in the protein interaction partners of PTPL1 based upon PTPL1 having 5 PDZ domains and a FERM domain capable of numerous protein-protein interactions. Therefore, PTPL1 functions as a bridging factor regardless of its catalytic activity.
VCP knock-out in mice is early embryonic lethal at the peri-implantation stage [30], suggesting a very fundamental and indispensable role of VCP in the cell. A central role for VCP is to direct ubiquitinated proteins to protosomal destruction or recycling, thus, the regulation of VCP by phosphorylation may control protein fate in the cell and the overall viability and fidelity of the cell. Higher VCP mRNA and/or protein is a poor prognostic factor in many types of carcinoma [31], however, there is no direct evidence that VCP is a tumor suppressor or a tumor promoter. However, there are a number of VCP mutations identified as the cause for an autosomal dominant disease called inclusion body myopathy with Paget disease of bone and frontotemporal dementia (IBMPFD), which affects multiple tissues, including muscle, bone, and the cerebral cortex [32]. How tyrosine phosphorylation of VCP fits into IBMPFD or cancer is not clear at this point.
Our work suggests that lack of VCP-Y805 phosphorylation prevents VCP localization to the midbody during cytokinesis. This could be due to lack of proper complex formation or simply due mislocalization of VCP in the cell. For the time being, the definite role for VCP tyrosine phosphorylation remains elusive, however, earlier studies demonstrated that phosphorylation does not affect VCP ATPase activity [24]. In addition our co-immunoprecipitation studies using the VCP-Y805 mutants show that these mutant protein are still able to complex with the endogenous VCP in forming the VCP hexamer [25] suggesting that they maintain structural functionality. Recent findings in the context of cell cycle and VCP revealed a direct link between VCP and Aurora B during various stages of the cell cycle. Our study also suggests a direct interaction between VCP and Aurora B during cytokinesis. In addition, VCP localization to the midbody might be regulated by its tyrosine phosphorylation. In addition, the precise special and temporal interplay between VCP and PTPL1 has yet to be revealed. Our data suggests a putative function in cytokinesis. In one scenario dephosphorylation of VCP could trigger events such as AuroraB degredation at the end of cell cycle [17]. Interplay between VCP and PTPL1 could reduce the threshold for a well controlled event such as cytokinesis to complete cell cycle. Such hypotheses need further investigation into the exact mechanism of VCP and PTPL1 interaction
The PTPL1 literature clearly demonstrates a complex and poorly defined role for PTPL1 in tumorigenesis. The conclusions from these studies suggest that each single observation could be indeed cell type specific. In our study, mouse embryonic fibroblasts that lack PTPL1 catalytic domain failed to transform in the presence of two oncogenes while PTPL1 wild-type fibroblasts did, supporting an oncogenic role for PTPL1 in this context. In contrast, normal mouse epithelial cells transfected with oncogenic Ras and depleted of PTPL1 were able to maintain anchorage-independent growth and grow in immuno-competent mice [33] supporting a tumor-suppressor role for PTPL1. Studies in cancers of epithelial origin line breast [8] and prostate [34] support the tumor-suppressor role. While in ES cells which are of mesenchymal origin we observe an oncogenic role for PTPL1. Therefore, there are clear physiological difference in PTPL1 function between epithelial and mesenchymal cells, which remain elusive, that confound the general role of PTPL1 in tumorigenesis. Thus data so far suggests a complex role for PTPL1. It is also important to point out that PTPH1 resembles PTPL1 in its domain structure and PTPH1 overexpression in NIH3T3 mouse fibroblasts result in growth inhibition while a DA mutant does not. This observation also demonstrated how fine-tuned these phosphatases are in cells of similar origin. Even-though VCP is a substrate for both PTPs, the exact biochemical link between VCP and PTPL1 or PTPH1 remains unknown.
Conclusions
In conclusion, we demonstrate that VCP is a novel, validated, substrate of PTPL1. Our data suggests that tyrosine phosphorylation of VCP may be responsible for its proper localization during cell cycle. In addition, the VCP kinase remains cryptic, further obscuring the regulatory role of VCP phosphorylation. Even though it is not evident how VCP dephosphorylation by PTPL1 regulates tumorigenesis, it is possible that such an event may be required to overcome a limiting step during cytokinesis in malignancy. There might be a yet to be discovered complex biochemical process that involves a major protein degradation controller, VCP, and a protein tyrosine phosphatase, such as PTPL1. Further studies into the role of VCP tyrosine phosphorylation in the context of cell cycle may clarify both PTPL1’s and VCP’s role in tumorigenesis.
Supplementary Material
Acknowledgments
We thank Dr. Steven Byers for critical reading the manuscript. We thank the Flow Cytometry Shared Resource especially Dr. Karen Creswell and Michelle Lombard, the Proteomics Shared Resource and Dr. Amrita Cheema for their technical help and for Mrs. Kerry Adam for editorial assistance. This work was generously supported by the Children’s Cancer Foundation of Baltimore, MD (J.A.T. and A.Ü.), Go4theGoal Foundation (to J.A.T.), Dani’s Foundation of Denver, CO (J.A.T.), the Liddy Shriver Sarcoma Initiative (J.A.T.), the Amschwand Sarcoma Cancer Foundation (J.A.T.), Burroughs-Wellcome Clinical Scientist Award in Translational Research (J.A.T.), and the National Institutes of Health, USA (to J.A.T. (CA88004)). Additional NIH support through the Cancer Center Support Grant P30 CA051008 for use of Proteomics, Flow Cytometry/Cell Sorting and Microscopy core facilities.
Footnotes
Authors’ contributions
ODA designed and carried out experiments and wrote the original manuscript, HVE carried out experiments, contributed significantly to the writing of the original manuscript, and rewrote the manuscript in response to the critique, WH contributed key reagents and writing of the manuscript, AU and JAT supervised the project and finalized the manuscript.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.yexcr.2012.09.003.
Contributor Information
Ogan D. Abaan, Email: oda@georgetown.edu.
Wiljan Hendriks, Email: W.Hendriks@ncmls.ru.nl.
Aykut Üren, Email: au26@georgetown.edu.
Jeffrey A. Toretsky, Email: jat42@georgetown.edu.
Hayriye V. Erkizan, Email: hve2@georgetown.edu.
References
- 1.Uren A, Toretsky JA. Ewing’s sarcoma oncoprotein EWS-FLI1: the perfect target without a therapeutic agent. Future Oncol. 2005;1:521–528. doi: 10.2217/14796694.1.4.521. [DOI] [PubMed] [Google Scholar]
- 2.Abaan OD, Levenson A, Khan O, Furth PA, Uren A, Toretsky JA. PTPL1 is a direct transcriptional target of EWS-FLI1 and modulates Ewing’s Sarcoma tumorigenesis. Oncogene. 2005;24:2715–2722. doi: 10.1038/sj.onc.1208247. [DOI] [PubMed] [Google Scholar]
- 3.Abaan OD, Toretsky JA. PTPL1: a large phosphatase with a split personality. Cancer Metastasis Rev. 2008;27:205–214. doi: 10.1007/s10555-008-9114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dromard M, Bompard G, Glondu-Lassis M, Puech C, Chalbos D, Freiss G. The putative tumor suppressor gene PTPN13/PTPL1 induces apoptosis through insulin receptor substrate-1 dephosphorylation. Cancer Res. 2007;67:6806–6813. doi: 10.1158/0008-5472.CAN-07-0513. [DOI] [PubMed] [Google Scholar]
- 5.Maekawa K, Imagawa N, Naito A, Harada S, Yoshie O, Takagi S. Association of protein-tyrosine phosphatase PTP-BAS with the transcription-factor-inhibitory protein IkappaBalpha through interaction between the PDZ1 domain and ankyrin repeats. Biochem. J. 1999;337(Pt 2):179–184. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu JH, Chen R, Yi W, Cantin GT, Fearns C, Yang Y, Yates JR, 3rd, Lee JD. Protein tyrosine phosphatase PTPN13 negatively regulates Her2/ErbB2 malignant signaling. Oncogene. 2007 doi: 10.1038/sj.onc.1210922. [DOI] [PubMed] [Google Scholar]
- 7.Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–176. doi: 10.1016/j.immuni.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Glondu-Lassis M, Dromard M, Lacroix-Triki M, Nirde P, Puech C, Knani D, Chalbos D, Freiss G. PTPL1/PTPN13 regulates breast cancer cell aggressiveness through direct inactivation of Src kinase. Cancer Res. 2010;70:5116–5126. doi: 10.1158/0008-5472.CAN-09-4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrmann L, Dittmar T, Erdmann KS. The protein tyrosine phosphatase PTP-BL associates with the midbody and is involved in the regulation of cytokinesis. Mol. Biol. Cell. 2003;14:230–240. doi: 10.1091/mbc.E02-04-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revillion F, Puech C, Rabenoelina F, Chalbos D, Peyrat JP, Freiss G. Expression of the putative tumor suppressor gene PTPN13/PTPL1 is an independent prognostic marker for overall survival in breast cancer. Int. J. Cancer. 2009;124:638–643. doi: 10.1002/ijc.23989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodman PG. p97, a protein coping with multiple identities. J. Cell. Sci. 2003;116:4283–4290. doi: 10.1242/jcs.00817. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Song C, Li CC. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J. Struct. Biol. 2004;146:44–57. doi: 10.1016/j.jsb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Rumpf S, Jentsch S. Functional division of substrate processing cofactors of the ubiquitin-selective Cdc48 chaperone. Mol. Cell. 2006;21:261–269. doi: 10.1016/j.molcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Elsasser S, Finley D. Delivery of ubiquitinated substrates to protein-unfolding machines. Nat. Cell. Biol. 2005;7:742–749. doi: 10.1038/ncb0805-742. [DOI] [PubMed] [Google Scholar]
- 15.Halawani D, Latterich M. p97: The cell’s molecular purgatory? Mol. Cell. 2006;22:713–717. doi: 10.1016/j.molcel.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Madeo F, Schlauer J, Zischka H, Mecke D, Frohlich KU. Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p. Mol. Biol. Cell. 1998;9:131–141. doi: 10.1091/mbc.9.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Cdc48/p97 promotes reformation of the nucleus by extracting the kinase Aurora B from chromatin. Nature. 2007;450:1258–1262. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- 18.Dobrynin G, Popp O, Romer T, Bremer S, Schmitz MH, Gerlich DW, Meyer H. Cdc48/p97-Ufd1-Npl4 antagonizes Aurora B during chromosome segregation in HeLa cells. J. Cell. Sci. 2011;124:1571–1580. doi: 10.1242/jcs.069500. [DOI] [PubMed] [Google Scholar]
- 19.Glondu-Lassis M, Dromard M, Chavey C, Puech C, Fajas L, Hendriks W, Freiss G. Downregulation of protein tyrosine phosphatase PTP-BL represses adipogenesis. Int. J. Biochem. Cell. Biol. 2009;41:2173–2180. doi: 10.1016/j.biocel.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchetot C, Chagnon M, Dube N, Halle M, Tremblay ML. Substrate-trapping techniques in the identification of cellular PTP targets. Methods. 2005;35:44–53. doi: 10.1016/j.ymeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem. J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wansink DG, Peters W, Schaafsma I, Sutmuller RP, Oerlemans F, Adema GJ, Wieringa B, Van Der Zee CE, Hendriks W. Mild Impairment of Motor Nerve Repair in Mice Lacking PTP-BL Tyrosine Phosphatase Activity. Physiol Genomics. 2004 doi: 10.1152/physiolgenomics.00079.2004. [DOI] [PubMed] [Google Scholar]
- 24.Egerton M, Samelson LE. Biochemical characterization of valosin-containing protein, a protein tyrosine kinase substrate in hematopoietic cells. J. Biol. Chem. 1994;269:11435–11441. [PubMed] [Google Scholar]
- 25.Pye VE, Beuron F, Keetch CA, McKeown C, Robinson CV, Meyer HH, Zhang X, Freemont PS. Structural insights into the p97-Ufd1-Npl4 complex. Proc. Natl. Acad. Sci. USA. 2007;104:467–472. doi: 10.1073/pnas.0603408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldisseri DM, Rustandi RR, Zhang Z, Tang C, Bair CL, Landar A, Zimmer DB, Weber DJ. 1 H, 13 C and 15 N NMR sequence-specific resonance assignments for rat apo- S100A1(alpha alpha) J. Biomol. NMR. 1999;14:91–92. doi: 10.1023/a:1008301518346. [DOI] [PubMed] [Google Scholar]
- 27.Utreras E, Jimenez-Mateos EM, Contreras-Vallejos E, Tortosa E, Perez M, Rojas S, Saragoni L, Maccioni RB, Avila J, Gonzalez-Billault C. Microtubule-associated protein 1B interaction with tubulin tyrosine ligase contributes to the control of microtubule tyrosination. Dev. Neurosci. 2008;30:200–210. doi: 10.1159/000109863. [DOI] [PubMed] [Google Scholar]
- 28.Freiss G, Chalbos D. PTPN13/PTPL1: An Important Regulator of Tumor Aggressiveness. Anticancer Agents Med. Chem. 2011;11:78–88. doi: 10.2174/187152011794941262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foehr ED, Lorente G, Vincent V, Nikolich K, Urfer R. FAS associated phosphatase (FAP-1) blocks apoptosis of astrocytomas through dephosphorylation of FAS. J. Neurooncol. 2005;74:241–248. doi: 10.1007/s11060-004-7202-x. [DOI] [PubMed] [Google Scholar]
- 30.Engelhardt M, Zeiser R, Ihorst G, Finke J, Muller CI. High-dose chemotherapy and autologous peripheral blood stem cell transplantation in adult patients with high-risk or advanced Ewing and soft tissue sarcoma. J. Cancer Res. Clin. Oncol. 2007;133:1–11. doi: 10.1007/s00432-006-0137-1. [DOI] [PubMed] [Google Scholar]
- 31.Tsujimoto Y, Tomita Y, Hoshida Y, Kono T, Oka T, Yamamoto S, Nonomura N, Okuyama A, Aozasa K. Elevated expression of valosin-containing protein (p97) is associated with poor prognosis of prostate cancer. Clin. Cancer Res. 2004;10:3007–3012. doi: 10.1158/1078-0432.ccr-03-0191. [DOI] [PubMed] [Google Scholar]
- 32.Ju JS, Weihl CC. Inclusion body myopathy, Paget’s disease of the bone and fronto-temporal dementia: a disorder of autophagy. Hum. Mol. Genet. 2010;19:R38–R45. doi: 10.1093/hmg/ddq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanos WC, Hoover A, Harris GF, Wu S, Strand GL, Anderson ME, Klingelhutz AJ, Hendriks W, Bossler AD, Lee JH. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss which allows anchorage-independent growth and synergizes with ras for invasive growth. J. Virol. 2008;82:2493–2500. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castilla C, Flores ML, Conde JM, Medina R, Torrubia FJ, Japon MA, Saez C. Downregulation of protein tyrosine phosphatase PTPL1 alters cell cycle and upregulates invasion-related genes in prostate cancer cells. Clin. Exp. Metastasis. 2012;29:349–358. doi: 10.1007/s10585-012-9455-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.