Abstract
Aggregation of α-synuclein (α-syn) in the brain is a defining pathological feature of neurodegenerative disorders classified as synucleinopathies. They include Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). Occupational and environmental exposure to manganese (Mn) is associated with a neurological syndrome consisting of psychiatric symptoms, cognitive impairment and parkinsonism. In this study, we examined α-syn immunoreactivity in the frontal cortex of Cynomolgus macaques as part of a multidisciplinary assessment of the neurological effects produced by exposure to moderate levels of Mn. We found increased α-syn-positive cells in the gray matter of Mn-exposed animals, typically observed in pyramidal and medium-sized neurons in deep cortical layers. Some of these neurons displayed loss of Nissl staining with α-syn-positive spherical aggregates. In the white matter we also observed α-syn-positive glial cells and in some cases α-syn-positive neurites. These findings suggest that Mn exposure promotes α-syn aggregation in neuronal and glial cells that may ultimately lead to degeneration in the frontal cortex gray and white matter. To our knowledge, this is the first report of Mn-induced neuronal and glial cell α-syn accumulation and aggregation in the frontal cortex of non-human primates.
Keywords: Synucleinopathies, Manganese, Frontal cortex, α-synuclein, Neurotoxicity, Non-human primates
1. Introduction
Exposure to elevated levels of manganese (Mn) in occupational settings is known to produce a complex neurological syndrome with the early appearance of neuropsychiatric symptoms and cognitive deficits followed by movement abnormalities with overlapping clinical features similar to those in idiopathic Parkinson's disease (PD) (Guilarte, 2010; Perl and Olanow, 2007). Experimental evidence indicates that Mn-induced parkinsonism has neuropathological features that are different from those in idiopathic PD (Guilarte, 2010; Perl and Olanow, 2007). For example, it is well documented that in subjects with idiopathic PD there is progressive degeneration of nigrostriatal dopaminergic neurons in the substantia nigra with marked loss of axonal projections to the caudate and putamen, conditions that do not occur in Mn-induced parkinsonism (Guilarte, 2010; Perl and Olanow, 2007). The most compelling human data in regards to Mn effects on dopaminergic neurons comes from recent studies in young drug addicts who inject very high levels of Mn from use of home-made psychostimulant preparations (ephedron) (Colisimo and Guidi, 2009; De Bie et al., 2007; Iqbal et al., 2012; Meral et al., 2007; Sanotsky et al., 2007; Selikhova et al., 2008; Sikk et al., 2010; Stepens et al., 2008, 2010; Yildirim et al., 2009). These individuals exhibited clinical parkinsonism (Colisimo and Guidi, 2009; De Bie et al., 2007; Iqbal et al., 2012; Meral et al., 2007; Sanotsky et al., 2007; Selikhova et al., 2008; Sikk et al., 2010; Stepens et al., 2008, 2010; Yildirim et al., 2009), that is not responsive to l-dopa therapy (Colisimo and Guidi, 2009; Sanotsky et al., 2007; Selikhova et al., 2008; Stepens et al., 2008), and have normal levels of dopamine terminals (dopamine transporters) in the striatum based on neuroimaging SPECT studies (Colisimo and Guidi, 2009; Iqbal et al., 2012; Selikhova et al., 2008; Sikk et al., 2010). More recently, diffusion tensor imaging of ephedron addicts has revealed white matter abnormalities underlying the ventral premotor cortex and the medial frontal cortex, brain regions that are involved in motor and executive function (Stepens et al., 2010). Consistent with these human studies, our laboratory has demonstrated a lack of nigrostriatal dopaminergic neuron degeneration in the striatum of Mn-exposed non-human primates (Guilarte et al., 2006a, 2008b). However, these Mn-exposed animals do express dopamine neuron dysfunction since there is marked inhibition of in vivo dopamine release in the striatum measured by PET (Guilarte et al., 2006a, 2008b), a finding that has been confirmed in rodent models of Mn exposure (Peneder et al., 2011; Vidal et al., 2005).
Historically, due to the overlapping clinical symptoms produced by occupational exposure to Mn with those observed in idiopathic PD, studies on the neurological consequences of Mn exposure have primarily focused on basal ganglia structures and movement abnormalities. However, in the last decade there has been an increasing number of reports indicating that occupational and environmental exposures to Mn produces deficits in working memory (Bowler et al., 2003; Klos et al., 2006) and poor cognitive function (Bowler et al., 2003, 2006, 2007; Klos et al., 2006; Mergler and Baldwin, 1997; Santos-Burgoa et al., 2001) as well as learning performance deficits in children (Khan etal., 2012; Wasserman etal., 2006). The detrimental effects of chronic Mn exposure on working memory and cognition suggest that cortical structures may be vulnerable to Mn-induced neurotoxicity.
We have recently shown that Mn-exposed non-human primates that exhibit impairment of in vivo dopamine release in the striatum measured by PET (Guilarte et al., 2008b) also have significant neuropathology in the frontal cortex (Guilarte et al., 2008a). Gene expression analysis revealed that in the frontal cortex of Mn-exposed non-human primates, 61 genes were increased and 4 genes were decreased relative to controls from a total of 6766 genes (Guilarte et al., 2008a). The biological functions of the genes altered by Mn-exposure were related to: (1) apoptosis, (2) cholesterol metabolism and transport, (3) axonal/vesicular transport, (4) inflammation/immune response, (5) cell cycle/DNA repair and biosynthesis and, (6) proteosome/protein folding/protein turnover. The most highly up-regulated gene was amyloid beta (Aβ) precursor-like protein 1 (APLP1) a member of the amyloid precursor protein (APP) family that has been associated with Alzheimer's disease (AD) pathology. Immunohistochemistry confirmed that APLP1 protein expression was increased and revealed the presence of diffuse amyloid-β plaques in the frontal cortex of Mn-exposed non-human primates (Guilarte et al., 2008a). The same Mn-exposed animals also expressed a significant degree of frontal cortex neuronal degeneration and glial cell activation with white matter involvement (Guilarte et al., 2008a). Further, they exhibited significant deficits in working memory (Schneider et al., 2009) with effects on motor function primarily affecting fine motor control (Schneider et al., 2006). These previous studies provided novel information on Mn-induced effects in the frontal cortex, a brain region that has not been previously associated with Mn neurotoxicity. Since we found diffuse amyloid-β aggregation in the frontal cortex of Mn-exposed animals, the goal of the present study was to assess whether processing of other amyloidogenic or intrinsically disordered proteins such as α-synuclein (α-syn) (Breydo and Uversky, 2011) are also affected. We report that Mn-exposed non-human primates exhibit α-syn aggregation in cellular elements of the frontal cortex gray matter and adjacent white matter similar to those observed in some human synucleinopathies.
2. Materials and methods
2.1. Manganese administration and animal care
Young adult male Cynomolgus macaques, 5–6 years of age were used in this study. All animal studies were reviewed and approved by the Johns Hopkins and the Thomas Jefferson University Animal Care and Use Committee and comply with current laws of the United States. Animals received injections of manganese sulfate into the saphenous vein under 1–3% isoflourane anesthesia once or twice a week. Animals were exposed to different weekly doses of Mn (3.3–5.0, 5.0–6.7, 8.3–10 mg Mn/kg BW) and several of their characteristics have been described in previous publications (Guilarte et al., 2006a, b, 2008a, b; Schneider et al., 2006, 2009). Data from two additional animals that have not been described previously are presented, as these are ongoing studies. The cumulative Mn dose and length of exposure for these animals (R805 and 9329) are presented in Table 1. All animals were euthanized by ketamine injection (20–30 mg/kg BW) followed by an overdose of pentobarbital (100mg/kg BW) and the brains were harvested as previously described (Guilarte et al., 2008a, b).
Table 1.
Descriptive animal data and α-synuclein assessment in the frontal cortex.
| Animal ID | Weekly dose (Mn, mg/kg) | Cummulative dose (Mn, mg/kg) | Exposure time (weeks) | Frontal cortex Mn (μg/g tissue) | α-syn cells and inclusions | |
|---|---|---|---|---|---|---|
|
| ||||||
| GM IR cells | WM IR cells | |||||
| 123146 | Control | N/A | N/A | 0.169 | − | + |
| 123193 | Control | N/A | N/A | 0.188 | − | + |
| 63-111 | Control | N/A | N/A | 0.265 | − | − |
| 001-1167 | Control | N/A | N/A | 0.119 | − | + |
| 6770 | Control | N/A | N/A | 0.190 | + | ± |
| 6499 | Control | N/A | N/A | 0.245 | − | + |
| R805a | 3.3–5.0 | 144.0 | 39 | 0.391 | +++ | +++ |
| 3154 | 3.3–5.0 | 173.8 | 45 | 0.343 | ++ | + |
| 75W | 3.3–5.0 | 151.7 | 44 | 0.378 | − | + |
| 107-705 | 3.3–5.0 | 166.7 | 42 | 0.220 | − | + |
| 144T | 3.3–5.0 | 171.7 | 50 | 0.485 | − | ++ |
| 9329a | 2 × 3.3–5.0b | 220.8 | 52 | 0.477 | +++ | ++ |
| 3114 | 5.0–6.7 | 206.4 | 46 | 0.336 | − | ++ |
| 7469 | 5.0–6.7 | 170.7 | 32 | 0.380 | ++ | + |
| 000-8001 | 5.0–6.7 | 173.9 | 34 | 0.549 | ± | ++ |
| 001-1099 | 5.0–6.7 | 173.9 | 32 | 0.328 | + | ++ |
| 9093 | 5.0–6.7 | 250.8 | 59 | 0.405 | + | + |
| 6697 | 8.3–10.0 | 68.3 | 7 | 2.163 | − | ++ |
| 7426 | 8.3–10.0 | 113.3 | 15 | 0.611 | ++ | ++ |
| 7839 | 8.3–10.0 | 218.3 | 38 | 0.444 | ++ | + |
Descriptive animal data and α-synuclein assessment in the frontal cortex. −, non-detectable; ±, very few; +, minimal; ++, significant; +++, abundant.
Not previously described.
Dose split in half and given twice a week.
2.2. Tissue preparation
Brains were removed from the skull, embedded into agarose and cut into 4 mm slabs in the coronal plane. Alternate brain tissue slabs from the right hemisphere (the left hemisphere was fresh frozen and kept at −80 °C) were processed for immunohistochemistry in two different ways for different purposes. The first slab (in a rostral to caudal direction) and every other slab was fixed in 4% paraformaldehyde, further embedded in paraffin and cut into 5 μm thick sections which were used in other studies not presented in the current report. Alternative tissue slabs (starting from the second frontal slab) were immersed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB) for 28 h, cryoprotected with 20% glycerol, 0.5% DMSO in PB, and frozen at −80°C. Fifty μm thick sections were cut using a freezing microtome, stored in cryoprotecting solution at −20 °C, and utilized for various immunohistochemical screenings. In the current study, two or three sections from anatomically comparable regions were selected from each animal for examination.
2.3. Immunohistochemistry
Tissue sections were processed free-floating. For α-syn immunolabeling, sections were processed with and without pre-treatment with 20% formic acid for 10 min to potentially enhance immunoreactivity of the insoluble α-syn. Sections were pre-treated with 3% H2O2 and 10% methanol in Tris-buffered saline (TBS) for 20 min, rinsed and incubated with 5% normal serum and 0.2% Triton X-100 in TBS for 1 h and then with two different primary antibodies against α-synuclein: polyclonal rabbit (1:5000; Chemicon) and monoclonal mouse antibodies (1:2000–1:5000; BD Transduction Laboratories). Sections were incubated in primary antibodies solution for 48–66 h at 4 °C followed by incubation with corresponding secondary biotinylated IgG (Vector, 1.5 h, at room temperature) and the avidin–biotin–peroxidase complex solution (1 h at room temperature). The reaction product was visualized with 0.25 mg/ml 3,3′-diaminobenzidine (DAB) and 0.03% H2O2. Sections were mounted on slides, stained with cresyl violet, dehydrated in graded ethanols and coversliped using DPX mounting media.
2.4. Evaluation of α-synuclein labeling
α-synuclein labeling in control and Mn-exposed animals was evaluated in the anterior cingulate cortex area 24, cortical areas 4, 6, 9, in the dorsolateral prefrontal cortex area 46, and in the adjacent white matter. We also examined α-syn labeling in the substantia nigra at the level of the third cranial nerve. Tissue sections from the substantia nigra of a confirmed PD subject were used as positive controls for α-syn immunolabeling kindly provided by Dr. Pletnikov (Department of Pathology, Johns Hopkins Hospital). Sections were examined using an Olympus BX51 microscope with a motorized stage (Proscan II, Prior, Rockland, MA, USA), allowing x,y and z-axes control, and linked to an Olympus DP70 video camera and a Visiopharm Integrator System (Visiopharm, Denmark). High-resolution imaging and contrast microscopy using oil immersion and 40× (numerical apertures 1.00) and 100× (numerical aperture 1.35) was used. The semi-quantitative analysis of tissue α-syn immunostaining and aggregation was performed by TV.
2.5. Manganese concentration in frontal cortex tissue
Brain samples were analyzed for metal content by high-resolution inductively-coupled plasma mass spectrometry using a Thermo (Finnigan) model Element 2 instrument (Bremen, Germany) as previously described (Guilarte et al., 2006a, 2008a, b).
3. Results
3.1. Brain Mn concentrations
Frontal cortex Mn levels (μg/g tissue) in control and Mn-exposed animals have been described elsewhere (Guilarte et al., 2008b) with the exception of animal numbers R805 and 9329 that have not been described in previous publications. Table 1 shows the frontal cortex Mn concentrations of all animals used for this study. The results clearly show a significant increase in the frontal cortex Mn content in exposed animals compared to controls. One Mn-exposed animal that was significantly above the range of frontal cortex Mn concentrations was animal 6697. This animal expressed severe movement abnormalities (hyperactivity, dyskinesia and dystonia) and died most likely as a result of liver failure and had the highest Mn concentration in the frontal cortex.
3.2. Tissue examination
Results of α-syn immunostaining are summarized in the Table 1 and images are provided in Figs. 1 and 2. The data indicate increased levels of α-syn aggregation in Mn-exposed animals relative to controls.
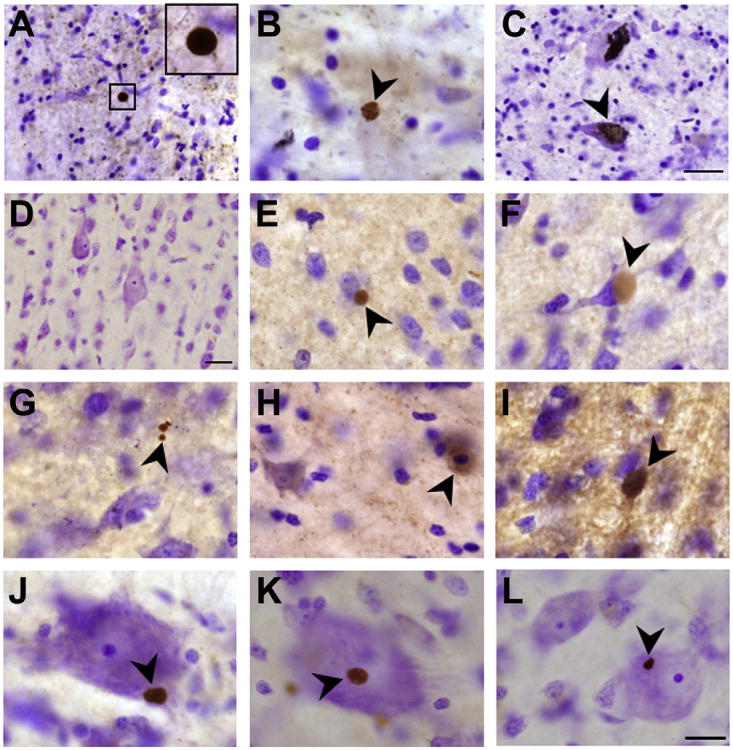
Fig. 1.
α-synuclein immunolabeling in frontal cortex gray matter. Panels A–C are representative of α-synuclein-positive Lewy bodies and α-synuclein intra-neuronal accumulation in the substantia nigra of a confirmed Parkinson's disease subject and this tissue was used as a positive control for staining and neuropathology. The immunostaining for this Parkinson's disease patient and frontal cortex tissue from control and Mn-exposed animals were performed at the same time. Panels A and B depict α-synuclein-positive Lewy bodies that appears to be present in the absence of surrounding neuronal morphology signifying neuronal loss. Panel C (arrow) depicts intra-neuronal α-synuclein accumulation and aggregation in a large DA neuron of the substantia nigra. Panel D is representative of α-synuclein immunostaining in the frontal cortex gray matter of a control animal. Panels E–L are from different manganese exposed animals. Panels E–L are representative of α-synuclein positive aggregates that were predominantly found in animals exposed to 5.0–6.7 and 8.3–10 mg Mn/kg body weight and were not observed in controls. Bar in panels A and D are 40 μm and bar in panels B, C and E–L are 20 μm.
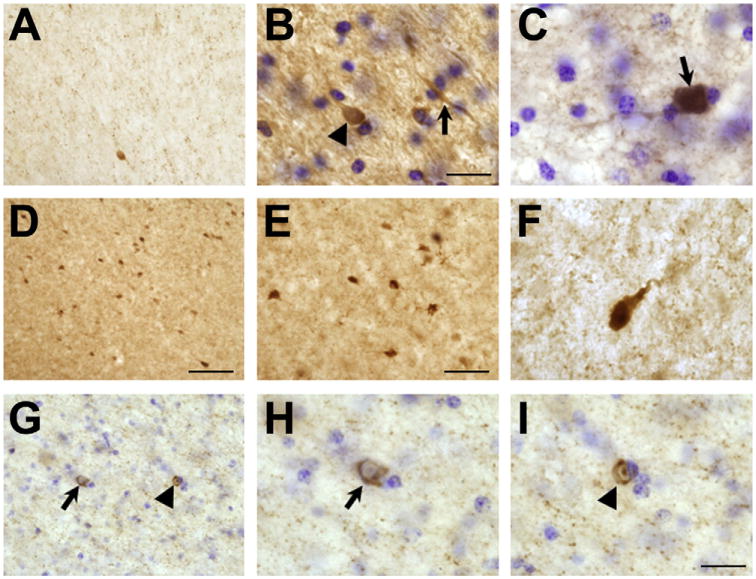
Fig. 2.
α-synuclein immunolabeling in frontal cortex white matter. Panel A is representative of immunolabeling in a control animal and shows a relative lack of cellular accumulation of α-synuclein with an occasional cell being labeled. Panels B–I are representative of α-synuclein immunolabeling in the frontal cortex white matter from Mn-exposed animals. Panel B is from a Mn-exposed animal that received a 8.3–10 mg Mn/kg dose. Note cellular α-synuclein accumulation (arrowhead) in cell body and process (arrow). Panel C is representative of another animal that received a 5.0–6.7 mg Mn/kg dose and shows intense α-synuclein accumulation in a cell. Panels D–F are from an animal that received 3.3–5.0 mg Mn/kg (R805). This particular animal exhibited a large number of cells with α-synuclein accumulation in the white matter. In Panel F, there is α-synuclein accumulation in a cell body and process. Panel G-I is from another animal that received 3.3–5.0 mg Mn/kg. Panel G shows α-synuclein inclusions in glial cells. The glial cell inclusion pointed by an arrow has a crescent cytoplasmic shape while the inclusion in the glial pointed by the arrowhead has a doughnut nuclear shape. Panels H and I are higher magnification images of cells in Panel G. Bar in panels A and E is 60 μm; panel D is 80 μm; panels C, F and G–I is 20 μm and panel B is 40 μm.
3.3. Evaluation of α-synuclein labeling
Animals treated with Mn displayed variable levels of cellular α-syn aggregation with control animals exhibiting little or no α-syn aggregation in the frontal cortex gray and white matter. There was no particular trend toward an increase in α-syn immunoreac-tivity/aggregation in the frontal cortex as a function of Mn dose, but there was a clear distinction in the level of α-syn aggregation between exposed and control animals (Table 1). Different patterns of α-syn labeling were recognizable in the gray matter of Mn-exposed animals. These included spherical aggregates of variable size that were frequently adjacent to cells (Fig. 1, panels E–L). In some cases, α-syn aggregates were associated with Nissl-negative cell indicating a degenerating cell (Fig. 1, panel G). We should note that the α-syn-positive aggregates observed in the frontal cortex gray matter of Mn-exposed animals were similar to Lewy bodies present in the substantia nigra of a confirmed idiopathic PD patient that was used as positive control (compare panels A and B to panels E–G and J–L in Fig. 1). We also observed intensely labeled cells with no apparent α-syn aggregation indicative of increased α-syn protein levels (Fig. 1, panel H). These types of α-syn aggregates were not observed in control animals (Fig. 1, panel D).
In the subjacent white matter from the frontal cortex of control animals, there was a relative lack of cellular α-syn immunoreactivity with occasionally detected labeled cells (Fig. 2A). On the other hand, in the white matter of Mn-exposed animals (Fig. 2, panels B–I), we detected different levels of α-syn labeling. There was one particular animal (R805) that expressed the highest level of α-syn-positive cells in the white matter with a pattern similar to that observed in MSA patients (Fig. 2, panels D–F). Panel B in Fig. 2 depicts the white matter from an animal that received 8.3–10 mg Mn/kg (6697). α-syn accumulation is apparent throughout the cell body (arrowhead) and in processes (arrow). Panel C represents intensely labeled α-syn cellular element in the white matter from an animal that received 5.0–6.7 mg Mn/kg (000–8001). Panels D–I are all from animal R805 and show an abundant number of labeled glial cells and glial cell inclusions in white matter with a crescent cytoplasmic α-syn labeling and a α-syn doughnut-like nuclear labeling, a pattern of cellular white matter staining observed in MSA subjects (Wakabayashi and Takahashi, 2006; Wenning and Jellinger, 2005; Yoshida, 2007).
4. Discussion
The present study provides evidence that exposure to Mn in non-human primates, with blood Mn levels in the upper range to those described in non-occupationally exposed populations, can produce α-syn aggregation in cellular elements of the gray and white matter in the frontal cortex. We were not able to find similar α-syn aggregation in the substantia nigra of the same animals (data not shown) suggesting a unique vulnerability of the frontal cortex at the levels and durations of Mn exposure used in this study.
The same animals used in the present study have been characterized from a behavioral, neuroimaging and neuropathological perspective and these findings have been communicated extensively in the literature (Burton et al., 2009; Guilarte, 2010; Guilarte et al., 2006a, b, 2008a, b; Schneider et al., 2006, 2009; Verina et al., 2011). From these previous studies we have found that the frontal cortex of these same Mn-expsoed animals also exhibit diffuse amyloid-β aggregation and significant neuropathology with glial cell activation (Guilarte et al., 2008a). The behavioral studies from the same animals provided evidence of deficits in working memory (Schneider et al., 2009) suggesting that the neuropathology in the frontal cortex of Mn-exposed animals is associated with significant deficits in cognitive domains mediated by the frontal cortex. These findings were the first to provide neuropathological evidence of frontal cortex involvement in Mn neurotoxicity. The neuropathology that we have described in the frontal cortex of Mn-exposed non-human primates have received support from a recent magnetic resonance spectroscopy study in smelter workers occupationally exposed to Mn (Dydak et al., 2011). The authors found a significant decrease in frontal cortex N-acetylaspartate (NAA)/creatine (Ct) ratio that was strongly correlated with cumulative Mn exposure. A decrease in the NAA/Cr ratio represents neuronal dysfunction or degeneration (Block et al., 2002; Clark, 1998) and may predict activation of frontal cortex regions involved in the execution of working memory tasks (Bertolino et al., 2000). These finding further support the vulnerability of the frontal cortex to Mn neurotoxicity.
We now report that Mn exposure also results in α-syn positive glial cells and cytoplasmic inclusions in the white matter of Mn-exposed animals, a condition that is present in MSA patients (Wakabayashi and Takahashi, 2006; Wenning and Jellinger, 2005; Yoshida, 2007). MSA is a neurodegenerative disease clinically expressed by varying degrees of progressive motor and cognitive decline. MSA has two major subtypes, cerebellar predominant and Parkinson-predominant with the later being characterized by levodopa-unresponsive parkinsonism (Fellner et al., 2011). The later resembles the levodopa unresponsive parkinsonism produced by Mn exposure (Guilarte, 2010). The role of environmental factors in MSA is not well characterized although epidemiological evidence suggests increased risk of MSA with exposure to solvents, metals and other toxins (Stefanova et al., 2009; Vanacore, 2005). We should note that increased neuronal α-syn immunoreactivity is also observed in the human and non-human primate brain as a function of age (Chu and Kordower, 2007). However, the animals used in our studies are young animals (5–6 years at the beginning of the study and at most 7–8 at the end of the study when the neuropathological examination was done) and it is unlikely that the observed increase in α-syn immunoreactivity or aggregation is related to aging.
The α-syn aggregation observed in the frontal cortex of Mn-exposed non-human primates differed from animal to animal despite the fact that in some cases the cumulative Mn dose was similar. This suggests the possibility of a genetic susceptibility in the animal-to animal variability that contributes to the differences in Mn-induced α-syn aggregation. There is evidence that the gene YPK9, the yeast ortholog of the PD-associated gene ATP13A2 (PARK9), not only suppresses α-syn toxicity, but yeast with a deletion of this gene are more sensitive to Mn toxicity (Chesi et al., 2012; Gitler et al., 2009) linking a PD-associated gene with Mn. Other studies provide evidence that ATP13A2 (PARK9) plays a role in sequestration and regulation of divalent heavy metal ions (Schmidt et al., 2009; Tan et al., 2011). Rentschler et al. (2012) have recently shown that polymorphisms of the ATP13A2 (PARK9) gene in humans may influence the neurotoxic effects of Mn. Since deficiency of ATP13A2 leads to lysosomal dysfunction, α-syn accumulation and toxicity (Usenovic et al., 2012) as well as increased Mn toxicity (Chesi et al., 2012; Gitler et al., 2009), it is possible that Mn exposure may decrease ATP13A2 levels and/or animals expressing specific ATP13A2 polymorphisms may be more susceptible to α-syn aggregation induced by Mn exposure.
What are putative mechanism(s) by which Mn exposure may increase α-syn protein aggregation? There is evidence in cell culture systems that exposure to Mn increases α-syn protein levels and oxidative stress markers and α-syn is able to modulate Mn neurotoxicity (Cai et al., 2010; Li et al., 2010; Pfil et al., 2004; Tong et al., 2009). Studies in cell-free systems have shown that Mn is able to induce partial folding, fibrillation and aggregation of α-syn via oxidation and di-tyrosine formation (Uversky et al., 2001). These observations together with our present in vivo findings in the non-human primate brain suggest that Mn and α-syn may interact to trigger neuronal cell death, possibly by increasing cellular levels of α-syn leading to the formation of α-syn aggregates.
We should note that one limitation of the present study is that the frontal cortex of the animals that were used in this study have also been used for the analysis of multiple other endpoints [e.g. gene array, metals analysis, receptor autoradiography and for the optimization of other histological and immunohistochemical methods to measure other endpoints which we have published (Burton et al., 2009; Guilarte et al., 2006a, b, 2008a, b; Schneider et al., 2006, 2009; Verina et al., 2011) ]. Therefore, we had limited tissue available to utilize other confirmatory assays such as α-syn Western blots and for more quantitative methods. Nevertheless, these preliminary results provide initial evidence of a significant difference in α-syn staining and aggregation between control and Mn-exposed animals. Since this is an ongoing project, future studies will provide a more extensive regional brain analysis and use multiple methodologies to investigate and confirm this initial finding.
Highlights.
Alpha-synuclein (α-syn) aggregation is a key feature of neurodegenerative disease.
Manganese exposure causes working memory deficits and parkinsonism.
Frontal cortex of manganese exposed non-human primates exhibit significant pathology.
α-syn aggregates are present in the frontal cortex of manganese-exposed animals.
α-syn aggregation is associated with frontal cortex pathology produced by manganese.
Acknowledgments
This work was performed with funding from the National Institute of Environmental Health Sciences Grant Number ES010975 to TRG.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- Bertolino A, Esposito G, Callicott JH, Mattay VS, Horn JD, Frank JA, Berman KF, Weinberger DR. Specific relationship between prefrontal neuronal N-acetylaspartate and activation of the working memory cortical network in schizophrenia. American Journal of Psychiatry. 2000;157:26–33. doi: 10.1176/ajp.157.1.26. [DOI] [PubMed] [Google Scholar]
- Block W, Traber F, Flacke S, Jessen F, Pohl C, Schild H. In vivo proton MR-spectroscopy of the human brain: assessment of N-acetylaspartate (NAA) reduction as a marker for neurodegeneration. Amino Acids. 2002;23:317–323. doi: 10.1007/s00726-001-0144-0. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. International Journal of Hygiene and Environmental Health. 2003;206:517–529. doi: 10.1078/1438-4639-00249. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Nakagawa S, Drezgic M, Roels HA. Manganese exposure: neuropsychological and neurological symptoms and effects in welders. Neurotoxicology. 2006;27:315–326. doi: 10.1016/j.neuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, Koller W, Bowler RP, Mergler D, Bouchard M, Smith D, Gwiazda R, Doty RL. Dose-effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occupational and Environmental Medicine. 2007;64:167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breydo L, Uversky VN. Role of metal ions in aggregation of intrinsically disordered proteins in neurodegenerative diseases. Metallomics. 2011;3:1163–1180. doi: 10.1039/c1mt00106j. [DOI] [PubMed] [Google Scholar]
- Burton NC, Schneider JS, Syversen T, Guilarte TR. Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the non-human primate brain. Toxicological Sciences. 2009;111:131–139. doi: 10.1093/toxsci/kfp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Yao T, Zheng G, Chen Y, Du K, Cao Y, Shen X, Chen J, Luo W. Manganese induces the overexpression of α-synuclein in PC12 cells via ERK activation. Brain Research. 2010;1359:201–207. doi: 10.1016/j.brainres.2010.08.055. [DOI] [PubMed] [Google Scholar]
- Chesi A, Kilaru A, Fang X, Cooper AA, Gitler AD. The role of the Parkinson's disease gene PARK9 in essential cellular pathways and the manganese homeostasis network in yeast. PLoS ONE. 2012;7:e34178. doi: 10.1371/journal.pone.0034178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is it the target for Parkinson's disease? Neurobiology of Disease. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Clark JB. N-acetylaspartate: a marker for neuronal loss or mitochondrial dysfunction. Developmental Neuroscience. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Colisimo C, Guidi M. Parkinsonism due to ephedrone neurotoxicity: a case report. European Journal of Neurology. 2009;16:e114–e115. doi: 10.1111/j.1468-1331.2009.02606.x. [DOI] [PubMed] [Google Scholar]
- De Bie RMA, Gladstone RM, Strafella AP, Ko JH, Lang AE. Manganese-induced parkinsonism associated with methcathinone (Ephedrone) abuse. Archives of Neurology. 2007;64:886–889. doi: 10.1001/archneur.64.6.886. [DOI] [PubMed] [Google Scholar]
- Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, Edden RAE, Hu S, Fu X, Long Z, Mo XA, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environmental Health Perspectives. 2011;119:219–224. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellner L, Jellinger KA, Wenning GK, Stefanova N. Glial dysfunction in the pathogenesis of α-synucleinopathies: emerging concepts. Acta Neuropathologica. 2011;121:675–693. doi: 10.1007/s00401-011-0833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. α-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nature Genetics. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson's disease: a critical review and new findings. Environmental Health Perspectives. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Experimental Neurology. 2006a;202:381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Degaonkar M, Chen MK, Barker PB, Syversen T, Schneider JS. Evidence of cortical dysfunction and widespread manganese accumulation in the non-human primate brain following chronic manganese exposure: a 1H-MRS and MRI study. Toxicological Sciences. 2006b;94:351–358. doi: 10.1093/toxsci/kfl106. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS. Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. Journal of Neurochemistry. 2008a;105:1948–1959. doi: 10.1111/j.1471-4159.2008.05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by presynaptic mechanism(s): implications to manganese-induced parkinsonism. Journal of Neurochemistry. 2008b;107:1236–1247. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M, Monaghan T, Redmond J. Manganese toxicity with ephedrone abuse manifesting as parkinsonism: a case report. Journal of Medical Case Reports. 2012;6:52. doi: 10.1186/1752-1947-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, Levy D, Mey J, van Geen A, Graziano JH, Factor-Litvak P. Manganese exposure from drinking water and children's academic achievement. Neurotoxicology. 2012;33:91–97. doi: 10.1016/j.neuro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos KJ, Chandler K, Kumar N, Ahiskog JE, Josephs KA. Neuropsychological profiles of manganese neurotoxicity. European Journal of Neurology. 2006;13:1139–1141. doi: 10.1111/j.1468-1331.2006.01407.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun L, Cai T, Zhang Y, Lv S, Wang Y, Ye L. α-synuclein overexpression during manganese-induced apoptosis in SH-SY5Y neuroblastoma cells. Brain Research Bulletin. 2010;81:428–433. doi: 10.1016/j.brainresbull.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Meral H, Kutukcu Y, Atmaca B, Ozer F, Hamancioglu K. Parkinsonism caused by chronic usage of intravenous potassium permanganate. Neurologist. 2007;13:92–94. doi: 10.1097/01.nrl.0000253089.20746.a8. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environmental Research. 1997;73:90–104. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Peneder TM, Scholze P, Berger ML, Riether H, Heinze G, Bertl J, Bauer J, Richfield EK, Hornykiewicz O, Pifl C. Chronic exposure to manganese decreases striatal dopamine turnover in human α-synuclein transgenic mice. Neuroscience. 2011;180:280–292. doi: 10.1016/j.neuroscience.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced parkinsonism. Journal of Neuropathology and Experimental Neurology. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Pfil C, Kjorchide M, Kattinger A, Reither H, Hardy J, Hornykiewicz O. α-synuclein selectively increases manganese-induced viability loss in SK-N-MC neuroblastoma cells expressing the human dopamine transporter. Neuroscience Letters. 2004;354:34–37. doi: 10.1016/j.neulet.2003.09.064. [DOI] [PubMed] [Google Scholar]
- Rentschler G, Covolo L, Haddad AA, Lucchini RG, Zoni S, Broberg K. ATP13A2 (PARK9) polymorphisms influence the neurotoxic effects of manganese. Neurotoxicology. 2012;33:697–702. doi: 10.1016/j.neuro.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanotsky Y, Lesyk R, Fedoryshyn L, Komnatska I, Matviyenko Y, Fahn S. Manganic encephalopathy due to ephedrone abuse. Movement Disorders. 2007;22:1337–1343. doi: 10.1002/mds.21378. [DOI] [PubMed] [Google Scholar]
- Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle F, Eden-Wynter RA, Texcalac-Sangrador JL, Villa-Boragar JP, Rodriguez-Agudelo Y, Montes S. Exposure to manganese: health effects on the general population, a pilot study in central Mexico. Environmental Research. 2001;85:90–104. doi: 10.1006/enrs.2000.4108. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Wolfe DM, Stiller B, Pearce DA. Cd2+, Mn2+, Ni2+and Se2+ toxicity to Saccharomyces cerevisiae lacking YPK9p the orthologue of human ATP13A2. Biochemical and Biophysical Research Communications. 2009;383:198–202. doi: 10.1016/j.bbrc.2009.03.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Koser AJ, Fritz S, Gonczi H, Syversen T, Guilarte TR. Effects of chronic manganese exposure on cognitive and motor functioning in non-human primates. Brain Research. 2006;1118:222–231. doi: 10.1016/j.brainres.2006.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Decamp E, Clark K, Bouquio C, Syversen T, Guilarte TR. Effects of manganese exposure on working memory in non-human primates. Brain Research. 2009;1258:86–95. doi: 10.1016/j.brainres.2008.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selikhova M, Fedoryshyn L, Matviyenko Y, Komnatska I, Kyrylchuk M, Krolicki L, Friedman A, Taylor A, Jager HR, Lees A, Sanotsky Y. Parkinsonism and dystonia caused by the illicit use of Ephedrone—a longitudinal study. Movement Disorders. 2008;23:2224–2231. doi: 10.1002/mds.22290. [DOI] [PubMed] [Google Scholar]
- Sikk K, Taba P, Haldre S, Bergquist J, Nyholm D, Askmark H, Danfors T, Sorensen J, Thurfjell L, Raininko R, Eriksson R, Flink R, Farnstrand C, Aquilonius SM. Clinical, neuroimaging and neuropsychological features in addicts with manganese-ephedrone exposure. Acta Neurologica Scandinavica. 2010;121:237–243. doi: 10.1111/j.1600-0404.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Bucke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurology. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- Stepens A, Logina I, Liguts V, Aldins P, Eksteina I, Platkajis A, Martinsone I, Terauds E, Rozentale B, Donaghy M. A parkinsonian syndrome in methcathinone users and the role of manganese. New England Journal of Medicine. 2008;358:1009–1017. doi: 10.1056/NEJMoa072488. [DOI] [PubMed] [Google Scholar]
- Stepens A, Stagg CJ, Platkajis A, Boudrias MH, Johansen-Berg H, Donaghy M. White matter abnormalities in methcathinone abusers with an extrapyramidal syndrome. Brain. 2010;133:3676–3684. doi: 10.1093/brain/awq281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Zhang T, Jiang L, Chi J, Hu D, Pan Q, Wang D, Zhang Z. Regulation of intracellular manganese homeostasis by Kufor-Rabek syndrome-associated ATP13A2 protein. Journal of Biological Chemistry. 2011;286:29654–29662. doi: 10.1074/jbc.M111.233874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Dong M, de la Monte SM. Brain-insulin like growth factor and neurotrophin resistance in Parkinson's disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. Journal of Alzheimer's Disease. 2009;16:585–599. doi: 10.3233/JAD-2009-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. Journal of Neuroscience. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein. Journal of Biological Chemistry. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- Vanacore N. Epidemiological evidence of multiple system atrophy. Journal of Neural Transmission. 2005;112:1605–1612. doi: 10.1007/s00702-005-0380-7. [DOI] [PubMed] [Google Scholar]
- Verina T, Kiihl SF, Schneider JS, Guilarte TR. Manganese exposure induces microglia activation and dystrophy in the substantia nigra of non-human primates. Neurotoxicology. 2011;32:215–226. doi: 10.1016/j.neuro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L, Alfonso M, Campos F, Faro LRF, Cervantes RC, Duran R. Effects of manganese on extracellular levels of dopamine in rat striatum: an analysis in vivo by brain microdialysis. Neurochemical Research. 2005;30:1147–1154. doi: 10.1007/s11064-005-7775-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–345. doi: 10.1111/j.1440-1789.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Lolacono NJ, Cheng Z, Zhang Y, Graziano JH. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environmental Health Perspectives. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning GK, Jellinger KA. The role of α-synuclein in the pathogenesis of multiple system atrophy. Acta Neuropathologica. 2005;109:129–140. doi: 10.1007/s00401-004-0935-y. [DOI] [PubMed] [Google Scholar]
- Yildirim EA, Essizoglu A, Koksal A, Dogu B, Baybas S, Gokalp P. Chronic manganese intoxication due to methcathinone (Ephedron) abuse: a case report. Turkish Journal of Psychology. 2009;20:294–298. [PubMed] [Google Scholar]
- Yoshida M. Multiple system atrophy: α-synuclein and neuronal degeneration. Neuropathology. 2007;27:484–493. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]