Abstract
Purpose
Most primary human ovarian tumors and peritoneal implants, as well as tumor vascular endothelial cells, express the CD44 family of cell surface proteoglycans, the natural ligand for which is hyaluronic acid (HA). Metronomic (MET) dosing, the frequent administration of chemotherapeutics at substantially lower than maximum tolerated doses (MTD), has been shown to result in reduced normal tissue toxicity and to minimize “off-treatment” exposure resulting in an improved therapeutic ratio.
Experimental Design
We tested the hypothesis that HA conjugates of paclitaxel (TXL; HA-TXL) would exert strong anti-tumor effects with MET dosing and induce anti-angiogenic effects superior to those achieved with MTD administration or with free TXL. Female nude mice bearing SKOV3ip1 or HeyA8 ovarian cancer cells were treated intraperitoneally (ip) with MET HA-TXL regimens (or MTD administration to determine therapeutic and biological effects.
Results
All MET HA-TXL-treated mice and the MTD group revealed significantly reduced tumor weights and nodules compared to controls (all p values < 0.05) in the chemotherapy-sensitive models. However, the MTD HA-TXL-treated mice showed significant weight loss compared to control mice, whereas body weights were not affected in the MET groups in HeyA8-MDR model, reflecting reduced toxicity. In the taxane-resistant HeyA8-MDR model, significant reduction in tumor weight and nodule counts was noted in the MET groups, whereas the response of the MTD group did not achieve significance. While both MTD and MET regimens reduced proliferation (Ki-67) and increased apoptosis (TUNEL), only MET treatment resulted in significant reductions in angiogenesis (CD31, microvessel density). Moreover, MET treatment resulted in substantial increases in thrombospondin-1 (Tsp-1), an inhibitor of angiogenesis.
Conclusions
This study demonstrated that MET HA-TXL regimens have substantial antitumor activity in ovarian carcinoma, likely via a predominant anti-angiogenic mechanism.
Keywords: Ovarian carcinoma, metronomic chemotherapy, and CD44
Introduction
Ovarian cancer is the most common cause of death from a gynecologic malignancy (1). The standard first-line management approach for patients with advanced ovarian cancer currently consists of surgery followed by chemotherapy. Unfortunately, most patients will develop recurrent cancer and die as a result of progressive disease. Therefore, new approaches to treatment are needed to improve the outcome for these patients. Recent clinical trial results have provided compelling evidence that intraperitoneal or more frequent administration of drugs results in markedly improved survival in small volume disease patients (2–4).
In the quest for more molecularly-targeted approaches for delivery of chemotherapy, a variety of approaches are being developed. Hyaluronic acid (HA), a linear polysaccharide of alternating D-glucuronic acid and N-acetyl-D-glucosamine units, is the only non-sulfated glycosaminoglycan and occurs primarily in vivo as sodium hyaluronate (5, 6). HA plays important roles in cell adhesion, growth, and migration (7, 8). HA also acts as a signaling molecule in cell motility, inflammation, wound healing, and cancer metastasis (8). Most malignant solid tumors contain elevated levels of HA, and these high levels of HA production provide a matrix that facilitates invasion (6, 9). HA internalization is mediated via matrix receptors, including the CD44 family, transmembrane receptors that can communicate cell-matrix interactions into cells and can alter the matrix in response to intracellular signals (6). HA is also closely related to angiogenesis in many types of tumors, in which HA receptors, primarily CD44, are abundantly overexpressed on the cell surface (10). Thus, malignant cells with high metastatic potential often exhibit enhanced binding and uptake of HA (7, 10, 11). Using HA as a drug carrier should thus combine the advantages of both the passive (11–16) and active targeting ability of a polymeric prodrug (6). Moreover, coupling of antitumor agents to HA can provide advantages in drug solubilization, stabilization, localization, and controlled release (6, 12).
The CD44 proteoglycan family is expressed in as high as 90% of fresh samples from primary human ovarian tumors or peritoneal implants (5, 13–19), and a hydrophilic HA backbone can give paclitaxel (TXL) aqueous solubility without use of an excipient, such as cremophor in paclitaxel (5). Elimination of cremophor is particularly important as it may interfere with the anti-angiogenic activity of taxanes (20, 21). Our earlier study indicated that HA-TXL, administrated intraperitoneally in a single injection at near maximum tolerated dose (MTD) levels in peritoneally-implanted CD44(+) human ovarian carcinoma mouse models, resulted in markedly reduced tumor burden (5). Standard chemotherapeutic regimens are designed to deliver the highest or maximum tolerated dose (MTD), which can be safely administered and is generally repeated in cycles. Usually, 3–4 weeks of rest periods are needed between treatments for recovery and to minimize additive toxicity, because of indiscriminant effects of chemotherapeutics on normal tissues. However, recent studies indicate that tumor-associated endothelial cells (EC) continue to proliferate and promote cancer growth between treatments (22–24), and may also be accompanied by recruitment of EC progenitors from the bone marrow to the tumor (25); thus, the rest periods may allow repair and re-growth of the tumor vasculature. Moreover, unexpected delays (“treatment holidays”) requiring dose-reduction or concomitant bone marrow support occur frequently in dose-intensive strategies. Hence, as an alternative to current MTD-based chemotherapy dosing schedules, metronomic (MET) dosing is being evaluated. MET dosing involves the frequent administration of chemotherapeutics at substantially lower doses than their MTDs. Ideally, the strategy would result in reduced normal tissue toxicity and minimize “off-treatment” exposure and risk of re-growth, resulting in an improved therapeutic ratio (22, 24). MET dosing of cytotoxic agents is believed to function in an anti-angiogenic manner, because the frequent, low-dose administration appears to target tumor associated ECs (26, 27).
We have previously demonstrated the superior efficacy/toxicity profile of single MTD HA-paclitaxel (HA-TXL) treatment compared to weekly dosing of paclitaxel in orthotopic human ovarian carcinomas (5, 28) and of weekly HA-TXL vs. paclitaxel in head and neck squamous cell carcinoma models (29). The advantages of MET vs. MTD dosing of free taxanes have also been shown in ovarian carcinoma models (30). Here, we demonstrate the superior antitumor activity of MET HA-TXL in multiple orthotopic mouse models of ovarian carcinoma.
Materials and Methods
Cell lines and culture
The human epithelial ovarian cancer cell lines SKOV3ip1, HeyA8 and HeyA8-MDR (taxane-resistant) have been described previously (31, 32). SKOV3ip1 and HeyA8 cells were cultured in RPMI-1640 medium supplemented with 15% fetal bovine serum (FBS) and 0.1% gentamicin sulfate (Gemini Bioproducts, Calabasas, CA). HeyA8-MDR cell lines were maintained in RPMI-1640 medium supplemented with 15% FBS, 0.1% gentamicin sulfate, and 300ng/ml of paclitaxel to sustain taxane-resistance.
Cytotoxicity analyses of response to continuous or MET HA-TXL
SKOV3ip1 cells were cultured overnight in 96-well plates in 100 μl of medium before drug treatment, as previously described (26). Based on preliminary experiments, cell numbers were adjusted to 1 × 104 cells/well to achieve subconfluent control cell monolayers at the end of the assay. The cytotoxic effects of HA-TXL were established using either continuous exposure for 144 hr to a dose range of drug up to 500 ng/ml (TXL equivalents), or to three cycles of 48 hr exposure to either 10 or 100 ng/ml HA-TXL (followed by washout each time), to mimic frequent, lower dose MET scheduling. Remaining viable cells were stained with MTT after 144 hr, and the percentage of control survival as measured by optical density of incorporated dye was determined.
HA-TXL Synthesis
HA (~40 kDa) was provided by K3 Corporation (Great Falls, VA). 1-Ethyl-3-[3V-(dimethylamino)propyl]carbodiimide (EDCI), diphenylphosphoryl chloride, adipic dihyrazide (ADH), succinic anhydride, N-hydroxysuccinimide (NHS), and triethylamine were purchased from Sigma-Aldrich Co. (Milwaukee, WI). Paclitaxel (TXL) was purchased from HandeTech Development Co. (Houston, TX). All solvents were of reagent or HPLC grade. HA-TXL was synthesized as previously described (5), using a modification of the previously published multi-step synthesis (5, (6, 33)
Orthotopic mouse models of human ovarian cancer
Female athymic nude mice (NCr-nu) were purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD) and housed in specific pathogen-free conditions. The mice were cared for in accordance with guidelines set forth by the American Association for Accreditation for Laboratory Animal Care and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. All mouse studies were approved and supervised by the MDACC Institutional Animal Care and Use Committee.
The development and characterization of the orthotopic models of advanced ovarian cancer used in these experiments have been previously described by our laboratory (34). Ovarian cancer (SKOV3ip1) cells harvested in log-phase growth were injected intraperitoneally (i.p.) in 200 μL of cell suspension. One week later, treatment was performed by i.p. injection according to the following groups (10 mice/group): 1) Control/PBS, 2) metronomic (MET) HA-TXL, 10 mg/kg, 3) MET HA-TXL, 20 mg/kg, 4) MET HA-TXL, 30 mg/kg, 5) MTD HA-TXL, single injection of 180 mg/kg, all HA-TXL doses being in TXL equivalents. Since the MET groups were to be given nine injections, the 20 mg/kg group was total dose-equivalent to the 180 mg/kg MTD group, and the lower dose was 50% of this level, and the higher, 150%. The HeyA8 and HeyA8-MDR groups were treated as follows: 1) Control/PBS, 2) MET HA-TXL, 5 mg/kg, 3) MET HA-TXL, 10 mg/kg, 4) MET HA-TXL, 20 mg/kg, 5) MTD HA-TXL, single injection of 180 mg/kg. Thus, the total doses for the MET groups corresponded to 25%, 50% and 100% of the MTD dose. To evaluate the effects of HA-TXL based therapy on bulkier disease, we also carried out experiments where treatment was started 12 days following tumor cell injection (HeyA8-MDR). To assess the role of the HA/CD44 axis in the specificity of anti-tumor effects resulting from HA-TXL therapy, mice were pre-treated with excess free HA immediately prior to injection of HA-TXL, both given i.p. For assessing effects on survival, treatment was continued until each animal became moribund (the experiment was terminated at 60 days post-tumor implantation). To compare the effects of HA-TXL to free paclitaxel (TXL), the free TXL was dosed either in MET (0.5 mg/kg) or MTD (10 mg/kg) fashion. Before injection, the initial weights of mice were measured and dose adjustments were performed according to mean weight of each group. MET therapy groups were injected i.p. every other day, beginning either on Day 7 or day 14.. Body weights were measured every week, beginning on Day 7. The aliquots of lyophilized HA-TXL were solubilized in PBS immediately prior to injection to ensure chemical stability. All animals were sacrificed and a necropsy performed when mice from any of the groups became moribund (typically 3–6 weeks after therapy initiation depending on the model). The individuals performing the necropsy were blinded to the different groups. Tumors were harvested and fixed in formalin for paraffin embedding or snap-frozen in optimal cutting medium (OCT; Miles, Inc., Elkhart, IN) for immunohistochemical analyses.
Immunohistochemistry
Immunohistochemical analyses of CD31, Ki-67, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), and thrombospondin-1 (Tsp-1) were conducted on 4-μm-thick frozen or formalin-fixed paraffin embedded tumor specimens. Primary antibodies used included: CD31 (rat monoclonal anti-mouse, 1:800 dilution; BD Bioscience, Pharmingen, San Jose, CA); Ki-67 (rabbit polyclonal anti-human, 1:200 dilution: Biocare Medical, Concord, CA); Tsp-1 (1:25 dilution, Abcam: Cambridge, MA); TUNEL (Promega, Madison, WI). Quantification of microvessel density (MVD/CD31), tumor cell proliferation (Ki-67), Tsp-1 and apoptosis (TUNEL) was performed on slides from each treatment group, as previously described (34, 35). For assessment of EC apoptosis, frozen sections were stained for CD31 (red) followed by TUNEL (green), as described previously (36).
Determination of cytokines
Multiplex ELISA was carried out based on the manufacturer’s instruction for detecting both human and mouse IL-6, G-CSF, and VEGF in plasma obtained from the control and treated groups. The MILLIPLEX® MAG Human Cytokine /Chemokine panel (Millipore Corporation, Billerica, MA) allows us to quantitative multiplex detection of dozens of analytes simultaneously, which can dramatically improve productivity. Mouse plasma was collected at Day 7 (basal), Day 14 (seven days after starting treatment) and at the time of euthanasia. Blood sampling was performed using a glass pipette coated with anticoagulant (EDTA) from the orbital sinus under anesthesia. Collected blood samples were centrifuged at 2000 rpm for 10 minutes at room temperature. Tsp-1 levels were detected using an ELISA kit (R&D Systems, IN., Minneapolis, MN).
Statistics
Continuous variables were compared with the Student’s t test (between two groups) or ANOVA (for all groups) if normally distributed, and the Mann-Whitney rank-sum test or Kruskal-Wallis test (for all groups) if non-parametric. Pairwise differences in normally distributed variables in our treatment groups were compared by the Tukey-Kramer statistic for multiple comparisons. A Bonferoni adjustment to a (default value 0.05) was made based on the number of pairwise comparisons within a treatment experiment using the formula: a(α) = 0.05/k, where k = number of comparisons against control. For in vivo therapy experiments, 10 mice in each group were used, as directed by a power analysis to detect a 50% reduction in tumor size (β-error 0.2). A p < 0.05 on two-tailed testing was considered significant.
Results
In vivo effects of HA-TXL therapy
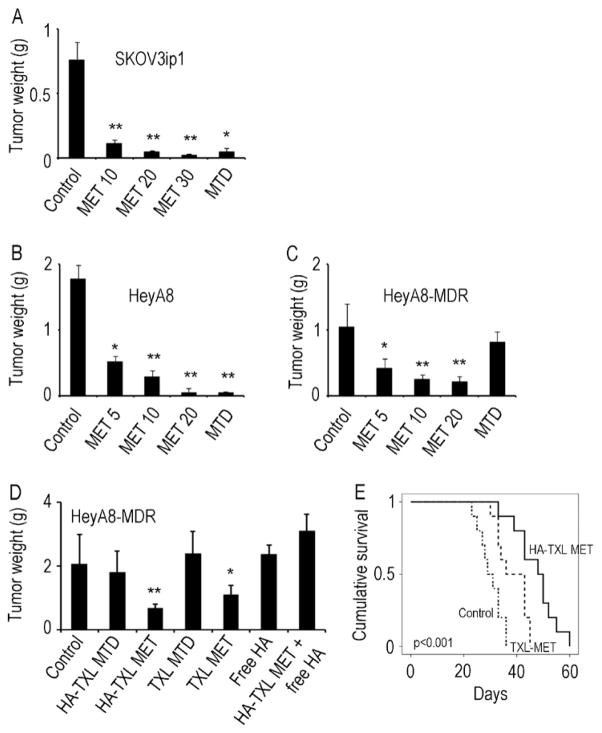
To compare the effects of MET versus MTD HA-TXL therapy in taxane-sensitive ovarian cancer models, we first utilized the human SKOV3ip1 model. The MET regimens (10 mg/kg, 20 mg/kg, and 30 mg/kg) and MTD regimen (180 mg/kg) all reduced tumor weights by 88–96% as compared to PBS-treated mice (p < 0.01 for all: Fig. 1A). There were no significant differences in tumor growth among the MET and MTD dosing groups. As with the tumor weights, the number of tumor nodules was reduced significantly when mice were treated with any of the MET regimens as well as with the MTD regimen compared to the PBS-treated control group (p < 0.01 for all; Supplementary Fig. S1). We also examined the effects of MET therapy in the HeyA8 model. Here, we also used a MET 5 mg/kg arm since there was substantial efficacy noted at the 10 mg/kg dose in the previous experiment, and dropped the 30 mg/kg group. There was significant reduction in tumor growth in all MET treatment groups following three weeks of therapy (Fig. 1B).
Figure 1.
Effects of metronomic (MET) dosing and maximal tolerated dosing (MTD) of HA-TXL on tumor growth in SKOV3ip1 (A), HeyA8 (B) and HeyA8-MDR (C) models of ovarian cancer. Mice were randomly allocated to five groups and underwent treatment with intraperitoneal injection as follows for the SKOV3ip1 model: 1) Control PBS, 2) MET HA-TXL, 10 mg/kg, 3) MET HA-TXL, 20 mg/kg, 4) MET HA-TXL, 30 mg/kg, 5) MTD HA-TXL 180 mg/kg, all HA-TXL doses being given as TXL equivalents. For the HeyA8 and HeyA8-MDR studies, the MET groups were lowered to 5, 10 and 20 mg/kg. For comparison of HA-TXL to TXL, the HeyA8-MDR model was used (D) according to the following groups: 1) Control, 2) HA-TXL MTD (180 mg/kg), 3) HA-TXL MET (20 mg/kg), 4) TXL MTD (10mg/kg), 5) TXL MET (0.5 mg/kg), 6) Free HA, 7) HA-TXL MET + free HA. Effects of TXL-MET or HA-TXL MET were assessed on animal survival in the HeyA8-MDR model (E). Values are means + SEM. *p<0.01; **p<0.001.
Given the clinical reality of tumor resistance to chemotherapy in women with relapsed ovarian cancer, we also examined the effects of HA-TXL therapy in the taxane-resistant HeyA8-MDR model. While there was only non-significant, modest reduction in tumor growth in the MTD group, there was 60 – 78% reduction in tumor growth in the MET dosing groups (p values < 0.05 Fig. 1C). The tumor nodule count generally mirrored the tumor weight pattern (Supplementary Fig. S1). There was ~15% reduction in body weight of mice in the MTD group, but no significant effects on body weight were noted in the HeyA8-MDR model with MET treatment (Supplementary Fig. S1D), suggesting that feeding habits and general health were not affected by highly efficacious MET therapy. Additional experiments were performed to characterize the anti-tumor effects of HA-TXL compared to free paclitaxel (TXL). Moreover, to examine the effects on bulky, drug-resistant disease, treatment was started 12 days following tumor cell injection. MTD HA-TXL or TXL had no significant effect on HeyA8-MDR tumor growth (Fig. 1D). MET dosing with either HA-TXL or TXL resulted in significant reduction in tumor growth. The reduction in tumor growth was greater (by 39%) in the HA-TXL MET group compared to the TXL MET group (p=0.03; Fig. 1D). To assess the specificity of the effects of HA-TXL and dependence on CD44 binding, we also used free HA alone or in combination with HA-TXL MET. Free HA completely blocked the efficacy of HA-TXL MET (Fig. 1D). Complete blood count analysis revealed that apart from a modest decrease in WBC with TXL-MTD, there were no significant effects on WBC or hemoglobin in the other groups (Supplementary Fig. S2).
Next, we examined potential effects of HA-TXL on animal survival in the HeyA8-MDR model. Since MTD regimens were ineffective, we focused on the MET regimens. Treatment was started 12 days following tumor cell injection and continued until each animal became moribund. The greatest improvement in survival was noted in the HA-TXL MET group (mean survival: control, 30.1 days, TXL-MET, 38.5 days; HA-TXL MET, 47.3 days; p<0.001; Fig. 1E).
Effects of MD and MTD HA-TXL therapy on tumor microenvironment
We next assessed the effects of treatment on the tumor microenvironment. Tumors from the above experiments were examined for proliferation (Ki67), apoptosis (TUNEL), microvessel density (MVD, CD31), and apoptosis of endothelial cells (CD31/TUNEL). For the SKOV3ip1 model, there was a significant reduction in proliferation in all of the treatment groups (p < 0.05), MET and MTD (Fig. 2A), but significantly reduced MVD was noted only in the MET groups (Fig. 2B, p < 0.05). Increased TUNEL staining, indicating tumor cell apoptosis, was noted in all of the treatment groups (Fig. 2C). Since reduced MVD was noted primarily in the MET groups, we also examined the effects of MET versus MTD dosing on EC apoptosis. For this experiment, mice with established HeyA8-MDR tumors (17 days following tumor cell injection) were treated with either a single dose of MTD HA-TXL or with three doses of MET HA-TXL prior to resecting the tumors for fluorescence microscopy. There were significantly greater effects on EC apoptosis in the higher dose MET HA-TXL groups compared to the MTD arm, reflecting an anti-angiogenic effect (Supplementary Fig. S2E).
Figure 2.
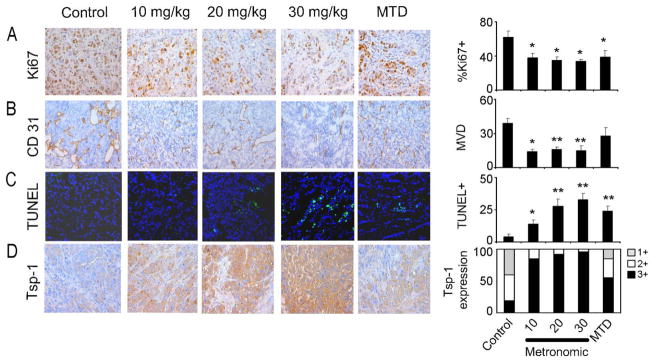
Effects of MET and MTD HA-TXL treatment in the SKOV3ip1 model on biological endpoints, including (A) cell proliferation (Ki67); (B) MVD (CD31); (C) apoptosis (TUNEL) and (D) TSP-1. Tumors harvested following 3–4 weeks of therapy were stained for Ki67, CD31, TSP-1 and TUNEL. All pictures were taken at original magnification × 200. The error bars represent SEM. *p<0.05; **p<0.001
Some studies have reported increases in anti-angiogenic factors, such as thrombospondin-1 (Tsp-1), following treatment with MET chemotherapy (37, 38). Tumors harvested from the SKOV3ip1 model were subjected to immunostaining for Tsp-1. There were marked increases in Tsp-1 expression following treatment with MET HA-TXL compared to the MTD group (Fig. 2D), which is consistent with the observed anti-angiogenic effects. Similar effects of MTD versus MET dosing were noted in the HeyA8-MDR model (Supplementary Fig. S3). By ELISA, there was a 35% increase in tumoral TSP-1 levels in the HA-TXL MTD group and a 275% increase in the HA-TXL MET group.
Effects of HA-TXL therapy on systemic cytokine production
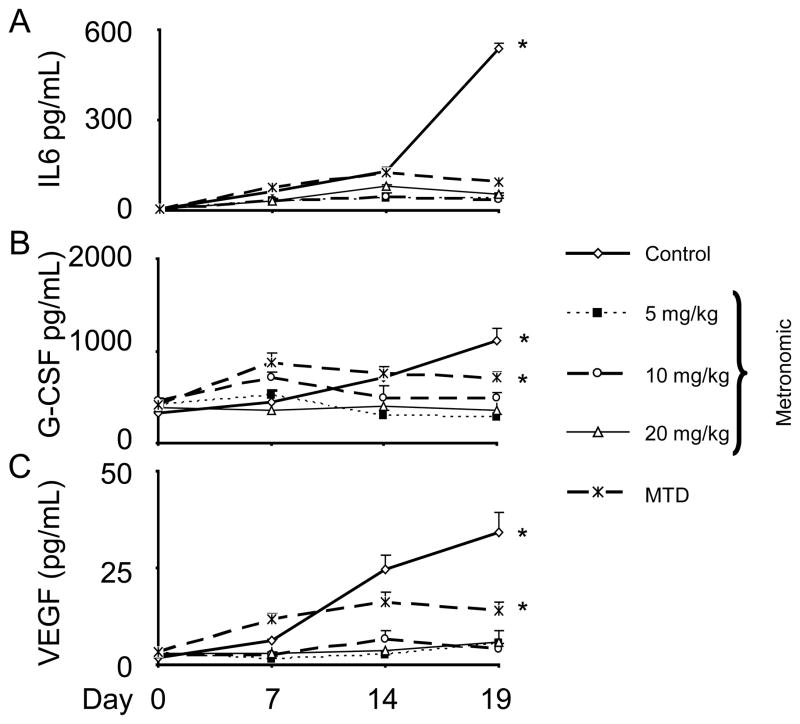
Next, we examined the impact on selected angiogenic factors longitudinally in the treatment groups described above. Specifically, we examined plasma IL-6, G-CSF, and VEGF levels prior to the start of treatment and at days 7, 14, and 19, during treatment. As expected, there was a progressive increase in these cytokines in the untreated controls (Fig. 3). Interestingly, the cytokine levels remained lower throughout in the MET dosing groups compared to the MTD treatment group.
Figure 3.
Effects of HA-TXL therapy on systemic cytokine production. Plasma was collected from HeyA8 model at day 7 post-tumor implantation (basal), day 14 (7 days after startingtreatment) and at the time of euthanasia. Multiplex ELISA was carried out based on the manufacturer’s instruction for detecting human and mouse IL-6 (A), G-CSF (B), and VEGF (C) in plasma obtained from the control and treated groups. There was a progressive increase in these cytokines in the untreated controls; however, the cytokine levels remained lower throughout in the MET dosing groups compared to the MTD treatment group. *p<0.05. Error bars represent SEM.
In vitro cytotoxic effects of continuous vs. pulsed HA-TXL treatment
We have previously shown that SKOV3ip1 cells are somewhat sensitive to brief (4 hr) treatment with HA-TXL, demonstrating ~20% cytotoxicity (~80% survival) at a drug concentration of 500 ng/ml after further incubation for 72 hr (12). Here SKOV3ip1 cells were either exposed continuously to a concentration range of up to 500 ng/ml of HA-TXL for a total of six days, or were treated with three consecutive 48 hr pulses of HA-TXL, to mimic in vivo MET scheduling, at either 10 or 100 ng/ml HA-TXL. Results indicated that the lower MET HA-TXL concentration had minimal effects (>90% survival); however, the higher MET HA-TXL concentration decreased survival to ~20% at this time point, markedly lower than the ~55% survival with continuous exposure to 500 ng/ml HA-TXL (data not shown). These data suggest that while there are modest direct effects of MET therapy on cancer cells, the robust in vivo efficacy is likely to reflect both direct (tumor cell apoptosis) and indirect (anti-angiogenic) effects.
Discussion
The key findings of this study are that MET HA-TXL therapy was generally more effective than MTD HA-TXL in reducing tumor growth in murine xenograft models of advanced TXL-sensitive human ovarian cancer, as well as being less toxic, resulting in a higher therapeutic index. Moreover, MET HA-TXL therapy also demonstrated such anti-tumor effects in a murine xenograft model bearing TXL-resistant human ovarian cancer, whereas the MTD arm was ineffective, both in terms of tumor volume and tumor nodule counts. All of the MET regimens were able to decrease cell proliferation and angiogenesis while increasing tumor cell apoptosis and markedly increasing expression of the inhibitor of angiogenesis, Tsp-1. We had hypothesized that MET HA-TXL therapy would be effective on both TXL-sensitive and TXL-resistant CD44(+) ovarian carcinoma cells, since drug resistance in the epithelial tumor compartment should not affect the susceptibility of the tumor ECs to MET treatment. Both body weights and plasma IL-6, G-CSF, and VEGF levels indicated that MET schedules were less toxic than the MTD arm.
In a recent study (5), we used orthotopic (i.p.) human ovarian carcinoma xenografts and administration of MTD HA-TXL locoregionally (also i.p.). HA could be regarded as a targeted backbone by which TXL might be delivered to CD44(+) tumor cells that would allow efficient and specific receptor-mediated prodrug uptake and internalization by CD44, as shown in in vitro binding studies (5). However, establishing the precise extent of the HA/CD44 interaction in the in vivo anti-tiumor activity of HA-TXL is rendered complex, due to the absence of established CD44(−) human ovarian carcinoma xenograft models. It has been suggested that HA binds best to parental CD44, and that the additional amino acid sequences neighboring the HA-binding domain in the splice variants may interfere with this binding. This has been postulated to reside in electrostatic repulsions between negatively-charged HA and sialic acid residues that tail the amino acids of the sequence. However, this is not a universal observation and remains rather controversial. For example, there is also evidence that such differences in rank order of binding efficiency are cell-type specific, and may even be influenced by cellular exposure to cytokines and activation of other signaling networks. Ongoing clinical trials with HA-encapsulated irinotecan have not revealed such issues in colorectal cancer patients (39).
Further, by using free HA alone or in combination with MET HA-TXL, we found that free HA completely blocked the efficacy of MET HA-TXL, underscoring a key role for receptor-specific uptake and internalization of the conjugate. The HA backbone itself might have a direct role in the anti-tumor effect of HA-TXL; that is, HA may induce anoikis by disrupting CD44(+) tumor cell-extracellular matrix interactions (5, 40). However, we did not observe any cytotoxicity from HA alone in in vitro assays (5), nor was an HA monotherapy arm active against orthotopic human head and neck squamous cell carcinoma xenografts (29), nor in the current study (Fig. 1E).
MET dosing of chemotherapeutics has been demonstrated to reduce normal tissue toxicity and minimize off-treatment exposure. Several clinical trials have documented that frequent lower dosing has low toxicity (41) and improved pathologic response rates in breast cancer patients (42). A previous pre-clinical study on MET taxane therapy reported superiority to MTD in both taxane-sensitive and –resistant ovarian cancer models (30). Another study on MET topotecan in ovarian cancer models showed the same effect as MTD in reducing tumor growth with tolerable toxicity (22). Of mechanistic significance, clinical studies in ovarian and breast cancer suggest that the major therapeutic impact of MET dosing may be on tumor ECs, and not on the tumor cells themselves (30, 42, 43). MET regimens may have an inhibitory effect on the mobilization and viability of bone marrow-derived circulating endothelial precursor cells (25).
In the current study, we demonstrated that all MET HA-TXL treatment groups reduced tumor weights and tumor nodule counts in the TXL-resistant HeyA8-MDR bearing model. We designed the study so that the MET 5, 10 and 20 mg/kg HA-TXL groups had 25%, 50% and 100%, respectively, of the TXL equivalents in the MTD 180 mg/kg group. Even the lowest dose MET HA-TXL (5 mg/kg) group was more effective than the MTD arm, indicating that MET HA-TXL treatment could overcome TXL-resistance in this ovarian carcinoma model, most likely due to the anti-vascular effects of MET HA-TXL chemotherapy. In that regard, the ability of even the lowest MET dose group to strongly induce Tsp-1 expression compared to the MTD arm is remarkable, and aligns well with the patterns of reduced MVD, which is consistent with other reports (37, 38). A possible explanation might be that activated endothelial cells and circulating endothelial precursors are directly killed by MET chemotherapy, but not by MTD treatment. The induction of TSP1 by MET dosing is likely related to p53 upregulation by DNA-damaging activity (38). However, whether Tsp-1 could be a surrogate marker to predict the response to MET treatment in ovarian cancer patients is still controversial (44).
Other formulations of HA-bound paclitaxel are also under development (45, 46), including evaluation in ovarian tumor models (47–49), and reaching initial clinical testing in BCG-refractory bladder cancer (50). The latter, ONCOFID-P-B™, uses a longer HA chain (~200 kDa) than employed in our studies (~40 kDa) and has a higher paclitaxel loading (~20% vs. 10–12%), and all of the pre-clinical tumors studies with this formulation have employed MTD or near-MTD dosing. This confounds direct comparisons to our results with HA-TXL.
In summary, we have shown the efficacy of MET HA-TXL to inhibit tumor growth using several orthotopic ovarian cancer models, and in particular, that MET HA-TXL could be effective against taxane-resistant ovarian carcinoma. These effects are thought to be mediated by anti-proliferative and pro-apoptotic effects against the epithelial tumor cells themselves of the regimen, as well as the anti-vascular effects of MET dosing. Assessment of Tsp-1 may implicate a non-invasive biomarker of therapeutic response. Our data suggest that MET HA-TXL is highly efficacious and should be considered for future clinical trials.
Supplementary Material
Translational Relevance.
Most patients with advanced ovarian cancer eventually develop resistance to conventional chemotherapy despite MTD regimens. Metronomic (MET) dosing of selected agents has been shown to result in reduced normal tissue toxicity and to minimize “off-treatment” exposure, resulting in an improved therapeutic ratio. In the present study, we have shown the efficacy of MET CD44-targeted, HA-conjugated paclitaxel (HA-TXL) to inhibit the growth of taxane-sensitive and -resistant ovarian carcinoma. These effects are thought to be mediated by anti-proliferative and pro-apoptotic effects against the epithelial tumor cells, as well as the anti-vascular effects of MET dosing. Our findings suggest that MET HA-TXL is highly efficacious and should be considered for future clinical trials.
Acknowledgments
Financial Support:
Portions of this work were also supported by the NIH (P50 CA083639, P50 CA098258, CA128797, RC2GM092599, U54 CA151668), the DOD (OC073399, W81XWH-10-1-0158, BC085265), the Marcus Foundation, a Program Project Development Grant from the Ovarian Cancer Research Fund, Inc., the Marcus Foundation, the University of Texas MD Anderson Cancer Center Institutional Research Grant (IRG) Program, and the Betty Anne Asche Murray Distinguished Professorship.
The authors thank Donna Reynolds and Dr. Robert Langley for their insightful discussions and expertise.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Lee JW, Han HD, Shahzad MM, Kim SW, Mangala LS, Nick AM, et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2009;101:1193–205. doi: 10.1093/jnci/djp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33:S3–11. doi: 10.1053/j.seminoncol.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Auzenne E, Ghosh SC, Khodadadian M, Rivera B, Farquhar D, Price RE, et al. Hyaluronic acid-paclitaxel: antitumor efficacy against CD44(+) human ovarian carcinoma xenografts. Neoplasia. 2007;9:479–86. doi: 10.1593/neo.07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Y, Ziebell MR, Prestwich GD. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules. 2000;1:208–18. doi: 10.1021/bm000283n. [DOI] [PubMed] [Google Scholar]
- 7.Entwistle J, Hall CL, Turley EA. HA receptors: regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61:569–77. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Xin D, Wang Y, Xiang J. The use of amino acid linkers in the conjugation of paclitaxel with hyaluronic acid as drug delivery system: synthesis, self-assembled property, drug release, and in vitro efficiency. Pharm Res. 27:380–9. doi: 10.1007/s11095-009-9997-9. [DOI] [PubMed] [Google Scholar]
- 9.Knudson W. Tumor-associated hyaluronan. Providing an extracellular matrix that facilitates invasion. Am J Pathol. 1996;148:1721–6. [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Lee K, Park TG. Hyaluronic acid-paclitaxel conjugate micelles: synthesis, characterization, and antitumor activity. Bioconjug Chem. 2008;19:1319–25. doi: 10.1021/bc8000485. [DOI] [PubMed] [Google Scholar]
- 11.Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. J Cell Sci. 1993;106 (Pt 1):365–75. doi: 10.1242/jcs.106.1.365. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Seymour LW, Miyamoto Y. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjug Chem. 1992;3:351–62. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- 13.Cannistra SA, Abu-Jawdeh G, Niloff J, Strobel T, Swanson L, Andersen J, et al. CD44 variant expression is a common feature of epithelial ovarian cancer: lack of association with standard prognostic factors. J Clin Oncol. 1995;13:1912–21. doi: 10.1200/JCO.1995.13.8.1912. [DOI] [PubMed] [Google Scholar]
- 14.Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53:3830–8. [PubMed] [Google Scholar]
- 15.Gardner MJ, Catterall JB, Jones LM, Turner GA. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis. 1996;14:325–34. doi: 10.1007/BF00123391. [DOI] [PubMed] [Google Scholar]
- 16.Kayastha S, Freedman AN, Piver MS, Mukkamalla J, Romero-Guittierez M, Werness BA. Expression of the hyaluronan receptor, CD44S, in epithelial ovarian cancer is an independent predictor of survival. Clin Cancer Res. 1999;5:1073–6. [PubMed] [Google Scholar]
- 17.Sillanpaa S, Anttila MA, Voutilainen K, Tammi RH, Tammi MI, Saarikoski SV, et al. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin Cancer Res. 2003;9:5318–24. [PubMed] [Google Scholar]
- 18.Stickeler E, Runnebaum IB, Mobus VJ, Kieback DG, Kreienberg R. Expression of CD44 standard and variant isoforms v5, v6 and v7 in human ovarian cancer cell lines. Anticancer Res. 1997;17:1871–6. [PubMed] [Google Scholar]
- 19.Yeo TK, Nagy JA, Yeo KT, Dvorak HF, Toole BP. Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol. 1996;148:1733–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44. [PubMed] [Google Scholar]
- 21.Ng SS, Sparreboom A, Shaked Y, Lee C, Man S, Desai N, et al. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res. 2006;12:4331–8. doi: 10.1158/1078-0432.CCR-05-2762. [DOI] [PubMed] [Google Scholar]
- 22.Merritt WM, Danes CG, Shahzad MMK, Lin YG, Kamat AA, Han LY, et al. Anti-angiogenic properties of metronomic topotecan in ovarian carcinoma. Cancer Biology & Therapy. 2009;8:1–8. doi: 10.4161/cbt.8.16.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–40. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 24.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 25.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–6. [PubMed] [Google Scholar]
- 26.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–7. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonini G, Schiavon G, Silletta M, Vincenzi B, Santini D. Antiangiogenic properties of metronomic chemotherapy in breast cancer. Future Oncol. 2007;3:183–90. doi: 10.2217/14796694.3.2.183. [DOI] [PubMed] [Google Scholar]
- 28.Klostergaard J, Auzenne E, Ghosh S, Farquhar D, Rivera B, Price RE. Magnetic resonance imaging-based prospective detection of intraperitoneal human ovarian carcinoma xenografts treatment response. Int J Gynecol Cancer. 2006;16 (Suppl 1):111–7. doi: 10.1111/j.1525-1438.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 29.Galer CE, Sano D, Ghosh SC, Hah JH, Auzenne E, Hamir AN, et al. Hyaluronic acid-paclitaxel conjugate inhibits growth of human squamous cell carcinomas of the head and neck via a hyaluronic acid-mediated mechanism. Oral Oncol. 47:1039–47. doi: 10.1016/j.oraloncology.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 30.Kamat AA, Kim TJ, Landen CN, Jr, Lu C, Han LY, Lin YG, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281–8. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 31.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 18:185–97. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug Chem. 1999;10:755–63. doi: 10.1021/bc9900338. [DOI] [PubMed] [Google Scholar]
- 34.Kim TJ, Ravoori M, Landen CN, Kamat AA, Han LY, Lu C, et al. Antitumor and antivascular effects of AVE8062 in ovarian carcinoma. Cancer Res. 2007;67:9337–45. doi: 10.1158/0008-5472.CAN-06-4018. [DOI] [PubMed] [Google Scholar]
- 35.Ioachim E, Michael MC, Salmas M, Damala K, Tsanou E, Michael MM, et al. Thrombospondin-1 expression in urothelial carcinoma: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. BMC Cancer. 2006;6:140. doi: 10.1186/1471-2407-6-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, et al. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–83. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- 37.Ng SS, Figg WD. Upregulation of endogenous angiogenesis inhibitors: a mechanism of action of metronomic chemotherapy. Cancer Biol Ther. 2004;3:1212–3. doi: 10.4161/cbt.3.12.1369. [DOI] [PubMed] [Google Scholar]
- 38.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci U S A. 2003;100:12917–22. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbs P, Brown TJ, Ng R, Jennens R, Cinc E, Pho M, et al. A pilot human evaluation of a formulation of irinotecan and hyaluronic acid in 5-fluorouracil-refractory metastatic colorectal cancer patients. Chemotherapy. 2009;55:49–59. doi: 10.1159/000180339. [DOI] [PubMed] [Google Scholar]
- 40.Herrera-Gayol A, Jothy S. Effect of hyaluronan on xenotransplanted breast cancer. Exp Mol Pathol. 2002;72:179–85. doi: 10.1006/exmp.2002.2437. [DOI] [PubMed] [Google Scholar]
- 41.Nardi M, Azzarello D, Maisano R, Del Medico P, Giannicola R, Raffaele M, et al. FOLFOX-4 regimen as fist-line chemotherapy in elderly patients with advanced gastric cancer: a safety study. J Chemother. 2007;19:85–9. doi: 10.1179/joc.2007.19.1.85. [DOI] [PubMed] [Google Scholar]
- 42.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–92. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 43.Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18:1212–9. doi: 10.1200/JCO.2000.18.6.1212. [DOI] [PubMed] [Google Scholar]
- 44.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 45.He M, Zhao Z, Yin L, Tang C, Yin C. Hyaluronic acid coated poly(butyl cyanoacrylate) nanoparticles as anticancer drug carriers. Int J Pharm. 2009;373:165–73. doi: 10.1016/j.ijpharm.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Rivkin I, Cohen K, Koffler J, Melikhov D, Peer D, Margalit R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials. 31:7106–14. doi: 10.1016/j.biomaterials.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 47.Banzato A, Bobisse S, Rondina M, Renier D, Bettella F, Esposito G, et al. A paclitaxel-hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin Cancer Res. 2008;14:3598–606. doi: 10.1158/1078-0432.CCR-07-2019. [DOI] [PubMed] [Google Scholar]
- 48.Banzato A, Rondina M, Melendez-Alafort L, Zangoni E, Nadali A, Renier D, et al. Biodistribution imaging of a paclitaxel-hyaluronan bioconjugate. Nucl Med Biol. 2009;36:525–33. doi: 10.1016/j.nucmedbio.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 49.De Stefano I, Battaglia A, Zannoni GF, Prisco MG, Fattorossi A, Travaglia D, et al. Hyaluronic acid-paclitaxel: effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother Pharmacol. 68:107–16. doi: 10.1007/s00280-010-1462-2. [DOI] [PubMed] [Google Scholar]
- 50.Bassi PF, Volpe A, D’Agostino D, Palermo G, Renier D, Franchini S, et al. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: results of a phase I study. J Urol. 185:445–9. doi: 10.1016/j.juro.2010.09.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.