Abstract
Nanomaterials play a significant role in biomedical research and applications due to their unique biological, mechanical, and electrical properties. In recent years, they have been utilised to improve the functionality and reliability of a wide range of implantable medical devices ranging from well-established orthopaedic residual hardware devices (e.g. hip implants) that can repair defects in skeletal systems to emerging tissue engineering scaffolds that can repair or replace organ functions. This review summarizes the applications and efficacies of these nanomaterials that include synthetic or naturally occurring metals, polymers, ceramics, and composites in orthopaedic implants, the largest market segment of implantable medical devices. The importance of synergistic engineering techniques that can augment or enhance the performance of nanomaterial applications in orthopaedic implants is also discussed,, the focus being on a low intensity direct electric current (LIDC) stimulation technology to promote the long-term antibacterial efficacy of oligodynamic metal-based surfaces by ionization, while potentially accelerating tissue growth and osseointegration. While many nanomaterials have clearly demonstrated their ability to provide more effective implantable medical surfaces, further decisive investigations are necessary before they can translate into medically safe and commercially viable clinical applications. The paper concludes with a discussion about some of the critical impending issues with the application of nanomaterials-based technologies in implantable medical devices, and potential directions to address these.
INTRODUCTION
Nanomaterials are defined as materials or material forms with at least one constituent dimension between 1 – 100 nm. They possess unique mechanical, electrical, optical, chemical and biological properties compared to their conventional (bulk) forms, largely on account of their increased surface area to volume ratio and quantum effects. Due to these unique properties, many nanomaterials have successful commercial applications in areas such as electronics1, energy2, and biotechnology3, and the nanomaterials market is projected to grow at the annual rate of approximately 23% until 2016.4 Over the past decade, a significant effort in nanomaterials research has been dedicated to applications in biomedicine.5,6 In addition to drugs, diagnostic and therapeutic technologies, nanomaterials that can significantly enhance the quality, reliability and functionality of implantable medical devices have emerged. The market for implantable medical devices is growing at a fast pace with the annual demand in the US estimated to reach $52 billion by 2015.7 A snapshot of this projected market is presented in Figure 1. Orthopaedic implants are the largest market segment, and are projected to continue leading over the next several years impelled by the scientific advancements in medical technologies, increased awareness in the population about the necessity and benefits of treatments and surgeries (e.g. total joint replacement) to treat musculoskeletal injuries and congenital defects, and the aging baby boomer generation.
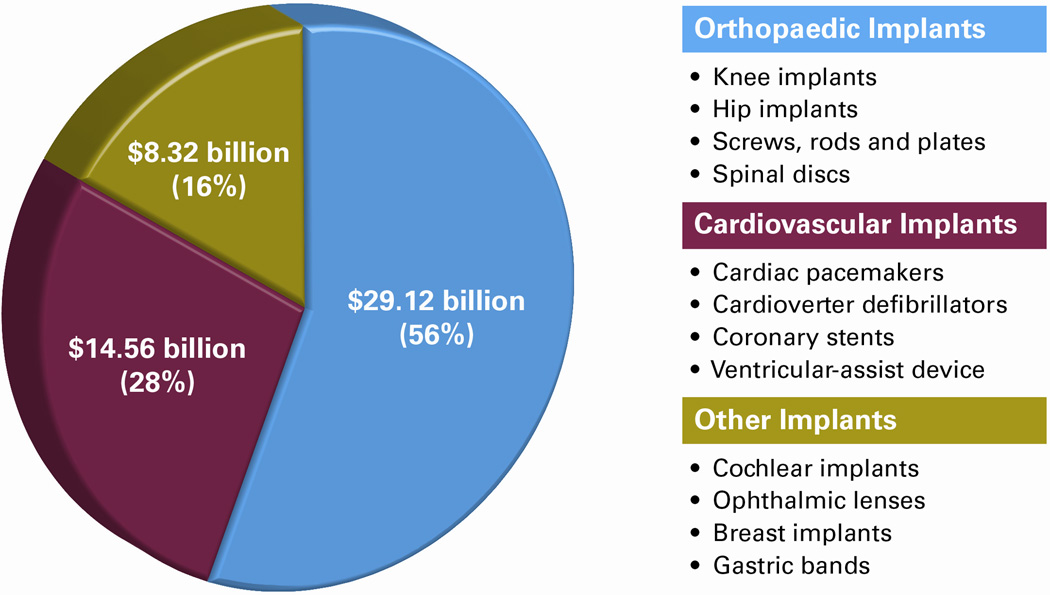
Figure 1.
This chart shows the projection of the 2015 US Market (in USD) and examples of implantable medical devices7. Orthopaedic implantable medical devices have the most significant market share (56%). Nanomaterials and synergistic engineering technologies have the potential to impact the unmet needs and shortcomings in this market.
The soaring implantable medical devices market has several existing issues and unmet needs that can potentially be resolved by appropriately engineering nanomaterials. Some of the key characteristics that make nanomaterials attractive especially for orthopaedic implant applications include their high strength-to-weight ratio, wear/corrosion resistance, antimicrobial/drug release potentials, and tissue integration/ regeneration capabilities among others. For example, current hip implants made of titanium or stainless steel alloys, although bioinert, lack the surface nanostructure of natural bone leading to a lack of bone adhesion to the implant surface. Nanophase coatings of titania or hydroxyapatite (HA) on contact surfaces of these implants can closely mimic natural bone structure, thereby favouring osteoblast proliferation and osseointegration, and long term implant stability.8,9 The various nanomaterials that are being explored for implantation within the human body to prevent or treat musculoskeletal deformities and injuries include metals, polymers, ceramics, carbon-based materials, and their composites.6,8–10 These could be synthetic or naturally occurring and in the form of nanoparticles, nanocrystals, nanofibers, nanotubes, nanofilms, or nanostructures.8–10 The efficacy of nanomaterials can also be augmented by synergistically coupling them with engineering technologies such as electric current stimulation,11 mechanical vibration,12 and pulsed electromagnetic activation.13 Such technologies in conjunction with nanomaterials can be utilized not only in orthopaedic implants but also in other medical devices such as cardiovascular implants and tissue engineering scaffolds.
This review provides an overview of the trends and applications of nanomaterials in orthopaedic implantable medical devices, and discusses the importance of complementary technologies to promote the performance of existing nanomaterials. In the later part, the focus is primarily on low intensity direct electric current (LIDC) stimulation as a means to promote the long-term antibacterial efficacy of oligodynamic metal-based surfaces by ionization, while also accelerating bone growth and osseointegration. While the advantages of nanomaterials have been well acknowledged, shortcomings such as their potential toxicity in in vivo applications have also been recognized.14 This review also highlights some of these critical issues that need addressal, and provides recommendations and future directions to accelerate the translation of nanomaterials technology to safe and affordable clinical applications.
NANOMATERIALS IN ORTHOPAEDIC IMPLANTS
Orthopaedic implants are primarily used as a treatment for bone fractures and diseases such as osteoarthritis to repair or replace the function of bones in the impaired or deteriorating joints. These medical devices that include hip and knee implants, bone rods and plates, fixator screws etc. are known to significantly improve the quality of life of patients. This is evident by the fact that the number of joint replacement surgeries has been steadily rising since the 1990s. According to a 2011 market research report, the global market for orthopaedic devices is estimated to increase from $21.1 billion in 2007 to $46.5 billion in 2017.15 This escalating market segment stands to gain from the distinctive characteristics of nanomaterials that can improve the quality and reliability of the medical devices. While nanomaterials can provide several benefits, a review of trends in recent literature shows that a majority of the investigations are mainly geared towards the application of nanomaterials to: (a) promote tissue-implant adhesion and tissue regeneration, and (b) provide antimicrobial prophylaxis. These trends are governed by the fact that lack of implant integration with bone tissue and infections are two of the most common causes of orthopaedic implant failures.
Table 1 summarizes the types of nanomaterials being investigated for orthopaedic implant applications. Some of these studies and their findings are discussed below.
Table 1.
Summary of nanomaterials for applications in orthopaedic implantable medical devices
| Materials & Examples | Material forms | Potential advantages |
|---|---|---|
| Metals: |
|
|
| Ceramics: | ||
| Polymers: |
Tissue-implant adhesion and tissue regeneration
Orthopaedic implants provide structural support as bone substitutes. In doing so, the implant surface essentially interacts with the host tissue and cells to provide a framework into which the host bone and vascular network regenerate.16 Thus, the implant materials play a vital role in governing its functionality and success. Biocompatible metals and alloys (e.g. titanium, 316L stainless steel) have traditionally been used as core materials for orthopaedic implants, while the components made of or coated with polymers/copolymers (e.g. polyethylene, polycarbonate-urethane) and ceramics (e.g. HA, titania) are not uncommon these days. While the utility and functional performance of these implants has been acceptable, critical issues such as the lack of osseointegration and eventual aseptic loosening have not been satisfactorily resolved yet.17–19 According to statistics from the American Academy of Orthopaedic Surgeons (AAOS), about 25% of all titanium hip implant surgeries require revisions to retrieve the failed implants, the lack of implant-tissue adhesion and osseointegration being a major factor.20,21
Over the last decade, scientists and engineers have started exploring nanomaterials for use in orthopaedic implants. With the advent of the field of biomimetics, a major emphasis is on mimicking the surface morphology of natural bone structures. That the porous bone matrix is composed of nano-HA crystals (2 – 3nm) dispersed within collagen type I fibers suggests that the physicochemical properties of the implant surface would be pertinent to bone regeneration.22–24 Several studies have highlighted the contribution of nanoscale topography and surface roughness in controlling tissue cell functions, independent of the specific surface chemistry.16,25–27 It is now understood that nanostructured surfaces promote the response (attachment, growth and proliferation) of bone tissue at the implant-tissue interface.28 In general, micro and nanostructured surfaces have been reported to favour apatite formation and result in a low bone resorption rate compared to smooth surfaces partially due to the higher bone-to-implant contact.29–33 In several studies that compared osseointegration on surfaces with smooth, micron/sub-micron and nanoscale topographies, nano-featured surfaces have consistently performed better, both in vitro and invivo.21,34–36 In such an in vitro comparative study, Khang et al.21 found nanometer-patterned surfaces created by electron beam deposition of pure titanium to be the most efficient in increasing the surface energy and cell (endothelial and osteoblasts) adhesion, making them appropriate candidates for long-term prostheses. Ballo et al.34 and Dauggard et al.35 found similar trends in osseointegration while investigating in vivo effects of titanium implants using a rat and canine model, respectively. These nano-scale effects are consistent across other materials including alloys (stainless steel, Ti6Al4V, CoCrMo), polymers (polyglycolic acid (PGA), poly(lactic-co-glycolic acid) (PLGA), poly(L-lactic acid) (PLLA)) and ceramics (titania, alumina, HA) as well.36–41 For example, Webster et al.42 observed higher osteoblast cell densities and greater than twice the increase in calcium content in extracellular matrices (ECM) of osteoblasts on nanophase alumina, titania and HA compared to their conventional grain size counterparts. Ergun et al.43 observed osteoblast adhesion to increase on calcium phosphate nanoparticles with decrease in average grain size, porosity and pore size. Likewise, improved osteoblast cell responses were reported from polymer casts of nanophase carbon fibres and polymer casts of polycarbonate urethane/carbon nanotube composites compared to their conventional forms, with the nanoscale topography contributing to the enhancement.44,45
In some cases, the lack of tissue-implant adhesion is a result of the implant surface promoting the excessive formation of connective tissue due to cells such as fibroblasts which can directly hinder osteogenesis. Excessive soft connective tissue leads to unstable fixation of implants that will render them unstable under physiological loading conditions.16,46 In terms of nanomaterials application, the positive news is that nanoscale topography and surface roughness can promote osteoclastic and osteoblastic cell responses while decreasing sub-confluent fibroblast proliferation, thus reducing soft tissue regeneration.16,25 As Balasundaram and Webster have noted, resisting the formation of soft fibrous tissue also contributes to wear and debris control from the implant surface; proper fixation of an implant into surrounding bone tissue leaves little to no room for wear debris to situate and cause bone death.16 Thus, in addition to improving the osteogenic properties of surfaces, some of these nanomaterials are also found to be useful for enhancing the mechanical and wear characteristics of implantable orthopaedic devices. Sol-gel-derived coatings such as nano-HA coatings have demonstrated their ability to provide improved mechanical properties owing to their nanocrystalline structure.47 Owing to their excellent mechanical properties, carbon and titania nanotubes and nanofibers, have been studied as potential reinforcement materials for implants; they have been observed to promote osseointegration as well.28,48–50 Preliminary studies with composites of carbon nanofibers and nanotubes with ceramics such as HA and alumina and polymers such as poly-ether-ether-ketone (PEEK) have also provided encouraging results in terms of improved mechanical wear properties.28,52–55
While the underlying mechanisms responsible for promoting the osteogenic activities, osseointegration, and subsequently, reduction in mechanical wear, on nanomaterial-based surfaces are not entirely understood yet, the hypothesis is that these materials create favourable surface energies that enhance the adsorption of ECM proteins such as laminin, vitronectin, fibronectin, and collagen which control cellular functions.56–58 Based on the results so far, it is clear that nanomaterials-based surfaces can significantly promote osseointegration and reduce aseptic loosening and associated problems. On account of the favourable scaling effects, there is a conjecture that nanomaterials-based surface treatments can prolong the life of orthopaedic implants to upwards of 40 years from the current 10–15 years.6,59
Antimicrobial prophylaxis
Nanomaterials are also being investigated for use in orthopaedic prostheses to prevent or treat infections. In addition to surface morphology and topography of nanoscale surfaces that is of prime importance in improving the implant-tissue adhesion, nanoparticles that can alter the surface chemistry are also of interest here. The goal is to have nanoscale coatings that can provide antimicrobial protection in addition to enhancement of other desired implant surface properties while reducing the need for pharmaceutical interventions.60 Infections such as osteomyelitis caused by biofilm-forming bacteria from implant surfaces have been a significant cause of concern in the global healthcare system. The infection rates in the US have been reported to be approximately 1–2% for primary hip and knee implants (2–6% after revisions) and 4–20% for fixator pins.61–65 Although these infection rates (percentages) are relatively small, they translate into large (absolute) number of infections due to the sheer volume of implants. Such implant infections can result in serious complications with damaging effects on bone and surrounding soft tissue.66–68 To make matters worse, a significant proportion of these infections are caused by antibiotic resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) which cannot be treated with conventional antibiotics. Infections associated with orthopaedic prostheses also have significant economic consequences. The annual cost of mitigating infected fracture fixation implants, not including the loss of productive time or the suffering by the patient, was estimated to be $1.5 billion in 2004.69,70 Treatment of hip prostheses infections alone costs approximately 5.23 times more compared to a non-infected prosthesis.11
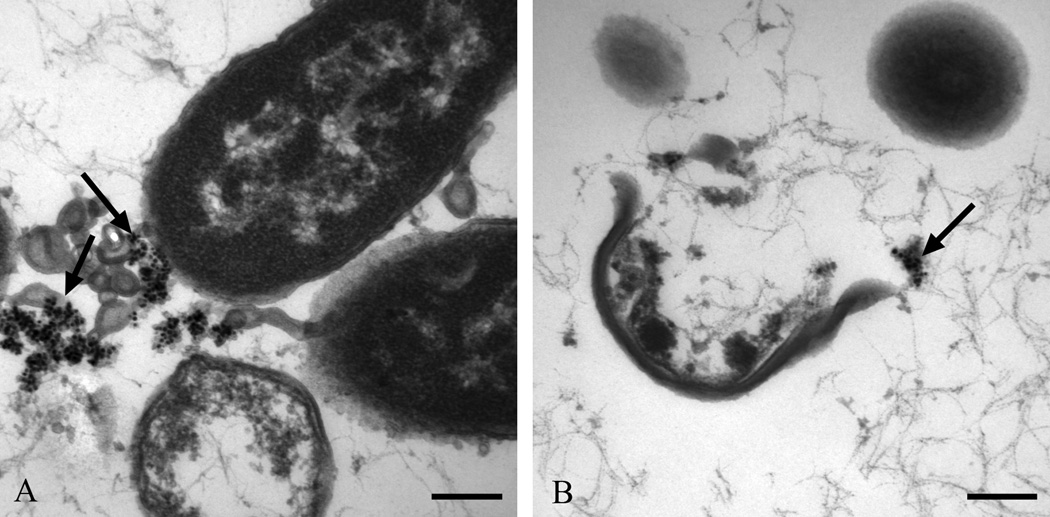
In order to curtail or treat osteomyelitic and surgical site infections, especially the ones caused by resistant microbes, ,the attention has shifted from antibiotics to alternatives such as oligodynamic metals (e.g. silver, copper, gold) that have been used in medicinal components for many years with anecdotal evidence of antimicrobial efficacy. Metallic and compound forms of these have already been utilized in medical devices such as catheters, vascular grafts and wound dressings, but the clinical results have not been significant.71–75 The nanoparticles of these metals, on the other hand, represent a promising strategy to combat pathogenic infections without being toxic to mammalian cells and tissues.76 Nanomaterials such as silver nanoparticles have been shown to be effective against a broad spectrum of regular and antibiotic-resistant bacteria (e.g. Staphylococcus epidermis, Klebsiella pneumoniea, MRSA, VRE), fungi (e.g. Candida albicans, Aspergillus niger), and viruses (e.g. HIV-1, Hepatitis B).77–83 Figure 2 depicts transmission electron micrographs of S. aureus and Escherichia coli treated with 10µg/ml of 20nm washed silver nanoparticles with evidence of whole bacteria that ruptured with silver nanoparticle agglomerates near the degenerate cells.83 Silver nanoparticles have also demonstrated a low potential for inducing antimicrobial resistance. As a result, several studies have looked at methods to create antibacterial surface coatings by incorporating silver nanoparticles within other materials, such as silver-doped hydroxyapatite, polymer-silver nanoparticles, and titanium-silver nanoparticles.84–87 For example, Wagener and Biogate have suggested using silver nanoparticle coatings on orthopaedic pins or dispersing them in polymethyl-methacrylate (PMMA) bone cement to prevent bacterial colonization.88 Juan et al. tested titanium surfaces deposited with silver nanoparticles against S. aureus and E. coli and observed 94% and 95% reduction, respectively, within 24hr.87 The first set of trauma products coated with silver nanoparticles is currently in clinical trials.5,28
Figure 2.
Transmission electron micrographs of (a) Escherichia coli (J53), and (b) Staphylococcus aureus (ATCC 25213) exposed to 10µg/ml of 20nm washed silver nanoparticles. Bar=200nm. Arrows depict agglomerated silver nanoparticles.83
Recently, Chang et al. reported titanium surfaces coated with modified zirconia-Ag to be effective against S. aureus and Actinobacillus actinomycetemcomitans.89 Bignozzi et al. developed antibacterial coatings for orthopaedic implants using modified titania and zirconia nanocrystals that showed effectiveness against eight species of bacteria and two species of fungi.90 The significance of this study was that these coatings also promoted osseointegration. Makhluf et al. documented the antibacterial activity of MgO nanoparticles and highlighted its size-dependence; the smallest (8nm) MgO nanoparticles demonstrated significantly higher antibacterial efficacy against S. aureus and E. coli compared to larger (23nm) nanoparticles.91 Single-walled and multi-walled carbon nanotubes (SWNTs and MWNTs) have also demonstrated antimicrobial efficacy that was dependent on the nanotube diameters.92,93 The application of carbon nanotubes to orthopaedic implant surfaces is very relevant since they can drastically improve the mechanical properties of implant due to their exceptional strength to weight ratio.94
Furthermore, nanomaterials-based approaches have been explored for their anti-adhesive anti-biofilm characteristics which are also critical for orthopaedic implants. Nanotextured surfaces of materials such as titania, ZnO and carbon nanotubes have been observed to lower microbial adhesion and biofilm formation.95 Biofilms, which are complex sessile micro-colonies of bacteria or yeast embedded within a microbially-derived protective extracellular matrix, are virtually inseparable from the implant and serve as a formidable antibiotic delivery barrier.11,69,70,96 Just as nanotopography affects the adhesion and proliferation of mammalian cells onto implant surfaces, it also has a strong influence on prokaryotic cell attachment and biofilm formation. Juan et al. demonstrated that their silver nanoparticles-based antibacterial surfaces also possessed anti-adhesive qualities compared to untreated titanium surfaces; this deters the formation of biofilms.87 In vitro experimentation by Singh et al. demonstrated that the relationship between bacterial adhesion/ biofilm formation and nanoroughness was non-monotonic.97 While studying the effects of nanostructured TiO2 on S. aureus and E. coli, they reported a linear increase of bacterial adhesion with nanoroughness for surfaces with Rq values less than 20 nm, and a significant decrease in bacterial biofilm formation and adhesion with further increase in roughness. They established the primary relationship between biofilm formation, surface morphology and the amount of protein adsorption. This is very critical since protein adsorption also governs the adhesion and proliferation of osteoblast cells onto the implant surface. The same nanostructured surface effect that improves protein adsorption and bioactivity and promotes subsequent mammalian cell functions can be synergistically utilized to prevent biofilm colonization.98 In general, the nanomaterials-based approaches to prevent biofilm formation are favoured because of their passivity. This attribute helps to circumvent the problems associated with the spread of antimicrobials/antibiotic drugs in the bone and surrounding tissue and the subsequent risk of inducing antimicrobial resistant pathogens.68
While the exact mechanism by which nanomaterials demonstrate antimicrobial efficacy is still under investigation, their increased surface area to volume ratio is believed to be the main enabler. The primary proposed antimicrobial mechanisms specifically for silver nanoparticles involve (a) interaction with sulphur and phosphorous in the cell proteins and DNA, (b) attack on the respiratory chain in the mitochondria, and (c) release of silver ions which induce oxidative stresses.75,99–103 It Is vital that these mechanisms be ascertained if antimicrobial nanomaterials are to be used in vivo clinically. It is also important to develop a quantitative understanding and in vitro and in vivo models of the effects of nanomaterial concentrations and morphologies on both eukaryotic and prokaryotic cell functions. This will help in devising strategies to avoid any potential microbial resistance to these materials in future. Since some of the antimicrobial nanomaterials including silver nanoparticles have shown toxicity to mammalian cells including osteoblasts and osteoclasts, it is imperative to investigate their toxicity mechanisms in details and conceive ways to control it.104–107
SYNERGISTIC TECHNOLOGIES FOR ENHANCING NANOMATERIAL CHARACTERISTICS AND PERFORMANCE
Based on this review, it is clear that nanomaterials have a significant potential to improve the functionality and performance of implantable medical devices. In addition to the continual progress in nanomaterials themselves, it is also possible to synergistically integrate other biomedical technologies that can augment the performance of nanoscale materials or nanoscale forms (e.g. ions) or overcome some of their existing drawbacks. For example, nanomaterials are being incorporated into a variety of sensors that can be applied to orthopaedic prostheses for diagnostic and therapeutic purposes. Feedback sensors have been designed using CNTs, such that the release of antibiotics is only triggered by the presence of bacteria cells.45,108,109 In addition to the desired mechanical characteristics imparted by CNTs, this technology also prevents the risk of inducing microbial resistance due to the excessive dosage of antibiotics. A second example is the potential use of controlled vibrational loading in conjunction with nanomaterials to promote tissue healing and osteogenesis. Mechanical, electromagnetic and ultrasound stimulations that have been shown to positively influence osseointegration, both in vitro and in vivo, can be coupled with nanostructured implant surfaces with great benefits.12,13,110,111 Recent investigations have also focused on a new prophylactic antimicrobial technology based on the controlled release of oligodynamic metal ions by LIDC stimulation. This technology can overcome some of the drawbacks associated with the uncontrolled release kinetics or insufficient concentration of antimicrobial nanoparticles.11,112–116 While antimicrobial nanoparticles of metals such as silver have demonstrated better antimicrobial efficacy than their elemental form, it is actually the ions released from these nanomaterials that possess the antimicrobial properties.117–120 LIDC stimulation results in ionization, and hence, can be a more effective mechanism to achieve or enhance the antimicrobial properties of metallic or nanoparticulate silver. The technology is discussed in detail the following sub-section.
LIDC-activated technology
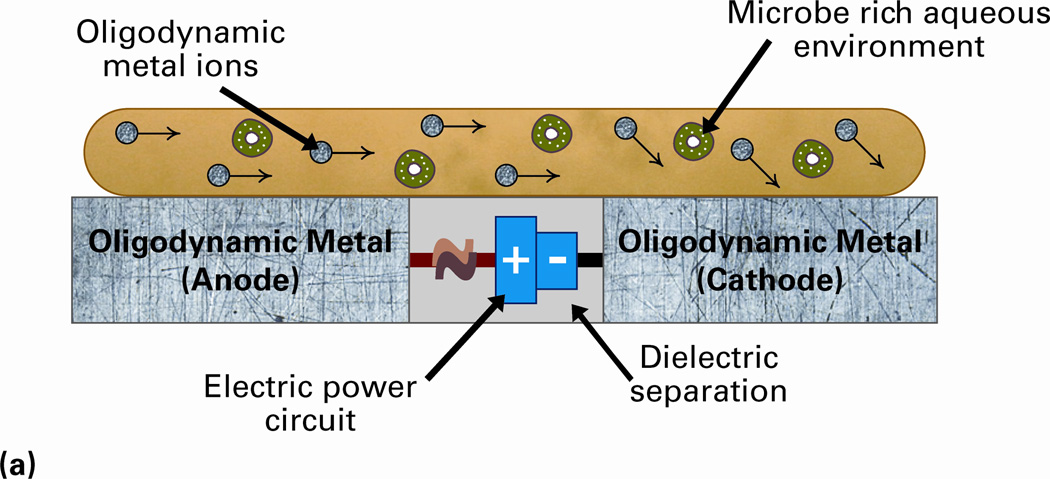
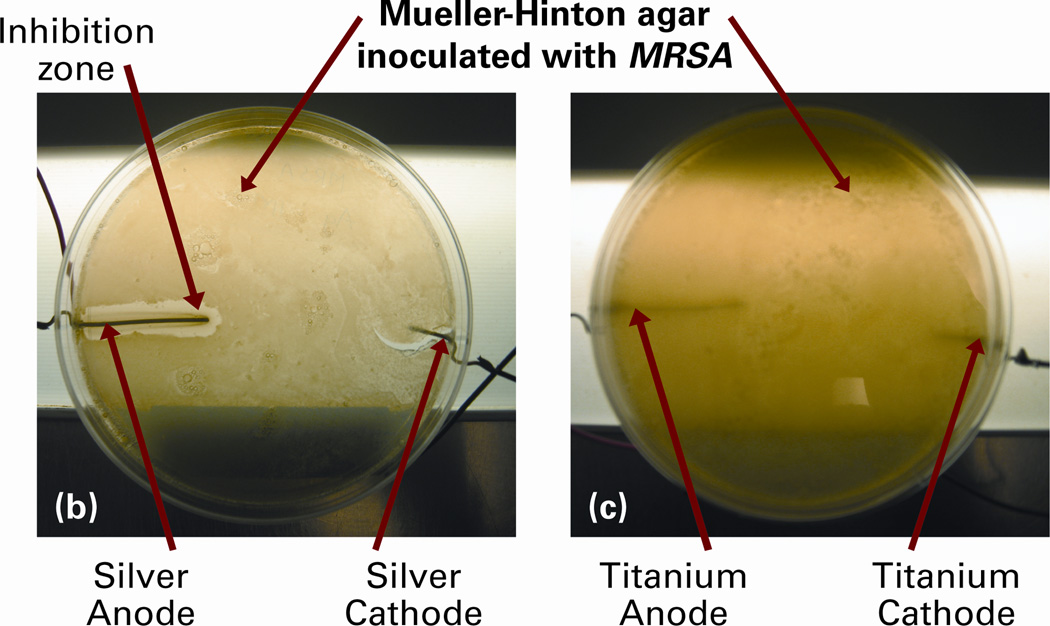
Different forms of electrical stimuli have been used in medical treatments for several years. Their most predominant clinical applications have been in the forms of direct and pulsed currents and voltages in chronic wound healing and ulcer treatments.121–125 Direct currents ranging from a few microamperes to milliamperes – LIDC – have also been explored for antimicrobial treatments based on electrochemical ionization mechanisms.112–114 Recently, our research group has developed multiple configurations of the LIDC stimulated oligodynamic metal ions-based technology that can be applied to the orthopaedic implantable medical devices.11, 115,116 These system configurations are stimulated by electric currents of up to 20µA and are capable of operating as a prophylactic that will inhibit infections from developing or as a system that can be activated after an infection has been discovered in order to eliminate it. The concept of this technology and results of its efficacy test against MRSA are shown in Figure 3. The ionization effect can be quantified using Faraday’s law of electrolysis.11
Figure 3.
(a) Concept of the prophylactic technology that uses low intensity direct electric current (LIDC) stimulation for the release of oligodynamic metal (e.g. silver) ions. The potential is created through the microbe rich environment, and the antimicrobial ions that are released disrupt the bacteria cells. To demonstrate its efficacy, Mueller-Hinton agar plates have been inoculated with MRSA and exposed to (b) silver electrodes, and (c) titanium electrodes, with 20µA system current. Note the clear zone of inhibition due to the antimicrobial silver ions that were released at the anode in (b). No such zone of inhibition was observed in case of LIDC stimulated titanium in (c).11,115,116
The system is comprised of one or more electrically isolated oligodynamic metal electrodes with opposite polarities charged by an external voltage source. Since alternate electrodes are separated by a dielectric, the system is in a passive state in absence of any conductive medium between them. A critical requirement in order to activate the system is getting the media that may contain the microbes to serve as the conductor between the electrodes; the oligodynamic ions then directly interact with the microbes to disrupt their cellular functions and actually kill them. While this technology has been extensively tested with multiple metals including 316L stainless steel, titanium, silver, copper, and gold, silver-based configurations have demonstrated the best antimicrobial efficacy. The have been found to effectively inhibit multiple pathogen species including S. aureus, E. coli, MRSA and C. albicans. Fuller et al. presented the design of an orthopaedic hip replacement stem employing this technology and confirmed its in vitro efficacy against P. aeruginosa.11 Wysk et al. demonstrated the in vivo efficacy of miniaturized versions of this hip implant stem using a rat model.115 Current results have been validated using elemental silver, but the technology has the capability to be used synergistically with nanosilver films or silver nanoparticles to enhance their inherent antimicrobial attributes.
Stimulation with LIDC also has other advantages. In addition to providing the antimicrobial efficacy, the LIDC stimulation is capable of disrupting biofilms.126–128 LIDC has also been shown to promote osteogenesis and bone growth, and improve bone fracture healing in studies with cultured cells as well as in vivo animal models.129–134 For example, in a clinical study of 175 human patients, LIDC stimulation demonstrated solid bone union in 83.7% patients.135 What is noteworthy is that the LIDC value used (20µa) was of the same magnitude needed for the antimicrobial technology, and the healing rate in patients with history of osteomyelitis was 74.4%. A few of such recent in vitro studies have also included nanomaterials including CNTs and titanium-based nanofibers.136,137 In summary, the LIDC stimulation technology when rightly configured with the appropriate nanomaterials has the multi-modal capabilities apt for implantable medical devices, and is worth further investigations. Furthermore, the technology can be extended not only to orthopaedic prostheses but also to other implantable medical devices including cardiovascular and orthodontic implants.
FUTURE DIRECTIONS IN IMPLANTABLE NANOMATERIALS
The applications of nanomaterials and associated technologies in medical devices are still in their developmental phase, but their scientific merit, utility and capabilities have been recognized and well acknowledged. The mechanical and biological responses required for demanding medical applications have significantly improved using nanomaterials in various forms. Orthopaedic, cardiovascular, dental and maxillofacial, ocular and other implantable medical device applications all benefit by these enhanced properties as well as new means of fabrication. Future work will likely develop enhanced design methodologies to take advantage of these materials and new fabrication technology. While making these advances, several unsolved questions and concerns will need to be addressed in order to accelerate the translation of implantable nanomaterials from research labs to clinics.
Due to the limited insights into their fundamental structure-property relationships and interactions with biological systems (e.g. cells, tissues, proteins), nanomaterials continue to present challenges that currently restrict them to ex vivo applications. Studies focusing on this fundamental understanding, identification of any undesirable effects, and their systematic characterization are much needed before the full potential of nanomaterials in implantable medical devices can be realized. The long term consequences of using nanomaterials and synergistic technologies such as LIDC stimulation within mammalian bodies are not well understood, but some trade-offs in biological and mechanical characteristics have already been reported in literature. For example, titanium surfaces with nanoscale pores that promote osteoblast adhesion and bone tissue healing in the peri-implant region have also demonstrated lower corrosion resistance. This can be attributed to the increased specific surface area that results in faster ionization.33,1388–141 Similarly, titania nanoparticles have also been shown to cause oxidative stress-mediated toxicity in multiple mammalian cell types.142–144 Hence, a more complete understanding of the role of surface texture coupled with improved processing techniques to achieve nanoscale structures on a macro scale implant surface is critical. More importantly, it is imperative to quantitatively analyse and model the tissue-nanomaterial interactions at the molecular and cellular levels.145 Specifically in case of LIDC stimulated implants, the ionization is a result of the surface oxidation, and its effect on the wear of the implants, or the long term biocompatibility of oxidized implant surfaces needs to be investigated in detail.
Research to date strongly suggests that long term in vivo studies with nanomaterials are necessary, and the effects must be investigated not only on the immediate surrounding tissue (e.g. femur in case of hip replacement stem,), but also at a systemic level in the body.146 Consider the following example. While nanoscale and ionic forms of silver are known to decrease infections, the adverse effects of their prolonged use such as argyria and argyrosis (discoloration of skin and eyes, respectively) have also been noted.147,148 The mechanisms by which this happens are still not recognized. In addition to determining those, it is critical to investigate the optimal size, surface conditions, synthesis methods and dosages such that the nanoparticles are antibacterial without being toxic to mammalian tissues and cells.83,102,149 Alongside, the continued development of suitable mechanisms to control the ion delivery so that only the appropriate dosages are served is necessary.11 In general, appropriately validated analytical methods and an in-depth understanding of the mechanism of nanotoxicty on different cell lines is a short coming that needs to be addressed to ensure the safe therapeutic use of these materials.5
As with all medical treatment options care must be taken to minimize cost for the consumer. This can only occur by taking a more holistic approach during product development to improve properties and biological and mechanical responses through effective employment of nanomaterials, innovative synthesis and fabrication techniques, and new design paradigms. We must synergistically target multiple desired characteristics (e.g. osseointegration, wear resistance and antimicrobial efficacy for orthopaedic implants), instead of focusing on each one in isolation. We have to better understand any detrimental effects and minimize their impacts through an approach or framework that integrates materials, design and fabrication considerations, if possible. Multidisciplinary collaborations between physical scientists, medical doctors and engineers supported by partnerships with the industry and regulatory organizations will be the most critical enabler in all of this.
ACKNOWLEDGEMENTS
The authors acknowledge the help of Mr. Pradeep Kumar Ravi Prakash from the Fitts Department of Industrial and Systems Engineering at North Carolina State University in the preparation of this manuscript.
Footnotes
Drs. Richard A. Wysk and Paul H. Cohen are Principals in ArgentumCidalElectrics Inc., Lewistown, PA, a company with multiple patents pending on the LIDC activated antimicrobial technology discussed in this article. Drs. Rohan A. Shirwaiker and Nancy A. Monteiro-Riviere have previously received a research grant from the company to study the cytotoxic effects of the LIDC activated antimicrobial technology.
Contributor Information
Rohan A. Shirwaiker, Fitts Department of Industrial and Systems Engineering and UNC-NCSU Joint Department of Biomedical Engineering, North Carolina State University; rashirwaiker@ncsu.edu
Meghan E. Samberg, Center for Chemical Toxicology Research and Pharmacokinetics, Department of Clinical Sciences, and UNC-NCSU Joint Department of Biomedical Engineering, North Carolina State University
Paul H. Cohen, Fitts Department of Industrial and Systems Engineering, North Carolina State University
Richard A. Wysk, Fitts Department of Industrial and Systems Engineering and UNC-NCSU Joint Department of Biomedical Engineering, North Carolina State University
Nancy A. Monteiro-Riviere, Center for Chemical Toxicology Research and Pharmacokinetics, Department of Clinical Sciences, and UNC-NCSU Joint Department of Biomedical Engineering, North Carolina State University, and Department of Anatomy and Physiology, Kansas State University
REFERENCES
- 1.Korkin A, Gusev E, Labanowski JK, Luryi S, editors. Nanotechnology for Electronic Materials and Devices. New York: Springer; 2007. p. 368. [Google Scholar]
- 2.Nalwa HS. Nanomaterials for energy storage applications. American Scientific Publishers; 2009. p. 350. [Google Scholar]
- 3.Gouma P(State University of New York, NY) Nanomaterials for Chemical Sensors and Biotechnology. Singapore: Pan Stanford Publishing; 2010. p. 159. [Google Scholar]
- 4.Growth opportunities in global nanomaterials market 2011–2016: Trend, forecast, and opportunity analysis. Dallas (TX): Lucintel (Global Market Research Firm); 2011. Nov, p. 153. [Google Scholar]
- 5.Saji SV, Choe HC, Yeung KWK. Nanotechnology in biomedical applications: A review. Int J Nano Biomaterials. 2010;3(2):119–139. [Google Scholar]
- 6.Roszek B, de Jong WH, Geertsma RE. (The National Institute for Public Health and the Environment (RIVM)). Nanotechnology in medical applications: state-of-the-art in materials and devices. Netherlands: Department of Pharmaceutical Affairs and Medical Technology of the Dutch Ministry of Health, Welfare and Sports; 2005. p. 123. Report No. 265001001/2005. [Google Scholar]
- 7.Implantable medical devices. Industry study with forecasts for 2015 and 2020, 2012. Cleveland (OH): The Fredonia Group; 2012. Mar, p. 395. [Google Scholar]
- 8.Tran N, Webster TJ. Nanotechnology for bone materials. WIREs Nanomed Nanobiotechnol. 2009;1(3):336–351. doi: 10.1002/wnan.23. [DOI] [PubMed] [Google Scholar]
- 9.Thomas JB, Peppas NA, Sato M, Webster TJ. Nanotechnology and Biomaterials. In: Gogotsi Y, editor. Nanomaterials handbook. 1st ed. Boca raton (FL): CRC Press; 2006. pp. 605–634. [Google Scholar]
- 10.Christenson EM, Anseth KS, van den Beucken JJJP, Chan CK, Ercan B, Jansen JA, Laurencin CT, Li WJ, Murugan R, Nair LS, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25(1):11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 11.Fuller T, Wysk RA, Charumani C, Kennett M, Sebastiennelli WJ, Abrahams RA, Shirwaiker RA, Voigt RC, Royer P. Developing an engineered antimicrobial/prophylactic system using electrically activated bactericidal metals. J Mater Sci Mater Med. 2010;21(7):2103–2114. doi: 10.1007/s10856-010-4071-z. [DOI] [PubMed] [Google Scholar]
- 12.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanicalvibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39(5):1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Fini M, Cadossi R, Canè V, Cavani F, Giavaresi G, Krajewski A, Martini L, Aldini NN, Ravaglioli A, Rimondini L, et al. The effect of pulsed electromagnetic fields on the osteointegration of hydroxyapatite implants in cancellous bone: a morphologic and microstructural in vivo study. J Orthop Res. 2002;20(4):756–763. doi: 10.1016/S0736-0266(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41:2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orthopedic Implants – A Global Market Overview. Hyderabad, India: Industry Experts; 2011. Nov, p. 197. [Google Scholar]
- 16.Balasundaram G, Webster TJ. A perspective on nanophase materials for orthopedic implant applications. J Mater Chem. 2006;16:3737–3745. [Google Scholar]
- 17.Johanson PE, Digas G, Herberts P, Thanner J, Karrholm J. Highly crosslinked polyethylene does not reduce aseptic loosening in cemented THA 10-year findings of a randomized study. Clin Orthop Relat Res. 2012:11. doi: 10.1007/s11999-012-2400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bahraminasab M, Sahari BB, Edwards KL, Farahmand F, Arumugam M, Hong TS. Aseptic loosening of femoral components – a review of current and future trends in materials used. Mater Des. 2012;42:459–470. [Google Scholar]
- 19.Wang W, Ouyang Y, Poh CK. Orthopaedic implant technology: biomaterials from past to future. Ann Acad Med Singapore. 2011;40:237–244. [PubMed] [Google Scholar]
- 20.American Academy of Orthopaedic Surgeons (AAOS) 2005 http://www.aaos.org/.
- 21.Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomater. 2008;29(8):970–983. doi: 10.1016/j.biomaterials.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Mann S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry. Oxford (UK): Oxford University Press; 2002. p. 210. [Google Scholar]
- 23.Einhorn TA, O’Keefe RJ, Buckwalter JA, editors. Orthopaedic Basic Science. 3rd ed. Rosemont (IL): American Academy of Orthopaedic Surgeons (AAOS); 2007. p. 465. [Google Scholar]
- 24.Balasundaram G, Webster TJ. Nanotechnology and biomaterials for orthopaedic medical applications. Nanomedicine. 2006;1(2):169–176. doi: 10.2217/17435889.1.2.169. [DOI] [PubMed] [Google Scholar]
- 25.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis ASG. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials. 2002;23(14):2945–2954. doi: 10.1016/s0142-9612(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 26.Dalby MJ, Childs S, Riehle MO, Johnstone H, Affrossman S, Curtis ASG. Fibroblast reaction to island topography: changes in cytoskeleton and morphology with time. Biomaterials. 2003;24(6):927–935. doi: 10.1016/s0142-9612(02)00427-1. [DOI] [PubMed] [Google Scholar]
- 27.Thapa A, Webster TJ, Haberstroh KM. Polymers with nano-dimensional surface features enhance bladder smooth muscle cell adhesion. J Biomed Mater Res A. 2003;67A(4):1374–1383. doi: 10.1002/jbm.a.20037. [DOI] [PubMed] [Google Scholar]
- 28.Streicher RM, Schmidt M, Fiorito S. Nanosurfaces and nanostructures for artificial orthopedic implants. Nanomedicine. 2007;2(6):861–874. doi: 10.2217/17435889.2.6.861. [DOI] [PubMed] [Google Scholar]
- 29.Chehroudi B, McDonnell D, Brunette DM. The effects of micromachined surfaces on formation of bonelike tissue on subcutaneous implants as assessed by radiography and computer image processing. J Biomed Mater Res. 1997;34:279–290. doi: 10.1002/(sici)1097-4636(19970305)34:3<279::aid-jbm2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Cochran DL, Buser D, ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, Peters F, Simpson JP. The use of reduced healing times on ITI implants with a sandblasted and acidetched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002;13:144–153. doi: 10.1034/j.1600-0501.2002.130204.x. [DOI] [PubMed] [Google Scholar]
- 31.Papalexiou V, Novaes AB, Jr, Grisi MFM, Souza SSLS, Taba M, Jr, Kajiwara JK. Influence of implant microstructure on the dynamics of bone healing around immediate implants placed into periodontally infected sites: a confocal laser scanning microscopic study. Clin Oral Implants Res. 2004;15:44–53. doi: 10.1111/j.1600-0501.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 32.Kasuga T. Formation of titanium oxide nanotubes using chemical treatments and their characteristic properties. Thin Solid Films. 2006;496:141–145. [Google Scholar]
- 33.Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28:3188–3197. doi: 10.1016/j.biomaterials.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Ballo A, Agheli H, Lausmaa J, Thomsen P, Petronis S. Nanostructured model implants for in vivo studies: influence of well-defined nanotopography on de novo bone formation on titanium implants. Int J Nanomedicine. 2011;6:3415–3428. doi: 10.2147/IJN.S25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daugaard H, Elmengaard B, Bechtold JE, Soballe K. The effect of dual acid texturing implants on bone ongrowth – an unloaded implant model in an experimental canine study. Trans 52nd Orth Res Soc Meeting. 2006:948. [Google Scholar]
- 36.Klein CP, Patka P, van der Lubbe HB, Wolke JG, de Groot K. Plasma-sprayed coatings of tetracalciumphosphate, hydroxylapatite and alpha-TCP on titanium allow: an interface study. J Biomed Mater Res. 1991;25:53–65. doi: 10.1002/jbm.820250105. [DOI] [PubMed] [Google Scholar]
- 37.Thapa A, Miller DC, Webster TJ, Haberstroh KM. Nano-structured polymers enhance bladder smooth muscle cell function. Biomaterials. 2003;24(17):2915–2926. doi: 10.1016/s0142-9612(03)00123-6. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 39.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25(19):4731–4739. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Demetrescu I, Pirvu DC, Mitran V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry. 2010;79:122–129. doi: 10.1016/j.bioelechem.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Han P, Ji W, Zhao C, Zhang X, Jiang Y. Improved osteoblast proliferation, differentiation and mineralization on nanophase Ti6Al4V. Chinese Med J. 2011;124(2):273–279. [PubMed] [Google Scholar]
- 42.Webster TJ, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21(17):1803–1810. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 43.Ergun C, Liu H, Webster TJ, Olcay E, Yilmaz S, Sahin FC. Increased osteoblast adhesion on nanoparticulate calcium phosphates with higher Ca/P ratios. J Biomed Mater Res. 2008;85A:236–241. doi: 10.1002/jbm.a.31555. [DOI] [PubMed] [Google Scholar]
- 44.Price RL, Ellison K, Haberstroh KM, Webster TJ. Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts. J Biomed Mater Res A. 2004;70(1):129–138. doi: 10.1002/jbm.a.30073. [DOI] [PubMed] [Google Scholar]
- 45.Webster TJ, Waid MC, McKenzie JL, Price RL, Ejiofor JU. Nano-biotechnology: carbon nanofibres as improved neural and orthopaedic implants. Nanotechnology. 2004;15(1):48–54. doi: 10.1088/0957-4484/15/1/009. [DOI] [PubMed] [Google Scholar]
- 46.Anderson JM, Gristina AG, Hanson SR. In: Biomaterial science: An introduction to materials in medicine. Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. San Diego (CA): Academic Press, Inc; 1996. pp. 165–214. [Google Scholar]
- 47.Besim BN, Choi AH. Sol-gel production of bioactive nanocoatings for medical applications. Part 1: an introduction. Nanomedicine-UK. 2006;1:311–319. doi: 10.2217/17435889.1.3.311. [DOI] [PubMed] [Google Scholar]
- 48.Das K, Bose S, Bandyopadhyay A. Titanium Surface Modification to Titania Nanotube for Next Generation Orthopedic Applications. In: Narayan R, Colombo P, editors. Advances in Bioceramics and Porous Ceramics: Ceramic Engineering and Science Proceedings. 7. Vol. 29. Hoboken (NJ): John Wiley & Sons, Inc; 2009. [Google Scholar]
- 49.Singh MK, Marques PAAP, Sousa ACM, Gracio J, Silva VS, et al. Biotoxicity study of bone cement based on a functionalised multi-walled carbon nanotube-reinforced PMMA/HAp nanocomposite. Int J Nano Biomater. 2009;2:442–453. [Google Scholar]
- 50.Shokuhfar T, Titus E, Cabral G, Sousa ACM, Gracio J, et al. Modelling on the mechanical properties of nanocomposite hydroxyapatite/PMMA/carbon nanotube coatings. Int J Nano Biomater. 2007;1:107–115. [Google Scholar]
- 51.Swami N, Cui Z, Nair L. Titania nanotubes: Novel nanostructures for improved osseointegration. J Heat Transfer. 2011 034002-1-7: 133. [Google Scholar]
- 52.Werner P, Altstaed V, Jaskulka R, Jacobs O, Sandler JKW, Shaffer MSP, Windle AH. Tribological behaviour of carbon-nanofibre-reinforced poly(ether ether ketone) Wear. 2004;257(9–10):1006–1014. [Google Scholar]
- 53.Zhan GD, Kuntz JD, Wan J, Mukherjee AK. Singlewalled carbon nanotubes as attractive toughening agents in alumina based nanocomposites. Nat Mater. 2003;2:38–42. doi: 10.1038/nmat793. [DOI] [PubMed] [Google Scholar]
- 54.Balani K, Anderson R, Laha T, Andara M, Tercero J, Crumpler E, Agarwal A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials. 2007;28(4):618–624. doi: 10.1016/j.biomaterials.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Zhang YQ, Gan CH, Yu G. Wear studies of hydroxyapatite composite coating reinforced by carbon nanotubes. Carbon. 2007;45:998–1004. [Google Scholar]
- 56.Davies JE. The importance of surface charge species in cell behaviour at the biomaterial interface. In: Ratner BD, editor. Surface characteristics of biomaterials. Elsevier, NY: 1988. [Google Scholar]
- 57.Webster TJ. Nanophase ceramics: the future of orthopedic and dental implant material. In: Ying JY, editor. Nanostructured Materials. New York: Academy Press; 2001. pp. 125–166. [Google Scholar]
- 58.Webster TJ, Schadler LS, Siegel RW, Bizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001;7(3):291–301. doi: 10.1089/10763270152044152. [DOI] [PubMed] [Google Scholar]
- 59.Catledge SA, Fries MD, Vohra YK, Lacefield WR, Lemons JE, Woodard S, Venugopalan R. Nanostructured ceramics for biomedical implants. J Nanosci Nanotechnol. 2002;2(3–4):293–312. doi: 10.1166/jnn.2002.116. [DOI] [PubMed] [Google Scholar]
- 60.Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed Nanotech Biol Med. 2011;7(1):22–39. doi: 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Sculco TP. Economic impact of infected total joint arthroplasty. Instr Course Lect. 1993;42:349–351. (AAOS) [PubMed] [Google Scholar]
- 62.Garvin KL, Hanssen AD. Infection after total hip arthroplasty. Past present, and future. J Bone Joint Surg Am. 1995;77(10):1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 63.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 64.Gosheger G. Antimicrobial coating. Abstract Symposium – Prevention and treatment of infection in orthopaedic surgery. 2003 [Google Scholar]
- 65.Williams H, Griffiths P. The effectiveness of pin site care for patients with external fixators. Br J Community Nurs. 2004;9(5):206–221. doi: 10.12968/bjcn.2004.9.5.12889. [DOI] [PubMed] [Google Scholar]
- 66.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 67.Montanaro L, Testoni F, Poggi A, Visai L, Speziale P, Arciola CR. Emerging pathogenetic mechanisms of the implant-related osteomyelitis by Staphylococcus aureus. Int J Artif Organs. 2011;34(9):781–788. doi: 10.5301/ijao.5000052. [DOI] [PubMed] [Google Scholar]
- 68.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant material. Biomater. 2012;33(26):5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 70.Ehrlich GD, Stoodley P, Kathju S, Zhao Y, McLeod BR, Balaban N, et al. Engineering approaches for the detection and control of orthopaedic biofilm infections. Clin Orthop Relat Res. 2005;437:59–66. doi: 10.1097/00003086-200508000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riley DK, Classen DC, Stevens LE, Burke JP. A large randomised clinical trial of a silverimpregnated urinary catheter: lack of efficacy and staphylococcal superinfection. Am J Med. 1995;98:349–356. doi: 10.1016/S0002-9343(99)80313-1. [DOI] [PubMed] [Google Scholar]
- 72.Walder B, Pittet D, Tramer MR. Prevention of bloodstream infections with central venous catheters treated with antiinfective agents depends on catheter type and insertion time: evidence from a meta-analysis. Infect Control Hosp Epidemiol. 2002;23(12):748–756. doi: 10.1086/502005. [DOI] [PubMed] [Google Scholar]
- 73.Crabtree JH, Burchette RJ, Siddiqi RA. The efficacy of silver-ion implanted catheters in reducing peritoneal dialysis-related infections. Perit Dial Int. 2003;23(4):368–374. [PubMed] [Google Scholar]
- 74.Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, et al. Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J Antimicrob Chemother. 2004;54:1019–1024. doi: 10.1093/jac/dkh478. [DOI] [PubMed] [Google Scholar]
- 75.Madhumathi K, Sudheesh Kumar PT, Abhilash S, Sreeja V, Tamura H. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J Mater Sci Mater Med. 2010;21:807–813. doi: 10.1007/s10856-009-3877-z. [DOI] [PubMed] [Google Scholar]
- 76.Travan A, Marsich E, Donati I, Paoletti S. Silver nanocomposites and their biomedical applications. In: Kumar CSSR, editor. Nanocomposites. Germany: Wiley-VCH; 2010. pp. 81–127. [Google Scholar]
- 77.Lago VD, de Oliveira LF, Goncalves KA, Kobarg J, Cardoso MB. Size-selective silver nanoparticles: future of biomedical devices with enhanced bactericidal properties. J Mater Chem. 2011;21:12267–12273. [Google Scholar]
- 78.Pinto RJB, Fernandes SCM, Freire CSR, Sadocco P, Causio J, Neto CP, Trindade T. Antibacterial activity of optically transparent nanocomposite films based on chitosan or its derivatives and silver nanoparticles. Carbohydrate Res. 2012;348:77–83. doi: 10.1016/j.carres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed Nanotech Biol Med. 2009;5(4):452–456. doi: 10.1016/j.nano.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 80.Elechiguerra J, Burt J, Morones J, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005;3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, Kuk E, Yu K, Kim J, Park S, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 82.Lu L, Sun R, Chen R, Hui C, Ho C, et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther. 2008;13:253–262. [PubMed] [Google Scholar]
- 83.Samberg ME, Orndorff PE, Monteiro-Riviere NA. Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology. 2011;5(2):244–253. doi: 10.3109/17435390.2010.525669. [DOI] [PubMed] [Google Scholar]
- 84.Arumugam SK, Sastry TP, Sreedhar SB, Mandal AS. One step synthesis of silver nanorods by autoreduction of aqueous silver ions with hydroxyapatite: an inorganic-inorganic hybrid nanocomposite. J Biomed Mater Res. 2007;80(2):391–398. doi: 10.1002/jbm.a.30895. [DOI] [PubMed] [Google Scholar]
- 85.Min Y, Akulut M, Kristairsen K, Golan Y, Israelachvili J. The role of interparticle and external forces in nanoparticle assembly. Nat Mater. 2008;7:527–538. doi: 10.1038/nmat2206. [DOI] [PubMed] [Google Scholar]
- 86.Zheng J, Hua Y, Xinjun L, Shanqing Z. Enhanced photocatalytic activity of TiO2 nano-structured thin film with a silver hierarchical configuration. Appl Surf Sci. 2008;254(6):1630–1635. [Google Scholar]
- 87.Juan L, Zhimin Z, Anchun M, Lei L, Jingchao Z. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int J Nanomedicine. 2010;5:261–267. doi: 10.2147/ijn.s8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagener M, Biogate AG. Silver based nanocomposites for antimicrobial medical devices. Proc Nano Europ. 2006;9(1):12–14. [Google Scholar]
- 89.Chang YY, Huang HL, Chen YC, Weng JC, Lai CH. Characterization and antibacterial performance of ZrNO–Ag coatings. Surf Coat Tech. 2012 http://dx.doi.org/10.1016/j.surfcoat.2012.05.084. [Google Scholar]
- 90.Bignozzi CA, Carinci F, Caramori S, Dissette V. Nanomaterial coatings for osteointegrated biomedical prosthesis. PCT Int Appl. 2008 WO 2008020460. [Google Scholar]
- 91.Makhluf S, Dror R, Nitzan Y, Abramovich Y, Jelinek R, Gedanken A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Adv Funct Mater. 2005;15(10):1708–1715. [Google Scholar]
- 92.Kang S, Pinault M, Pfefferle LD, Elimelech M. Single-walled carbon nanotubes exhibit strong antimicrobial activity. Langmuir. 2007;23(17):8670–8673. doi: 10.1021/la701067r. [DOI] [PubMed] [Google Scholar]
- 93.Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24(13):6409–6413. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- 94.Smart SK, Cassady AI, Lu GQ, Martin DJ. The biocompatibility of carbon nanotubes. Carbon. 2006;44(6):1034–1047. [Google Scholar]
- 95.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomedicine. 2011;6:1463–1473. doi: 10.2147/IJN.S22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schierholz JM, Rump AF, Pulverer G, Beuth J. Anti-infective catheters: novel strategies to prevent nosocomial infections in oncology. Anticancer Res. 1998;18:3629–3638. [PubMed] [Google Scholar]
- 97.Singh AV, Vyas V, Patil R, Sharma V, Scopelliti PS, Bongiorno G, et al. Quantitative Characterization of the Influence of the Nanoscale Morphology of Nanostructured Surfaces on Bacterial Adhesion and Biofilm Formation. PLoS ONE. 2011;6(9):e25029. doi: 10.1371/journal.pone.0025029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Colon G, Ward BC, Webster TJ. Increased osteoblast and decreased Staphylococcus epidermidis functions on nanophase ZnO and TiO2. J Biomed Mater Res Part A. 2006;78A(3):595–604. doi: 10.1002/jbm.a.30789. [DOI] [PubMed] [Google Scholar]
- 99.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res A. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 101.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface. 2007;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 102.Morones JR, Elechiguerra JL, Camacho A, Ramirez JT. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 103.Song HY, Ko KK, Oh LH, Lee BT. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cells Mater. 2006;11:58. [Google Scholar]
- 104.Albers CE, Hofstetter W, Siebenrock KA, Landmann R, Klenke FM. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicol. 2011 doi: 10.3109/17435390.2011.626538. 22013878. [DOI] [PubMed] [Google Scholar]
- 105.Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicol. 2010;4(3):319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- 106.Zhao J, Castranova V. Toxicology of nanomaterials used in nanomedicine. J Toxicol Environ Health B. 2011;14(8):593–632. doi: 10.1080/10937404.2011.615113. [DOI] [PubMed] [Google Scholar]
- 107.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sinha N, Yeow JTW. Carbon nanotubes for biomedical applications. IEEE Trans Nanobiosci. 2005;4(2):180–195. doi: 10.1109/tnb.2005.850478. [DOI] [PubMed] [Google Scholar]
- 109.Sirivisoot S, Pareta R, Webster TJ. Electrically controlled drug release from nanostructured polypyrrole coated on titanium. Nanotechnology. 2011;22(8):085101. doi: 10.1088/0957-4484/22/8/085101. [DOI] [PubMed] [Google Scholar]
- 110.Eberson CP, Hogan K, Moore D, Ehrlich MG. Effect of Low-Intensity Ultrasound Stimulation on Consolidation of the Regenerate Zone in a Rat Model of Distraction Osteogenesis. J Ped Orthop. 2003;23(1):46–51. [PubMed] [Google Scholar]
- 111.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 112.Barranco SD, Spadaro JD, Berger TJ, Becker RO. In vitro effect of weak direct current on Staphylococcus aureus. Clin Orthop. 1974;100:250–255. [PubMed] [Google Scholar]
- 113.Berger TJ, Spadaro JA, Chapin SE, Becker RO. Electrically generated silver ions: quantitative effects on bacterial and mammalian cells. Antimicrob Agents Chemother. 1976;9:357–358. doi: 10.1128/aac.9.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Becker RO, Spadaro JA. Treatment of orthopedic infections with electrically generated silver ions. J Bone Joint Surg. 1978;60:871–881. [PubMed] [Google Scholar]
- 115.Wysk RA, Sebastianelli WJ, Shirwaiker RA, Bailey GM, Charumani C, Kennett M, Kaucher A, Abrahams R, Fuller TA, Royer P, et al. Prophylactic bactericidal orthopedic implants – animal testing study. J Biomed Sci Eng. 2010;3(9):917–926. [Google Scholar]
- 116.Shirwaiker RA, Wysk RA, Kariyawasam S, Carrion H, Voigt RC. Micro-scale fabrication and characterization of a silver-polymer based electrically activated antibacterial surface. Biofabrication. 2011;3(1):015003. doi: 10.1088/1758-5082/3/1/015003. [DOI] [PubMed] [Google Scholar]
- 117.Mader JT, Nordern C, Nelson JD, Calandra GB. Evaluation of new anti-infective drugs for the treatment of osteomyelitis in adults. Clin Infect Dis. 1992;15(Suppl. 1):S155–S161. doi: 10.1093/clind/15.supplement_1.s155. [DOI] [PubMed] [Google Scholar]
- 118.Slawson RM, Vandyke MI, Lee H, Trevors JT. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid. 1992;27:72–77. doi: 10.1016/0147-619x(92)90008-x. [DOI] [PubMed] [Google Scholar]
- 119.Burrell R, Heggers J, Wright J. Efficacy of silver coated dressings as bacterial barriers in a rodent burn sepsis model. Wounds. 1999;11:64–71. [Google Scholar]
- 120.Stobie N, Duffy B, Hinder SJ, McHale P, McCormack DE. Silver doped perfluoropolyetherurethane coatings: antibacterial activity and surface analysis. Colloids Surf. 2009;72:62–67. doi: 10.1016/j.colsurfb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 121.Gardner SE, Frantz RA, Schmidt FL. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen. 1999;7(6):495–503. doi: 10.1046/j.1524-475x.1999.00495.x. [DOI] [PubMed] [Google Scholar]
- 122.Kloth LC, Feedar JA. Acceleration of wound-healing with high-voltage, monophasic, pulsed current. Phys Ther. 1988;68(4):503–508. doi: 10.1093/ptj/68.4.503. [DOI] [PubMed] [Google Scholar]
- 123.Feedar JA, Kloth LC, Gentzkow GD. Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther. 1991;71(9):639–649. doi: 10.1093/ptj/71.9.639. [DOI] [PubMed] [Google Scholar]
- 124.Lundeberg TCM, Eriksson SV, Malm M. Electrical nerve stimulation improves healing of diabetic ulcers. Ann Plast Surg. 1992;29:328–331. doi: 10.1097/00000637-199210000-00009. [DOI] [PubMed] [Google Scholar]
- 125.Baker LL, Chambers R, DeMuth SK, Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care. 1997;20(3):405–412. doi: 10.2337/diacare.20.3.405. [DOI] [PubMed] [Google Scholar]
- 126.Wellman N, Fortun SM, McLeod BR. Bacterial biofilms and the bioelectric effect. Antimicrob Agents Chemother. 2006;40:2012–2014. doi: 10.1128/aac.40.9.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Van der Borden AJ, van der Werf H, van der Mei C, Busscher HJ. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl Environ Microbiol. 2004;70(11):6871–6874. doi: 10.1128/AEM.70.11.6871-6874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of staphylococcus and pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother. 2009;53(1):41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yonemori K, Matsunaga S, Ishidou Y, Maeda TS, Yoshida H. Early effects of electrical stimulation on osteogenesis. Bone. 1996;19(2):173–180. doi: 10.1016/8756-3282(96)00169-x. [DOI] [PubMed] [Google Scholar]
- 130.Yarington CT, Jaquiss GW. Electrical control of bone growth in ossicles. Arch Otolaryngol. 1969;89:856–860. doi: 10.1001/archotol.1969.00770020858011. [DOI] [PubMed] [Google Scholar]
- 131.Korenstein R, Somjen D, Fischler H, Binderman I. Capacitative pulsed electric stimulation of bone cells. Induction of cyclic-AMP and DNA synthesis. Biochem Biophys Acta. 1984;803:302–307. doi: 10.1016/0167-4889(84)90121-6. [DOI] [PubMed] [Google Scholar]
- 132.Friedenberg ZB, Zerasky LM, Pollis RP, Brighton CT. The response of non-tranmatized bone to direct current. J Bone Jt Surg. 1974;56A:1023–1030. [PubMed] [Google Scholar]
- 133.Lavine LS, Lustrin I, Shamos MH, Moss ML. The influence of electric current on bone regeneration in vivo. Acta Orthop Scand. 1971;42:305–314. doi: 10.3109/17453677108989050. [DOI] [PubMed] [Google Scholar]
- 134.Uysal T, Amasyali M, Olmez H, Karslioglu Y. Stimulation of bone formation by direct electrical current in an orthopedically expanded suture in the rat. Korean J Orthod. 2010;40(2):106–114. [Google Scholar]
- 135.Brighton CT, Black J, Friedenberg ZB, Esterhai JL, Day LJ, Connolly JF. A multicenter study of the treatment of non-union with constant direct current. J Bone Joint Surg Am. 1981;63(1):2–13. [PubMed] [Google Scholar]
- 136.Ercan B, Webster TJ. The effect of biphasic electrical stimulation on osteoblast function at anodized nanotubular titanium surfaces. Biomaterials. 2010;31(13):3684–3693. doi: 10.1016/j.biomaterials.2010.01.078. [DOI] [PubMed] [Google Scholar]
- 137.Shao S, Zhou S, Li L, Li J, Luo C, Wang J, et al. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials. 2011;32(11):2821–2833. doi: 10.1016/j.biomaterials.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 138.Gao L, Feng B, Wang J, Lu X, Liu D, Qu S, Weng J. Micro/Nanostructural Porous Surface on Titanium and Bioactivity. J Biomed Mater Res Part B: Appl Biomater. 2008;89(2):335–341. doi: 10.1002/jbm.b.31221. [DOI] [PubMed] [Google Scholar]
- 139.Le Gu’ehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 140.Wong M, Eulenberger J, Schenk R, Hunziker E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res. 1995;29:1567–1575. doi: 10.1002/jbm.820291213. [DOI] [PubMed] [Google Scholar]
- 141.Becker W, Becker BE, Ricci A, Bahat O, Rosenberg E, Rose LF, Handelsman M, Israelson H. A prospective multicentre clinical trial comparing one-and two-stage titanium screwshaped fixtures with one-stage plasma-sprayed solid-screw fixtures. Clin Implant Dent Relat Res. 2000;2:159–165. doi: 10.1111/j.1708-8208.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 142.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 143.Auffan M, Rose J, Bottero JV, Lowry GV, Jolivet JP, et al. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat Nanotechnol. 2009;4(10):634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 144.Ramires PA, Romito A, Cosentino F, Milella E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials. 2001;22(12):1467–1474. doi: 10.1016/s0142-9612(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 145.Laurencin CT, Kumbar SG, Nukavarapu SP. Nanotechnology and orthopedics: a personal perspective. WIREs Nanomed Nanobiotechnol. 2009;1:6–10. doi: 10.1002/wnan.25. [DOI] [PubMed] [Google Scholar]
- 146.Mehta S, Parvizi J. Nanotechnology in orthopaedic surgery. US Musculoskeletal Review. 2009;4(1):8–10. [Google Scholar]
- 147.Panyala NR, Pena-Mendez EM, Havel J. Silver or silver nanoparticles: a hazardous threat to the environment and human health? J App Biomed. 2008;6:117–129. [Google Scholar]
- 148.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18:225103. doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]