Abstract
Glycosylphosphatidylinositol (GPI) anchors are critical for the membrane attachment of a wide variety of essential signaling and cell adhesion proteins. The GPI anchor is a complex glycolipid structure that utilizes glycosylphosphatidylinositol-mannosyltransferases (GPI-MTs) for the addition of three core mannose residues during its biosynthesis. Here, we demonstrate that Drosophila GPI-MT2 is required for the GPI-mediated membrane attachment of several GPI-anchored proteins, including the photoreceptor-specific cell adhesion molecule, chaoptin. Mutations in gpi-mt2 lead to defects in chaoptin trafficking to the plasma membrane in Drosophila photoreceptor cells. In gpi-mt2 mutants, loss of sufficient chaoptin in the membrane leads to microvillar instability, photoreceptor cell pathology, and retinal degeneration. Finally, using site-directed mutagenesis, we have identified key amino acids that are essential for GPI-MT2 function and cell viability in Drosophila. Our findings on GPI-MT2 provide a mechanistic link between GPI anchor biosynthesis and protein trafficking in Drosophila and shed light on a novel mechanism for inherited retinal degeneration.
Keywords: Drosophila photoreceptor, Protein trafficking, Retinal degeneration, GPI anchor biosynthesis, Secretory pathway
Introduction
Many eukaryotic proteins are attached to the outer leaflet of the plasma membrane via a glycosylphosphatidylinositol (GPI) anchor. GPI-anchored proteins serve essential functions as cell adhesion molecules (CAMs), hydrolytic enzymes, extracellular receptors in signal transduction, antigens for T cell activation, inhibitors of the complement cascade, and key regulators of embryogenesis (Paulick & Bertozzi, 2008; Varma & Hendrickson, 2010). Defects in the surface expression of two critical GPI-anchored proteins in the complement cascade, decay accelerating factor (DAF) and complement defense 59 (CD59), have been implicated in the pathogenesis of a wide array of human diseases, including age-related macular degeneration (AMD), Alzheimer’s disease (AD), multiple sclerosis (MS), and systemic lupus erythematosus (Lopez-Pedrera et al., 2010; Khandhadia et al., 2011; Veerhuis, 2011; Veerhuis et al., 2011). Accordingly, in the last decade, chronic local inflammation and activation of the complement cascade have become a central focus in retinal disease research. Not only are many forms of AMD linked to inflammation and the complement system (Sheffield & Stone, 2011) but also gene therapy approaches using DAF and CD59 show promise in slowing down the progression of AMD (Ramo et al., 2008; Ma et al., 2010). Furthermore, DAF and CD59 have been implicated in human diabetic retinopathy (Zhang et al., 2002; Ruiz et al., 2006). Another GPI-anchored protein that is critical for retinal function is nyctalopin (NYX), which is a leucinerich glycoprotein. NYX is predominantly expressed in the retina and kidney, and mutations in NYX have been shown to cause the complete form of X-linked congenital stationary night blindness (CSNB1) (Bech-Hansen et al., 2000; Pusch et al., 2000; Zeitz et al., 2003; O’Connor et al., 2005).
Outside the retina, GPI-anchored proteins such as the pathogenic prion protein (PrPSc) and the urokinase-type plasminogen activator receptor (uPAR) play central roles in prion disease progression and tumorigenesis, respectively (Radford & Mallucci, 2010; Varma & Hendrickson, 2010). GPI anchors are also abundant on the surface of parasitic protozoa, such as Trypanosoma brucei and Plasmodium falciparum and have been implicated in the pathogenesis of sleeping sickness (Nagamune et al., 2003) and malaria (Arrighi & Faye, 2010), respectively. Despite the importance of GPI-anchored proteins and their extensive involvement in human disease, very little is known about the mechanistic role of the GPI anchor in protein function and the role of GPI biosynthesis as a culprit in animal models of human disease.
Mutations in the enzymes responsible for GPI anchor biosynthesis have profound negative consequences for GPI-anchored proteins and lead to additional human diseases, including hyperphosphatasia mental retardation syndrome, paroxysomal nocturnal hemoglobinuria, multiple congenital anomalies-hypotonia-seizures syndrome, and a syndrome characterized by venous thrombosis and seizures (Bessler et al., 1994; Almeida et al., 2006; Maydan et al., 2011). The absolute requirement for proper GPI biosynthesis is reflected in the fact that complete loss of the GPI structure is lethal in mice and yeast (Kawagoe et al., 1996; Leidich et al., 1994; Orlean et al., 1994; Nozaki et al., 1999; Tremml et al., 1999). Accordingly, the human GPI diseases mentioned above are caused by either somatic X-linked mutations or autosomomal recessive hypomorphic mutations, in which there is some functional GPI in some cells.
Despite the clear role of hypomorphic mutations in the progression of human GPI diseases, to date, studies on the GPI enzymes have focused almost exclusively on the characterization of null mutations. Therefore, defects in GPI biosynthesis have been limited to investigations in yeast, where conditional mutants can be studied, or in mammalian cell culture, since GPI is not essential for cell viability in these cases. The identification of hypomorphic mutations in GPI enzymes represents a yet unexplored method for studying the consequences of defective GPI anchor biosynthesis in whole animals. Here, we show that hypomorphic mutations in the GPI enzyme, GPI-MT2, lead to retinal degeneration in Drosophila. Our data complement the previous work done in cell culture (Fabre et al., 2005; Kang et al., 2005) and highlight the importance of GPI anchor biosynthesis in the retina. Thus, studies in animals with hypomorphic mutations represent an invaluable addition to the field of GPI research, by circumventing the challenges associated with animal lethality and allowing for the characterization of defects associated with partial disruption of the GPI biosynthesis cascade in vivo.
The GPI anchor is a highly complex glycolipid structure that is thought to serve diverse biological functions beyond membrane anchoring. For example, the GPI anchor has been implicated in the proper folding, sorting, and trafficking of proteins to the apical surface of polarized cells (Maeda & Kinoshita, 2011). The GPI anchor is also thought to mediate protein association with specialized sphingolipid- and cholesterol-enriched lipid microdomains, or lipid rafts (Fujita & Jigami, 2008; Levental et al., 2010). Interestingly, these lipid microdomains have been implicated as the main site for production of the pathogenic β-amyloid peptide (Aβ) in AD. Not only do changes in cholesterol homeostasis influence the production of Aβ but also attachment of a GPI anchor to β-secretase (BACE1) leads to a significant increase in the production of Aβ (Cordy et al., 2003; Vetrivel et al., 2011).
Finally, the GPI anchor can be cleaved by phosphatidylinositol (PI)-specific phosholipase C (PI-PLC), allowing for secretion of certain GPI-anchored proteins (Sharom & Lehto, 2002). Release of GPI-anchored proteins from the plasma membrane may facilitate unique functional properties, such as regulation of synaptic transmission in the case of acetylcholinesterase (Hicks et al., 2011). In contrast, cleavage and release of certain GPI-anchored proteins can downregulate their activity, as is the case for the major histocompatibility class 1-specific CD160 receptor, for which secretion inhibits cell-mediated cytotoxicity (Ortonne et al., 2011).
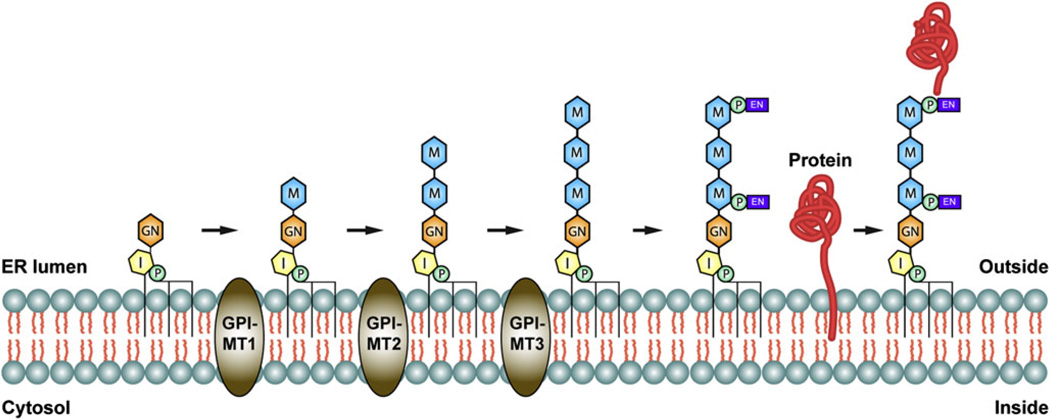
The GPI anchor is synthesized in the endoplasmic reticulum (ER) by the sequential addition of carbohydrates and lipids to PI (Fig. 1). The biosynthetic pathway for GPI is highly conserved between Drosophila and humans and utilizes a wide array of resident ER enzymes (Tiede et al., 1999; Kinoshita et al., 2008). Of particular importance, three mannose residues are transferred from dolicholphosphate-mannose (Dol-P-Man) to the growing GPI structure by a group of glycosylphosphatidylinositol-mannosyltransferases (GPI-MTs) termed GPI-MT1 (PIG-M in mammals/Gpi14p in yeast), GPI-MT2 (PIG-V/Gpi18p), and GPI-MT3 (PIG-B/Gpi10p) (Fig. 1). In some cases, a fourth mannose residue is attached as an α1,2-linked side branch of the third core mannose by GPI-MT4 (PIG-Z/SMP3), but this additional mannose residue is mostly found in yeast (Grimme et al., 2001). Ethanolamine phosphate (EthN-P) is then transferred to the third mannose in order to form the mature GPI anchor (Fig. 1). In many cases, the GPI structure is also modified with EthN-P on other mannose residues, most commonly the first mannose (Orlean & Menon, 2007). Nonetheless, the GPI structure is attached to the C-terminus of newly synthesized proteins in the ER via the EthN-P on its third mannose. Attachment of GPI to nascent ER proteins requires the presence of a hydrophobic C-terminal signal sequence, which is cleaved prior to GPI anchor attachment. (Maxwell et al., 1995a,b; Spurway et al., 2001) (Fig. 1).
Fig. 1.
The GPI-MTs in GPI anchor biosynthesis. Graphic representation of key steps in the GPI biosynthesis pathway, showing how GPI-MT1, GPI-MT2, and GPI-MT3 function in the sequential addition of the core mannose residues to the growing GPI precursor. P = Phosphate (green), I = Inositol (yellow), GN = Glucosamine (orange), M = mannose (blue), EN = ethanolamine (purple).
Although the GPI anchor was first discovered and characterized in the 1980s, the GPI-MT2 enzyme responsible for addition of the second mannose was not identified until 2005 (Fabre et al., 2005; Kang et al., 2005). GPI-MT2 remained elusive because it displays little amino acid identity with other known mannosyltransferases. GPI-MT2 is present in a wide variety of eukaryotes, from yeast to humans, and contains at least two highly conserved domains that are thought to contribute to its catalytic sites (Kang et al., 2005). The role of GPI-MT2 has been investigated in Chinese hamster ovary (CHO) cells. CHO cells defective in gpi-mt2 (pig-v) displayed reduced surface expression of two critical human GPI-anchored proteins, DAF and CD59, which were stably transfected into the CHO cells (Kang et al., 2005). In humans, these proteins play essential roles in the regulation of the complement cascade, which has now been implicated in a host of neurodegenerative diseases (Lopez-Pedrera et al., 2010; Khandhadia et al., 2011; Veerhuis, 2011; Veerhuis et al., 2011), highlighting the importance of understanding GPI-MT2 function in GPI anchor biosynthesis in vivo.
One critical GPI-anchored protein in Drosophila is chaoptin (Chp). Chp is a photoreceptor-specific CAM required for microvillar membrane organization in the rhabdomere (Krantz & Zipursky, 1990). In Drosophila photoreceptor cells, rhabdomeres are the photosensitive organelles containing rhodopsin and the other components of phototransduction. They are comprised of numerous tightly packed microvilli and are functionally equivalent to the outer segments of the vertebrate rods and cones (Yau & Hardie, 2009; Colley, 2010; Fain et al., 2010). The GPI anchor is thought to play a critical role in the attachment of chaoptin to the extracellular microvillar membrane, where chaoptin displays homotypic interactions via leucine-rich repeat (LRR) domains in order to stabilize two opposing microvilli (Van Vactor et al., 1988; Krantz & Zipursky, 1990). Several GPI-anchored CAMs have been identified in humans. For example, GPI-anchored neural CAMs are involved in complex processes including axon guidance, neuronal migration, neurite outgrowth, synapse formation, and synaptic plasticity (Karagogeos, 2003). Accordingly, GPI-anchored CAMs are essential during various stages of development, explaining why defects in GPI biosynthesis lead to embryonic lethality.
Chaoptin has 38 LRR domains encompassing almost 90% of the entire polypeptide (Kanie et al., 2009) and is a member of the LRR superfamily of proteins. These proteins share a nonglobular horseshoe-shaped tertiary structure and function as hormone receptors, tyrosine kinase receptors, bacterial virulence factors, enzymes, extracellular matrix-binding glycoproteins, and CAMs (Hirai-Fujita et al., 2008). Several human LRR proteins, including NYX and the Nogo receptor (NgR), are linked to the membrane via GPI anchors and have been associated with human disease. As mentioned above, NYX is involved in stationary night blindness, while NgR plays a dual role in neuronal plasticity and inflammation and has been implicated as a therapeutic target for MS and AD (David et al., 2008).
Here, we establish a role for Drosophila GPI-MT2 in the biosynthesis of a functional GPI anchor. We show that hypomorphic mutations in gpi-mt2 lead to defects in the GPI anchor-mediated membrane attachment of chaoptin, preventing its proper surface expression in photoreceptor cells. Loss of sufficient chaoptin expression in the rhabdomeres causes severe microvillar instability, leading to retinal degeneration in the gpi-mt2 mutants. We have identified key amino acid residues that are essential for the function of GPI-MT2 in Drosophila. Taken together, our results show that GPI-MT2 is required for the membrane association and targeting of chaoptin as well as for photoreceptor cell survival.
Materials and methods
Genetic screen and Drosophila strains
Drosophila melanogaster stocks were reared on standard media at 25°C, on a 12:12 light:dark cycle. To isolate the gpi-mt2A276V mutant, we screened approximately 12,000 ethyl methyl sulfonate (EMS)-mutagenized lines from the Zuker collection (Koundakjian et al., 2004) for the presence or absence of the deep pseudopupil (DPP), as previously described (Rosenbaum et al., 2006). The wild-type strain used in this study was the parental stock from the EMS mutagenesis: w+; cn,bw; + (cn,bw). The deficiency stocks used for mapping the gpi-mt2 EMS-mutation were obtained from the Bloomington Drosophila Stock Center (Indiana University). The gpi-mt2 P-element allele, Pbac{WH}vegf05618, was obtained from the Exelixis Collection at the Harvard Medical School. This is a null allele and is homozygous lethal. The original veg alleles used for complementation testing were obtained from G. Tear (vegk03402, vegZ322) and the Bloomington Drosophila Stock Center (vegk07202). Other fly stocks in this study include a null allele of chaoptin, chp2 (Van Vactor et al., 1988), and several transgenic stocks (see Site-Directed Mutagenesis and Transgenic Animals).
Sequencing and alignment
Genomic DNA was isolated from the EMS-mutagenized Drosophila line (gpi-mt2A276V) and the wild-type parental line (cn,bw) using the DNeasy Blood and Tissue Kit, according to the manufacturer’s instructions (QIAGEN Inc., Valencia, CA). Primer pairs spanning numerous loci between 53D14 and 53F1 were designed based on their GenBank sequence accession numbers. Polymerase chain reaction (PCR)-amplified DNA sequences were determined by the DNA Sequencing Facility at the University of Wisconsin Biotechnology Center.
Site-directed mutagenesis and transgenic animals
A full-length gpi-mt2 cDNA clone (RE16378) was obtained from the Berkeley Drosophila Genome Project through the Drosophila Genomics Resource Center (Bloomington, IN). We used the QuikChange Lighting Site-Directed Mutagenesis Kit (Agilent Technologies, Inc., Santa Clara, CA) to introduce PCR-generated nucleotide substitutions into the gpi-mt2 coding sequence, producing the following four amino acid substitutions in GPI-MT2: W62L, D63A, Q306A, and W310L. The wild-type cDNA, as well as the four mutant cDNAs, were cloned into the pGaSpeR transformation vector using an EcoRI/BamHI fragment. This DNA was injected into wild-type embryos (Rainbow Transgenic Flies, Inc., Camarillo, CA) for the production of transgenic flies expressing the gpi-mt2 cDNAs under the control of the Rh1 (ninaE) promoter: p[ninaE-gpi-mt2]. Each transgene was crossed into the gpi-mt2A276V mutant background using standard Drosophila techniques to test for rescue of the existing phenotypes. For all transgenic animals used in this study, PCR and sequencing were employed to verify the presence of both the gpi-mt2A276V genomic mutation as well as the appropriate site-directed mutation in each transgenic copy of gpi-mt2.
Generation of anti-chaoptin and anti-Gqα antibodies
Polyclonal antibodies directed to chaoptin (Chp) and the Gq-protein alpha subunit (Gqα) were generated using peptides at the C-terminus of each protein. The Chp peptide: NH2-YGRHNC-DEHDDDIRETRCENKGGQQK-COOH, corresponded to amino acid numbers 1255–1279. The Gqα peptide consisted of NH2-KYLACNPDPERQCYSK-COOH, corresponding to amino acid numbers 306–320. Both peptides carried a terminal lysine residue to facilitate glutaraldehyde conjugation to the immunogenic reagent, keyhole limpet hemocyanin (Pierce Biotechnology, Inc., Rockford, IL). The antibodies were generated in rabbits by Cocalico Biologicals (Reamstown, PA).
Western blotting
One-day-old fly heads were separated by electrophoresis in sodium dodecyl sulfate (SDS) polyacrylamide gels and electroblotted onto nitrocellulose membranes as previously described (Colley et al., 1991). The Rh1 (4C5) and β-tubulin (E7) mouse monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, University of Iowa. Polyclonal antibodies directed to Chp and Gqα are described above and polyclonal antibodies directed to Cnx were previously described (Rosenbaum et al., 2006). Antibodies directed to QUASIMODO (QSM) and contactin (CONT) were provided by R. Stanwesky and M. Bhat, respectively (Faivre-Sarrailh et al., 2004; Chen et al., 2011). The immunoreactive proteins were visualized using horseradish peroxidase-conjugated goat anti-mouse, goat anti-rabbit, goat anti-rat, or rabbit anti-guinea pig immunoglobulin G (IgG) (Invitrogen Corporation, Carlsbad, CA) followed by ECL detection (Amersham Pharmecia Biotech, Piscataway, NJ).
Immunocytochemistry
Experiments were carried out on 1–2-day-old flies, as previously described (Colley et al., 1991). Briefly, heads were fixed in phosphate buffered saline (PBS) with 3% formaldehyde and 0.05% glutaraldehyde for 45 min and infiltrated with 2.3 m sucrose overnight. Frozen 0.5-µm sections were immunolabeled with the following antibodies: Mouse monoclonal antibodies directed to Rh1 (4C5) and Chp (24B10) were obtained from the Developmental Studies Hybridoma Bank, University of Iowa. Mouse monoclonal antibodies to Rh3 and Rh4 were provided by S. Britt, and polyclonal antibodies directed to Cnx were previously described (Rosenbaum et al., 2006). Primary antibody labeling was detected using DyLight 488-conjugated goat anti-mouse or DyLight 549-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Nuclei were labeled with To-Pro-3 nucleic acid stain (Molecular Probes, Inc., Eugene, OR). All samples were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Sections were viewed at room temperature using a point-scanning confocal microscope (Bio-Rad Radiance 2100 MP Rainbow) with a 60× PlanApo, NA 1.40, lens. Imaging data were acquired using Bio-Rad LaserSharp 6.0 and processed using Adobe Photoshop C3S, Adobe Illustrator CS3, and Adobe InDesign CS3. For each experiment, at least three individual heads were sectioned and between 50 and 100 ommatidia were observed in each eye.
Electron microscopy
Adult heads were fixed and processed according to a modification of the methods of Baumann and Walz, as previously described (Colley et al., 1991, 1995). Briefly, heads were fixed in cacodylate buffer containing 2% formaldehyde and 2% glutaraldehyde for 4 h, followed by incubation in the same fixative containing 1% tannic acid overnight at 4°C. Tissue was rinsed in cacodylate buffer and postfixed in 2% osmium tetroxide for 2 h. Heads were dehydrated through an ethanol series, rinsed with propylene oxide, and infiltrated and embedded in Spurr’s resin (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections were stained with 2% uranyl acetate and Reynold’s lead citrate and viewed at 80 kV on a Phillips CM120 electron microscope (University of Wisconsin Medical School Electron Microscope Facility). Images were captured using a MegaView III camera (Olympus Soft Imaging Systems, Center Valley, CA) and iTEM Olympus Soft Imaging Systems (v. 5.0 build 1243) at a magnification of 4400× to 11,500×. For each experiment, at least three individual heads were sectioned and between 50 and 100 ommatidia were observed per eye.
Biochemical procedures
All reagents for the endoglycosidase H (Endo H) and peptide N-glycosidase F (PNGase F) digestions were obtained from New England BioLabs (Ipswich, MA). For each reaction, 40 wild-type heads (or 100 gpi-mt2A276V mutant heads) were homogenized into 100 µl denaturing buffer, sonicated, and processed according to a modification of the manufacturer’s instructions; we included 1% SDS in the reaction buffer and incubated for longer times. For membrane preparations and PI-PLC digestions, 150 wild-type heads (or 300 gpi-mt2A276V mutant heads) were homogenized into 300 µl 20 mM Tris, pH 7.5. The total cell homogenate (TCH) was sonicated and lightly spun to remove chitin. The homogenates were then incubated for 1 h at 37°C in the presence or absence of PI-PLC enzyme (Invitrogen Corporation, Carlsbad, CA) and centrifuged at 100,000× g for 60min at 4°C. For Endo H, PNGase F, and PI-PLC digestions, all samples were mixed with appropriate volumes of sample buffer (Laemmli, 1970) and assessed via Western blot analysis.
Results
Identification of Drosophila GPI-MT2
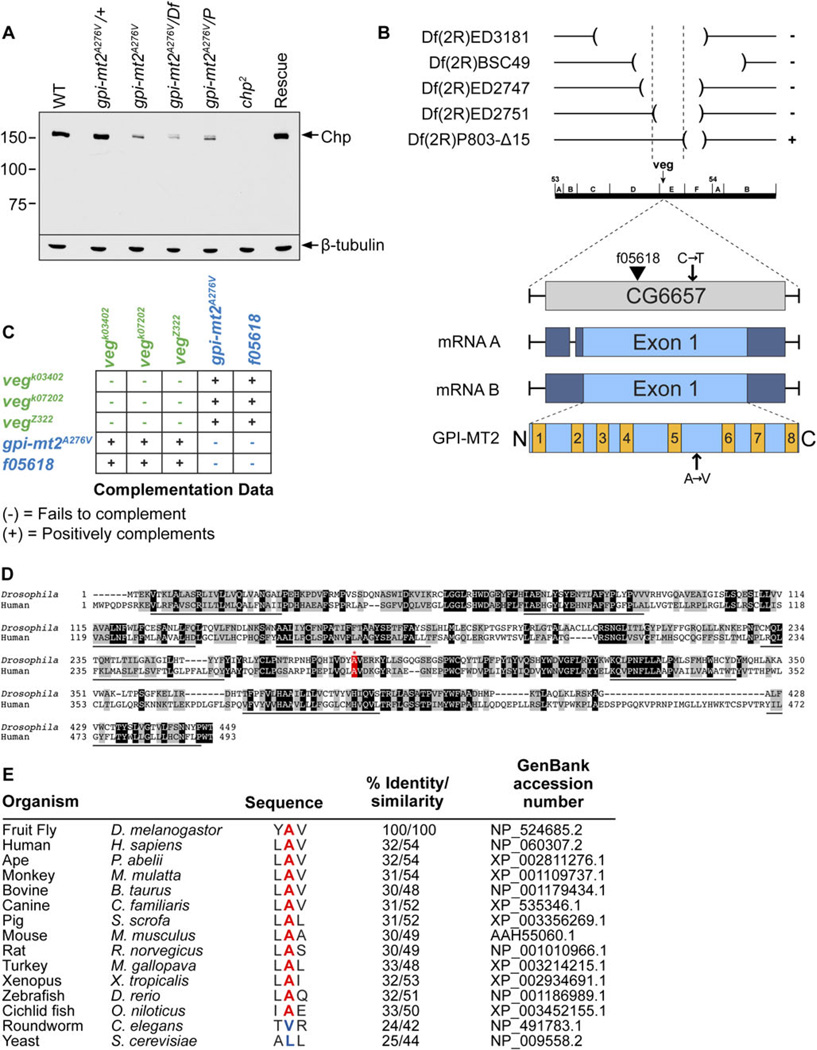
To identify GPI-MT2, we screened the Zuker collection of EMS-mutagenized Drosophila (Koundakjian et al., 2004) for genetic loci that are critical for photoreceptor cell structure and function. This was accomplished by monitoring the presence or absence of the DPP, which is a reliable indicator of the structural integrity of the photoreceptor cells (Franceschini & Kirschfeld, 1971; Franceschini, 1972). We identified a mutant that displayed a severe attenuation of the DPP, as well as a reduction in chaoptin (Chp) protein levels (Fig. 2A, lane 3) compared to wild type (Fig. 2A, lane 1). The EMS-mutation is recessive, as the heterozygotes displayed wild-type levels of Chp (Fig. 2A, lane 2).
Fig. 2.
Drosophila GPI-MT2. (A) Western blot of proteins isolated from 1-day-old fly heads, labeled with a polyclonal antibody directed to Chaoptin (Chp). From left to right: Wild type (cn,bw), gpi-mt2A276V heterozygotes, gpi-mt2A276V homozygotes, gpi-mt2A276V/Df(2R) ED2751, gpi-mt2A276V/Pbac{WH}vegf05618, chp2 (null allele), GPI-MT2 rescue line. Western blot was reprobed for β-tubulin as a loading control. Two heads were loaded per lane. (B) Deficiency mapping the gpi-mt2 locus. The cytogenetic locations of four deficiencies that failed to complement (−) and one deficiency that positively complemented (+) the gpi-mt2A276V allele are shown. The gpi-mt2 locus is comprised of a single exon that encodes both a 1778-base pair messenger RNA (mRNA) transcript (isoform A) and a 1858-base pair mRNA transcript (isoform B). Both transcripts encode the same 449 amino acid (aa) protein product. A proposed domain structure for the Drosophila GPI-MT2 protein is shown, indicating eight putative TM domains and very short cytosolic N-terminal (aa1–4) and C-terminal (aa448–449) domains. TM1 = aa5–27, TM2 = aa70–90, TM3 = aa112–132, TM4 = aa151–173, TM5 = aa231–253, TM6 = aa321–342, TM7 = aa369–392, and TM8 = aa426–447. Protein topology was based on analysis of the human GPI-MT2 protein expressed in CHO cells (Kang et al., 2005). (C) Complementation data were obtained by performing standard genetic crosses between each veg allele to generate transheterozygotes. The vegk03402, vegk07202, and vegZ322 mutants were previously shown to belong to a single complementation group (Kania et al., 1995; Prokopenko et al., 2000). In the current study, we demonstrate that these alleles positively complement the gpi-mt2A276V and vegf05618 alleles, using Western blot analysis (unpublished observations). (D) Amino acid alignment between Drosophila GPI-MT2 (NP_524685.2) and human GPI-MT2 (NP_060307.2), generated with the UniProt Align program. Dark shading indicates identity, light shading indicates similarity, and TM domains are underlined. Numbers refer to aa residues. The alanine mutated in the gpi-mt2A276V allele is highlighted in red. (E) UniProt aa alignment between Drosophila GPI-MT2 and other GPI-MT2 proteins from a variety of species, demonstrating that alanine276 (red) is highly conserved in most animals, but not in C. elegans or S. cerevisiae. % Identity/similarity calculated relative to D. melanogastor sequence.
To identify the gpi-mt2 mutant locus, we first used deficiency mapping to narrow the cytogenetic location to 53D14-53F1 on the second chromosome, corresponding to 37 genes (Fig. 2A, lane 4, and Fig. 2B). We sequenced prioritized candidate genes in this region and identified a C to T substitution at nucleotide position 827 within the coding region of CG6657. This mutation corresponds to a substitution at amino acid position 276, from a highly conserved alanine to a valine (A276V). CG6657 consists of a single exon that encodes a resident ER α1,6-mannosyltransferase enzyme (GPI-MT2) involved in the biosynthesis of GPI (Figs. 1 and 2B). We obtained a homozygous lethal P-element allele of CG6657 (Pbac {WH}vegf05618) and demonstrated that chaoptin protein levels were significantly reduced in flies harboring the P-element in trans to the A276V EMS-mutation (Fig. 2A, lane 5). To further confirm that CG6657 was indeed the correct locus, we performed genetic rescue experiments. We introduced a wild-type copy of CG6657 into the genome of flies harboring the A276V mutation and showed that the DPP, as well as chaoptin protein levels, were restored to normal (Fig. 2A, lane7). These results demonstrate that CG6657, encoding GPI-MT2, is the responsible gene.
The CG6657 locus was originally named vegetable (veg) based on analysis of a genetic complementation group comprised of several embryonic lethal P-element alleles and one EMS allele that all caused severe neuronal loss and fasciculation defects in the peripheral nervous system (PNS) (Kania et al., 1995; Prokopenko et al., 2000) (Fig. 2C, green). Plasmid rescue was used to isolate and sequence a genomic fragment flanking the insertion site of one of these P-elements, which was determined to be CG6657 (Prokopenko et al., 2000). Since these initial findings, the P-elements have been more precisely mapped to numerous cytological locations outside of the CG6657 locus. Furthermore, we have demonstrated that several of the original veg alleles positively complement both the A276V EMS allele and the f05618 P-element allele, which inserts directly within the coding sequence of CG6657 (Fig. 2B and 2C). Based on these findings, we propose that the embryonic PNS phenotypes described for the original veg alleles were not due to defects in CG6657, but rather due to defects in a neighboring locus. From this point forward, we will refer to the CG6657 locus as gpi-mt2, based on the identity of the encoded protein as a GPI-MT2 enzyme involved in GPI anchor biosynthesis. In some mammalian species, this enzyme is also referred to as PIG-V for phosphatidylinositol glycan class V (Kang et al., 2005).
The GPI-MT2 family of proteins is conserved from yeast through humans, and Drosophila GPI-MT2 displays 32% amino acid identity with human GPI-MT2 (Fig. 2D). The alanine residue mutated in our gpi-mt2A276V allele is highly conserved among GPI-MT2 proteins in a variety of animals, indicating its functional importance (Fig. 2E). Interestingly, this alanine is not conserved in Caenorhabditis elegans (C. elegans) or Saccharomyces cerevisiae (S. cerevisiae). GPI-MT2 is predicted to have eight transmembrane (TM) domains (Fig. 2B and 2D), with both its N- and C-terminus facing the cytoplasmic side of the ER, placing alanine276 within the lumen of the ER. This topology, together with the fact that this residue is highly conserved in most animals, suggests that alanine276 serves an important role in the function of GPI-MT2 during GPI biosynthesis. The A276V mutation is hypomorphic, as the gpi-mt2A276V mutant flies are homozygous viable.
GPI-MT2 is critical for GPI-mediated ER exit and surface expression of chaoptin
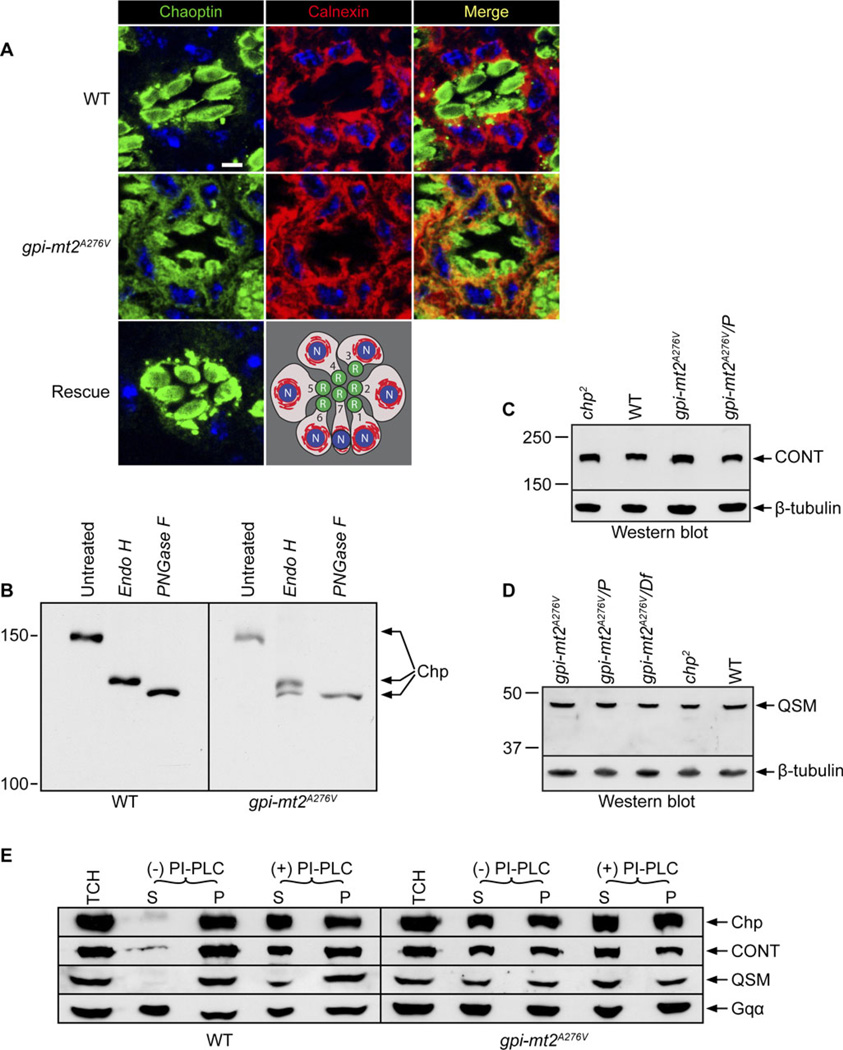
In addition to its function in membrane attachment, the GPI anchor is thought to play an important role in protein sorting and trafficking (Maeda & Kinoshita, 2011). Therefore, we examined the role of GPI-MT2 in the production of a functional GPI anchor by investigating the surface expression of the GPI-anchored protein, chaoptin. Consistent with defects in GPI anchor biosynthesis, chaoptin protein was severely mislocalized in the gpi-mt2A276V mutant (Fig. 3A). Chaoptin was abnormally retained in the ER and throughout the secretory pathway, where it colocalized with the ER chaperone, calnexin. Some chaoptin was also present in the rhabdomeres. This is in contrast to the highly specific targeting of chaoptin to the cell surface within the rhabdomeres of wild-type flies (Fig. 3A), for which we do not see colocalization between chaoptin and calnexin in the secretory pathway. The finding that some chaoptin protein was successfully targeted to the rhabdomeres in the gpi-mt2A276V mutant is consistent with the mutation being hypomorphic. Chaoptin localization was completely restored to wild type in the GPI-MT2 rescue line (Fig. 3A), further demonstrating that CG6657 is indeed the correct locus.
Fig. 3.
GPI-MT2 is essential for the GPI-anchored protein, chaoptin. (A) Confocal images of 0.5-µm cross-sections from wild type (cn,bw), gpi-mt2A276V, and GPI-MT2 rescue flies, labeled with a monoclonal antibody (24B10) directed to chaoptin (green) and a polyclonal antibody directed to calnexin (red). Colocalization of chaoptin and calnexin is shown in the merge (yellow). Nuclei were stained with To-Pro-3 (blue). Bar, 1 µm. A schematic of a cross-section from the R1–7 photoreceptor cells is shown to illustrate the rhabdomeres (R, green), ER (red), and nuclei (N, blue). (B) Western blot of proteins isolated from wild-type (cn,bw) or gpi-mt2A276V mutant heads treated with either Endo H or PNGase F enzyme and labeled with a polyclonal antibody directed to chaoptin. The samples of 10 µl (WT) or 25 µl (gpi-mt2A276V) were loaded per lane. (C) Western blot of proteins isolated from 1-day-old fly heads, labeled for CONT. From left to right: chp2 (null allele), wild type (cn,bw), gpi-mt2A276V homozygotes, gpi-mt2A276V/Pbac{WH}vegf05618. (D) Western blot of proteins isolated from 1-day-old fly heads, labeled for QSM. From left to right: gpi-mt2A276V homozygotes, gpi-mt2A276V/Pbac{WH}vegf05618, gpi-mt2A276V/Df(2R)ED2751, chp2 (null allele), wild type (cn,bw). For (C) and (D), Western blots were reprobed for β-tubulin as a loading control and 10 heads were loaded per lane. (E) Western blots of protein fractions generated from wild-type (cn;bw) and gpi-mt2A276V mutant heads following centrifugation of a TCH that was incubated in the presence (+) or absence (−) of PI-PLC enzyme. Blots were labeled with polyclonal antibodies directed to chaoptin (Chp), CONT, and QSM. S=supernatant, P=pellet. Western blots were reprobed for the Gq-protein α-subunit (Gqα) as a loading control. We loaded a 15 µl (WT) or 20 µl (gpi-mt2A276V) sample per lane.
Chaoptin is N-glycosylated at 13 different sites, and the oligosaccharides at each site have been well characterized by MALDI-TOF-MS (Kanie et al., 2009). To determine whether the trafficking defects in gpi-mt2A276V were accompanied by alterations in the N-glycosylation state of chaoptin, we performed Endo H and PNGase F digestions. In wild-type flies, Endo H digestion resulted in a partially deglycosylated form of chaoptin, whereas PNGase F treatment produced the fully deglycosylated form of chaoptin (Fig. 3B). This is explained by the fact that 10 of the N-glycosylation sites on chaoptin lie within the interior β-strand region of an arch in chaoptin’s tertiary structure and are thus protected from enzymatic processing as chaoptin traffics through the secretory pathway (Kanie et al., 2009). As a result, these sites remain occupied by high-mannose-type glycans that are sensitive to Endo H treatment (Fig. 3B). There are, however, three N-glycosylation sites on chaoptin that lie outside the arch, on the more exposed surface of the protein (Kanie et al., 2009). These sites are occupied by complex-type glycans that have been processed in the Golgi and are thus Endo H insensitive. This explains why, in wild-type flies, PNGase F treatment was required to produce the fully deglycosylated form of chaoptin (Fig. 3B).
Unlike wild-type tissue, treatment of the gpi-mt2A276V mutant tissue with Endo H produced two bands: the partially deglycosylated form that was detected in wild-type tissue and the fully deglycosylated form that is normally only detected with PNGase F treatment (Fig. 3B). These results indicate that about half of the chaoptin protein in the gpi-mt2A276V mutant is retained in the ER, while the other half is properly modified in the Golgi. Again, these results demonstrate that gpi-mt2A276V is a hypomorphic allele. These biochemical data are also consistent with the chaoptin immunolabeling in the gpi-mt2A276V mutant (Fig. 3A), showing that while some chaoptin is properly trafficked to the rhabdomere, a significant amount of protein is also retained in the secretory pathway due to defects in GPI anchor biosynthesis. Therefore, GPI-MT2 is required for the GPI-mediated ER exit and proper targeting of chaoptin to the cell surface.
GPI-MT2 is required for GPI-mediated membrane attachment of chaoptin
Genetic elimination of gpi-mt2, in both yeast and CHO cells, leads to the accumulation of a truncated GPI intermediate possessing only a single mannose residue, modified with a side-branching EthN-P (Fabre et al., 2005; Kang et al., 2005). In both studies, metabolic radiolabeling of GPI was utilized to demonstrate that this truncated anchor was unable to attach to its protein substrates (Fabre et al., 2005; Kang et al., 2005). To determine if the incomplete GPI anchor is attached to chaoptin, we assessed whether chaoptin was soluble or membrane bound in the gpi-mt2A276V mutant tissue.
In wild-type flies, chaoptin was almost entirely associated with the membrane (Fig. 3E, top left). This association was shown to occur via the GPI anchor, as treatment with PI-PLC released a substantial amount of chaoptin into the soluble fraction (Fig. 3E). Our results agree with those of Krantz and Zipursky (1990) that in wild-type tissue, about half of the Chp is released by PI-PLC. The inability of PI-PLC to release all the Chp may be due to the fact that some of the protein is inaccessible to the enzyme in the TCH. In the gpi-mt2A276V mutant, significantly more chaoptin protein was present in the soluble fraction compared with wild-type tissue (Fig. 3E, top right). These results demonstrate that defects in GPI-MT2 lead to the inability of chaoptin to be associated with the plasma membrane. Failure in the attachment of the GPI anchor to chaoptin is likely responsible for the defects in the ER exit, transport, and targeting of chaoptin to the cell surface.
CONT and QSM are defective in the gpi-mt2 mutant
GPI-MT2 is expressed ubiquitously throughout the body, as well as during development. To determine whether there were defects in other GPI-anchored proteins in the gpi-mt2A276V mutant, we investigated the Drosophila circadian rhythm protein, QSM, and another CAM, CONT (Faivre-Sarrailh et al., 2004; Chen et al., 2011). Both CONT and QSM were present at wild-type levels in the gpi-mt2A276V mutant (Fig. 3C and 3D). However, there were significantly higher concentrations of QSM and CONT in the soluble fraction of gpi-mt2A276V mutant tissue, compared to wild type (Fig. 3E, middle rows). These results show that like Chp, QSM and CONT require GPI-MT2 for their GPI-mediated membrane attachment. Therefore, GPI-MT2 plays an important role in other tissues in addition to the eye.
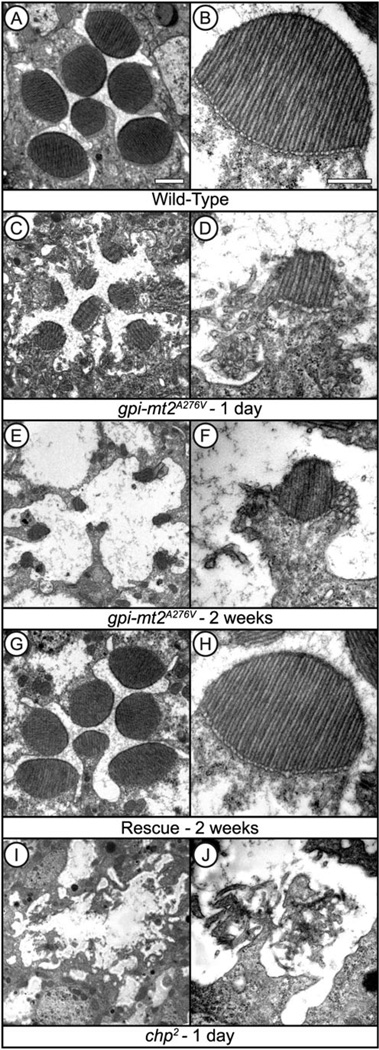
GPI-MT2 is essential for microvillar stability in the rhabdomere
Mutations in chaoptin lead to a severe early onset retinal degeneration due to the lack of microvillar structural integrity (Van Vactor et al., 1988). To investigate the morphological defects in the gpi-mt2A276V mutant, we performed detailed ultrastructural analyses. In 1-day-old gpi-mt2A276V mutants, the rhabdomeres were reduced in size and highly “chaoptic” in appearance (Fig. 4C). The microvilli were disorganized and collapsing from the periphery of the rhabdomere (Fig. 4D). By 2 weeks of age, the gpi-mt2A276V mutants displayed further defects in the overall structural integrity of the photoreceptor cells and the rhabdomeres were nearly absent (Fig. 4E and 4F). The pathology in the gpi-mt2A276V mutant at 2 weeks was less severe than that observed in the null allele of chaoptin at 1 day (Fig. 4I and 4J). These results confirm that some functional chaoptin does traffic to the rhabdomeres in the gpi-mt2A276V mutant (Fig. 3A and 3B), showing once again that the mutation in gpi-mt2A276V is hypomorphic. The cellular pathology was completely reverted back to wild type in the GPI-MT2 rescue line (Fig. 4G and 4H), demonstrating that defects in CG6657 are responsible for the microvillar disorganization and retinal degeneration.
Fig. 4.
GPI-MT2 is required for microvillar stability in the rhabdomere. Electron micrographs of cross-sections through the Drosophila compound eye. Flies were raised on a 12:12 light-dark cycle for either 1 day or 2 weeks. (A, B) Wild type (cn,bw), (C–F) gpi-mt2A276V, (G,H) GPI-MT2 rescue, (I, J) chp2 (null) mutant. Bar, 1 µm (A, C, E, G, I). Bar, 500 nm (B, D, F, H, J).
GPI-MT2 and the Drosophila opsins
Phototransduction in Drosophila is a G protein-coupled phosphoinositide-mediated signaling cascade in which light stimulation of rhodopsin (Rh1) leads to the opening of cation-selective transient receptor potential (TRP) and TRP-like (TRPL) channels (Hardie & Raghu, 2001; Wang & Montell, 2007; Hardie & Postma, 2008; Katz & Minke, 2009). In wild-type flies, Rh1 specifically resides within the rhabdomeres for its function in phototransduction (Fig. 5B). Consistent with defects in rhabodomere morphology, Rh1 was severely reduced and mislocalized in both the chp2 and gpi-mt2A276V mutants (Fig. 5A and 5B). Interestingly, Rh1 was not found in the secretory pathway, indicating that the initial biosynthetic trafficking of Rh1 proceeded normally in both mutants. Instead, most Rh1 was detected in or around the defective rhabdomeres. Therefore, Rh1 is mislocalized and reduced in the gpi-mt2A276V mutant due to reductions in chaoptin and microvillar instability.
Fig. 5.
GPI-MT2 and the Drosophila opsins. (A) Western blot of proteins isolated from 1-day-old fly heads, labeled for Rh1. From left to right: Wild type (cn,bw), chp2 (null allele), gpi-mt2A276V. Western blot was reprobed for β-tubulin as a loading control. Two heads were loaded per lane. (B) Confocal images of 0.5-µm cross-sections from wild type (cn,bw), chp2 (null allele), gpi-mt2A276V, and GPI-MT2 rescue flies, labeled for Rh1 (green). Nuclei were stained with To-Pro-3 (blue). Bar, 1 µm. (C) Confocal images of 0.5-µm cross-sections from wild-type (cn,bw) and gpi-mt2A276V mutant eyes, labeled for Rh3 or Rh4 (green). Nuclei were stained with To-Pro-3 (blue). A schematic of a cross-section from the R1–7 photoreceptor cells is shown to illustrate the R1–6 cell rhabdomeres (R, black), R7 cell rhabdomere (R, green), ER (black), and nuclei (N, blue). Bar, 1 µm.
Rh1 serves as the major photopigment in the R1–6 photoreceptor cells, whereas the R7 cells express the minor opsins, Rh3 and Rh4 (Wang & Montell, 2007). As with Rh1, we determined that Rh3 and Rh4 were mislocalized and not entirely restricted to the rhabdomeres of the R7 photoreceptor cells in the gpi-mt2A276V mutant (Fig. 5C). Taken together, these data demonstrate that defects in rhabdomere morphology have a detrimental effect on the expression and localization of the opsins in the photoreceptor cells.
Functional analysis of GPI-MT2
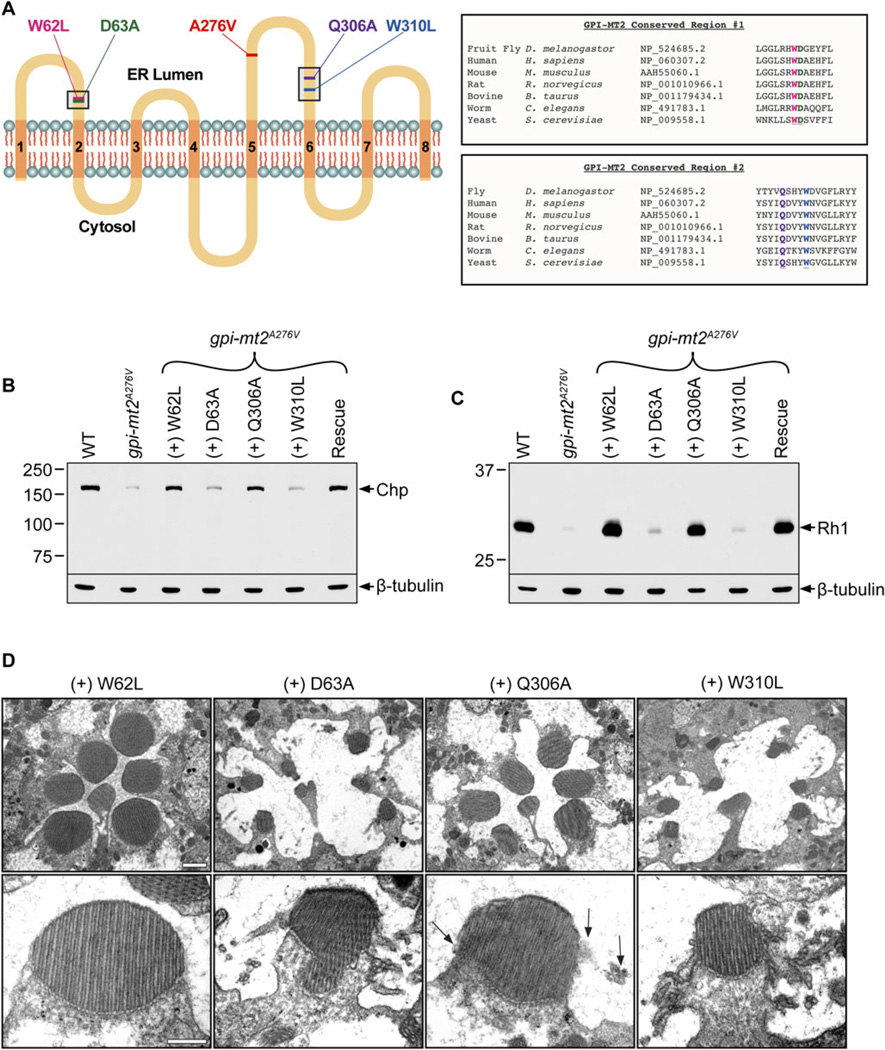
Addition of the second mannose residue to the growing GPI precursor is one of the least characterized steps in GPI anchor biosynthesis. This is partially due to the fact that GPI-MT2 was the last enzyme in the pathway to be identified and also because it displays little amino acid identity with other known mannosyltransferases (Kang et al., 2005). Previously, four amino acids were identified as critical for the function of human GPI-MT2 in CHO cells (Kang et al., 2005). To determine if these residues are also important in Drosophila, we conducted site-directed mutagenesis and produced transgenic animals harboring the same four amino acid substitutions in GPI-MT2 (Fig. 6A).
Fig. 6.
Functional analysis of GPI-MT2. (A) Predicted topology of the Drosophila GPI-MT2 protein, showing eight TM domains (orange) and two highly conserved regions (black boxes) where site-directed mutagenesis was performed. The location of the EMS-generated mutation (A276V) and the four site-directed mutations (W62L, D63A,Q306A, and W310L) are also shown. Protein topology was based on analysis of the human GPI-MT2 protein expressed in CHO cells (Kang et al., 2005). UniProt amino acid alignment between Drosophila GPI-MT2 and other GPI-MT2 proteins from a variety of species demonstrates that W62 (pink), D63 (green), Q306 (purple), andW310 (blue) are all highly conserved residues. (B, C) Western blots of proteins isolated from 1-day-old fly heads, labeled for Chp (B) and Rh1 (C). From left to right: Wild type (cn,bw), gpi-mt2A276V, gpi-mt2W62L, gpi-mt2D63A, gpi-mt2Q306A, gpi-mt2W310L, GPI-MT2 rescue line. Western blots were reprobed for β-tubulin as a loading control. Two heads were loaded per lane. (D) Electron micrographs of cross-sections through gpi-mt2W62L, gpi-mt2D63A, gpi-mt2Q306A, and gpi-mt2W310L mutant fly eyes. Flies were raised on a 12:12 light-dark cycle for 2 weeks. Arrows designate minor chaoptic rhabdomere morphology in the gpi-mt2Q306A mutant. Bar, 1 µm (top row). Bar, 500 nm (bottom row).
GPI-MT2 is thought to have at least two functionally important domains facing the ER lumen, where highly conserved amino acid residues have been implicated in catalytic sites (Fig. 6A). The first domain is a hydrophilic sequence along the first loop on the lumenal side of the ER membrane, between TM domains 1 and 2 (Fig. 6A). This region is conserved among the entire family of ER-resident Dol-P-Man-utilizing glycosyltransferase enzymes, including those involved in N-glycosylation (Oriol et al., 2002). Here, we generated two mutations, namely W62L and D63A. The second highly conserved region in GPI-MT2 lies along the third loop on the lumenal side of the ER membrane, between TM domains 5 and 6 (Fig. 6A). This region is conserved only among the smaller family of GPI-MT2 proteins. Here, we generated two additional mutations, namely Q306A and W310L. The gpi-mt2W62L, gpi-mt2D63A, gpi-mt2Q306A, and gpi-mt2W310L cDNAs were expressed in flies harboring the genomic A276V EMS-mutation. In this genetic background, we assessed the ability of each mutant form of GPI-MT2 to rescue the existing phenotypes in the gpi-mt2A276V mutant.
The W62L mutation had no effect on the function of Drosophila GPI-MT2, as the gpi-mt2W62L transgenic construct was able to rescue chaoptin and Rh1 expression in the gpi-mt2A276V mutant (Fig. 6B and 6C). Consistent with the immunoblotting results, the gpi-mt2W62L transgenic flies exhibited a wild-type DPP and normal rhabdomere morphology at 2 weeks (Fig. 6D). In contrast to the W62L mutation, the D63A and W310L mutations completely inhibited the function of GPI-MT2, as the gpi-mt2D63A and gpi-mt2W310L transgenic mutants still displayed a severe reduction in both chaoptin and Rh1 protein levels (Fig. 6B and 6C). Consistent with the reduction in chaoptin and Rh1, the gpi-mt2D63A and gpi-mt2W310L transgenic mutants displayed chaoptic rhabdomeres and severe retinal degeneration at 2 weeks (Fig. 6D), comparable to that observed in the original gpi-mt2A276V mutant (Fig. 4E and 4F). These results demonstrate that the D63A and W310L mutant forms of GPI-MT2 were nonfunctional and thus unable to rescue the EMS-generated phenotypes in gpi-mt2A276V.
Finally, the Q306A mutation rendered a partially functional form of GPI-MT2, as the gpi-mt2Q306A transgenic mutants displayed minor defects. Chaoptin and Rh1 were expressed at wild-type levels in flies harboring the Q306A form of GPI-MT2 (Fig. 6B and 6C), but there were noticeable ultrastructural defects at 2 weeks of age. The rhabdomeres showed signs of microvillar instability, and there was an overall disruption in the organization of the individual photoreceptor cells, compared to wild type (Fig. 6D). Like the A276V EMS-mutation, the Q306A mutation is hypomorphic, as there was only partial rescue of the phenotypes in the gpi-mt2A276V mutant background. It is possible that these two partially functional forms of GPI-MT2 (A276V and Q306A) are able to compensate for one another when expressed in the same cell, explaining the rescued chaoptin levels and mostly normal rhabdomere morphology in these transgenic flies. Taken together, these results suggest that amino acids D63 and W310 are essential, while amino acid Q306 is partially required for Drosophila GPI-MT2 function in vivo.
Discussion
The GPI anchor is a highly complex and unique structure for attaching proteins to the membrane. Despite almost 30 years of investigation into the biosynthesis and remodeling of the GPI structure, little is known regarding the requirements for GPI by specific substrate proteins in animal models. Here, we utilize hypomorphic mutations in gpi-mt2 to demonstrate that addition of the second mannose residue on GPI is required, in vivo, for the membrane attachment of chaoptin. These results are consistent with previous reports that only fully formed GPI anchors are attached, en bloc, to newly synthesized proteins and suggest that there may be mechanisms in place to prevent the premature attachment of GPI during its biosynthesis.
Here, we show that for Drosophila chaoptin, failure in GPI attachment has severe consequences for sorting and trafficking to the photoreceptor cell surface. These results are consistent with previous findings that the GPI anchor is essential for the ER exit and apical targeting of proteins (Maeda & Kinoshita, 2011). Most GPI-anchored proteins are additionally modified by N-glycosylation, and N-glycans have also been reported to act as apical sorting signals for GPI-anchored proteins (Vagin et al., 2009). Therefore, there has been some debate regarding the relative contribution of the GPI anchor and the N-glycan for the intracellular targeting of proteins. One study, on the membrane dipeptidase protein, demonstrated that it was the N-glycan, not the GPI anchor, that mediated the apical targeting of the protein in kidney epithelial cells (Pang et al., 2004). However, our results show that when chaoptin lacks the GPI anchor, it is N-glycosylated and yet remains in the Endo H-sensitive ER form. Therefore, ER exit and cell surface expression are defective. In the gpi-mt2 mutant, chaoptin fails to be targeted to the rhabdomere for its function in microvillar stability, indicating that while the N-glycan may be necessary, it is not sufficient for apical targeting.
There remains much to be learned regarding the structure, enzymology, and regulation of the complex GPI-MTs that function in GPI anchor biosynthesis. Here, we extend previous studies carried out in CHO cells (Kang et al., 2005) and identify key amino acid residues in GPI-MT2 that are critical for its function in Drosophila. Our findings on the necessity of specific residues for the function of Drosophila GPI-MT2 largely agree with those of human GPI-MT2 in CHO cells, indicating that while D63 and W310 are absolutely required, Q306 is only partially required. Mutations in Q306 were hypomorphic in both Drosophila GPI-MT2 (Q306A) and human GPI-MT2 (Q308A), resulting in a partially functional enzyme (Kang et al., 2005). The finding that amino acid D63 was absolutely required for Drosophila GPI-MT2 function was not surprising, as the corresponding aspartic acid in several other glycosyltransferases in the same family is thought to be involved in stabilization of a metal ion during catalysis (Kang et al., 2005).
It is interesting that amino acid W62 was not essential for the function of Drosophila GPI-MT2, given that the corresponding mutation in human GPI-MT2 (W66L) disrupted enzymatic activity in CHO cells (Kang et al., 2005). While this difference may reflect distinct amino acid requirements in humans compared with flies, it could also be due to the fact that the original experiments were carried out in CHO cells. Unlike photoreceptor cells, which have a highly defined apical domain, CHO cells are not neuronal in origin and are not polarized cells. These differences in cell type may explain the conflicting findings. Given the central role of GPI anchors in the apical targeting of proteins, studies involving polarized cells in their native environments will be key for elucidating the precise role of the GPI anchor during protein trafficking in these cell types.
Defects in GPI-anchored proteins lead to numerous human diseases. For example, DAF and CD59 have been implicated in the inflammatory pathogenesis of a wide variety of neurodegenerative disorders including AMD, MS, lupus erythematosus, and AD (Lopez-Pedrera et al., 2010; Khandhadia et al., 2011; Veerhuis, 2011; Veerhuis et al., 2011). In addition, defects in NYX are associated with the complete form of congenital stationary night blindness (Bech-Hansen et al., 2000; Pusch et al., 2000; Zeitz et al., 2003; O’Connor et al., 2005). In other cases, expression of GPI-anchored proteins can activate oncogenic pathways or facilitate the aggregation of the pathogenic prion protein, implicating the GPI anchoring system as a potential therapeutic target for human cancers and prion diseases. As of yet, mutations in the GPI biosynthesis enzymes have not been identified in these diseases, but it is possible that their role has been overlooked. The identification of viable hypomorphic mutations in GPI enzymes, such as the one presented here, provide insight into novel loci and mechanisms underlying the pathogenesis of human disease and may be key in age-related degenerative disease.
In addition to being highly amenable to a wide array of genetic manipulations, Drosophila displays strong genetic similarity to humans, and the highly conserved pathway for GPI anchor biosynthesis is no exception. Therefore, Drosophila represents an exceptional system for studying the molecular genetics of complex hereditary diseases involving GPI-anchored proteins. Additional genetic studies, such as epistasis experiments and modifier screens in the fly, will be extremely useful for obtaining insights into additional constituents of the GPI anchor biosynthesis pathway that are critical for retinal function and could point to novel loci in retinal diseases. Our experiments on Drosophila GPI-MT2 represent an important step towards elucidating the role of GPI biosynthesis in photoreceptor cell survival and point to defective GPI biosynthesis as a possible culprit in retinal degeneration disorders such as stationary night blindness and AMD.
Acknowledgments
The authors thank S. Haroon, N. Jaeger, N. Rozas, and M. Sookochoff for their expert technical assistance, as well as B. Krieber and Dr. B. Ganetzky for assistance with fly stocks. We thank Dr. J. O’Tousa for providing the pGaSpeR expression vector. We thank Dr. S. Britt for providing the Rh3 and Rh4 antibodies, Dr. R. Stanewsky for providing the QSM antibody, and Dr. M. Bhat for providing the CONT antibody. We thank R. Kalil, L. Rodenkirch, and M. Hendrickson of the W.M. Keck Laboratory for Biological Imaging and B. August and R. Massey of the University of Wisconsin Medical School Electron Microscope Facility. We are grateful to C. Vang and T. Moua for their assistance with the computer graphics. We thank C. Berg for discussions on the veg locus. We thank Dr. C.S. Zuker, who generously provided us with the opportunity to screen the EMS-generated alleles from the Zuker Collection. This work was supported by funding from NIH EY008768 (N.J.C.), NIH AG321762 (E.E.R.), National Institute of General Medical Sciences (NIGMS) T32GM007507 (E.E.R.), as well as from the Retina Research Foundation and the RRF/Walter H. Helmerich Research Chair (N.J.C.). We acknowledge support from P30 EY016665 core grant and the Research to Prevent Blindness Foundation (Department of Ophthalmology & Visual Sciences, N.J.C.).
References
- Almeida AM, Murakami Y, Layton DM, Hillmen P, Sellick GS, Maeda Y, Richards S, Patterson S, Kotsianidis I, Mollica L, Crawford DH, Baker A, Ferguson M, Roberts I, Houlston R, Kinoshita T, Karadimitris A. Hypomorphic promoter mutation in PIGM causes inherited glycosylphosphatidylinositol deficiency. Nature Medicine. 2006;12:846–851. doi: 10.1038/nm1410. [DOI] [PubMed] [Google Scholar]
- Arrighi RB, Faye I. Plasmodium falciparum GPI toxin: A common foe for man and mosquito. Acta Tropica. 2010;114:162–165. doi: 10.1016/j.actatropica.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Sparkes RL, Koop B, Birch DG, Bergen AA, Prinsen CF, Polomeno RC, Gal A, Drack AV, Musarella MA, Jacobson SG, Young RS, Weleber RG. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nature Genetics. 2000;26:319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- Bessler M, Mason PJ, Hillmen P, Miyata T, Yamada N, Takeda J, Luzzatto L, Kinoshita T. Paroxysmal nocturnal haemoglobinuria (PNH) is caused by somatic mutations in the PIG-A gene. The EMBO Journal. 1994;13:110–117. doi: 10.1002/j.1460-2075.1994.tb06240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KF, Peschel N, Zavodska R, Sehadova H, Stanewsky R. QUASIMODO, a Novel GPI-anchored zona pellucida protein involved in light input to the Drosophila circadian clock. Current Biology. 2011;21:719–729. doi: 10.1016/j.cub.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Colley NJ. Retinal degeneration through the eye of the fly. Encyclopedia of the Eye. 2010;4:54–61. [Google Scholar]
- Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Colley NJ, Cassill JA, Baker EK, Zuker CS. Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3070–3074. doi: 10.1073/pnas.92.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Fry EJ, Lopez-Vales R. Novel roles for Nogo receptor in inflammation and disease. Trends in Neurosciences. 2008;31:221–226. doi: 10.1016/j.tins.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Fabre AL, Orlean P, Taron CH. Saccharomyces cerevisiae Ybr004c and its human homologue are required for addition of the second mannose during glycosylphosphatidylinositol precursor assembly. The FEBS Journal. 2005;272:1160–1168. doi: 10.1111/j.1742-4658.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Fain GL, Hardie R, Laughlin SB. Phototransduction and the evolution of photoreceptors. Current Biology. 2010;20:R114–R124. doi: 10.1016/j.cub.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Franceschini N. Pupil and pseudopupil in the compound eye of Drosophila. In: Wehner R, editor. Information Processing in the Visual Systems of Arthropods. Berlin, Germany: Springer-Verlag; 1972. pp. 75–82. [Google Scholar]
- Franceschini N, Kirschfeld K. Pseudopupil phenomena in the Drosophila compound eye. Kybernetik. 1971;9:159–182. doi: 10.1007/BF02215177. [DOI] [PubMed] [Google Scholar]
- Fujita M, Jigami Y. Lipid remodeling of GPI-anchored proteins and its function. Biochimica et Biophysica Acta. 2008;1780:410–420. doi: 10.1016/j.bbagen.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Grimme SJ, Westfall BA, Wiedman JM, Taron CH, Orlean P. The essential Smp3 protein is required for addition of the side-branching fourth mannose during assembly of yeast glycosylphosphatidylinositols. The Journal of Biological Chemistry. 2001;276:27731–27739. doi: 10.1074/jbc.M101986200. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Postma M. Phototransduction in Microvillar Photoreceptors of Drosophila and Other Invertebrates. Vol. 1. San Diego, CA: Academic Press; 2008. [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hicks D, John D, Makova NZ, Henderson Z, Nalivaeva NN, Turner AJ. Membrane targeting, shedding and protein interactions of brain acetylcholinesterase. Journal of Neurochemistry. 2011;116:742–746. doi: 10.1111/j.1471-4159.2010.07032.x. [DOI] [PubMed] [Google Scholar]
- Hirai-Fujita Y, Yamamoto-Hino M, Kanie O, Goto S. N-Glycosylation of the Drosophila neural protein Chaoptin is essential for its stability, cell surface transport and adhesive activity. FEBS Letters. 2008;582:2572–2576. doi: 10.1016/j.febslet.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Kang JY, Hong Y, Ashida H, Shishioh N, Murakami Y, Morita YS, Maeda Y, Kinoshita T. PIG-V involved in transferring the second mannose in glycosylphosphatidylinositol. The Journal of Biological Chemistry. 2005;280:9489–9497. doi: 10.1074/jbc.M413867200. [DOI] [PubMed] [Google Scholar]
- Kania A, Salzberg A, Bhat M, D’Evelyn D, He Y, Kiss I, Bellen HJ. P-element mutations affecting embryonic peripheral nervous system development in Drosophila melanogaster. Genetics. 1995;139:1663–1678. doi: 10.1093/genetics/139.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanie Y, Yamamoto-Hino M, Karino Y, Yokozawa H, Nishihara S, Ueda R, Goto S, Kanie O. Insight into the regulation of glycan synthesis in Drosophila chaoptin based on mass spectrometry. PLoS One. 2009;4:e5434. doi: 10.1371/journal.pone.0005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagogeos D. Neural GPI-anchored cell adhesion molecules. Frontiers in Bioscience. 2003;8:s1304–s1320. doi: 10.2741/1214. [DOI] [PubMed] [Google Scholar]
- Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Frontiers in Cellular Neuroscience. 2009;3:2. doi: 10.3389/neuro.03.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe K, Kitamura D, Okabe M, Taniuchi I, Ikawa M, Watanabe T, Kinoshita T, Takeda J. Glycosylphosphatidylinositol-anchor-deficient mice: Implications for clonal dominance of mutant cells in paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:3600–3606. [PubMed] [Google Scholar]
- Khandhadia S, Cipriani V, Yates JR, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology. 2011;217:127–146. doi: 10.1016/j.imbio.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: Recent progress. Journal of Biochemistry. 2008;144:287–294. doi: 10.1093/jb/mvn090. [DOI] [PubMed] [Google Scholar]
- Koundakjian EJ, Cowan DM, Hardy RW, Becker AH. The Zuker collection: A resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics. 2004;167:203–206. doi: 10.1534/genetics.167.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz DE, Zipursky SL. Drosophila chaoptin, a member of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. The EMBO Journal. 1990;6:1969–1977. doi: 10.1002/j.1460-2075.1990.tb08325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leidich SD, Drapp DA, Orlean P. A conditionally lethal yeast mutant blocked at the first step in glycosyl phosphatidylinositol anchor synthesis. The Journal of Biological Chemistry. 1994;269:10193–10196. [PubMed] [Google Scholar]
- Levental I, Grzybek M, Simons K. Greasing their way: Lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrera C, Aguirre MA, Barbarroja N, Cuadrado MJ. Accelerated atherosclerosis in systemic lupus erythematosus: Role of proinflammatory cytokines and therapeutic approaches. Journal of Biomedicine & Biotechnology. 2010 doi: 10.1155/2010/607084. pii: 607084, Epub 2010 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma KN, Cashman SM, Sweigard JH, Kumar-Singh R. Decay accelerating factor (CD55)-mediated attenuation of complement: Therapeutic implications for age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2010;51:6776–6783. doi: 10.1167/iovs.10-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Kinoshita T. Structural remodeling, trafficking and functions of glycosylphosphatidylinositol-anchored proteins. Progress in Lipid Research. 2011;50:411–424. doi: 10.1016/j.plipres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Ramalingam S, Gerber LD, Brink L, Udenfriend S. An active carbonyl formed during glycosylphosphatidylinositol addition to a protein is evidence of catalysis by a transamidase. The Journal of Biological Chemistry. 1995a;270:19576–19582. doi: 10.1074/jbc.270.33.19576. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Ramalingam S, Gerber LD, Udenfriend S. Cleavage without anchor addition accompanies the processing of a nascent protein to its glycosylphosphatidylinositol-anchored form. Proceedings of the National Academy of Sciences of the United States of America. 1995b;92:1550–1554. doi: 10.1073/pnas.92.5.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydan G, Noyman I, Har-Zahav A, Neriah ZB, Pasmanik-Chor M, Yeheskel A, Albin-Kaplanski A, Maya I, Magal N, Birk E, Simon AJ, Halevy A, Rechavi G, Shohat M, Straussberg R, Basel-Vanagaite L. Multiple congenital anomalieshypotonia-seizures syndrome is caused by a mutation in PIGN. Journal of Medical Genetics. 2011;48:383–389. doi: 10.1136/jmg.2010.087114. [DOI] [PubMed] [Google Scholar]
- Nagamune K, Ohishi K, Ashida H, Hong Y, Hino J, Kangawa K, Inoue N, Maeda Y, Kinoshita T. GPI transamidase of Trypanosoma brucei has two previously uncharacterized (trypanosomatid transamidase 1 and 2) and three common subunits. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10682–10687. doi: 10.1073/pnas.1833260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Ohishi K, Yamada N, Kinoshita T, Nagy A, Takeda J. Developmental abnormalities of glycosylphosphatidylinositolanchor-deficient embryos revealed by Cre/loxP system. Laboratory Investigation. 1999;79:293–299. [PubMed] [Google Scholar]
- O’Connor E, Eisenhaber B, Dalley J, Wang T, Missen C, Bulleid N, Bishop PN, Trump D. Species specific membrane anchoring of nyctalopin, a small leucine-rich repeat protein. Human Molecular Genetics. 2005;14:1877–1887. doi: 10.1093/hmg/ddi194. [DOI] [PubMed] [Google Scholar]
- Oriol R, Martinez-Duncker I, Chantret I, Mollicone R, Codogno P. Common origin and evolution of glycosyltransferases using Dol-P-monosaccharides as donor substrate. Molecular Biology and Evolution. 2002;19:1451–1463. doi: 10.1093/oxfordjournals.molbev.a004208. [DOI] [PubMed] [Google Scholar]
- Orlean P, Leidich SD, Drapp DA, Colussi P. Isolation of temperature-sensitive yeast GPI-anchoring mutants. Brazilian Journal of Medical and Biological Research. 1994;27:145–150. [PubMed] [Google Scholar]
- Orlean P, Menon AK. GPI anchoring of protein in yeast and mammalian cells, or: How we learned to stop worrying and love glycophospholipids. Journal of Lipid Research. 2007;48:993–1011. doi: 10.1194/jlr.R700002-JLR200. [DOI] [PubMed] [Google Scholar]
- Ortonne N, Ram-Wolff C, Giustiniani J, Marie-Cardine A, Bagot M, Mecheri S, Bensussan A. Human and mouse mast cells express and secrete the GPI-anchored isoform of CD160. The Journal of Investigative Dermatology. 2011;131:916–924. doi: 10.1038/jid.2010.412. [DOI] [PubMed] [Google Scholar]
- Pang S, Urquhart P, Hooper NM. N-glycans, not the GPI anchor, mediate the apical targeting of a naturally glycosylated, GPI-anchored protein in polarised epithelial cells. Journal of Cell Science. 2004;117:5079–5086. doi: 10.1242/jcs.01386. [DOI] [PubMed] [Google Scholar]
- Paulick MG, Bertozzi CR. The glycosylphosphatidylinositol anchor: A complex membrane-anchoring structure for proteins. Biochemistry. 2008;47:6991–7000. doi: 10.1021/bi8006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopenko SN, He Y, Lu Y, Bellen HJ. Mutations affecting the development of the peripheral nervous system in Drosophila: A molecular screen for novel proteins. Genetics. 2000;156:1691–1715. doi: 10.1093/genetics/156.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch CM, Zeitz C, Brandau O, Pesch K, Achatz H, Feil S, Scharfe C, Maurer J, Jacobi FK, Pinckers A, Andreasson S, Hardcastle A, Wissinger B, Berger W, Meindl A. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nature Genetics. 2000;26:324–327. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- Radford HE, Mallucci GR. The role of GPI-anchored PrP C in mediating the neurotoxic effect of scrapie prions in neurons. Current Issues in Molecular Biology. 2010;12:119–127. [PubMed] [Google Scholar]
- Ramo K, Cashman SM, Kumar-Singh R. Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Investigative Ophthalmology & Visual Science. 2008;49:4126–4136. doi: 10.1167/iovs.08-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum EE, Hardie RC, Colley NJ. Calnexin is essential for rhodopsin maturation, Ca2+ regulation, and photoreceptor cell survival. Neuron. 2006;49:229–241. doi: 10.1016/j.neuron.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz P, Weppler D, Tryphonopoulos P, Nishida S, Moon J, Kato T, Selvaggi G, Levi D, Madariaga J, DelaGarza J, Tuteja S, Garcia M, Tzakis A. CD55 and CD59 deficiency in transplant patient populations: Possible association with paroxysmal nocturnal hemoglobinuria-like symptoms in Campath-treated patients. Transplantation Proceedings. 2006;38:1750–1752. doi: 10.1016/j.transproceed.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Lehto MT. Glycosylphosphatidylinositol-anchored proteins: Structure, function, and cleavage by phosphatidylinositol-specific phospholipase C. Biochemistry and Cell Biology. 2002;80:535–549. doi: 10.1139/o02-146. [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Stone EM. Genomics and the eye. The New England Journal of Medicine. 2011;364:1932–1942. doi: 10.1056/NEJMra1012354. [DOI] [PubMed] [Google Scholar]
- Spurway TD, Dalley JA, High S, Bulleid NJ. Early events in glycosylphosphatidylinositol anchor addition. substrate proteins associate with the transamidase subunit gpi8p. The Journal of Biological Chemistry. 2001;276:15975–15982. doi: 10.1074/jbc.M010128200. [DOI] [PubMed] [Google Scholar]
- Tiede A, Bastisch I, Schubert J, Orlean P, Schmidt RE. Biosynthesis of glycosylphosphatidylinositols in mammals and unicellular microbes. Biological Chemistry. 1999;380:503–523. doi: 10.1515/BC.1999.066. [DOI] [PubMed] [Google Scholar]
- Tremml G, Dominguez C, Rosti V, Zhang Z, Pandolfi PP, Keller P, Bessler M. Increased sensitivity to complement and a decreased red blood cell life span in mice mosaic for a nonfunctional Piga gene. Blood. 1999;94:2945–2954. [PubMed] [Google Scholar]
- Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. American Journal of Physiology. Renal Physiology. 2009;296:F459–F469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vactor D, Jr, Krantz DE, Reinke R, Zipursky SL. Analysis of mutants in Chaoptin, a photoreceptor cell-specific glycoprotein in Drosophila, reveals its role in cellular morphogenesis. Cell. 1988;52:281–290. doi: 10.1016/0092-8674(88)90517-x. [DOI] [PubMed] [Google Scholar]
- Varma Y, Hendrickson T. Methods to study GPI anchoring of proteins. Chembiochem. 2010;11:623–636. doi: 10.1002/cbic.200900704. [DOI] [PubMed] [Google Scholar]
- Veerhuis R. Histological and direct evidence for the role of complement in the neuroinflammation of AD. Current Alzheimer Research. 2011;8:34–58. doi: 10.2174/156720511794604589. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Molecular Immunology. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Barman A, Chen Y, Nguyen PD, Wagner SL, Prabhakar R, Thinakaran G. Loss of cleavage at beta’-site contributes to apparent increase in beta-amyloid peptide (Abeta) secretion by beta-secretase (BACE1)-glycosylphosphatidylinositol (GPI) processing of amyloid precursor protein. The Journal of Biological Chemistry. 2011;286:26166–26177. doi: 10.1074/jbc.M111.260471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflügers Archiv. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139:246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, Scherthan H, Freier S, Feil S, Suckow V, Schweiger S, Berger W. NYX (nyctalopin on chromosome X), the gene mutated in congenital stationary night blindness, encodes a cell surface protein. Investigative Ophthalmology & Visual Science. 2003;44:4184–4191. doi: 10.1167/iovs.03-0251. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–3504. doi: 10.2337/diabetes.51.12.3499. [DOI] [PubMed] [Google Scholar]