Abstract
Introduction
Staging of node negative (N0) non-small cell lung cancer is modified in the 7th edition TNM classification. Here, we pool data from JBR.10 and CALGB-9633 to explore the prognostic and predictive effects of the new T-size descriptors and KRAS mutation status.
Methods
Node negative patients were reclassified as T2a (>3-≤5cm), T2b (>5-≤7cm), T3 (>7cm) or T≤3 cm (≤3cm but other T2 characteristics).
Results
Of 538 eligible patients, 288 (53.5%) were T2a, 111 (21%) T2b, 62 (11.5%) T3, while 77 (14%) T≤3cm were excluded to avoid confounding. KRAS mutations were detected in 104/390 (27%) patients. T-size was prognostic for disease-free survival (DFS; p=0.03), but borderline for overall survival (OS; p=0.10), on multivariable analysis. Significant interaction between the prognostic value of KRAS and tumor size was observed for OS (p=0.01), but not DFS (p=0.10). There was a non-significant trend (p=0.24) for increased chemotherapy effect on OS with advancing T-size (HR T2a 0.90, [0.63-1.30]; T2b 0.69, [0.38-1.24]; and T3 0.57, [0.28-1.17]). The HR for chemotherapy effect on OS in T2a patients with KRAS wild-type tumors was 0.81 (p=0.36), while a trend for detrimental effect was observed in those with mutant tumors (HR 2.11; p=0.09; interaction p=0.05). Similar trends were observed in T2b-T3 patients with wild-type (HR 0.86; p=0.62), and KRAS mutant tumors (HR 1.16; p=0.74; interaction p=0.58).
Conclusion
Chemotherapy effect appears to increase with tumor size. However, this small study could not identify subgroups of patients who did or did not derive significant benefit from adjuvant chemotherapy based on T-size or KRAS status.
Introduction
Recently, several randomised clinical trials and two individual patient data meta-analyses have confirmed a survival benefit for adjuvant cisplatin-based chemotherapy in stage II-IIIA non-small cell lung cancer (NSCLC), with absolute improvements in 5-year survival of 4-15% 1-5. Unplanned retrospective subset analyses of some trials also suggest potential benefit in node negative patients with tumors ≥4 cm 6,7. Importantly, all adjuvant chemotherapy trials reported to date are based on an outdated staging system, and preceded adoption of the Union Internationale Contre le Cancer/American Joint Cancer Committee (UICC/AJCC) 7th Edition Staging Classification of Lung Cancer 8-10. The implications of these changes on recommendations for adjuvant chemotherapy use remain to be determined.
Clinical trials suggesting a potential benefit for adjuvant chemotherapy in node negative NSCLC patients with tumors ≥4 cm include the JBR.10 and CALGB-9633 trials 6,7. However, the 4 cm tumor cut-point was reached arbitrarily. Furthermore, as this size does not correspond to the T-size descriptors employed in either the old or new TNM staging systems, practical questions are raised regarding its clinical application and how best to utilize this finding in future studies.
The UICC/ AJCC 7th edition particularly alters the stage classification of stage I tumors 8,9. T1 tumors now are sub-classified as T1a (≤2 cm) and T1b (>2- ≤3 cm), T2 tumors as T2a (>3- ≤5 cm) and T2b (>5- ≤7 cm), with reclassification of tumors >7 cm as T3. This results in upstaging of pT2bN0 from IB to IIA, and pT3N0 to IIB. Whether the new staging system better stratifies patients for benefit from adjuvant chemotherapy remains to be determined.
The selection of NSCLC patients for adjuvant chemotherapy on the basis of stage alone, however, is suboptimal, with high rates of relapse observed. This has led to attempts to identify other potential predictive markers of chemotherapy benefit. Analyses of both JBR.10 and CALGB-9633 suggested that the presence of KRAS mutations may be associated with resistance to platinum-based chemotherapy, although neither study could demonstrate a significant interaction 11,12. We questioned, therefore, whether the interaction of tumor size and KRAS status might predict for adjuvant chemotherapy benefit in node negative NSCLC patients.
In this retrospective study, we pooled data from JBR.10 and CALGB-9633 to provide the first exploratory analysis of the effect of the new T-size descriptors on survival benefit from adjuvant platinum-based chemotherapy in node negative NSCLC patients. Furthermore, we explored the interaction between T-size and KRAS mutation status in predicting benefit from adjuvant chemotherapy. Finally, we evaluated the interaction between the prognostic values of T-size and KRAS mutation status on overall (OS) and disease-free survival (DFS).
Methods
Study Population
All node negative (N0) patients randomised to receive either adjuvant chemotherapy or observation as part of JBR.10 and CALGB-9633 were eligible. Methodology of both clinical trials has been described previously 4,7. Pathological confirmation of negative lymph nodes at mediastinoscopy and/or surgery was mandatory for inclusion in the CALGB-9633 study; while for JBR.10, intraoperative mediastinal lymph node resection or biopsy of nodes ≥1.5 cm was required. CALGB-9633 was limited to patients with pT2N0 NSCLC, while JBR.10 included patients with completely resected pT2N0, pT1N1, or pT2N1 NSCLC. In CALGB-9633, carboplatin/paclitaxel was administered for four postoperative cycles, and in JBR.10, cisplatin/vinorelbine was administered, also for four cycles.
KRAS Gene Mutation Assay
Pre-treatment tumor specimens were collected prospectively in both trials. Available specimens were evaluated for the presence of KRAS mutation (codons 12, 13, 61) using allelespecific oligonucleotide hybridization followed by confirmation by sequencing in JBR.10 and mass spectrometry-based genotyping in CALGB-9633, as previously described 11,12.
Statistical analysis
Individual patient data including tumor size and survival status were collected for all eligible patients. Node negative patients were reclassified by tumor size as T2a (>3- ≤5 cm), T2b (>5- ≤7 cm), T3 (>7 cm) or T≤3 cm (tumor size ≤3 cm but with other T2 defining characteristics: involvement of the bronchus ≥2 cm distal to the carina; the presence of visceral pleural invasion; atelectasis or pneumonitis extending to the hilar region but not involving the entire lung). The T≤3 cm subgroup represented a potential source of confounding as it included patients upstaged to T2 by virtue of factors other than tumor size. As it was not possible to study the influence of these factors on outcome, due to insufficient cases and incomplete data, the T≤3 cm subgroup was excluded from analyses to avoid bias. The primary end point of the study was overall survival (OS). The secondary end point was disease-free survival (DFS), defined as time to recurrence, or death from any cause in the absence of recurrence.
Median follow-up was calculated using the reverse Kaplan-Meier method 13. Analyses comparing the chemotherapy and control arms used an intention-to-treat principle. Survival analyses were performed using the log-rank test method and the Cox model stratified by trial and adjusted for age, sex, histology and type of surgery. The Hazard Ratios corresponding to univariable analyses are displayed in all survival curves shown, while the results of multivariable analyses are reported within the results section. The main analysis was the multivariable analysis.
The treatment effect variation by T-size for survival was studied using tests for trend. The T2b and T3 subgroups were pooled for analyses evaluating the effect of KRAS mutations due to the limited number of events observed. We planned to evaluate the prognostic value of T-size, and its interaction with KRAS mutation status, in the control group of the relevant study cohort, except in the absence of interaction of these variables with treatment effect, in which case, an analysis using both chemotherapy and control arms, stratified by treatment arm, would be performed.
Statistical analyses were performed using SAS Software, Version 9.1 (SAS Institute, Cary, NC). Survival curves were performed using R software, version 2.13.0 (copyright 2011 the R Foundation for Statistical Computing). All p-values are two-sided.
Results
Study Population
JBR.10 included 482 patients with completely resected stage IB (T2N0, n=219) or II (T1-2N1, n=263) NSCLC. CALGB-9633 included 344 stage IB (T2N0) NSCLC patients. Following pathological review, 218/219 JBR.10 and 331/344 CALGB-9633 N0 patients remained eligible for study. Tumor size data were available for 538/549 N0 patients (Figure 1). Among these, 288 were T2a, 111 T2b, and 62 T3 by T-size criteria based on the new 7th Edition TNM Staging Classification.
Figure 1.
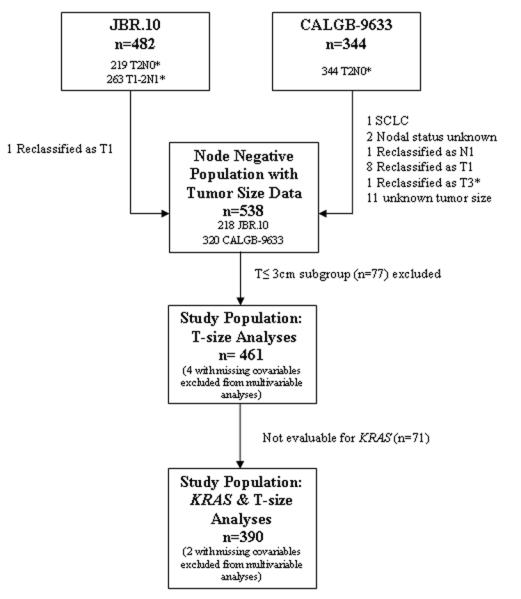
Study Population
Baseline characteristics of the study population, by tumor size, are presented in Table 1. CALGB-9633 included a higher proportion of patients with larger tumors (p<0.0001). Adenocarcinomas tended to be smaller relative to other histological subtypes (p=0.03). Large tumors more often required pneumonectomy (p<0.0001). There were no significant differences among the subgroups with respect to the treatment received (p=0.10). Median follow up was 5.3 years for JBR.10 and 7.5 years for CALGB-9633 (6.5 years for all study participants).
Table 1.
Baseline demographics of patients by T-size according to 7th edition staging classification
| T2a (>3-≤5cm) n=288 |
T2b (>5-≤7cm) n=111 |
T3 (>7cm) n=62 |
T≤3cm (≤3cm*) n=77 |
P Value |
|
|---|---|---|---|---|---|
|
| |||||
| No. | No. | No. | No. | ||
| Clinical trial | |||||
| JBR.10 | 128 | 38 | 19 | 33 | <.0001 |
| CALGB 9633 | 160 | 73 | 43 | 44 | |
| Treatment | |||||
| JBR.10 | |||||
| Chemotherapy | 72 | 21 | 9 | 8 | 0.10 |
| Observation | 56 | 17 | 10 | 25 | |
| CALGB-9633 | |||||
| Chemotherapy | 80 | 34 | 25 | 21 | |
| Observation | 80 | 39 | 18 | 23 | |
| Age at diagnosis (years) | |||||
| Median | 61 | 61 | 59 | 60 | 0.73 |
| Range | 34-81 | 37-76 | 42-78 | 40-78 | |
| Age groups (years) | |||||
| < 55 | 83 | 33 | 18 | 18 | 0.91 |
| 55 – 64 | 98 | 39 | 20 | 32 | |
| > 64 | 107 | 39 | 24 | 27 | |
| Sex | |||||
| Male | 184 | 71 | 41 | 45 | 0.79 |
| Female | 104 | 40 | 21 | 32 | |
| ECOG performance status | |||||
| 0 | 167 | 59 | 27 | 41 | 0.21** |
| 1-2 | 120 | 51 | 35 | 35 | |
| Unknown | 1 | 1 | 0 | 1 | |
| Type of surgery | |||||
| Pneumonectomy | 26 | 15 | 19 | 3 | <.0001** |
| Lobectomy | 259 | 96 | 43 | 74 | |
| Unknown | 3 | 0 | 0 | 0 | |
| Histological subtype | |||||
| Adenocarcinoma | 145 | 50 | 28 | 52 | 0.03** |
| Squamous | 99 | 37 | 24 | 13 | |
| Other | 43 | 24 | 10 | 12 | |
| Unknown | 1 | 0 | 0 | 0 | |
T≤3cm but with other T2 defining characteristics (excluded from analyses)
excluding unknown category
KRAS Mutations
KRAS mutation testing was successful for 390/461 (85%) patients (174/185 [94%] JBR.10 and 216/276 [78%] CALGB-9633). In total 104/390 (27%) patients had tumors with KRAS mutation (Table 2). There was no significant association between the distribution of KRAS mutations and tumor size according to the new T-size descriptor categories (p=0.49).
Table 2.
Distribution of KRAS mutations by tumor size among 390 T2a-T3 patients with available T-size and KRAS data
| T-size category | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| T2a | T2b | T3 | All | ||||||
| Number of patients |
% | Number of patients |
% | Number of patients |
% | Number of patients |
% | ||
| Trial | KRAS | 87 | 35 | 25 | 27 | 14 | 27 | 126 | 32 |
| JBR 10 | Wild type | ||||||||
| Mutated | 35 | 14 | 9 | 10 | 4 | 8 | 48 | 12 | |
| CALGB -9633 |
Wild type | 94 | 38 | 46 | 51 | 20 | 38 | 160 | 41 |
| Mutated | 31 | 13 | 11 | 12 | 14 | 27 | 56 | 15 | |
| All | KRAS | 181 | 73 | 71 | 78 | 34 | 65 | 286 | 73 |
| Wild type | |||||||||
| Mutated | 66 | 27 | 20 | 22 | 18 | 35 | 104 | 27 | |
| All | 247 | 100 | 91 | 100 | 52 | 100 | 390 | 100 | |
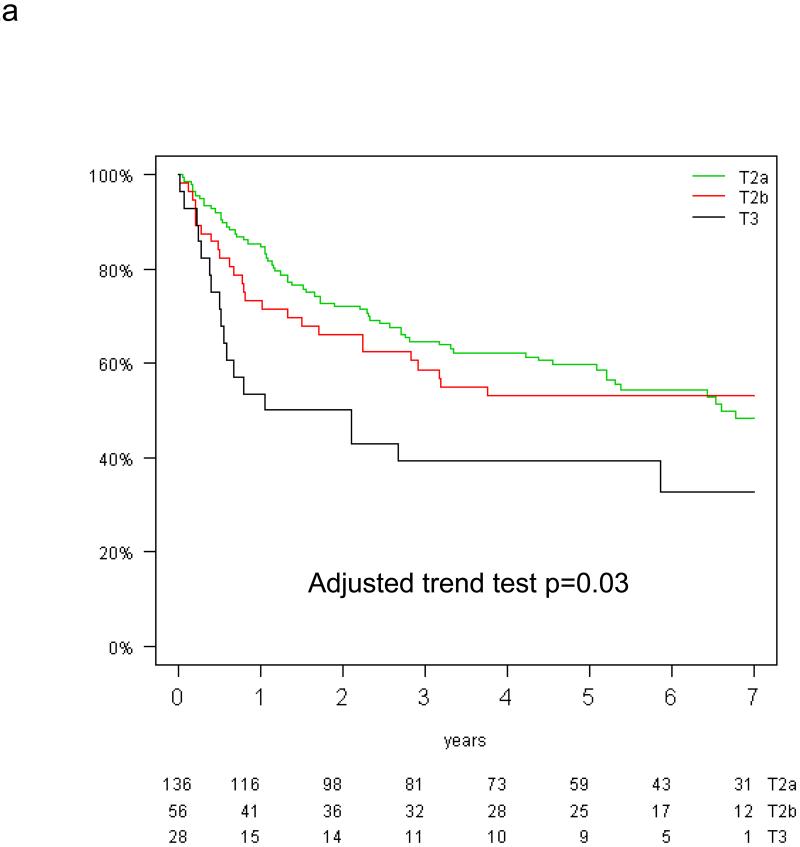
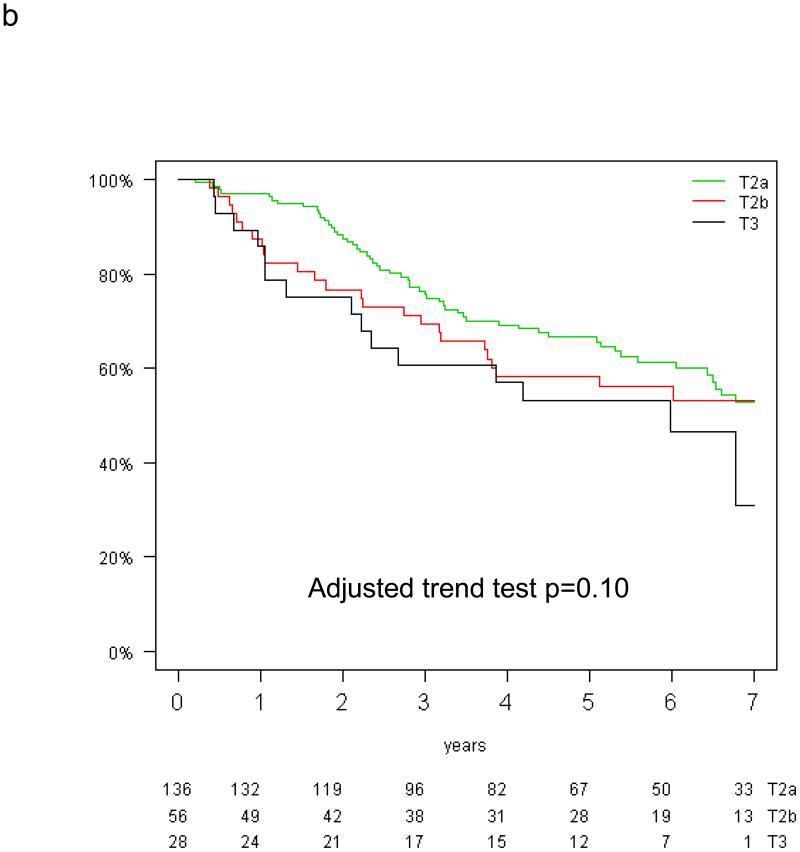
Prognostic Effect of Tumor Size for Overall and Disease-Free Survival
The prognostic value of tumor size for overall and disease-free survival was analysed in the control group of the T-size population because of a trend for interaction between tumor size and treatment effect on DFS (p=0.10). In multivariable analysis, tumor size was significantly prognostic for DFS (test for trend, p=0.03), but only borderline for OS (p=0.10; Figure 2a&b). Compared with T2a patients, the HR for recurrence was 1.09 (95% CI 0.70-1.71) for T2b and 2.07 (95% CI 1.20-3.59) for T3, while the HR for death was 1.19 (95% CI 0.75-1.89) and 1.64 (95% CI 0.91-2.97), respectively (reported in Table, Supplemental Digital Content 1).
Figure 2.
Disease-free (a) and overall survival (b) by tumor size in the control arm of the T-size population
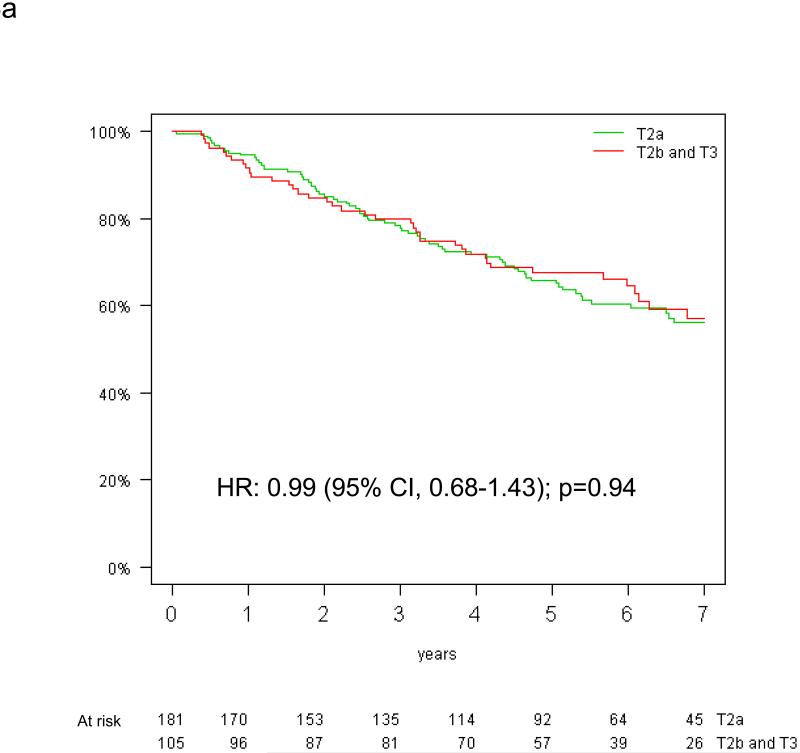
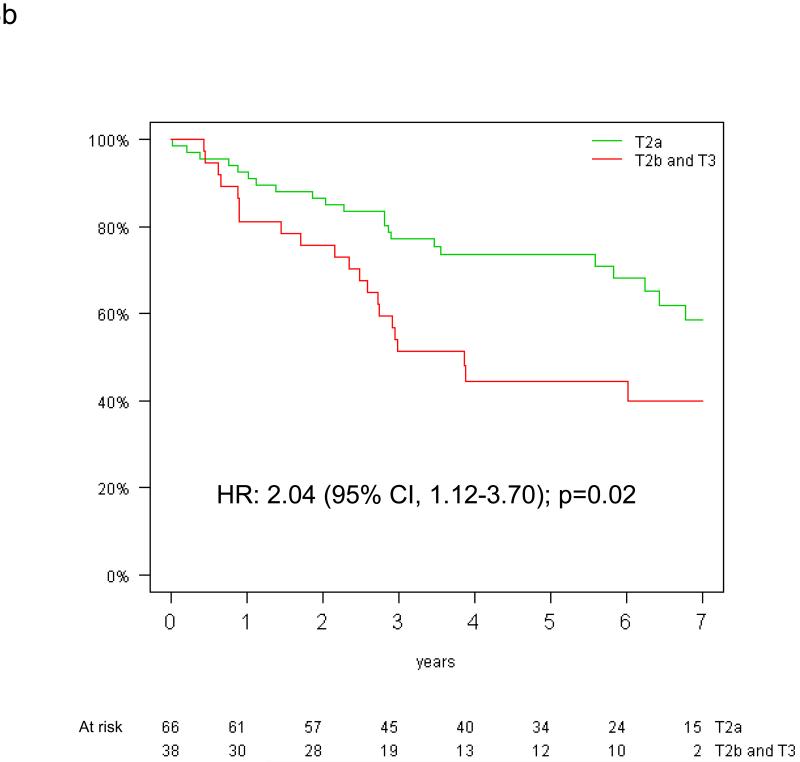
Prognostic Effect of Tumor Size by KRAS Mutation Status for Overall and Disease-Free Survival
The prognostic effect of tumor size on overall survival by KRAS mutation status is shown in Figure 3. Among patients with KRAS mutant tumors, those with tumors >5 cm had a significantly worse survival (HR=2.38, 95% CI: 1.30-4.35, p=0.005) (Figure 3b). In contrast, there was no significant difference in the risk of death according to tumor size among patients with KRAS wild-type tumors (HR 0.96, 95% CI 0.66-1.40; p=0.82) (Figure 3a). Significant interaction between the prognostic values of T-size and KRAS mutation was observed for overall (interaction p=0.01), but not disease-free survival (interaction p=0.10).
Figure 3.
Overall survival by tumor size among the 390 patients with both T-size and KRAS data available: (a) KRAS wild-type and (b) KRAS mutated tumors (HR for univariable analysis).
Predictive Value of Tumor Size for Survival Benefit from Adjuvant Chemotherapy
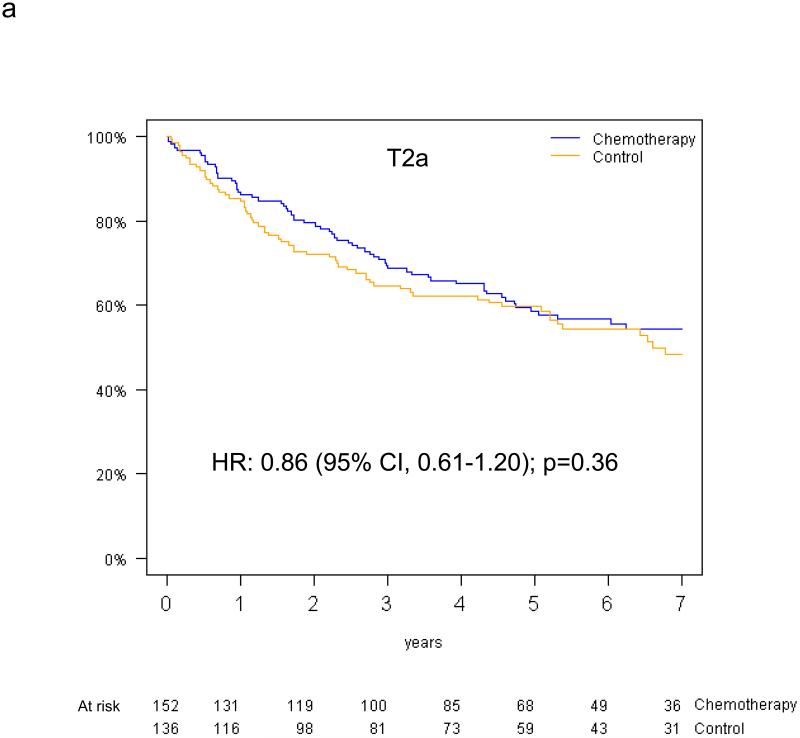
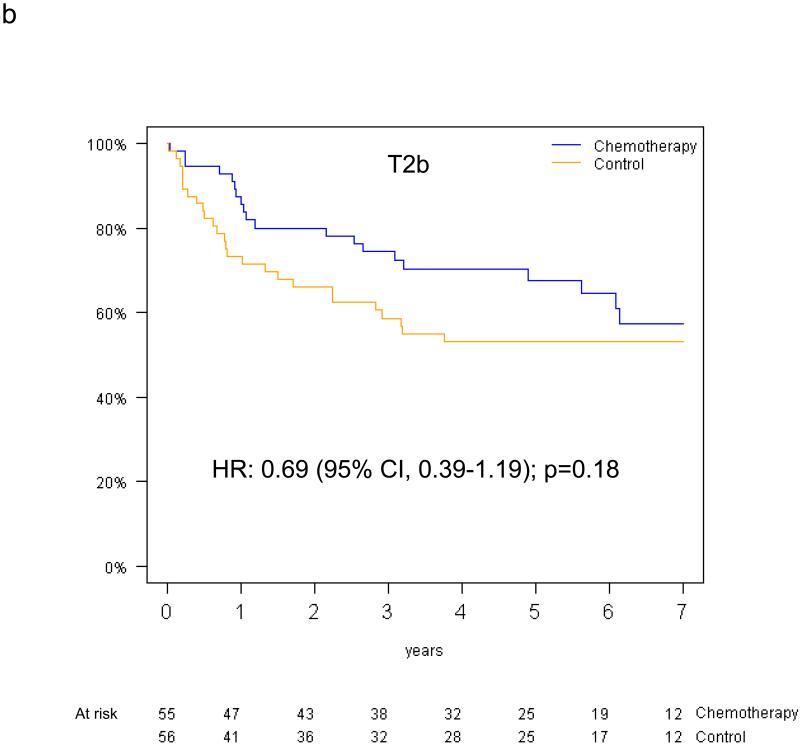
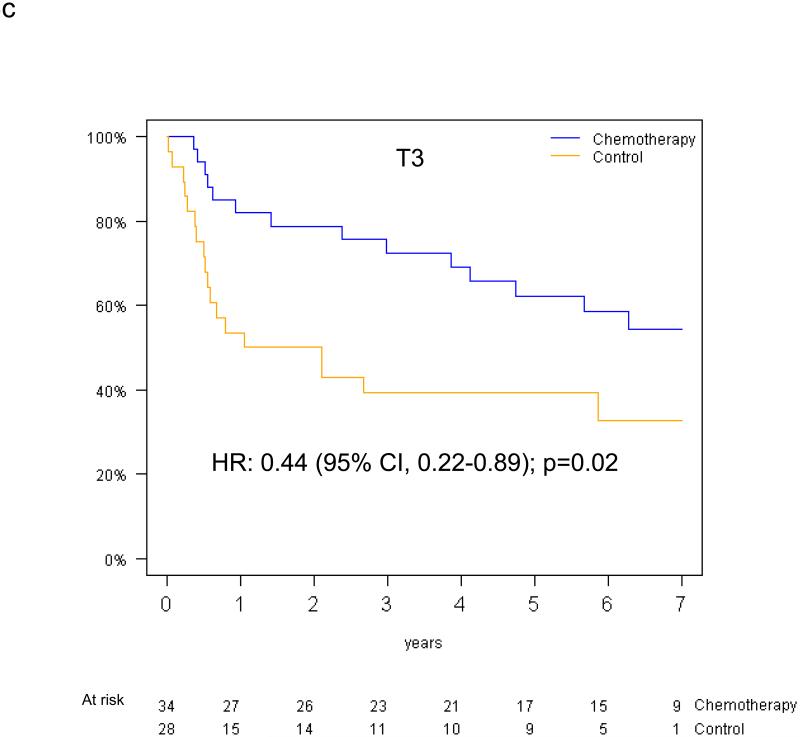
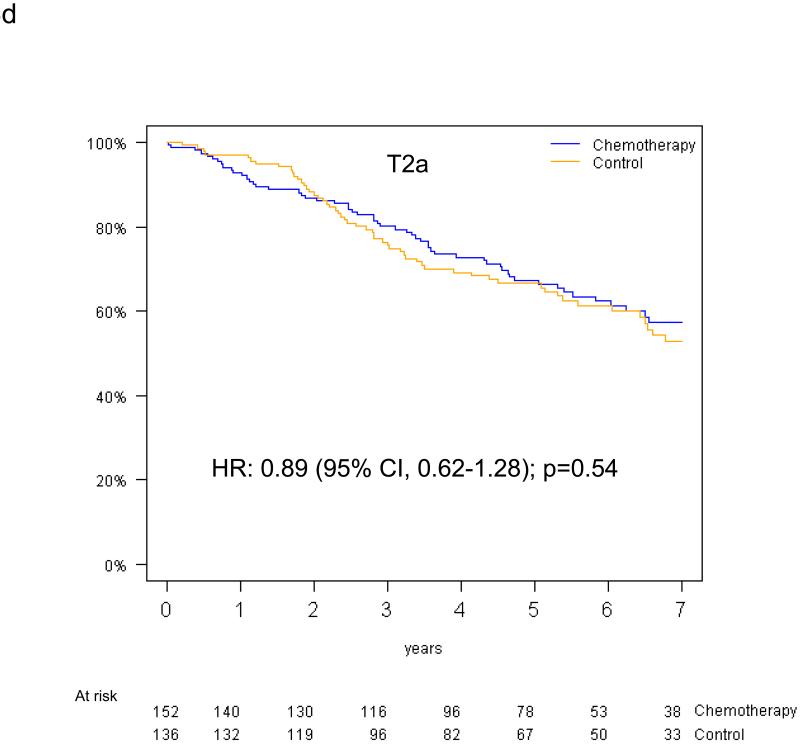
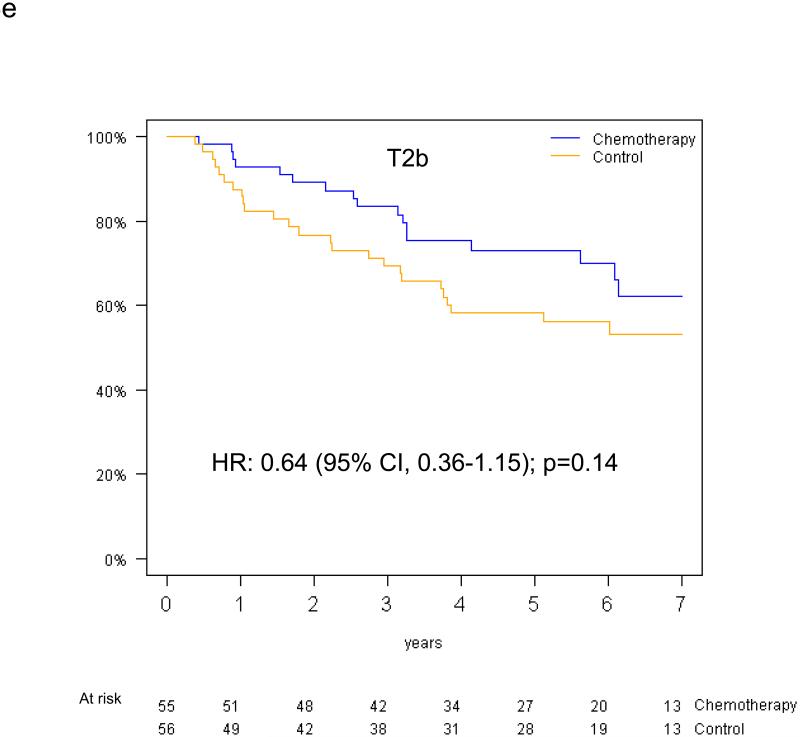
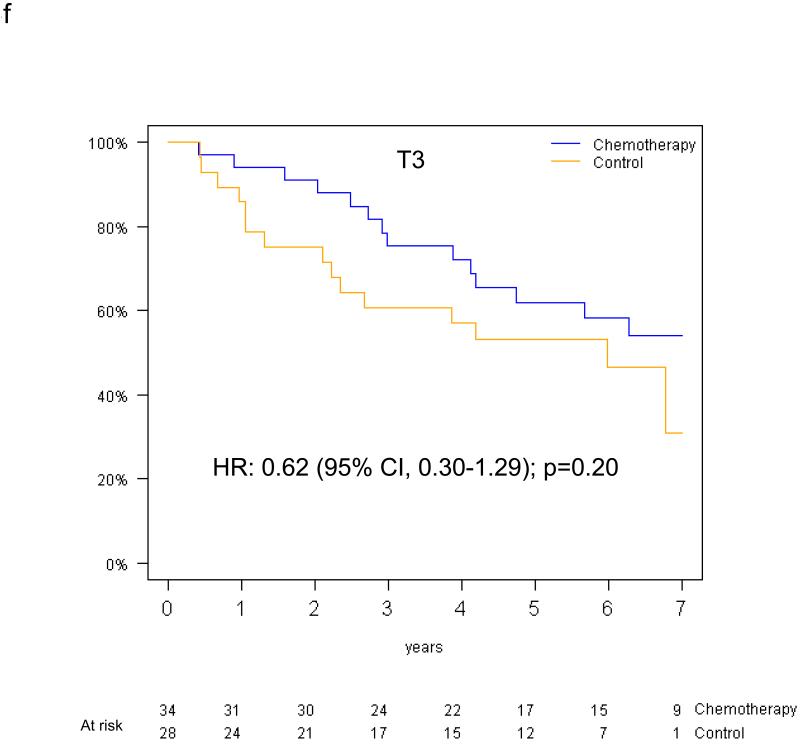
Pooled analysis of the 461 patients in the T-size population revealed an overall significant beneficial effect of adjuvant chemotherapy for DFS (HR 0.75, 95% CI 0.57-0.98, p=0.04), and a slightly smaller effect for OS (HR 0.80, 95% CI 0.60-1.06, p=0.13). In multivariable analysis, a non-significant trend towards increased DFS benefit from adjuvant chemotherapy was observed with increasing tumor size: HR 0.85 (95% CI 0.61-1.20) for T2a, 0.73 (95% CI 0.42-1.28) for T2b and 0.41 (95% CI 0.21-0.82) for T3 (test for trend p=0.10) (Figure 4a-c). Similarly, we observed a non-significant increase in effect of adjuvant chemotherapy on OS with advancing tumor size: HR 0.90 (95% CI 0.63-1.30) for T2a, 0.69 (95% CI 0.38-1.24) for T2b, and 0.57 (95% CI 0.28-1.17) for T3 (test for trend p= 0.24) (Figure 4d-f).
Figure 4.
Disease-free survival (a-c) and overall survival (d-f) (chemotherapy versus control) in the T2a, Tb2 and T3 subgroups.
Predictive Value of KRAS Mutation Status for Survival Benefit from Adjuvant Chemotherapy by Tumor Size
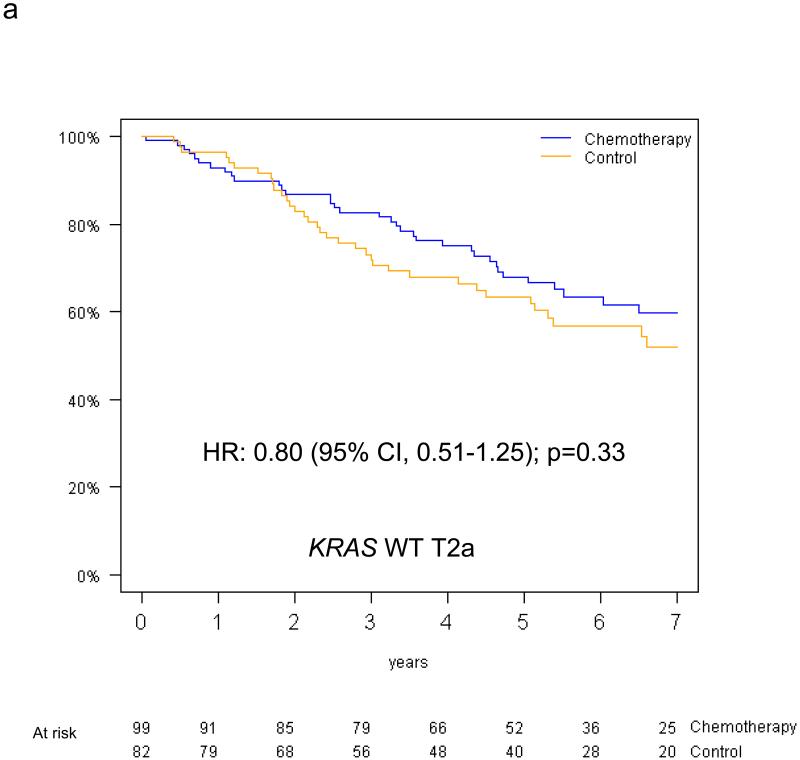
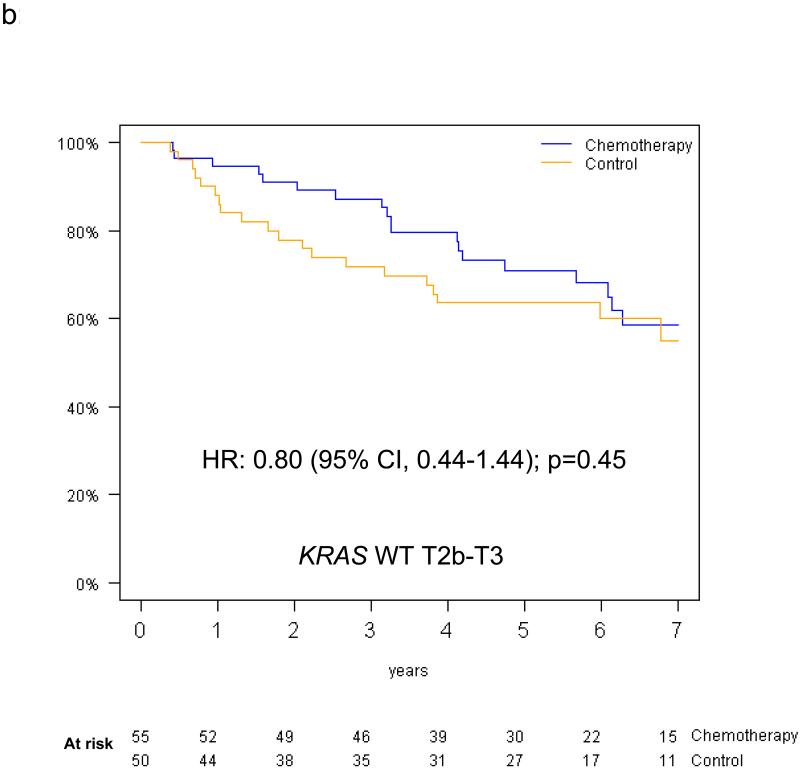
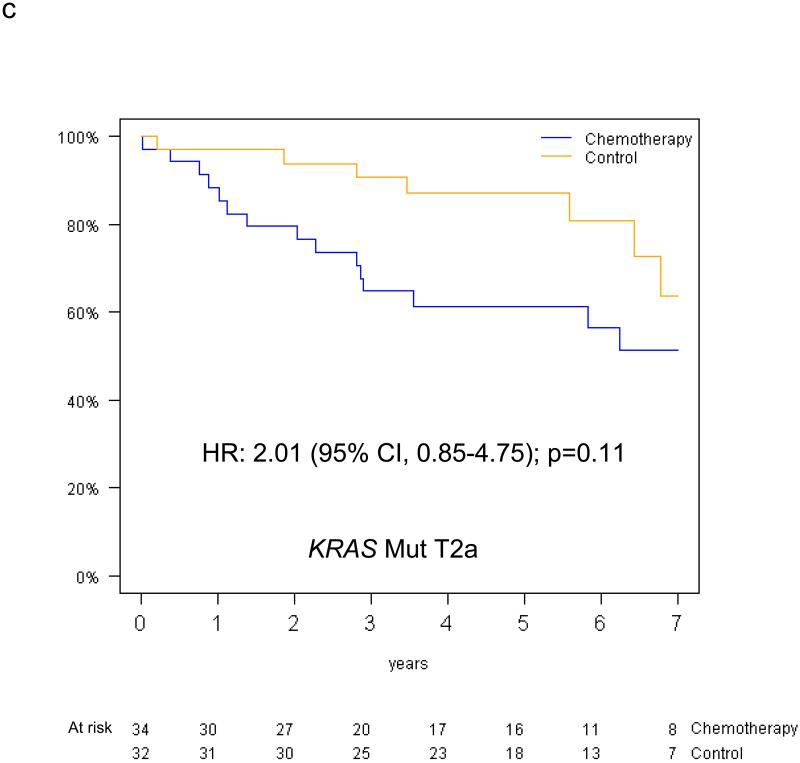
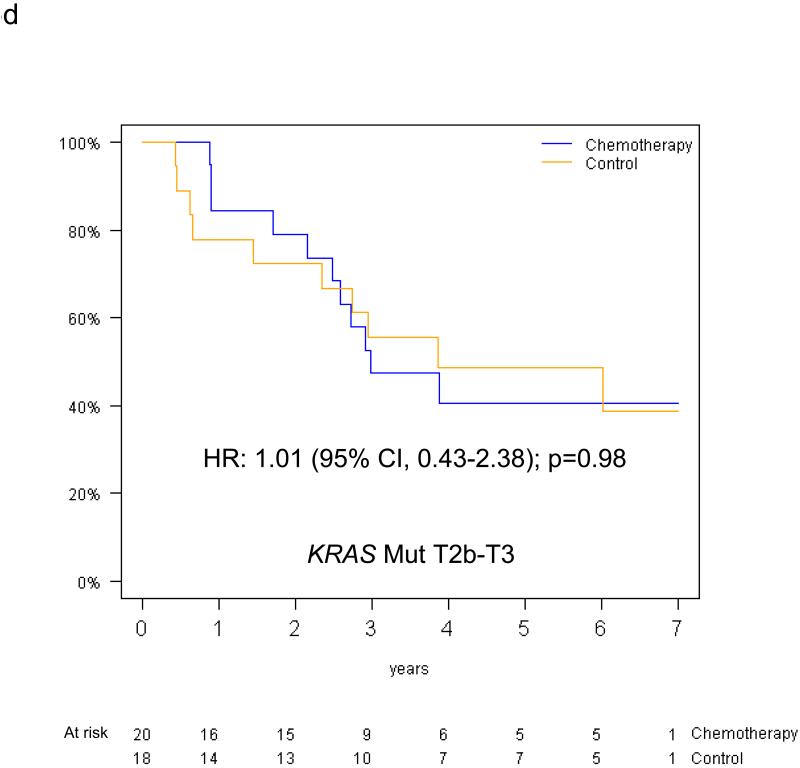
The predictive value of KRAS status for survival benefit from adjuvant chemotherapy by tumor size among the 390 evaluable patients is summarised in Figure 5. Among T2a patients, the HR for chemotherapy effect on OS was 0.81 (95% CI 0.51-1.28; p=0.36) in those with KRAS wild-type tumors, while a trend for detrimental effect was observed in those with KRAS mutations (OS HR=2.11, 95% CI 0.89-5.00; p=0.09); this interaction was of borderline significance (p=0.05). Among T2b-T3 patients, trends were in the same direction in those with KRAS wild-type tumors (OS HR=0.86, 95% CI 0.47-1.56, p=0.62), and in those with KRAS mutations (OS HR=1.16, 95% CI 0.49-2.78, p=0.74); however, this interaction was not significant (interaction p=0.58). Similar results were obtained for DFS (reported in Table, Supplemental Digital Content 2). Finally, a three-way interaction between T-size, KRAS mutation status and chemotherapy was not significant for either OS (p=0.37) or DFS (p=0.83). (DFS survival curves illustrated as Figure, Supplemental Digital Content 3).
Figure 5.
Overall survival curves (chemotherapy versus control) for the T2a and T2b-T3 subgroups among patients with KRAS wild type tumors (a,b) and patients with KRAS mutant tumors (c,d).
Discussion
Clinical trials supporting the use of adjuvant platinum-based chemotherapy in completely resected NSCLC are based on a now outdated staging classification. The UICC/AJCC 7th Edition Staging Classification of Lung Cancer dramatically alters the staging of node negative NSCLC patients, and was incorporated into clinical practice without knowledge of the potential impact of these changes on recommendations for adjuvant chemotherapy in the new subgroups. The current Cancer Care Ontario and the American Society of Clinical Oncology guidelines for adjuvant chemotherapy in NSCLC are based on the 6th edition and recommend use of adjuvant platinum-based chemotherapy in good performance patients with completely resected stage II-IIIA NSCLC, while citing insufficient evidence to endorse its routine use in stage IB 14. However, given that the new staging system results in upstaging of node negative NSCLC patients with tumors >5 cm from IB to IIA (>5-≤7 cm) and IIB (>7 cm), there is resultant uncertainty as to how these subsets of patients should be treated. This has prompted calls for further information regarding the impact of the new T-size descriptors on chemotherapy effect 15. To our knowledge, this retrospective study is the first to address this question by using pooled data from two pivotal adjuvant chemotherapy studies. Furthermore, we examine the potential of KRAS mutations as markers of resistance to adjuvant platinum-based therapy to evaluate whether the interaction of T-size and KRAS mutation status might better predict for treatment effect.
In this study, reclassification of patients using the 7th edition T-size descriptors, led to upstaging of one third of the node negative population; 111 pT2b N0 patients from IB to IIA, and 62 pT3N0 patients from IB to IIB. This finding highlights the importance of the new T-size descriptors in influencing stage shifts and is consistent with those of Boffa et al, who recently reported that 5.5% of all participants in the IASLC staging database were upstaged from IB based on tumor size alone 16. Thus, our study reinforces the pressing need for improved understanding of the impact of the new T-size descriptors on adjuvant chemotherapy effect. This is particularly valid when we consider that up to 77% of surveyed lung cancer physicians would alter patient management in response to a change in stage designation 16.
In this retrospective study, we have shown an increasing effect of adjuvant chemotherapy with advancing tumor size; however, the interaction was not significant for OS, and was only borderline for DFS. Although not statistically significant, our findings are consistent with the pooled analysis, performed by Douillard et al, which showed an increase in treatment effect with tumor stage in patients randomized to cisplatin/vinorelbine versus observation as part of the LACE-vinorelbine meta-analyses which was based on the 6th edition staging system 17. Unfortunately, the other participating studies in the LACE meta-analyses were not eligible for inclusion in our study as they lacked sufficient T-descriptor data, including tumor size. The power of this study is, therefore, limited which likely impacted on the ability to detect a significant differences or interaction from adjuvant chemotherapy based on the new T-size descriptors. Nonetheless, a clear trend for increased chemotherapy effect in the T2b and T3 subgroups was observed, with a suggestion of clinically meaningful benefit from adjuvant chemotherapy in this population. Indeed, the hazard ratios for mortality of 0.69 for pT2bN0 and 0.57 for pT3N0 patients, while not statistically significant, are not dissimilar to those observed for stage II patients (HR=0.83 [0.73-0.95]) in the LACE meta-analysis 5. Caution must be exercised in interpreting these results, however, as there was no statistically significant interaction between treatment and tumor size in our relatively small study. Confirmatory data of the benefit of adjuvant chemotherapy in node negative NSCLC from large scale prospective trials are, therefore, warranted.
In this exploratory analysis, patients with completely resected pT2aN0 NSCLC did not appear to derive significant benefit from adjuvant platinum-based chemotherapy. This is not surprising, given that this subgroup includes node negative patients with tumors 3-5 cm in size, and individual retrospective analyses of the CALGB-9633 and JBR.10 trials have suggested previously that node negative patients with tumors <4 cm do not benefit from adjuvant chemotherapy. Nonetheless, the question still remains as to how to treat patients with tumors ≥4-5 cm in size as this study lacked sufficient power to examine this extremely small subgroup.
Previous analyses of JBR.10 and CALGB-9633 suggested that the presence of KRAS mutations may be associated with resistance to platinum-based adjuvant chemotherapy 11,12. We undertook exploratory analyses, therefore, to determine whether there might be interaction of KRAS mutations and tumor size on treatment effect in this population. We identified KRAS mutations in the tumors of 27% of evaluable patients, a rate higher than that observed in a meta-analysis of literature conducted by Mascaux et al. 18. Consistent with previously reported studies, there was a suggestion, albeit not significant, that patients with KRAS wild-type tumors may benefit from adjuvant chemotherapy, while those with KRAS mutant tumors did not appear to benefit from treatment. However, this relatively small study could not identify any particular subgroups of patients who did or did not derive significant benefit from adjuvant chemotherapy based on T-size and KRAS mutation and so this cannot be recommended as a means of selecting patients for receipt of adjuvant chemotherapy.
Tumor size was significantly prognostic for DFS, and borderline for OS in this study. The power of our study was limited, however, with the results highly dependent on a small group of T3 patients. As such, we could only validate the 7th edition T-size descriptors partially in this node negative population. Indeed, the prognostic significance of the T size descriptors, proposed by the IASLC staging committee, was based on analyses of more than 7,000 NSCLC patients who underwent complete surgical resection without prior induction therapy 8. A recent single centre review of 1,805 cases of resected NSCLC also confirmed the prognostic significance of the 2, 3 and 7 cm cut-points in the 7th edition, although the 5 cm cut-point could not be validated 19. Similarly, a separate single institution review of 1,393 NSCLC patients independently confirmed the prognostic significance of all the new T-size descriptors in the total study population, although the prognostic significance of the 5 cm cut-point was lost when analyses were confined to the node negative subgroup 20. Interestingly, a recent study has suggested that microscopic vascular invasion is a stronger prognostic indicator than T-size in T1a-T2b categories 21.
A significant interaction between the prognostic value of KRAS mutations and tumor size was observed for OS, but not DFS in this study. This was an unexpected finding given the larger number of events included in the disease-free survival analyses. However, the presence of KRAS mutations significantly increased the risk of death only in patients with T2b-T3 tumors. Previous studies evaluating the prognostic value of KRAS mutations in NSCLC have shown mixed results. The meta-analysis, conducted by Mascaux et al, identified the presence of RAS mutations as a negative prognostic factor in NSCLC (HR=1.40, 95% CI 1.18-1.65) 18. However, there was no significant prognostic effect for RAS mutation status in a previous retrospective analysis of stage Ib-II (by the 6th edition) NSCLC patients in JBR.10 11, or for KRAS mutation status in a pooled analysis of 1751 NSCLC patients included in the LACE-bio analysis 22. Furthermore, in a randomised trial comparing postoperative radiation therapy to radiation therapy and chemotherapy in stage II-IIA NSCLC, the presence of KRAS mutations was not independently prognostic on multivariable analysis 23.
The T≤3 cm subgroup represented a potential source of confounding in this study as it included patients who were classified as T2 by virtue of T2-defining characteristics other than tumor size. Meaningful conclusions can, therefore, be drawn only from the analyses which excluded this subgroup. These T2-defining descriptors include: involvement of the bronchus ≥2 cm distal to the carina; the presence of visceral pleural invasion; and atelectasis or pneumonitis extending to the hilar region but not involving the entire lung. Several studies have suggested that the presence of one or more of these T-descriptors confers poor prognosis in NSCLC 24-28, although the recent IASLC staging project was unable to address this issue due to insufficient clinical data 8. Nonetheless, if is accepted that these T-descriptors are prognostic for poor outcome, one can postulate that they may potentially also predict independently for adjuvant chemotherapy effect. Unfortunately, data regarding the co-existence of T-descriptors other than size were incomplete for the T2a-T3 subgroups, thereby, precluding analysis of their effect in multivariable analyses.
Prospective data from large adjuvant chemotherapy trials are necessary before clinical guidelines regarding management of surgically resected node negative NSCLC can be updated to reflect the changes introduced by the 7th edition staging system. The results of ongoing studies that prospectively are recording all T-descriptor data hopefully will provide valuable information in this regard. However, until these results become available, this retrospective exploratory analysis supports treatment of pT2bN0 and pT3N0 NSCLC patients with adjuvant platinum-based chemotherapy as per existing guidelines for stage II patients. While chemotherapy should not be recommended in node negative patients with tumor size <4 cm based on previous reports, optimal treatment of patients with tumor size 4-5 cm remains unclear. Despite the trends observed in this relatively small study, at this time, KRAS mutational status cannot be recommended as a means of identifying node negative NSCLC patients who may or may not benefit from adjuvant chemotherapy. Finally, it should be remembered that evolving technologies such as gene prognostic signatures have shown promise in predicting for adjuvant chemotherapy benefit and may aid clinical decision making in the future 29.
Supplementary Material
Acknowledgments
Source of Funding
Supported by the French Ligue Nationale Contre le Cancer and an unrestricted grant from Sanofi.
The JBR.10 trial was supported by the Canadian Cancer Society and the National Cancer Institute of the United States.
The research for CALGB 9633 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Monica M. Bertagnolli M.D., Chair) and to the CALGB Statistical Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute.
Footnotes
Conflicts of Interest
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NSCLC Meta-analyses Collaborative Group Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–77. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 5.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 6.Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol. 2010;28:29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B. J Clin Oncol. 2008;26:5043–51. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 9.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 10.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 11.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5240–7. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 12.Capelletti M, Wang XF, Gu L, et al. Impact of KRAS mutations on adjuvant carboplatin/paclitaxel in surgically resected stage IB NSCLC: CALGB 9633. J Clin Oncol. 2010;28(suppl) abstr 7008. [Google Scholar]
- 13.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 14.Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 15.De Pas T, de Braud F, Veronesi G. Adjuvant chemotherapy in new stage II pN0 non-small cell lung cancer: a new issue for a case-by-case decision making process. J Thorac Oncol. 2010;5:754. doi: 10.1097/JTO.0b013e3181d6bc50. [DOI] [PubMed] [Google Scholar]
- 16.Boffa DJ, Detterbeck FC, Smith EJ, et al. Should the 7th edition of the lung cancer stage classification system change treatment algorithms in non-small cell lung cancer? J Thorac Oncol. 2010;5:1779–83. doi: 10.1097/JTO.0b013e3181ee80c7. [DOI] [PubMed] [Google Scholar]
- 17.Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–8. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]
- 18.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–9. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruffini E, Filosso PL, Bruna MC, et al. Recommended changes for T and N descriptors proposed by the International Association for the Study of Lung Cancer - Lung Cancer Staging Project: a validation study from a single-centre experience. Eur J Cardiothorac Surg. 2009;36:1037–44. doi: 10.1016/j.ejcts.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 20.Yano T, Morodomi Y, Ito K, et al. Verification of the newly proposed T category (seventh edition of the tumor, node, and metastasis classification) from a clinicopathological viewpoint in non-small cell lung cancer-special reference to tumor size. J Thorac Oncol. 2010;5:45–8. doi: 10.1097/JTO.0b013e3181c0996c. [DOI] [PubMed] [Google Scholar]
- 21.Ruffini E, Asioli S, Filosso PL, et al. Significance of the Presence of Microscopic Vascular Invasion After Complete Resection of Stage I-II pT1-T2N0 Non-small Cell Lung Cancer and Its Relation with T-Size Categories: Did the 2009 7th Edition of the TNM Staging System Miss Something? J Thorac Oncol. 2011;6(2):319–26. doi: 10.1097/JTO.0b013e3182011f70. [DOI] [PubMed] [Google Scholar]
- 22.Tsao MS, Hainaut P, Bourredjem A, et al. LACE-Bio Pooled Analysis of the Prognostic and Predictive Value of KRAS Mutation in Completely Resected Non-Small Cell Lung Cancer (NSCLC) Ann Oncol. 2010;21(suppl 8):viii63. [Google Scholar]
- 23.Schiller JH, Adak S, Feins RH, et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: a Laboratory Ancillary Study on an Eastern Cooperative Oncology Group Prospective Randomized Trial of Postoperative Adjuvant Therapy. J Clin Oncol. 2001;19:448–57. doi: 10.1200/JCO.2001.19.2.448. [DOI] [PubMed] [Google Scholar]
- 24.Manac’h D, Riquet M, Medioni J, et al. Visceral pleura invasion by non-small cell lung cancer: an underrated bad prognostic factor. Ann Thorac Surg. 2001;71:1088–93. doi: 10.1016/s0003-4975(00)02649-7. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu K, Yoshida J, Nagai K, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:160–5. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Hsu CP, Hsia JY, Chang GC, et al. Surgical-pathologic factors affect long-term outcomes in stage IB (pT2 N0 M0) non-small cell lung cancer: a heterogeneous disease. J Thorac Cardiovasc Surg. 2009;138:426–33. doi: 10.1016/j.jtcvs.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Osaki T, Nagashima A, Yoshimatsu T, et al. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg. 2004;77:1769–73. doi: 10.1016/j.athoracsur.2003.10.058. discussion 1773. [DOI] [PubMed] [Google Scholar]
- 28.Mountain CF, Carr DT, Anderson WA. A system for the clinical staging of lung cancer. Am J Roentgenol Radium Ther Nucl Med. 1974;120:130–8. doi: 10.2214/ajr.120.1.130. [DOI] [PubMed] [Google Scholar]
- 29.Zhu CQ, Ding K, Strumpf D, et al. Prognostic and predictive gene signature for adjuvant chemotherapy in resected non-small-cell lung cancer. J Clin Oncol. 2010;28:4417–24. doi: 10.1200/JCO.2009.26.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.