Graphical abstract
Highlights
► Only one drug (praziquantel) is available for treatment of schistosomiasis. ► Two compounds (plumbagin and sanguinarine) have shown to possess potent antischistosomal activity. ► In vitro antischistosomal effects meet the WHO’s criterion of “hit” compounds for the control of schistosomiasis. ► Morphological changes and tegumental alternations of worms treated by the two compounds are quite different.
Keywords: Plumbagin, Sanguinarine, Chemotherapy, Schistosomiasis
Abstract
Schistosomiasis, a snail-borne parasitic disease, affects more than 200 million people worldwide. Currently the treatment of schistosomiasis relies on a single therapy of praziquantel, a drug developed over 30 years ago. Thus, there is an urgent need to develop alternative antischistosomal drugs. In the pursuit of novel antischistosomal drugs, we examined the antischistosomal activities of 45 compounds that had been reported to exhibit antimicrobial and/or antiparasitic activities. Two plant-derived compounds, plumbagin and sanguinarine, were found to possess potent antischistosomal activities in vitro. For both the compounds, a concentration of 10 μM (equivalent to 1.88 μg/ml for plumbagin and 3.68 μg/ml for sanguinarine) resulted in 100% mortality at 48 h, which meets the World Health Organization’s (WHO) criterion of “hit” compounds for the control of schistosomiasis. Morphological changes and tegumental alterations of the dead worms treated by the two compounds were quite different. The significant morphological changes of worms after treatment by the two compounds suggest the two compounds target different biological pathways, both of which result in parasite’s death. This study provides evidence to suggest plumbagin and sanguinarine have real potential as effective alternative chemotherapeutic agents for the treatment of schistosomiasis.
1. Introduction
Schistosomiasis is a chronic, debilitating disease caused by blood-dwelling trematodes of the genus Schistosoma. The global health impact of schistosomiasis is second only to malaria. According to a recent World Health Organization (WHO) report, 239 million people were infected with schistosomiasis (WHO, 2012). In sub-Saharan Africa alone, an estimated 150,000 deaths per year were attributable to schistosomiasis (van der Werf et al., 2003). In addition, people infected with schistosomes may have increased susceptibility to other infectious diseases such as HIV/AIDS (Secor, 2012). It resulted in up to 70 million disability-adjusted life years (DALYs) lost annually. This number exceeds that of malaria and tuberculosis, and nearly equivalent to the DALYs lost from HIV/AIDS (Hotez and Fenwick, 2009). Despite the impacts described, the aggregate health impact of schistosomiasis is often underestimated because of the complexity of evaluating the disease (King, 2010).
Considerable effort has been made in vaccine development, which has been unsuccessful so far (McWilliam et al., 2012). Current treatment of schistosomiasis relies on the drug praziquantel (PZQ), which was developed in the late 1970s (Seubert et al., 1977). PZQ has been widely used as an effective means to control schistosomiasis. However, PZQ does not treat early infection or prevent reinfection (Magnussen, 2003). In addition, available evidence indicates the emergence of PZQ resistance by schistosomes (Cioli et al., 1993; Fallon and Doenhoff, 1994; Ismail et al., 1999). For example, investigations in Egypt, Senegal and Kenya revealed different degrees of PZQ drug resistance in Schistosoma mansoni (Ismail et al., 1999; Melman et al., 2009; van den Enden, 2009). Moreover, recent efforts to expand mass chemotherapy administrations using PZQ (Webster et al., 2009) might accelerate the emergence of drug resistance in schistosomes.
Some antiparasitic drugs like artemether and mefloquine, both antimalarial drugs, as well as miltefosine, an antileishmanial drug, have shown antischistosomal activity (Caffrey and Secor, 2011). Administration of these drugs with or without PZQ is currently under evaluation (Eissa et al., 2011; Keiser et al., 2011; Xiao et al., 2011a). However, it is important to keep in mind the multi-use of antimalarial drugs may put the control of malaria at risk. This is likely due to the co-occurrence of schistosomes and malaria in most endemic areas and also the well-documented ability of Plasmodium to develop drug resistance. An older drug, oxamniquine, is no longer manufactured because it is only effective against one species of schistosome, S. mansoni. Additionally, it has been found to possess undesired side effects and promote drug resistance (Fallon and Doenhoff, 1994). Some new drug candidates such as the antioxidant inhibitors, oxadiazoles (Sayed et al., 2008), and some protease inhibitors (Abdulla et al., 2007) have promising potentials against a schistosome infection, but have yet to reach clinical trials. Developing drugs for neglected tropical diseases is largely ignored; i.e., out of 1599 new drugs developed worldwide from 1975 to 2004, only 21 were for patients with neglected tropical diseases (Renslo and McKerrow, 2006) despite the large numbers of infected individuals worldwide. Developing a new drug in general is very complicated, only one in ten drug projects at the discovery phase progress to clinical development (Brown and Superti-Furga, 2003). Thus to alleviate these challenges, efforts to explore and discover novel “hit” and “lead” chemotherapeutic compounds are continually undertaken.
In the search of new candidates of alternative antischistosomal drugs, we tested the antischistosomal activities of 45 compounds that had been reported to possess antiparasitic and/or antimicrobial activities. We found that two plant-derived compounds, plumbagin and sanguinarine, possess potent antischistosomal activities. In this paper we describe the antischistosomal activities of the two compounds as well as the morphological changes and tegumental changes observed in the worms after treatment with the two compounds.
2. Materials and methods
2.1. Parasites
NIH strain S. mansoni (NIH-SM-PR2) was maintained in the laboratory using the M line snail Biomphalaria glabrata and mice as intermediate and definitive hosts, respectively (Zhang and Coultas, 2011). All procedures of schistosome infection and mouse perfusion were conducted in accordance with protocols approved by the University of New Mexico Institutional Animal Care and Use Committee.
2.2. Compounds
If not otherwise stated, all chemical reagents were purchased from Sigma (http://www.sigmaaldrich.com/united-states.html) and are listed below. The number in parenthesis is the catalogue number of the product: albendazole (A4673), amodiaquin dihydrochloride dehydrate (A2799), anacardic acids (A7236), apicidin (A8851), arachidonic acid (A9673), artemether (A9361), atovaquone (A7986), berberine chloride hydrate (14050), bisdemethoxycurcumin (B6938), chelidonine (54274), chloroquine diphosphate salt (C6628), closantel (34093), curcumin (C1386), diclofenac sodium salt (D6899), dipyridamole hydrochloride (D9766), docosahexaenoic acid (D2534), doxycycline hyclate (D9891), ethacrynic acid, etazolate hydrochloride (E1896), halofantrine hydrochloride (H9414), humic acid (53680), 2-hydroxy-1,4-naphthoquinone (H46805), ibuprofen (I4883), lapachol (I2905), levamisol hydrochloride (31742), macrocyclic lactone (437026), mebendazole (M2523), mefloquine hydrochloride (M2319), methylene blue (M9140), metronidazole (M3761), miltefosine (M5571), 2-methoxy-1,4-naphthoquinone (189162), 1,2-naphthoquinone (346616), oxantel pamoate (O4755), oxfendazole (34176), praziquantel (P4668), pyrantel pamoate (P6210), piperazine (80621), plumbagin (P7262), pyrimethamine (46706), primaquine bisphosphate (160393), quine (22620), sanguinarine (S5890), sulfadoxine (S7821), sulfadiazine (S8626), and trichostatin A (T8552).
2.3. Culture of schistosome worms with compounds
All adult schistosome worms were collected from mice after 42–45 days post exposure to S. mansoni. Stock solutions of individual chemical reagents were prepared in dimethylsufoxide (DMSO) (EMD Chemicals) and stored in −30 °C. The final concentration of DMSO in all treatments, including control, was 1% (v/v). The enriched RPMI (eRPMI) 1640 culture medium contained 25 mM HEPES, 10% fetal bovine serum (FBS), 125 units/ml each penicillin and streptomycin (all from Gibco) (Coultas and Zhang, 2012). The worms were washed with eRPMI three times and placed into a 6-well plate. In each well, 20–50 worms were cultured in 5 ml culture medium. For the control, the worms were treated with equal amounts of DMSO alone. The worms were cultured at 37 °C in an atmosphere of 5% CO2. The criterion for classifying dead worms was made according to Eissa et al. (2011) and Manneck et al. (2010) with a more stringent modification as described below. The plate was agitated slightly to ensure better parasitic visibility and then placed under a stereomicroscope. Worms that did not exhibit motility, particularly in the mouth region, for two minutes were considered dead.
The measurement of length of worms was conducted under a Zeiss Axioskop 2 Plus Mot Plus microscope. A high-resolution microscopy camera, AxioCam HRc, was used to photograph the worms. After photography, the curve line in the middle of worm body was drawn to represent the actual length of the worm and exact length of the worm was calculated by a computer connected to the microscope.
With regard to effect of compounds on mortality of cercariae, 50–120 cercariae were placed into a 24-well plate. For a given compound, triplicates at each concentration were performed. Live cercariae show very active movement, thus if no movement was observed, cercariae exhibiting no movement for one minute were considered dead under the Zeiss stereo microscope (Discovery V8). In addition, the death of cercariae as described above was also verified by the propidium iodide staining method (Coultas and Zhang, 2012).
2.4. Scanning electron microscopy (SEM) study
After the adult worms were incubated in the medium that contained either compounds to be tested or a DMSO control, worm samples were collected and washed with fresh eRPMI two times. Worm samples were then washed two more times with phosphate buffer saline (PBS) for 10 min. each at room temperature (RT) to remove any residual compounds and/or media before fixing. Next, samples were fixed in 2.5% glutaraldehyde in PBS buffer (0.1 mol/L, pH 7.4) overnight at 4 °C. After fixing for 24 h, samples were washed with PBS four times for 10 min. each at RT. Samples were dehydrated in a series of ethanol washes (25%, 50%, 70%, 75%, 85%, 95% and 100% ethanol) for 10 min each at room temperature. The 100% ethanol wash was performed two times. Samples were critically point dried, mounted on carbon paste and coated with gold/palladium before examining with the JEOL 5800LV scanning electron microscope.
3. Results
3.1. Plumbagin and sanguinarine exhibit potent antischistosomal activities in vitro
The compounds that resulted in 100% mortality at a concentration of 50 μM after four days were further investigated. Among the 45 compounds tested (as listed in the Section 2), six compounds (PZQ, plumbagin, sanguinarine, curcumin, mefloquine and miltefosine) met the criterion and received further investigation.
Among the six compounds depicted in Fig. 1, the antischistosomal activity of PZQ was the most effective. In addition, the efficacy of plumbagin and sanguinarine were better than that of curcumin, mefloquine or miltefosine.
Fig. 1.
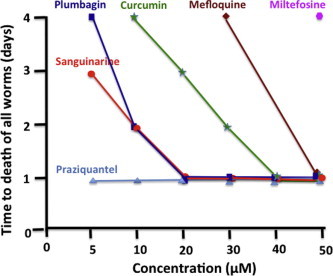
In vitro antischistosomal activities. Effect of compounds at various concentrations on the mortality of schistosome worms under culture conditions. The data was generated from three independent studies and the time (day) of death was determined based on two or three consistent experiments. No obvious mortality in control groups (DMSO) was observed within 4 days of worm culture or 4 h of cercariae culture. The final concentration of DMSO is 1% (v/v) for all experiments. Note that the intervals on the X-axis are not equal.
Given the relatively minor antischistosomal effect of curcumin, mefloquine and miltefosine compared to plumbagin and sanguinarine, as well as the data published for the antischistosomal activity of the compounds, we focused in the subsequent studies on the effect of different concentrations of plumbagin and sanguinarine on the mortality of the worms (Fig. 2). Investigation revealed that all worms were killed by either of the two compounds within 6 h at 50 μM. At a concentration of 30 μM, the time required for 100% mortality for sanguinarine and plumbagin we found to be 6 and 12 h respectively. At a concentration of 10 and 5 μM, the time to reach full mortality for sanguinarine was 24 and 72 h, respectively and the time to reach full mortality for plumbagin at these concentrations was 48 and 96 h, respectively. Taken together, this study demonstrates that both compounds possess potent antischistosomal effects and that sanguinarine is more effective against adult worms than plumbagin.
Fig. 2.
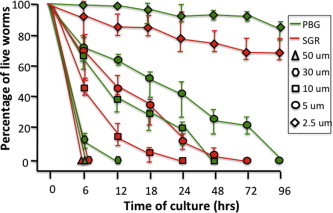
Effect of various concentrations of plumbagin and sanguinarine on the survival of worms. The average percent of live worms is shown. The average percentages were generated from three independent experiments and the bars show the range of live worm percentages in the experiments. Note that the intervals on the X-axis are not equal.
Finally, we compared the effects of plumbagin and sanguinarine and other compounds described in Fig. 1 on the killing of cercariae (Fig. 3A). Our study indicated that cercariae are much more sensitive to the chemicals than adult worms. Similarly, the efficacies of the compounds tested on killing cercariae were somewhat consistent with those of the worm experiments described above. We found that sanguinarine displayed the strongest anticercariae effect amongst the compounds investigated. Notable still, plumbagin possessed similar schistosomicidal effects to that of PZQ. Moreover, we found the plumbagin caused a high percentage of separation of cercariae heads from tails whereas sanguinarine resulted in fewer separations. While plumbagin caused muscle contractions, which lead to the splitting of the head from the tail, sanguinarine seemed to simply paralyze the cercariae (Fig. 3B).
Fig. 3.

Effect of compounds on the survival of cercariae. (A) The effect of various concentrations of compounds on cercariae mortality. The data was generated from three independent studies and the time (hour) of death was determined based on two or three consistent experiments. Note that the intervals on the X-axis are not equal. (B) Upper (a) and lower (b) images showing cercariae after treatment with plumbagin and sanguinarine, respectively.
3.2. Plumbagin leads to worm contraction, but sanguinarine does not
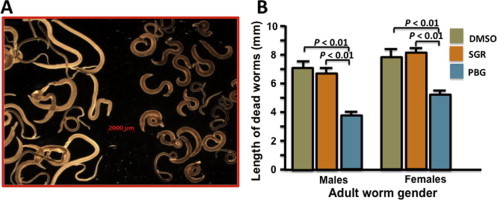
In addition to the profound in vitro schistosomicidal effects described above, we also noted that the morphological appearance of the dead adult worms treated by the two compounds was quite different (Fig. 4). Adult worms treated with plumbagin became contracted, immobile and at times, appeared tightly coiled. The length of dead male and female worms after treatment with plumbagin was respectively about 54% and 70% of the length of worms incubated with DMSO (P < 0.01). Conversely, no such morphological changes were observed in the sanguinarine-treated worms in both sexes, as compared to the control group (Fig. 4A and B).
Fig. 4.

Comparison of morphological differences of the dead worms after treatment with plumbagin (PBG) and sanguinarine (SGR). (A) Morphological appearance of dead schistosome worms after treatment with sanguinarine (left) and plumbagin (right) (under a microscopic field). (B) Actual average size of dead worms (male and female) after treatment with either sanguinarine, plumbagin or DMSO (control). No significant difference was found between sanguinarine and DMSO control in either sex.
3.3. Both compounds can cause tegumental alterations, but each compound exhibits different effects on the tegument
To better understand the effects of the compounds on the worm tegument, the interface between host and parasite, we used SEM to examine the surface membrane of worms under different treatments. We found that both compounds significantly damaged the worm’s tegument (Fig. 5). Interestingly, in most cases, plumbagin showed prominent alterations of the tegumental surfaces, usually with disintegration of tubercles and often times accompanied by a decrease in the number of spines. Emergence of holes on the surface was also observed (Fig. 5C and D). Sanguinarine treatment resulted in severe erosion and disintegration of the tegumental surface between tubercles, however the tubercles and spines were relatively intact. In fact, some tubercles did not display spine loss while other tubercles simply had fewer spines (Fig. 5E and F). For both compounds, the tegument of female worms after treatment are shown to varying degrees of swelling and cracking ranging from minimal to moderate throughout the anterior and posterior tegumental ridges/fissures.
Fig. 5.

SEM images showing teguments of male (left panel) and female worms (right panel) after culture with DMSO (A, B), plumbagin (C, D) and sanguinarine (E, F). (A) and (B) show SEM image of the normal tegument of male and female worms. Male teguments shows hill-shaped tubercles covered with pointed spines and a convoluted surface of the tegumental membrane between tubercles. The female tegument does not have tubercles and spines and appears like a field plowed surface. (C) Shows a SEM image of a male worm exposed in vitro to plumbagin at a concentration of 10 μM for 24 h. Tegumental surfaces are damaged usually with disintegration of tubercles accompanied by a decrease in the number of spines. In addition, there were varying degrees of disintegration of the tegumental surfaces including peeling, wrinking and blebbing. (D) Among the female worms treated with plumbagin at a concentration of 10 μM for 24 h, the worms displayed moderate damage when compared to male damage of the tegumental surface. Holes and divots are prevalent throughout the tegument. (E) SEM image shows the tegument of an adult worm exposed in vitro to sanguinarine at a concentration of 10 μM for 24 h. Distortions of the tegumental surfaces can be seen usually with severe erosion and disintegration of the tegumental surface between tubercles. The holes and broken surface are visible. (F) Female worms treated with 30 μM sanguinarine for 8 h displayed varying degrees of swelling and cracking ranging from minimal to moderate throughout the anterior and posterior tegumental ridges/fissures.
4. Discussion
In this study, 45 compounds that had been reported to possess antiparasitic and/or antimicrobial activities were selected for our antischistosomal experiments. Among them, six compounds (plumbagin, sanguinarine, PZQ, curcumin, mefloquine and miltefosine) were found to possess schistosomicidal activity based on the criterion of our experiment (e.g., 100% mortality at a concentration of 50 μM for four days). The antischistosomal activity of PZQ was the most profound amongst all compounds tested and the efficacy of the two compounds identified (plumbagin and sanguinarine) were better than that of curcumin, mefloquine and miltefosine, the three compounds known to possess schistosomicidal activity. Curcumin, a plant-derived compound, has been recently studied as a potential antischistosomal drug (Allam, 2009; Magalhães et al., 2009; Luz et al., 2012). The antimalarial drug, mefloquine, (Keiser et al., 2011; Xiao et al., 2011b) and the antileishmanial drug, miltefosine (Eissa et al., 2011) have also recently been tested as alternative drugs for schistosomiasis based on animal models or small clinic trails. Thus our comparative study suggests plumbagin and sanguinarine have significant potentials as novel antischistosomal chemotherapeutics.
Although we did not observe discernible antischistosomal activity in many agents (listed in the Section 2, but not shown in Fig. 1), it does not necessarily mean those compounds do not have such an effect. For example, artemether has been reported to possess an antischistosomal effect (Abdul-Ghani et al., 2011; Xiao et al., 2011a). Others, such as pyrantel pamoate, oxantel pamoate, levamisol, mebendazole, albendazole, oxfendazole, metronidazole and piperazine, have also been reported to possess certain levels of anthelmintic effects, particularly in antinematode responses (Martin and Robertson, 2010; Beech et al., 2011; Müllner et al., 2011; van den Enden, 2009), but they showed very limited antischistosomal activities in our in vitro comparative study. The reason we did not find apparent antischistosomal effects in our experiments for these compounds may be due to our use of relatively low concentrations of the chemicals, which might have reduced the chance of observing antischistosomal activity. Another plausible explanation for why we did not observe antischistosomal effects for the compounds that others did, especially in nematodes, may be due to a high phylogenetic divergence between nematodes and trematodes. Nematodes and trematodes belong to the two superphyla of protostomes, Ecdysozoa and Lophotrochozoa, respectively. It is predicted that molecular structure of drug-related receptors and relevant pathways are quite different, which will affect the efficiency of a given drug. Nevertheless, our study suggests that plumbagin and sanguinarine have better efficacy against schistosomiasis than all the other compounds tested in our investigation.
Our study shows that a concentration of 10 μM (equivalent to 1.88 μg/ml for plumbagin and 3.68 μg/ml for sanguinarine) of both compounds resulted 100% mortality at 48 h. WHO’s definition and activity criteria for hits and leads sets inhibition of 100% motility of adult worms at a concentration of 5 μg/ml (in vitro) and 80% worm reduction after five injections at a dosage 100 mg/kg body-weight/day (in vivo) (Hwaka and Hudson, 2006). Clearly, plumbagin and sanguinarine meet WHO’s criterion for “hit” compounds and are effective against schistosomes. Moreover, our comparative studies revealed that the in vitro antischistosomal effects of the two compounds is better than other antischistosomal drugs such as artemether, mefloquine, miltefosine and curcumin that are under current investigation as alternative therapies (Allam, 2009; Sissoko et al., 2009; Eissa et al., 2011; Keiser et al., 2011; Xiao et al., 2011a,b; Luz et al., 2012). These exciting findings provide a strong impetus to investigate the in vivo efficacy of plumbagin and sanguinarine on schistosomes in the future.
In addition to the antischistosomal activity described, it is intriguing that the morphological appearance of the dead worms after treatment with plumbagin and sanguinarine is quite different. Worms treated with plumbagin become withered while worms treated with sanguinarine show no apparent changes in appearance after death. It seems that plumbagin causes muscle contraction, but sanguinarine does not. This change is similarly observed in experiments of cercariae, where muscle contraction caused by plumbagin may be responsible for the high rate of head separation observed (Fig. 3B). Taken together, these differences imply there are at least two different mechanisms involved in the parasite’s death. Based on the observations, it seems that plumbagin affects muscle function because of the muscle contraction observed. In the case of sanguinarine, it paralyzes the worms, potentially through the nervous system. Due to the complexity of drug mechanisms and their mode of action, comparative investigation of these differences using molecular approaches may reveal the fundamental mechanism(s) of killing the parasites, which in turn, can facilitate the design of new drugs to combat schistosomiasis.
Another intriguing phenomenon we observed is that plumbagin and sanguinarine, although possessing different effects on schistosomes, can both alter the tegumental structure of the worms; i.e., both compounds damage the tegument, thus implying their antischistosomal effect may increase in the host. This is due to the fact that the tegument is the interface between the host and parasite and harbors large amounts of molecules that constitute very complicated structures. It is well recognized that the parasite’s surface membrane and tegumental integrity play a vital role in immune evasion, modulation and nutrient uptake and thus ensure worm survival in the host. Profound damage caused by the two compounds observed here could alter tegumental structure and stability. In addition to the direct effect on the survival of the worms, tegumental alteration might result in exposing the typically unexposed parasite antigens to the host’s immune system. As a result, treatment with plumbagin and sanguinarine might increase the vulnerability of parasites in the host and thus further enhance the efficiency of the compounds at killing schistosomes in vivo.
Plumbagin and sanguinarine are plant-derived products (Reuter et al., 2011). Exploring natural products has long been considered an ideal alternative for the development of new drugs (Geary et al., 2012). According to data of all approved agents from 1981 to 2006, about 50% of new drugs were directly or indirectly derived from natural products (Newman and Cragg, 2007). With respect to parasitic diseases, many compounds isolated from plants have proven to be the mainstay in anthelmintics and antimalarial therapy. For example, there are notable plant-derived antiparasitic drugs such as quinolone alkaloids against Leishmania amazonensis and indole alkaloids against Plasmodium and Entamoeba (Kayser et al., 2003). Three major antimalarial drugs, chloroquine, atovaquone and artemisinin, are derived from plants (Oliveira et al., 2009). Chloroquine was synthesized based on the structure of quinine, a component of the tree bark Cinchona spp. that was used to treat malaria 400 years ago (Achan et al., 2011). Atovaquone was structurally derived from lapachol, originally purified from Tabebuia spp. Artemisinin was extracted from Artemisia annua and many more effective drugs such as artemether were designed based on the artemisinin (Miller and Su, 2011). In light of these discoveries, similar efforts on schistosomiasis should be emphasized (Ribeiro-dos-Santos et al., 2006). Although a few plant products have been screened for activity against schistosomes (Cichewicz et al., 2002; Sanderson et al., 2002; Allam, 2009; Magalhães et al., 2010), presently no antischistosomal compounds have been purified and reported.
Plumbagin is one of the simplest secondary plant metabolites of the three major families, Plumbaginaceae, Droseraceae and Ebenaceae. It exhibits many highly potent biological activities including activation of apoptosis, induction of redox cycling and modification of chromatin structure. Plumbagin and its derivatives can act as an antioxidant, anti-inflammatory, anticancer, antibacterial and antifungal agents (Padhye et al., 2012). Additionally, plumbagin is capable of inhibiting the drug efflux mechanism in drug-resistant bacteria, thereby allowing intracellular accumulation of potent drug molecules. With regard to an antiparasitic role, evidence showed that plumbagin possesses antifilarial activity by inhibiting trypanothione and glutathione S-transferases (Srinivasan et al., 2009; Sharma et al., 2012).
During the process of submission of our manuscript, we noted that a paper describing the antischistosomal role of plumbagin was published (Lorsuwannarat et al., 2012). Although the effect of plumbagin on mortality of larvae (cercariae) and the morphology of adult worms was not described in the paper, the published paper and ours provide independent evidence supporting an antischistosomal role of plumbagin on adult worms. The slight differences observed between the two reports in terms of efficacy in vitro and tegumental changes may be due to the different strains of S. mansoni used in each investigation.
Sanguinarine is derived from the root of Sanguinaria spp and possesses a wide spectrum of biological assets including antimicrobial, antioxidant and anti-inflammatory properties. Sanguinarine can induce apoptosis in malignant cell types and interact with chromatin and modulate it epigenetically (Selvi et al., 2009). Active efforts have been made to develop sanguinarine and its derivatives as anticancer agents (Sun et al., 2010; Pica et al., 2012). A recent study has also revealed sanguinarine’s role in killing the fish parasite Ichthyophthirius multifiliis (Yao et al., 2010). Our data presented here provides the first evidence suggesting an antischistosomal role of sanguinarine.
Previous studies have shown that plumbagin and sanguinarine have a wide array of medical and health applications, hence it would not be surprising if they additionally possess potent antischistosomal activities. Both compounds belong to naphthoquinones, a large family of chemical compounds. A recent study suggested that a slight modification of some napththoquinone compounds could increase antileishmanial activity (Ali et al., 2011). This might imply that the compounds, if designed properly, may be an effective antischistosomal therapy. In fact, we have already confirmed that some analogs of the two compounds have a similar antischistosomal activity (data not shown), implying the two chemicals identified in this study can serve as basic compounds for development of novel chemotherapeutics to control schistosomiasis in the future. Given the broad spectrum of documented pharmacological activities exhibited by the compounds, they continue to be the subject of extensive studies to evaluate possible therapeutic applications in human health. This will make future development of the two compounds as antischistosomal therapy relatively easy.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Abdul-Ghani R., Loutfy N., Sheta M., Hassan A. Artemether shows promising female schistosomicidal and ovicidal effects on the Egyptian strain of Schistosoma mansoni after maturity of infection. Parasitol. Res. 2011;108:1199–1205. doi: 10.1007/s00436-010-2163-9. [DOI] [PubMed] [Google Scholar]
- Abdulla M.H., Lim K.C., Sajid M., McKerrow J.H., Caffrey C.R. Schistosomiasis mansoni: novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007;4:e14. doi: 10.1371/journal.pmed.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achan J., Talisuna A.O., Erhart A., Yeka A., Tibenderana J.K., Baliraine F.N., Rosenthal P.J., D’Alessandro U. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar. J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Assimopoulou A.N., Papageorgiou V.P., Kolodziej H. Structure/antileishmanial activity relationship study of naphthoquinones and dependency of the mode of action on the substitution patterns. Planta Med. 2011;77:2003–2012. doi: 10.1055/s-0031-1280092. [DOI] [PubMed] [Google Scholar]
- Allam G. Immunomodulatory effects of curcumin treatment on murine Schistosomiasis mansoni. Immunobiology. 2009;214:712–727. doi: 10.1016/j.imbio.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Beech R.N., Skuce P., Bartley D.J., Martin R.J., Prichard R.K., Gilleard J.S. Anthelmintic resistance: markers for resistance, or susceptibility? Parasitology. 2011;138:160–174. doi: 10.1017/S0031182010001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Superti-Furga G. Rediscovering the sweet spot in drug discovery. Drug Discov. Today. 2003;8:106–1077. doi: 10.1016/s1359-6446(03)02902-7. [DOI] [PubMed] [Google Scholar]
- Caffrey C.R., Secor W.E. Schistosomiasis: from drug deployment to drug development. Curr. Opin. Infect. Dis. 2011;24:410–417. doi: 10.1097/QCO.0b013e328349156f. [DOI] [PubMed] [Google Scholar]
- Cichewicz R.H., Lim K.V., McKerrow J.H., Nair M.G. Kwanzoquinones A-G and other constituents of Hemerocallis fulva ‘Kwanzo’ roots and their activity against the human pathogenic trematode Schistosoma mansoni. Tetrahedron. 2002;58:8597–8606. [Google Scholar]
- Cioli D., Pica-Mattoccia L., Archer S. Drug resistance in schistosomes. Parasitol. Today. 1993;9:162–166. doi: 10.1016/0169-4758(93)90138-6. [DOI] [PubMed] [Google Scholar]
- Coultas K.A., Zhang S.-M. In vitro cercariae transformation: comparison of mechanical and nonmechanical methods and observation of morphological changes of detached cercariae tails. J. Parasitol. 2012 doi: 10.1645/GE-3072.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa M.M., El-Azzouni M.Z., Amer E.I., Baddour N.M. Miltefosine, a promising novel agent for Schistosomiasis mansoni. Int. J. Parasitol. 2011;41:235–242. doi: 10.1016/j.ijpara.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Fallon P.G., Doenhoff M.J. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Chibale K., Abegaz B., Andrae-Marobela K., Ubalijoro E. A new approach for anthelmintic discovery for human. Trends Parasitol. 2012;28:176–181. doi: 10.1016/j.pt.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl. Trop. Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwaka S., Hudson A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- Ismail M., Botros S., Metwally A., William S., Farghally A., Tao L.F., Day T.A., Bennett J.L. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- Kayser O., Olbrich C., Croft S.L., Kiderlen A.F. Formulation and biopharmaceutical issues in the development of drug delivery systems for antiparasitic drugs. Parasitol. Res. 2003;2:S63–S70. doi: 10.1007/s00436-002-0769-2. [DOI] [PubMed] [Google Scholar]
- Keiser J., Manneck T., Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J. Antimicrob. Chemother. 2011;66:1791–1797. doi: 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
- King C.H. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsuwannarat N., Saowakon N., Ramasoota P., Wanichanon C., Sobhon P. The anthelmintic effect of plumbagin on Schistosoma mansoni. Exp. Parasitol. 2012 doi: 10.1016/j.exppara.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Luz P.P., Magalhães L.G., Pereira A.C., Cunha W.R., Rodrigues V., Andrade E., Silva M.L. Curcumin-loaded into PLGA nanoparticles: preparation and in vitro schistosomicidal activity. Parasitol. Res. 2012;110:593–598. doi: 10.1007/s00436-011-2527-9. [DOI] [PubMed] [Google Scholar]
- Magalhães L.G., Machado C.B., Morais E.R., Moreira E.B., Soares C.S., da Silva S.H., da Silva Filho A.A., Rodrigues V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009;104:1197–1201. doi: 10.1007/s00436-008-1311-y. [DOI] [PubMed] [Google Scholar]
- Magalhães L.G., Kapadia G.J., da Silva Tonuci L.R., Caixeta S.C., Parreira N.A., Rodrigues V., da Silva Filho A.A. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol. Res. 2010;106:395–401. doi: 10.1007/s00436-009-1674-8. [DOI] [PubMed] [Google Scholar]
- Magnussen P. Treatment and re-treatment strategies for schistosomiasis control in different epidemiological settings: a review of 10 years’ experiences. Acta Trop. 2003;86:243–254. doi: 10.1016/s0001-706x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Manneck T., Haggenmüller Y., Keiser J. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology. 2010;137:85–98. doi: 10.1017/S0031182009990965. [DOI] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P. Control of nematode parasites with agents acting on neuro-musculature systems: lessons for neuropeptide ligand discovery. Adv. Exp. Med. Biol. 2010;692:138–154. doi: 10.1007/978-1-4419-6902-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H.E., Driguez P., Piedrafita D., McManus D.P., Meeusen E.N. Novel immunomic technologies for schistosome vaccine development. Parasite Immunol. 2012;34:276–284. doi: 10.1111/j.1365-3024.2011.01330.x. [DOI] [PubMed] [Google Scholar]
- Melman S.D., Steinauer M.L., Cunningham C., Kubatko L.S., Mwangi I.N., Wynn N.B., Mutuku M.W., Karanja D.M., Colley D.G., Black C.L., Secor W.E., Mkoji G.M., Loker E.S. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L.H., Su X. Artemisinin: discovery from the Chinese herbal garden. Cell Host Microbe. 2011;6:855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner A., Helfer A., Kotlyar D., Oswald J., Efferth T. Chemistry and pharmacology of neglected helminthic diseases. Curr. Med. Chem. 2011;18:767–789. doi: 10.2174/092986711794480096. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Oliveira A.B., Dolabela M.F., Braga F.C., Jácome R.L., Varotti F.P., Póvoa M.M. Plant-derived antimalarial agents: new leads and efficient phythomedicines. Part I. Alkaloids. An. Acad. Bras. Cienc. 2009;81:715–740. doi: 10.1590/s0001-37652009000400011. [DOI] [PubMed] [Google Scholar]
- Padhye S., Dandawate P., Yusufi M., Ahmad A., Sarkar F.H. Perspective on medicine properties of plumbagin and its analogs. Med. Res. Rev. 2012;32:1131–1158. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- Pica F., Balestrieri E., Serafino A., Sorrentino R., Gaziano R., Moroni G., Moroni N., Palmieri G., Mattei M., Garaci E., Sinibaldi-Vallebona P. Antitumor effects of the benzophenanthridine alkaloid sanguinarine in a rat syngeneic model of colorectal cancer. Anticancer Drugs. 2012;23:32–42. doi: 10.1097/CAD.0b013e32834a0c8e. [DOI] [PubMed] [Google Scholar]
- Renslo A.R., McKerrow J.U. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Park B., Goel A., Aggarwal B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93–108. doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-dos-Santos G., Verjovski-Almeida S., Leite L.C. Schistosomiasis – a century searching for chemotherapeutic drugs. Parasitol. Res. 2006;99:505–521. doi: 10.1007/s00436-006-0175-2. [DOI] [PubMed] [Google Scholar]
- Sanderson L., Bartlett A., Whitfield P.J. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. J. Helminthol. 2002;76:241–247. doi: 10.1079/JOH2002116. [DOI] [PubMed] [Google Scholar]
- Sayed A.A., Simeonov A., Thomas C.J., Inglese J., Austin C.P., Williams D.L. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 2008;14:407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secor W.E. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr. Opin. HIV AIDS. 2012;7:254–259. doi: 10.1097/COH.0b013e328351b9e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvi B.R., Pradhan S.K., Shandilya J., Das C., Sailaja B.S., Shankar G.N., Gadad S.S., Reddy A., Dasgupta D., Kundu T.K. Sanguinarine interacts with chromatin, modulates epigenetic modifications, and transcription in the context of chromatin. Chem. Biol. 2009;16:203–216. doi: 10.1016/j.chembiol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Seubert J., Pohlke R., Loebich F. Synthesis and properties of Praziquantel, a novel broad spectrum anthelmintic with excellent activity against Schistosomes and Cestodes. Experientia. 1977;33:1036–1037. doi: 10.1007/BF01945954. [DOI] [PubMed] [Google Scholar]
- Sharma N., Shukla A.K., Das M., Dubey V.K. Evaluation of plumbagin and its derivative as potential modulators of redox thiol metabolism of Leishmania parasite. Parasitol. Res. 2012;110:341–348. doi: 10.1007/s00436-011-2498-x. [DOI] [PubMed] [Google Scholar]
- Sissoko M.S., Dabo A., Traore H., Diallo M., Traore B., Konate D., Niare B., Diakite M., Kamate B., Traore A., Bathily B., Tapily A., Toure O.B., Cauwenbergh S., Jansen H.F., Doumbo O.K. Efficacy of artesunate + sulfamethoxypyrazine/pyrimethamine versus praziquantel in the treatment of Schistosoma haematobium in children. PLoS One. 2009:e6732. doi: 10.1371/journal.pone.0006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L., Mathew N., Muthuswamy K. In vitro antfilarial activity of glutathione S-transferase inhibitors. Parasitol. Res. 2009;105:1179–1183. doi: 10.1007/s00436-009-1534-6. [DOI] [PubMed] [Google Scholar]
- Sun M., Lou W., Chun J.Y., Cho D.S., Nadiminty N., Evans C.P., Chen J., Yue J., Zhou Q., Gao A.C. Sanguinarine suppresses prostate tumor growth and inhibits survivin expression. Genes Cancer. 2010;1:283–292. doi: 10.1177/1947601910368849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Enden E. Pharmacotherapy of helminth infection. Expert Opin Pharmacother. 2009;10:435–451. doi: 10.1517/14656560902722463. [DOI] [PubMed] [Google Scholar]
- van der Werf M.J., de Vlas S.J., Brooker S., Looman C.W., Nagelkerke N.J., Habbema J.D., Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Webster J.P., Koukounari A., Lamberton P.H., Stothard J.R., Fenwick A. Evaluation and application of potential schistosome-associated morbidity markers within large-scale mass chemotherapy programmes. Parasitology. 2009;136:1789–1799. doi: 10.1017/S0031182009006350. [DOI] [PubMed] [Google Scholar]
- WHO, 2012. Accelerating work to overcome the global impact of neglected tropical disease: a roadmap for implementation. <http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf>.
- Xiao S.H., Mei J.Y., Jiao P.Y. Effect of mefloquine administered orally at single, multiple, or combined with artemether, artesunate, or praziquantel in treatment of mice infected with Schistosoma japonicum. Parasitol. Res. 2011;108:399–406. doi: 10.1007/s00436-010-2080-y. [DOI] [PubMed] [Google Scholar]
- Xiao S.H., Xue J., Zhang H.B. Further studies on mefloquine and praziquantel alone or interaction of both drugs against Schistosoma japonicum in vitro. Parasitol. Res. 2011;110:1239–1248. doi: 10.1007/s00436-011-2621-z. [DOI] [PubMed] [Google Scholar]
- Yao J.Y., Shen J.Y., Li X.L., Xu Y., Hao G.J., Pan X.Y., Wang G.X. Effect of sanguinarine from the leaves of Macleaya cordata against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idella) Parasitol. Res. 2010;107:1035–1042. doi: 10.1007/s00436-010-1966-z. [DOI] [PubMed] [Google Scholar]
- Zhang S.-M., Coultas K.A. Identification and characterization of five transcription factors that are associated with evolutionarily conserved immune signaling pathways in the schistosome-transmitting snail Biomphalaria glabrata. Mol. Immunol. 2011;48:1868–1881. doi: 10.1016/j.molimm.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


