Abstract
The present study examined the impact of cognitive reserve in maintaining intact neuropsychological (NP) function among older HIV-positive individuals, a uniquely at-risk subgroup. Participants included 129 individuals classified by HIV serostatus, age group, and NP impairment. A three-way analysis of variance (ANOVA) followed by a series of within-group ANOVA and multiple regression analyses were conducted to investigate the pattern of cognitive reserve (vs. other protective) influence among groups with varying risks of NP impairment. Results indicated a significant age ×HIV status interaction, with older HIV-positive individuals demonstrating higher cognitive reserve than subgroups with less risk for NP compromise (younger age and/or HIV-negative). Results demonstrated higher cognitive reserve specific to NP-intact older HIV-positive individuals. Within this group, the interaction of younger age and higher cognitive reserve independently contributed to cognitive status when controlling for psychiatric, immunological, and psychosocial protective mechanisms, suggesting the importance of cognitive reserve beyond other protective mechanisms in maintaining optimal NP functioning in those individuals most at risk. Alongside younger age, factors contributing to cognitive reserve (i.e., education and estimated premorbid intelligence) may provide substantial benefit for older HIV-positive adults who are at high risk for NP compromise.
Keywords: aging, cognitive reserve, HIV, neuropsychologically intact, protective factors
A number of studies have demonstrated that factors such as education, estimated premorbid intellectual function, occupational complexity, and intracranial volume (collectively, “cerebral reserve”), may increase the threshold for neuropsychological (NP) dysfunction in the presence of cognitive risk factors such as advancing age and neurological disease (Andel, Vigen, Mack, Clark, & Gatz, 2006; Richards & Deary, 2005; Roe, Xiong, Miller, & Morris, 2007; Satz et al., 1993; Staff, Murray, Deary, & Whalley, 2004; Stern et al., 2005; Whalley, Deary, Appleton, & Starr, 2004). These cognitive reserve factors can be differentiated into passive and active variants (Richards & Deary), with the passive form (i.e., “brain reserve”) reflecting differences in physiological aspects of the brain, such as brain weight and neuroplasticity, and the active form (i.e., “cognitive reserve”) reflecting differences in patterns of brain functioning related to educational and occupational attainment and complexity. The synergistic effects of active cognitive reserve and passive brain reserve may explain the potential for preserved NP function in some individuals or groups despite the presence of neuropathology (Christensen et al., 2007; Richards & Deary; Roe et al.; Staff et al.; Vance & Struzick, 2007; Whalley et al.). Cognitive reserve in particular has been posited to serve as a protective factor with the function of preserving NP skills in the face of nonpathological, “normal” effects of aging (Richards & Deary; Staff et al.; Whalley et al.).
The study of cerebral reserve has also been applied to neurodegenerative disorders of aging (Andel et al., 2006; Roe et al., 2007), particularly Alzheimer’s disease (AD). These studies have offered evidence that individuals with greater cognitive reserve may be better able to compensate for NP deficits resulting from the neuropathological effects of neurodegeneration.
Recent investigation has also begun to consider the effects of cognitive reserve with respect to NP decline in patients with HIV/AIDS, because a high proportion (approximately 50%) of HIV-positive individuals will experience some form of NP decline during the course of their illness (Bloom & Rausch, 1997). However, some individuals appear to demonstrate NP resiliency in the face of HIV infection, suggesting that the role of cognitive reserve in maintaining NP function may also extend to HIV-related neurodegeneration (Basso & Bornstein, 2000; Satz et al., 1993; Stern, Silva, Chaisson, & Evans, 1996).
Cognitive reserve most simply incorporates innate intelligence alongside enriching life experiences (Stern, 2009), and thus, state-of-the-art operational requirements for evaluating cognitive reserve have entailed psychosocial factors that reflect engagement in or exposure to cognitively stimulating activity throughout the lifespan and that include simple word reading and educational attainment in years (Brickman et al., 2009). However, a uniform method for evaluating level of cognitive reserve fails to exist, and therefore, studies examining cognitive reserve often utilize somewhat discrepant definitions of proxies for cognitive reserve (e.g., education and occupational attainment [Stern et al., 1996], education only [Satz et al., 1993], estimated premorbid intellectual functioning [Basso & Bornstein, 2000], word reading and educational attainment [Brickman et al.]), making cross-study comparisons or a concrete understanding of the fundamental nature of cognitive reserve somewhat more challenging. More recent examinations (Brickman et al.), however, have begun to utilize the combined proxies of education and word reading to better capture the phenomenon of cognitive reserve while reducing Type I error.
In a study examining NP performance among HIV-positive subjects, results showed that a group with low cognitive reserve scores (based upon education and occupational attainment) demonstrated poorer executive function, attention, information processing speed, and verbal learning and memory (Stern et al., 1996). Other work has similarly demonstrated that HIV-positive individuals with a high school education or less appear to have nearly 3 times the NP impairment of HIV-positive individuals with higher education. These findings were evident even when controlling for the effects of age, ethnicity, CD4 count, and psychiatric/substance abuse (Satz et al., 1993). Another study (Pereda et al., 2000) similarly indicated that HIV-positive subjects with low cognitive reserve performed more poorly on tests of attention, psychomotor abilities, memory, motor function, and verbal fluency than both HIV-positive individuals with high cerebral reserve and HIV-negative control subjects with low cerebral reserve. HIV-positive individuals with high cognitive reserve performed similarly to HIV-negative groups with both high and low cognitive reserve in virtually all areas of function. This phenomenon appears to be consistent over time (e.g., results supported in longitudinal work; Basso & Bornstein, 2000) and irrespective of HIV disease stage (late stage, Basso & Bornstein; early stage, Pereda et al.). In particular, one study (Pereda et al.) examined the unique subgroup of early-stage HIV-positive individuals and found that low reserve capacity in conjunction with other factors, such as lack of treatment and older age, results in a lower threshold for executive dysfunction. Basso and Bornstein also supported the role of cognitive reserve as a protective mechanism for executive function during later stages of illness, at which time dysfunction becomes more pronounced. In this study, subjects with above-average premorbid ability, irrespective of their disease status, performed consistently with, or better than, their performance 12 months prior. In contrast, subjects with only average levels of cognitive reserve (reflected by premorbid ability) exhibited a decline in performance that correlated with the severity of illness.
Research has not yet begun to address the impact of cognitive reserve in older HIV-positive individuals, although it has been documented that this subgroup may be at particular risk for NP compromise (Goodkin et al., 2001; Hinkin et al., 2004; Valcour, Shikuma, Watters, & Sacktor, 2004) when compared with their younger counterparts, particularly in the areas of executive functioning, verbal and visual memory, and motor speed (Sacktor et al., 2007). It is well established that clinical outcome is worse in older HIV-positive adults relative to younger patients, because older HIV-positive individuals demonstrate a shortened time between HIV infection and AIDS diagnosis, a higher mortality rate following an AIDS diagnosis, and a briefer latency between AIDS diagnosis and onset of dementia (Butt et al., 2001). Aging also appears to exacerbate the NP effects of HIV infection, particularly among patients who have progressed to AIDS (Hardy et al., 1999), and the combined effects of older age and NP decline appear to be associated with poorer medication adherence (Hinkin et al., 2004) and other functional capacities. Therefore, in conjunction with the effects of aging, NP decline as a result of HIV can pose a great threat to everyday functioning.
Although these studies provide solid evidence that older HIV-positive individuals are at higher risk for NP impairment, it is interesting to note that some aging HIV-positive individuals fail to demonstrate the expected reduction in cognition (e.g., 13% of older HIV-positive adults in Hardy et al., 1999; and 12% of older HIV-positive adults in Valcour et al., 2004). Therefore, research is clearly needed to investigate the contributions of reserve capacity to the maintenance of NP function in later-life HIV.
The aforementioned investigations have demonstrated the benefit of high reserve capacity on NP function following the onset and progression of neurologic insults including HIV infection. However, we found no studies to date with a primary focus upon the unique subgroup of older HIV-positive individuals. The present study seeks to identify whether cognitive reserve raises the threshold for NP dysfunction among individuals who might be expected to be susceptible to compromise but who do not show notable impairment (i.e., HIV-positive individuals of advancing age). This group is of special interest due to the “double assault” caused by the impact of both neurologic processes (HIV infection and advanced age) leading to increased risk for NP decline. In addition, the independent effects of cognitive reserve relative to other protective factors among this unique subgroup (e.g., HIV biomarker levels, psychiatric function, age, and psychosocial status) have yet to be addressed. In the current study, we hypothesized that the level of cognitive reserve for older HIV-positive individuals who have not experienced NP decline will be greater than that for other groups demonstrating fewer neurologic risks. We further hypothesized that among NP-intact older HIV-positive adults, the contribution of cognitive reserve will outweigh other protective factors described above.
METHOD
Participants
Participants included 129 adults who were recruited from community agencies and medical centers within the Los Angeles area. For the total sample, 102 participants were HIV-positive and 27 were HIV-negative controls. For the HIV-positive group, mean age was 49.8 years (SD = 10.0) and mean years of education was 13.5 years (SD = 2.3). Males comprised 81.4% of the HIV-positive sample, and most were represented by African American (45.6%), Hispanic (15.5%), and Caucasian (33.0%) ethnic groups. History of opportunistic infection was present in 43.1% of the HIV-positive sample, and 71.4% of the sample met criteria for AIDS at the time of the study based upon history of an opportunistic infection or CD4 count less than 200. The mean log 10 CD4 count was 2.61 (SD = 0.29), mean Ln viral load was 5.9 (SD = 2.3), and 30.3% of HIV-positive subjects demonstrated a detectable viral load. For the HIV-negative control group, the mean age was 45.8 years (SD = 15.6) and the mean education level was 12.7 years (SD = 2.0). Males comprised 48.1% of the HIV-negative control sample, and most were represented by African American (59.3%) and Caucasian (18.5%) ethnic groups.
Data concerning demographic and cognitive descriptive statistics for the HIV status groups, age groups, and NP impairment groups are included in Table 1. For the age-group comparisons, significant differences were found for age (p <.01, older >younger) and education (p = .03, older >younger). There were no differences in NP ability between the age groups. For the NP impairment group comparisons, there were significant differences in education (p = .03, NP intact >NP impaired) and for all seven cognitive domains (p ≤.01), including language, attention and working memory, processing speed, executive function, learning, memory, and motor function. For the HIV-status group comparisons, there were differences in percentage of males (p <.01, HIV positive >HIV negative) and for the motor function domain (p = .04, HIV positive >HIV negative) on NP comparisons. No additional group differences were found.
TABLE 1.
Descriptive Statistics per Group
|
Age Group
|
F/χ2 | p |
Impairment Group
|
F/χ2 | p |
HIV Status
|
F/χ2 | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Y | U | I | + | − | |||||||
| N = 94 | N = 35 | N = 96 | N = 33 | N = 102 | N = 27 | |||||||
| Demographic | ||||||||||||
| Age | 55.0 | 32.6 | 402.14 | <.01 | 48.7 | 48.7 | 0.00 | 1.00 | 49.8 | 45.8 | 2.72 | .10 |
| Education | 13.6 | 12.7 | 4.69 | .03 | 13.6 | 12.6 | 5.16 | .03 | 13.5 | 12.7 | 2.856 | .09 |
| Male (%) | 78.9 | 62.9 | 5.42 | .07 | 78.1 | 65.6 | 4.37 | .11 | 81.4 | 48.1 | 13.73 | <.01 |
| Ethnicity (%) | 10.51 | .06 | 2.64 | .76 | 5.29 | .38 | ||||||
| Caucasian | 22.9 | 32.6 | 28.1 | 34.4 | 33.0 | 18.5 | ||||||
| AA | 42.9 | 50.5 | 51.0 | 43.8 | 45.6 | 59.3 | ||||||
| Hispanic | 20.0 | 12.6 | 12.5 | 18.8 | 15.5 | 11.1 | ||||||
| Other | 14.3 | 4.3 | 8.4 | 3.1 | 5.8 | 11.1 | ||||||
| Cognitive | ||||||||||||
| Attention | 47.0 | 45.5 | 0.56 | .46 | 48.8 | 38.8 | 32.68 | <.01 | 47.0 | 45.0 | 1.00 | .32 |
| Processing Sp | 45.2 | 47.2 | 2.30 | .13 | 47.3 | 40.7 | 28.69 | <.01 | 46.1 | 44.1 | 2.11 | .15 |
| Language | 47.8 | 47.1 | 0.15 | .70 | 49.8 | 41.2 | 27.82 | <.01 | 47.8 | 47.3 | 0.07 | .80 |
| Learning | 39.9 | 40.8 | 0.17 | .68 | 43.7 | 31.6 | 45.28 | <.01 | 40.6 | 36.8 | 1.56 | .22 |
| Memory | 38.0 | 43.0 | 2.54 | .12 | 45.2 | 25.9 | 64.17 | <.01 | 40.0 | 37.1 | 0.41 | .52 |
| Executive | 46.6 | 47.6 | 0.47 | .49 | 48.5 | 41.7 | 21.38 | <.01 | 47.5 | 44.5 | 3.28 | .07 |
| Motor | 42.9 | 44.0 | 0.32 | .57 | 44.9 | 38.5 | 11.67 | <.01 | 44.1 | 40.0 | 4.13 | .04 |
| Global | 44.5 | 45.3 | 0.29 | .59 | 47.3 | 37.8 | 77.69 | <.01 | 44.9 | 43.2 | 0.69 | .41 |
p ≤.05.
p ≤.01.
p ≤.001.
OI = Opportunistic Infection; AIDS diagnosis = based upon OI/CD4 <200; AA = African American; I = Impaired; U = Intact; O = Older; Y = Younger; += HIV positive; – = HIV negative.
Procedure
All participants completed comprehensive NP test batteries administered by trained psychometrists and supervised by a board-certified neuropsychologist (CHH).
Age
Age was coded dichotomously (younger, <40 years old; older ≥50 years old) for analysis of variance (ANOVA) tests. Individuals between the ages of 40 and 50 years old were excluded from the study to allow for greater differentiation between the age groups. For the HIV-positive participants, there were 25 individuals in the younger group and 77 individuals in the older group. For the HIV-negative controls, there were 10 individuals in the younger group and 17 individuals in the older group.
Cognitive reserve composite
A cognitive reserve composite was created that consisted of an averaged score of years of formal education and word-reading ability on the Wide Range Achievement Test-Third Edition, Reading subtest (Wilkinson, 1993). Although studies on cognitive reserve have employed varying measurements for examining this phenomenon, combining simple word reading with educational achievement appears to be the most widely used, robust, and state-of-the-art method and has been most commonly employed in recent studies (Brickman et al., 2009; Rentz et al., 2010) because it incorporates both elements (i.e., psychosocial exposures measured by educational attainment and intrinsic intelligence measured by simple word reading) that have been demonstrated to be most critical for the assessment of cognitive reserve (Stern, 2009). Each value was normalized to the total study sample using the following formula to create a consistent reference scale for equal weighting of number of years of education and word-reading ability: ([subject raw score − sample mean]/standard deviation ×15 +100), i.e., RS − M/SD × 15 +100. These sample-standardized values were then averaged into a single combined composite score equally reflecting educational attainment and word-reading ability based upon M = 100 (SD = 15). Using a single cognitive reserve composite score including equal weights of education and word reading allowed us to reduce Type I error and increase the robustness of the cognitive reserve measurement.
NP assessment
We assessed seven domains of NP function, including language, attention and working memory, processing speed, executive function, learning, memory, and motor function. See Table 2 for NP tests comprising each domain.
TABLE 2.
Cognitive Tests Administered
| Cognitive Domain | Tests Administered | Normative Source |
|---|---|---|
| Language | ||
| Controlled Oral Word Association Test (FAS and Animal Naming) | Heaton et al. (1991) | |
| Boston Naming Test | Heaton et al. (1991) | |
| Executive Functioning | ||
| Trail-Making Test (Part B) | Heaton et al. (1991) | |
| Stroop Color and Word Test (Interference) | Selnes et al. (1991) | |
| Wisconsin Card-Sorting Test (Perseverative Errors) | Kongs et al. (2000) | |
| Processing Speed | ||
| Trail-Making Test (Part A) | Heaton et al. (1991) | |
| Stroop Color and Word Test (Word and Color) | Selnes et al. (1991) | |
| Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Digit Symbol Coding and Symbol Search) | Wechsler (1997) | |
| Attention and Working Memory | ||
| Paced Auditory Serial Addition Test (Trial 1) | Stuss et al. (1988) | |
| WAIS-III (Letter Number Sequencing) | Wechsler (1997) | |
| Learning | ||
| Hopkins Verbal Learning Test-Revised (Trial 1 & Total Trials 1 through 3) | Brandt & Benedict (1997) | |
| Brief Visual Memory Test-Revised (Trial 1 & Total Trials 1 through 3) | Benedict (1997) | |
| Memory | Hopkins Verbal Learning Test-Revised (Delayed Recall) | Brandt & Benedict (1997) |
| Brief Visual Memory Test-Revised (Delayed Recall) | Benedict (1997) | |
| Fine Motor | ||
| Grooved Pegboard (Dominant and Nondominant Hands) | Heaton et al. (1991) | |
Data transformations and impairment classification for NP scores
For NP impairment classifications only, raw scores from all measures were converted to T scores using published, demographically adjusted norms that included corrections for normal aging and other demographic characteristics relevant to interpretation of performance on each test. Domain scores were calculated by averaging T scores for each test. A global cognition score consisted of averaged performances across each of the seven domains of NP function. Participants were classified as NP impaired if their global cognition score fell below T = 40 and were classified as NP intact if their global cognition score fell at or above T = 40. Unstandardized (rather than demographically corrected) variables were used in primary study analyses to avoid the circularity that would otherwise be associated with equivalent/overlapping NP impairment group classification and study analytic methods (e.g., ANOVA, regression) and to allow for a better appreciation of the varying influences of protective mechanisms on cognition among a distinctly at-risk NP intact group.
However, for secondary regression analyses on cognitive function, scores were normalized to the total sample using: ([raw score − M]/SD) × 15 +100. We did not apply demographic corrections because age was an independent predictor for those subsequent analyses.
Secondary predictors of cognitive functioning
To assess the extent to which cognitive reserve was predictive of overall NP function beyond the effects of other supportive variables, we conducted follow-up analyses using measures of HIV status (CD4 count), psychosocial status, and psychiatric function. We created a continuous psychosocial status variable, which was comprised of several support variables including current living arrangement, relationship status, and employment status, with higher values indicating increased psychosocial support. Measures of psychiatric functioning included the Beck Anxiety Inventory (BAI; Beck & Steer, 1993) and the Beck Depression Inventory-Second Edition (BDI-II; Beck, Steer, & Brown, 1996).
Statistical Analyses
A three-way ANOVA was conducted for age (younger, <40 years old; older, ≥50 years old), HIV status (positive/negative), and NP impairment (presence/absence), with the cognitive reserve composite score as the dependent variable. See Table 1 for demographic descriptive statistics for the study subgroups. Standard and hierarchical multiple regression analyses were conducted for the older NP-intact HIV-positive group to evaluate the independent contributions of cognitive reserve when controlling for other protective mechanisms.
RESULTS
Results of the 3 × 2 ANOVA for age (older vs. younger), HIV status (HIV positive vs. HIV negative), and NP impairment (NP intact vs. NP impaired) groups indicated a significant main effect for NP impairment, F(1,1033) = 7.26, p = .008, with the NP-intact group showing higher levels of cognitive reserve than the NP-impaired group. Main effects for age and HIV status failed to reach statistical significance. Results also indicated a significant two-way interaction effect for age group × HIV status, F(1, 612) = 4.30, p = .04, partial eta2 = .035, power = .539, on cognitive reserve, reflecting higher cognitive reserve in older HIV-positive participants. Due to smaller cell sizes among subgroups leading to insufficient power, the three-way interaction for age group × HIV status × NP impairment group failed to reach significance. Post-hoc pairwise comparisons revealed higher cognitive reserve among older HIV-positive individuals when compared with both older HIV-negative subjects (p = .002) and younger HIV-positive subjects (p = .04).
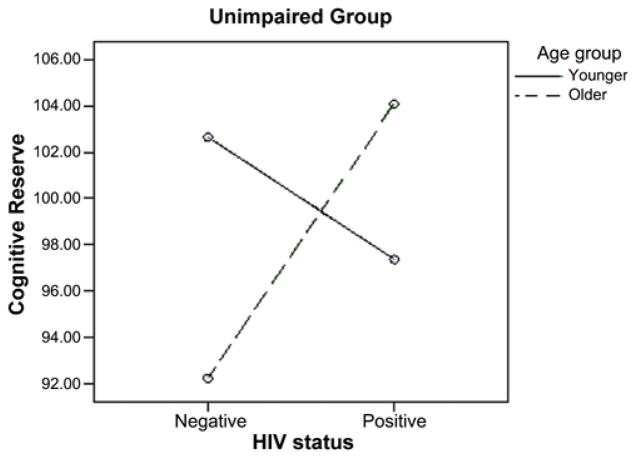
To further explore potential subgroup effects, we examined the age group × HIV-status group interaction effect within each of the NP impairment groups separately. We expected to find significantly higher cognitive reserve among the older HIV-positive NP-intact group when compared with groups characterized by fewer risks for cognitive compromise. As expected, results revealed a significant age group × HIV status interaction for the NP-intact group only, F(1,1077.6) = 8.43, p = .005. Post-hoc pairwise comparisons revealed significantly higher cognitive reserve for the older NP-intact HIV-positive subjects when compared with the older NP-intact HIV-negative subjects (p = .002) and the younger NP-intact HIV-positive subjects (p = .031). See Figure 1 for a graphical illustration of these results.
FIGURE 1.
Interaction of HIV status × age for the intact group. Cognitive reserve = averaged score of years of formal education and word-reading ability on the Wide Range Achievement Test-Third-Edition, Reading subset. Both values were normalized to the total sample to create a consistent reference scale for equal weighting of education and word reading. (Color figure available online.)
To further assess the pattern of group differences and evaluate hypotheses regarding higher levels of cognitive reserve among the at-risk NP-intact individuals, we then conducted a series of follow-up ANOVAs among subgroups characterized by age group, HIV status, or NP impairment level. These analyses were conducted to test whether individuals with a greater number of NP risks and who continue to maintain normal NP functioning have higher levels of cognitive reserve. We hypothesized that given their higher levels of NP function despite risks for decline, the older NP-intact HIV-positive individuals would demonstrate higher levels of cognitive reserve than other groups characterized by lower levels of NP risk.
Results of these analyses provided further support for our hypothesis. These analyses revealed significant differences in the anticipated direction between older HIV-positive NP impairment groups (NP intact >NP impaired, p = .003), NP-intact HIV-positive age groups (older >younger, p = .026), and older NP-intact HIV-status groups (HIV >controls, p = .001) on cognitive reserve. No additional pairwise comparisons reached significance.
Across these comparisons, a pattern emerged, with older HIV-positive individuals demonstrating significantly higher cognitive reserve than other subgroups characterized by lower risk for cognitive decline. These findings are consistent with expectation and suggest higher levels of cognitive reserve among NP-intact individuals at particular risk for compromise. In contrast, significant effects were not revealed for groups with little or no susceptibility to NP compromise, demonstrating the specificity of these results (see Figure 2). In summary, the results from this series of analyses demonstrated that subgroups characterized by risk for NP impairment (older, HIV positive) and those who remain NP intact (versus impaired) demonstrate the highest levels of cognitive reserve capacity. Figure 2 illustrates the global NP capacities of individuals characterized by varying levels of risk for NP impairment (i.e., no risks [younger, HIV-negative status], one risk [i.e., HIV-positive status or older age], or two risks [i.e., HIV-positive status and older age]). As can be seen in Figure 2, the subgroup at greatest risk for NP compromise (i.e., presenting with both risk factors) surprisingly demonstrates NP function of a level that is consistent with the subgroup presenting without risks and that is superior to subgroups presenting with only a single risk factor. This unique finding highlights the importance of cognitive reserve function for individuals who are particularly vulnerable to impairment and further implies that individuals minimally susceptible to NP compromise may receive less benefit from such cognitive protective mechanisms.
FIGURE 2.
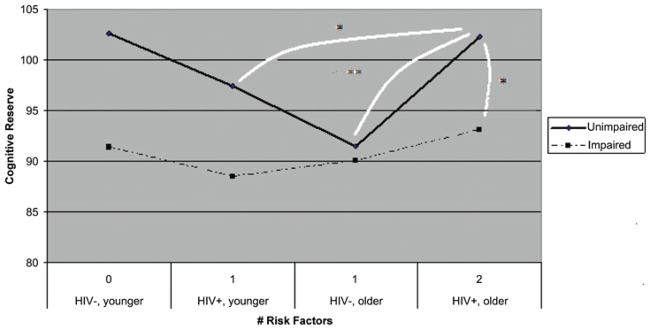
Cognitive reserve by risk level. Subjects with the greatest number of risk factors for cognitive decline but who do not demonstrate notable NP impairment also show the highest level of cognitive reserve. Connective lines indicate the presence of significant group differences. *p <.05. **p <.01. (Color figure available online.)
As a follow-up to the significant findings for the older NP-intact HIV-positive participants, we conducted standard multiple regression analyses for this group on global cognition, while entering cognitive reserve, age, psychosocial status, psychiatric function, and HIV status as predictor variables to determine whether an independent contribution of cognitive reserve exists beyond the beneficial impacts of other protective mechanisms that are believed to positively mediate NP function. Results indicated a significant full model, R-squared = .45, F(6,200.0) = 6.92, p <.001, and significant main effects for cognitive reserve (B = 0.63, t = 5.83, p <.001) and age (B = −0.44, t = −4.05, p < .001). To assess whether an age × cognitive reserve interaction was present, we conducted a hierarchical multiple regression analysis, with the age × cognitive reserve interaction variable entered last. Results indicated a significant full model, R-squared change = .71, F change (1,91) = 365.73, p <.001, with a significant age × cognitive reserve interaction term (B = 1.45, t = 9 2.05, p <.001. Psychosocial, psychiatric, and immunological measures, including BDI-II and BAI scores, psychosocial status, and CD4 count, were not significant predictors of global cognition. These results provide evidence for the relative importance of cognitive reserve over other putative protective variables in sustaining or maintaining sufficiently high levels of NP capacity, with a particularly striking effect of these results for those who are at high risk for NP decline (namely, HIV-positive individuals of advanced age).
DISCUSSION
The present study sought to examine the impact of cognitive reserve versus other protective factors upon the uniquely at-risk subgroup of older HIV-positive individuals. We compared groups subcategorized by HIV status, age, and NP impairment level. We further considered the independent contributions of cognitive reserve within the NP-intact older HIV-positive subgroup when controlling for other protective mechanisms. We hypothesized that cognitive reserve would play a significant role in NP maintenance and that its contribution would be greater than that of HIV biomarkers, psychiatric distress, or psychosocial support.
In accordance with prior investigations, our findings suggested that a significant proportion (n = 57) of older HIV-positive individuals remains NP intact despite being at particular risk for compromise. Although previous work has suggested that the combined effects of HIV and advancing age may significantly compromise NP function (Becker, Lopez, Dew, & Aizenstein, 2004; Hardy et al., 1999), investigation has also documented that some aging HIV-positive individuals fail to demonstrate the expected NP reductions resulting from the combined effects of both neurologic processes (Pereda et al., 2000).
More importantly, our results indicated that these older NP-intact HIV-positive individuals demonstrated significantly higher cognitive reserve than other subgroups characterized by varying levels of risk for NP compromise. Higher levels of cognitive reserve thus appear to protect against NP deterioration and may be especially important in maintaining adequate NP capacity for this particularly susceptible group. While prior HIV work has suggested that NP capability is related to levels of cognitive reserve (Basso & Bornstein, 2000; Pereda et al., 2000; Satz et al., 1993; Stern et al., 1996), our findings extend the existing literature base to suggest a heightened role of reserve capacity among older HIV-positive individuals in particular.
Our results further demonstrated that the subgroup at greatest risk for NP compromise (i.e., presenting with both risk factors) surprisingly demonstrates NP function of a level that was consistent with the subgroup presenting without risks and that was superior to other subgroups presenting with only a single risk factor (i.e., either older age or HIV-positive status). This unique finding highlights the importance of cognitive reserve function for individuals who are particularly susceptible to impairment and further implies that individuals minimally at risk for NP compromise may receive less benefit from such cognitive protective mechanisms. Therefore, it appears that cognitive reserve successfully and preferentially protects individuals affected with the double assault of HIV infection and advanced age.
Results also advance previous findings regarding the role of active (vs. passive) cognitive reserve in preserving neurocognitive function in the face of neurodegeneration (Andel et al., 2006; Roe et al., 2007) and normal aging (Richards & Deary, 2005; Staff et al., 2004; Whalley et al., 2004). In both cases, cognitive reserve capacity has demonstrated benefit for NP function among individuals at risk for decline. AD in particular has been of special interest in this regard. Several studies have offered support that individuals with greater cognitive reserve may be better able to compensate for NP deficits resulting from the neuropathological effects of AD. Even in the presence of significant levels of amyloid beta deposition and neurofibrillary tangle lesions, high levels of formal educational attainment or occupational complexity may attenuate the deleterious impact of neuropathology on cognition (Andel et al.; Roe et al.). Furthermore, in normal aging, prior research suggests that cognitive reserve confers mitigating effects, reducing the typical consequences of aging on NP function (Stern, 2002). These studies offer support that greater cognitive reserve may in fact serve as a defense against normal aging and the neuropathological effects of neurodegenerative diseases. Our findings and those of prior studies therefore suggest that the role of educational attainment and premorbid intellectual function in the maintenance of intact NP function may also extend to the HIV-positive population (Basso & Bornstein, 2000; Stern et al., 1996).
However, in contrast to prior HIV work (Basso & Bornstein, 2000; Pereda et al., 2000), the role of cognitive reserve as a protective factor for AD appears to diminish as the degree of neuropathology worsens, with a more rapid rate of decline becoming apparent following reserve depletion. In our study, opportunistic infections were present in roughly 43% of subjects, and approximately 71% of subjects met criteria for AIDS diagnosis, suggesting that our subjects continued to benefit from cognitive reserve even in spite of greater immunosuppression. Further work employing more vulnerable HIV-positive individuals (i.e., later disease stages, poorer CD4 count and viral load, presence of neuropathological markers) should be conducted to better discern whether the impact of cognitive reserve capacity on this unique neurodegenerative process may attenuate at increasingly compromised disease stages, as has been shown in the AD literature.
Results of this study also extend prior research by suggesting that the contribution of cognitive reserve on NP function among older NP-intact HIV-positive individuals is independent of other effects purported to provide neuroprotection and NP benefit. Previous research has suggested that HIV-positive individuals involved in stable interpersonal relationships and adherent to anti-retroviral medications may demonstrate a slower rate of disease progression and an increase in CD4 cells (Young et al., 2004). Prior work has also shown that various psychological resources, such as positive affect and low depressive symptoms, are inversely associated with HIV-related mortality and time of death (Ickovics et al., 2006). Although these previous studies have offered evidence for advantageous roles of biological, psychosocial, and psychiatric protective factors in bolstering or maintaining optimal NP function, our results did not suggest significant and independent benefit of these factors beyond the effects of cognitive reserve. Our findings therefore support the role of active cognitive reserve over other protective mechanisms and are in line with the work of Staff et al. (2004), who found a beneficial impact of only active reserve (education and occupational complexity) but not the more biologically driven passive reserve (total intracranial volume) for NP function in old age.
Questions have been raised concerning the means by which protective mechanisms of cognitive reserve may contribute to a higher threshold for NP dysfunction. It is commonly believed that proxies for cerebral reserve support maintenance of neuroplasticity in cognitive aging. Although aging is accompanied by relatively high brain tissue volume loss, recent studies (Whalley et al., 2004) attribute the NP effects of brain aging primarily to a reduction in synaptic plasticity. Neuroplasticity allows neuronal connections to be generated, maintained, and repaired, and stimulating cognitive activities, such as education and complex occupational roles, may support these neuroplastic mechanisms. Thus, active cognitive reserve is produced by NP-challenging activities, improving the physiological integrity of the brain and thereby enhancing functional ability (Vance & Struzick, 2007).
Our results also revealed an independent contribution of younger age, as well as a significant interaction for younger age and cognitive reserve, upon cognition within the NP-intact older HIV-positive group only. It is well established that advancing age is associated with poorer NP function among healthy aging adults, but age effects are particularly notable among older individuals with neurodegenerative conditions such as HIV (Becker et al., 2004; McArthur et al., 2003; Valcour et al., 2004). The age effects in our study can be explained by the lack of age corrections for our primary cognitive data; follow-up analyses conducted with demographically corrected normative data expectedly yielded nonsignificant age results. Nevertheless, our findings suggesting that the combined effects of younger age and cognitive reserve capacity contribute to NP capability are well in line with previous work (Pereda et al., 2000), which found that low cognitive reserve capacity in conjunction with other factors including older age result in a lower threshold for executive dysfunction. Current findings also suggest NP benefit among individuals who are particularly susceptible to NP deterioration yet do not demonstrate the expected level of NP impairment. Our findings also suggest that younger age and higher cognitive reserve collectively exert a neuroprotective effect over and above other biological, psychiatric, and psychosocial variables.
Summary
Investigation into the contributions of varying protective mechanisms for this uniquely vulnerable subgroup (i.e., HIV-positive older adults without NP impairment) has become an increasingly important objective. Results of the present study suggest that individuals at particular risk for NP compromise who remain NP intact demonstrate higher levels of cognitive reserve, which acts as a neuroprotective factor in preventing NP deterioration. Our findings also suggest that the combined neuroprotective effects of younger age and higher cognitive reserve are over and above those explained by biological, psychiatric, and psychosocial variables. Because cognitive reserve appears to be NP protective for individuals at risk, it follows that individuals who are NP intact at any cross-sectional slice in time are likely to be those with higher cognitive reserve, and individuals with lower levels of cognitive reserve protection are likely to end up within impaired groups. Future studies including longitudinal tracking of individuals with high versus low cognitive reserve will be necessary to test this relationship more directly. The finding of higher levels of NP function among the most particularly vulnerable individuals (HIV-positive individuals and subjects of advancing age) with adequate levels of cognitive reserve has not previously been demonstrated. Of further interest is that the most at-risk individuals with high cognitive reserve have levels of NP function that are similar to younger HIV-negative individuals and that are significantly superior to all other subgroups with varying risk for NP impairment, suggesting that cognitive reserve successfully and preferentially protects individuals affected with the double assault of HIV infection and advancing age.
Acknowledgments
This study was conducted at the University of California Los Angeles and the West Los Angeles Veteran’s Affairs Medical Center and was supported by VA Merit Review: Aging & HIV/AIDS: Neurocognitive Sequelae and Functional Consequences to CHH, and 5 T32 MH19535: Neuropsychology of HIV/AIDS to CHH.
Contributor Information
Jessica M. Foley, Department of Psychiatry and Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles, California and Department of Psychiatry, VA Boston Healthcare System/Harvard Medical School, Boston, Massachusetts
Mark L. Ettenhofer, Department of Psychiatry and Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior, Los Angeles, California and Department of Medical and Clinical Psychology, Uniformed Services University of the Health Sciences, Bethesda, Maryland
Michelle S. Kim, Department of Psychiatry, West Los Angeles Veteran’s Affairs Medical Center, Los Angeles, California
Nina Behdin, Department of Psychology, UCLA, Los Angeles, California.
Steven A. Castellon, Department of Psychiatry and Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior and Department of Psychiatry, West Los Angeles Veterans’ Affairs Medical Center, Los Angeles, California
Charles H. Hinkin, Department of Psychiatry and Biobehavioral Sciences, UCLA Semel Institute for Neuroscience and Human Behavior and Department of Psychiatry, West Los Angeles Veterans’ Affairs Medical Center, Los Angeles, California
References
- Andel R, Vigen C, Mack WJ, Clark LJ, Gatz M. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. Journal of the International Neuropsychological Society. 2006;12(1):147–152. doi: 10.1017/S1355617706060206. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. Journal of Clinical and Experimental Neuropsychology. 2000;22(2):208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R. Beck Anxiety Inventory manual. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. manual. [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl 1):S11–8. [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test-Revised. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Bloom FE, Rausch DM. HIV in the brain: Pathology and neurobehavioral consequences. Journal of Neurovirology. 1997;3:102–109. doi: 10.3109/13550289709015800. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Brickman AM, Siedlecki KL, Muraskin J, Manly JJ, Luchsinger JA, Yeung LK, Stern Y. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiology of Aging. 2011;32(9):1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AA, Dascomb KK, DeSalvo KB, Bazzano L, Kissinger PJ, Szerlip HM. Human immunodeficiency virus infection in elderly patients. Southern Medical Journal. 2001;94:397–400. [PubMed] [Google Scholar]
- Christensen H, Anstey KJ, Parslow RA, Maller J, Mackinnon A, Sachdev P. The brain reserve hypothesis, brain atrophy, and aging. Gerontology. 2007;53(2):82–95. doi: 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, Eisdorfer C. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. Journal of Clinical Epidemiology. 2001;54(Suppl 1):S35–S43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore L. Age differences and neurocognitive performance in HIV-infected adults. New Zealand Journal of Psychology. 1999;28:94–101. [Google Scholar]
- Heaton R, Grant I, Matthews C. Comprehensive norms for an expanded Halstead-Reitan battery: Demographic corrections, research findings, and clinical implications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M. Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18:S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics JR, Milan S, Boland R, Schoenbaum E, Shuman P, Vlahov D HIV Epidemiology Research Study (HERS) Group. Psychological resources protect health: 5-year survival and immune function among HIV-infected women from four US cities. AIDS. 2006;20(14):1851–1860. doi: 10.1097/01.aids.0000244204.95758.15. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test—64 Card Computerized Version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: An evolving disease. Journal of Neurovirology. 2003;9(2):205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Pereda M, Ayuso-Mateos JL, Gómez del Barrio A, Echevarria S, Farinas MC, García Palomo D, Vásquez-Barquero JL. Factors associated with neuropsychological performance in HIV-seropositive subjects. Psychological Medicine. 2000;30(1):205–217. doi: 10.1017/s0033291799001348. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Deary IJ. A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller P, Morris JC. Education and Alzheimer’s disease without dementia: Support for the cognitive reserve hypothesis. Neurology. 2007;68:223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Skolasky R, Selnes OA, Watters M, Poff P, Shiramizo B, Valcour V. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of Neurovirology. 2007;13:203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- Satz P, Morgenstern H, Miller EN, Selnes OA, McArthur JC, Cohen BA, D’Elia LF. Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the multicenter AIDS Cohort Study (MACS) Journal of Acquired Immunodeficiency Syndromes. 1993;6:503–511. [PubMed] [Google Scholar]
- Selnes OA, Jacobson L, Machado AM, Becker JT, Wesch J, Miller EN, McArthur JC. Normative data for a brief neuropsychological screening battery. Multicenter AIDS Cohort Study. Perception and Motor Skills. 1991;73(2):539–550. doi: 10.2466/pms.1991.73.2.539. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, Evans DL. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Archives of Neurology. 1996;53(2):148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: An extension. Clinical Neuropsychologist. 1988;2(3):246–250. [Google Scholar]
- Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS. 2004;18:79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Struzick TC. Addressing risk factors of cognitive impairment in adults aging with HIV: A social work model. Journal of Gerontological Social Work. 2007;49(4):51–77. doi: 10.1300/J083v49n04_04. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Aging Research Reviews. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Young J, Bucher HC, Battegay M, De Geest S, Spirig R, Flepp M, Vernazza P. Stable partnership and progression to AIDS or death in HIV-infected patients receiving highly active antiretroviral therapy: Swiss HIV cohort study. British Medical Journal. 2004;328:15–20. doi: 10.1136/bmj.328.7430.15. [DOI] [PMC free article] [PubMed] [Google Scholar]