SUMMARY
Neutrophils are typically the first responders in host defense against invading pathogens, which they destroy by both oxidative and nonoxidative mechanisms. However, despite a longstanding recognition of neutrophil presence at disease sites in tuberculosis, their role in defense against mycobacteria is unclear. Here we exploit the genetic tractability and optical transparency of zebrafish to monitor neutrophil behavior and its consequences during infection with Mycobacterium marinum, a natural fish pathogen. In contrast to macrophages, neutrophils do not interact with mycobacteria at initial infection sites. Neutrophils are subsequently recruited to the nascent granuloma in response to signals from dying infected macrophages within the granuloma, which they phagocytose. Some neutrophils then rapidly kill the internalized mycobacteria through NADPH oxidase-dependent mechanisms. Our results provide a mechanistic link to the observed patterns of neutrophils in human tuberculous granulomas and the susceptibility of humans with chronic granulomatous disease to mycobacterial infection.
INTRODUCTION
The crucial importance of neutrophils in host defenses is highlighted by the extreme susceptibility of patients with congenital or drug-induced neutropenia to a variety of bacterial and fungal infection (Amulic et al., 2012). These highly motile cells are rapidly recruited to infection sites and can destroy invading pathogens by both oxidative and nonoxidative mechanisms, whether through phagocytosis of the microorganisms themselves or through extracellular release of microbicidal granules (Amulic et al., 2012). In mycobacterial infections, the role of neutrophils remains poorly understood, despite a longstanding recognition of their presence at disease sites in tuberculosis (TB), for example, in the cerebrospinal fluid of TB meningitis patients (Lowe et al., 2011; Thwaites et al., 2002). In the most common pulmonary form of TB, infected neutrophils are present in the characteristic macrophage-predominant granulomas, both in newly forming ones and advanced cavitary lesions (Canetti, 1955; Eum et al., 2010). Consistent with these histological findings, a neutrophil-driven transcriptional signature has been identified in the blood of TB patients (Berry et al., 2010).
Reports on the mycobactericidal capacity of isolated human neutrophils are conflicting, perhaps because of the inherent variability of these terminally differentiated, short-lived cells in culture (Lowe et al., 2011). Some studies found that they kill Mycobacterium tuberculosis (Brown et al., 1987; Jones et al., 1990; Kisich et al., 2002), while others reported no effect (Corleis et al., 2012; Reyes-Ruvalcaba et al., 2008). Animal models have also yielded conflicting results. While earlier work identified a protective role for neutrophils in early infection (Pedrosa et al., 2000; Sugawara et al., 2004), recent work has focused on their pathological role, particularly with excessive neutrophil accumulation in advanced infection (Desvignes and Ernst, 2009; Eruslanov et al., 2005; Nandi and Behar, 2011).
Zebrafish larvae infected with their natural pathogen Mycobacterium marinum (Mm), a close genetic relative of M. tuberculosis, have proved a useful and relevant model for the mechanistic dissection of human TB (Tobin et al., 2010, 2012; Volkman et al., 2010). Their genetic tractability and optical transparency have allowed dissection of mycobacterium-macrophage interactions not amenable in traditional animal models (Clay et al., 2007; Davis et al., 2002; Davis and Ramakrishnan, 2009). Here, we have sought to understand the role of neutrophils in mycobacterial infection using a zebrafish transgenic line with fluorescently labeled neutrophils (Hall et al., 2007). We show that zebrafish larval neutrophils share functional characteristics with human neutrophils and are rapidly recruited to Pseudomonas aeruginosa (Pa), against which they exert full protection. In contrast, they are rarely recruited to initial sites of Mm infection, and even the few are seldom infected. Neutrophil interactions with mycobacteria are initiated only when granulomas form. Signals from dead or dying granuloma macrophages recruit neutrophils, which then phagocytose infected macrophages. Using genetically engineered neutropenic zebrafish and zebrafish deficient in phagocytic oxidase activity, we show that neutrophils exert a protective effect through oxidative killing of the mycobacteria they phagocytose from dead granuloma macrophages.
RESULTS
Zebrafish lyz:EGFP Transgene Specifically Labels Neutrophils
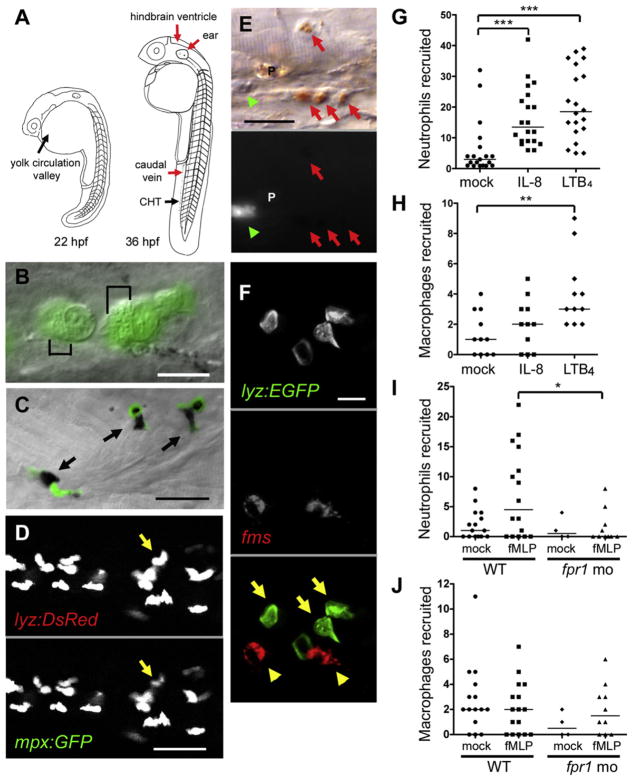
lyz, encoding zebrafish lysozyme C, marks both neutrophils and macrophages at 32 hr postfertilization (hpf); at this developmental stage of exclusively primitive hematopoiesis from the yolk area, it is coexpressed with both the neutrophil-specific marker myeloperoxidase (mpx) and the macrophage-specific csf1 receptor (fms) (Figure 1A) (Herbomel et al., 1999; Liu and Wen, 2002). Accordingly, zebrafish fluorescent transgenic lines using the lyz promoter were thought to label both phagocyte lineages (Hall et al., 2007). However, by 48 hpf, when the site of hematopoiesis switches over to the caudal hematopoietic tissue (CHT) (Murayama et al., 2006), multiple lines of evidence led us to conclude that lyz exclusively marks neutrophils: (1) high-resolution differential interference contrast (DIC) microscopy of the Tg(lyz:EGFP)nz117 (lyz-green) line showed that the GFP-positive cells contain the distinctive small motile granules specific for teleost neutrophils (Le Guyader et al., 2008) (Figure 1B); (2) the neutrophil-specific dye Sudan black stained only and all GFP-positive cells in this line (Figure 1C) (Le Guyader et al., 2008); (3) in a double transgenic line, Tg(lyz:DsRED2)nz50 (lyz-red) and Tg(mpx:GFP)i114 (rendering neutrophils green fluorescent), all red fluorescent, lyz-expressing cells also expressed GFP (Renshaw et al., 2006) (Figure 1D); (4) in the lyz-green line, the GFP-positive cells did not stain with neutral red, a macrophage-specific dye (Figure 1E) (Herbomel et al., 1999); and (5) in the lyz-green line, whole-mount in situ hybridization (WISH) revealed no overlap in macrophage-specific marker fms RNA expression and anti-GFP antibody staining (Figure 1F). Thus, the lyz promoter is a reliable and specific marker for neutrophils in zebrafish larvae from 48 hpf on. The combination of DIC and fluorescence microscopy using the lyz fluorescent lines allows the visualization of both neutrophils and macrophages as illustrated in Figure 1E.
Figure 1. Zebrafish Neutrophils Are Marked by GFP Expression in the lyz-Green Line and Are Functionally Similar to Mammalian Neutrophils.
(A) Cartoon of the zebrafish at two developmental stages showing sites of primitive and definitive hematopoiesis (black arrows) and injection sites used in this study (red arrows). CHT, caudal hematopoietic tissue.
(B) GFP-positive cells in lyz-green fish showing well-defined granules (black brackets). Scale bar, 10 μm.
(C) Colocalization of GFP-expressing cells (black arrows) with Sudan black. Scale bar, 20 μm.
(D) Individual fluorescence channels of a single image of the lyz-red – mpx:gfp double transgenic larvae. Arrow identifies a cell with differential expression of the two transgenes. Scale bar, 20 μm.
(E) DIC (top) and GFP fluorescence channel (bottom) of a single image of a lyz-green larva stained with neutral red. Macrophages, red arrows; neutrophil, green arrowhead; pigment cell, P. Scale bar, 20 μm.
(F) Whole-mount in situ hybridization analysis of 48 hpf lyz-green larva with fms probe (red fluorescence) combined with GFP antibody staining (green fluorescence). Macrophages, yellow arrowheads; neutrophils, yellow arrows. Scale bar, 10 μm.
(G–J) Median phagocyte recruitment into the ear (G) or hindbrain ventricle (H–J) of 48 hpf larvae in response to injection of vehicle (mock), IL-8, LTB4, or fMLP. In (I) and (J), fpr1 mo refers to larvae with morpholino knockdown of the formyl peptide receptor 1. Results in (G)–(J) are representative of two experiments. Significance testing performed by Kruskal-Wallis test with Dunn’s posttest in (G)–(J). *p < 0.05; **p < 0.01; ***p < 0.0001.
Zebrafish Larval Neutrophils and Macrophages Respond to Distinct Signals
In zebrafish larvae, neutrophil migration to the ear and macrophage migration to the hindbrain ventricle (HBV) in response to injected compounds or bacteria can be readily assessed (Tobin et al., 2010). Using these two phagocyte recruitment assays, we found that recombinant human interleukin-8 (IL-8 or CXCL8), a neutrophil-specific chemotactic cytokine (Stillie et al., 2009), recruited neutrophils but not macrophages (Figures 1G and 1H). In contrast, human leukotriene B4 (LTB4) recruited neutrophils and macrophages, consistent with its activity as a dual neutrophil and macrophage chemoattractant in humans (Crooks and Stockley, 1998) (Figures 1G and 1H). Finally, the synthetic N-formylated peptide N-formyl-methionine-leucine-phenylalanine (fMLP), a potent neutrophil chemoattractant (Schiffmann et al., 1975), recruited neutrophils, but not macrophages (Figures 1I and 1J). Neutrophil recruitment by fMLP was abolished by modified antisense oligonucleotide (morpholino) knockdown of its predicted receptor (formyl peptide receptor 1) (Williams et al., 1977) while leaving baseline macrophage recruitment unchanged (Figures 1I and 1J). These findings suggest functional conservation of neutrophil chemotaxis between zebrafish and humans, and that larval zebrafish neutrophils and macrophages already have distinct chemotactic responses.
Neutrophils Do Not Interact with Mm at Initial Sites of Infection
Previously we had observed very few infected neutrophils at 1 day postinfection (dpi) into the caudal vein upon staining infected larvae with an anti-mpx antibody (Clay et al., 2007). This finding suggests either that neutrophils do not phagocytose mycobacteria or that neutrophils destroy mycobacteria rapidly upon phagocytosis. To distinguish between these possibilities, we injected red fluorescent Mm into the caudal vein of 48 hpf lyz-green fish and monitored infection serially by DIC and fluorescence microscopy. At 10 min postinfection, we observed several infected macrophages but no infected neutrophils (Figure 2A). Again at 1 hour postinfection (hpi), no infected neutrophils were found despite many uninfected neutrophils being clustered around the injection site, probably reflecting its proximity to the CHT site of their origin (Figure 2B) (Hall et al., 2007). To rule out the possibility that zebrafish neutrophils are developmentally immature at 48 hpf, similar experiments were conducted in 5 dpf larvae, and no infected neutrophils were observed at 1 dpi (see Figure S1A online). Thus, neutrophils do not phagocytose extracellular mycobacteria, even when in close proximity.
Figure 2. Neutrophils Are Poorly Recruited to the Initial Site of Mycobacterial Infection and Do Not Phagocytose Mycobacteria.
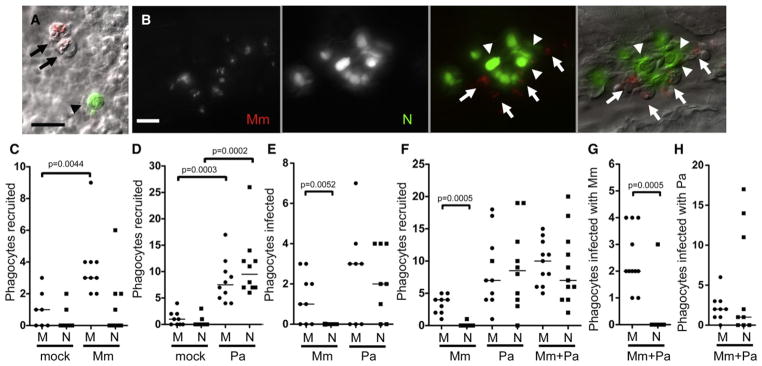
(A) Fluorescence and DIC overlay of macrophages infected with red fluorescent Mm (arrows) and a green fluorescent neutrophil (arrowhead) 10 min after caudal vein injection of 48 hpf larva. Scale bar, 10 μm.
(B) Infected macrophages (arrows) and uninfected neutrophils (arrowheads) 24 hr after Mm injection into caudal vein of 48 hpf larva. Scale bar, 20 μm.
(C–H) Median number of phagocytes recruited to, and infected in, the HBV of 48 hpf larvae 6 hr postinfection of vehicle (mock), (C) 108 Mm, (D) 45 Pa, (E) 108 Mm or 18 Pa, and (F–H) 27 Mm and 22 Pa together. M, macrophage, N, neutrophil. Results in (C)–(H) are each representative of more than three experiments. p value determined by Mann-Whitney rank test. (Also see Figure S1 and Movie S1.)
Mycobacterial infection is characterized by rapid macrophage recruitment (Dannenberg, 1993); in the zebrafish larva, phagocyte recruitment is readily observed by injection of bacteria into the HBV (Figure 1A), a site that is normally devoid of phagocytes (Clay et al., 2007). Injection of red fluorescent Mm into the HBV of 48 hpf lyz-green fish consistently produced a significant influx of macrophages, but not neutrophils (Figure 2C). In contrast, Pa, a pathogen that interacts with both neutrophils and macrophages in humans and zebrafish (Brannon et al., 2009; Cheung et al., 2000; Lyczak et al., 2000), recruited similar numbers of both cell types (Figure 2D). In the rare case where a few neutrophils were recruited to the HBV following Mm infection, only macrophages were infected (Figure 2E). This finding is consistent with our observations during caudal vein infection (Figure 2A). In contrast, both neutrophils and macrophages became infected with Pa (Figure 2E). The differential phagocytic capacity of macrophages and neutrophils for Mm was further highlighted in time-lapse DIC images showing a macrophage actively moving toward and phagocytosing Mm in the HBV (Movie S1A); in contrast, the few neutrophils arriving in the HBV did not interact with Mm even when in close proximity; they continued their rapid migration past the bacteria (Movie S1B).
We explored the possibility that mycobacteria actively inhibit neutrophil recruitment. If so, the presence of Mm should reduce neutrophil migration to Pa. However, neutrophil recruitment in response to coinjection of Mm and Pa into the HBV was indistinguishable from Pa infection alone (Figure 2F). Strikingly, the recruited neutrophils did not phagocytose Mm even while retaining their capacity to phagocytose Pa (Figures 2G and 2H).
In vitro, complement-mediated opsonization of mycobacteria increases their phagocytosis by neutrophils (Jones et al., 1990; Majeed et al., 1998). We asked if complement opsonization facilitated the phagocytosis of Mm by the neutrophils brought into proximity to the mycobacteria by coinfection with Pa. Opsonization with fetal bovine serum or fresh adult zebrafish serum did not increase neutrophil phagocytosis of Mm (Figures S1B–S1E). In sum, mycobacteria appear to be invisible to neutrophils in terms of both chemotaxis and phagocytosis.
Neutrophils Become Infected in Early Granulomas through Phagocytosis of Infected Macrophages
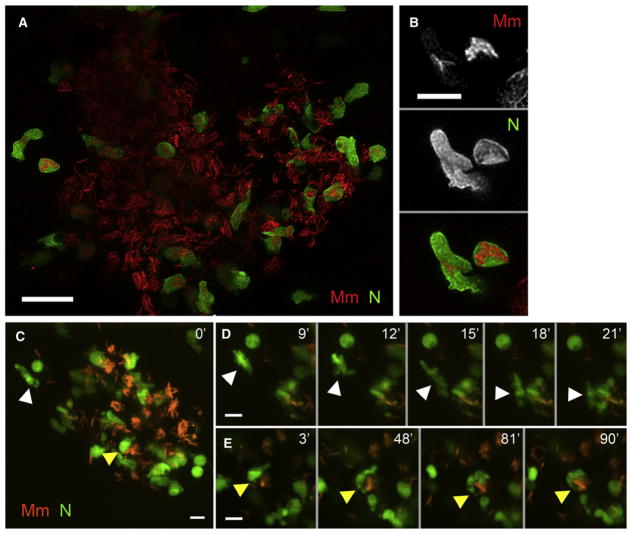
While no infected neutrophils were observed at the initial infection site, serial monitoring of infection in the lyz-green larvae consistently revealed infected neutrophils within the granulomas that form by 2–4 dpi (Figures 3A and 3B) (Davis et al., 2002). High-resolution spinning-disc confocal microscopy of individual granulomas over 2 hr showed uninfected neutrophils trafficking into granulomas and moving rapidly among its infected macrophages (Figures 3C and 3D, and Movie S2A). Many interacted closely with infected macrophages, recapitulating behaviors of macrophages arriving at granulomas (Davis and Ramakrishnan, 2009), and eventually engulfed their bacterial contents (Figures 3C and 3E, Movies S2B, and Movie S3). We also observed neutrophils that migrated through the granuloma and left without becoming infected (Movie S4). Since most granuloma neutrophils at any given time were infected (Figure 3A), infected neutrophils may be preferentially retained within these structures. In sum, neutrophils arriving in the granuloma become infected through phagocytosis of infected macrophages, similar to newly arriving macrophages (Davis and Ramakrishnan, 2009).
Figure 3. Neutrophils Are Present in the Early Granuloma.
(A) High-resolution laser-scanning confocal image showing a mycobacterial granuloma containing uninfected and infected neutrophils. Scale bar, 20 μm.
(B) Two GFP-positive neutrophils infected with red fluorescent Mm from granuloma in (A). Scale bar, 10 μm.
(C–E) Series of time-lapse spinning-disc confocal images highlighting cellular dynamics of neutrophils of the early granuloma. (C) A snapshot of a granuloma with the two neutrophils (arrowheads) monitored by time-lapse imaging. (D) Uninfected neutrophil (white arrowhead) arriving at the granuloma shown in (C). (E) Uninfected neutrophil (yellow arrowheads) engulfing infected macrophage to become infected. Elapsed minutes indicated in upper right corner of (D) and (E). Green, neutrophil (N); red, Mm. Scale bars in (C)–(E), 15 μm. (Also see Movie S2, Movie S3, and Movie S4.)
Neutrophil Recruitment into Granulomas Is Mediated by Cell Death Signals from Infected Macrophages
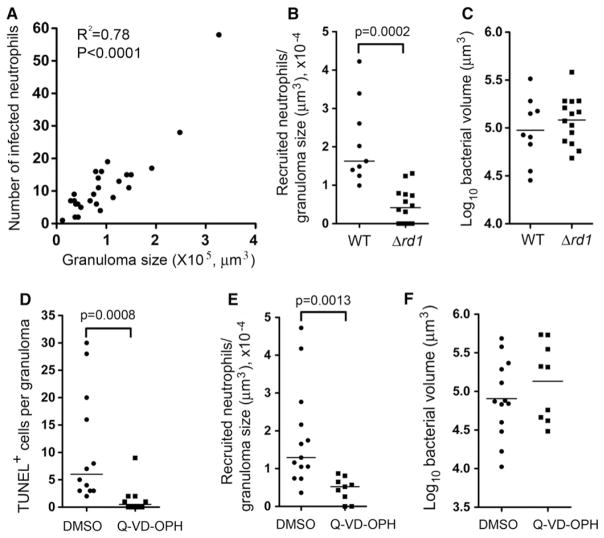
Why might neutrophils be attracted to Mycobacterium-containing granulomas but not directly to the infecting mycobacteria? We observed that the number of infected neutrophils increased with granuloma size (Figure 4A), representing a constant proportion of the total number of infected cells (15%–18%), and of the total bacterial volume in granulomas over a range of sizes (1.27 ± 0.12 × 10−4/μm3). Given that larger granulomas contain more dying infected macrophages, we wondered if these were providing the signals for neutrophil recruitment. To test this possibility, we infected fish with Mm that lack the RD1 locus (Δrd1), one of whose encoded secreted proteins, ESAT-6, promotes the apoptotic death of macrophages (Davis and Ramakrishnan, 2009; Derrick and Morris, 2007; Volkman et al., 2004). Owing to its reduced ability to form granulomas, 5-fold more Δrd1 bacteria were required to create similar sized granulomas to wild-type (WT) (Davis et al., 2002). A 4.5-fold reduction of recruited neutrophils in Δrd1 granulomas was accompanied by a 5.2-fold reduction of infected neutrophils within them (Figures 4B and 4C and Figure S2A). In contrast, granulomas formed by an Mm mutant defective in the secreted protein Erp that specifically mediates intramacrophage mycobacterial growth (Clay et al., 2007; Cosma et al., 2006a) had no reduction in infected neutrophils despite being slightly smaller (Figures S2B and S2C). Together, these results suggested that neutrophils respond to signals emanating from dead cells in granulomas. To test this directly, we treated Mm-infected larvae with Q-VD-OPH, a pancaspase inhibitor that blocks apoptosis in zebrafish (Walters et al., 2009). Q-VD-OPH treatment produced a 7.2-fold reduction in the number of TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)-positive cells (Figure 4D). Q-VD-OPH-treated fish had a 3.5-fold reduction in neutrophil recruitment to granulomas corresponding to a similar (3.7-fold) reduction in infected granuloma neutrophils (Figures 4E and 4F and Figure S2D). Thus signals from dying macrophages appear to recruit neutrophils.
Figure 4. Neutrophil Recruitment to Granu-lomas Is Mediated by Death Signals.
(A) Number of infected neutrophils in granuloma versus granuloma size as determined by log bacterial volume (μm3). R2 = 0.78; two-tailed p < 0.0001, Pearson correlation.
(B) Median neutrophils recruited to WT (from 9 animals) and Δrd1 Mm granulomas (from 12 larvae) in the HBV at 3 dpi, normalized to granuloma size.
(C) Mean size of WT and Δrd1Mm containing granulomas in (B). Results in (B) and (C) are representative of two experiments.
(D) Median TUNEL+ cells in WT Mm containing granulomas treated with DMSO (vehicle control) or Q-VD-OPH, normalized to granuloma size, nine larvae per treatment.
(E) Median neutrophils recruited to granulomas in (D), normalized to granuloma size.
(F) Mean size of granulomas in (D). Results in (D)–(F) are representative of two experiments. Recruited neutrophils in (B) and (E) represent the sum of infected and uninfected neutrophils within or closely surrounding the granuloma. p value by Student’s unpaired t test (C and F), and by Mann-Whitney rank test (B, D, and E). Also see Figure S2.
Neutrophils Play a Protective Role in the Early Granuloma
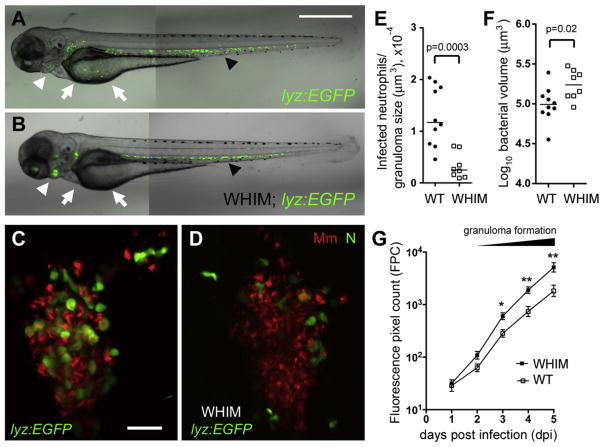
We next asked if neutrophil recruitment to granulomas influences the course of infection. We took advantage of the zebrafish WHIM transgenic line, Tg(-8mpx:cxcr4.1-EGFP)uwm3, developed to model the human WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome caused by mutations in the neutrophil chemokine receptor, CXCR4, and characterized by chronic neutropenia owing to neutrophil retention in the hematopoietic tissues (Walters et al., 2010). In the zebrafish WHIM line, a mutated zebrafish cxcr4 is under control of the mpx promoter. Its neutrophils are retained in the CHT and pronephros, and neutrophil migration to wound sites is impaired (Figures 5A and 5B) (Walters et al., 2010).
Figure 5. Neutrophils Play a Protective Role upon Granuloma Formation.
(A and B) Fluorescence and DIC overlay of 3 dpf WT (A) and WHIM (B) zebrafish showing neutrophil distribution. Arrows, yolk; white arrowheads, pronephros; black arrowheads, CHT. Scale bar, 300 μm.
(C and D) High-resolution laser confocal images of representative 3 dpi granulomas in WT (C) and WHIM (D) larvae. Green, neutrophil (N), red, Mm. Scale bar, 30 μm.
(E) Median infected neutrophils per granuloma, normalized to granuloma size. Granulomas were from ten WT and seven WHIM larvae. p value by Mann-Whitney rank test.
(F) Mean size of granulomas in (E). p values were determined by Student’s unpaired t test.
(G) Serial bacterial burdens (mean ± SEM) of 2 dpf WT (n = 13) or WHIM (n = 10) fish infected by caudal vein with 189 Mm measured by fluorescent pixel count (FPC). Representative of three experiments. *p < 0.05; **p < 0.01 (one-way ANOVA with Bonferroni’s multiple comparison test; all other comparisons not significant). Also see Figure S3.
We found WHIM fish to display the expected neutrophil migration defect in response to Pa infection: HBV infection of 48 hpf fish revealed a drastic reduction in neutrophil recruitment that resulted in increased susceptibility of these animals (Figures S3A and S3B). Only neutrophil migration was defective; macrophage migration to both Pa and to Mm was normal (Figures S3A and S3C).
WHIM granulomas created by HBV injection had 4-fold fewer infected neutrophils than WT (Figures 5C–5E), accompanied by a 1.7-fold increase in bacterial burdens (Figures 5C, 5D, and 5F). This increased bacterial burden in individual WHIM granulomas was confirmed at the whole-animal level by injection of Mm into the caudal vein. An increase in bacterial burdens in the WHIM fish became apparent at 2 dpi coincident with granuloma formation and progressed to a 2.3-fold difference at 5 dpi (Figure 5G). These results confirm a protective effect of neutrophils during early infection, which is temporally linked to the granuloma formation that allows them access to the mycobacteria.
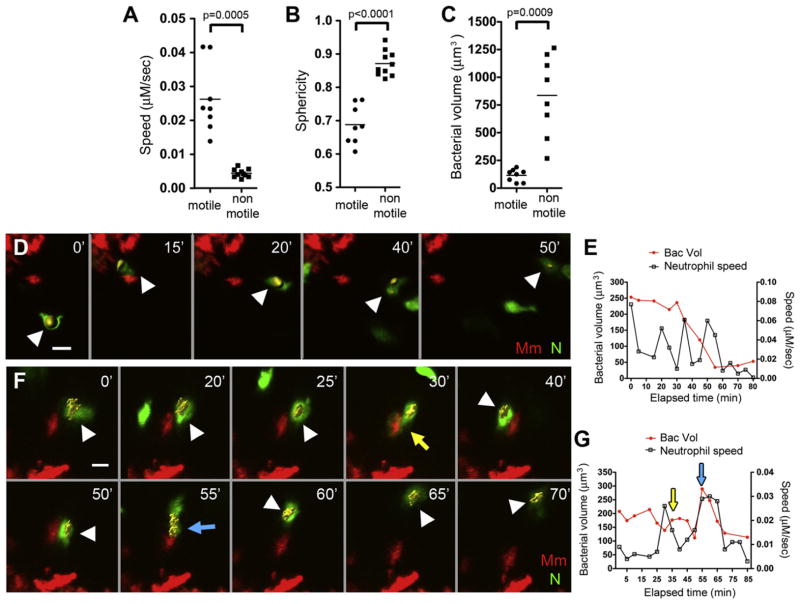
A Subpopulation of Neutrophils Can Kill Their Phagocytosed Mycobacteria
The most parsimonious explanation for our findings was that granuloma neutrophils directly kill the bacterial contents of the macrophages they phagocytose. To determine if this was the case, we first characterized the motility dynamics of infected neutrophils by real-time, high-resolution imaging at 3–5 min intervals over 5–8 hr. Two discrete populations were discerned based on velocity: 80% were relatively nonmotile (speed <0.01 μM/s), and 20% moved through the granuloma at speeds comparable to infected macrophages (mean speed 0.026 μM/s) (Figure 6A) (Davis and Ramakrishnan, 2009). Representative motile and non-motile cells are shown in Movie S5. The nonmotile cells were more spherical, as a consequence of being more highly infected than the motile cells (Figure 6B and 6C); this relationship is exemplified in Figure 3B. The inverse correlation between motility and infection level has also been observed for granuloma macrophages (Davis and Ramakrishnan, 2009). These findings posed the following questions: Does the progression of infection reduce the motility of all neutrophils so that infected motile cells all progress to become heavily infected, nonmotile cells? Alternatively, do the less infected cells represent a discrete subset of neutrophils with increased mycobactericidal activity?
Figure 6. Neutrophils Phagocytose and Kill Mycobacteria from Infected Granuloma Macrophages.
(A–C) Mean speed (A), sphericity (B), and bacterial volume (C) of infected neutrophils in 3 dpi granulomas, sampled from three granulomas each from different larvae. p value by Student’s unpaired t test (B) and by Student’s unpaired t test with Welch’s correction (A and C).
(D) Representative frames of time-lapse laser confocal images of infected motile neutrophils in larva showing a neutrophil (white arrowhead) phagocytosing bacteria from a granuloma macrophage.
(E) Bacterial burden over time and interval speed of neutrophil in (D).
(F) Representative frames of time-lapse laser confocal images of infected motile neutrophil in larva showing neutrophil (white arrowhead) repeatedly phagocytosing bacteria (yellow and blue arrows) from a granuloma macrophage.
(G) Bacterial burden over time and interval speed of neutrophil in (F) with yellow and blue arrowheads indicating respective phagocytic events in (F). In (D) and (F), green, neutrophil (N); red, Mm, minutes in upper right corner. Yellow objects (white arrowheads) showing bacteria within neutrophils are built by 3D surface rendering with Imaris. Also see Figure S4 and Movie S5, Movie S6, and Movie S7.
To address these questions, we quantified neutrophil bacterial burdens every 3–5 min for at least 1 hr. In the motile neutrophils, there was a reduction in intracellular bacterial fluorescence over time, which was indicative of bacterial killing; the example in Figures 6D and 6E and Movie S6 shows a 50% reduction in bacterial fluorescence over 50 min. Furthermore, motile neutrophils could phagocytose and kill bacteria multiple times as exemplified in Figures 6F and 6G and Movie S7. From the time it was first visualized, the bacterial fluorescence inside this neutrophil decreased over the first 30 min. While in the process of killing its intracellular bacteria, this neutrophil moved into close proximity to an infected macrophage forming membrane protrusions that wrapped around it (Figure 6F, 30 min time point). After the resolution of the tethering between the infected neutrophil and macrophage, we observed increased bacterial fluorescence in the neutrophil (Figure 6F, 40 min). A second close encounter with the same infected macrophage at 55 min resulted in another increase in bacterial fluorescence at 60 min, followed by a 60% decrease at 85 min.
Only motile infected neutrophils appeared to have this microbicidal capacity. The nonmotile cells did not kill their bacteria over a 7 hr observation period and even appeared to support intracellular bacterial replication (Figure S4 and data not shown). Thus, a minority of neutrophils can kill mycobacteria effectively through repeated rounds of phagocytosis. It is not clear if these neutrophils comprise a distinct neutrophil lineage.
Neutrophils Kill Intracellular Mycobacteria through Oxidative Mechanisms
To determine if neutrophils depend on the production of reactive oxygen species (ROS) to kill their internalized mycobacteria, we used morpholino knockdowns to create larvae doubly deficient in two key subunits of NADPH oxidase, gp91phox and p22phox (Niethammer et al., 2009). These phox morphants exhibited the expected increase in susceptibility to Pa infection (Figure S5A) (Speert et al., 1994). With Mm, similar numbers of infected neutrophils were present in phox morphant and WT granulomas, suggesting that recruitment and phagocytosis were unaffected (Figure S5B). However, the morphant neutrophils had a greatly reduced capacity to kill their internalized mycobacteria (Figures 7A–7C). Thus, neutrophils appear to play a protective role by intracellular killing through NADPH-dependent mechanisms.
Figure 7. Neutrophil Killing of Intracellular Mycobacteria Is Mediated by NADPH Oxidase.
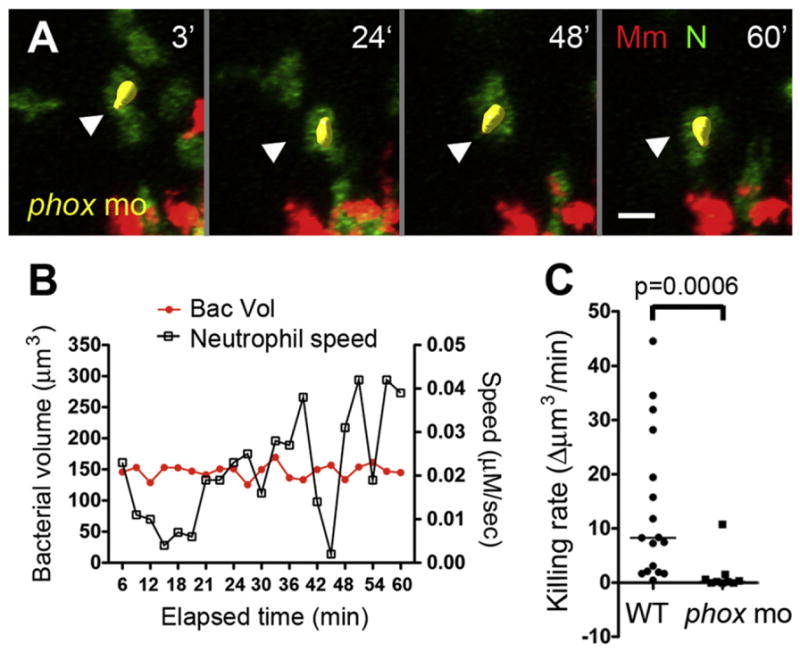
(A) Representative frames of time-lapse laser confocal images of a motile infected neutrophil (white arrowhead) in phox morphants (phox mo). Green, neutrophil (N); red, Mm. Elapsed minutes in upper right corner.
(B) Bacterial burden over time and interval speed of neutrophil in (A).
(C) Median killing rate (decrease in bacterial volume of infected neutrophil over time) of WT and phox morphant neutrophils. Infected neutrophils were sampled from three granulomas in three different infected larvae for each group. p values by Mann-Whitney rank test. Also see Figure S5.
DISCUSSION
From early in development, zebrafish have functionally mature neutrophils that share several key characteristics with adult human neutrophils. They are highly motile and exhibit the expected specific responses to chemotactic signals. They are recruited to Pa infection, against which they protect by oxidative killing. Thus validating the zebrafish larval model for the study of neutrophils, we have explored neutrophils’ role in mycobacterial infection. We find that, like many other serious human pathogens, mycobacteria initially evade neutrophil phagocytic mechanisms. However, tuberculous granuloma formation later circumvents this mycobacterial evasion strategy by cloaking the pathogen within macrophages. Signals from dying granuloma macrophages appear to beckon neutrophils, which upon arrival can collect mycobacteria from macrophages by phagocytosis and kill them by oxidative mechanisms.
The mechanism of neutrophil function in granulomas revealed here provides a possible explanation for longstanding observations of the susceptibility of chronic granulomatous disease (CGD) patients to mycobacterial infection. CGD is characterized by decreased respiratory burst production owing to genetic deficiencies in phagocytic NADPH oxidase (Amulic et al., 2012). Along with well-documented susceptibilities to other catalase-producing organisms, susceptibility to TB in highly endemic areas has been documented (Dogru et al., 2010; Lee et al., 2008), and is likely underreported, given the improbability of diagnosing CGD in these areas. What is striking is the widely documented susceptibility of CGD sufferers to disseminated disease following vaccination with the live attenuated Bacille Calmette Guerin (BCG) strain, which is derived from Mycobacterium bovis (Movahedi et al., 2003; Reichenbach et al., 2001). BCG lacks the RD1 locus, and we find that granulomas formed by RD1-deficient bacteria recruit fewer neutrophils, presumably because they contain fewer dead macrophages. Given the convincing role of an intact phagosomal oxidase in human BCG infection, we speculate that even the fewer neutrophils in RD1 mutant granulomas are capable of clinically significant mycobacterial killing.
How do our findings relate to the published findings on neutrophils in TB? While recent studies have focused on the pathological role caused by excessive neutrophil accumulation in late disease (Desvignes and Ernst, 2009; Nandi and Behar, 2011), some older work supports our finding of an early protective role for them (Martineau et al., 2007; Pedrosa et al., 2000). Several in vitro and in vivo models have been proposed for the protective mechanism of neutrophils during mycobacterial infection. Collectively, these models attribute the beneficial nature of neutrophils to their indirect enhancement of macrophage microbicidal activity, either via cytokine production or by macrophages acquiring neutrophilic granules upon phagocytosis of apoptotic neutrophils (Silva et al., 1989; Tan et al., 2006). These models are based on indirect or in vitro evidence, and our observations do not rule them out as additional mechanisms of protection by neutrophils. Importantly, our results suggest direct oxidative killing as a substantial protective mechanism.
Then, contrary to our findings, direct phagocytosis of mycobacteria by mammalian neutrophils has been reported. Many of these studies used isolated neutrophils (Majeed et al., 1998; Persson et al., 2008), or animals with high infection doses that may not represent the low bacterial numbers thought to result in human infection (Abadie et al., 2005). In the zebrafish too, we found that very high inocula (>250 bacteria per animal) did produce a low level of direct neutrophil infection, <5% of the total infected phagocytes at the initial infection site. Conversely, with low infection doses in mice, the earliest infected neutrophils were observed only at 2 weeks postinfection (Pedrosa et al., 2000; Tsai et al., 2006). Importantly, our data are in accordance with extensive observations of tuberculous lesions in the human lung, where neutrophils were seldom found to be directly infected, except after what appeared to be an unusually high inoculum that produced tuberculous bronchopneumonia (Canetti, 1955).
Our findings place mycobacteria among the group of notorious pathogens that cause severe systemic disease, particularly meningitis, by evading neutrophil phagocytosis at least partially (Tunkel and Scheld, 1993). These organisms often use their capsule or the functional capsular equivalents in their cell wall to avoid phagocytosis, and mycobacterium may similarly employ one or multiple components of its complex cell wall (Brennan, 2003).
Finally, our data suggest a role for neutrophils as macrophage scavengers, a reversal of the well-recognized arrangement in which macrophages act as scavengers that efficiently remove apoptotic neutrophils and cell debris to resolve inflammation (Amulic et al., 2012; Mosser and Edwards, 2008; Serhan, 2007). Previous studies in a lipopolysaccharide-induced lung inflammation model in the mouse have reported a similar scavenger behavior of neutrophils and proposed it as a backup mechanism when the scavenging capacity of macrophages is insufficient (Rydell-Tormanen et al., 2006). While our data suggest that caspase-mediated cell death recruits neutrophils, the precise viability status of the cells phagocytosed by neutrophils awaits deeper examination.
A teleological consideration of our findings may serve as a useful perspective on the host-mycobacterium interface. Mycobacteria may have evolved to avoid neutrophils while possessing multiple mechanisms to gain macrophage entry (Ernst, 1998). The macrophage may provide mycobacteria a safe haven, sheltering them from other immune cells, while transporting them into deeper tissues, potentially as a means to minimize competition with mucosal organisms (Clay et al., 2007). Once within the host tissues, mycobacteria additionally benefit from inducing macrophage death and recruitment to promote granuloma expansion through phagocytosis of dead macrophages by multiple newly arriving ones (Davis and Ramakrishnan, 2009). However, macrophage death—the very mechanism mycobacteria use to promote their expansion in granulomas—also causes neutrophil recruitment that results in mycobacterial killing. Identification of mycobacterial neutrophil evasion strategies should be of interest and may be useful in the context of vaccination strategies. Moreover, in terms of therapeutic strategies, further investigation is warranted into how the dichotomous mycobactericidal capacity of granuloma neutrophils can be tipped to favor the host.
EXPERIMENTAL PROCEDURES
Zebrafish and Bacterial Strains
The WT zebrafish AB line from the Zebrafish International Resource Center (ZIRC), along with transgenic lines Tg(lyz:EFGP)nz117, Tg(lyz:DsRED2)nz50, Tg(mpx:GFP)i114, and Tg(-8mpx:cxcr4.1-EGFP)uwm3 (Hall et al., 2007; Renshaw et al., 2006; Walters et al., 2010) were maintained, infected, and imaged as described (Cosma et al., 2006b). Fluorescent derivatives of Mm strain M (ATCC #BAA-535) and mutants derived from it were used (Clay et al., 2008; Cosma et al., 2006b). Pa PAO1 strain and the fluorescent derivatives used have been previously described (Brannon et al., 2009). Pa burdens were determined by plating lysed larvae for colony-forming units (CFUs), and Mm burdens were determined by bacterial fluorescent pixel counts (FPCs) in live animals (Adams et al., 2011). All zebrafish husbandry and experiments were conducted in accordance with the University of Washington Institutional Animal Care and Use Committee regulation.
Quantification of Phagocyte Recruitment and Phagocytosis of Bacteria
IL-8 (15–25 pg) (rhIL-8, R&D Systems, Inc.), 5–15 pg of LTB4 (Cayman Chemicals), 10–15 nl of 0.2 μM fMLP (Sigma), and/or specified bacterial doses were injected into the HBV of 44–52 hpf larvae. Six hours after injection, the identity and quantity of phagocytes and their infection status per larvae were determined using DIC and fluorescence microscopy on Nikon’s Eclipse E600. Objectives used in this assay included 20× Plan Fluor 0.5 NA and 40× Plan Fluor 0.75 NA (Davis and Ramakrishnan, 2009).
Opsonization of Bacteria
A mixture of Mm and Pa was incubated at room temperature for 20 min in 70% FBS (heat inactivated at 56°C for 30 min or not) or in 70% adult zebrafish serum. Zebrafish adult serum was obtained using a protocol modified from MacCarthy et al. (2008) as follows: blood was collected from the aorta of 8- to 12-month old adults using a capillary tube, transferred to a glass tube, allowed to clot at room temperature for 2 hr, and then incubated at 4°C for 4 hr to contract the clot. Serum was collected by centrifugation.
Morpholino Injection
Splice-blocking morpholinos purchased from Gene Tools, LLC (Eugene, OR, USA), were injected into one to eight cell stage embryos and the efficacy of gene knockdown confirmed by reverse transcription polymerase chain reaction (RT-PCR) (Nasevicius, 2000 #214). Morpholino concentrations that achieved maximal gene knockdown with low toxicity were chosen. The fpr1 (XM_003200188) morpholino sequence is 5′-GATCCATCATTGTACCTTCAAACCT-3′. Morpholinos targeting zebrafish gp91phox and gp22phox were used as described (Niethammer et al., 2009).
Whole-Mount In Situ Hybridization and Antibody Staining
WISH with the fms probe was combined with fluorescence detection of GFP with an anti-GFP antibody as described (Clay and Ramakrishnan, 2005; Clay et al., 2008).
Analysis of Cellular Dynamics of Neutrophils in Granulomas
For single time point static assessment of neutrophil recruitment to, and infection in, granulomas, lyz-green larvae infected with red fluorescent Mm were imbedded in 1% agarose (low melting point) for imaging (Davis and Ramakrishnan, 2009). A series of z stack images with a 2.5 μm step size was generated of the granuloma, using the galvo scanner (laser scanner) of the Nikon A1 confocal microscope with a 20× Plan Apo 0.75 NA objective. The size of the given granuloma was evaluated by its bacterial burden determined by the 3D surface rendering feature of Imaris (Bitplane Scientific Software).
Cellular dynamics of neutrophils in granulomas were monitored by time-lapse confocal imaging. A series of z stack images with a 2.5 μm step size was taken at 3–5 min intervals over 5–8 hr using the high-speed scanner (resonant scanner) with a 20× Plan Apo 0.75 NA objective. Neutrophils were tracked using the 3D-tracking feature and their bacterial burdens determined using the 3D surface rendering feature, both in Imaris.
Q-VD-OPH Treatment and TUNEL Staining
Larvae were incubated for 24 hr in 50 μM of Q-VD-OPH (R&D Systems, Inc.) dissolved in DMSO or the corresponding concentration of 0.5% DMSO as a control (Walters et al., 2009), after which TUNEL staining was performed as described (Volkman et al., 2004).
Statistics
Statistical analyses were performed using Prism 5.01 (GraphPad).
Supplementary Material
Acknowledgments
We thank A. Huttenlocher for suggesting and providing the WHIM fish, K. Winglee for initiating observations of neutrophils, D. Beery for Movie S1A, H. Volkman for suggesting the Q-VD-OPH reagent, J. Cameron for fish husbandry, C. Cosma and P. Edelstein for advice on statistical analyses, and D. Tobin and F. Chu for manuscript review. This work was supported by a postdoctoral fellowship from the Taiwan National Science Council (NSC97-2917-I-564-109) (C.-T.Y.), a National Science Foundation predoctoral fellowship (C.J.C.), an American Society for Engineering Education predoctoral fellowship (J.M.D.), and National Institute of Health R01 grants A154503 and A136396 (L.R.). L.R. is a recipient of the NIH Director’s Pioneer Award.
Footnotes
ACCESSION NUMBERS
The GenBank accession number for the fpr1 sequence reported in this paper is XM_003200188.
Supplemental Information includes five figures and seven movies and can be found with this article at http://dx.doi.org/10.1016/j.chom.2012.07.009.
References
- Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon MK, Davis JM, Mathias JR, Hall CJ, Emerson JC, Crosier PS, Huttenlocher A, Ramakrishnan L, Moskowitz SM. Pseudomonas aeruginosa Type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell Microbiol. 2009;11:755–768. doi: 10.1111/j.1462-5822.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2003;83:91–97. doi: 10.1016/s1472-9792(02)00089-6. [DOI] [PubMed] [Google Scholar]
- Brown AE, Holzer TJ, Andersen BR. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156:985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- Canetti G. The Tubercle Bacillus in the Pulmonary Lesion of Man; Histobacteriology and Its Bearing on the Therapy of Pulmonary Tuberculosis. New York: Springer; 1955. [Google Scholar]
- Cheung DO, Halsey K, Speert DP. Role of pulmonary alveolar macrophages in defense of the lung against Pseudomonas aeruginosa. Infect Immun. 2000;68:4585–4592. doi: 10.1128/iai.68.8.4585-4592.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Ramakrishnan L. Multiplex fluorescent in situ hybridization in zebrafish embryos using tyramide signal amplification. Zebrafish. 2005;2:105–111. doi: 10.1089/zeb.2005.2.105. [DOI] [PubMed] [Google Scholar]
- Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corleis B, Korbel D, Wilson R, Bylund J, Chee R, Schaible UE. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell Microbiol. 2012;14:1109–1121. doi: 10.1111/j.1462-5822.2012.01783.x. [DOI] [PubMed] [Google Scholar]
- Cosma CL, Klein K, Kim R, Beery D, Ramakrishnan L. Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect Immun. 2006a;74:3125–3133. doi: 10.1128/IAI.02061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma CL, Swaim LE, Volkman H, Ramakrishnan L, Davis JM. Zebrafish and frog models of Mycobacterium marinum infection. Curr Protoc Microbiol. 2006b;Chapter 10(Unit 10B):12. doi: 10.1002/0471729256.mc10b02s3. [DOI] [PubMed] [Google Scholar]
- Crooks SW, Stockley RA. Leukotriene B4. Int J Biochem Cell Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- Dannenberg AM., Jr Immunopathogenesis of pulmonary tuberculosis. Hosp Pract (Off Ed) 1993;28:51–58. doi: 10.1080/21548331.1993.11442738. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693–702. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- Derrick SC, Morris SL. The ESAT6 protein of Mycobacterium tuberculosis induces apoptosis of macrophages by activating caspase expression. Cell Microbiol. 2007;9:1547–1555. doi: 10.1111/j.1462-5822.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogru D, Kiper N, Ozcelik U, Yalcin E, Tezcan I. Tuberculosis in children with congenital immunodeficiency syndromes. Tuberk Toraks. 2010;58:59–63. [PubMed] [Google Scholar]
- Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, Apt AS. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73:1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., 3rd Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebra-fish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- Jones GS, Amirault HJ, Andersen BR. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162:700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002;70:4591–4599. doi: 10.1128/IAI.70.8.4591-4599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Chan KW, Jiang L, Chen T, Li C, Lee TL, Mak PH, Fok SF, Yang X, Lau YL. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis J. 2008;27:224–230. doi: 10.1097/INF.0b013e31815b494c. [DOI] [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, Mordelet E, Zapata A, Shinomiya H, Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- Liu F, Wen Z. Cloning and expression pattern of the lysozyme C gene in zebrafish. Mech Dev. 2002;113:69–72. doi: 10.1016/s0925-4773(01)00658-x. [DOI] [PubMed] [Google Scholar]
- Lowe DM, Redford PS, Wilkinson RJ, O’Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2011;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- MacCarthy EM, Burns I, Irnazarow I, Polwart A, Greenhough TJ, Shrive AK, Hoole D. Serum CRP-like protein profile in common carp Cyprinus carpio challenged with Aeromonas hydrophila and Escherichia coli lipopolysaccharide. Dev Comp Immunol. 2008;32:1281–1289. doi: 10.1016/j.dci.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Majeed M, Perskvist N, Ernst JD, Orselius K, Stendahl O. Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain of Mycobacterium tuberculosis in human neutrophils. Microb Pathog. 1998;24:309–320. doi: 10.1006/mpat.1997.0200. [DOI] [PubMed] [Google Scholar]
- Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi M, Aghamohammadi A, Farhoudi A, Moin M, Pourpak Z, Gharagozlou M, Mansouri D, Babaei Jandaghi A, Shahnavaz N, Rezaei N, et al. Respiratory manifestations of chronic granulomatous disease; a clinical survey of patients from Iranian primary immunodeficiency registry. Iran J Allergy Asthma Immunol. 2003;2:45–51. [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Nandi B, Behar SM. Regulation of neutrophils by interferon-gamma limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson YA, Blomgran-Julinder R, Rahman S, Zheng L, Stendahl O. Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect. 2008;10:233–240. doi: 10.1016/j.micinf.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Reichenbach J, Rosenzweig S, Doffinger R, Dupuis S, Holland SM, Casanova JL. Mycobacterial diseases in primary immunodeficiencies. Curr Opin Allergy Clin Immunol. 2001;1:503–511. doi: 10.1097/00130832-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DM, Elworthy S, Ingham PW, Whyte MK. A transgenic zebrafish model of neutrophilic inflammation. Blood. 2006;108:3976–3978. doi: 10.1182/blood-2006-05-024075. [DOI] [PubMed] [Google Scholar]
- Reyes-Ruvalcaba D, Gonzalez-Cortes C, Rivero-Lezcano OM. Human phagocytes lack the ability to kill Mycobacterium gordonae, a non-pathogenic mycobacteria. Immunol Lett. 2008;116:72–78. doi: 10.1016/j.imlet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Rydell-Tormanen K, Uller L, Erjefalt JS. Neutrophil cannibalism—a back up when the macrophage clearance system is insufficient. Respir Res. 2006;7:143. doi: 10.1186/1465-9921-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E, Corcoran BA, Wahl SM. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci USA. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Silva MT, Silva MN, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989;6:369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Speert DP, Bond M, Woodman RC, Curnutte JT. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J Infect Dis. 1994;170:1524–1531. doi: 10.1093/infdis/170.6.1524. [DOI] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- Sugawara I, Udagawa T, Yamada H. Rat neutrophils prevent the development of tuberculosis. Infect Immun. 2004;72:1804–1806. doi: 10.1128/IAI.72.3.1804-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- Thwaites GE, Chau TT, Stepniewska K, Phu NH, Chuong LV, Sinh DX, White NJ, Parry CM, Farrar JJ. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet. 2002;360:1287–1292. doi: 10.1016/s0140-6736(02)11318-3. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Vary JC, Jr, Ray JP, Walsh GS, Dunstan SJ, Bang ND, Hagge DA, Khadge S, King MC, Hawn TR, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell. 2010;140:717–730. doi: 10.1016/j.cell.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, Ko DC, Zou Y, Bang ND, Chau TT, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, Tufariello J, Flynn J, Chan J. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8:218–232. doi: 10.1111/j.1462-5822.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- Tunkel AR, Scheld WM. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman HE, Clay H, Beery D, Chang JC, Sherman DR, Ramakrishnan L. Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2004;2:e367. doi: 10.1371/journal.pbio.0020367. http://dx.doi.org/10.1371/journal.pbio.0020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KB, Dodd ME, Mathias JR, Gallagher AJ, Bennin DA, Rhodes J, Kanki JP, Look AT, Grinblat Y, Huttenlocher A. Muscle degeneration and leukocyte infiltration caused by mutation of zebrafish Fad24. Dev Dyn. 2009;238:86–99. doi: 10.1002/dvdy.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LT, Snyderman R, Pike MC, Lefkowitz RJ. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci USA. 1977;74:1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.