Abstract
Social difficulties during adolescence influence life-span health. To elucidate underlying mechanisms, we examined whether a noxious social event, targeted rejection (TR), influences the signaling pathways that regulate inflammation, which is implicated in a number of health problems. For this study, 147 adolescent women at risk for developing a first episode of major depression were interviewed every 6 months for 2.5 years to assess recent TR exposure, and blood was drawn to quantify leukocyte messenger RNA (mRNA) for nuclear factor-κB (NF-κB) and inhibitor of κB (I-κB) and the inflammatory biomarkers C-reactive protein and interleukin-6. Participants had more NF-κB and I-κB mRNA at visits when TR had occurred. These shifts in inflammatory signaling were most pronounced for adolescents high in perceived social status. These findings demonstrate that social rejection upregulates inflammatory gene expression in youth at risk for depression, particularly for those high in status. If sustained, this heightened inflammatory signaling could have implications for life-span health.
Keywords: health, life experiences, psychological stress, genetics, interpersonal relationships
Emerging research has shown that lifelong patterns of health are influenced by experiences during childhood and adolescence (Collins, Maccoby, Steinberg, Hetherington, & Bornstein, 2000; Dahl, 2004; Patton & Viner, 2007; Sawyer et al., 2012; Smetana, Campione-Barr, & Metzger, 2006; Steinberg & Morris, 2001). Adolescence is when recurrent and sometimes chronic psychiatric disorders like major depression first manifest (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Cyranowski, Frank, Young, & Shear, 2000; Paus, Keshavan, & Giedd, 2008) and also when physical conditions such as obesity, diabetes, and other cardiovascular risk factors become more prevalent (Berenson et al., 1998; Berenson & Srnivasan, 2005; Gluckman, Hanson, Cooper, & Thornburg, 2008; Shonkoff, Boyce, & McEwen, 2009). The presence of these conditions suggests the initiation of preclinical disease processes, such as persistent mild inflammation, which over time foster chronic diseases of aging that are associated with substantial morbidity and mortality (Chung et al., 2009).
Although the health of all individuals is influenced by events occurring during the teenage years, adolescents who have a depressed parent and those who exhibit persistently negative styles of thinking are especially vulnerable for developing mental and physical health conditions that tend to emerge early and continue over the life course. Adolescents with a depressed parent, for example, are 3 to 5 times more likely to experience depression than adolescents without a depressed parent (Foland-Ross, Hardin, & Gotlib, 2012; S. H. Goodman, 2007). In addition, these individuals are more susceptible to a wide range of somatic complaints and physical health problems, including asthma, bronchitis, obesity, pain, and several dermatological disorders (National Research Council and Institute of Medicine, 2009). These health problems lead to high rates of health care utilization and are associated with enormous personal and economic costs resulting from psychosocial impairment, chronic disease management, emergency room visits, reduced productivity, and suicide (Greenberg et al., 2003).
Adolescence is a developmentally sensitive period for lifelong health in part because it is a time of great biological and social flux. Specifically, neurohormonal systems that play an important role in orchestrating the body’s response to stress are highly plastic during puberty, making them malleable to a variety of social-environmental influences (Charmandari, Kino, Souvatzoglou, & Chrousos, 2003; Romeo et al., 2006). In addition, adolescence is a time when individuals begin assigning greater value to their status within peer social networks and reduce their involvement with family (Larson & Richards, 1991; Spear, 2000). With this combination of malleable physiology, focus on status within the peer group, and withdrawal from the family, adolescents may be especially vulnerable to the negative consequences of social stressors.
Although many kinds of stressors can alter biological and behavioral responding in a way that enhances disease risk (Cohen, Janicki-Deverts, & Miller, 2007; Miller, Chen, & Cole, 2009), stressors that threaten a person’s social standing appear to be particularly impactful (Kemeny, 2009). The most noxious experience of this type is targeted rejection, which involves the active and intentional rejection of an individual by another person or group (Slavich, Thornton, Torres, Monroe, & Gotlib, 2009). Targeted rejection precipitates depression 3 times faster than do other comparably severe life events. Furthermore, it has been proposed that inflammatory processes may mediate this effect and increase risk for the development of an array of mental and physical health problems that involve excessive innate immune activation (Raison, Capuron, & Miller, 2006; Slavich, O’Donovan, Epel, & Kemeny, 2010; Slavich, Way, Eisenberger, & Taylor, 2010). To our knowledge, however, no studies to date have directly investigated whether rejection-related life events affect inflammatory activity or the molecular signaling pathways that regulate these dynamics.
Laboratory-based stressors that involve social rejection have been found to activate systems that regulate inflammatory activity, including the hypothalamic-pituitary-adrenal axis and sympathetic nervous system (Dickerson, Gable, Irwin, Aziz, & Kemeny, 2009; Dickerson & Kemeny, 2004; Gruenewald, Kemeny, Aziz, & Fahey, 2004; Irwin & Cole, 2011; Mendes, Major, McCoy, & Blascovich, 2008). This work suggests a biologically plausible scenario whereby rejection-related life events could initiate a neurohormonal cascade that modulates the activity of pro- and anti-inflammatory signaling pathways. Inflammation is orchestrated by innate immune cells, particularly monocytes and macrophages and dendritic cells, and is regulated at the level of gene expression. Nuclear factor-κB (NF-κB) is a transcription factor that regulates the expression of genes encoding various proteins that drive inflammation. Normally, inactive NF-κB is sequestered in the cytoplasm of cells by a family of proteins called inhibitor of κB (I-κB). However, signals emanating from sites of tissue damage cause I-κB to break down, freeing NF-κB to translocate into the nucleus and activate genes that drive pro-inflammatory signaling (Webster, Tonelli, & Sternberg, 2002). Stress hormones evoked by targeted rejection, like glucocorticoids and catecholamines, modulate the activity of the genes encoding NF-κB and I-κB (Bierhaus et al., 2003; Irwin & Cole, 2011; Wolf, Rohleder, Bierhaus, Nawroth, & Kirschbaum, 2009). Although inflammation is adaptive in the context of injury and infection, it must be closely regulated because prolonged, excessive cytokine responses are involved in the pathogenesis of a variety of mental illnesses (e.g., depression, dementia) and physical disease states (e.g., diabetes, cardiovascular disease, and some cancers; Chung et al., 2009).
Evidence for the possibility that targeted rejection affects inflammatory processes is provided by several related lines of research on immunological responses to social stress. For example, laboratory-based studies of social evaluation and rejection have shown that giving a speech in front of a panel of nonresponsive, socially rejecting evaluators triggers an increase in pro-inflammatory cytokine activity (Dickerson et al., 2009; Slavich, Way, et al., 2010). Similar increases in inflammation have been found in laboratory studies of spouses who are asked to engage in a conflictual conversation (Kiecolt-Glaser, Gouin, & Hantsoo, 2010; Kiecolt-Glaser et al., 2005). In addition, naturalistic studies have shown that experiencing social difficulties with friends and family members is associated with elevated pro-inflammatory cytokine levels and activity (Fuligni et al., 2009; Miller, Rohleder, & Cole, 2009). Finally, a large body of work has shown that loneliness and social isolation, both of which are possible outcomes of social rejection, are associated with the activation of inflammatory pathways (Cacioppo & Hawkley, 2003; Cacioppo, Hawkey, & Berntson, 2003; Cacioppo et al., 2002; Cole et al., 2007). Taken together, these studies provide strong evidence that experiences involving social rejection and exclusion upregulate inflammatory activity.
Despite these findings, several important questions remain unanswered. First, what are the inflammatory consequences of experiencing a real-life targeted rejection event? Laboratory studies of acute social conflict and naturalistic studies of chronic social difficulties show that these stressors can provoke inflammatory activity. However, it remains unclear whether targeted rejection, perhaps the most noxious social stressor of all, can also provoke changes in inflammation. If so, this could provide clues about the mechanism by which targeted rejection increases risk for depression and how it might affect other mental and physical health disorders. Second, much of the research on social stress and inflammation has focused on adults who differ cross-sectionally in terms of their exposure to, or experience of, social stress (e.g., socially isolated vs. socially integrated adults). As such, it remains unclear whether social rejection alters inflammatory activity in a developmentally sensitive period like adolescence and, if so, how these dynamics play out prospectively. Finally, are there individual differences that moderate adolescents’ inflammatory responses to targeted rejection? Previous work has largely focused on the effects of social rejection, irrespective of possible moderating factors. However, responses to social rejection may differ as a function of where people believe they stand in their peer social hierarchies. Indeed, a recent study found that individuals who reported being above the sample median in social status exhibited more pronounced cortisol responses to a social-evaluative stressor than did their counterparts who were below the sample median in status (Gruenewald, Kemeny, & Aziz, 2006). As such, it seems likely that the effects of social rejection depend on contextual factors, specifically where the targets of rejection experiences perceive they reside within their social hierarchy. Two scenarios are plausible here. On the one hand, individuals who sit atop their social hierarchies could show the most pronounced boosts in inflammatory signaling following targeted rejection, by virtue of having relatively more status to lose in such an encounter and having less experience managing salient threats to their public stature. Alternatively, the most pronounced increases in inflammation could occur in relatively low-status individuals, who have fewer peer resources to draw on for support and friendship during social rejection.
To address these questions, we conducted a six-wave prospective study over 2.5 years of 147 healthy adolescent women. These participants had no personal history of any major psychiatric disorder. However, they were at elevated risk for experiencing a first episode of major depression by virtue of either (a) having a depressed parent or (b) exhibiting cognitive styles that confer increased risk for depression. Based on the research summarized earlier showing that targeted rejection-related stressors upregulate inflammatory activity, we hypothesized that targeted rejection would provoke increased expression of NF-κB and a compensatory increase in expression of I-κB. We expected these shifts to result in higher levels of the systemic inflammatory markers C-reactive protein (CRP) and interleukin-6 (IL-6). In addition to these main effects, as discussed earlier, we hypothesized that the effects of targeted rejection on inflammatory responding would be moderated by differences in perceived social status, as indexed by where a participant believed she stood within the social hierarchy of her peer group at school.
Method
Participants
Data were collected as part of a longitudinal study of adolescent women at risk for developing a first onset of depression (see other reports from this data set, e.g., Miller & Cole, in press; Murphy, Miller, & Wrosch, 2012; Ross, Martin, Chen, & Miller, 2011). Participants were recruited from the Vancouver, British Columbia, community through advertisements in schools, newspapers, and magazines. Eligibility criteria included being (a) between 15 and 19 years old; (b) fluent in English; (c) free of acute and chronic medical conditions; (d) without a current or lifetime history of any major psychiatric disorder; and (e) at risk for developing a first episode of major depression, where high risk was defined as having a first-degree relative with a history of major depression or having an elevated score on either the Dysfunctional Attitudes Scale (Weissman & Beck, 1978) or the Adolescent Cognitive Style Questionnaire (Hankin & Abramson, 2002). A total of 147 high-risk participants were enrolled in the study between 2004 and 2007 (see Table 1). Written consent was obtained from all participants. A parent also provided consent for each participant under age 18. The Research Ethics Board of the University of British Columbia approved this project.
Table 1.
Descriptive Characteristics of the Sample (N = 147)
| Variable | M | SD |
|---|---|---|
| Baseline age (years) | 17.01 | 1.33 |
| Alcohol use (number of drinks per week) | 2.65 | 5.44 |
| Waist-to-hip ratio | 0.75 | 0.05 |
| Oral contraceptive use | n = 66 | |
| Perceived social status at school (range 1–10) | 6.47 | 1.03 |
| Depressive symptoms (range 0–63) | 6.64 | 5.65 |
| Severe life events | n = 165 | |
| Targeted rejection life events | n = 20 | |
| Lymphocytes (× 109 cells per L) | 1.82 | 0.51 |
| Monocytes (× 109 cells per L) | 0.41 | 0.13 |
| Neutrophils (× 109 cells per L) | 3.50 | 1.24 |
| CRP (mg/L) | 0.84 | 1.33 |
| IL-6 (pg/mL) | 0.78 | 0.84 |
| NF-κB (relative expression) | 9.09 | 1.62 |
| I-κB (relative expression) | 7.96 | 1.99 |
| NF-κB to I-κB ratio | 1.17 | 0.18 |
Note: Except for baseline age, severe life events, and targeted rejection life events, all descriptive statistics are based on data averaged across all visits of the study. CRP = C-reactive protein; IL-6 = interleukin-6; NF-κB = nuclear factor κB; I-κB = inhibitor of κB.
Procedure
Participants were assessed every 6 months for 2.5 years. At each visit, they completed questionnaires and were administered the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 2002) to determine psychiatric diagnoses and another interview to evaluate their recent stress exposure (see the next section). Participants also had a variety of physical attributes assessed, and each provided a blood sample through antecubital venipuncture. Participants fasted overnight prior to each visit, and blood was drawn between 8:00 a.m. and 11:00 a.m. to control for diurnal variations in immune function. Overall, 134 participants (91%) completed at least three visits, and the majority of participants (n = 94, 64%) completed all six visits. Analyses were based on all available data.
Life stress and targeted rejection
Stressful life events were assessed using the Life Stress Interview (Hammen, 1991), a semistructured interview designed to identify acute life events and chronic stressors occurring over the past 6 months. Interviewers determined the nature of each life event and its contextual features. This information was subsequently presented to an independent panel of raters who, following a discussion regarding the person’s biographical circumstances and each event’s contextual features, made consensual ratings regarding each event’s long-term threat. Raters were kept blind to participants’ emotional responses to the events to prevent this information from biasing the ratings. Ratings of all life events were made in half-point increments on a scale from 1 (no negative impact) to 5 (severe impact).
Following criteria outlined by Slavich et al. (2009), events that received a severity score of 2.5 or higher were subsequently coded for targeted rejection. An event was judged to be targeted rejection if it (a) happened primarily to the participant, (b) involved the rejection of the participant by another person or group, (c) involved a clear intent to actively reject the participant, (d) directly affected the participant, and (e) resulted in the severing of a relational tie between the participant and the other person or group. Coding was done by M.L.M.M. and G.E.M., and transcripts of a subset of rated events were evaluated by G.M.S., who coded the events blind to the original ratings. The team showed excellent interrater agreement (n = 21 team coded events; intraclass correlation coefficient [ICC] = 0.92).
Perceived social status
Participants’ perceived social status was assessed at each visit using the MacArthur Scale of Subjective Social Status Youth Version (E. Goodman et al., 2001). This scale measures where adolescents feel they fit into their local peer group hierarchy. Participants were shown a picture of a ladder with 10 rungs and told that lower rungs corresponded to people in their school who were not respected, did not have friends, and received poor grades and that higher rungs corresponded to people who were respected, had friends, and did well academically. They were then instructed to select where they perceived themselves to be on the ladder. Scores on this measure were stable over the study (n = 703; ICC = 0.53). Thus, for analyses, we formed individual difference scores for each participant by averaging ratings over the six assessments.
Pro- and anti-inflammatory signaling
The expression of inflammatory signaling molecules was quantified through real-time reverse transcriptase polymerase chain reaction (RT-PCR). We assessed the NF-κB p105 subunit as a marker of pro-inflammatory signaling and I-κB as a marker of anti-inflammatory signaling. Peripheral blood was collected into PAXgene Blood RNA tubes. Total RNA was later extracted using PAXgene Blood RNA kits (Pre-Analytix, Hombrechtikon, Switzerland). RT-PCRs were carried out on an Applied Biosystems (ABI) Sequence Detection System using commercially available assays from ABI, as described in an earlier article (Rohleder, Marin, Ma, & Miller, 2009). Results were expressed as relative quantities of each target messenger RNA (mRNA) after correction for expression of the housekeeping gene β-actin. Higher values indicate greater expression of the respective mRNA. We also calculated a ratio of pro- to anti-inflammatory signaling by dividing each participant’s NF-κB mRNA value by her I-κB mRNA value. Higher values on this variable indicate more pro-inflammatory signaling.
Indicators of low-grade inflammation
CRP and IL-6 were quantified in serum as indicators of ongoing inflammation. For this, blood was collected in Serum-Separator Tubes (Becton-Dickinson, Franklin Lanes, NJ), centrifuged at 1,000 × g for 25 min, and the serum was aspirated and frozen at −30°C until analysis. CRP was assessed by high-sensitivity chemiluminescence on an IMMULITE 2000 (Diagnostic Products Corporation, Los Angeles, CA). This assay has a detection threshold of 0.20 mg/L and an interassay coefficient of variation of 2.2%. IL-6 was measured in duplicate using commercially available high-sensitivity enzyme-linked immunosorbent assay kits (HS600B; R&D Systems, Minneapolis, MN), which have a minimum detection threshold of 0.039 pg/ml and inter- and intraassay variability of less than 10%.
Alternative explanations
To examine alternative explanations for any observed associations, we collected demographic data on each participant’s age at baseline and her ethnicity (coded as Caucasian or other). In addition, given that having more central adiposity and using oral contraceptives are both associated with greater inflammatory activity (Pirkola et al., 2010), we assessed each participant’s waist-to-hip ratio and whether she was taking oral contraceptives at any point during the study. Alcohol consumption can be either pro- or anti-inflammatory, depending on amount used (Imhof et al., 2001). As such, we evaluated each participant’s typical weekly alcohol consumption. Finally, to examine the possibility of confounding by stress-related changes in the composition of the circulating leukocyte pool, we conducted a complete blood count with differential at each visit, using standard methods (ADVIA 70 Hematology System, GMI Inc., Holiston, MA).
Statistical analyses
We tested hypotheses by using the software HLM 6.08 (Raudenbush, Bryk, & Congdon, 2004) to evaluate a series of multilevel models. In all models, for the Level 1 equations, the outcome variable of interest was predicted by within-person changes in whether targeted rejection had occurred at each visit. Time and waist-to-hip ratio were also included. The variable for targeted rejection was person centered, and the variable for waist-to-hip ratio was grand mean centered. For the Level 2 equations, the Level 1 slopes and intercept were predicted by individual differences in perceived social status, as well as age, ethnicity, oral contraceptive use, and alcohol use. The variables for perceived social status, age, and alcohol use were grand mean centered. The key coefficient for these analyses was the cross-level interaction between perceived social status and targeted rejection. A significant interaction indicated that the nature of the association between targeted rejection and the outcome variable of interest depended on the participant’s perceived social status.
Results
Targeted rejection and pro- and anti-inflammatory signaling
We used multilevel modeling to test whether targeted rejection is associated with altered expression of NF-κB and I-κB. We found significant within-person associations between targeted rejection and these signaling molecules. As predicted, at visits when participants experienced a recent targeted rejection life event, they exhibited higher quantities of mRNA for both NF-κB (b = 1.24, SE = 0.47, p = .009) and I-κB (b = 1.71, SE = 0.59, p = .005) and a lower ratio of NF-κB to I-κB (b = −0.11, SE = 0.05, p = .04) compared with visits when they had not experienced a recent targeted rejection event. These associations were independent of waist-to-hip ratio, age, ethnicity, oral contraceptive use, and alcohol consumption.
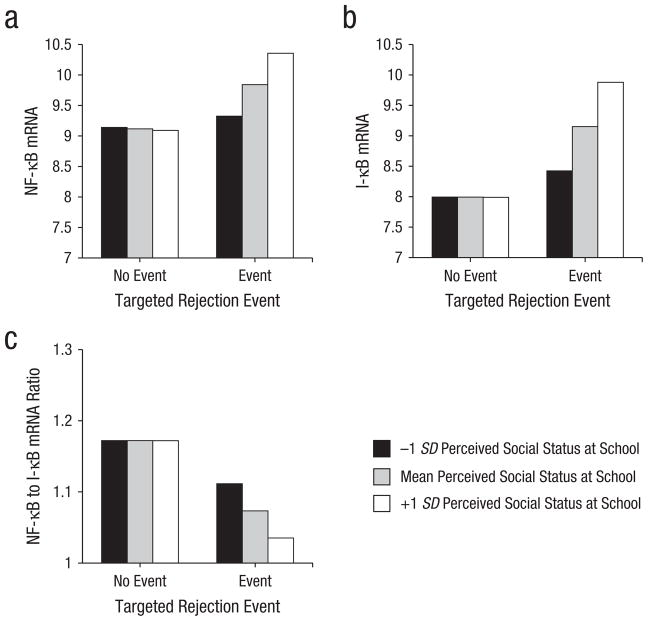
As expected, individual differences in perceived social status moderated the association of targeted rejection with NF-κB (b = 0.52, SE = 0.18, p = .004), I-κB (b = 0.71, SE = 0.59, p = .001), and the ratio of NF-κB to I-κB (b = −0.04, SE = 0.02, p = .046) and did so in a manner consistent with a scenario wherein targeted rejection has more pronounced effects on inflammatory signaling in higher status individuals. Specifically, following a recent targeted rejection life event, higher status participants expressed 11% more NF-κB mRNA and 17% more I-κB mRNA than their lower status peers (see Fig. 1). However, following targeted rejection, higher status participants also had a 7% smaller ratio of NF-κB to I-κB than their lower status counterparts, suggesting they mounted a more effective compensatory response. As Table 2 shows, these associations were independent of waist-to-hip ratio, age, ethnicity, oral contraceptive use, and alcohol consumption.
Fig. 1.
Differences in messenger RNA (mRNA) expression for (a) nuclear factor-κB (NF-κB), (b) inhibitor of κB (I-κB), and (c) the ratio of NF-κB to I-κB, following a recent targeted rejection life event for different levels of perceived social status. Participants had more mRNA for NF-κB (p = .009) and I-κB (p = .005) and a lower ratio of NF-κB to I-κB (p = .036) at visits when a recent targeted rejection life event had occurred compared with visits when targeted rejection had not occurred. These effects were moderated by perceived social status (ps = .001–.046), with high-status adolescents showing the greatest increases in inflammatory gene expression following targeted rejection (N = 147).
Table 2.
Level 2 Hierarchical Linear Modeling Coefficients for the Cross-Level Interactions Between Perceived Social Status and Targeted Rejection in Predicting the Inflammatory Molecules NF-κB, I-κB, NF-κB to I-κB ratio, CRP, and IL-6 (N = 147)
| Inflammatory marker | Unstandardized coefficient (b) | Standard error | p value |
|---|---|---|---|
| NF-κB | |||
| Intercept | 1.239 | 0.466 | .009 |
| Age | −0.403 | 0.215 | .063 |
| Ethnicity | −0.829 | 0.584 | .158 |
| Oral contraceptive use | −0.238 | 0.473 | .615 |
| Alcohol use | −0.195 | 0.059 | .002 |
| Perceived social status | 0.524 | 0.179 | .004 |
| I-κB | |||
| Intercept | 1.714 | 0.593 | .005 |
| Age | −0.176 | 0.277 | .527 |
| Ethnicity | −0.926 | 0.753 | .221 |
| Oral contraceptive use | −0.234 | 0.544 | .668 |
| Alcohol use | −0.175 | 0.089 | .050 |
| Perceived social status | 0.707 | 0.191 | .001 |
| NF-κB to I-κB ratio | |||
| Intercept | −0.107 | 0.051 | .036 |
| Age | −0.031 | 0.022 | .160 |
| Ethnicity | 0.019 | 0.067 | .771 |
| Oral contraceptive use | −0.001 | 0.054 | .990 |
| Alcohol use | 0.000 | 0.007 | .951 |
| Perceived social status | −0.037 | 0.018 | .046 |
| CRP | |||
| Intercept | 0.056 | 0.213 | .792 |
| Age | 0.199 | 0.094 | .036 |
| Ethnicity | −0.194 | 0.246 | .431 |
| Oral contraceptive use | −0.494 | 0.273 | .072 |
| Alcohol use | 0.123 | 0.054 | .024 |
| Perceived social status | 0.083 | 0.126 | .508 |
| IL-6 | |||
| Intercept | −0.098 | 0.126 | .439 |
| Age | −0.039 | 0.068 | .561 |
| Ethnicity | −0.388 | 0.279 | .166 |
| Oral contraceptive use | 0.434 | 0.270 | .110 |
| Alcohol use | −0.050 | 0.030 | .095 |
| Perceived social status | −0.019 | 0.060 | .756 |
Note: The intercept reflects the direct effect of targeted rejection on the given outcome holding the other variables constant. The other coefficients reflect the cross-level interaction between the indicated variable and targeted rejection in predicting the given outcome holding the other variables constant. NF-κB = nuclear factor κB; I-κB = inhibitor of κB; CRP = C-reactive protein; IL-6 = interleukin-6.
Targeted rejection and systemic inflammation
Next, we tested whether the effects of targeted rejection extended to the circulating biomarkers of inflammation, CRP and IL-6. Exposure to targeted rejection was unrelated to levels of either CRP (b = 0.06, SE = 0.21, p = .79) or IL-6 (b = −0.10, SE = 0.13, p = .44). Furthermore, there was no evidence that perceived social status moderated the effects of targeted rejection on CRP (b = 0.08, SE = 0.13, p = .51) or IL-6 (b = −0.02, SE = 0.06, p = .76; see Table 2).
Depression as a mediator of targeted rejection–related inflammatory signaling
Targeted rejection is known to precipitate depression, and depression, in turn, is strongly associated with inflammation (Raison et al., 2006; Slavich et al., 2009). Thus, we examined whether depressive symptoms mediated the effects of targeted rejection on NF-κB and I-κB mRNA expression among high-status adolescents. We tested this hypothesis by adding participants’ depression scores from each visit to the multilevel models as a time-varying covariate. On a within-person basis, depressive symptoms were positively related to NF-κB (b = 0.04, SE = 0.02, p = .02) and I-κB (b = 0.06, SE = 0.02, p = .004) and negatively related to the ratio of NF-κB to I-κB (b = −0.003, SE = 0.001, p = .02). Adjusting for depressive symptoms, however, did not influence the significance of the interactions between social status and targeted rejection for any of the three signaling outcomes (all ps remained < .02). Thus, it does not appear that targeted rejection influences inflammatory signaling in high-status participants via depressive symptoms.
Effects of circulating leukocyte distribution
Stress alters the composition of the circulating leukocyte pool, increasing the proportion of granulocytes and monocytes. Because gene expression profiles vary across cell populations (Irwin & Cole, 2011), we examined whether variations in leukocyte distribution might explain the observed associations. Statistical models were recomputed after incorporating time-varying covariates reflecting counts of neutrophils, lymphocytes, and monocytes (in separate equations). In these models, all of the reported cross-level interactions for NF-κB and I-κB remained significant (ps < .02). However, controlling for cell counts attenuated the magnitude of the cross-level interactions for the ratio of NF-κB to I-κB, in some cases to marginal significance (ps < .01–.09). These results suggest that little of the interaction between social status and targeted rejection in predicting inflammatory signaling is due to stress-induced changes in leukocyte distribution.
Discussion
These data demonstrate that exposure to a recent targeted rejection life event activates the molecular signaling pathways that regulate inflammation. Specifically, in a six-wave prospective study of adolescent women at risk for developing a first episode of major depression who were interviewed every 6 months for 2.5 years, we found that participants had more mRNA for both NF-κB and I-κB, and a lower ratio of NF-κB to I-κB, following visits when a recent targeted rejection life event had occurred compared with visits when no targeted rejection life event had occurred. Because social status is increasingly salient during adolescence, we subsequently examined how the effects of targeted rejection on inflammatory signaling differed as a function of adolescents’ perceived social standing. Compared to low-status adolescents, high-status adolescents displayed greater increases in mRNA for NF-κB and I-κB, and a lower ratio of NF-κB to I-κB, following recent targeted rejection. Considered together, these data are the first to show that targeted rejection upregulates inflammatory gene expression and that these effects are moderated by adolescents’ perceptions of their social standing in their peer group.
Why might inflammatory responses to targeted rejection be especially pronounced for adolescents who exhibit higher perceived social status? One possibility involves the potential adaptive value that such a response would have conferred for individuals atop their social hierarchy in the ancestral context. In both animals and humans, abrasive social encounters are potent activators of pro-inflammatory signaling, especially when such interactions involve conflict (Kiecolt-Glaser et al., 2005; Stark et al., 2001). The upregulation of inflammatory processes under these circumstances may represent a proactive response that serves to accelerate wound healing and pathogen clearance following physical injury, which is more likely to occur during conflictual encounters. Although such responses can enhance fitness regardless of one’s status, a pronounced anticipatory response may be especially adaptive for high-status organisms to the extent that they are more frequently the targets of attacks by conspecifics attempting to displace them in the social hierarchy. Consistent with this formulation, animals at the top of primate social hierarchies are subject to more frequent aggressive actions than their lower status peers, and they also display the largest glucocorticoid responses to these challenges (Gesquiere et al., 2011; Sapolsky, 2011). Similarly, controlled laboratory studies with humans have shown that high-status individuals exhibit greater adrenocortical responses to social threat than do low-status individuals (Gruenewald et al., 2006).
Although increased pro-inflammatory signaling is adaptive during social conflict, this response must be closely regulated to minimize the potential for collateral tissue damage that results from sustained inflammation. We see evidence of these counterregulatory dynamics in that high-status individuals exhibited increases in I-κB mRNA following targeted rejection. NF-κB and I-κB are frequently coexpressed in the genome of leukocytes, and this ensures an appropriate counterbalancing of pro- and anti-inflammatory signaling (Baldwin, 1996). When viewed from this perspective, high-status adolescents can be regarded as having mounted a balanced transcriptional response to targeted rejection wherein a boost in pro-inflammatory levels of NF-κB was countered by a compensatory increase in anti-inflammatory I-κB. The present data are also consistent with this counterregulatory interpretation in the sense that targeted rejection–related increases in NF-κB did not manifest into heightened levels of CRP and IL-6 in circulation. Even among high-status individuals, therefore, rejection-related increases in pro-inflammatory signaling are unlikely to have immediate consequences for health. These regulatory processes are very sensitive, though, and exposure to additional social adversity coupled with aging-related breakdowns in these counterregulatory mechanisms could lead to an increasingly pro-inflammatory phenotype that enhances an individual’s susceptibility to physical illnesses with an inflammatory component, including diabetes, cardiovascular disease, and some cancers (Chung et al., 2009). Furthermore, excessive inflammation is increasingly recognized as a contributor to affective disorders (Raison et al., 2006), raising the possibility that the patterns of upregulated inflammatory signaling seen here may contribute to the pathogenesis of depressive episodes that often follow targeted rejection (Slavich, O’Donovan, et al., 2010; Slavich et al., 2009).
Compared with their high-status peers, low-status adolescents in the present study exhibited relatively small changes in NF-κB and I-κB mRNA following targeted rejection. At least three explanations are possible. First, to the extent that low-status individuals experience rejection on a more frequent basis than high-status individuals do, they may be relatively more accustomed to such experiences and may have developed strategies for minimizing the impact that rejection has on them. Second, individuals who are lower in the social hierarchy may have less status to lose than do high-status individuals and, as such, may view rejection experiences as being relatively less threatening to their social standing or well-being. Finally, it is possible that low-status individuals have higher basal levels of inflammatory activity and thus simply exhibit less pronounced changes in inflammatory gene expression following targeted rejection. Contrary to this possibility, however, high- and low-status participants in this study had nearly identical levels of NF-κB and I-κB mRNA under no-stress conditions and exhibited differential levels of inflammatory gene expression only after experiencing a recent targeted rejection life event (see Fig. 1).
Some readers may be surprised by the pattern of findings here, particularly if they view the MacArthur Scale as an index of socioeconomic position and expected rejection to most strongly affect low-status participants. However, we emphasize that the adolescent version of the instrument used here—which inquires about where youth stand in the local social hierarchy in terms of respect, friendship, and academics—captures something more akin to social esteem than material resources do. Indeed, as the scale’s authors suggest, this instrument captures an adolescent’s developing sense of self within a local social network. In adolescence, this dimension of status may be a more important contributor to health than concrete material resources are (E. Goodman et al., 2001).
A limitation of this study is that the associations observed between targeted rejection and inflammatory signaling are correlational. We followed participants longitudinally and adjusted for plausible confounds when testing for within-person changes in rejection-related inflammatory signaling, but we still cannot be certain about the causal structure of the observed associations. In addition, because participants were assessed every 6 months, the amount of time that transpired between when participants experienced a targeted rejection event and when they completed an inflammation assessment was not equivalent for each participant. Although differences in this time lag are not responsible for our findings (there was no correlation between perceived social status and duration from targeted rejection to blood draw; p = .89), this design feature may have added measurement error to the assessment of inflammation. Furthermore, we observed relatively few targeted rejection events during the study. Because identification of these events was based on the careful rating of more than 800 in-depth interviews, it is unlikely that we missed targeted rejection events. A more plausible explanation is that targeted rejection occurs somewhat rarely but that when it does occur it has a significant impact. To capture greater numbers of targeted rejection life events, future studies could interview more individuals or selectively sample for increased likely exposure to recent social rejection.
It is also important to note that this study was carried out on adolescent women who, although healthy, were at elevated risk for developing a first episode of major depression. This aspect of the study may have affected our findings in a couple of ways. For example, one potential manifestation of elevated risk for depression might be an increased propensity to engage in behaviors that elicit rejection from others. Consistent with this possibility, adult women with depression have been shown to experience higher levels of interpersonal life stress compared with women without the disorder (Hammen, 1991). Similarly, longitudinal research on school bullying has demonstrated that children who are depressed are more likely to become targets of bullying than their nondepressed counterparts (Fekkes, Pijpers, Fredriks, Vogels, & Verloove-Vanhorick, 2006). In addition to being more likely to experience social rejection, participants in our study may also be more sensitive to social rejection, and thus more likely to exhibit potentiated biological responses to rejection, than adolescents at low risk for depression. Although we cannot fully evaluate these hypotheses without a low-risk comparison group, secondary analyses of the present data lead us to believe that the findings are likely to be generalizable. In our data, for example, we did not find any evidence that changes in depressive symptoms explained the observed associations between targeted rejection and inflammatory signaling or that these relations varied according to whether participants entered the study based on their high cognitive vulnerability status, positive family history of depression status, or both. Taken together, these results suggest that our findings may generalize at least to other high-risk populations of adolescents. Of course, further research with diverse community samples will be necessary to fully evaluate the generalizability of these observations.
The next steps in this program of research will be to first replicate the effects we have reported here with a more rigorous experimental design and then, if successful, evaluate their contribution to subsequent mental and physical health problems. For the experimental corroboration, future studies could make use of any number of laboratory paradigms developed in social psychology to elicit feelings of social rejection, exclusion, and ostracism (for a review, see Williams, 2007). If the observations we report here reflect a causal association, then experimentally inducing feelings of targeted rejection should elicit heightened inflammatory processes in participants and should do so most prominently for high-status individuals. The next step would then be to examine what implications targeted rejection has for health outcomes presumed to have inflammatory causes. To do that, future studies might longitudinally follow young individuals and track how experiences of targeted rejection relate to the development of mental health conditions such as anxiety and depression and physical health problems such as obesity, metabolic syndrome, and infectious diseases. Although these associations could certainly be examined in adult samples, identifying such effects in adolescents may be advantageous insofar as it could lead to methods for reducing processes such as heightened sensitivity to social stress before they become engrained in a way that affects a person’s mental and physical health.
To summarize, several studies have now shown that negative social experiences upregulate inflammatory activity (Irwin & Cole, 2011; Miller, Rohleder, et al., 2009). The present study extends this work by demonstrating for the first time that acute life events involving targeted rejection are associated with increased expression of the genes encoding NF-κB and I-κB, especially for adolescents who perceive themselves to be high in social status. These findings converge with a growing number of transcriptome-wide studies showing enhanced expression of pro-inflammatory immune response genes for individuals confronting a range of adverse social experiences including anticipated bereavement, low socioeconomic status, traumatic life events, and the diagnosis of a life-threatening illness (Slavich & Cole, 2012). The emerging account, therefore, is that inflammation and the molecular signaling pathways that regulate inflammation are influenced to a significant degree by the external social world. These findings have important implications for understanding how social conditions increase risk for a variety of inflammation-related diseases, including obesity, diabetes, cardiovascular disease, certain types of cancer, and depression (Miller, Chen, et al., 2009; Slavich, O’Donovan, et al., 2010). They also challenge fundamental notions about the self as a biologically stable entity. For example, although the structure of human DNA changes relatively little over the life course, the activity of our genome is quite fluid and more permeable to external social influence than we realize (Slavich & Cole, 2012).
Acknowledgments
Funding
This research was supported by grants from the Canadian Institutes of Health Research (67191), the National Alliance for Research on Schizophrenia and Depression, the Heart and Stroke Foundation of Canada, and the National Institute of Child Health and Human Development (HD058502) to Gregory E. Miller and by a Society in Science: Branco Weiss Fellowship to George M. Slavich.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declare that they have no conflicts of interest with respect to their authorship or the publication of this article.
References
- Baldwin AS. The NF-κB and IκB proteins: New discoveries and insights. Annual Review of Immunology. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srnivasan SR. Cardiovascular risk factors in youth with implications for aging: The Bogalusa Heart Study. Neurobiology of Aging. 2005;26:303–307. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. New England Journal of Medicine. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences, USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspectives in Biology and Medicine. 2003;46:S39–S52. doi: 10.1353/pbm.2003.0063. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkey LC, Berntson GG. The anatomy of loneliness. Current Directions in Psychological Science. 2003;12:71–74. doi: 10.1111/1467-8721.01232. [DOI] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Berntson GG. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Charmandari E, Kino T, Souvatzoglou E, Chrousos GP. Pediatric stress: Hormonal mediators and human development. Hormone Research. 2003;59:161–179. doi: 10.1159/000069325. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Research Reviews. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. doi: 10.1037//0003-066X.55.2.218. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear K. Adolescent onset of the gender difference in lifetime rates of major depression. Archives of General Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation. Psychological Science. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Fekkes M, Pijpers FIM, Fredriks AM, Vogels T, Verloove-Vanhorick SP. Do bullied children get ill, or do ill children get bullied? A prospective cohort study on the relationship between bullying and health-related symptoms. Pediatrics. 2006;117:1568–1574. doi: 10.1542/peds.2005-0187. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, research version, non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Foland-Ross LC, Hardin MG, Gotlib IH. Neurobiological markers of familial risk for depression. Current Topics in Behavioral Neuroscience. 2012 doi: 10.1007/7854_2012_213. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, Irwin MR. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. Life at the top: Rank and stress in wild male baboons. Science. 2011;333:357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, Colditz GA. Adolescents’ perceptions of social status: Development and evaluation of a new indicator. Pediatrics. 2001;108:e31. doi: 10.1542/peds.108.2.e31. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: How did it change between 1990 and 2000? Journal of Clinical Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N. Subjective social status moderates cortisol responses to social threat. Brain Behavior and Immunity. 2006;20:410–419. doi: 10.1016/j.bbi.2005.11.00510.1016/j.bbi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosomatic Medicine. 2004;66:915–924. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037/0021-843X.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child and Adolescent Psychology. 2002;31:491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/s0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME. Psychobiological responses to social threat: Evolution of a psychological model in psychoneuroimmunology. Brain, Behavior, and Immunity. 2009;23:1–9. doi: 10.1016/j. bbi.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neuroscience and Biobehavioral Reviews. 2010;35:33–38. doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Larson R, Richards MH. Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Development. 1991;62:284–300. doi: 10.2307/1131003. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology. 2008;94:278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry. doi: 10.1016/j.biopsych.2012.02.034. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosomatic Medicine. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MLM, Miller GE, Wrosch C. Conscientiousness and stress exposure and reactivity: A prospective study of adolescent females. Journal of Behavioral Medicine. 2012 doi: 10.1007/s10865-012-9408-2. Advance online publication. [DOI] [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine. Depression in parents, parenting, and children: Opportunities to improve identification, treatment, and prevention. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkola J, Vaarasmaki M, Ala-Korpela M, Bloigu A, Canoy D, Hartikainen AL, Pouta A. Low-grade, systemic inflammation in adolescents: Association with early-life factors, gender, and lifestyle. American Journal of Epidemiology. 2010;171:72–82. doi: 10.1093/aje/kwp320. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] (Version 6.08) Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Rohleder N, Marin TJ, Ma R, Miller GE. Biologic cost of caring for a cancer patient: Dysregulation of pro- and anti-inflammatory signaling pathways. Journal of Clinical Oncology. 2009;27:2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Ross K, Martin T, Chen E, Miller GE. Social encounters in daily life and 2-year changes in metabolic risk factors in young women. Development and Psychopathology. 2011;23:897–906. doi: 10.1017/S0954579411000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Sympathy for the CEO. Science. 2011;333:293–294. doi: 10.1126/science.1209620. [DOI] [PubMed] [Google Scholar]
- Sawyer SM, Afifi RA, Bearinger LH, Blakemore SJ, Dick B, Ezeh AC, Patton GC. Adolescence: A foundation for future health. Lancet. 2012;379:1630–1640. doi: 10.1016/s0140-6736(12)60072-5. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce T, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emergence of human social genomics. 2012. Manuscript submitted for publication. [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neuroscience and Biobehavioral Reviews. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH. Targeted rejection predicts hastened onset of major depression. Journal of Social and Clinical Psychology. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proceedings of the National Academy of Sciences, USA. 2010;107:14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana JG, Campione-Barr N, Metzger A. Adolescent development in interpersonal and societal contexts. Annual Review of Psychology. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. American Journal of Physiology- Regulatory Integrative and Comparative Physiology. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annual Review of Immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Weissman A, Beck AT. Development and validation of the Dysfunctional Attitudes Scales: A preliminary investigation. Paper presented at the Meeting of the American Educational Research Association; Toronto, Ontario, Canada. 1978. Mar, [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Rohleder N, Bierhaus A, Nawroth PP, Kirschbaum C. Determinants of the NF-kappa B response to acute psychosocial stress in humans. Brain Behavior and Immunity. 2009;23:742–749. doi: 10.1016/j.bbi.2008.09.009. [DOI] [PubMed] [Google Scholar]