Abstract
Background
Recurrence is a common problem in bladder cancer; this has been attributed to cancer stem cells. In this study, we characterized potential cancer stem cell populations isolated from three cell lines that demonstrate different responses to cisplatin.
Materials and Methods
The ALDEFLUOR® assay was used to isolate cells from TCCSUP, T24, and 5637 cell lines, and these cells were evaluated for their ability to form colonies, differentiate, migrate and invade.
Results
The cell lines demonstrate a spectrum of aldehyde dehydrogenase high (ALDHHigh)populations that correlate with resistance to cisplatin. In the two resistant cell lines, T24 and 5637, the ALDHHigh cells demonstrate increased colony formation, migration, invasion, and ability to differentiate. The resistant T24 and 5637 cell lines may serve as models to investigate alternative therapies for bladder cancer.
Keywords: Bladder cancer, aldehyde dehydrogenase, cisplatin
Recurrence is a major issue in bladder cancer. Approximately 70% of diagnosed bladder carcinomas are non-invasive and often treated with transurethral resection, yet these cases have a 50–70% recurrence rate (1). Invasive cases of bladder cancer are often treated with radical cystectomy, and there is a 30% recurrence rate in these patients (2). Neoadjuvant and adjuvant chemotherapy with agents, including cisplatin, is sometimes used with cystectomy, however, the extent to which the addition of chemotherapy improves survival is debated (1). Additionally, in cases where metastasis is present at diagnosis, responses to chemotherapy agents, including cisplatin, are not durable and recurrence occurs in the majority of these patients (3). This high rate of recurrence requires thorough follow-up care and lifetime surveillance. The cost of this surveillance, along with the cost of treating recurrences, makes bladder cancer the malignancy where the most lifetime dollars per patient are spent (4).
Current research suggests that resistance to commonly used chemotherapy agents may be due to a population of cells within a tumor, referred to as cancer stem cells (CSCs). The persistence of these CSCs after radical surgery or chemotherapy may help to explain the high rates of recurrence of the disease (5). CSCs have been defined as a small subset of cells within a tumor that possess the property of self-renewal and can give rise to the heterogeneous lineages of cancer cells that comprise a tumor (6).
A variety of surface markers have been proposed for use in the isolation of CSCs, however, there is controversy over the effectiveness of these surface markers for stem cell identification (6). Another proposed method to isolate CSCs is the functional assay, ALDEFLUOR®, that measures the ability of CSCs to evade cytotoxic insults with an enzyme-based detoxification system (7). This enzyme, aldehyde dehydrogenase (ALDH), is a member of the NAD(P)+ family and is involved in the detoxification of a wide variety of aldehydes (8). Hematopoietic stem cells express high levels of ALDH, also the enzyme required for differentiation through conversion of retinol to retinoic acid (9). Chemoresistance has been attributed to ALDH activity, and the ALDEFLUOR® assay has been used to isolate the CSC population from tumors of several types of cancer (10–16).
The overall aim of this study was to investigate the cell populations isolated by the ALDEFLUOR® assay in three invasive bladder cancer cell lines, TCCSUP, 5637, and T24, which have a spectrum of responses to the commonly used chemotherapy agent, cisplatin. Vinall et al. showed TCCSUP cells respond to cisplatin, while T24 and 5637 cells possess increased levels of resistance to cisplatin, respectively (17). This study sought to determine if ALDH is a marker for CSCs in bladder cancer and explores the potential of these cell lines to serve as models for studying CSCs in bladder cancer.
Materials and Methods
Cell culture
T24, TCCSUP and 5637 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA).
ALDEFLUOR® assay and cell sorting
The ALDEFLUOR® assay (Stemcell Technologies, Vancouver, BC, Canada) was used according to the manufacturer’s instructions. Cells were incubated with the ALDEFLUOR® reagent, with and without specific ALDH inhibitor diethylaminobenzaldehyde (DEAB) at 37°C for 45 minutes. (1 μg/ml) Propidium iodide (Sigma-Aldrich, St Louis, MO, USA) was then added to the sample. Cells were sorted on a MoFlo Sorter (Dako Cytomation, Carpinteria, CA, USA). Cells were gated on scatter and pulse width to identify single cells. Propidium iodide was used to exclude dead cells. A negative control sample was incubated with DEAB to allow for accurate determination of the gate separating the ALDHLow and ALDHHigh populations. This ALDEFLUOR® assay was repeated on each cell line at least three times using a different passage at each time.
Colony formation
Sorted cells were seeded at 200 cells per well in a 6-well plate. One full plate was used for each population, ALDHLow and ALDHHigh, for TCCSUP, T24, and 5637 cells. Cells were cultured for two weeks and then fixed with 6% glutaraldehyde and stained with 0.5% crystal violet. Colony-forming efficiency (CFE) is reported and is the percentage of plated cells that formed colonies of approximately 50 or more cells. This experiment was repeated using three individual sorts of different passage cells to account for variation in cell line passage and sorting.
Differentiation
Cells were sorted and then cultured for two weeks. After two weeks, the ALDEFLUOR® assay was used to stain the cells as described above, and the cells were analyzed on an LSR II instrument (Becton Dickinson, Franklin Lakes, NJ, USA).
Cell migration and invasion
Sorted cells (25000) in RPMI-1640 with 5% FBS were plated per well of a 24-well chamber (Becton Dickinson, Franklin Lakes, NJ, USA). The chamber contained a polyester membrane with 8 μm pores uncoated for migration or coated with Matrigel® for invasion. RPMI-1640 with 20% FBS was added to the lower chamber. Cells were cultured for 36 hours, and then scraped from the upper side of the filter. Filters were stained with the Hema-3 staining system (Fisher Scientific, Pittsburgh, PA, USA) according to the manufacturer’s instructions. Four random fields per filter were counted at x150 and three chambers were counted for each population.
Statistical analysis
Differences in colony formation, migration and invasion between the ALDHLow and ALDHHigh populations for each line were tested for significance using a two-sided t-test.
Results
Identification of ALDHLow and ALDHHigh populations
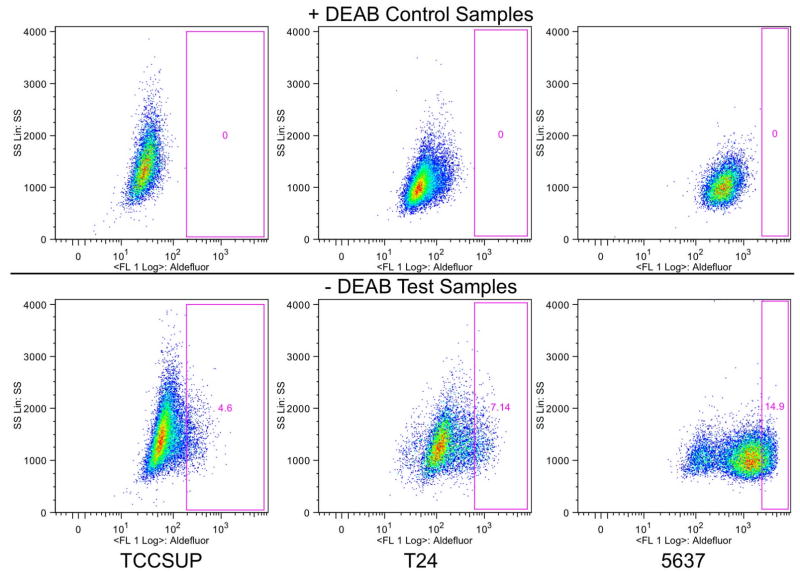
The ALDEFLUOR® assay was used to characterize the ALDHLow and ALDHHigh populations in cell lines shown to respond differently to cisplatin treatment. The data presented in Figure 1 are representative of the ALDEFLUOR® populations in the three cell lines. The DEAB-negative control sample is shown to verify the gating strategy. The gate was set to exclude all cells present in the control sample and was then applied to the test sample. Cells that fell outside of this gate are referred to as ALDHHigh. Each of the three cell lines contains a small population of cells within the ALDHHigh gate, although the percentage of ALDHHigh cells varies among the three cell lines. Table I shows the average and standard deviation for the ALDHHigh population present in each cell line based on at least three analyses. 5637 cells comprised 9.64% ALDHHigh cells and demonstrated the most resistance to cisplatin with an IC50 value of 1.7 μM. T24 cells had slightly fewer ALDHHigh cells at 8.84%, and were slightly less resistant to cisplatin with an IC50 value of 1.5 μM. TCCSUP cells comprised a small population of ALDHHigh cells at 3.27%, and remained responsive to cisplatin with an IC50 value of 0.2 μM (17).
Figure 1.
Identification of the ALDHLow and ALDHHigh populations. The ALDEFLUOR® assay was used to identify the ALDHLow and ALDHHigh populations in these three cell lines. The ‘+DEAB’ control samples were used to set the gate where all cells were excluded. This gate was then applied to the ‘ DEAB’ test sample, identifying the ALDHHigh population. The number on each plot demonstrates the percentage of ALDHHigh cells. A representative plot for each cell line is shown.
Table I.
The ALDHHigh population found in each cell line.
| TCCSUP | T24 | 5637 | |
|---|---|---|---|
| % ALDHHigh | 3.27 | 8.84 | 9.64 |
| Standard Deviation | 1.91 | 1.81 | 4.81 |
Colony formation
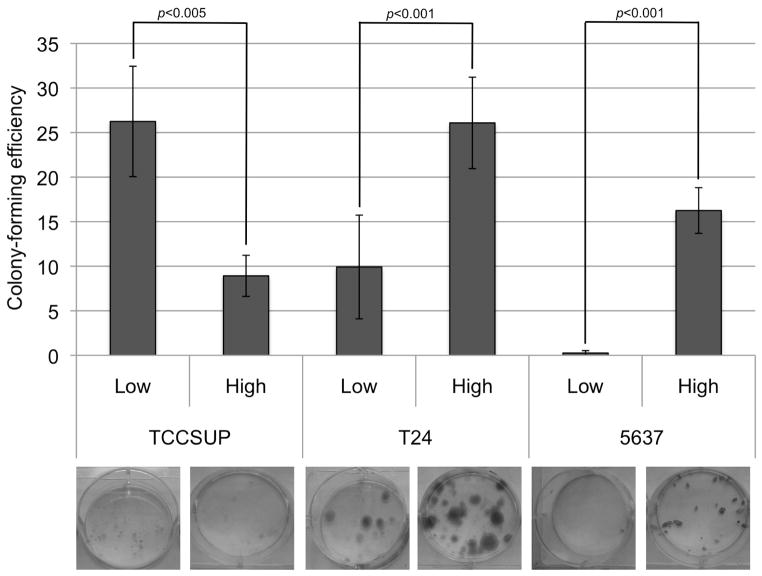
The cell lines were sorted into the ALDHLow and ALDHHigh populations and then seeded at a low density in 6-well plates. Each population was cultured for two weeks after sorting and their ability to form colonies was assessed and can be seen in Figure 2. Table II provides the average and standard deviation of the CFE for three assays.
Figure 2.
Colony-forming ability of the ALDHLow and ALDHHigh populations. ALDHLow and ALDHHigh populations were sorted for each line and then cultured at a low density for two weeks. Colonies were stained and counted. The colony-forming efficiency is the percentage of cells plated that formed a colony. This graph displays the average of six wells. An image of an example well for each population is shown.
Table II.
The colony forming efficiency (CFE) of the cell lines sorted by the ALDEFLUOR® assay.
| TCCSUP | T24 | 5637 | ||||
|---|---|---|---|---|---|---|
| High | Low | High | Low | High | Low | |
| CFE | 3.87 | 9.58 | 21.3 | 10.8 | 16.7 | 3.11 |
| Standard Deviation | 3.92 | 12.6 | 6.15 | 3.91 | 1.89 | 3.89 |
Differentiation
To investigate the ability of the ALDEFLUOR® sorted cells to differentiate, cells were sorted, cultured for two weeks, and then the population was analyzed with the ALDEFLUOR® assay. Table III shows the number of cells that fell into the ALDHHigh gate after two weeks for each sorted population. In the cisplatin-responsive TCCSUP cells, both ALDHLow and ALDHHigh sorted populations were able to differentiate and give rise to both ALDHLow and ALDHHigh cells. In cisplatin-resistant T24 and 5637 cells, the sorted ALDHLow population gave rise to very few ALDHHigh cells, 0.127% and 0.143%, respectively. The ALDHHigh population in these cell lines gave rise to both ALDHLow and ALDHHigh cells, with 2.43% ALDHHigh for T24 cells and 8.89% ALDHHigh for 5637 cells.
Table III.
The percentage of ALDHHigh cells after cell lines were sorted into ALDHHigh and ALDHLow populations and cultured for two weeks.
| TCCSUP | T24 | 5637 | ||||
|---|---|---|---|---|---|---|
| High | Low | High | Low | High | Low | |
| High | 16.9 | 2.01 | 2.43 | 0.127 | 8.89 | 0.143 |
Cell migration and Invasion
The three bladder cancer cell lines were again sorted into ALDHLow and ALDHHigh populations and evaluated for the ability to migrate and invade. Figure 3A displays the migratory abilities and Figure 3B displays the invasion abilities of the populations.
Figure 3.
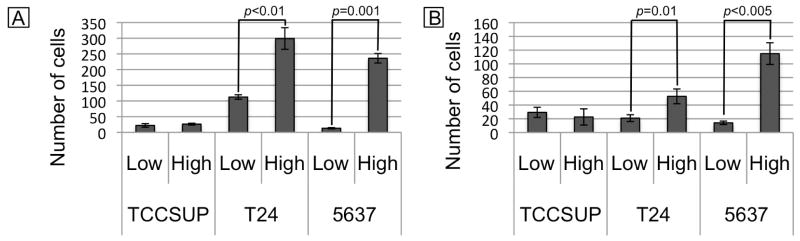
Migration and invasion abilities of the ALDHLow and ALDHHigh populations. ALDHLow and ALDHHigh cells were sorted from each cell line and cultured on uncoated (A) or Matrigel-coated (B) transwell filters. Cells that migrated (A) or invaded (B) were counted at x150. These graphs display the average of four random fields from three different chambers.
Discussion
The percentage of cells in the ALDHHigh gate for each cell line was relatively consistent for each of the sorting replicates performed, despite using cells at different passages. The average size of the ALDHHigh population within the three different cell lines coincided with the response of the cell lines to cisplatin, as demonstrated by their IC50 value determined previously (17). These findings support what has been found in other types of cancer with chemoresistance attributed to ALDH activity (12–14). A larger population with high ALDH activity is seen in the cell lines that demonstrated resistance to cisplatin.
Several functional assays were carried out to investigate if the ALDHHigh cells in these cell lines demonstrated stem cell-like behavior as has been shown in other types of cancer (10–16). The clonogenic potential of the populations and their ability to proliferate and self-renew was investigated with a colony-formation assay. In the two bladder cancer cell lines that demonstrate resistance to cisplatin, T24 and 5637, the ALDHHigh population consistently exhibited an increased ability to form colonies compared to the ALDHLow population consistently. The cisplatin-responsive cell line, TCCSUP, provided inconsistent results.
Another behavior often attributed to cells with high levels of ALDH activity is the ability to asymmetrically divide and differentiate into a population of cells that reconstitutes the parental cell line (13,15). The ALDHHigh cells in these two lines demonstrate the stem cell property of asymmetric division and differentiation, giving rise to both ALDHLow and ALDHHigh cells, while the ALDHLow cells primarily gave rise to more ALDHLow cells.
In order to grow and metastasize in the body, tumors must possess the properties of migration and invasion. Cells with high levels of ALDH activity have been shown to possess an increased ability to migrate and invade (12,13). The cisplatin-responsive TCCSUP ALDHLow and ALDHHigh populations demonstrated similarly low levels of migration and invasion. The cisplatin-resistant T24 and 5637 ALDHHigh populations demonstrated increased migration and invasion compared to the respective ALDHLow population.
5637 and T24 cells have been investigated previously for their ALDH populations. Su et al. found 5637 cells to have an ALDHHigh population of 8.2±2.0%, and T24 cells 7.9±1.9% (18). This study also found that ALDHHigh populations demonstrate a higher CFE and are 100 times more potent in in vivo tumorigenicity assays than are the ALDHLow cells (18). Our results are consistent with the results published in the study by Su et al.
It is unclear if high ALDH activity is functionally involved in stemness or if it is useful as a biomarker to identify CSCs (12). The ALDEFLUOR® assay does show more promise for isolating the CSC population than does the use of surface markers. CD44 and CD47 have been proposed as cell surface markers for isolating the CSC population in bladder cancer (19). The CD44+/CD47+ population in all three cell lines was large and did not show increased CSC behavior compared to the CD44−/CD47− population (data not shown). The ALDHHigh population identified in all three of our cell lines is relatively small, and only in the resistant cell lines does this ALDHHigh population consistently display behavior characteristic of CSCs. This provides evidence that high ALDH activity is not the sole marker for the CSC population in bladder cancer. There is likely another marker within the ALDHHigh population that is needed to isolate a pure CSC population. The ALDEFLUOR® assay allows for the initial separation, but further investigation of this population may prove fruitful for a second marker of CSCs. One important factor to keep in mind is that the ALDEFLUOR® assay has only been validated for ALDH1, while there are 19 other known isoforms of the enzyme (12). It is unlikely that there will be a single assay with the ability to isolate a truly pure population (15). It is possible that another isoform of ALDH may more specifically select for the CSC population.
In vivo limited dilution assays typically performed in CSC studies were not undertaken in this study and this may be seen as a possible limitation. However, these limited dilution assays may not necessarily identify human CSCs if the mouse microenvironment is not conducive to growth (16, 20), leading to equivocal results. An additional limitation is the use of established cell lines instead of primary cells. While studies with primary cells have the strength of maintaining the original features of the tissue they came from, they are difficult to obtain and often yield very small samples with a limited lifetime (21, 22). There is also great heterogeneity due to genetic and epigenetic differences between patients (23). This study and others have shown that cell lines represent reproducible and cost-effective alternatives to primary cell lines for studying CSCs because they give rise to heterogeneous and hierarchical populations similar to those seen in a tumor (21, 23).
The T24 and 5637 cell lines may serve as future in vitro tools to study chemoresistance in bladder cancer and for use in drug development assays. These two cell lines demonstrate resistance to cisplatin and harbor an ALDHHigh population that shows characteristics of stem cell-like behavior. Our results with these cell lines are consistent with what other groups have found (18). The responsive TCCSUP cell line displayed high variability in our assays. Generally the ALDHHigh population in the TCCSUP cell line did not demonstrate stem cell-like behavior, limiting its use as a model. It will be of great interest to determine if the ALDHHigh population in the resistant T24 and 5637 cell lines are the cells specifically responsible for the resistance to cisplatin. These cells could then be investigated with alternative therapies. Using ALDHHigh cells from cell lines that display resistance to cisplatin for drug discovery offers a reproducible and cost-effective way to identify therapies that target a CSC-like population. The consistent results obtained by different laboratories with these cell lines confirms the stability of these lines in culture, making them ideal models to use for drug development.
Acknowledgments
This project was supported by grants from Lawrence Livermore National Laboratory (LLNL) LDRD 10-LW-033 and the National Center for Research Resources (5P41RR013461-14) and the National Institute of General Medical Sciences (8 P41 GM103483-14) from the National Institutes of Health. This work performed under the auspices of the U.S. Department of Energy by LLNL under Contract DE-AC52-07NA27344. We thank the University of California Davis Cancer Center Flow Cytometry Shared Resource for assistance with cell sorting and analysis. We also thank Kristen Kulp for helpful comments in the writing of this article.
References
- 1.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol. 2006;24:296–304. doi: 10.1007/s00345-006-0061-7. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal N, Hussain M. Management of bladder cancer, current and emerging strategies. Drugs. 2009;69:1173–1187. doi: 10.2165/00003495-200969090-00003. [DOI] [PubMed] [Google Scholar]
- 4.Shah JB, McConkey DJ, Dinney CPN. New strategies in muscle-invasive bladder cancer: on the road to personalized medicine. Clin Cancer Res. 2011;17:2608–2612. doi: 10.1158/1078-0432.CCR-10-2770. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg B. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem—cells perspective on current studies and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 7.Storms RW, Trujillo AP, Springer JB, Shah L, Colvin OM, Ludeman SM, Smith C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sladek NE. Human aldehyde dehydrogenases: potential pathological, pharmacological, and toxicological impact. J Biochem Mol Toxicol. 2003;17:7–23. doi: 10.1002/jbt.10057. [DOI] [PubMed] [Google Scholar]
- 9.Chute JP, Muramoto GG, Whitesides J, Colvin M, Safi R, Chao NJ, McDonnell DP. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc Natl Acad Sci USA. 2006;103:11707–11712. doi: 10.1073/pnas.0603806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreb JS, Gabr A, Vartikar GR, Gowda S, Zucali JR, Mohuczy D. Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroxycyclophosphamide and acetaldehyde. J Pharm Exp Ther. 1998;312:339–345. doi: 10.1124/jpet.104.072496. [DOI] [PubMed] [Google Scholar]
- 11.Croker AK, Allan AL. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- 12.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzman-Ramirez N, Hamdy FC, Eaton CL, Thalmann GN, Cecchini MG, Pelger RCM, van der Pluijm G. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70:5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 13.Jiang F, Qiu Q, Khanna A, Todd NW, Deppak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honoki K, Fujii H, Kubo A, Kido A, Mori T, Tanaka Y, Tsujiuchi T. Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncol Rep. 2010;24:501–505. doi: 10.3892/or_00000885. [DOI] [PubMed] [Google Scholar]
- 15.Awad O, Yustein JT, Shah P, Gul N, Katuri V, O’Neill A, Kong Y, Brown ML, Toretsky JA, Loeb DM. High ALDH activity identifies chemotherapy-resistant Ewing’s sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLOS One. 2010;5:e13943. doi: 10.1371/journal.pone.0013943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Park P, Zhang H, La Marca F, Lin C. Prospective identification of tumorigenic osteosarcoma cancer stem cells in OS99-1 cells based on high aldehyde dehydrogenase activity. Int J Cancer. 2011;128:294–303. doi: 10.1002/ijc.25331. [DOI] [PubMed] [Google Scholar]
- 17.Vinall RL, Ripoll AZ, Pan CX, Devere White RW. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of P53-Rb pathway status. Int J Cancer. 2011 doi: 10.1002/ijc.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su Y, Qiu Q, Zhang X, Jiang Z, Leng Q, Liu Z, Stass SA, Jiang F. Aldehyde dehydrogenase 1 A1-positive cell population is enriched in tumor-initiating cells and associated with progression of bladder cancer. Cancer Epidemiol Biomark Prev. 2010;19:327–337. doi: 10.1158/1055-9965.EPI-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Jr, Chang HY, van de Rijn M, Shortliffe L, Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Adad Sci USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shmelkov SV, Butler JM, Hooper AT, Kushner J, Milde T, St Clair R, Balijevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafli S. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C, Yao Z, Dai J, Zhang H, Escara-Wilke J, Zhang Z, Keller ET. ALDH activity indicates increased tumorigenic cells, but not cancer stem cells, in prostate cancer cell lines. In Vivo. 2011;25:69–76. [PubMed] [Google Scholar]
- 22.Crallan RA, Georgopoulos NT, Southgate J. Experimental models of human bladder carcinogenesis. Carcinogenesis. 2006;27:374–381. doi: 10.1093/carcin/bgi266. [DOI] [PubMed] [Google Scholar]
- 23.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]