Abstract
Though Type 2 diabetes (DM) is a recognized risk factor for development of tuberculosis (TB), the impact on treatment is unclear. Among the few published reports, some suggest that mycobacterial clearance during treatment is delayed in TB patients with DM. Using survival analysis on data from 469 culture-positive TB patients retrospectively identified in South Texas, we found DM to be an independent risk factor for a five-day delay in mycobacterial clearance within the first 60 days of treatment.
SHORT REPORT
The association of diabetes (DM) with tuberculosis (TB) is a re-emerging problem due to the rapid rise world-wide of type 2 DM.1–7 There is evidence that TB patients with DM may have higher bacillary loads than patients with TB without DM, but others have not confirmed these observations.8–12 The impact of DM on reduction in bacillary load during TB treatment and the potential for transmission is also unclear. In the light of this uncertainty we sought to determine whether TB patients with DM patients were more likely to take longer to clear the bacterium from sputum during the first phase of treatment than those with TB patients without DM.
We accessed a large retrospective dataset containing sociodemographic and culture data from all TB patients reported to South Texas between 1996–2002 and selected all TB patients recorded with positive sputum culture. Ethical approval for the study was obtained from all participating institutions.7 All patients received standard ‘directly observed therapy, short course’ (DOTS): isoniazid (5–10 mg/kg), rifampin (10 mg/kg), pyrazinamide (15–30 mg/kg) and ethambutol (15–25 mg/kg) during the first two months (first phase), and isoniazid and rifampin for the following four months (second phase). At 2–4 weeks most patients are switched from daily to twice weekly dosing. Sputum specimens were taken at the time of diagnosis and monthly thereafter provided the patient had a productive cough. Culture was performed on Lowenstein-Jensen medium and either Bactec radiometric method or the Mycobacteria Growth Indicator Tube (MGIT™ Becton-Dickinson, Franklin Lakes, NJ). Demographic information, self-reported social and medical risk factors for TB, clinical and radiological characteristics of TB disease at diagnosis, and treatment outcome for each TB patient were recorded. Microbiology records were generated by the laboratory only when a specimen was received.
Since our preliminary data indicated that impaired clearance in TB patients with DM was concentrated in the first phase of DOTS, we focused on the first 60 days of treatment.7 The dates of treatment initiation and the dates of collection of specimens which yielded the last positive and first negative cultures for a given patient were established.7 We calculated the time (in days) for culture conversion from positive to negative (‘time to clearance’ or TTC) for each patient, that is days between the treatment initiation date and the date of collection of the first negative culture, provided there were no subsequent positive cultures (no censoring). When records showed only positive cultures or the last culture was positive the data were censored, and the TTC was calculated as the number of days between the last positive culture and the treatment start date. We removed 69 patients with positive HIV serology since this infection independently impairs clearance, and 66 non-compliant patients who did not complete treatment, for the same reason. The dataset thus generated contained 469 adult TB patients (age ≥ 20 years).7
All data analyses were performed using SAS version 9.1 (Cary, North Carolina). We first used descriptive statistics to compare TB patients with DM and TB patients without DM, followed by the Kaplan-Meier method and the log-rank test to compare the distribution of TTC between the two groups.13 Potential associations between the culture TTC and other independent variables were also explored. Since TTC is not normally distributed and estimation of means may be biased due to censoring, we report median TTC. The Cox proportional hazard mode was then used to identify any interactions and potential associations after adjusting for other covariates.14 Variables entered into the final model included age, gender, and any variable from the univariate analysis with a p-value < 0.1. Then we conducted step-wise removal of the least significant independent variable leaving only those with a p-value < 0.05. We estimated adjusted hazard ratios and their 95% confidence intervals as a measure of association between DM status and culture TTC.
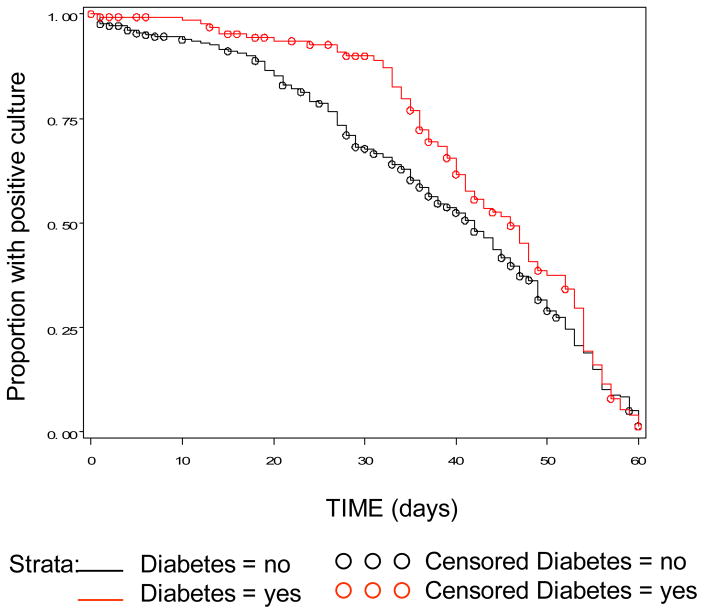
The characteristics of the TB patients did not differ from those described previously (Table 1).7 Briefly, DM patients were more likely to be older females with no classical social risks for TB (e.g. jail, drug and alcohol abuse), and were also more likely to be smear positive at diagnosis. Table 2 shows the comparison of median time to clearance using univariate and a stepwise multiple regression model. During the first 60 days of treatment the median culture TTC was longer for patients with DM (42 days for TB patients with DM versus 37 days for TB patients without DM; p = 0.03; table 2, figure). DM status continued to be significantly associated with delayed bacterial clearance in TB patients with DM, when compared to TB patients without DM (table 2), after controlling for possible confounders.
Table 1.
Characteristics of TB patients with culture conversion within 60 days of treatment follow-up during 1996–2002*
| Total (n=469) | Diabetes (n=152) | No Diabetes (n=317) | p value† | OR (95% CI) | |
|---|---|---|---|---|---|
| Sociodemographics | n(%)‡ | n(%)‡ | n(%)‡ | ||
| Female | 146 (31.1) | 62 (40.8) | 84 (26.5) | 0.002 | 1.9 (1.3, 2.9) |
| Male | 323 (68.9) | 90 (59.2) | 233 (73.5) | 1.0 | |
| Age group (yrs) | |||||
| 20–39 | 160 (34.1) | 20 (13.2) | 140 (44.2) | <0.001 | 0.3 (0.14, 0.50) |
| 40–69 | 218 (46.5) | 100 (65.8) | 118 (37.2) | 1.5 (0.94, 2.6) | |
| 70+ | 91 (19.4) | 32 (21.1) | 59 (18.6) | 1.0 | |
| Hispanic | 425 (90.6) | 146 (96.1) | 279 (88.0) | 0.008 | 3.3 (1.4, 8.0) |
| Risks for TB (other than diabetes) | |||||
| Incarceration | 39 (8.3) | 4 (2.6) | 35 (11.0) | 0.005 | 0.2 (0.08, 0.62) |
| Homeless | 15 (3.2) | 0 (0) | 15 (4.7) | NA | NA |
| Alcohol abuse | 89 (19.0) | 136 (89.5) | 244 (77.0) | 0.002 | 0.4 (0.22, 0.70) |
| Drug abuse | 54 (11.5) | 8 (5.2) | 46 (14.5) | 0.005 | 0.33 (0.15, 0.71) |
| Characteristics of TB disease | |||||
| Smear positive | 257 (55.8) | 95 (63.8) | 162 (51.9) | 0.02 | 1.6 (1.1, 2.4) |
| Pulmonary TB | 444 (95.1) | 144 (95.7) | 300 (95.2) | 0.81 | 0.90 (0.37, 2.17) |
| Cavitations | 167 (48.7) | 51 (44.4) | 116 (50.9) | 0.25 | 1.3 (0.82, 2.04) |
| Relapsed TB | 23 (4.9) | 8 (5.3) | 15 (4.8) | 1.1 | 1.11 (0.46, 2.68) |
| Multi-drug resistance | 6 (1.3) | 3 (2.0) | 3 (1.0) | 0.36 | 2.1 (0.42, 10.57) |
p value estimated by chi square;
percent based on total number of cases with available information
Table 2.
Comparison of median time to clearance of M. tuberculosis by culture during the first 60 days of treatment
| Factor | Time to culture conversion in days
|
|||
|---|---|---|---|---|
| Univariate
|
Multivariate (final model)
|
|||
| median (95% CI) | P value* | Hazard ratio (95% CI) | P value* | |
|
|
|
|||
| Sociodemographics | ||||
| Gender | ||||
| Female | 40 (37, 45) | 0.042 | 1.4 (1.13, 1.77) | 0.002 |
| Male | 37 (33, 41) | 1 | ||
| Age groups (years) | ||||
| 20–39 | 42 (38, 45) | 0.002 | 0.52 (0.38, 0.70) | 0.0001 |
| 40–69 | 40 (36, 44) | 0.75 (0.59, 0.96) | ||
| 70+ | 33 (27, 38) | 1 | ||
| Hispanic | ||||
| Yes | 40 (37, 42) | 0.03 | ||
| No | 33 (20, 39) | |||
| Risks for TB | ||||
| Diabetes status | ||||
| Yes | 42 (38, 46) | 0.03 | 0.75 (0.59, 0.96) | 0.02 |
| No | 37 (35, 41) | 1 | ||
| Incarceration | ||||
| Yes | 50 (42, 55) | 0.01 | ||
| No | 38 (36, 41) | |||
| Homeless | ||||
| Yes | 40 (31, 56) | 0.54 | ||
| No | 39 (37, 42) | |||
| Alcohol abuse | ||||
| Yes | 42 (38, 50) | 0.008 | ||
| No | 38 (35, 41) | |||
| Drug abuse | ||||
| Yes | 48 (40, 51) | 0.01 | ||
| No | 38 (36, 41) | |||
p value for comparing distribution of TTC between study groups using log-rank test for univariate model and the Cox proportional Hazard model for the final multivariate model
Figure.
Survival curve for time to culture conversion from positive to negative within 60 days of treatment by diabetes status of patient.
Using a powerful analytic technique on a large TB database we show here that DM is an independent risk factor for delayed culture clearance of mycobacteria during the first phase of DOTS; the time during which treatment is considered to render the patient non-infectious.15 Though infectiousness is thought to be reduced before smear or culture conversion, a delay in clearance measured by culture positivity could translate into increased exposure in the community, particularly one where there are large numbers of DM patients.16;17 In a population such as ours where we now find prospectively that over 35% of TB patients have DM (unpublished data), a median increase of 5 days translates into 175 additional days of potential infectivity for every 100 patients with newly-diagnosed TB. Since TB patients with DM tend to be older females with few if any social risk factors for TB, their closest contacts are likely to be family members. A strong risk factor for DM is family history, so exposed household members of these female TB patients with DM may also be at increased risk of TB due to DM.18–20
Our findings are consistent with our previous observation and those of others, of higher bacterial load in patients with TB patients with DM at diagnosis and during the first or second months of treatment, but not thereafter.7;21 It is likely that the observed delay in TTC during the first two months of treatment among DM patients is related to the increased likelihood of their being smear-positive at diagnosis, (a well-recognized predictor of delayed bacterial clearance during treatment).20–22 Consistent with this observation we found that TB patients who did not clear until the second month were more likely to have DM (p=0.001) and be smear-positive (p=0.02) than those who cleared in the first month. Indeed, the association of smear-positivity with delayed TTC was so strong that it masked all other effects. For this reason we excluded smear positivity from our final model so that we could determine the importance of DM as an underlying pathology.
Possible biological explanations for delayed TTC in the first phase of treatment in DM patients include higher bacterial burden at diagnosis, which could be related to slower kinetics in the immune response in DM patients. Delayed IFN-γ response has been observed in a mouse model of TB and DM, and in TB patients with DM.23–25 Altered metabolism of rifampin in TB patients with DM is also a possible explanation.26
We examined the effect of MDR-TB, but in patients who clear M. tuberculosis (MTB) within two months rates of MDR-TB did not differ by diabetes status. Not unexpectedly, however, if we look at the effect of MDR-TB between 2 and 9 months of initiation of treatment, then we do see significant delay in MTB clearance among patients with MDR-TB (data not shown). A report from Indonesia found a delay in culture clearing in TB patients with DM patients at 6-month follow-up but they took no account of MDR-TB which may have biased their results.4
The major limitation of this study is that it used retrospective data, in which DM classification is based on self-reporting. However this would likely lead to some undiagnosed DM patients being included in the group not reporting DM. This would tend to reduce the observed difference. Missing follow-up data was also a limitation for survival analysis particularly the fact that failure to produce sputum as the patient improved was not recorded. Nevertheless we censored less than 50% of the data, which is acceptable for survival analysis for estimating the median TTC. Shorter time intervals between specimens would also have provided more precision.
Despite the goal of global TB eradication by the year 2050, in 2004 there were an estimated 8.9 million new cases of TB worldwide.27 Our data show that DM appears to interfere with sterilization of pulmonary TB by drug therapy. By 2030 it is estimated that 336 million of the worlds’ population will have DM, many in TB endemic countries.1 DM on this scale may impact TB control. Prospective studies are needed to define more clearly the consequences for transmission among DM patients and the measures which might be taken to lessen the effect.
Acknowledgments
We thank the members of the NSTT (www.nstt.info) in south Texas and the members of the Division of Tuberculosis Elimination at the Texas Department of State Health Services who contributed to this study by collecting and entering data and by processing and reporting routine microbiological reports. We also acknowledge the statistical consulting work performed on this paper through a subcontract with Universal Statistical Technology and Training (USTAT), based in Okemos, Michigan.
This study was supported by NIAID 1 R21 AIO56207-01 and by the Hispanic Health Research Center EXPORT grant NIHMHD P20 MD000170 020.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Oscarsson N, Silwer H. Incidence of pulmonary tuberculosis among diabetics. Acta Med Scand. 1958;160(suppl 335):23–48. [PubMed] [Google Scholar]
- 3.Ponce-De-Leon A, Garcia-Garcia Md ML, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, Rogas R, Ferreyra-Reyes L, Cano-Arellano B, Bobadilla M, Small PM, Sifuentes-Osornia J. Tuberculosis and diabetes in southern Mexico. Diabetes Care. 2004;27:1584–1590. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 4.Alisjahbana B, van CR, Sahiratmadja E, den HM, Maya A, Istriana E, Danusantoso H, Ottenhoff TH, Nelwan RH, van der Meer JW. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 5.Coker R, McKee M, Atun R, Dimitrova B, Dodonova E, Kuznetsov S, Drobniewski F. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ. 2006;332:85–87. doi: 10.1136/bmj.38684.687940.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty N, Shemko M, Vaz M, D’Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis. 2006;10:80–86. [PubMed] [Google Scholar]
- 7.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, McCormick JB. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2006;135:1–9. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–79. [PubMed] [Google Scholar]
- 9.Wang JY, Lee LN, Hsueh PR. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2005;9:777–783. [PubMed] [Google Scholar]
- 10.Bacakoglu F, Basoglu OO, Cok G, Sayiner A, Ates M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration. 2001;68:595–600. doi: 10.1159/000050578. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Guzman C, Torres-Cruz A, Villarreal-Velarde H, Vargas MH. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am J Respir Crit Care Med. 2000;162:1738–1740. doi: 10.1164/ajrccm.162.5.2001040. [DOI] [PubMed] [Google Scholar]
- 12.Lee KM, Choe KH, Kim SJ. Clinical investigation of cavitary tuberculosis and tuberculous pneumonia. Korean J Intern Med. 2006;21:230–235. doi: 10.3904/kjim.2006.21.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1956;53:457–481. [Google Scholar]
- 14.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall; 1984. [Google Scholar]
- 15.American Thoracic Society, Infectious Diseases Society of America, Centers for Disease Control and Presention. Treatment of Tuberculosis. MMWR. 2003;20:1–77. [Google Scholar]
- 16.Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, Riley MC, Wells WF. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. Am J Epidemiol. 1959;142:3–14. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad D, Morgan WK. How long are TB patients infectious? CMAJ. 2000;25:157–158. [PMC free article] [PubMed] [Google Scholar]
- 18.Cantor AB, Krischer JP, Cuthbertson DD, Schatz DA, Riley WJ, Malone J, Schwartz S, Quattrin T, Maclaren NK. Age and family relationship accentuate the risk of insulin-dependent diabetes mellitus (IDDM) in relatives of patients with IDDM. J Clin Endocrinol Metab. 1995;80:3739–1343. doi: 10.1210/jcem.80.12.8530627. [DOI] [PubMed] [Google Scholar]
- 19.O’Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307:370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 20.Hallman DM, Boerwinkle E, Gonzalez VH, Klein BE, Klein R, Hanis CL. A genome-wide linkage scan for diabetic retinopathy susceptibility genes in Mexican Americans with type 2 diabetes from Starr County, Texas. Diabetes. 2007;56:1167–1173. doi: 10.2337/db06-1373. [DOI] [PubMed] [Google Scholar]
- 21.Guler M, Unsal E, Dursun B, Aydln O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract. 2007;61:231–235. doi: 10.1111/j.1742-1241.2006.01131.x. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 23.Martens GW, Arikan MC, Lee J, Ren F, Greiner D, Kornfeld H. Tuberculosis Susceptibility of Diabetic Mice. Am J Respir Cell Mol Biol. 2007;37:518–524. doi: 10.1165/rcmb.2006-0478OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stalenhoef JE, Alisjahbana B, Nelwan EJ, van d V, Ottenhoff TH, van der Meer JW, Nelwan RH, Netea MG, van Crevel R. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis. 2007;27:97–103. doi: 10.1007/s10096-007-0395-0. [DOI] [PubMed] [Google Scholar]
- 25.Tsukaguchi K, Yoneda T, Okamura H, Tamaki S, Takenaka H, Okamoto Y, Narita N. Defective T cell function for inhibition of growth of Mycobacterium avium-intracellulare complex (MAC) in patients with MAC disease: restoration by cytokines. J Infect Dis. 2000;182:1664–1671. doi: 10.1086/317601. [DOI] [PubMed] [Google Scholar]
- 26.Nijland HM, Ruslami R, Stalenhoef JE, Nelwan EJ, Alisjahbana B, Nelwan RH, van der V, Danusantoso H, Aarnoutse RE, van Crevel R. Exposure to rifampicin is strongly reduced in patients with tuberculosis and type 2 diabetes. Clin Infect Dis. 2006;43:848–854. doi: 10.1086/507543. [DOI] [PubMed] [Google Scholar]
- 27.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–9. 40. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]