Novel HRV Predicts CV Mortality in the Elderly
Background
It is unknown whether abnormal heart rate turbulence (HRT) and abnormal fractal properties of heart rate variability identify older adults at increased risk of cardiovascular death (CVdth).
Methods
Data from 1,172 community-dwelling adults, ages 72 ± 5 (65–93) years, who participated in the Cardiovascular Health Study (CHS), a study of risk factors for CV disease in people ≥65 years. HRT and the short-term fractal scaling exponent (DFA1) derived from 24-hour Holter recordings. HRT categorized as: normal (turbulence slope [TS] and turbulence onset [TO] normal) or abnormal (TS and/or TO abnormal). DFA1 categorized as low (≤1) or high (>1). Cox regression analyses stratified by Framingham Risk Score (FRS) strata (low = <10, mid = 10–20, and high >20) and adjusted for prevalent clinical cardiovascular disease (CVD), diabetes, and quartiles of ventricular premature beat counts (VPCs).
Results
CVdths (N = 172) occurred over a median follow-up of 12.3 years. Within each FRS stratum, low DFA1+abnormal HRT predicted risk of CVdth (RR=7.7 for low FRS; 3.6, mid FRS; 2.8, high FRS). Among high FRS stratum participants, low DFA1 alone also predicted CVdth (RR = 2.0). VPCs in the highest quartile predicted CVdth, but only in the high FRS group. Clinical CV disease predicted CVdth at each FRS stratum (RR = 2.9, low; 2.6, mid; and 1.9, high). Diabetes predicted CVdth in the highest FRS group only (RR = 2.2).
Conclusions
The combination of low DFA1 + abnormal HRT is a strong risk factor for CVdth among older adults even after adjustment for conventional CVD risk measures and the presence of CVD.
Keywords: risk factor, heart rate variability, elderly, heart rate turbulence, ventricular ectopy
Abnormal heart rate variability (HRV) is an independent risk factor for cardiovascular mortality in clinical1-4 and population-based studies.5,6 These studies have generally used time domain (statistical) or frequency domain (power spectral or autoregressive) measures of HRV that quantify how much HRV is present. More recently, novel measures of HRV have been developed. Among them are the short-term fractal scaling exponent (alpha 1 or DFA1) that quantifies the regularity of the heart rate. Another novel measure, heart rate turbulence (HRT), characterizes the response of heart rate to the perturbations associated with ventricular premature beats. Both of these measures have provided greater predictive power for CVD mortality than traditional HRV measures like SDNN (the standard deviation of normal-to-normal interbeat intervals) in clinical studies of patients with heart failure and in patients postmyocardial infarction.7-10
The ability of these two novel HRV measures to predict CVdth in an older population, with or without CVD, has not been tested. The Cardiovascular Health Study (CHS), a multicenter, population-based study of risk factors for cardiovascular disease (CVD) in older adults offers the opportunity to test the impact of these measures.11 HRV measures were derived from 24-hour Holter monitoring in 1,429 participants ≥65 years old who volunteered to have a recording at the baseline examination and were followed for a median of more than 12 years.
Methods
Study Population
Recruitment methods for the CHS have been published.19 In brief, a random sample of individuals ≥65 years of age, derived from Medicare eligibility lists, and other household members ≥65 years, were invited to participate in the study. Potential participants were excluded if they were institutionalized, were unable to attend clinic visits, or had illnesses that were expected to lead to early death. Five thousand two hundred and one participants were recruited in 1989–1990 (original cohort) and 687 in 1992–1993 to provide additional representation of African Americans (new cohort). All participants signed informed consent upon entry into the study.
The Holter cohort for this study (N=1,429) was recruited from the original cohort at the time of the baseline examination (year 2). Our prior analysis has shown that the demographic and clinical factors in the Holter cohort and the total CHS cohort were similar.12
Baseline Examination
At baseline examination, participants completed standardized interviews and answered questions regarding past medical history.13 They also underwent standardized examinations that included: electrocardiograms, measurement of the anklearm index, blood pressure, and fasting laboratory tests.14 Diabetes was defined as fasting glucose >125 mg/dL or use of hypoglycemic medications. Subjects were followed twice yearly following enrollment.
Covariates
In order to assess the impact of DFA1 and HRT on CVD mortality relative to traditional risk factors for CVD, we adjusted our analyses by the Framingham Risk Score (FRS) that includes CVD risk factors such as age, gender, blood pressure, and cholesterol. The FRS was calculated for each participant based on separate formulas for men and women.15 In addition, because diabetes, a strong risk factor for CVD mortality, is not included in the FRS, the presence of diabetes was added to the models.
Prevalent CVD was defined as a confirmed diagnosis of coronary heart disease, stroke, or heart failure.11 Confirmation of deaths was ascertained through reviews of obituaries, medical records, (including hospital and nursing home records, as well as physician questionnaires), death certificates, and the National Death Index and Health Care Finance Administration (HCFA) health care utilization database for hospitalizations. Through these methods, there was 100% ascertainment of vital status.16 Cause of death was defined as cardiovascular if the underlying cause of mortality was determined to be cardiac or cerebrovascular.16
Ambulatory ECG Monitoring and Assessment of HRV
Holter tapes were recorded on Del Mar Avionics recorders that have a calibrated timing signal and processed by research technicians at the Washington University School of Medicine Heart Rate Variability Laboratory (St. Louis, MO, USA), using a GE Marquette MARS 8000 Holter analyzer (GE-Marquette, Milwaukee, WI, USA). All Holter analyses were reviewed in detail by one of us (PKS), with special attention paid to ensuring that only normal-to-normal (N-N) beats with uniformly detected onsets were included in the HRV analyses. The longest and shortest true N-N intervals were identified for each tape, and intervals outside of these limits, including blocked atrial premature contractions (APCs) as well as ectopic beats, were excluded from all HRV calculations. HRV was calculated from beat-to-beat files exported to a Sun Enterprise 450 server (Sun Microsystems, Santa Clara, CA, USA) using validated research software.
Of the 1,384 tapes with usable ECG data, 36 were excluded because of atrial fibrillation or pacemakers. Forty-eight recordings with a wandering atrial pacemaker or a cardiac rhythm too irregular to accurately identify normal beats were also excluded. Of the remaining tapes, 1,198 were acceptable for 24-hour nonlinear HRV analysis that required at least 18 hours of data with ≥80% normal-to-normal (N-N) intervals. Only four participants were excluded from the analysis because excessive ventricular premature contractions (VPCs) caused them to have <80% N-N intervals.
Heart Rate and Time Domain HRV
To compare the relative ability of traditional and novel HRV measures to predict CV mortality, 24-hour average heart rate and the standard deviation of the intervals between normal heart beats over 24-hours (SDNN; in ms)1 were included in the analyses. Decreased SDNN is an established risk factor for mortality among patients post-MI.1 The number of VPCs was determined for each recording. A high number of VPCs is also a risk factor for post-MI mortality.1
Short-Term Fractal Scaling Exponent
Nonlinear HRV analysis quantifies the randomness or degree of self-similarity of heart rate patterns at different time scales. Detrended fluctuation analysis (DFA) quantifies the short-term self-similar properties of the R-R interval time series.17,18 The short-term fractal scaling exponent (DFA1) is determined for 4–11 beat sequences of R-R interval data.19 DFA1 of 0.5 would indicate a totally random signal and a DFA1 of 1.5 would indicate one that is totally correlated. In the current analysis, DFA1 was calculated from N-N intervals only.
HRT
HRT quantifies the response of the sinus node to VPCs.20 Two indices are calculated: turbulence onset (TO) and turbulence slope (TS). In healthy hearts, there is generally a brief sinus tachycardia after a VPC. TO measures the magnitude of this tachycardia as the percentage of change in N-N interval of the sinus rhythm two beats after the VPC compared to the two beats before. In healthy hearts, this index is negative or zero. Thus, a TO > 0 is abnormal (no immediate tachycardia or possibly bradycardia). TS quantifies the slower oscillation in heart rate (tachycardia, bradycardia, then return to baseline) that follows a VPC as the largest fitted slope of the N-N intervals between any 5 beats within 15 beats of the VPC. These indices require ≥5 VPCS for calculation and are determined as an average of all of the VPCs on the recording. HRT indices are usually analyzed as categorical variables.
The usual cutpoint for abnormal TS is < 2.5 ms/beat and for TO is > 0 based on data from post-MI patients in the ATRAMI study.7 Because we are studying the population-based elderly, we explored other cutpoints for TS and TO, using univariate Cox regression analysis, and found the optimal cut point that separated those who died of CVD causes and those who did not in the CHS to be TS <3 and TO >0. Thus, participants were categorized as having abnormal HRT if either TS or TO was abnormal using these cutpoints. Participants with <5 qualifying ventricular premature beats (in whom HRT could not be calculated) were categorized as having normal HRT, and those with >5 VPCs in whom HRT could not be calculated (N = 20) were excluded from the analysis.
Statistical Analysis
Univariate Cox regression analyses tested the relationship of the FRS (categorized as <10, 10–20, or >20), presence of clinical CVD, quartiles of VPC counts, diabetes, heart rate, SDNN, high versus low DFA1, and normal versus abnormal HRT with CVD mortality. Predictors that were significant in the univariate analysis were combined in a multivariate Cox regression analysis and then the analyses was stratified by categories of FRS. A P < 0.05 was considered statistically significant. Population attributable risks in percent, associated with abnormal HRT and DFA1, were calculated as: 100* [PABN(RR – 1)]/[1 + PABN (RR – 1)], where PABN is the prevalence of the abnormal HRV risk factor and RR is the relative risk associated with that risk factor. SPSS 14 (SPSS Inc, Chicago, IL, USA) software was used for these analyses.
Results
Table 1 describes clinical and demographic covariates. Mean age was 72±5 years and the majority was female (55%) and white (96%). Fifteen percent had diabetes. Relatively few (9%) smoked, 44% had hypertension, and 22% had prevalent CVD.
TABLE 1.
Clinical and Demographic Characteristics of the Cohort (N = 1,198)
| Variable | Mean ± SD or Percent |
|---|---|
| Age (years) | 72 ± 5 |
| Females (%) | 55 |
| Diabetes (%) | 15 |
| Current cigarette smoker (%) | 9 |
| Clinical Cardiovascular disease (%) | 29 |
| High-density lipoprotein (mg/dL) | 53 ± 15 |
| Total cholesterol (mg/dL) | 215 ± 38 |
| Systolic blood pressure (mmHg) | 134 ± 21 |
| Diastolic blood pressure (mmHg) | 70 ± 11 |
| FRS (%) | 15 ± 10 |
| Heart rate (bpm) | 73 ± 9 |
| VPCs (median and interquartile range) | 10 [118] |
| SDNN (ms) | 122 ± 34 |
| DFA1 | 1.04 ± 0.18 |
| HRT | |
| Normal (TS and TO) (%) | 73 |
| Any abnormality (TS and/or TO) (%) | 27 |
DFA1 = short-term fractal scaling exponent; SDNN = standard deviation of normal-to-normal interbeat intervals; TO = heart rate turbulence onset; TS=heart rate turbulence slope; VPC=ventricular premature contractions; FRS, Framingham Risk Score.
Unadjusted Relationship of Predictors to Cardiovascular Mortality
There were 451 deaths during follow-up (median 12.3 years). Of these deaths, 172 were due to CVD. CVD death occurred in 10% (N=94) of participants without prevalent CVD and in 30% (N=81) of participants with prevalent CVD. Because the FRS was designed to estimate risk of CVD mortality among people without CVD, we separately tested whether the FRS predicted CVD mortality among participants with and without prevalent CVD. Although the relationship was weaker among those with prevalent CVD, FRS was significantly related to CVD death in both groups (data not shown). They were therefore combined for all further analyses.
Table 2 shows the univariate relationships of covariates with CVD mortality. Higher FRS category, prevalent clinical CVD, decreased SDNN, increased VPC counts, presence of diabetes, lower DFA1, and abnormal HRT all predicted CVD mortality.
TABLE 2.
Univariate Predictors of CVD Mortality (N = 1,198, 172 CV deaths)
| RR (95% CI) | P-Value | |
|---|---|---|
| FRS < 10 (reference category) | 1.0 | |
| FRS = 10–20 | 1.6 (1.05–2.3) | 0.028 |
| >20 | 3.3 (2.2–4.9) | <0.001 |
| Diabetes | 2.4 (1.8–3.2) | <0.001 |
| Heart rate (bpm) | NS | NS |
| SDNN (ms) | 0.99 (0.99–1.00) | 0.001 |
| VPC count (quartiles) | ||
| 0–1 (reference) | 1.0 | |
| 2–13 | 1.8 (1.1–3.1) | 0.017 |
| 14–152 | 2.5 (1.5–4.0) | <0.001 |
| >153 | 3.9 (2.4–6.1) | <0.001 |
| DFA1 ≥ 1 (reference category) | 1.0 | |
| DFA1 < 1.0 (Low) | 2.7 (2.0–3.6) | <0.001 |
| HRT | ||
| TS> = 3 and TO < 0 (reference) | 1.0 | |
| TS < 3 and/or TO > 0 (abnormal) | 3.0 (2.2–4.0) | <0.001 |
DFA1 = short term fractal scaling exponent; FRS = Framingham Risk Score; SDNN = standard deviation of normal-to-normal interbeat intervals; TO = heart rate turbulence onset; TS = heart rate turbulence slope; VPC = ventricular premature contractions.
Multivariate Relationships of Predictors to Cardiovascular Mortality (Table 3)
TABLE 3.
Multivariate Predictors of CVD Mortality with Full Model and Stratified by Framingham Risk Score*
| Full Model |
Framingham Score < 10 (N = 396, 38 Deaths) |
Framingham Score 10–20 (N = 532, 72 Deaths) |
Framingham Score > 20 (N = 250, 62 Deaths) |
|||||
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) |
P-value | RR (95% CI) |
P-value | RR (95% CI) |
P-value | RR (95% CI) |
P-value | |
| Clinical CVD | 2.2 (1.6–3.0) |
<0.001 | 2.9 (1.6–5.1) |
<0.001 | 2.6 (1.6–4.3) |
<0.001 | 1.9 (1.2–3.1) |
0.008 |
| Diabetes | 1.7 (1.2–2.5) |
0.005 | NS | NS | 2.2 (1.4–3.5) |
0.001 | ||
| VPC count (quartiles) 1st–3rd quartiles (0–152) |
NS | NS | NS | NS | ||||
| 4th quartile (>153) | 1.7 (1.2–2.3) |
0.001 | NS | NS | 2.2 (1.4–3.6) |
0.001 | ||
| HRT and DFA combined | ||||||||
| Normal HRT and Normal DFA1 | 1.0 | – | 1.0 | – | 1.0 | – | 1.0 | – |
| Normal HRT and Abnormal DFA1 | NS | NS | NS | 2.0 (1.1–3.8) |
0.034 | |||
| Abnormal HRT and Normal DFA1 | NS | NS | NS | NS | ||||
| Abnormal HRT and Abnormal DFA1 | 3.6 (2.4–5.3) |
<0.001 | 7.7 (3.7–16.0) |
<0.001 | 3.6 (1.8–7.4) |
<0.001 | 2.8 (1.5–5.1) |
0.001 |
N for multivariate model = 1,178 because HRT could not be categorized in 20 participants.
CVD = cardiovascular disease; DFA1 = short-term fractal scaling exponent; TO = heart rate turbulence onset; TS = heart rate turbulence slope; VPC = ventricular premature contractions.
Univariate predictors of CVD mortality were combined into multivariate models for CVD mortality risk, and then the model was categorized by stratum of the FRS. SDNN was no longer associated with CVD mortality after adjustment for covariates. Clinical CVD remained independently associated with CVD mortality at each level of the FRS. Diabetes was associated with CVD mortality only in the group with the highest FRS. Notable is the group with both low DFA1 and abnormal HRT. At each stratum, these participants were at increased risk of CVD mortality. The strongest independent effect on CVD mortality was in the lowest FRS stratum (RR 7.7, 95% CI 3.7–16.0, P < 0.001). The combination of low DFA1 and normal HRT was also associated with increased risk of mortality, but only in the group with the highest FRS. VPC counts in the highest quartile were associated with CVD mortality in the group with the highest FRS only.
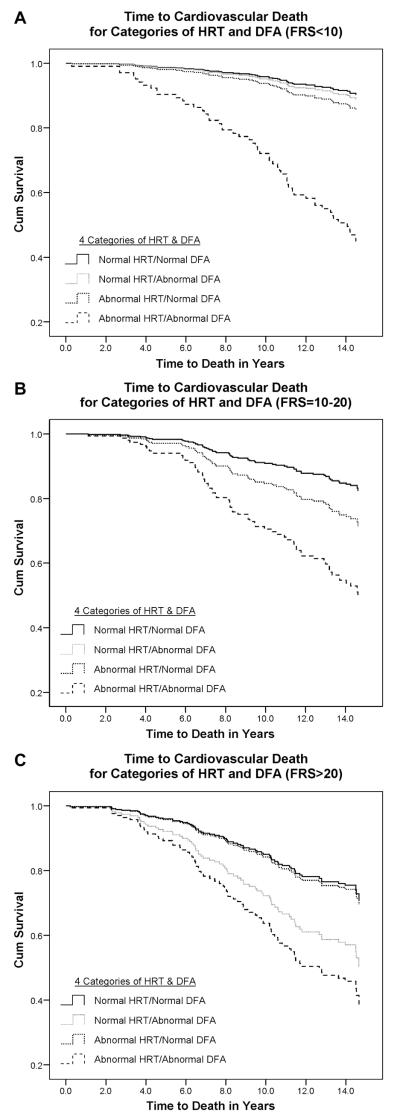
Figure 1 shows covariate-adjusted survival curves for CVD mortality at each level of the FRS. In the low FRS group, only participants with abnormal HRT and low DFA1 were at high risk of CVD mortality. In the intermediate FRS group, there was a trend for those with low DFA1 and normal HRT to have increased mortality (P nonsignificant) and a markedly poorer survival among those with abnormal HRT and decreased DFA1. In the high FRS group, there was poorer survival of participants with low DFA1, independent of HRT.
Figure 1.
Survival curves for cardiovascular death among participants with normal values of DFA1 and HRT compared with those who had: Abnormal DFA1 and normal HRT, abnormal HRT and normal DFA1, or abnormal DFA1 and abnormal HRT. Analyses were stratified at different levels of the Framingham Risk Score (FRS). (A) is for FRS < 10, (B) is for FRS=10–20, and (C) is for FRS >20.
To put these findings into perspective, we calculated the population attributable risk (PAR) associated with abnormal DFA1 and HRT for each stratum of FRS. In the low FRS stratum, the PAR of having both these HRV measures abnormal was 23%. In the middle FRS stratum, the PAR was 19%. Among those with high FRS, the PAR for having only DFA1 abnormal was 11%, while that for having both measures abnormal was 17%, so the combined PAR for abnormal HRT was 28%.
Discussion
In this study of older adults, the novel HRV measures DFA1 and HRT had a strong relationship with cardiovascular mortality over approximately 12 years follow-up. This relationship was stronger than that of traditional time domain HRV or the number of VPCs over 24 hours. Further, this association was present after adjustment for prevalent CVD, diabetes, and the FRS. At each stratum of the FRS, the combination of lower DFA1 with abnormal HRT was a stronger risk factor for CVD mortality than the presence of diabetes or clinically diagnosed CVD.
Decreased DFA1 may be viewed as a marker of erratic heart rate patterns, that is, sinus arrhythmia that is highly irregular and does not reflect respiration-associated heart rate changes. It is unclear whether decreased DFA1 reflects sub-clinical SA-nodal dysfunction, a loss of integration of the multiple feedback loops involved in cardiovascular regulation, or both. In the Rotterdam study, erratic heart rate patterns were associated with increased mortality from ischemic heart disease.21 In a different analysis, we have shown that a higher prevalence of erratic heart rate patterns, identified using a semiquantitative method, was associated with an increased risk of mortality in the CHS22 and that more erratic heart rate patterns in association with antiarrhythmic therapy predicted mortality in the Cardiac Arrhythmia Suppression Trial (CAST).23 In post-MI studies, DFA1 has been shown to be a better predictor of mortality than traditional HRV measures.8,9
Abnormal HRT is a predictor of mortality post-MI in both retrospective and prospective analyses.7 It is believed to be a marker for autonomically mediated baroreceptor functioning.24 It reflects the sensitivity of the cardiovascular system to the loss of cardiac output that follows the diminished filling during a VPC and the compensatory increase in cardiac output with the following beat.25 Thus, abnormal HRT may reflect a loss of the adaptive ability of the cardiovascular system to changes in blood pressure or heart rate.
Another point regarding our results should be noted. The finding that an increased VPC count over 24 hours was associated with CVD mortality in the elderly, but only in those with the highest FRS scores, has not previously been reported. A recent study from ARIC (Atherosclerosis Risk In Communities) reported an increased risk of CVD mortality in middle-aged adults who had VPCs on a 2-min rhythm strip, compared with those who did not.26 An association of increased ventricular ectopy and CVD mortality on short recordings was reported for men without coronary heart disease in the Framingham Heart Study.27 In a different study, among subjects over 85 years old, a markedly increased risk of mortality was found among subjects with a high VPC count on 24-hour recordings.28 Finally, in a study of 678 healthy older and middle-aged men, having ≥30 VPCs/hour was a significant independent predictor of an increased cardiac event rate over 5-year follow-up.29 In contrast, our highest quartile of VPCs had >153 VPCs over 24 hours, or about 6 events/hour, and elevated VPC-associated risk was found only among those with a high FRS.
We found that combining DFA1, HRT, and VPC count better identified high-risk participants, as compared with using any one index alone. This suggests that multiple autonomic disturbances are a strong marker for physiologic dysfunction. This conclusion has precedence in two other studies. In the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) study, decreased SDNN (standard deviation of N-N intervals) in association with abnormal HRT was significantly associated with mortality over a mean of 20 months of post-MI follow-up. In the CAST (Cardiac Arrhythmia Suppression Trial), abnormal circadian HRV together with increased randomness of HRV predicted mortality.10
The limited applicability of our results should be noted. HRT is calculated from a signal-average of at least 5 VPCs and is relatively robust. Some commercial Holter systems are able to calculate HRT, and the algorithm is available online for research purposes.30 Having fewer than five VPCs may be considered equivalent to normal HRT. Identification of patients with a high number of VPCs is already possible from Holter recordings. Accurate values for DFA1 require scanning of Holter recordings to research standards to ensure that erratic HR patterns are not due to uneven beat detection by the scanning software. At the present time, although the algorithm for DFA1 is available online,31 DFA1 is not available in any commercial system. Furthermore, it is not yet clear whether calculations of DFA1 from R-R or N-N intervals would be optimal. However, accurate Holter scanning technology is evolving rapidly, and the potential of measures like DFA1 to improve risk stratification should result in their being incorporated into commercial Holter systems.
Conclusion
Holter-based factors reflecting erratic rhythm, abnormal baroreflex function, and increased ventricular ectopy identify older adults at increased risk of CVD mortality over a median of approximately 12 years of follow-up. This increased risk is independent of prevalent CVD, diabetes, and traditional risk factors for CVD. The use of a combination of novel Holter-based measures improves the identification of at-risk individuals compared to the use of any single measure and also compared to the use of SDNN, a traditional HRV measure. Finally, Holter-based risk stratification for CVD mortality among the elderly using measures of HRV is not limited to people with increased CVD risk or those with prevalent CVD.
Acknowledgments
(All Investigators here listed have provided signed permission to be acknowledged.) Steering Committee Chairman: Curt D. Furberg, MD, PhD, Wake Forest University School of Medicine. NHLBI Project Office: Jean Olson, MD, MPH. Wake Forest University School of Medicine: Gregory L. Burke, MD. Wake Forest University — ECG Reading Center: Ronald Prineas, MD, PhD. University of California, Davis: John Robbins, MD, MHS. The Johns Hopkins University: Linda P. Fried, MD, MPH. The Johns Hopkins University—MRI Reading Center: David Yousem, MD, MBA. University of Pittsburgh: Lewis H. Kuller, MD, DrPH. University of California, Irvine—Echocardiography Reading Center (baseline): Julius M. Gardin, MD.
Georgetown Medical Center—Echocardiography Reading Center (follow-up): John S. Gottdiener, MD. New England Medical Center, Boston—Ultrasound Reading Center: Daniel H. O’Leary, MD. University of Vermont—Central Blood Analysis Laboratory: Russell P. Tracy, PhD. University of Arizona, Tucson—Pulmonary Reading Center: Paul Enright, MD. Retinal Reading Center–University of Wisconsin: Ronald Klein, MD. University of Washington—Coordinating Center: Richard A. Kronmal, PhD.
Also, Nelson Hui for his tireless assistance with data analyses.
The research reported in this article was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, and U01 HL080295, and also by R0-1 HL62181 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke.
Footnotes
A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
References
- 1.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 2.Cripps TR, Malik M, Farrell TG, Camm AJ. Prognostic value of reduced heart rate variability after myocardial infarction: Clinical evaluation of a new analysis method. Br Heart J. 1991;65:14–19. doi: 10.1136/hrt.65.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintana M, Storck N, Lindblad LE, Lindvell K, Ericson M. Heart rate variability as a means of assessing prognosis after acute myocardial infarction: A 3-year follow-up study. Eur Heart J. 1997;18:789–797. doi: 10.1093/oxfordjournals.eurheartj.a015344. [DOI] [PubMed] [Google Scholar]
- 4.Zuanetti G, Neilson JM, Latini R, Santoro E, Maggioni AP, Ewing DJ. Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era: The GISSI-2 results. Circulation. 1996;94:432–436. doi: 10.1161/01.cir.94.3.432. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 6.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 7.Ghuran A, Reid F, La Rovere MT, Schmidt G, Bigger JT, Jr, Camm AJ, Schwartz PJ, Malik M. ATRAMI Investigators: Heart rate turbulence-based predictors of fatal and nonfatal cardiac arrest (The Autonomic Tone and Reflexes After Myocardial Infarction substudy) Am J Cardiol. 2002;89:184–190. doi: 10.1016/s0002-9149(01)02198-1. [DOI] [PubMed] [Google Scholar]
- 8.Huikuri HV, Mäkikallio TH, Peng C-K, Goldberger AL, Hintze U, Møller M, for the DIAMOND Study Group Fractal correlation properties of the R-R interval dynamics and mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation. 2000;101:47–53. doi: 10.1161/01.cir.101.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Tapanainen JM, Bloch-Thomsen PE, Kober L, Torp-Pedersen C, Mäkikallio TH, Still AM, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–352. doi: 10.1016/s0002-9149(02)02488-8. [DOI] [PubMed] [Google Scholar]
- 10.Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE, CAST Investigators Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:113–120. doi: 10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: Results from the Cardiovascular Health Study. Circulation. 2006;24:388–393. doi: 10.1161/CIRCULATIONAHA.105.570903. [DOI] [PubMed] [Google Scholar]
- 12.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RM. Heart rate variability and its relationship to glucose disorders and metabolic syndrome: The Cardiovascular Health Study. Diabet Med. 2007;24:855–863. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 13.Psaty BM, Kuller LH, Hermanson B, Manolio TA, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1991;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the. Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 15.National Heart, Lung, and Blood Institute Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/risk_tbl.htm.
- 16.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 17.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar N, Peng C-K, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol. 1996;271:R1078–R1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- 19.Mäkikallio TH, Seppänen T, Airaksinen KEJ, Koistinen J, Tulppo MP, Peng CK, Goldberger AL, Huikuri HV. Dynamic analysis of heart rate may predict subsequent ventricular tachycardia after myocardial infarction. Am J Cardiol. 1997;80:779–783. doi: 10.1016/s0002-9149(97)00516-x. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt G, Malik M, Barthel P, Schneider R, Ulm K, Rolnitzky L, Camm AJ, Bigger JT, Jr, Schömig A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353:1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- 21.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrographic recordings predicts mortality from all-causes in middle-aged and elderly men. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 22.Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: Results of graphical and non–linear analyses. J Cardiovasc Electrophysiol. 2005;16:1–6. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- 23.Stein PK, Le QC, Domitrovich PP, for the Cast Investigators Development of more erratic heart rate patterns is associated with mortality post-MI. J Electrocardiol. 2008;41:110–115. doi: 10.1016/j.jelectrocard.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voss A, Baier V, Schirdewan A, Leder U. Physiological hypotheses on heart rate turbulence. In: Malik M, Camm AJ, editors. Dynamic Electrocardiography. Blackwell; Oxford: 2004. pp. 203–210. [Google Scholar]
- 25.Watanabe MA, Schmidt G. Heart rate turbulence: A 5-year review. Heart Rhythm. 2004;1:732–738. doi: 10.1016/j.hrthm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Massing MW, Simpson RJ, Jr, Rautaharju PM, Schreiner PJ, Crow R, Heiss G. Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the Atherosclerosis Risk In Communities cohort) Am J Cardiol. 2006;98:1609–1612. doi: 10.1016/j.amjcard.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 27.Bikkina M, Larson MG, Levy D. Prognostic implications of asymptomatic ventricular arrhythmias: The Framingham Heart Study. Ann Intern Med. 1992;117:990–996. doi: 10.7326/0003-4819-117-12-990. [DOI] [PubMed] [Google Scholar]
- 28.Raiha IJ, Piha SJ, Seppanen A, Puukka P, Sourander LB. Predictive value of continuous ambulatory electrocardiographic monitoring in elderly people. BMJ. 1994;12:1263–1267. doi: 10.1136/bmj.309.6964.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Frederiksen BS, Davanlou M, Hansen JF. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age > or = 55 years. Am J Cardiol. 2006;97:1351–1357. doi: 10.1016/j.amjcard.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 30. http://www.h-r-t.com/hrt/en/index.html.
- 31.Goldberger AL, Amaral LAN, Glass L, Hausdorff JM, Ivanov PCh, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation. 2000 Jun 13;101(23):e215–e220. doi: 10.1161/01.cir.101.23.e215. [Circulation Electronic Pages; http://circ.ahajournals.org/cgi/content/full/101/23/e215]; [DOI] [PubMed] [Google Scholar]