Abstract
WalRK (YycFG) two-component systems (TCSs) of low-GC Gram-positive bacteria play critical roles in regulating peptidoglycan hydrolase genes involved in cell division and wall stress responses. The WalRK (VicRK) TCSs of Streptococcus pneumoniae (pneumococcus) and other Streptococcus species show numerous differences with those of other low-GC species. Notably, the pneumococcal WalK sensor kinase is not essential for normal growth in culture, unlike its homologues in Bacillus and Staphylococcus species. The WalK sensor kinase possesses histidine autokinase activity and mediates dephosphorylation of phosphorylated WalR~P response regulator. To understand the contributions of these two WalK activities to pneumococcal growth, we constructed and characterized a set of walK kinase and phosphatase mutants in biochemical reactions and in cells. We identified an amino acid substitution in WalK that significantly reduces phosphatase activity, but not other activities. Comparisons were made between WalRK regulon expression levels and WalR~P amounts in cells determined by Phos-tag SDS-PAGE. Reduction of WalK phosphatase activity resulted in nearly 90% phosphorylation to WalR~P, consistent with the conclusion that WalK phosphatase is strongly active in exponentially growing cells. WalK phosphatase activity was also shown to depend on the WalK PAS domain and to limit crosstalk and the recovery of WalR~P from walK+ cells.
Keywords: WalR response regulator, WalK sensor kinase, WalK phosphatase activity, pneumococcus, Phos-tag SDS-PAGE
Introduction
The WalRK (YycFG) two-component regulatory system (TCS) plays important roles in maintaining peptidoglycan and surface homeostasis and in responding to cell wall stresses in low-GC Gram-positive bacteria (reviewed in (Dubrac et al., 2008, Jordan et al., 2008, Winkler & Hoch, 2008). Although the WalRK TCS shows distinctive features in each bacterial species, several unifying patterns have emerged about this TCS. The WalR response regulator is generally essential and required for growth in culture (Fabret & Hoch, 1998, Hancock & Perego, 2004, Kadioglu et al., 2003, Martin et al., 1999, Senadheera et al., 2005, Throup et al., 2000, Wagner et al., 2002), although bypass suppressors can be identified that uncouple regulon expression from WalR control (Delaune et al., 2011, Ng et al., 2003, Winkler & Hoch, 2008). WalR proteins are members of the PhoB family of response regulators and consist of phosphorylated receiver domains and winged-helix DNA-binding effector domains that contain characteristic structural features (see (Barbieri et al., 2010, Bent et al., 2004, Doi et al., 2010, Okajima et al., 2008)).
The core of all WalRK regulons consists of genes encoding peptidoglycan hydrolases involved in cell division (Bisicchia et al., 2010, Bisicchia et al., 2007, Dubrac et al., 2007, Dubrac & Msadek, 2004, Liu & Burne, 2011, Ng et al., 2003, Ng et al., 2005, Senadheera et al., 2012). Other gene classes in the regulon vary among different bacteria and include genes that mediate wall teichoic acid biosynthesis, exopolysaccharide production, or virulence (Botella et al., 2011, Duque et al., 2011, Liu et al., 2006, Mohedano et al., 2005, Ng et al., 2005, Senadheera et al., 2005). With the exception of pcsB homologues in some Streptococcus species (Barendt et al., 2009, Ng et al., 2004, Ng et al., 2003), the essentiality of WalR is caused by regulation of multiple genes (Bisicchia et al., 2007, Delaune et al., 2011, Dubrac et al., 2008). Mutations that activate WalR function lead to the formation of wall-less L-form cells (Dominguez-Cuevas et al., 2012), and other amino acid changes in WalR result in vancomycin-intermediate resistance of Staphylococcus aureus (VISA) laboratory strains and clinical isolates (Howden et al., 2011, Shoji et al., 2011) .
In contrast to WalR, WalK sensor kinases are divided into two distinct classes (Fukushima et al., 2008, Ng & Winkler, 2004, Turck & Bierbaum, 2012). WalK homologues of Bacillus, Staphylococcus, Enterococcus, and most other species contain two transmembrane domains flanking an extracytoplasmic domain (Fukushima et al., 2008, Szurmant et al., 2007b), which is the typical arrangement for many histidine kinases (see (Gao & Stock, 2009)). The transmembrane domains of Bacillus subtilis WalKBsu interact with the membrane domains of the auxiliary WalHI (YycHI) proteins away from the division septum to negatively regulate phosphorylation levels of WalRBsu (Fukushima et al., 2011, Szurmant et al., 2008, Szurmant et al., 2007a). In contrast, WalKSpn from Streptococcus pneumoniae (pneumococcus), which exemplifies the other class, contains a single transmembrane domain connected to an extracellular peptide of only 12 amino acids, and S. pneumoniae lacks homologues of WalHI (Fig. 1) (Ng & Winkler, 2004). Another major difference between the two WalK classes is that members of the majority class, exemplified by WalKBsu, are generally essential for bacterial growth in culture, whereas Streptococcal WalK sensor kinases are dispensable, although their absence may impair growth (Fabret & Hoch, 1998, Ng et al., 2003, Senadheera et al., 2012).
Fig. 1.
Genetic organization of the walRKJ operon in S. pneumoniae and model of WalRKSpn TCS function in exponentially growing and stressed cells. (A). The co-transcribed walR (response regulator), walK (histidine kinase/phosphatase) and walJ (auxiliary protein) genes are drawn to scale at left. walR, but not walK or walJ, is essential under normal growth conditions. Domains in one subunit of the WalK histidine kinase are shown at right, including the single membrane-spanning domain, the HAMP (linker) and PAS (signaling) domains, the DHp (dimerization/histidine phosphorylation) domain, and the CA (ATPase) catalytic domain. Both WalK and WalJ are membrane associated, whereas WalR is cytoplasmic (Wayne et al., 2010). (B) Model for WalRK signaling and gene regulation from this study. In exponentially growing cells, ≈26% WalR is phosphorylated, and functions to stimulate a basal level of WalRK regulon expression that maintains normal growth. In unstressed cells, WalK functions mainly as a phosphatase that sets the amount of WalR~P and limits crosstalk by non-cognate sensor kinases. In cells subjected to wall stresses or limitation of the pcsB regulon gene (signal arrow), the phosphatase activity of WalK is reduced, possibly by binding of a ligand or protein to the PAS domain of WalK, and the WalK autokinase/phosphoryltransferase reactions lead to increased amounts of WalR~P and regulon expression. See text for additional details.
The cytoplasmic domains of both classes of WalK sensors are similar and contain a HAMP (linker) domain followed by a PAS (potential signal binding or protein interaction) domain, which precedes the DHp (dimerization/histidine phosphorylation) and catalytic CA (ATPase) domains, characteristic of sensor kinases (Fig. 1) (Gao & Stock, 2009, Henry & Crosson, 2011). In addition, both classes of WalRK TCS involve an auxiliary protein, called WalJ (YycJ), which is a predicted member of the metallo-β-lactamase superfamily (Ng & Winkler, 2004, Turck & Bierbaum, 2012, Winkler & Hoch, 2008). Although its enzymatic function is unknown, accumulated data suggests links among WalJ, WalRK TCS function, and peptidoglycan metabolism that if perturbed, disrupt accurate coordination of cell division and DNA replication (Biller et al., 2011, Ng et al., 2003).
Cell wall damage by antibiotics is believed to be a signal that induces formation of WalR~P (Dubrac et al., 2008), although the mechanisms of signal transduction by WalRK and their auxiliary proteins remain largely unknown. WalK is randomly distributed in the membrane of dividing S. pneumoniae cells (Wayne et al., 2010), in contrast to the septal location of WalKBsu in dividing B. subtilis cells (Fukushima et al., 2011). Changing the conserved, phosphorylated Asp52 residue of pneumococcal WalR to Asn, Gln, or Ala is not tolerated, implying that WalR~P formation is required for pcsB expression (Ng et al., 2003). Even the Asp52Glu change, which can mimic aspartyl phosphate, is barely tolerated, except in ΔwalK mutants, suggesting additional interactions between WalK and WalR (Ng et al., 2003). Titration experiments indicated that WalR is present in excess in exponentially growing S. pneumoniae (Ng et al., 2003). Consistent with this expectation, WalR is an abundant protein present in ≈6,000 monomers per cell, which is a ≈13-fold molar excess over WalK dimer amount (Wayne et al., 2010).
Recently, we reported that the WalK sensor kinase of S. pneumoniae exhibits a strong phosphatase activity that mediates dephosphorylation of WalR~P in biochemical reactions (Gutu et al., 2010, Huynh & Stewart, 2011). Optimal WalK phosphatase activity in reactions depended on its PAS domain (Gutu et al., 2010). In this study, we examine the roles of the WalK autokinase and phosphatase activities in setting WalRK regulon expression and WalR~P amount in exponentially growing S. pneumoniae cells. To this end, we identified an amino acid change in WalK that specifically reduces its phosphatase activity, and we modified the Phos-tag SDS-PAGE method (Barbieri & Stock, 2008) for use in S. pneumoniae. Our results support the conclusions that WalK phosphatase is the default activity in exponentially growing cells and that WalK prevents phosphorylation of WalR by non-cognate sensor kinases. Yet, we show that crosstalk phosphorylation of WalR is partly responsible for the viability of pneumococcal walK mutants. This work also demonstrates the utility of the Phos-tag SDS-PAGE method to determine response regulator phosphorylation levels in bacterial cells and a way to deal with a potential limitation of this method.
Results
WalKT222A is defective in phosphatase activity, but not in other WalK activities
To understand more about the physiological roles of WalK phosphatase activity in S. pneumoniae, we needed to identify a phosphatase-deficient mutant of WalK that retained nearly normal autokinase and phosphoryltransferase activities. Huynh and Stewart recently proposed a reaction mechanism for the phosphatase activity mediated by transmitter domains of HisKA-family sensor kinases (Huynh et al., 2010, Huynh & Stewart, 2011). Based on this model and on other previous studies of phosphatase deficient mutants of sensor kinases (Capra et al., 2010, Dutta et al., 2000, Hsing & Silhavy, 1997, Zhu et al., 2000), we expressed and purified 13 mutant WalK proteins with amino acid changes in the DHp domain near the phosphorylated His218 residue (Fig. S1). The V216G, S217D, P223A, P223S, R221D, or T222D (Fig. S2) or T222R (Gutu et al., 2010) amino acid changes severely diminished autokinase activity and were not studied further.
Combined autokinase-phosphoryltransferase-phosphatase assays were performed to screen for decreased phosphatase activity of the remaining WalK mutant proteins (Fig. 2 and S3) (Gutu et al., 2010). In combined assays, little WalR~P accumulated in Mg2+ buffer because of the WalK phosphatase activity (Gutu et al., 2010). More WalR~P accumulated in Ca2+ than Mg2+ buffer because of the strong dependence of the phosphatase activity on Mg2+ ion (Gutu et al., 2010, Huynh & Stewart, 2011, Zhu et al., 2000). WalK with the T225A, S217A, or R221K amino acid changes (Fig. S3C, S3D, and S3H) showed similar low-level accumulation of WalR~P as wild-type WalK+ in Mg2+ buffer and were not ostensibly defective in phosphatase or other WalK activities. The T222A, R221A, R221S, or T222Y amino acid changes in WalK resulted in increased WalR~P accumulation in Mg2+ buffer (Fig. 2 and S3B, S3F, and S3G), characteristic of a defect in WalK phosphatase activity (Gutu et al., 2010). Of these, WalKT222A showed the strongest effect (Fig. 2) and was characterized further.
Fig. 2.

Combined assays of the autokinase, phosphoryltransfer, and phosphatase activities mediated by truncated WalK+ or WalKT222A. Reactions were performed at 25°C in reaction mixtures containing Mg2+ or Ca2+ buffer as described in Experimental procedures. Autophosphorylation reactions containing 2.0 μM of purified (A) (N)-Sumo-ΔN35-WalK+ or (B) (N)-Sumo-ΔN35-WalKT222A protein proceeded for 30 min before (N)-His-WalR (final concn = 6.6 μM) was added without removal of ATP (t=0). The experiment was repeated >3 times, and representative phosphorimages of time courses are shown.
The increase in WalR~P accumulation in combined assays containing WalKT222A could be the result of increased autokinase or phosphoryltransferase activity or decreased phosphatase activity. Michaelis-Menton kinetic analysis of the autokinase reaction showed that WalKT222A has a similar Km (ATP) to that of wild-type WalK+, and a slightly lower kcat (Fig. S4), which would not account for greater accumulation of WalR~P in combined assays (Fig. 2). We estimated the catalytic efficiency of the phosphoryltransfer reaction containing WalKT222A or WalK+ by determining the half-life of WalK~P after WalR addition (Fig. S5) (see (Gutu et al., 2010)). The catalytic efficiency of phosphoryltransfer to WalR was greater in Mg2+ buffer than Ca2+ buffer as we reported before (Fig. S5) (Gutu et al., 2010). However, in both Mg2+ and Ca2+ buffers, the kinetic preference of phosphoryltransfer from wild-type WalK+~P was slightly greater than from WalKT222A~P (Fig. S5). The lower efficiency of phosphoryltransfer from WalKT222A~P again would not account for the increase in WalR~P formation in the combined assays (Fig. 2). Finally, we assayed the phosphatase activity of WalKT222A using a direct HPLC-based assay (Fig. S6 and S7) (Gutu et al., 2010). The phosphatase activity mediated by WalKT222A was at least 13-fold lower than that of WalK+. We conclude that greatly reduced phosphatase activity accounts for the accumulation of WalR~P in combined assays containing WalKT222A (Fig. 2), which has comparatively minor defects in its autokinase and phosphoryltransferase activities. This conclusion was confirmed by determination of the cellular amounts of WalR~P in a walKT222A mutant as described below.
WalK autokinase activity is required for optimal pneumococcal growth in statically aerated cultures
Four isogenic mutants were studied to learn more about the roles of the WalK autokinase and phosphatase activities in growth of encapsulated S. pneumoniae strain D39 (Table S1). The ΔwalK, walKH218A, and walKΔPAS mutants were reported previously and contain an in-frame markerless deletion of the central region of the walK gene, a nucleotide substitution that changes the phosphorylated His218 to Ala in WalK, and an internal in-frame deletion that removes the PAS domain of WalK, respectively (Gutu et al., 2010). In biochemical assays using truncated WalK derivatives, WalKH218A has no autokinase activity and shows ≈12-fold less phosphatase activity than wild-type WalK+ (Gutu et al., 2010). WalKΔPAS has ≈12-fold less phosphatase activity, but is kinetically defective in that its Km (ATP) in the autokinase reaction is increased by ≈6-fold compared to that of wild-type WalK+, while its kcat is similar (Gutu et al., 2010). However, the increased Km (ATP) (≈300 μM) of WalKΔPAS remains below the total ATP concentration (≈2,000 μM) in exponentially growing S. pneumoniae D39 cells (Ramos-Montanez, et al., 2010). The walKT222A mutant was constructed for this study (see above).
Repeated growth determinations showed that the autokinase-deficient ΔwalK and walKH218A mutants consistently grew slightly (≈14%) slower and reached a lower (≈45%) final OD620 (growth yield) than the walK+ parent strain in cultures grown statically in BHI broth in an atmosphere of 5% CO2 without additional aeration (Fig. 3A and S8A) (Gutu et al., 2010). In comparison, the walKΔPAS and walKT222A phosphatase-deficient mutants grew at the same rate as the walK+ parent strain, although the growth yield of the walKT222A mutant was consistently slightly lower (≈15%) than that of the parent (Fig. 3A and S8A). The decreased growth yield of the walKH218A mutant compared to the walK+ and walKT222A strains could not be attributed to cell death or premature autolysis during exponential phase or the transition to stationary phase (Fig. S8 and S9).
Fig. 3.
Representative growth curves of mutant strains used in this study. (A) walK mutant strains and (B) walK pnpRS mutant strains were grown in static BHI broth cultures at 37°C in an atmosphere of 5% CO2 as described in Experimental procedures.
Importantly, the walKH218A point mutation should not be polar on downstream walJ expression, and similar amounts of WalKΔPAS, WalKH218A, and WalK+ were detected by Western blotting in cultures during exponential growth (Fig. S8C). The ΔwalK mutation, which retained 60 bp from each end of the gene, was also not polar on downstream transcription of walJ as determined by microarray analyses (Table 1). Notably, inactivation of walK caused decreased relative transcript amounts confined predominantly to members of the WalRK regulon, including spd_0104 (−3.1 fold; LysM protein), spd_0703 (−1.9 fold; putative SEDS protein), spd_1874 (−4.4; putative N-acetylmuramidase), and pcsB (−2.6 fold; putative peptidoglycan hydrolase) (Barendt et al., 2011, Ng et al., 2005, Sham et al., 2011).
Table 1.
Changes in relative transcript amounts in strain IU1896 (ΔwalK) compared to IU1781 (walK+) grown exponentially in BHI brotha
| Gene tag | Gene descriptionb | Fold changes |
Bayesian P value |
|---|---|---|---|
| Decreased relative expression | |||
| spd_0104 | LysM domain-containing protein | −3.1 | 8.6E-07 |
| spd_0391 | Hypothetical protein | −1.9 | 2.5E-05 |
| spd_0392 | Hypothetical protein | −1.8 | 5.2E-05 |
| spd_0447 |
glnR; transcriptional repressor of the glutamine synthetase gene |
−1.7 | 4.3E-04 |
| spd_0451 | hsdS; type I restriction enzyme | −2.5 | 1.5E-06 |
| spd_0703 | Hypothetical protein, putative SEDS protein | −1.9 | 3.0E-06 |
| spd_0853 | lytB, N-acetylglucosaminidase | −1.6 | 6.9E-04 |
| spd_1824 | Putative transmembrane protein with conserved FtsX superfamily domain |
−1.6 | 1.4E-04 |
| spd_1870 |
pcp-truncation; pyrrolidone carboxyl
peptidase, truncation |
−1.7 | 4.8E-05 |
| spd_1871 | Conserved hypothetical protein | −2.0 | 5.4E-06 |
| spd_1872 |
marR; transcriptional regulator - MarR
family |
−2.0 | 5.6E-05 |
| spd_1874 |
LysM domain-containing protein, putative
N-acetylmuramidase |
−4.4 | 2.7E-07 |
| spd_2043 | pcsB, putative peptidoglycan hydrolase | −2.6 | 1.6E-06 |
| Increased relative expression | |||
| spd_0094 | Hypothetical protein | 1.6 | 4.7E-04 |
| spd_0453 |
hsdS; type I site-specific deoxyribonuclease chain S |
2.8 | 6.0E-07 |
Strain construction, growth, and microarray analysis are described in Experimental procedures. RNA was prepared from exponential cultures grown to OD620 ≈ 0.2. Fold changes and Bayesian P-values are based on 3 independent biological replicates, including a dye swap. Cutoffs for this table were 1.6-fold and P<0.001. A complete data set is deposited in GEO (accession GSE19752).
Members of the WalRKSpn regulon are shown in bold type.
WalKT222A increases WalRK regulon expression and cellular WalR~P amount
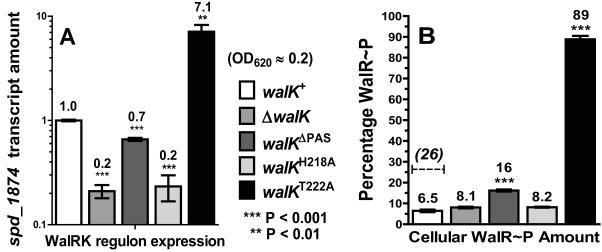
QRT-PCR assays confirmed the microarray results (Table 1) by showing that the relative transcript amounts of WalRK regulon genes spd_1874 and pcsB dropped by 5- and 3-fold in the ΔwalK and walKH218A mutants, respectively, compared to the walK+ parent (Fig. 4A and S10A). Control experiments indicated that the drop in the relative amount of spd_1874 transcript was complemented by ectopic expression of the walK+ gene (Fig. S10B). In subsequent experiments, we used relative amounts of spd_1874 transcript from QRT-PCR assays as a read out of regulon expression, because spd_1874 responds the most strongly to the WalRK TCS (Table 1) (Ng et al., 2005). We conclude that WalK autokinase activity and phosphoryltransfer to WalR are necessary to maintain normal WalRK regulon expression in exponentially growing cells.
Fig. 4.
WalRK regulon expression in different walK mutant strains during exponential growth and corresponding cellular amounts of WalR~P. Strains were grown statically in BHI broth at 37°C in an atmosphere of 5% CO2 to OD620 ≈ 0.2. RNA samples for QRT-PCR analysis and protein samples for Phos-tag SDS-PAGE were prepared and analyzed as described in Experimental procedures. walK+ parent strain (IU1781); ΔwalK (IU1896); walKΔPAS (IU2306); walKH218A (IU3102); walKT222A (IU5401). (A) Amounts of spd_1874 transcript (a representative gene in the WalRK regulon) were normalized to that of gyrA. Transcript amounts are expressed relative to that of the walK+ parent (≡ 1.0) and represent averages from duplicate samples from at least 2 independent experiments. Unpaired two-tailed t tests of relative transcript amounts compared to that of the WalK+ parent were performed using GraphPad Prism 5 software. (B) Percentage of WalR~P compared to total WalR detected in extracts of walK+ parent and walK mutant strains determined by Phos-tag SDS-PAGE and quantitative Western blotting with anti-WalRSpn antibody as described in Supplemental Information. The corrected amount of WalR~P in the walK+ parent strain is indicated by the dotted line (see text). Averages are from 2 to 5 independent biological samples resolved on 50 and 75 μM Phos-tag acrylamide gels (see Fig. 5 and S12).
The WalK PAS domain is required for optimal phosphatase activity in biochemical reactions, but WalKΔPAS also has a kinetic defect in the autokinase activity (see above) (Gutu et al., 2010). The walKΔPAS mutant showed ≈30% less WalRK regulon expression compared to the walK+ parent (Fig. 4A). In contrast, the walKT222A phosphatase mutant showed a large (7-fold) increase in WalRK regulon expression (Fig. 4A), consistent with an increased cellular amount of WalR~P.
To understand these regulation patterns further, we determined the cellular amounts of WalR and WalR~P in these mutants (Fig. 4B and 5) by a modification of the Phos-tag SDS-PAGE method reported by Barbieri and Stock (Barbieri & Stock, 2008). The acid extraction procedure used in (Barbieri & Stock, 2008) gave poor protein yields in Gram-positive S. pneumoniae. We overcame this problem by optimizing and validating a rapid extraction procedure for quantitative determinations of pneumococcal WalR and WalR~P amounts by Phos-tag SDS-PAGE (Experimental procedures; Supplemental Information; Fig. 5, S11, and S12). In these experiments, the total amount of WalR detected by Western blotting remained constant in each strain tested (data not shown).
Fig. 5.
Representative Western blots of Phos-tag gels containing extracts of walK and pnpRS mutant strains grown exponentially in BHI broth. Growths, rapid preparation of protein extracts, 75 μM Phos-tag SDS-PAGE, and quantitative Western blotting with anti-WalRSpn antibody are described in Experimental procedures and Supplemental Information. The positions of unphosphorylated WalR, WalR~P, and a very faint contaminant band are indicated. (A) walK+ parent strain (IU1781); ΔwalK (IU1896); walKΔPAS (IU2306); walKH218A (IU3102); walKT222A (IU5401); and control heated sample of walKT222A (IU5401) showing heat lability of WalR~P. (B) walK+ ΔpnpR (IU3483); walK+ ΔpnpRS (IU4086); ΔwalK ΔpnpR (IU5720); ΔwalK ΔpnpRS (IU5728); and control heated sample of ΔwalK ΔpnpR (IU5720). Additional heated controls for extracts of other strains are shown in Fig. S12. The faint contaminant band was at the limit of detection and still present in extracts of a ΔwalR Pc-pcsB+ strain (EL1472), which lacks WalR and WalR~P (see Fig S11).
Three main results were obtained from these experiments. First, the percentage of WalR~P clearly increases in the walKΔPAS and walKT222A mutants compared to the other strains (Fig. 4B and 5A). Remarkably, the percentage of WalR~P is ≈90% in the walKT222A mutant. As a control, heating samples from the walKT222A mutant converted all WalR~P to WalR, consistent with the heat-lability of aspartyl-phosphate bonds (Fig. 5A, right). Second, there is a correlation between WalRK regulon expression and percentage of WalR~P in mutants (ΔwalK; walKΔPAS, walKH218A, walKT222A, ΔwalK ΔpnpR, and ΔwalK ΔpnpRS) defective in WalK phosphatase activity (Fig. 4, 5, and 6 (see next section)). This correlation can be visualized by fitting values on a non-linear exponential curve (not shown). Third, unlike the mutants, the correlation between regulon expression and percentage of WalR~P breaks down in the WalK+ parent strain, which contained a barely detectable amount of WalR~P (Fig. 4 and 5A). The most likely explanation for this lack of correlation is loss of WalR~P during cell lysis of strains with active WalK phosphatase activity (see Discussion). Together, these results support the conclusion that WalK phosphatase is strongly active in exponentially growing pneumococcal cells and that mutational inactivation of WalK phosphatase activity results in high levels of WalR~P, accompanied by a large increase in WalRK regulon expression.
Fig. 6.
Dephosphorylation of WalR~P by WalK and WalKT222A in combined assays containing physiologically relevant molar ratios of WalK sensor kinase to WalR response regulator (1:6.7) (Wayne et al., 2010). Combined assays were performed in buffers containing Mg2+ or Ca2+ ion as described in Experimental procedures, and amounts of WalR~P remaining after 30 min of incubation are shown. Similar trends were observed after 1 min and 60 min incubations (Fig. S13). Means with standard errors are indicated from 3 independent experiments.
The idea that WalK phosphatase can predominate over autokinase activity was further supported by in vitro experiments. In cells, most response regulators are present in large molar excess over their cognate sensor kinases (see (Goulian, 2010)). It was proposed that for certain TCSs, the importance of phosphatase activity was overestimated, because sensor kinases were present in molar excess over their cognate response regulators in coupled biochemical reactions (Kenney, 2010). Our experiments do not support this idea for the WalRKSpn TCS. In exponentially growing strain D39, ≈7-fold more WalR monomer is present than WalK monomer (i.e., 13-fold more WalR monomer than WalK dimer) (Wayne et al., 2010). At comparable molar ratios, WalR~P did not accumulate in coupled reactions containing WalK+ in Mg2+ buffer (Fig. 7 and S13). In contrast, WalR~P did accumulate in reactions containing WalK+ and Ca2+ buffer or in reactions containing phosphatase-deficient WalKT222A (Fig. 6). We conclude that WalK functions primarily as a phosphatase in biochemical reactions, consistent with the strong WalK phosphatase activity detected in unstressed growing cells (Fig. 4 and 5).
Fig. 7.
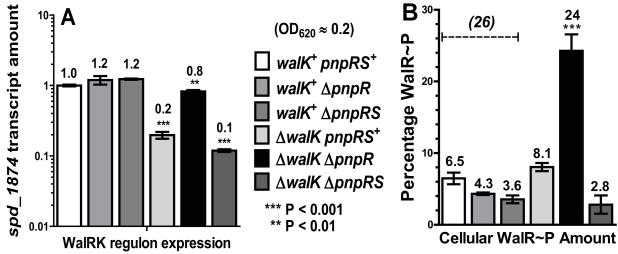
WalRK regulon expression in walK and pnpRS mutant strains during exponential growth and corresponding cellular amounts of WalR~P. Strains were grown statically in BHI broth at 37°C in an atmosphere of 5% CO2 to OD620 ≈ 0.2. RNA samples for QRT-PCR analysis and protein samples for Phos-tag SDS-PAGE were prepared and analyzed as described in Experimental procedures and Supplemental Information. walK+ pnpRS+ parent strain (IU1781); walK+ ΔpnpR (IU3483); walK+ ΔpnpRS (IU4086); ΔwalK (IU1896); ΔwalK ΔpnpR (IU5720); ΔwalK ΔpnpRS (IU5728). (A) Amounts of spd_1874 transcript were normalized to that of gyrA. Transcript amounts are expressed relative to that of the walK+ parent (≡ 1.0) and represent averages from duplicate samples from at least 2 independent experiments. Unpaired two-tailed t tests of relative transcript amounts compared to that of the walK+ parent were performed using GraphPad Prism 5 software. (B) Percentage of WalR~P compared to total WalR detected in extracts of walK+ parent and walK and pnpRS mutant strains determined by Phos-tag SDS-PAGE and quantitative Western blotting with anti-WalRSpn antibody as described in Supplemental Information. The corrected amount of WalR~P in the walK+ parent strain is indicated by the dotted line (see text). Averages are from 2 to 5 independent biological samples resolved on 50 and 75 μM Pho-tag acrylamide gels (see Fig. 5 and S12). The walK+ pnpRS+ and ΔwalK pnpRS+ data are from Fig. 4.
WalK phosphatase activity limits crosstalk in exponentially growing cells
The amount of WalR~P in the ΔwalK and walKH218A mutants was close to the limit of detection of this method (Fig. 4B and 5A). The residual WalR~P detected in ΔwalK and walKH218A mutants likely reflects crosstalk. One source of crosstalk is phosphorylation by non-cognate histidine kinases in the absence of WalK function. Alignment of other HisKA-family sensor kinases revealed that the DHp recognition helix of WalK (HK02) has a similar amino acid sequence to that of the PnpS (HK04), HK06, VncS (HK10), CiaH (HK05), and HK08 senor kinases (Fig. S14). We expressed and purified soluble truncated derivatives of these other 5 histidine kinases to test whether they could phosphorylate non-cognate WalR (Table S3; Fig. S14 and S15). In coupled reactions containing cognate WalK~P, WalR~P was detected rapidly within 1 min, despite WalK phosphatase activity (Fig. S7). In contrast, a low level of crosstalk phosphorylation of WalR to WalR~P was detected for non-cognate PnpS~P, HK08~P, and CiaH~P after long incubation times of >10 min (Fig. S15B, S15C, and S15D). No WalR~P was detected for non-cognate VncS~P and HK06~P (Fig. S15E and S15F), which phosphorylated their cognate response regulators, VncR and RR06 (Fig. S15G and S15H). We conclude that crosstalk between PnpS~P, HK08~P, and CiaH~P and non-cognate WalR requires long incubation times in biochemical reactions and is not kinetically favored compared to cognate WalK~P (see (Laub et al., 2007, Laub & Goulian, 2007)).
Of the pneumococcal sensor kinases, the DHp recognition domains of WalK and PnpS are the most closely matched (Fig. S14); therefore, PnpS was a likely candidate for crosstalk to WalR in cells. The pneumococcal PnpRS TCS likely regulates phosphate uptake in S. pneumoniae (Moreno-Letelier et al., 2011, Novak et al., 1999). Moreover, in B. subtilis, crosstalk was detected between the PhoRBsu histidine kinase, which is the homologue of PnpSSpn, and WalRBsu (Bisicchia et al., 2010, Botella et al., 2011, Howell et al., 2006). Indeed, we detected significant phosphorylation of WalR in a ΔwalK ΔpnpR mutant, which lacks WalK and the PnpR response regulator (Fig. 5B and 7B). WalR~P accumulation in the ΔwalK ΔpnpR mutant led to ≈4-fold greater WalRK regulon expression than in the ΔwalK mutant (Fig. 7A) and corrected the growth defect of the ΔwalK mutant (Fig. 3B).
The increase in percentage of cellular WalR~P was abrogated in a ΔwalK ΔpnpRS mutant, indicating that PnpS phosphorylated WalR in the absence of WalK in the ΔwalK ΔpnpR mutant (Fig. 5B and 7B). WalRK regulon expression was reduced in the ΔwalK ΔpnpRS mutant compared to the ΔwalK mutant (Fig. 7A), showing that the PnpS sensor kinase contributed to the crosstalk that allowed growth of the ΔwalK mutant under these conditions. Finally, for the three walK+ strains with corresponding alleles of pnpRS (walK+ pnpRS+; walK+ ΔpnpR; and walK+ ΔpnpRS), there was again a lack of correlation between WalRK regulon expression and the percentage of WalR~P (Fig. 7A and 7B). WalRK regulon expression was maintained around the wild-type level, whereas apparent WalR~P amounts were low compared to the ΔwalK ΔpnpR mutant (Fig. 7A and 7B). As discussed below, maintenance of wild-type levels of regulon expression in the walK+ strains is consistent with a role of WalK phosphatase in preventing crosstalk from PnpS and other non-cognate sensor kinases during exponential growth.
Discussion
WalK phosphatase is the default activity in biochemical reactions and exponentially growing pneumococcal cells
In this study, we further analyzed the contributions of the WalK autokinase and phosphatase activities in setting WalRK regulon expression and WalR~P amounts in non-stressed, exponentially growing cells of S. pneumoniae. We also examined the role of crosstalk as the basis for the non-essentiality of pneumococcal WalK. To carry out these studies, we identified the WalKT222A mutant sensor kinase that is deficient for WalK mediated dephosphorylation of WalR~P, but proficient in autokinase or phosphoryltransferase activities (Fig. 2, S4-S7). We also optimized the Phos-tag SDS-PAGE method to quantitate WalR phosphorylation levels in the walKT222A and other walK mutants (Fig. 5, S11, and S12). We identified WalKT222A by a biochemical screen based on amino acids predicted to be involved in the WalK-mediated phosphatase reaction (Results; Fig. S1). According to a recent model proposed by Huynh and Steward (Huynh et al., 2010, Huynh & Stewart, 2011), Thr222 of WalKSpn should coordinate a water molecule for nucleophilic hydrolysis of the aspartyl-phosphate group of WalR~P. Our finding that the T222A (and to a lesser extent the Thr222Y (Fig. S3G)) substitution results in a phosphatase-deficient, autokinase-proficient WalK supports this role for Thr222. However, other changes made at Thr222 (e.g., T222D and T222R) strongly decreased WalK autokinase activity (Fig. S3) (Gutu et al., 2010), possibly by perturbing the structure of the DHp domain.
Our combined data support the idea that the WalK phosphatase is the default activity in biochemical reactions and in unstressed pneumococcal cells growing exponentially. In coupled biochemical reactions containing physiological ratios of WalR to WalK, WalK phosphatase activity predominated over autokinase activity and prevented accumulation of WalR~P (Fig. 6). WalR~P only accumulated to appreciable amounts in reactions containing phosphatase-deficient WalKT222A instead of WalK+. Most importantly, the walKT222A mutation greatly increased WalRK regulon expression by ≈7-fold (Fig. 4A) and increased the percentage of WalR~P to ≈90% in exponentially growing cells (Fig. 4B). Constitutive overexpression of the WalRK regulon in the walKT222A mutant did not decrease growth rate or significantly alter yield (Fig. 3A and S8A) or strongly affect cell morphology or viability (Fig. S8B and S9). Most members of the WalRK regulon likely function as murein hydrolases, whose activities are allosterically regulated by cell division proteins (Sham et al., 2011, Sham et al., 2012, Uehara & Bernhardt, 2011, Yang et al., 2011). Therefore, overexpression alone of these proteins may not be deleterious to growing cells. For example, overexpressed PcsB would likely be secreted into the growth medium, because the amount of PcsB bound to the cell surface is set by the amount of its interaction partner, the FtsX integral membrane protein (Sham et al., 2011). Taken together, these results support the idea that WalK phosphatase activity plays a major role in setting the amount of WalR~P and the level of WalRK regulon expression in exponentially growing S. pneumoniae cells. If the WalK phosphatase activity is ablated, WalR becomes nearly completely phosphorylated to WalR~P, even in unstressed exponentially growing cells.
WalRK regulon expression correlates with cellular WalR~P amount in mutants deficient in WalK phosphatase activity, but not in walK+ cells
WalRK regulon expression was correlated with percentage of WalR~P for walK mutants deficient in phosphatase activity, but not for phosphatase-proficient walK+ strains (see Results). The simplest and most likely explanation for this non-correlation is that WalR~P was lost during cell lysis of walK+ strains due to WalK phosphatase activity. More complicated explanations, such as function of unphosphorylated WalR, require additional assumptions to account for other trends in the data and are difficult to reconcile with the requirement for the aspartate residue that becomes phosphorylated in WalR (Introduction). Initial tests limited loss of WalR~P from walK+ bacteria to the lysis step. Mixing equal densities of walK+ (7% WalR~P) and walKT222A (90% WalR~P) bacteria before extraction resulted in ≈50% WalR~P, indicating that loss of WalR~P was not occurring in the extracts after cell disruption (data not shown). Longer chilling of cultures before centrifugation and addition of commercially available phosphatase inhibitor cocktails (from Pierce or Roche) did not increase the proportion of WalR~P in walK+ strains (data not shown). Additional extraction protocols need to be tested to address this potential limitation of the Phos-tag SDS-PAGE method. Nevertheless, we can use the correlation in the phosphatase-deficient strains and the regulon expression levels in walK+ strains to calculate relative WalR~P amounts. Based on this correction, ≈26% WalR~P is present in the non-stressed, exponentially growing walK+ strains (dotted lines in Fig. 4 and 7).
The assumption that relative WalR~P amount is underestimated in phosphatase-proficient walK+ strains allows interpretation of other trends in these data. The walKΔPAS mutant showed modestly decreased (≈30%) WalRK regulon expression compared to the walK+ strain (Fig. 4A). This decreased regulon expression, which was insufficient to affect growth (Fig. 3A), implied that the autokinase kinetic defect of WalKΔPAS (see Results) may have decreased WalR~P amount in cells. Consistent with this interpretation, the ≈16% WalR~P recovered from the walKΔPAS mutant is less than the ≈26% WalR~P estimated for the walK+ strain (Fig. 4B). Moreover, the fact that we recovered increased WalR~P (≈2.5-fold) from the walKΔPAS mutant compared to the walK+ parent (Fig. 4B) supports the idea that WalKΔPAS is deficient in phosphatase activity in cells as well as in biochemical reactions (Gutu et al., 2010). Another important implication of this interpretation comes from the increased recovery of WalR~P from antibiotic treated walK+ bacteria (Fig. S11C) (K. Kazmierczak, in preparation). Recovery of a substantial amount of WalR~P from a walK+ strain implies that the WalK phosphatase activity is somehow blocked during cell wall stress, perhaps by interaction with a ligand or another protein.
WalK autokinase activity is required for normal pneumococcal growth and cell morphology
The absence of WalK autophosphorylation in the walKH218A or ΔwalK mutant caused moderate growth defects that did not reflect a severe drop in cell viability (Fig. 3, S8, and S9). These phenotypes contrast with the severe drop in cell viability reported recently for ΔwalKSmu mutants of S. mutans (Senadheera et al., 2012), which is distantly related to S. pneumoniae (Kawamura et al., 1995, Sultana et al., 1998). This difference in growth phenotypes may reflect the composition of the WalRK regulons in these two bacterial species. In S. mutans, inactivation of walKSmu led to changes in relative transcription of genes from many functional classes, including bacteriocin and competence genes (Senadheera et al., 2012). In contrast, we show here that inactivation of walKSpn decreased expression of a small number of genes that primarily mediates peptidoglycan hydrolysis (Table 1). Expression of this same limited set of genes was increased when pcsB was underexpressed, suggesting feedback control (Fig. 1B) (Barendt et al., 2009). Likewise, expression of this core set of murein hydrolase genes was increased or decreased when walRSpn expression was increased or decreased, respectively (Mohedano et al., 2005, Ng et al., 2003, Ng et al., 2005). Lack of WalKSpn autokinase activity resulted in ≈3-fold less pcsB gene transcription (Table 1; Fig. S10). Previous work shows that this degree of pcsB underexpression is sufficient to account for the slight decrease in growth rate, markedly lower growth yield, and moderate defects in cell morphology of the ΔwalK and walKH218A mutants (Barendt et al., 2009).
These combined observations suggest an attractive model that will be tested in future experiments (Fig. 1B and S16). In exponentially growing S. pneumoniae, the PAS domain regulates WalK phosphatase activity to set the basal WalR~P amount and WalRK regulon expression level. Cell wall damage or limitation of PcsB amount induces a ligand or another protein to bind to the PAS domain and turn off WalK phosphatase activity, thereby increasing relative WalR~P amount and WalRK regulon expression. It has not been determined whether the PAS domain of WalKBsu might play a similar role in B. subtilis, but the PAS domain of WalKBsu does have an additional function compared to its counterpart in WalKSpn. The PAS domain of WalKBsu interacts with the divisome and directs the localization of WalKBsu to the septum of dividing B. subtilis cells (Fukushima et al., 2011). In contrast, WalKSpn is randomly distributed in the membrane of dividing S. pneumoniae cells (Wayne et al., 2010).
Crosstalk accounts for the non-essentiality of walK mutations in S. pneumoniae
ΔwalK and walKH218A mutants, which are deficient in both autokinase and phosphatase activities (see Results), contained ≈8% WalR~P (Fig. 4B, Fig. 5, Fig. 7B) and were moderately impaired in growth (Fig. 3 and S8). The ΔwalK ΔpnpR mutant strikingly exhibited crosstalk phosphorylation of WalR by PnpS; the percentage of WalR~P increased from 8% to 24% (Fig. 5B and 7B), WalRK regulon expression increased by ≈4-fold (Fig. 7A), and wild-type growth was restored (Fig. 3B) compared to the ΔwalK mutant. These increases disappeared in a ΔwalK ΔpnpRS mutant demonstrating that crosstalk in the ΔwalK ΔpnpR mutant depended on PnpS function (Fig. 7). Furthermore, the 2-fold drop in WalRK regulon expression, decrease in relative WalR~P amount, and impaired growth of the ΔwalK ΔpnpRS mutant compared to the ΔwalK mutant (Fig. 3B and 7B) shows that crosstalk to WalR by PnpS contributes to the viability of walK mutants. In comparison, a comparable analysis indicated that acetyl phosphate, which is plentiful (≈3 mM) in S. pneumoniae, contributed negligibly (≈20%) to maintaining WalRK regulon expression in a ΔwalK mutant (Ramos-Montanez et al., 2010). Presumably, the barely detectable, residual (<3%) amount of WalR~P left in the ΔwalK ΔpnpRS mutant is provided by CiaH and HK08, which phosphorylated WalR inefficiently in vitro (Fig. S15C and S15D). It remains to be determined whether a ΔwalK ΔpnpRS ΔciaH Δhk08 mutant is viable in the absence of constitutive PcsB expression (see (Ng et al., 2003, Ng et al., 2005)). Since the WalR response regulator is abundant (≈6,000 monomers per cell), it is also possible that unphosphorylated WalR may activate sufficient expression of the WalRK regulon (see (Bourret, 2010, Gao et al., 2007, Gao & Stock, 2010)) to allow some level of growth in the absence of WalR phosphorylation by crosstalk.
In contrast, no level of crosstalk seems to occur in walK+ S. pneumoniae growing exponentially. Expression of the WalRK regulon was the same in the walK+ pnpRS+ (parent), walK+ ΔpnpR, and walK+ ΔpnpRS strains (Fig. 7A). As discussed above, the low recovery of WalR~P from these walK+ strains likely reflects WalK phosphatase activity during cell lysis. Following the correction described above, the percentage of WalR~P is ≈26% in each of these strains (dashed line, Fig. 7B). Thus, WalK+ function sets the level of WalRK regulon expression and presumably WalR~P amount. Notably, no crosstalk was detected in the walK+ ΔpnpR mutant, despite the capacity of PnpS to phosphorylate WalR in the ΔwalK ΔpnpR mutant (Fig. 7B). In B. subtilis, the PhoRBsu sensor kinase, which responds to phosphate limitation, phosphorylates WalRBsu in walK+ strains during phosphate limitation to provide a form of physiological cross regulation that links cell wall maintenance to the phosphate stress response (Botella et al., 2011, Howell et al., 2003, Howell et al., 2006). It remains to be determined whether certain combinations of stress conditions, such as cell wall damage and phosphate limitation, elicit similar cross regulation in S. pneumoniae. Paradoxically, both PhoRBsu of B. subtilis and PnpSSpn of S. pneumoniae seem to have the capacity to phosphorylate their WalR homologues in ΔwalK mutants. Yet, this crosstalk only occurs in S. pneumoniae walK mutants (Fig. 7A), and walK mutations are lethal to B. subtilis (Fabret & Hoch, 1998). Perhaps the additional function of WalKBsu in cell division described above (Fukushima et al., 2011), which is lacking in S. pneumoniae (Wayne et al., 2010), accounts for this difference in the essentiality of WalKBsu and WalKSpn.
Experimental procedures
Pneumococcus strains and growth conditions
Bacterial strains used in this study are listed in Supplemental Table S1. S. pneumoniae cultures were grown statically in Brain-Heart Infusion broth (BHI; Becton-Dickinson BD) or on plates containing Trypticase Soy Agar II modified (BD) and 5% (v/v) defibrinated sheep blood (Remel) (TSAII BA) at 37°C in an atmosphere of 5% CO2. Growth was monitored directly by determining OD620 using a Spectronic 20 Genesys spectrophotometer. For overnight cultures, strains were inoculated from frozen stocks into 5 ml of BHI broth in 17-mm-diameter polystyrene plastic tubes, serially diluted over five tubes, and grown for 11 to 13 h. Cultures at OD620 ≈0.1 to 0.3 were adjusted to OD620 ≈0.1 with fresh BHI and diluted 1/100 into BHI broth to start final cultures, which lacked antibiotics. For strain constructions, antibiotics were present at concentrations indicated in Table S1.
Viability determinations by live-dead staining
Overnight and final cultures were grown as described above. 500 μL of culture were removed at the indicated stages of growth. Cells were collected by centrifugation at 16,100 × g for 2 min at 25°C, suspended in 50 μl of fresh BHI broth with gentle pipetting, and stained without fixation by adding 2 μL of a 1:1 (v/v) mixture of Syto-9 and propidium iodide (LIVE/DEAD BacLight Bacterial Viability Kit, Molecular Probes) for 5 min in the dark at 25°C. Stained bacteria were examined using a Nikon E-400 epifluorescence phase-contrast microscope with FITC and Texas Red bandpass filters, and phase-contrast and fluorescent images were captured using a cooled digital SPOT camera as described before (Barendt et al., 2009). At least 100 and as many as 1,300 bacteria from 2 to 7 microscopic fields were examined for each time point. The experiment was performed 3 times with similar results.
Strain constructions
Oligonucleotide primers used in strain constructions are listed in Table S2. walK mutants in S. pneumoniae were constructed by the Janus method of allele replacement used previously (Table S1) (Gutu et al., 2010, Ramos-Montanez et al., 2008, Sung et al., 2001, Wayne et al., 2010). walK mutants were described previously (Gutu et al., 2010), except for walKT222A, which was constructed by crossing a walKT222A linear amplicon into strain IU1885 (Table S1). Mutations in the pnpRS locus were constructed by replacing the pnpR or pnpRS reading frames with an ErmR antibiotic cassette in strains IU1896 and IU1781 (Table S1).
RNA extraction
Overnight cultures were grown as described above and inoculated into 30 ml BHI broth cultures contained in 50 ml conical centrifuge tubes to a starting OD620 ≈0.001. At OD620 ≈0.2, RNA was extracted from 4 ml of exponentially growing cultures by a hot lysis-acid phenol extraction followed by purification using an RNAeasy minikit (Qiagen) and on-column DNaseI treatment as described previously (Barendt et al., 2009, Kazmierczak et al., 2009). For QRT-PCR assays, 5 μg of total RNA was further digested with DNase using a DNA-free kit (Ambion).
Microarray analysis
Microarray analyses were performed as described in (Barendt et al., 2009) to compare relative transcript amounts of strains IU1896 (ΔwalK) and IU1781 (walK+). Total RNA was prepared as described above from 10 ml cultures. Microarrays were purchased from Ocimum Biosolutions. Synthesis, labeling, hybridization, scanning, normalization, and analysis using the Cyber-T web interface were performed as described previously (Barendt et al., 2009). Fold changes and Bayesian P-values were based on 3 independent biological replicates, including a dye swap. Intensity and expression ratio data for all transcripts are deposited in the GEO database, accession number GSE19752.
Construction of recombinant protein-expression plasmids
Plasmids used in this study are listed in Table S1. Genomic DNA used to construct plasmids was isolated from S. pneumoniae laboratory strain R6, which has the same DNA sequences of the cloned genes as strain D39 (Lanie et al., 2007). PCR amplicons generated with the primers in Table S2 were cloned into the BamHI and BsaI sites of plasmid pSumo (LifeSensors, Inc.) to generate protein expression plasmids. Plasmids were transformed into E. coli strain DH5α (Bioline) for storage and into strain Rosetta 2(DE3) (Novagen) or Tuner (EMD) for protein expression and purification (Tables S1 and S3). Deletion and point mutations in cloned histidine kinases were generated by fusion PCR as described previously (Gutu et al., 2010) using PCR primers listed in Table S2.
Protein expression and purification
Recombinant (N)-His-WalR, other response regulators, and truncated histidine kinases (Table S3) were purified as described previously (Gutu et al., 2010), with the following modifications. E. coli strains were grown with shaking at 37°C to OD620 ≈0.4 to 0.6 in Difco LB broth (BD) supplemented with antibiotics required for plasmid maintenance (see Table S1). Protein expression was induced by addition of 0.2 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG; Calbiochem), followed by incubation at 16°C overnight with shaking (220 rpm). Cells were collected by centrifugation at 8,000 x g at 4°C for 30 min and stored at −80°C.
Cell pellets were suspended in cold buffer A (20 mM NaPO4, 0.5 M NaCl, 40 mM imidazole, pH 7.4) supplemented with 50 μl per g of cell pellet of Protease Inhibitor set III (Calbiochem). Cells were lysed by two passes through a chilled French pressure cell (20,000 lb in−2) and insoluble material was removed by two centrifugations at 12,000 x g for 20 min at 4°C. Lysates were filtered through a 0.2-μm pore-size membrane (Pall) before loading onto a HiTrap Chelating HP column (GE Healthcare) charged with 0.1 M NiSO4 (Sigma) according to the manufacturer’s instructions and equilibrated with buffer A. Filtrates were loaded at a flow rate of 1 ml per min at 4°C. Loaded columns were washed with cold buffer A for 15 min and proteins were eluted by imidazole gradients as described previously (Gutu et al., 2010). Fractions containing purified recombinant protein were pooled, concentrated, and exchanged into the optimized storage buffers listed in Table S3. Purified protein samples were centrifuged at 100,000 x g for 15 min at 4°C to remove any remaining insoluble material and distributed to several tubes for storage at −80 °C. The concentration of purified proteins was determined by using the DC protein assay kit (Bio-Rad) with bovine serum albumin (Sigma Fraction V) as the standard. The purity of the protein samples was >95% based on Coomassie-stained gels (data not shown).
Autophosphorylation of WalK mutant proteins
(N)-Sumo-ΔN35-WalK proteins (2.0 μM) containing amino acid changes in the DHp recognition helix (Fig. S1) were equilibrated in Mg2+ reaction buffer (50 mM Tris-HCl (pH 7.6), 200 mM KCl, 5.0 mM MgCl2) for 10 min at 25°C. Autophosphorylation reactions were started by the addition of 100 μM of [γ-33P]ATP (0.25 Ci/ mmol, Perkin-Elmer). Samples were removed at 10, 30, and 60 min, analyzed by SDS-PAGE, and quantified by phosphorimaging as described in (Gutu et al., 2010).
Combined autophosphorylation-phosphoryltransfer-phosphatase assay
Combined assays were carried out as described previously (Gutu et al., 2010) with the following modifications. Purified truncated (N)-Sumo-ΔN35-WalK (final concn = 2.0 μM) or mutant derivatives of WalK containing amino acid substitutions (Fig. S1) were equilibrated in 100 μl of Mg2+ or Ca2+ reaction buffer (50 mM Tris-HCl (pH 7.6), 200 mM KCl, 5 mM MgCl2 or 5 mM CaCl2) for 10 min at 25 °C. Reducing agent was omitted from reaction buffers as described previously (Gutu et al., 2010). Autophosphorylation reactions were started by the addition of [γ-33P] ATP (0.25 Ci/ mmol, Perkin-Elmer) to 100 μM. A 15 μl sample was removed after 30 min and mixed with an equal volume of 2X Laemmli sample buffer (Bio-Rad) containing 5% (vol/vol) β-mercaptoethanol (Sigma) to stop the reaction. At this time point (t=0), purified (N)-His-WalR was added (final concn = 6.6 μM) to the remaining reaction mixture to start the phosphoryltransfer reaction. Samples (15 μl) were removed 1, 10, and 30 min after WalR addition, stopped as above, and analyzed by 10% Tris-glycine SDS-PAGE as described in (Gutu et al., 2010). Dried gels were exposed to a storage phosphor screen (GE Healthcare) for at least 16 h and analyzed using ImageQuant and GraphPad Prism 5 software as described previously (Gutu et al., 2010).
Combined assays at different WalK:WalR ratios
Purified (N)-Sumo-ΔN35-WalK and (N)-His-WalR proteins were equilibrated in Mg2+ or Ca2+ reaction buffer for 10 min at 25°C. Reactions containing the following final protein concentrations: 6.0 μM WalK and 6.0 μM WalR (1:1); 1.0 μM WalK and 6.0 μM WalR (1:6); and 0.6 μM WalK and 6.0 μM WalR (1:10), were started by the addition of [γ-33P] ATP (0.25 Ci/ mmol, Perkin-Elmer) to 100 μM. Samples were removed after 1, 30, and 60 min, analyzed by SDS-PAGE, and WalR~P amounts were quantitated by phosphorimaging as described above.
Determination of WalKT222A autophosphorylation kinetic parameters
Autophosphorylation kinetic parameters were determined as described previously (Gutu et al., 2010) with the following modifications. Purified (N)-Sumo-ΔN35-WalK+ or (N)-Sumo-ΔN35-WalKT222A protein (final concn = 1.0 μM) was equilibrated with Mg2+ reaction buffer (50 mM Tris-HCl (pH 7.6), 200 mM KCl, 5.0 mM MgCl2) for 10 min at 25°C. Autophosphorylation reactions were started by addition of [γ-33P] ATP (0.25 Ci/ mmol, Perkin-Elmer) to 6.0 μM, 12.5 μM, 50 μM, 100 μM, or 250 μM. Samples were removed after 15, 30, 45, and 60 sec, analyzed by SDS-PAGE, and quantified by phosphorimaging as described above. Quantitation of WalK~P in each lane and calculation of reaction rates and Michaelis-Menton kinetic parameters was performed as described in (Gutu et al., 2010).
Estimation of phosphoryltransfer efficiency between WalK~P constructs and WalR
The basis for the assay was described in (Gutu et al., 2010), and the assays were carried out with the following modifications. (N)-Sumo-ΔN35-WalK+ or (N)-Sumo-ΔN35-WalKT222A protein (final concn = 3.0 μM) was equilibrated in Mg2+ or Ca2+ reaction buffer (50 mM Tris-HCl (pH 7.6), 200 mM KCl, 5.0 mM MgCl2 or CaCl2, 15% (vol/vol) glycerol) at 25°C for 5 min. Autophosphorylation reactions were started by the addition of [γ-33P]ATP (0.25 Ci/ mmol, Perkin-Elmer) to 500 μM. After 20 min, excess ATP was removed from the reaction by using spin desalting columns (Pierce), and a sample of desalted protein was removed to determine the amount of WalK~P by SDS-PAGE. (N)-His-WalR (final concn = 0.5 μM or 1.0 μM) was added to the remaining desalted sample to initiate phosphoryltransfer. Samples were removed after 30, 60, 120, and 240 sec, analyzed by SDS-PAGE, and quantified by phosphorimaging. Phosphoryltransfer efficiency between WalK~P constructs and WalR was evaluated as described previously (Gutu et al., 2010).
Quantification of WalKT222A-catalyzed dephosphorylation of WalR~P
Dephosphorylation activity of the (N)-Sumo-ΔN35-WalKT222A protein was determined as reported previously (Gutu et al., 2010) with the following modifications. (N)-His-WalR (final concn = 6.0 μM) was phosphorylated with 40 mM acetyl phosphate (Sigma) in reaction buffer (50 mM Tris-HCl (pH 7.4), 200 mM KCl, 4.0 mM MgCl2) at 37°C for 70 min. Excess acetyl phosphate was removed by using spin desalting columns (Thermo Scientific). Desalted WalR~P was incubated with 13.2 μM ADP (Sigma) in the presence or absence of 2.0 μM (N)-Sumo-ΔN35-WalKT222A protein at 25°C. Samples were removed at time points ranging from 30 min to 240 min, mixed with eluent A (20% (vol/vol) acetonitrile and 0.1% (vol/vol) trifluoroacetic acid (EM Science) in water), and analyzed by reversed-phase HPLC using a Phenomenex Jupiter 300A C4 column and a Shimadzu 10A HPLC system as described in (Gutu et al., 2010). The relative amount of WalR~P remaining at each time point was normalized to the starting amount of WalR~P, and half-lives were calculated as described previously (Gutu et al., 2010).
Autophosphorylation of other pneumococcal HisKA-family histidine kinases followed by phosphoryltransfer to WalR or cognate response regulators
Combined assays of autophosphorylation of truncated (N)-Sumo-ΔN206-PnpS, (N)-Sumo-ΔN58-HK08, (N)-Sumo-ΔN210-CiaH, (N)-Sumo-ΔN165-HK06, or (N)-Sumo-ΔN164-VncS and phosphoryltransfer to (N)-His-WalR were performed as described above for (N)-Sumo-ΔN35-WalK. Similar combined assays were performed with cognate pair (N)-Sumo-ΔN165-HK06 (final concn = 2.0 μM) and (N)-His-RR06 (final concn = 4.6 μM). Reactions containing (N)-Sumo-ΔN164-VncS (1.0 μM) were started by addition of [γ-32P] ATP (0.5 Ci/mmol; Perkin-Elmer) to 250 μM. After 3 min (t=0), (N)-His-WalR or (N)-Sumo-VncR (final concn = 6.7 μM) was added, and samples were removed at the times indicated in Fig. S15.
QRT-PCR analysis
QRT-PCR analysis was performed as described in (Kazmierczak et al., 2009, Ramos-Montanez et al., 2008) with the following changes. First-strand cDNA was synthesized using a qScript Flex cDNA synthesis kit (Quanta Biosciences) according to the manufacturer’s protocol. cDNA was diluted 6-fold and serially diluted in 5-fold steps 1 to 3 times more. QRT-PCR reactions contained 10 μL of 2× Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent), 2.0 μL each of 2 μM QPCR primers (Table S2), 0.3 μL of a 1:500 dilution of ROX reference dye, and 6.0 μL of diluted cDNA. Data were collected using an MX3000P thermocycler (Stratagene) running the SYBR Green with dissociation curve program modified as per the manufacturer’s recommendations and converted to the Comparative Quantitation with calibrator experiment type for analysis (MxPro v. 3.0). Reactions were performed in duplicate using cDNA from at least two independent biological samples, and transcript amounts were normalized to gyrA RNA amount (Kazmierczak et al., 2009). Normalized transcript amounts were compared to that of the wild-type parent strain by performing pairwise unpaired two-tailed t tests (GraphPad Prism 5).
Phos-tag SDS-PAGE and quantitative Western blotting
The Phos-tag SDS-PAGE method was based on (Barbieri & Stock, 2008) with the additions and modifications described in Supplemental Information.
Validation of rapid extraction and Phos-tag SDS-PAGE
Several validation experiments were performed. Purified WalR and WalR~P phosphorylated by acetyl phosphate in biochemical reactions (see above) (Gutu et al., 2010) were resolved by 25 μM Phos-tag SDS-PAGE, although the resolution was not optimal (Fig. S11A and S11B). WalR~P and WalR were resolved and detected on Western blots of extracts prepared from exponentially growing cells (Fig. S11C). The percentage of WalR~P significantly increased in response to antibiotic stress conditions that stimulated WalRK regulon expression in the walR+ pcsB+ parent strain (Fig. S11C) (K. Kazmierczak, in preparation), and the WalR and WalR~P bands were not detected for a ΔwalR Pc-pcsB+ mutant, where the essential pcsB gene is expressed from a constitutive promoter (Fig. S11C) (Ng et al., 2003). Our anti-WalRSpn antibody cross reacted with a faint contaminant band in Western blots that sometimes interfered with quantitation of WalR~P amounts. Consequently, the Phos-tag acrylamide was increased to 50 μM and finally 75 μM, which moved WalR~P away from the contaminant band (Fig. 5 and S11C). In addition, we obtained the same results shown in Fig. 5 for strains expressing WalR-L-FLAG3, which is detected with anti-FLAG antibody (see (Wayne et al., 2010)). Additional controls demonstrated that the WalR~P band was completely converted to WalR upon heating, as expected for the aspartyl-phosphate residue in WalR~P (Fig.5 and S12). Results from three control experiments argue against significant loss of WalR~P in extracts after cell disruption. We spiked purified WalR~P, which was phosphorylated in biochemical reactions, into suspensions of walK+ parent cells right before they were disrupted. Since the amount of WalR~P recovered was extremely low in these cells (Fig. 4B and 5A), we were able to follow loss of the added WalR~P. We found minimal loss of WalR~P by Phos-tag SDS-PAGE (data not shown). Second, the amount of WalR~P is ≈90% in the walKT222A strain (Fig. 4B and 5A), indicating minimal loss of cellular WalR~P in this modified procedure. Third, as mentioned in the text, mixing cultures of the walK+ (WalR~P ≈7%) with walKT222A (WalR~P ≈90%) mutants before the first centrifugation yielded ≈50% WalR~P on Phos-tag gels, consistent with minimal phosphatase activity in the extracts after cell disruption.
Supplementary Material
Acknowledgments
We thank Chris Sham and anonymous reviewers for detailed, critical comments about this work. We also thank Alina Gutu, Carl Bauer, Dan Kearns, David Kehoe, and Clay Fuqua for information and helpful discussions. K.J.W. was a predoctoral trainee on NIH Grant F31FM082090. This work was supported by NIAID grants AI060744 and AI095814 to M.E.W. and by funds from the Indiana University Bloomington METACyt Initiative, funded in part by a major grant from the Lilly Endowment, to M.E.W. The contents of this paper are solely the responsibility of the authors and do not necessarily represent official views of the funding agencies.
Footnotes
Dedicated to the memory of Professor Howard Gest (Indiana University Bloomington)
References
- Barbieri CM, Mack TR, Robinson VL, Miller MT, Stock AM. Regulation of response regulator autophosphorylation through interdomain contacts. J Biol Chem. 2010;285:32325–32335. doi: 10.1074/jbc.M110.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri CM, Stock AM. Universally applicable methods for monitoring response regulator aspartate phosphorylation both in vitro and in vivo using Phos-tag-based reagents. Analyt Biochem. 2008;376:73–82. doi: 10.1016/j.ab.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendt SM, Land AD, Sham LT, Ng WL, Tsui HC, Arnold RJ, Winkler ME. Influences of capsule on the cell shape and chaining of wild-type and pcsB mutants of serotype 2 Streptococcus pneumoniae. J Bacteriol. 2009;191:3024–3040. doi: 10.1128/JB.01505-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendt SM, Sham LT, Winkler ME. Characterization of mutants deficient in the L,D-carboxypeptidase (DacB) and WalRK (VicRK) regulon, involved in peptidoglycan maturation of Streptococcus pneumoniae serotype 2 strain D39. J Bacteriol. 2011;193:2290–2300. doi: 10.1128/JB.01555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent CJ, Isaacs NW, Mitchell TJ, Riboldi-Tunnicliffe A. Crystal structure of the response regulator 02 receiver domain, the essential YycF two-component system of Streptococcus pneumoniae in both complexed and native states. J Bacteriol. 2004;186:2872–2879. doi: 10.1128/JB.186.9.2872-2879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller SJ, Wayne KJ, Winkler ME, Burkholder WF. The putative hydrolase YycJ (WalJ) affects the coordination of cell division with DNA replication in Bacillus subtilis and may play a conserved role in cell wall metabolism. J Bacteriol. 2011;193:896–908. doi: 10.1128/JB.00594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisicchia P, Lioliou E, Noone D, Salzberg LI, Botella E, Hubner S, Devine KM. Peptidoglycan metabolism is controlled by the WalRK (YycFG) and PhoPR two-component systems in phosphate-limited Bacillus subtilis cells. Mol Microbiol. 2010;75:972–989. doi: 10.1111/j.1365-2958.2009.07036.x. [DOI] [PubMed] [Google Scholar]
- Bisicchia P, Noone D, Lioliou E, Howell A, Quigley S, Jensen T, Jarmer H, Devine KM. The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis. Mol Microbiol. 2007;65:180–200. doi: 10.1111/j.1365-2958.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- Botella E, Hubner S, Hokamp K, Hansen A, Bisicchia P, Noone D, Powell L, Salzberg LI, Devine KM. Cell envelope gene expression in phosphate-limited Bacillus subtilis cells. Microbiology. 2011;157:2470–2484. doi: 10.1099/mic.0.049205-0. [DOI] [PubMed] [Google Scholar]
- Bourret RB. Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol. 2010;13:142–149. doi: 10.1016/j.mib.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra EJ, Perchuk BS, Lubin EA, Ashenberg O, Skerker JM, Laub MT. Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet. 2010;6:e1001220. doi: 10.1371/journal.pgen.1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune A, Poupel O, Mallet A, Coic YM, Msadek T, Dubrac S. Peptidoglycan crosslinking relaxation plays an important role in Staphylococcus aureus WalKR-dependent cell viability. PloS One. 2011;6:e17054. doi: 10.1371/journal.pone.0017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Okajima T, Gotoh Y, Tanizawa K, Utsumi R. X-ray crystal structure of the DNA-binding domain of response regulator WalR essential to the cell viability of Staphylococcus aureus and interaction with target DNA. Biosci Biotech Biochem. 2010;74:1901–1907. doi: 10.1271/bbb.100307. [DOI] [PubMed] [Google Scholar]
- Dominguez-Cuevas P, Mercier R, Leaver M, Kawai Y, Errington J. The rod to L-form transition of Bacillus subtilis is limited by a requirement for the protoplast to escape from the cell wall sacculus. Mol Microbiol. 2012;83:52–66. doi: 10.1111/j.1365-2958.2011.07920.x. [DOI] [PubMed] [Google Scholar]
- Dubrac S, Bisicchia P, Devine KM, Msadek T. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol. 2008;70:1307–1322. doi: 10.1111/j.1365-2958.2008.06483.x. [DOI] [PubMed] [Google Scholar]
- Dubrac S, Boneca IG, Poupel O, Msadek T. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol. 2007;189:8257–8269. doi: 10.1128/JB.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrac S, Msadek T. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J Bacteriol. 2004;186:1175–1181. doi: 10.1128/JB.186.4.1175-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque C, Stipp RN, Wang B, Smith DJ, Hofling JF, Kuramitsu HK, Duncan MJ, Mattos-Graner RO. Downregulation of GbpB, a component of the VicRK regulon, affects biofilm formation and cell surface characteristics of Streptococcus mutans. Infect Immun. 2011;79:786–796. doi: 10.1128/IAI.00725-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Yoshida T, Inouye M. The critical role of the conserved Thr247 residue in the functioning of the osmosensor EnvZ , a histidine kinase/phosphatase, in Escherichia coli. J Biol Chem. 2000;275:38645–38653. doi: 10.1074/jbc.M005872200. [DOI] [PubMed] [Google Scholar]
- Fabret C, Hoch JA. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J Bacteriol. 1998;180:6375–6383. doi: 10.1128/jb.180.23.6375-6383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Furihata I, Emmins R, Daniel RA, Hoch JA, Szurmant H. A role for the essential YycG sensor histidine kinase in sensing cell division. Mol Microbiol. 2011;79:503–522. doi: 10.1111/j.1365-2958.2010.07464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Szurmant H, Kim EJ, Perego M, Hoch JA. A sensor histidine kinase co-ordinates cell wall architecture with cell division in Bacillus subtilis. Mol Microbiol. 2008;69:621–632. doi: 10.1111/j.1365-2958.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Mack TR, Stock AM. Bacterial response regulators: versatile regulatory strategies from common domains. Trends Biochem Sci. 2007;32:225–234. doi: 10.1016/j.tibs.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Stock AM. Molecular strategies for phosphorylation-mediated regulation of response regulator activity. Curr Opin Microbiol. 2010;13:160–167. doi: 10.1016/j.mib.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M. Two-component signaling circuit structure and properties. Curr Opin Microbiol. 2010;13:184–189. doi: 10.1016/j.mib.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutu AD, Wayne KJ, Sham LT, Winkler ME. Kinetic characterization of the WalRKSpn (VicRK) two-component system of Streptococcus pneumoniae: dependence of WalKSpn (VicK) phosphatase activity on its PAS domain. J Bacteriol. 2010;192:2346–2358. doi: 10.1128/JB.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock LE, Perego M. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J Bacteriol. 2004;186:7951–7958. doi: 10.1128/JB.186.23.7951-7958.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JT, Crosson S. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu Rev Microbiol. 2011;65:261–286. doi: 10.1146/annurev-micro-121809-151631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden BP, McEvoy CR, Allen DL, Chua K, Gao W, Harrison PF, Bell J, Coombs G, Bennett-Wood V, Porter JL, Robins-Browne R, Davies JK, Seemann T, Stinear TP. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Path. 2011;7:e1002359. doi: 10.1371/journal.ppat.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A, Dubrac S, Andersen KK, Noone D, Fert J, Msadek T, Devine K. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol Microbiol. 2003;49:1639–1655. doi: 10.1046/j.1365-2958.2003.03661.x. [DOI] [PubMed] [Google Scholar]
- Howell A, Dubrac S, Noone D, Varughese KI, Devine K. Interactions between the YycFG and PhoPR two-component systems in Bacillus subtilis: the PhoR kinase phosphorylates the non-cognate YycF response regulator upon phosphate limitation. Mol Microbiol. 2006;59:1199–1215. doi: 10.1111/j.1365-2958.2005.05017.x. [DOI] [PubMed] [Google Scholar]
- Hsing W, Silhavy TJ. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TN, Noriega CE, Stewart V. Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc Nat Acad Sci USA. 2010;107:21140–21145. doi: 10.1073/pnas.1013081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh TN, Stewart V. Negative control in two-component signal transduction by transmitter phosphatase activity. Mol Microbiol. 2011;82:275–286. doi: 10.1111/j.1365-2958.2011.07829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:107–146. doi: 10.1111/j.1574-6976.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Kadioglu A, Echenique J, Manco S, Trombe MC, Andrew PW. The MicAB two-component signaling system is involved in virulence of Streptococcus pneumoniae. Infect Immun. 2003;71:6676–6679. doi: 10.1128/IAI.71.11.6676-6679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Hou XG, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Internat J System Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- Kazmierczak KM, Wayne KJ, Rechtsteiner A, Winkler ME. Roles of rel in stringent response, global regulation and virulence of serotype 2 Streptococcus pneumoniae D39. Mol Microbiol. 2009;72:590–611. doi: 10.1111/j.1365-2958.2009.06669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney LJ. How important is the phosphatase activity of sensor kinases? Curr Opin Microbiol. 2010;13:168–176. doi: 10.1016/j.mib.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanie JA, Ng WL, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol. 2007;189:38–51. doi: 10.1128/JB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laub MT, Biondi EG, Skerker JM. Phosphotransfer profiling: systematic mapping of two-component signal transduction pathways and phosphorelays. Meth Enzymol. 2007;423:531–548. doi: 10.1016/S0076-6879(07)23026-5. [DOI] [PubMed] [Google Scholar]
- Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- Liu M, Hanks TS, Zhang J, McClure MJ, Siemsen DW, Elser JL, Quinn MT, Lei B. Defects in ex vivo and in vivo growth and sensitivity to osmotic stress of group A Streptococcus caused by interruption of response regulator gene vicR. Microbiology. 2006;152:967–978. doi: 10.1099/mic.0.28706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Burne RA. The major autolysin of Streptococcus gordonii is subject to complex regulation and modulates stress tolerance, biofilm formation, and extracellular-DNA release. J Bacteriol. 2011;193:2826–2837. doi: 10.1128/JB.00056-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PK, Li T, Sun D, Biek DP, Schmid MB. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J Bacteriol. 1999;181:3666–3673. doi: 10.1128/jb.181.12.3666-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohedano ML, Overweg K, de la Fuente A, Reuter M, Altabe S, Mulholland F, de Mendoza D, Lopez P, Wells JM. Evidence that the essential response regulator YycF in Streptococcus pneumoniae modulates expression of fatty acid biosynthesis genes and alters membrane composition. J Bacteriol. 2005;187:2357–2367. doi: 10.1128/JB.187.7.2357-2367.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Letelier A, Olmedo G, Eguiarte LE, Martinez-Castilla L, Souza V. Parallel evolution and horizontal gene transfer of the pst operon in Firmicutes from oligotrophic environments. Internat J Evol Biol. 2011;2011:781642. doi: 10.4061/2011/781642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL, Kazmierczak KM, Winkler ME. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Mol Microbiol. 2004;53:1161–1175. doi: 10.1111/j.1365-2958.2004.04196.x. [DOI] [PubMed] [Google Scholar]
- Ng WL, Robertson GT, Kazmierczak KM, Zhao J, Gilmour R, Winkler ME. Constitutive expression of PcsB suppresses the requirement for the essential VicR (YycF) response regulator in Streptococcus pneumoniae R6. Mol Microbiol. 2003;50:1647–1663. doi: 10.1046/j.1365-2958.2003.03806.x. [DOI] [PubMed] [Google Scholar]
- Ng WL, Tsui HC, Winkler ME. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J Bacteriol. 2005;187:7444–7459. doi: 10.1128/JB.187.21.7444-7459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL, Winkler ME. Singular structures and operon organizations of essential two-component systems in species of Streptococcus. Microbiology. 2004;150:3096–3098. doi: 10.1099/mic.0.27550-0. [DOI] [PubMed] [Google Scholar]
- Novak R, Cauwels A, Charpentier E, Tuomanen E. Identification of a Streptococcus pneumoniae gene locus encoding proteins of an ABC phosphate transporter and a two-component regulatory system. J Bacteriol. 1999;181:1126–1133. doi: 10.1128/jb.181.4.1126-1133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima T, Doi A, Okada A, Gotoh Y, Tanizawa K, Utsumi R. Response regulator YycF essential for bacterial growth: X-ray crystal structure of the DNA-binding domain and its PhoB-like DNA recognition motif. FEBS Lett. 2008;582:3434–3438. doi: 10.1016/j.febslet.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Ramos-Montanez S, Kazmierczak KM, Hentchel KL, Winkler ME. Instability of ackA (acetate kinase) mutations and their effects on acetyl phosphate and ATP amounts in Streptococcus pneumoniae D39. J Bacteriol. 2010;192:6390–6400. doi: 10.1128/JB.00995-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Montanez S, Tsui HC, Wayne KJ, Morris JL, Peters LE, Zhang F, Kazmierczak KM, Sham LT, Winkler ME. Polymorphism and regulation of the spxB (pyruvate oxidase) virulence factor gene by a CBS-HotDog domain protein (SpxR) in serotype 2 Streptococcus pneumoniae. Mol Microbiol. 2008;67:729–746. doi: 10.1111/j.1365-2958.2007.06082.x. [DOI] [PubMed] [Google Scholar]
- Senadheera DB, Cordova M, Ayala EA, Chavez de Paz LE, Singh K, Downey JS, Svensater G, Goodman SD, Cvitkovitch DG. Regulation of bacteriocin production and cell death by the VicRK signaling system in Streptococcus mutans. J Bacteriol. 2012;194:1307–1316. doi: 10.1128/JB.06071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera MD, Guggenheim B, Spatafora GA, Huang YC, Choi J, Hung DC, Treglown JS, Goodman SD, Ellen RP, Cvitkovitch DG. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J Bacteriol. 2005;187:4064–4076. doi: 10.1128/JB.187.12.4064-4076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham LT, Barendt SM, Kopecky KE, Winkler ME. Essential PcsB putative peptidoglycan hydrolase interacts with the essential FtsXSpn cell division protein in Streptococcus pneumoniae D39. Proc Nat Acad Sci USA. 2011;108:E1061–1069. doi: 10.1073/pnas.1108323108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham LT, Tsui HC, Land AD, Barendt SM, Winkler ME. Recent advances in pneumococcal peptidoglycan biosynthesis suggest new vaccine and antimicrobial targets. Curr Opin Microbiol. 2012;15:194–203. doi: 10.1016/j.mib.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Cui L, Iizuka R, Komoto A, Neoh HM, Watanabe Y, Hishinuma T, Hiramatsu K. walK and clpP mutations confer reduced vancomycin susceptibility in Staphylococcus aureus. Antimicrob Agent Chemother. 2011;55:3870–3881. doi: 10.1128/AAC.01563-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana F, Kawamura Y, Hou XG, Shu SE, Ezaki T. Determination of 23S rRNA sequences from members of the genus Streptococcus and characterization of genetically distinct organisms previously identified as members of the Streptococcus anginosus group. FEMS Microbiol Lett. 1998;158:223–230. doi: 10.1111/j.1574-6968.1998.tb12824.x. [DOI] [PubMed] [Google Scholar]
- Sung CK, Li H, Claverys JP, Morrison DA. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 2001;67:5190–5196. doi: 10.1128/AEM.67.11.5190-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurmant H, Bu L, Brooks CL, 3rd, Hoch JA. An essential sensor histidine kinase controlled by transmembrane helix interactions with its auxiliary proteins. Proc Natl Acad Sci USA. 2008;105:5891–5896. doi: 10.1073/pnas.0800247105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurmant H, Mohan MA, Imus PM, Hoch JA. YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis. J Bacteriol. 2007a;189:3280–3289. doi: 10.1128/JB.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurmant H, White RA, Hoch JA. Sensor complexes regulating two-component signal transduction. Curr Opin Struct Biol. 2007b;17:706–715. doi: 10.1016/j.sbi.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throup JP, Koretke KK, Bryant AP, Ingraham KA, Chalker AF, Ge Y, Marra A, Wallis NG, Brown JR, Holmes DJ, Rosenberg M, Burnham MK. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol Microbiol. 2000;35:566–576. doi: 10.1046/j.1365-2958.2000.01725.x. [DOI] [PubMed] [Google Scholar]
- Turck M, Bierbaum G. Purification and activity testing of the full-length YycFGHI proteins of Staphylococcus aureus. PloS One. 2012;7:e30403. doi: 10.1371/journal.pone.0030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Bernhardt TG. More than just lysins: peptidoglycan hydrolases tailor the cell wall. Curr Opinion Microbiol. 2011;14:698–703. doi: 10.1016/j.mib.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Saizieu Ad A, Schonfeld HJ, Kamber M, Lange R, Thompson CJ, Page MG. Genetic analysis and functional characterization of the Streptococcus pneumoniae vic operon. Infect Immun. 2002;70:6121–6128. doi: 10.1128/IAI.70.11.6121-6128.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne KJ, Sham LT, Tsui HC, Gutu AD, Barendt SM, Keen SK, Winkler ME. Localization and cellular amounts of the WalRKJ (VicRKX) two-component regulatory system proteins in serotype 2 Streptococcus pneumoniae. J Bacteriol. 2010;192:4388–4394. doi: 10.1128/JB.00578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler ME, Hoch JA. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J Bacteriol. 2008;190:2645–2648. doi: 10.1128/JB.01682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DC, Peters NT, Parzych KR, Uehara T, Markovski M, Bernhardt TG. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc Nat Acad Sci USA. 2011;108:E1052–1060. doi: 10.1073/pnas.1107780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qin L, Yoshida T, Inouye M. Phosphatase activity of histidine kinase EnvZ without kinase catalytic domain. Proc Nat Acad Sci USA. 2000;97:7808–7813. doi: 10.1073/pnas.97.14.7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.