Abstract
Summary
Background
Each year, millions of platelet transfusions save the lives of cancer patients and patients with bleeding complications. However, between 10 and 30% of all platelet transfusions are clinically ineffective as measured by corrected count increments, but no test is currently used to identify and avoid these transfusions. ThromboLUX® is the first platelet test intended to routinely characterize platelet concentrates prior to transfusion.
Methods
ThromboLUX is a non-invasive, optical test utilizing dynamic light scattering to characterize a platelet sample by the relative quantity of platelets, microparticles, and other particles present in the sample. ThromboLUX also determines the response of platelets to temperature changes. From this information the ThromboLUX score is calculated. Increasing scores indicate increasing numbers of discoid platelets and fewer microparticles. ThromboLUX uses calibrated polystyrene beads as a quality control standard, and accurately measures the size of the beads at multiple temperatures.
Results
Results from apheresis concentrates showed that ThromboLUX can determine the microparticle content in unmodified samples of platelet concentrates which correlates well with the enumeration by flow cytometry. ThromboLUX detection of microparticles and microaggregates was confirmed by microscopy.
Conclusion
ThromboLUX provides a comprehensive and novel analysis of platelet samples and has potential as a noninvasive routine test to characterize platelet products to identify and prevent ineffective transfusions.
KeyWords: Blood platelets, Dynamic light scattering, DLS, Microparticles, Transfusion, Particle size distribution, Doppler shift, Photon correlation spectroscopy
Introduction
Platelets are blood cells that prevent and stop bleeding. In North America every year 4.2 million platelet transfusions are a life-saving part of cancer therapy and the treatment of bleeding patients [1, 2]. However, up to 30% of all platelet transfusions are reported to be ineffective as determined by 24-hour corrected count increments < 5 × 109/l and do not have the desired clinical outcome [3, 4]. While clinical outcome can be influenced by many factors, the characteristics of the transfused platelet product have been shown to play an important role [5]. However, currently no in vitro test is used routinely to characterize platelet transfusions and distinguish between effective and ineffective product [6]. Instead, platelets are transfused without pre-transfusion characterization, and the outcome in the patient is the only indicator of platelet effectiveness. This practice is time-consuming, costly, and a significant risk to patients. It was our objective to solve this problem in developing ThromboLUX®, a quick, easy, and inexpensive optical platelet test intended for routine use prior to administering a platelet transfusion.
The ThromboLUX system uses the principle of dynamic light scattering (DLS) to determine what kind of particles are in the platelet concentrate, how many of the particles are present in the sample, and how they respond to temperature stress by measuring their relative size change when activated by cooling from 37 °C (body temperature) to 20 °C [7]. DLS, also known as quasi-elastic light scattering, is used to determine the sizes of particles in suspension by observing their inherent, random Brownian motion. The relative light scattering intensity of a particular particle population is determined by its contribution to the total light scattering intensity. The particles of a homogeneous, monodisperse population contribute 100% of the total light scattering intensity.
Platelets respond to temperature variation and activate when cooled. Platelet activation can manifest as shape change, fragmentation and/or microaggregation. During shape change, platelets contract and form pseudopods [8] – protrusions that extend from the cell surface and reduce the speed of the platelets due to increased resistance – and appear to increase in size. Microaggregates are larger, slow-moving particles. Viable platelets might activate cell-to-cell interactions and form microaggregates when cooled [9]. Upon re-warming this interaction might be reversible [10].
Activation of platelets is associated with fragmentation into microparticles; however, the role and function of microparticles is still unclear and topic of ongoing research [11, 12]. The most widely used tool to investigate microparticles is flow cytometry. Microparticles range in size from about 50 nm to 1,000 nm. Flow cytometers have manufacturer-defined detection limits within this size range. With certain instrument and parameter settings and the use of internal calibration, flow cytometry has been shown to adequately measure microparticles. However, standardization of pre-analytical and analytical procedures for isolating, enumerating, and fluorescently labeling microparticles remains a challenge [13]. In platelet concentrates, microparticles may facilitate cell-to-cell interactions and could therefore affect both the quality of the product and transfusion outcome [14].
In a previous study using a research prototype we have shown that the ThromboLUX score correlates with the clinical outcome of platelet transfusions [3]. Further studies are underway to substantiate the clinical correlation, which will provide incentive to use this new technology. Here, the performance data from the clinic-ready ThromboLUX system are presented using calibrated polystyrene microspheres and apheresis concentrates. ThromboLUX results were confirmed with microscopy and flow cytometry to determine the microparticle content.
Material and Methods
Dynamic Light Scattering
DLS uses the principles of Brownian motion and Doppler shift to determine the size and number of particles in a suspension. The particles in the suspension undergo continuous random movement called Brownian motion. Larger particles travel more slowly, whereas smaller particles travel faster.
To characterize this motion, laser light is focused into the suspension, and a single photon counting detector is positioned at an angle with respect to the laser. As the particles move through the suspension, they pass through the laser beam, which causes some of the light to scatter. Because the particles are moving, the scattered light undergoes a Doppler shift, which is a small frequency change of the scattered light compared to the unscattered light. Larger particles move slowly, and exhibit a small Doppler shift, whereas small particles move quickly and exhibit a large Doppler shift.
The photon counter detects the light scattered in its direction and measures the fluctuations in light intensity that result from the Doppler shift. The intensity fluctuations are measured for a period of time, and the data is processed into a correlation function – a mathematical function that identifies the patterns in the measured intensity data. The exponential decay of the correlation function is characterized by the translational diffusion constant DT. Thus, the diffusion constant is determined by measuring the Doppler shift of the laser light scattered by the moving particle [15]. The diffusion constant of a particle in suspension is used to calculate its hydrodynamic radius RH, which is the radius of a sphere that would move at the same speed as the particle [16]. The Stokes-Einstein equation relates the diffusion constant DT to the hydrodynamic radius RH of the particles (Boltzmann constant kB, temperature T, viscosity η).
Thus, DLS is an absolute sizing technique that determines size indirectly from the measured speed of the particles moving in suspension. When a sample such as a platelet concentrate consists of a mixture of particles of different sizes, a polydispersity analysis is performed on the correlation function. The polydispersity analysis identifies the size distributions of particles in the suspension and the relative number of particles of each size.
ThromboLUXPlatelet Characterization
The ThromboLUX® system (LightIntegra Technology Inc., Vancouver, BC, Canada) consists of a bench-top instrument with integrated analysis software and a disposable sterile sampling set used to obtain platelets from a platelet storage bag. To avoid a breach of sterility, the sampling set is sterilely connected to the platelet bag, and the sample is automatically drawn into a small plastic capillary that is integrated into the sampling set and functions as the measurement container. In cases where sampling from the platelet bag is already performed and for laboratory use, an alternative option to manually fill the capillary portion of the sampling set and load it into the ThromboLUX console is available.
ThromboLUX Score
The particle size distributions obtained with ThromboLUX are displayed on the screen and are used to calculate the ThromboLUX score.
The large particle size distribution with hydrodynamic radii above 550 nm stems from platelets. The width of the distribution indicates the degree of heterogeneity of the platelet population. Exposure to low temperature activates platelets, which gives rise to pseudopod formation and microaggregation, both of which reduce the Brownian motion; the change in speed results in a broadening and right-shift of the platelet size distribution.
The overall quality of a platelet concentrate can therefore be determined by considering the platelet count and heterogeneity, the temperature response of the platelets, and the microparticle content. The ThromboLUX score was developed to take all of these factors into account.
where RPl = mean radius of particles above 550 nm, SDPl = standard deviation of the RPl particle distribution, IPl = normalized intensity of the RPl particle distribution, RMP = mean radius of particles between 50 and 550 nm, SDMP = standard deviation of the RMP microparticle distribution, IMP = normalized intensity of the RMP microparticle distribution, IMP = sum over multiple temperatures; for example: i = 1 is 37 °C, i = 2 is 20 °C, i = 3 is 37 °C, which is referred to as the ‘Temperature Response’.
When the platelet count of the sample is known, the concentration of microparticles can be calculated from the comparison of the microparticle and platelet signals.
Sample Preparation
ThromboLUX performance was determined by measuring platelet-sized polystyrene microspheres as well as mixtures of microparticle-sized and platelet-sized microspheres that mimic the composition of platelet concentrates.
The following Polybead® Polystyrene Microspheres (Polysciences Inc., Warrington, PA, USA) were used: 2 micron (Cat# 19814, Lot# 637436) – referred to as PS1900, and 0.35 micron diameter (Cat# 07306, Lot# 618898) – referred to as PS356. The stock suspensions contain 2.62% solid latex. The supplier determined the bead diameter of each lot by differential centrifugation and provided the following corrected sizes: 1,925 ± 0.042 µm for the PS1900 and 0.356 ± 0.014 µm for the PS356. PS1900 were diluted 1:200 v/v with distilled water (Gibco, Life Technologies Inc., Burlington, ON, Canada) for a final concentration of 3.5 × 1010 PS1900/l or mixed with PS356 for final concentrations of 5 × 1010 PS1900/l and 3 × 1012 PS356/l. Hands-on time for sample preparation and starting the ThromboLUX measurement is approximately 2 min. ThromboLUX is a walk-away test and completes in about 20 min.
Apheresis platelet concentrates were obtained from deferred normal volunteer donors at the Canadian Blood Services netCAD Research Donor Clinic. The study protocol met the standards of the Declaration of Helsinki and was approved by the Canadian Blood Services ethics review committee. In accordance with the Declaration of Helsinki, informed consent was obtained from all donors. Apheresis platelet concentrates were prepared using the Trima Accel® (Terumo BCT, Lakewood, CO, USA) automated blood collection system. For the Mirasol (Terumo BCT) study 12 double platelets were collected, rested for a minimum of 2 h and then divided into 2 equal components by weight. One component underwent the Mirasol process: transfer to a Mirasol storage bag, addition of 35 ml of riboflavin solution, and exposure to a metered dose of UV light in the Mirasol illuminator. The control component was transferred to a Mirasol storage bag and received 35 ml of a 0.9% saline solution, the same carrier solution that dissolves the riboflavin to ensure that both components had the same platelet concentration. Components were sampled and tested on days 1, 2, 5, and 7 post collection.
Platelet Assessment by Microscopy
Differential interference contrast (DIC) microscopy on a Leica DMRA2 microscope (Leica, Richmond Hill, ON, Canada) equipped with a 100× oil-immersion objective (NA = 1.4) and a digital camera (Retiga EXi Fast Cooled Mono 12-bit; QImaging, Surrey, BC, Canada) was used to obtain high-resolution images of microparticles and platelets. For morphology scoring we used a Nikon Eclipse 50i phase contrast microscope (100× oil-immersion objective NA = 1.25, DS-Fi1 digital camera) and followed the modified Kunicki protocol for fixed cells as described by Devine et al. [17]. A morphology score was calculated after counting 100 platelets and assigning them to one of three categories: i) Discs (Δ) – platelets that have maintained the characteristic discoid shape of fresh platelets; ii) Spiny spheres (Σ) – those platelets with spherical body and protrusions (pseudopods) of various lengths extending from the central body; iii) Balloons (B) – platelets that have undergone swelling after losing the capacity to maintain an osmotic gradient across their membrane.
Particles which did not resemble single platelets such as microparticles, microaggregates or any contaminants were ignored.
Detection of Microparticles by Flow Cytometry
Flow cytometry was performed on a FACS Canto II flow cytometer (BD Biosciences, Mississauga, ON, USA) using the FACSDiva software. For internal calibration, fluorescently labeled polystyrene beads were added. 10,000 events from fluorescently labeled 1 micron polystyrene beads (Flu-oresbriteTM Carboxylate YG Microspheres, Polysciences catalog #15702) were counted to provide normalization of the platelet and microparticle counts, which were gated separately by their forward and side scatter. The following flow cytometer settings were used: FSC-H: 700 V, SSC-H: 400 V, FL1 PMT: 320 V, threshold (FSC and SSC): 200, flow rate: low, bi-exponential display: ON.
Results
ThromboLUX
Based on a research prototype [7, 18], a user friendly device was designed to allow quick and easy testing of platelet concentrates. ThromboLUX can be used in capillary mode where the sample is manually drawn into a capillary and placed into the capillary holder, or the system draws the sample into a special sterile, disposable sampling set that is connected to the bag. ThromboLUX performs the DLS measurement and, according to equation 2, automatically calculates and displays the ThromboLUX score on the touch screen. A rendering of the alpha prototype is shown (fig. 1).
Fig. 1.
Rendering of the ThromboLUX device for automated platelet testing.
ThromboLUX Performance Verification
ThromboLUX performance was tested with aliquots of PS1900 polystyrene microspheres, with a nominal diameter of 1,925 nm. These are platelet-sized microspheres suspended in water. Each of the 50 tests was performed on a fresh sample by the same operator with the same ThromboLUX instrument.
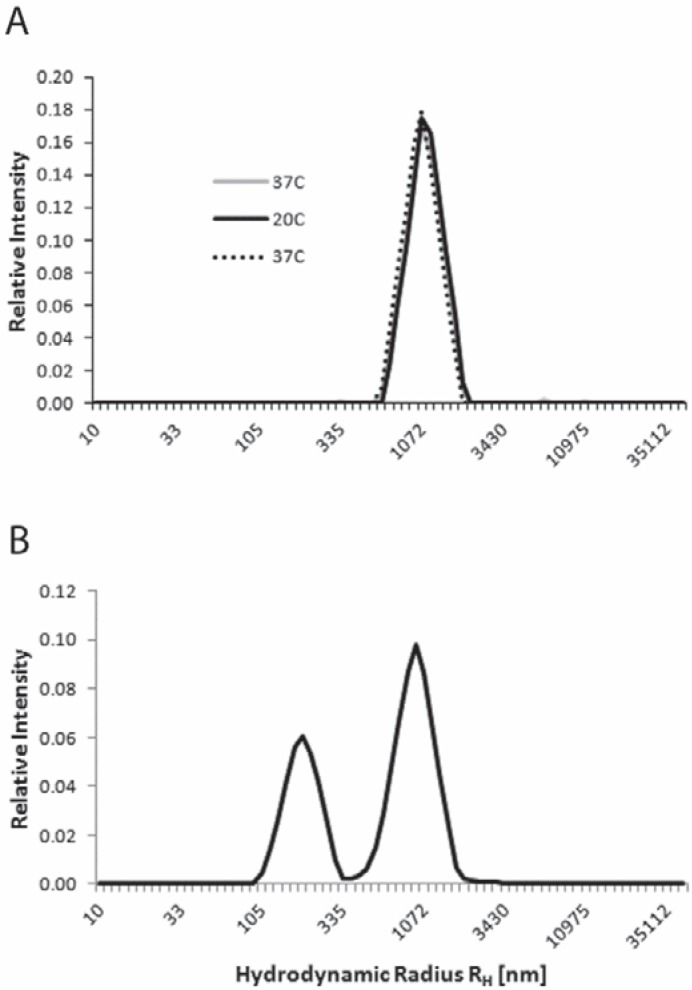
The polystyrene microspheres gave a score of 21.8 ± 2.5 (CV = 11.5%, table 1) and an average hydrodynamic radius for the beads of 1,115 ± 100 nm with an average scattering intensity of 234 ± 48 kHz. The size of the beads cannot change with temperature; therefore, obtaining overlapping size distributions for the size measurement of beads at 37 °C, 20 °C and 37 °C verifies that the temperature control performs within specification and that the settings (temperatures, viscosities) are entered correctly (fig. 2A). Measurement of bead suspensions was therefore used to insure best ThromboLUX performance.
Table 1.
Sizes and ThromboLUX scores of monodisperse and bidisperse polystyrene microsphere suspensions
| MP-sized beads |
PLT-sized beads |
||||||
|---|---|---|---|---|---|---|---|
| 37 °C | 20 °C | 37 °C | 37 °C | 20 °C | 37 °C | ||
| Hydrodynamic radius RH, nm | 1,115 | 1,147 | 1,100 | ||||
| Standard deviation | 100 | 105 | 80 | ||||
| CV, % | 8.9 | 9.1 | 7.3 | ||||
| ThromboLUX score (N = 50) | 21.8 ± 2.5 (CV 11.5%) | ||||||
| Hydrodynamic radius RH, mm | 223 | 218 | 228 | 1,091 | 1,096 | ||
| Standard deviation | 13 | 10 | 23 | 118 | 183 | ||
| CV, % | 5.7 | 4.7 | 10.1 | 10.8 | 16.7 | ||
| ThromboLUX score (N = 5) | 11.6 ± 0.7 (CV6.0%) | ||||||
Fig. 2.
ThromboLUX results of performance verification testing with polystyrene microspheres. A Size distributions of platelet-sized microspheres at three analysis temperatures (37 °C, 20 °C, 37 °C) representative of 50 tests. B Mixture of microspheres in the size range of both microparticles and platelets averaged over the 3 temperatures representative of 5 tests.
Aliquots of a bidisperse mixture of microparticle-sized and platelet-sized beads were measured 5 times by the same operator with the same ThromboLUX instrument and obtained an average score of 11.6 ± 0.7 (CV = 6.0%, table 1). This mixture of microspheres mimicked a platelet concentrate with a high concentration of microparticles (fig. 2B).
Comparison of ThromboLUX Results to Microscopy
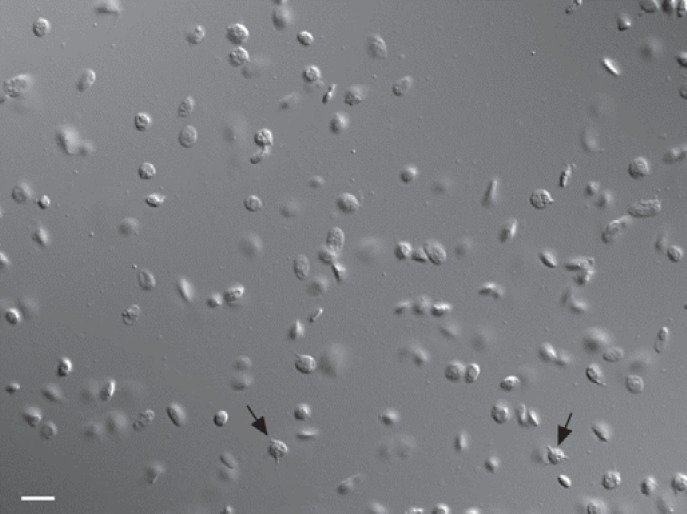
To properly view platelets on a microscope, a 100x oil-immersion objective and contrast enhancement were required. DIC microscopy is optimal to view both platelets and microparticles (fig. 3). In a sample taken from a 1-day-old platelet concentrate microparticles, discoid platelets, and a few activated platelets (marked by black arrows) can be seen.
Fig. 3.
DIC microscopy of platelets and microparticles in a sample from an apheresis platelet concentrate. The scale bar represents 5 microns. The arrows point to activated ‘spiny sphere’ platelets.
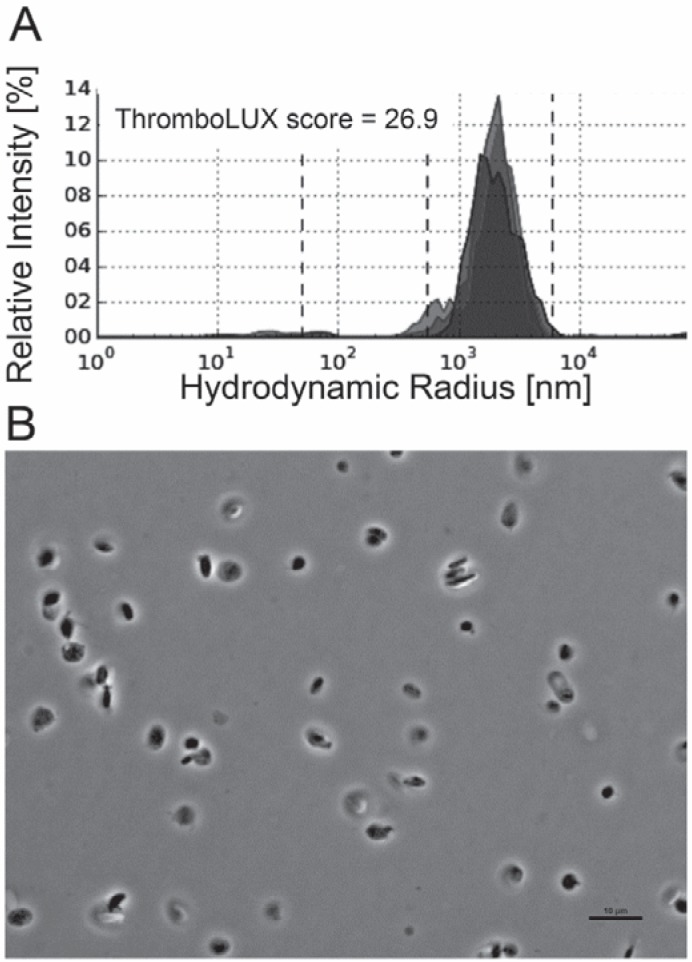
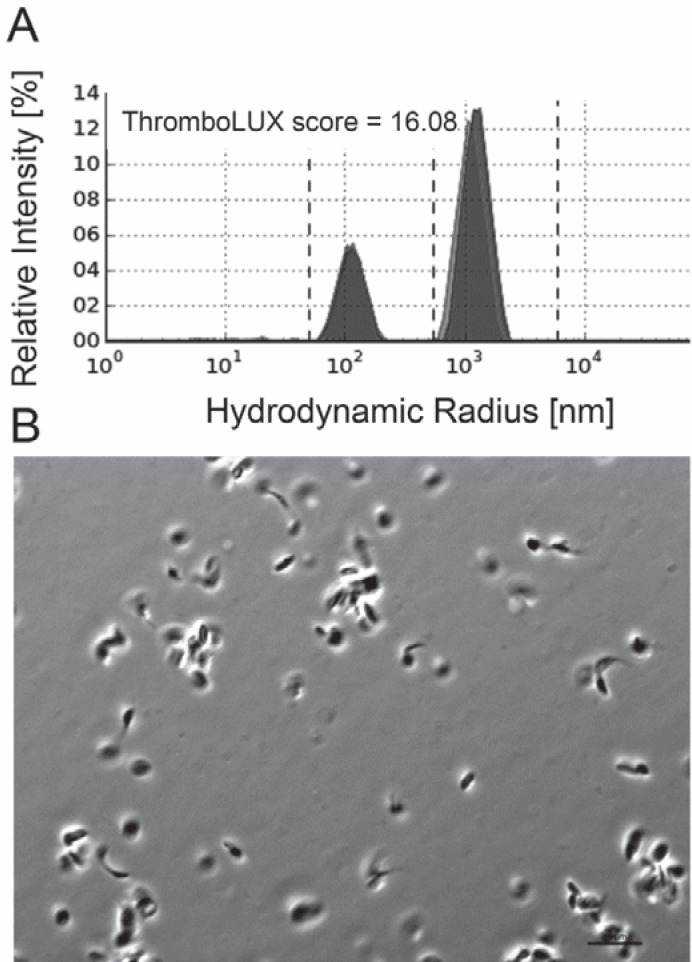
The morphology of platelets in concentrates was assessed by phase contrast microscopy which has traditionally been used for morphology scoring of platelets [19]. Microparticles are difficult to see using this microscopy technique. Platelets with high ThromboLUX score (fig. 4A) showed more discoid platelets, qualitatively fewer microparticles, and fewer/smaller microaggregates (fig. 4B). Platelets with low ThromboLUX score (fig. 5A) showed more spiny sphere platelets, more microparticles, and larger microaggregates (fig. 5B). While qualitatively the microscopy images agreed with the ThromboLUX scores as shown in the two representative examples, no significant correlation of the ThromboLUX score with the corresponding morphology score was found.
Fig. 4.
A High-scoring platelet concentrate (26.9) showing B many discoid platelets but also some small groups of double and triple platelets in the phase contrast micrograph. These are light enough to stay in the observation volume of ThromboLUX and contribute to the wide platelet population. Very few microparticles are present.
Fig. 5.
A Example of a low-scoring platelet concentrate (16.08) showing B a high content of microparticles and small platelets in the ThromboLUX result. Many aggregates 10 microns or larger are seen in the phase contrast micrograph. Aggregates of this size settle quickly and are not detected by ThromboLUX.
Comparison of ThromboLUX Results with Flow Cytometry
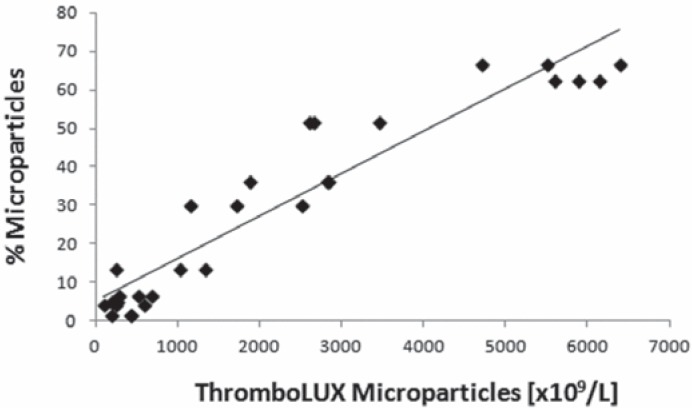
Microparticles are identified by flow cytometry using static light scattering and gating on the smallest particle population that appears above the electronic noise threshold. The microparticles are gated in the forward versus side scatter dot plot. In flow cytometry, the concentration of microparticles is determined relative to a known concentration of fluorescently labeled microspheres with a size of 1,000 nm as previously described [20]. In apheresis platelets the concentration of microparticles calculated from the ThromboLUX score strongly correlated with the microparticle content determined by flow cytometry (fig. 6) with a Pearson correlation coefficient r = 0.948.
Fig. 6.
Correlation of the microparticle concentration calculated from the ThromboLUX score with microparticle content determined by flow cytometry for 10 apheresis platelet concentrates, each measured three times.
Comparison of ThromboLUX Results with and without Mirasol Treatment
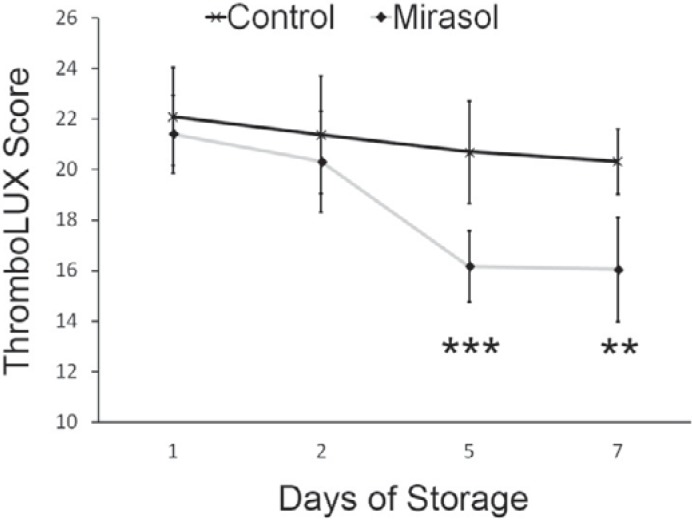
The average ThromboLUX scores of 12 double apheresis platelets which were split into control and Mirasol-treated components and sampled over 7 days of storage were compared. The means of the two treatment groups were compared by two-tailed paired t-test (α = 0.05) that showed a significant difference between control and Mirasol-treated platelets on day 5 (p < 0.001) and on day 7 (p < 0.005) (fig. 7).
Fig. 7.
Average ThromboLUX scores of 12 double apheresis platelets split into control and Mirasol-treated components sampled over 7 days of storage with the difference between the means significant at p < 0.001 on day 5 (***) and significant at p < 0.005 on day 7 (**; two-tailed paired t-test with α = 0.05).
Discussion
Blood transfusions are of critical importance to patients with both acute and chronic medical conditions in which blood is depleted or non-functioning. US statistics indicate that more than 4.5 million Americans would die each year without blood transfusions [1].
Historically, platelet concentrates have been considered ‘good’ if the platelet count was within an acceptable range for a given volume. Samples would be taken from the platelet concentrate and analyzed using automated cell counters to determine the platelet count [21, 22]. Counting platelets, however, does not test the functionality of the cells and does not account for microparticles arising from platelet fragmentation.
ThromboLUX was developed to measure two characteristics related to platelet viability: the presence of microparticles and the temperature response of the platelets. The device can be used with minimal training and does not require reagents, special techniques, or any other laboratory equipment, except for a sterile docking device and a heat sealer when the closed system sampling sets are used. Test results are displayed within 20 min.
ThromboLUX performance is verified with standard microspheres. As with all DLS measurements, ThromboLUX can determine the size of suspended particles as long as the parameters required to solve the Stokes-Einstein equation (equation 1) are known and stable. Measurements of bead suspensions are therefore used to verify that ThromboLUX performs within specifications (fig. 2, table 1). The repeatability of ThromboLUX compares well with other DLS devices used to measure the size of bead standards. Two micron beads sized by the Coulter principle were reported with a standard deviation of 10%, and submicron bead sizes determined with a Brookhaven instrument were reported with a standard deviation of 25% (according to package inserts for traceable beads).
Despite its wide use in platelet research, manual morphology scoring is not used routinely to test platelet transfusions. The characterization of platelet samples by microscopy is time-consuming, subjective, operator-dependent, and limited to a small number of cells that can be evaluated [23]. Both platelet viability and the number of microparticles are very challenging to assess by microscopy. Microparticles are extremely difficult to capture due to their small diameter of about 200–400 nm and their quick movement in and out of focus [20]. Furthermore, microscopy scoring methods such as the modified Kunicki morphology score ignore potentially important characteristics of platelet concentrates such as microparticle content, microaggregates, and other unclassified particles [3]. However, all these particles scatter light and therefore contribute to the ThromboLUX score, which is why microscopy images reflect the ThromboLUX score but morphology scores and ThromboLUX scores do not correlate. In addition, morphology scores have not correlated well with clinical outcome [6, 24].
The microparticle population in platelet concentrates is of particular interest: microparticles can derive from the donor and increase during storage of platelet concentrates as a consequence of platelet activation and aging [25]. Microparticle formation during storage occurs as small pieces of the platelet pseudopods fracture from the cells. The presence of microparticles might therefore be an indicator of diminished platelet quality. Changes of platelet characteristics in concentrates have been reported as a function of storage time and treatments such as pathogen reduction [26]. We were able to show the decrease in ThromboLUX scores as a consequence of the degradation of apheresis platelets during storage as well as an additional effect with the Mirasol pathogen reduction treatment. Our results are in agreement with other studies [26].
Because it has been difficult to include microparticle enumeration in clinical study protocols, little is known about the clinical effect of platelet transfusions with high microparticle load. However, high levels of platelet-derived microparticles were associated with adverse transfusion reactions [27, 28]. It remains to be demonstrated whether platelet concentrates containing fewer microparticles perform better after transfusion. In this context it might be hypothesized that substitution of plasma with platelet-additive solution could potentially reduce the microparticle concentration in the final component, thus leading to higher ThromboLUX scores and better clinical outcome. Studies are required to support this hypothesis.
Flow cytometry is not suitable for routine quantitation of microparticles in platelet concentrates, as this technique requires sophisticated and expensive instrumentation, highly trained personnel to perform the test and interpret the results, and, most importantly, several steps of sample preparation [29]. In contrast, ThromboLUX does not require any sample preparation and determines microparticle content as part of the ThromboLUX score.
ThromboLUX could reduce the risk of ineffective transfusions that are given primarily to cancer and bleeding patients. Consequently, the complications associated with ineffective transfusions would be reduced. Characterizing platelets to gain improved clinical effectiveness could dramatically lower the cost of platelet transfusion therapy for hospitals and health care systems.
Conclusion
Prior to ThromboLUX, no single in vitro test existed to evaluate characteristics of platelet transfusions that could be indicative of in vivo effectiveness [30, 31]. As hospitals currently cannot predict which transfusions will be effective, they transfuse all platelet products they acquire and only learn which ones were ineffective retrospectively by seeing patient responses.
ThromboLUX can measure the concentration of microparticles directly from platelet concentrates and does not require separation, dilution, or concentration steps. The instrument was designed to combine several important indicators of platelet quality and viability in one quick and easy-to-perform test that produces a simple output in the form of a numerical score. The ThromboLUX score does not correlate significantly with the modified Kunicki morphology score; however, the morphology score does not accurately reflect important characteristics of the platelet sample. Qualitatively, the ThromboLUX score correlates with platelet morphology observed by microscopy: a higher ThromboLUX score parallels a higher number of discoid platelets, a lower number of microparticles, and a more pronounced temperature response of platelets. The ThromboLUX score reflects the deterioration of platelet components over time and after pathogen reduction treatment.
Disclosure Statement
Elisabeth Maurer is a shareholder and director and Audrey Labrie is an employee of LightIntegra Technology.
Acknowledgements
We thank Keddie Brown for the initial development of the DLS platelet monitor. The work was supported by research grants from Canadian Blood Services and the Canadian National Research Council Industrial Research Assistance Program (IRAP).
References
- 1.Sahoo A. The Worldwide Market for Blood Products, Blood Testing, Blood Equipment and Synthetic Blood Products. New York: Kalorama Information; 2008. pp. 21–22. [Google Scholar]
- 2.Department of Health and Human Services USA: The 2009 National Blood Collection and Utilization Survey Report, 2011.
- 3.Maurer-Spurej E, Labrie A, Pittendreigh C, Chipperfield K, Smith C, Heddle N, Liu Y, Yi QL, Barnett M. Platelet quality measured with dynamic light scattering correlates with transfusion outcome in hematologic malignancies. Transfusion. 2009;49:2276–2284. doi: 10.1111/j.1537-2995.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 4.Sigle JP, Medinger M, Stern M, Infanti L, Heim D, Halter J, Gratwohl A, Buser A. Prospective change control analysis of transfer of platelet concentrate production from a specialized stem cell transplantation unit to a blood transfusion center. J Clin Apheresis. 2012;27:178–182. doi: 10.1002/jca.21214. [DOI] [PubMed] [Google Scholar]
- 5.Delaflor-Weiss E, Mintz PD. The evaluation and management of platelet refractoriness and alloimmunization. Transfus Med Rev. 2000;14:180–196. doi: 10.1016/s0887-7963(00)80007-3. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich RP, Li J, Pieters H, Crookes R, Roodt J, Heyns AP. Correlation of in vitro platelet quality measurements with in vivo platelet viability in human subjects. Vox Sang. 2006;90:279–285. doi: 10.1111/j.1423-0410.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 7.Maurer-Spurej E, Brown K, Labrie A, Marziali A, Glatter O. Portable dynamic light scattering instrument and method for the measurement of blood platelet suspensions. Phys Med Biol. 2006;51:3747–3758. doi: 10.1088/0031-9155/51/15/010. [DOI] [PubMed] [Google Scholar]
- 8.Bennett WF, Lynch G. Low-temperature induction of calcium-dependent protein phosphorylation in blood platelets. J Cell Biol. 1980;86:280–285. doi: 10.1083/jcb.86.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder EL, Bookbinder M. Role of microaggregate blood filtration in clinical medicine. Transfusion. 1983;23:460–470. doi: 10.1046/j.1537-2995.1983.23684074264.x. [DOI] [PubMed] [Google Scholar]
- 10.Maurer-Spurej E, Pfeiler G, Maurer N, Lindner H, Glatter O, Devine DV. Room temperature activates human blood platelets. Lab Invest. 2001;81:581–592. doi: 10.1038/labinvest.3780267. [DOI] [PubMed] [Google Scholar]
- 11.Viera AJ, Mooberry M, Key NS. Microparticles in cardiovascular disease pathophysiology and outcomes. J Am Soc Hypertens. 2012;6:243–252. doi: 10.1016/j.jash.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17:578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010;77:502–514. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Spurej E, Glatter O, Pfeiler G. Shape change of human blood platelets: reliable and fast detection by quasi-elastic light scattering. Experientia. 1992;48:71–79. doi: 10.1007/BF01923612. [DOI] [PubMed] [Google Scholar]
- 16.Ford NC. Light scattering apparatus. In: Pecora R, editor. Dynamic Light Scattering Applications of Photon Correlation Spectroscopy. New York: Plenum; 1985. pp. 7–58. [Google Scholar]
- 17.Devine DV, Bradley AJ, Maurer E, Levin E, Chahal S, Serrano K, Gyongyossy-Issa MI. Effects of prestorage white cell reduction on platelet aggregate formation and the activation state of platelets and plasma enzyme systems. Transfusion. 1999;39:724–734. doi: 10.1046/j.1537-2995.1999.39070724.x. [DOI] [PubMed] [Google Scholar]
- 18.Maurer-Spurej E, Pittendreigh C, Yakimec J, De Badyn MH, Chipperfield K. Erroneous automated optical platelet counts in 1-hour post-transfusion blood samples. Int J Lab Hematol. 2010;32:e1–e8. doi: 10.1111/j.1751-553X.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 19.Kunicki TJ, Tuccelli M, Becker GA, Aster RH. A study of variables affecting the quality of platelets stored at ‘room temperature’. Transfusion. 1975;15:414–421. doi: 10.1046/j.1537-2995.1975.15576082215.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Nakane N, Maurer-Spurej E. Novel test for microparticles in platelet-rich plasma and platelet concentrates using dynamic light scattering. Transfusion. 2011;51:363–370. doi: 10.1111/j.1537-2995.2010.02819.x. [DOI] [PubMed] [Google Scholar]
- 21.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. I. Factors influencing the harvest of viable platelets from whole blood. Br J Haematol. 1976;34:395–402. doi: 10.1111/j.1365-2141.1976.tb03586.x. [DOI] [PubMed] [Google Scholar]
- 22.Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. II. Storage variables influencing platelet viability and function. Br J Haematol. 1976;34:403–419. doi: 10.1111/j.1365-2141.1976.tb03587.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Plaza I. Modern Transfusion Medicine: A Practical Approach. New York: CRC Press; 1995. [Google Scholar]
- 24.Mintz PD, Anderson G, Avery N, Clark P, Bonner RF. Assessment of the correlation of platelet morphology with in vivo recovery and survival. Transfusion. 2005;45:72S–80S. doi: 10.1111/j.1537-2995.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 25.Maurer-Spurej E, Chipperfield K. Past and future approaches to assess the quality of platelets for transfusion. Transfus Med Rev. 2007;21:295–306. doi: 10.1016/j.tmrv.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Middelburg RA, Roest M, Ham J, Coccoris M, Zwaginga JJ, van der Meer PF: Flow cytometricassessment of agonist-induced P-selectin expression as a measure of platelet quality in stored platelet concentrates. Transfusion 2012; doi: 10.1111/trf.12001. [DOI] [PubMed]
- 27.Gilstad CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003;10:419–423. doi: 10.1097/00062752-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Nomura S, Okamae F, Abe M, Hosokawa M, Yamaoka M, Ohtani T, Onishi S, Matsuzaki T, Teraoka A, Ishida T, Fukuhara S. Platelets expressing P-selectin and platelet-derived microparticles in stored platelet concentrates bind to PSGL-1 on filtrated leukocytes. Clin Appl Thromb Hemost. 2000;6:213–221. doi: 10.1177/107602960000600406. [DOI] [PubMed] [Google Scholar]
- 29.Barnard MR, MacGregor H, Mercier R, Ragno G, Pivacek LE, Hechtman HB, Michelson AD, Valeri CR. Platelet surface P-selectin, platelet-granulocyte heterotypic aggregates, and plasma-soluble P-selectin during plateletpheresis. Transfusion. 1999;39:735–741. doi: 10.1046/j.1537-2995.1999.39070735.x. [DOI] [PubMed] [Google Scholar]
- 30.Murphy S, Rebulla P, Bertolini F, Holme S, Moroff G, Snyder E, Stromberg R. In vitro assessment of the quality of stored platelet concentrates. The BEST (Biomedical Excellence for Safer Transfusion) Task Force of the International Society of Blood Transfusion. Transfus Med Rev. 1994;8:29–36. doi: 10.1016/s0887-7963(94)70095-x. [DOI] [PubMed] [Google Scholar]
- 31.Vostal JG. Efficacy evaluation of current and future platelet transfusion products. J Trauma. 2006;60:S78–S82. doi: 10.1097/01.ta.0000199921.40502.5d. [DOI] [PubMed] [Google Scholar]