Abstract
The ability of pluripotent stem cells to self-renew and differentiate into all somatic cell types brings great prospects to regenerative medicine and human health. However, prior to clinical applications, much translational research is required to ensure that their therapeutic progenies are functional and non-tumorigenic, that they are stable and do not de-differentiate, and that they do not elicit immune responses that could threaten their survival in vivo. For this, an in-depth understanding of their biology, genetic and epigenetic makeup, and their antigenic repertoire is critical for predicting their immunogenicity and for developing strategies needed to assure successful long-term engraftment. More recently, the expectation that reprogrammed somatic cells would provide an autologous cell therapy for personalized medicine has been questioned. Induced pluripotent stem (iPS) cells display several genetic and epigenetic abnormalities that could promote tumorigenicity and immunogenicity in vivo. Understanding the persistence and effects of these abnormalities in iPS cell derivatives is critical to allow clinicians to predict graft fate following transplantation, and to take requisite measures to prevent immune rejection. With clinical trials of pluripotent stem cell therapy on the horizon, the importance of understanding immunological barriers and devising safe, effective strategies to bypass them is further underscored. This approach to overcome immunological barriers to stem cell therapy can take advantage of the validated knowledge acquired from decades of hematopoietic stem cell transplantation.
Keywords: immunogenicity, embryonic stem cells, induced pluripotent stem cells, transplantation, tolerance, patient-specific therapy, stem cell therapeutics
Introduction
Pluripotent stem cells can differentiate into cell types of the three primary embryonic germ layers, and therefore have extraordinary potential for regenerative medicine. James A. Thomson1 and Benjamin E. Reubinoff2 pioneered the development and differentiation of human embryonic stem (ES) cell lines over a decade ago. Following their discovery, these cells have been under intense investigation as a source of functional cells to augment damaged tissue function and to treat degenerative diseases. In animal models, differentiated ES cells have demonstrated regenerative capabilities in treating spinal cord injury3, diabetes4, Parkinson’s disease5, liver failure6, and myelin disease.7 Pluripotent stem cells have also generated great excitement for cardiovascular regenerative medicine, as they can be differentiated to functional cardiomyocytes8–10 and, as a part of biological pacemakers, can be grafted into injured myocardium.11, 12 Despite these promising results, immunological constraints associated with the transplantation of pluripotent stem cell derivatives have not been adequately addressed and remain one of the greatest obstacles to cell replacement therapy.
It was originally thought that pluripotent stem cells would be capable of evading immune surveillance and rejection due to their low expression of major histocompatibility complex (MHC) class I, MHC class II, and costimulatory molecules,13 and due to the expression of immunomodulatory molecules such as perforin-deactivating Serpin-6 (endogenous inhibitor of granzyme B)14 and transforming growth factor-β (TGF-β), which both inhibit T cell proliferation.15 However, this initial enthusiasm was dampened by evidence that pluripotent stem cells do elicit a donor-specific immune response in immunocompetent mice.16, 17 Indeed, transplanted allogeneic and xenogeneic ES cells and their derivatives are not immune-privileged, and therefore may encounter the same immunological barriers as any other grafts.
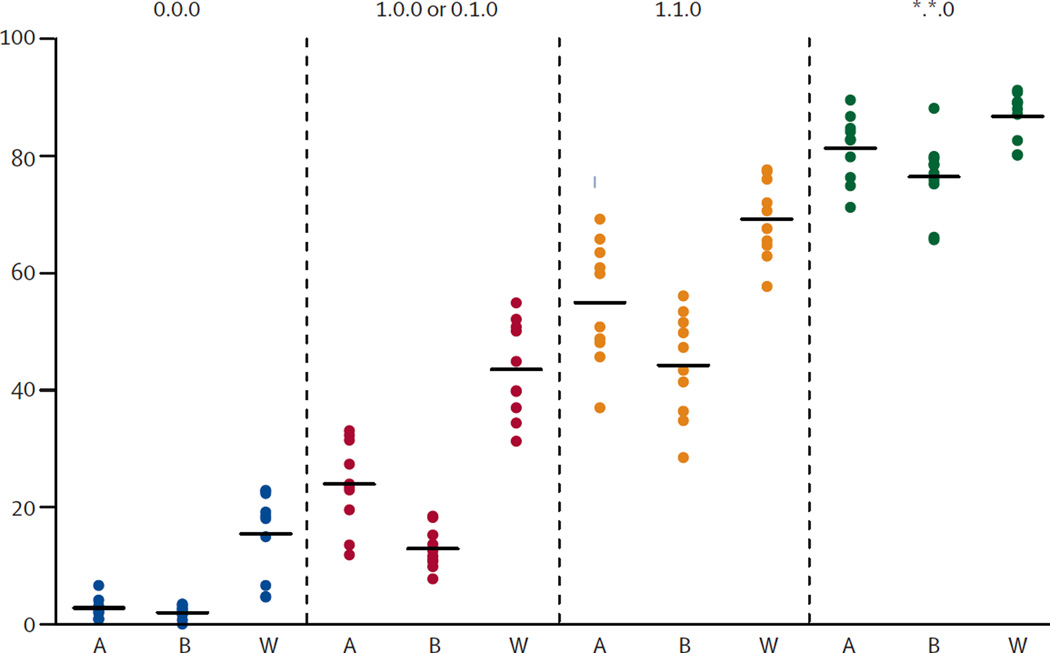
In an effort to minimize immunological rejection of transplanted ES cell derivatives, Taylor and colleagues18 devised a strategy to create a human ES cell bank from donated surplus embryos with sufficient HLA diversity to provide a HLA match for a reasonable percentage of the population in the United Kingdom. They predicted that a bank of 150 human ES cell lines would provide a full match at HLA-A, HLA-B, and HLA-DR for 20% of potential recipients; a beneficial match (defined as one HLA-A or one HLA-B mismatch only) or better for 37.9% of recipients; and an HLA-DR match or better for 84.9% of recipients (Figure 1). The results were calculated based on a criteria used clinically for kidney and heart transplantation, in which matching of blood group and of three out of nine MHC loci is considered sufficient and acceptable. Predictions such as these, however, have limited clinical value, and it remains unclear what level of disparity in MHC loci would warrant acceptance versus rejection of stem cell-derived grafts in humans. Moreover, recent research has indicated that matching MHC molecules alone is insufficient to guarantee tolerance to in vitro differentiated ES cells, as variance at the minor histocompatibility loci alone has been shown to induce rejection.19 A better understanding of how varying levels of compatibility elicit varying levels of immune responses to pluripotent stem cells and their derivatives in vivo is required to validate or revise cell line banking predictions, and would further aid in the progression of stem cell therapy toward clinical translation. Here, we review current knowledge of the immunogenicity of pluripotent stem cells and their progenies, discuss mechanisms of graft rejection, and present possible strategies to prevent immunological rejection.
Figure 1.
Percentage of Asian (A, n=797), Black (B, n=441), and White (W, n=5087) patients HLA matched using ten cohorts of 150 cadaveric organ donors. HLA mismatch grades was based on criteria used for allocation of cadaveric kidney donors in the UK: 1) zero HLA-A, HLA-B, and HLA-DR mismatch (0.0.0); 2) zero HLA-DR mismatch with no more than a single HLA-A or HLA-B mismatch (1.0.0 or 0.1.0); 3) zero HLA-DR mismatch with no more than a single HLA-A and a single HLA-B mismatch (1.1.0); 4) zero HLA-DR mismatch (*.*. 0). Reprint with permission.18
Hope for Immunocompatible Pluripotent Stem Cell Therapy
Hurdles associated with ES cell-based therapy have led to interest in a more readily accessible alternative with potential to be immunologically matched to the recipient. In 2006, Takahashi and Yamanaka narrowed down a list of transcription factors over-expressed in ES cells to four factors: octamer-binding transcription factor 4 (Oct4), SRY (sex determining region Y)-box 2 (Sox2), Krueppel-like factor 4 (Klf4), and c-myelocytomatosis viral oncogene homolog (c-Myc). When expressed retrovirally, these transcription factors were capable of reprogramming fibroblasts to an embryonic-like state.20, 21 Known as induced pluripotent stem (iPS) cells, they have revolutionized the field of stem cell research by demonstrating somatic cell plasticity and offering an appealing solution to the problem of immune rejection for stem cell-derived therapeutics. The derivation of ES-like cells from somatic tissues ignited the possibility of pursuing exciting avenues for patient-specific cell therapy, and as a platform for drug screening and disease modeling.22–24 Moreover, these cells represent a possible solution to the ethical objections that have been raised against the use of human ES cells.
Initial studies looking at the biology of iPS cells compared to ES cells showed they have similar morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity.20, 25 Nevertheless, a rapidly accumulating body of work suggests that considerable differences exist between these two pluripotent cell types, including important aspects such as their global gene expression,26 single cell transcription signature,27 epigenetic landscape,28, 29 genomic imprinting,30 and somatic mutations.31 These deficiencies represent a significant hurdle to the clinical value of iPS cells as therapeutics. For example, genomic alterations acquired during the reprogramming of somatic cells and also during the differentiation of iPS cells to a desired cell type may increase not only the tumorigenicity of these cells,32 but also generate potentially immunogenic “neoantigens” that could elicit immune responses even in a MHC-matched context.33 In support of this premise, a recent study has demonstrated that iPS cells carry a high incidence of duplications on chromosome 1234, resulting in significant enrichment of cell cycle-related genes. Such aneuploidy may affect the differentiation capacity of iPS cells, and also increase their tumorigenicity and possibly their immunogenicity.33
Very limited research has been done to determine whether clinically relevant therapeutic cells derived from autologous iPS cells are non-immunogenic or whether they possess some level of “autogenicity” (ability of a particular autologous substance to provoke an immune response in the body of a human or animal). If proven autogenic, the high costs and the length of time needed to produce adequate quantities of patient-specific iPS cell-derived therapeutics may not justify their use over allogeneic ES cells. As more systematic investigations into the immunobiology of iPS cells begin, the goal of bypassing immunologic barriers—even when transplanted autologously—remains only a possibility rather than a reality. The recent demonstration by Zhao and colleagues35 that mouse iPS cells are rejected in syngeneic recipients suggests that stringent screening for incompatibilities between the donors and recipients of stem cell-derived cellular therapeutics may be required not only for transplantation of allogeneic cells but also autologous cells.
Immunogenic Molecules of Pluripotent Stem Cells
Major Histocompatibility Antigens
The major histocompatibility complex, termed Human Leukocyte Antigen in humans, consists of glycoproteins encoded by highly polymorphic genes on chromosome 6 that are co-dominantly expressed on the surface of almost all vertebrate cells. MHC encodes the main molecular targets of allograft rejection and MHC-associated incompatibilities between donors and recipients are responsible for almost all acute rejection. MHC is critical for the development of an adaptive immune response against pathogenic and foreign antigens as it contains a groove into which the antigen binds and is presented to T cells. In most species, each class of MHC is represented by more than one locus (polygeny). In humans, the class I loci are HLA-A, -B and –C, which are expressed on every nucleated somatic cell. The class II loci, including HLA-DR, -DQ and -DP, are expressed mostly on antigen presenting cells (APCs) such as dendritic cells and macrophages. It is unclear why T cells should ever recognize foreign HLA molecules as they do in an allogeneic transplantation setting, but an estimated 1% to 10% of the T cell pool can react with intact allogeneic HLA during direct T cell allorecognition. As with any other tissue type, histocompatibility appears to be an important factor in the rejection of undifferentiated ES cells. ES cell rejection is accelerated when MHC molecules are upregulated during differentiation,36 interferon (IFN)�γ stimulation,37 or after teratoma formation.9, 17, 38–40 These results suggest that ES cells and potentially their progeny can become more immunogenic if transplanted into an environment that promotes upregulation of MHC (e.g., inflammatory environment). HLA matching as a criterion for transplantation of stem cell-derived grafts may reduce the possibility of eliciting an immune response, but may not be sufficient to promote graft acceptance. Manipulation of HLA expression on stem cells has recently shown promise as a strategy for generating hypoimmunogenic grafts.41 The same strategy has been previously used to facilitate transplantation of hematopoietic stem cells (HSCs).42 The clinical applicability of this strategy, however, remains questionable, as it requires genetic manipulation to knock down a gene, and may lead to the introduction of genetic variations. Knocking down HLA is yet another double-edged sword, as it can increase the susceptibility of cells to NK cell-mediated killing.43–45
Minor Histocompatibility Antigens
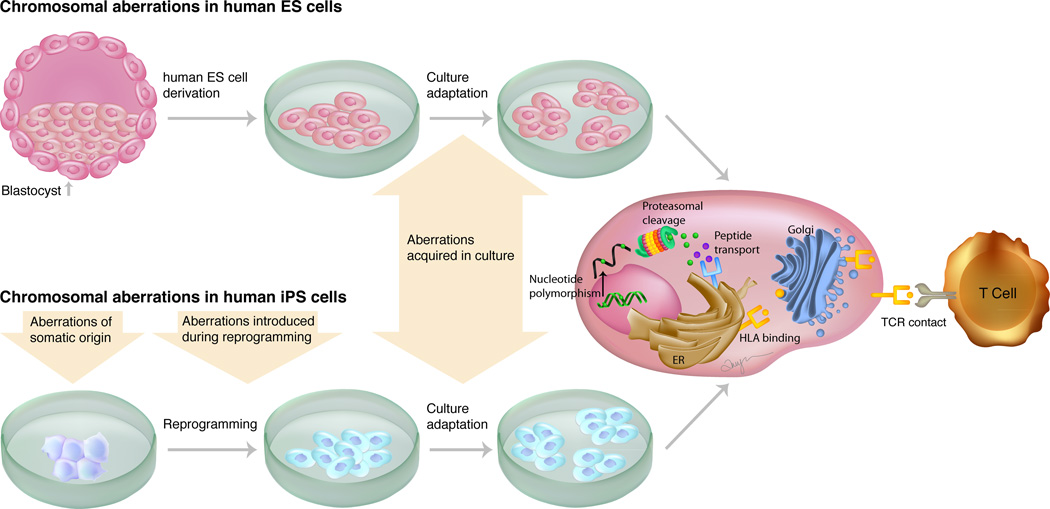
Identical HLA phenotype is not sufficient to guarantee graft survival. The role of non-HLA histocompatibility antigens such as minor histocompatibility antigens (miHA) in the context of immunological rejection of pluripotent stem cells and their derivatives remains murky. miHAs are peptides derived from normal cellular proteins that show polymorphism among related and unrelated individuals, and when transplanted, can be sufficiently antigenic to induce CD4+ and CD8+ T cells alloresponses.46, 47 The importance of miHA in human transplantation is proven by the observation that even MHC-identical sibling pairs can develop T cell-mediated graft failure,48 severe graft-versus-host disease (GVHD),49, 50 and graft-versus-leukemia (GVL) effect.51 Robertson and colleagues19 transplanted murine ES cell-derived embryoid bodies (EB) in MHC-matched mice that differed in their expression of miHAs. They demonstrated that full concordance at the MHC loci between donor and recipient mice was insufficient to promote acceptance of murine ES cell-derived EB as they were vigorously rejected at a rate similar to fully allogeneic EB. In transplantation of ES or iPS cell-derived therapeutics, natural miHA incompatibilities between donor and recipient may be accentuated by factors such as residual expression of embryonic antigens (e.g., Oct4)52 present in the graft and ectopic expression of miHAs acquired during reprogramming stress or during in vitro culture adaptation (Figure 2).35, 52 Additionally, pluripotent stem cell-derived therapeutics may incorporate immunogenic miHA as a results of exposure to animal product-containing media and/or to media containing non-physiologic constituents. Epitopes derived from these incorporated neoantigens may augment immunogenicity.53 For example, a recent study demonstrated that prolonged culture of human ES cells in animal-free knockout serum and high ascorbate levels resulted in ectopic expression of CD30.54 Incompatible miHA peptides can be presented directly on self-MHC class I to CD8+ T cells that destroy the therapeutic graft or through APCs that process and present miHA peptides to T cells, eliciting an alloresponse.55 However, the extent and severity by which miHA will influence immune response against pluripotent stem cell-derived therapeutics remains to be determined. If proven important, the optimization of culture conditions and reprogramming technique could prove crucial to the clinical translation of this technology. Also, the identification of potentially immunodominant miHA among pluripotent stem cell-derived progenies would facilitate screening for incompatibilities between donors and recipients and prediction of immunogenicity in vivo.
Figure 2.
Mechanisms for generating minor histocompatibility antigens in pluripotent stem cells. Polymorphisms induced in ES and iPS cells can result in expression of proteins and peptides that are distinct from those in the donor cells. Upon proteolytic degradation, these peptides are transported by the peptide transporter into the endoplasmic reticulum (ER), where they can bind to HLA molecules and pass through the Golgi apparatus to be presented at the cell surface as a complex with HLA and be recognized as foreign by donor T cells.
The ABO Blood Group
Primates express ABO blood group antigens that are displayed on the surface of red blood cells, epithelial cells, and vascular endothelial cells.56 Bacteria colonizing the gastrointestinal tract display carbohydrate structures that are similar to the oligosaccharide structures which comprise the ABO. To confer host protection against gastrointestinal bacteria, there is a natural production of immunoglobulin (Ig)M and IgG antibodies. These naturally occurring antibodies can cause antibody-mediated rejection of ABO-incompatible organ transplants. Recent studies have shown that human ES cells, as well as differentiated hepatocytes and cardiomyocyte-like cells,57 express ABO antigens.58 Therefore, the transplantation of an ABO-expressing stem cell derived-graft in an ABO-mismatched recipient could prompt an antibody-mediated hyperacute rejection by activating the complement cascade, thereby eliciting a complement-dependent target cell injury.59 Information about ABO expression in the various stem cell progenies is required prior to clinical translation, and the use of therapeutics derived from human ES and iPS cells from blastocysts of blood group O should be prioritized.60
Killer Immunoglobulin-like Receptors (KIR)
Natural Killer (NK) cells are key innate immune lymphocytes that play a critical role in recognizing self-MHC class I through a unique class of receptors called NK cell receptors (NKRs). While in an autologous setting, NK cells can kill cells that express low HLA class I molecules; in an allogeneic environment, they can kill cells that express HLA class I that are not recognized by their inhibitory KIRs.61, 62 The importance of the KIR family of NKRs for transplantation and its role in the rejection of MHC-matched organs and cells are becoming increasingly evident, especially for HSC transplantation. The great variations in gene content, gene copy number, and allelic polymorphism within individual KIR genes result in significant diversity in KIR haplotypes among individuals. It remains to be seen whether KIR-matching between donors and recipients will need to be considered prior to the transplantation of stem cell derivatives.
Pluripotent Stem Cells Meet the Immune System: Pathways to Allorecognition
The human immune system evolved in a hostile environment inhabited by pathogens, and consequently has developed productive responses against both pathogens and foreign cells. The best-known cell types responsible for the direct killing of pathogenic cells are cytotoxic CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), and NK cells. During an infection, both NK cells and CD8+ T cells are activated via antigen-specific receptors and by pro-inflammatory cytokines produced by auxiliary CD4+ T cells (also known as helper T cells) and APCs. The success of transplantation is largely limited by the activation of some of these same mechanisms. CTLs, helper T cells, and NK cells have been shown to hinder the survival of undifferentiated stem cells and embryonic stem cell-derived vascular progenitors in vivo,17, 45, 63 suggesting that these same mechanisms will pose an obstacle for stem cell-based therapy. However, the exact pathway(s) leading to immune reactivity against these cells remains unknown.
The adaptive immune response is usually necessary and sufficient to reject allografts and this also seems to be the case in rejection of stem cells and their cellular derivatives, where T cells emerge as pivotal players.17, 19, 36 Donor-derived MHC antigens expressed by an allograft almost always trigger T cell allorecognition. T cell receptor (TCR)-mediated recognition of the MHC antigens can occur essentially by two distinct pathways: (1) T cells recognize peptides complexed to donor MHC molecules displayed on the surface of the transplanted cells (direct pathway); or (2) T cells interact with processed donor-derived peptides bound to MHC molecules on self APCs (indirect pathway) (Figure 3).64, 65 Increasing evidence supports the role of the indirect pathway in acute and chronic rejection of various grafts,66, 67 and most recently, in rejection of stem cells. Rejection of pluripotent stem cells has been proposed to involve 3 main developmental stages. The first stage is intra-graft delayed-type hypersensitivity, during which recipient MHC class II-restricted CD4+ T cells recognize alloantigens presented by recipient APCs and release graft-damaging pro-inflammatory cytokines. The second stage is CTL response, during which self-restricted CD4+ T cells help generate CTLs that can recognize intact allogeneic MHC class I molecules. The third stage is alloantibody response, during which alloantigen-primed CD4+ T cells deliver activating signals to B cells. However, considering the many layers of redundancy in the immune system, the pathways that lead to rejection may be diverse.
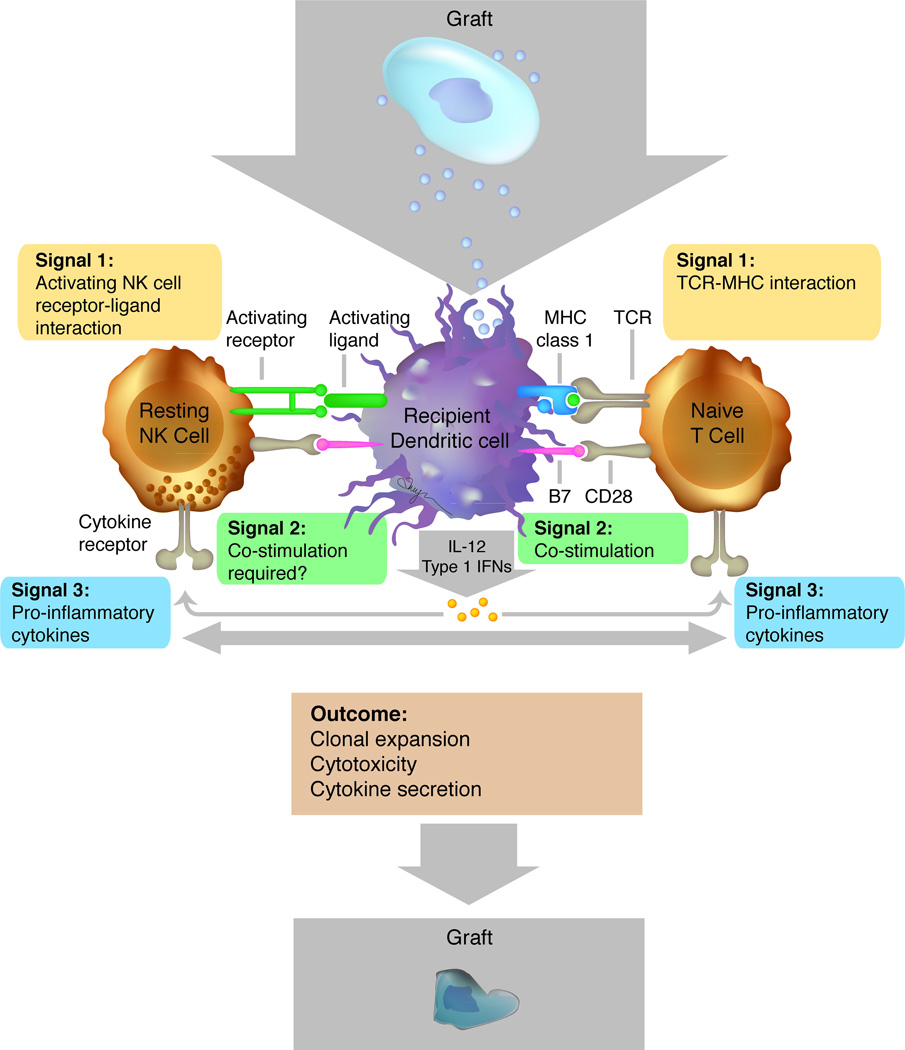
Figure 3.
Simplified schema exemplifying an immune response to a stem cell-derived cellular therapeutic. Dendritic cells acquire antigens from the graft for presentation to T cells and NK cells, which mount specific responses following antigen receptor activation (Signal 1). Upon TCR–MHC interactions, co-stimulation (Signal 2) and pro-inflammatory cytokines (Signal 3), such as interleukin-12 (IL-12) and type I IFNs, can promote the activation and clonal expansion of T cells. Similarly, resting NK cells may also receive signals via activating receptors (Signal 2) and pro-inflammatory cytokine receptors (Signal 3). Activated T cells and NK cells can generate cytotoxic responses against the graft, resulting in rejection. Figure adapted from Sun & Lanier (2011).109
It was originally proposed that the direct pathway of allorecognition could be easily mitigated by eliminating APCs from the graft prior to transplantation.68 However, certain types of pluripotent stem cell derivatives such as endothelial cells may elicit an immune response by interacting with T cells in an antigen-specific manner.69 Endothelial cells are capable of acting as non-professional APCs and, upon upregulation of MHC class II molecules, can present antigens in a MHC-restricted fashion. Consequently, these cells can mediate the direct pathway of allorecognition. Such a pathway of allorecognition has been demonstrated to compromise the survival of allografts.70, 71 Therapeutic use of ES or iPS cell-derived endothelial cells will likely require addressing this pathway. This example underscores how the mechanisms leading to allorecognition may vary depending on the nature of the graft, further emphasizing the fact the need for graft-specific strategies to adequately manipulate the immune system and prevent rejection.
Several investigators claim that undifferentiated ES cells are resistant to NK cell attack in vitro and in vivo,17, 40, 72 while others report that stem cells do express NK cell-activating ligands and are susceptible to NK cell attack.38, 44 In a study that compared the susceptibility of undifferentiated versus differentiated mouse ES cell-derived cardiomyocytes to NK cell-mediated destruction, undifferentiated ES cells were deemed susceptible to NK cell destruction in a perforin-dependent manner.44 This was attributed to the expression of the NK cell receptor, natural-killer group 2 member D (NKG2D), and the expression of the intercellular adhesion molecule 1 (ICAM-1). In support of these findings, NKG2D has also been detected in many other mouse pluripotent stem cells.38, 43 Interestingly, mouse ES cell-derived cardiomyocytes were not susceptible to NK cell killing, even after stimulation with IFN-γ and retinoic acid, which is known to mediate expression of NKG2D ligands.44 Whether the hypoxic and inflammatory microenvironment of the infarcted heart plays a role in damping the NK cell response remains to be investigated.
Innate vs Adaptive Immune Responses to Pluripotent Stem Cells Grafts: Insights From Immunodeficient Animal Models
Pluripotent mouse72, 73 and human74, 75 stem cells have been studied extensively in immunodeficient recipients. Although the immunobiology of stem cells was not the primary focus, these studies indirectly provided evidence for the absence of specific immune cells in promoting graft survival, and conversely for their role in clearing teratomas. The pivotal role of T cells in immune rejection of stem cells has been demonstrated using T cell-deficient mice76, 77 and rats.39, 78 These studies showed that injection of ES cells is readily followed by teratoma formation. Drukker and colleagues77 comprehensively investigated immune responses to ES cells using NOD/SCID (T- and B cell-deficient), BALB-nude (T cell-deficient), C57BL/6-Lystbg (NK cell-deficient), and CBA/CaHN Btkxid (B cell-deficient) mice. Five weeks after transplantation of ES cells, teratoma formation was detected only in the T and B cell-deficient NOD/SCID mice. By contrast, transplantation of human ES cells to Lystbg (NK cell-deficient) and Btkxid (B cell-deficient) mice led to vigorous rejection and failure to develop teratoma. These experiments demonstrated that xenorejection of human ES cells is T cell-mediated, and that NK or B cells play only a minor role in this process. The role of the complement pathway has also been investigated using an immunodeficient mouse model. Using complement 3 (C3)-deficient mice, Koch and colleagues showed that the homologous complement delays the formation and growth of mouse ES cell-derived teratomas and can completely prevent teratoma formation when a low number of mouse ES cells are implanted (1 × 105).79 The authors attributed the susceptibility of ES cells to the complement pathway to low expression of sialic acid. Koch and colleagues also investigated the susceptibility of ES cells to B cells and antibodies using JH−/− mice (B cell-deficient). Their results confirmed that B cells and antibodies are not critical for immune rejection of ES cells.
The innate immune response to human ES cells80 and mouse ES cells38 has also been studied using immunodeficient mice. Among the cells of the innate immune system, NK cells are the best characterized in the context of stem cell rejection. Mouse ES cell-derived teratomas grew significantly faster in SCID/beige mice (T, B, and NK cell-deficient) compared to SCID (T and B cell-deficient).38 NK cell responses to ES cells have been shown to modulate teratoma growth in syngeneic, allogeneic, and xenogeneic immunological conditions.43 Upon activation, NK cells have been shown to prevent teratoma formation in 50% of SCID recipients, and teratoma growth, where present, was decelerated.43 Studies in knockout mice null for the recombination-activating gene-2 and cytokine receptors that contain the common γ-chain (RAG2−/−γc−/−) have implicated NK cells in rejection of hematopoietic stem cells.81 Conversely, ES cell-derived cardiomyocytes44 and vascular progenitor cells45 were not deemed susceptible to NK cell killing. This observation was attributed to the upregulation in MHC-I expression that occurs with differentiation. Conflicting results in susceptibility of NK cell killing described between hematopoietic cells versus pluripotent stem cell-derived cardiomyocytes and endothelial cells suggest that the immune response pathways involved in rejection of the various stem cell derivatives cannot be generalized.
Rejection of Pluripotent Stem Cells in Different Histocompatibility Settings
Xenogeneic
ES cells transplanted xenogeneically at various anatomical sites (e.g., intra-muscularly, under the kidney capsule, subcutaneously, etc.) elicit both innate and adaptive immune responses. Upon intra-muscular injection, macrophages, neutrophils, B cells, and CD4+ and CD8+ T cells infiltrate human ES cell grafts.17, 36 Evidence indicates that rejection of undifferentiated human ES cells is largely T cell-mediated, while NK cells and B cells play only a minor role.17 However, in a rat model, NK cells have been implicated in the killing of undifferentiated mouse ES cells.38 In this study, susceptibility of mouse ES cells to NK killing was attributed to the expression of ligands of the activating NK receptor NKG2D, which was downregulated in differentiated cells. More recently, evidence that implicates the involvement of T cells in the rejection of a clinically relevant cell type was brought up by Pearl et al.,82 who demonstrated that a regimen consisting of a short-term course of costimulatory blockers that mitigates T cell alloresponses could extend survival of the human ES cell-derived endothelial cells. In another study, human ES cell-derived cardiomyocytes were injected into immunocompetent rats after myocardial infarction and were shown to survive, proliferate, and integrate with host cardiac tissues.9 In this study, a combination of cyclosporine A and methylprednisone was used as immunosuppressive therapy and the viability of grafted cells were monitored for up to 8 weeks.
Allogeneic
The rejection of allogeneic undifferentiated mouse pluripotent stem cells has been analyzed by several studies, but limited information is available regarding alloresponses to progenies differentiated from these cells. Allogeneic undifferentiated mouse ES cells (2 × 105) injected into ischemic hearts of mice have demonstrated the ability to mobilize innate and adaptive immune responses that resulted in graft destruction 4 weeks post-transplantation.83 The allograft rejection coincided with infiltration of IFN-γ-producing CD3+ T cells and CD11c+ dendritic cells. In a similar study, a higher number of mouse ES cells (1 × 106) was injected into ischemic hearts, and immune cell infiltration was monitored over time at 1, 2, 4, and 8 weeks post-transplantation. A progressive infiltration of CD4+ and CD8+ T cells as well as macrophages, dendritic cells, and granulocytes was observed, predominantly at weeks 4 and 8 after cell implantation.40 Similar results were reported by a different study showing that at 5 weeks following implantation into the myocardium, mouse ES cells were rejected, coinciding with lymphocytic infiltrates.72 The survival of allogeneic mouse ES cells (1 × 106) implanted in the gastrocnemius muscle was also limited to approximately 4 weeks as monitored by longitudinal bioluminescence imaging.16 Furthermore, this study showed that rejection of allogeneic mouse ES cells was accelerated in mice that had been previously pre-sensitized with mouse ES cells, suggesting that an immunological memory specific to antigens expressed in mouse ES cells had developed. Strategies to abrogate memory T cells may have to be taken into account upon consecutive implantation of stem cell therapeutics. Similar to human endothelial cell progeny, rejection of allogeneic mouse iPS-derived neural progenitor cells has been abrogated by blockers of costimulatory molecules in T cells.82
In an attempt to investigate allogeneic immune responses to human ES cells, Drukker et al. utilized a “humanized” mouse model termed “Trimera.”17 Essentially, Trimera mice are immunodeficient mice reconstituted with human peripheral blood mononuclear cells. Transplantation of undifferentiated human ES cells (1 × 106), or differentiated teratoma fragments, or a teratoma-derived primary cell line, resulted in tumor formation in all three settings. By contrast, Trimera mice were able to completely eliminate Burkitt's lymphoma cells. The authors attributed these findings to the hypo-immunogenic nature of human ES cells and their derivative tissues (Figure 4). This hypo-immunogenic phenotype might limit the activation of direct allospecific T cell responses. Humanized mouse models represent a very promising platform to study human immune responses in vivo, but results from studies using these models need to be cautiously interpreted, as there is evidence that aspects of immune responses in this model may be dysfunctional (e.g., defective cytotoxic T cells and NK cells).84, 85 As a result of the lack of robust humanized mouse models, the allogeneic immune responses to human ES cells remain unknown. The opportunity to address some of these questions in immunologically healthy humanized animal models will contribute greatly to our understanding of immune responses that thus far have impaired the survival of transplanted pluripotent stem cells in vivo.
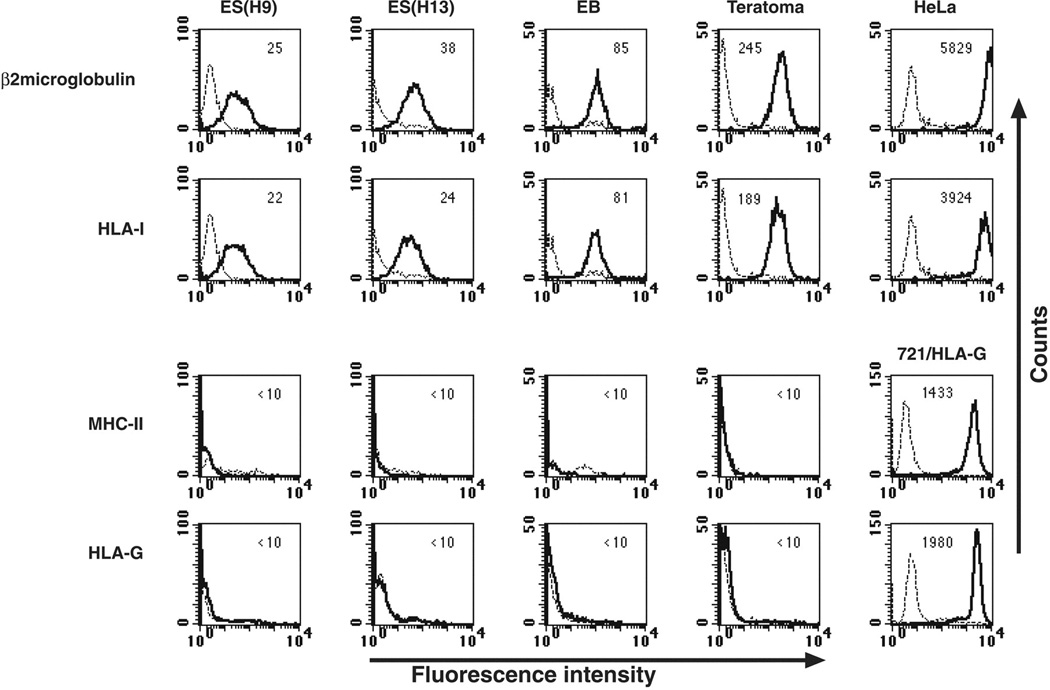
Figure 4.
Mean fluorescence intensity of various HLA proteins in various undifferentiated and differentiated human ES cell lines. The expression of HLA class I, HLA class II (HLA-DP,-DQ,-DR), and the non-classical HLA-I HLA-G was determined in two undifferentiated human ES cell lines (H9 and H13), embryonic bodies from in vitro differentiated human ES cells, in vivo differentiated human ES cells-teratomas; cervix epithelial cell line (HeLa). Dashed lines represent background control staining and solid lines demonstrate expression of specific antigens. Median fluorescence intensity staining is indicated at the top of each box. Reprint with permission.37
Syngeneic
Numerous studies have demonstrated that transplantation of mouse ES cells into syngeneic recipients leads to teratoma formation.40, 72 However, the characterization of immune responses to syngeneic and clinically relevant stem cell derivatives is absent or minimal at best. T and B cells as well as macrophages have been shown to infiltrate teratomas in syngeneic mice. While they were incapable of preventing tumor growth, these lymphocyte infiltrates did decelerate tumor growth.38 Interestingly, teratomas did not develop when a small number of ES cells were implanted.38, 86, 87 Implantation of 5 × 105 undifferentiated or differentiated syngeneic ES cells has been shown to result in teratoma formation in only 33% and 17% of recipients, respectively. On the other hand, implantation of high numbers of undifferentiated or differentiated cells (2 × 106) produced teratoma formation in 100% of recipients.38, 87 These results suggest that when low numbers of cells are implanted, cells either die immediately following transplantation or are rejected. Implantation of a higher number of cells may overcome immune responses due to intense proliferation or stem cell-mediated immunosuppression (e.g., release of TGF-β and IL-10).
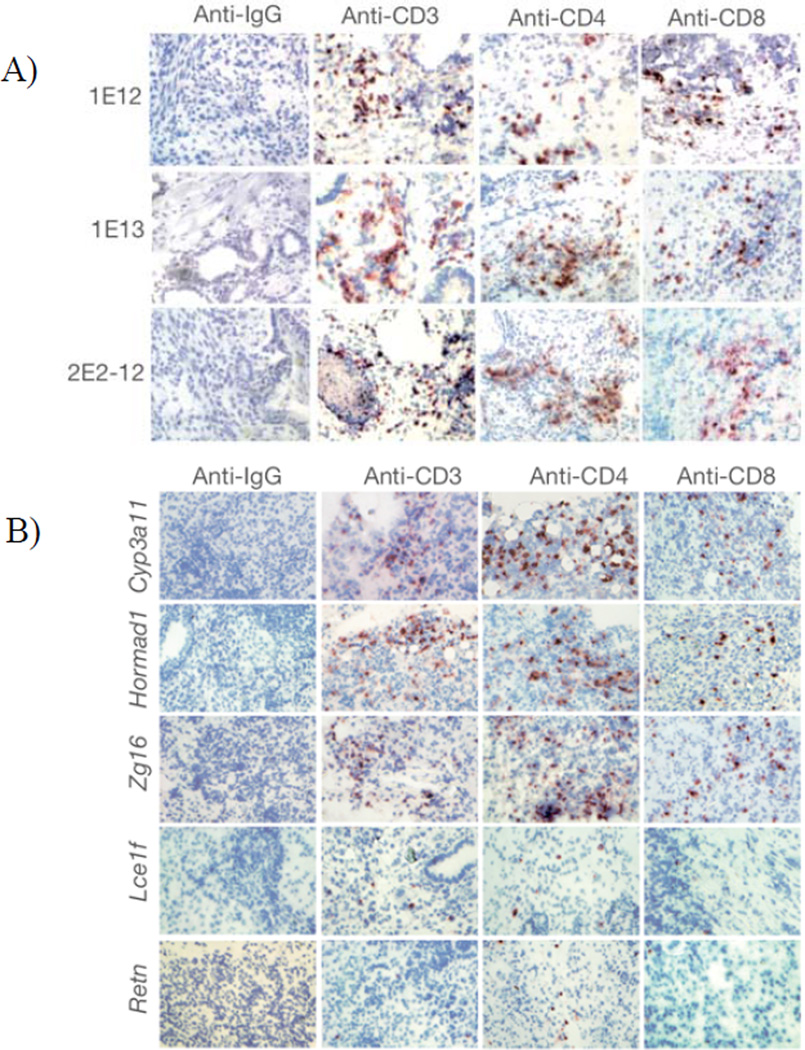
A recent study demonstrated that iPS cells are immunogenic and can be rejected even in MHC-matched recipients.35 CD4+ and CD8+ T cells were implicated in the rejection of syngeneic iPS cell grafts. They attributed their findings to the expression of aberrant genes (i.e., Hormad1, Zg16, and Cyp3a11) in iPS cells. They further validated their results by demonstrating specific T cell responses to Hormad1 and Zg16 in vitro and in vivo (Figure 5). Hormad1 and Zg16 have been identified as human tumor-associated antigens,88, 89 though no information was presented regarding the expression of these so-called immunogenic genes in human iPS cells. Although this study presented important information regarding the immunobiology of undifferentiated iPS cells, it did not evaluate whether clinically relevant iPS cell therapeutics possess similar immunogenicity. Another caveat was the use of only one syngeneic murine ES cell line as control. These omissions make it difficult to assess the significance of their findings and impossible to determine: (i) if “autogenicity” is a property exclusive to iPS cells or also exist in autologous somatic cells maintained in vitro for similar amount of time and in similar culture conditions; and (ii) whether there are any immunological benefits of using autologous iPS cells versus allogeneic ES cells. Results from this study are not entirely surprising considering that syngeneic iPS cells do express several oncofetal antigens, some of which are already known to be immunogenic (e.g., Oct4).52 Moreover, these cells are highly susceptible to chromosomal abnormalities due to the reprogramming process itself33 and culture adaptations.90 Nevertheless, results from this study suggest that no degree of matching between donors and recipients may be able to prevent rejection of stem cell therapeutics in the absence of immune intervention, and that screening for aberrant antigen expression in pluripotent stem cell therapeutics may be critical for predicting the risk of immunological rejection and immune intervention required.
Figure 5.
Extensive infiltration of T cells in regressing teratomas formed by syngeneic iPS and transgenic ES cells. A) T-cell infiltration in teratomas formed by syngeneic episomal-derived iPS cells from two different passages (1E-12, 1E-13) and after LoxP/Cre-mediated deletion of the reprogramming factor expression cassette from the integrated copy of episomal vector (2E2-12). B) Ectopic expression of Cyp3a11, Hormad1, and Zg16 in syngeneic mouse ES cells elicited infiltration of T cells in the teratomas. Few infiltrating T cells were detectable in the teratomas formed by Lce1f- and Retn-B6-expressing mouse ES cells. Reprint with permission.35
Prospects for Circumventing Immunogenicity
Evidence obtained from studies that used pluripotent stem cells and their progeny suggests that even full MHC concordance between donor and recipient may not guarantee survival stem cell therapeutics.19, 43 Some level of immunosuppression or tolerizing regimen will likely be required but it remains unknown the extent of immunological conditioning that will warrant acceptance of stem cell-derived therapeutics. In many transplantation scenarios, life-long immunosuppression has been used to accommodate residual antigen disparities between donor and recipient, ensuring the survival of the therapeutic graft. However, this approach can produce devastating toxic side effects, promote opportunistic infections, and increase patients’ susceptibility to malignancies. Because these adverse side effects can outweigh the potential curative benefits of stem cell-derived therapy, strategies to promote long-term tolerance with minimal immunosuppressive therapy are essential.
In a clinical setting, the benchmark for the establishment of tolerance means the complete and successful withdrawal of immunosuppressive drugs. Although tremendous progress has been made in the field of transplantation tolerance during the past half century (Table 1), durable donor-specific tolerance to prolong transplant survival in humans in the absence of immunosuppressants has not been consistently achieved. Mixed chimerism induced by HSC transplantation is known to promote donor-specific tolerance for over 40 years. In mixed chimeric animals, the continued presence of the organ donor’s bone marrow-derived cells in the recipient’s thymus and peripheral lymphoid tissue promotes and maintains immune tolerance by eliminating T cell clones that react to alloantigens of the graft.91 However, the use of HSCs to induce tolerance in the clinical setting has been unfeasible thus far, largely due to the unacceptable toxicity, morbidity, and mortality associated with the conditioning needed to achieve engraftment of allogeneic bone marrow, as well as complications such as GVHD. Significant efforts have been devoted to developing non-myeloablative methods for inducing mixed chimerism.92, 93 Short-term fractionated total lymphoid irradiation (TLI) has been successfully used to achieve mixed chimerism without GVHD in MHC mismatched mice strains.94 TLI radiotherapy is non-myeloablative and targets major lymphoid organs such as the spleen, thymus, and peripheral lymph nodes. This conditioning has been shown to promote engraftment of bone marrow cells and to promote immunological tolerance to skin and heart allografts that were transplanted concomitantly with donor bone marrow cells. When combined with anti-thymocyte serum (ATG), TLI has also achieved impressive results in post-transplant conditioning to promote organ allograft tolerance in rats.95 In humans, Samuel Strober has tested this regimen at Stanford University for induction of tolerance to kidney transplants. Out of 25 kidney transplant recipients conditioned with TLI and ATG, 15 patients had long-term functional grafts with minimal requirement for chronic immunosuppression.96
Table 1.
Examples of short-course regimens known to promote successful long-term acceptance of allogeneic or xenogeneic stem cells in mice. WBI = whole body irradiation; TI = thymic irradiation.
| Tolerance Regimen |
Treatment Course |
Mechanism of Action |
Findings | References |
|---|---|---|---|---|
| Indefinite survival of xenogeneic ES cells in testis but not in heart. | ||||
| CTLA4-Ig anti-CD40L anti-LFA1 |
6 to 8 days | Indefinite survival of xenogeneic ES and iPS cells, allogeneic ES and iPS cells in the leg muscle. | 82,110 | |
| CTLA4-Ig anti-CD40L 3Gy WBI |
2 days | Inhibits T cell activation by blocking CD28, CD40L, and LFA1 expressed on T cells. | Prolonged survival of xenogeneic ES cell-derived ECs and allogeneic iPS cell-derived NSCs. Permanent engraftment of fully MHC-mismatched allogeneic HSCs. Tolerance to donor skin. |
98,111 |
| Anti-CD40L 200cGy WBI |
14 days | Engraftment of fully MHC-mismatched HSCs. Long-term tolerance to donor skin. |
112 | |
| Non-depleting anti-CD8 & anti-CD4 |
4 days | Blocks CD8+ and CD4+ T cells from TCR-specific activation. Treg recruitment. |
Indefinite survival of allogeneic mouse ES cells and EBs. | 19,97 |
| Depleting anti-CD8 & anti-CD4 7Gy TI |
3 days | Depletes CD8+ and CD4+ T cells. | Permanent engraftment of fully MHC-mismatched allogeneic HSCs. Tolerance to donor skin. |
113 |
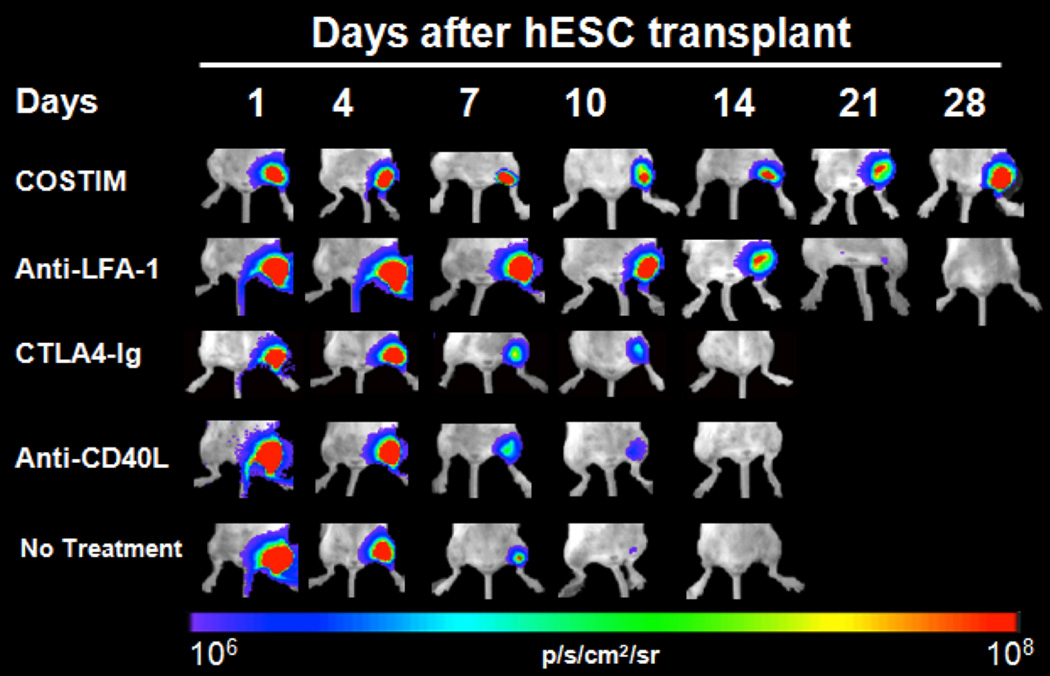
Some clinically available immunosuppressive regimens have been tested for enhancing the survival of undifferentiated human ES in vivo. Interestingly, the calcineurin inhibitor Tacrolimus, the target of rapamycin (TOR) inhibitor Sirolimus, and the anti-proliferative Mycophenolate Mofetil have been shown to provide only marginal improvements in the survival of human ES grafts.36 By contrast, the target-specific suppression of T cells has been much more effective in this context. The use of minimal host conditioning with non-depleting monoclonal antibodies against CD4 and CD8 was shown to induce long-term acceptance of ES cell-derived EBs implanted under the kidney capsule.19, 97 Costimulatory blockade in combination with a low dose total body irradiation (TBI) (3Gy) has been shown to promote stable mixed chimerism with high levels of engraftment of fully MHC-mismatched HSCs and to enhance tolerance to donor skin grafts.98 The costimulatory pathway is mediated by interactions of CD28 and CD40 ligand (CD40L, also called CD154) on T cells, and by B7 (CD80 and CD86) and CD40 on APCs. This pathway is of central importance for T cell-dependent immune responses, and its manipulation has improved survival of various grafts.99, 100 Optimal results have been obtained when CD28 and CD40 were blocked simultaneously.101, 102 A short-course blockade of the costimulatory receptors CD28, CD40 ligand (CD40L), and lymphocyte associated antigen-1 (LFA-1) on T cells alone has been recently shown to induce long-term acceptance of pluripotent stem cells as well as endothelial and neural progenitors (Figure 6).107, 108, 103 Interestingly, this regimen was remarkably more efficient in preventing rejection of pluripotent stem cell grafts than the conventional immunosuppressants Tacrolimus and Sirolimus. Despite these promising results, it is important to keep in mind that regimens proved efficient in preventing rejection of undifferentiated ES or iPS cells will not necessarily work for transplanting differentiated stem cell therapeutics.
Figure 6.
Longitudinal bioluminescence imaging of human ES cells implanted intramuscularly in mice demonstrating that triple-costimulatory blockade therapy (COSTIM) administered at days 0, 2, 4, and 6 prevented rejection of human ES cells. COSTIM refers to a combination of CTLA4-Ig, anti-LFA-1, and anti-CD40L. COSTIM was remarkably more efficient than monotherapy with anti-LFA-1, CTLA4-Ig, or anti-CD40L. Reprint with permission.82
Though promising in small animal models, the aforementioned drug regimens have not been able to induce donor-specific tolerance to kidney or to skin allografts in non-human primates.104, 105 Considering the insights gained from various transplantation models in primates, a more holistic approach using different immunomodulatory strategies simultaneously (e.g., costimulatory blockade, TLI, mixed chimerism, etc.) may be better for achieving tolerance with permanent acceptance while reducing or eliminating the need for long-term immunosuppressive therapy. At present, studies focused on immune responses to stem cell therapeutics in primates are still lacking and the immunological conditioning necessary to guarantee survival of stem cell-derived grafts may prove to be much more modest than expected. Addressing these questions will be critical for the advancement of stem cell technology to clinics.
Conclusions
As more diverse pluripotent stem cell derivatives become available, investigators have learned much about their in vivo behavior, functional properties, and immunogenicity. A comprehensive screening process for antigen repertoire variations among independent stem cell derived-therapeutics will be vital prior to translation of pluripotent stem cell therapy. Such information, combined with accurate interpretation of data, may facilitate the prediction of immunological responses and the development of risk scoring for optimal donor-recipient matching. Further understanding of the immunological pathways triggered by these putative therapeutic cells combined with efficient tracking of immune response is necessary to drive the development of safe strategies to bypass immune rejection. Progress made in these arenas should accelerate the development and clinical application of transiently administered, well-tolerated treatment regimens that exploit the specificity of the immune system while promoting long-term, rejection-free graft survival. More recently, the possibility of cell transdifferentiation, by directly converting or reprogramming one cell type to another while bypassing a pluripotent intermediate,106–108 introduces both additional complexity and exciting prospects for regenerative medicine. The investigation into how such an approach compares to pluripotent stem cells in terms of therapeutic repertoire of cell types, and whether transdifferentiated cells are functional or non-immunogenic in the host, will be a new frontier in this fast-paced field.
Acknowledgement
We thank Amy Morris for preparing the illustrations and Dr. Everett Meyer for valuable advice and constructive critical input. Due to space constraints, we are unable to include all relevant and important studies regarding the immunological barriers to pluripotent stem cell therapy; we apologize to those investigators whose valuable work we have omitted in this review.
Sources of Funding: This work was in part supported by National Institutes of Health AG036142, AI085575, HL099117, EB009689 (J.C.W.) and the International Society for Heart & Lung Transplantation (P.E.A.).
Non-standard Abbreviations and Acronyms
- APC
Antigen Presenting Cell
- C3
Complement Component 3
- CD40L
Cluster of Differentiation 40 Ligand
- CTL
Cytotoxic T Lymphocyte
- CTLA4
Cytotoxic T Lymphocyte Antigen-4
- EB
Embryoid Body
- ES
Embryonic Stem
- GVHD
Graft-Versus-Host Disease
- HSC
Hematopoietic Stem Cell
- HLA
Human Leukocyte Antigen
- IFN
Interferon
- Ig
Immunoglobulin
- IL-12
Interleukin-12
- iPS
Induced Pluripotent Stem
- KIR
Killer Immunoglobulin-Like Receptor
- Klf4
Krueppel-like factor 4
- LFA-1
Lymphocyte Associated Antigen-1
- MHC
Major Histocompatibility Complex
- miHA
Minor Histocompatibility Antigen
- NK
Natural Killer
- NKG2D
Natural-Killer Group 2 Member D
- NOD
Non-Obese Diabetic
- Oct4
Octamer-Binding Transcription Factor 4
- SCID
Severe Combined Immunodeficiency
- Sox2
SRY (sex determining region Y)-box 2
- SSEA1
Stage Specific Embryonic Antigen 1
- TCR
T Cell Receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, Pera MF, Fong C-Y, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotech. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Soundararajan P, Lindsey BW, Leopold C, Rafuse VF. Easy and rapid differentiation of embryonic stem cells into functional motoneurons using sonic hedgehog-producing cells. Stem Cells. 2007;25:1697–1706. doi: 10.1634/stemcells.2006-0654. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1:495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 5.Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- 6.Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, Tanaka K, Narushima M, Miki A, Ueda T, Jun H-S, Yoon J-W, Lebkowski J, Tanaka N, Fox IJ. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotech. 2006;24:1412–1419. doi: 10.1038/nbt1257. [DOI] [PubMed] [Google Scholar]
- 7.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 8.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, Kamp TJ. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonice stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. JACC. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 10.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 11.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 12.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magliocca JF, Held IK, Odorico JS. Undifferentiated murine embryonic stem cells cannot induce portal tolerance but may possess immune privilege secondary to reduced major histocompatibility complex antigen expression. Stem Cells Dev. 2006;15:707–717. doi: 10.1089/scd.2006.15.707. [DOI] [PubMed] [Google Scholar]
- 14.Abdullah Z, Saric T, Kashkar H, Baschuk N, Yazdanpanah B, Fleischmann BK, Hescheler J, Kronke M, Utermohlen O. Serpin-6 expression protects embryonic stem cells from lysis by antigen-specific CTL. J Immunol. 2007;178:3390–3399. doi: 10.4049/jimmunol.178.6.3390. [DOI] [PubMed] [Google Scholar]
- 15.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26:89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 16.Swijnenburg RJ, Schrepfer S, Cao F, Pearl JI, Xie X, Connolly AJ, Robbins RC, Wu JC. In vivo imaging of embryonic stem cells reveals patterns of survival and immune rejection following transplantation. Stem Cells Dev. 2008;17:1023–1029. doi: 10.1089/scd.2008.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 18.Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 19.Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H, Fairchild PJ. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci U S A. 2007;104:20920–20925. doi: 10.1073/pnas.0710265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 22.Narsinh K, Narsinh KH, Wu JC. Derivation of human induced pluripotent stem cells for cardiovascular disease modeling. Circ Res. 2011;108:1146–1156. doi: 10.1161/CIRCRESAHA.111.240374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis RP, Casini S, van den Berg CW, Hoekstra M, Remme CA, Dambrot C, Salvatori D, Oostwaard DW, Wilde AA, Bezzina CR, Verkerk AO, Freund C, Mummery CL. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation. 2012;125:3079–3091. doi: 10.1161/CIRCULATIONAHA.111.066092. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, Jan T, Wilson KD, Leong D, Rosenberg J, Yao M, Robbins RC, Wu JC. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121:1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pick M, Stelzer Y, Bar-Nur O, Mayshar Y, Eden A, Benvenisty N. Clone- and gene-specific aberrations of parental imprinting in human induced pluripotent stem cells. Stem Cells. 2009;27:2686–2690. doi: 10.1002/stem.205. [DOI] [PubMed] [Google Scholar]
- 31.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 33.Mullally A, Ritz J. Beyond HLA: the significance of genomic variation for allogeneic hematopoietic stem cell transplantation. Blood. 2007;109:1355–1362. doi: 10.1182/blood-2006-06-030858. [DOI] [PubMed] [Google Scholar]
- 34.Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT, Plath K, Lowry WE, Benvenisty N. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 36.Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, Wu JC. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dressel R, Schindehutte J, Kuhlmann T, Elsner L, Novota P, Baier PC, Schillert A, Bickeboller H, Herrmann T, Trenkwalder C, Paulus W, Mansouri A. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response. PLoS One. 2008;3:e2622. doi: 10.1371/journal.pone.0002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–I172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 41.Deuse T, Seifert M, Phillips N, Fire A, Tyan D, Kay M, Tsao PS, Hua X, Velden J, Eiermann T, Volk HD, Reichenspurner H, Robbins RC, Schrepfer S. Immunobiology of naive and genetically modified HLA-class-I-knockdown human embryonic stem cells. J Cell Sci. 2011;124:3029–3037. doi: 10.1242/jcs.087718. [DOI] [PubMed] [Google Scholar]
- 42.Hacke K, Falahati R, Flebbe-Rehwaldt L, Kasahara N, Gaensler KM. Suppression of HLA expression by lentivirus-mediated gene transfer of siRNA cassettes and in vivo chemoselection to enhance hematopoietic stem cell transplantation. Immunol Res. 2009;44:112–126. doi: 10.1007/s12026-008-8088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dressel R, Nolte J, Elsner L, Novota P, Guan K, Streckfuss-Bomeke K, Hasenfuss G, Jaenisch R, Engel W. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. Faseb J. 2010;24:2164–2177. doi: 10.1096/fj.09-134957. [DOI] [PubMed] [Google Scholar]
- 44.Frenzel LP, Abdullah Z, Kriegeskorte AK, Dieterich R, Lange N, Busch DH, Kronke M, Utermohlen O, Hescheler J, Saric T. Role of natural-killer group 2 member D ligands and intercellular adhesion molecule 1 in natural killer cell-mediated lysis of murine embryonic stem cells and embryonic stem cell-derived cardiomyocytes. Stem Cells. 2009;27:307–316. doi: 10.1634/stemcells.2008-0528. [DOI] [PubMed] [Google Scholar]
- 45.Ma M, Ding S, Lundqvist A, San H, Fang F, Konoplyannikov M, Berry C, Beltran LE, Chen G, Kovacic JC, Boehm M. Major histocompatibility complex-I expression on embryonic stem cell-derived vascular progenitor cells is critical for syngeneic transplant survival. Stem Cells. 2010;28:1465–1475. doi: 10.1002/stem.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallny HJ, Rammensee HG. Identification of classical minor histocompatibility antigen as cell-derived peptide. Nature. 1990;343:275–278. doi: 10.1038/343275a0. [DOI] [PubMed] [Google Scholar]
- 47.Roopenian D, Choi EY, Brown A. The immunogenomics of minor histocompatibility antigens. Immunol Rev. 2002;190:86–94. doi: 10.1034/j.1600-065x.2002.19007.x. [DOI] [PubMed] [Google Scholar]
- 48.Goulmy E, Termijtelen A, Bradley BA, van Rood JJ. Alloimmunity to human H-Y. Lancet. 1976;2:1206. doi: 10.1016/s0140-6736(76)91727-x. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–1576. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 51.Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371–380. doi: 10.1038/nrc1365. [DOI] [PubMed] [Google Scholar]
- 52.Dhodapkar KM, Feldman D, Matthews P, Radfar S, Pickering R, Turkula S, Zebroski H, Dhodapkar MV. Natural immunity to pluripotency antigen OCT4 in humans. Proc Natl Acad Sci U S A. 2010;107:8718–8723. doi: 10.1073/pnas.0915086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang C, Drukker M. Potential barriers to therapeutics utilizing pluripotent cell derivatives: intrinsic immunogenicity of in vitro maintained and matured populations. Semin Immunopathol. 2011;33:563–572. doi: 10.1007/s00281-011-0269-5. [DOI] [PubMed] [Google Scholar]
- 54.Chung TL, Turner JP, Thaker NY, Kolle G, Cooper-White JJ, Grimmond SM, Pera MF, Wolvetang EJ. Ascorbate promotes epigenetic activation of CD30 in human embryonic stem cells. Stem Cells. 2010;28:1782–1793. doi: 10.1002/stem.500. [DOI] [PubMed] [Google Scholar]
- 55.Fernandes E, Goold HD, Kissenpfennig A, Malissen B, Dyson J, Bennett CL. The role of direct presentation by donor dendritic cells in rejection of minor histocompatibility antigen-mismatched skin and hematopoietic cell grafts. Transplantation. 2011;91:154–160. doi: 10.1097/TP.0b013e318201ac27. [DOI] [PubMed] [Google Scholar]
- 56.Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 57.Molne J, Bjorquist P, Andersson K, Diswall M, Jeppsson A, Strokan V, Rydberg L, Breimer ME. Blood group ABO antigen expression in human embryonic stem cells and in differentiated hepatocyte- and cardiomyocyte-like cells. Transplantation. 2008;86:1407–1413. doi: 10.1097/TP.0b013e31818a6805. [DOI] [PubMed] [Google Scholar]
- 58.Paul LC, Baldwin WM. Humoral rejection mechanisms and ABO incompatibility in renal transplantation. Transplant Proc. 1987;19:4463–4467. [PubMed] [Google Scholar]
- 59.Springer GF, Horton RE. Blood group isoantibody stimulation in man by feeding blood group-active bacteria. J Clin Invest. 1969;48:1280-&. doi: 10.1172/JCI106094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859–871. doi: 10.1038/nri934. [DOI] [PubMed] [Google Scholar]
- 61.Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, Parham P. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 62.Moretta L, Locatelli F, Pende D, Marcenaro E, Mingari MC, Moretta A. Killer Ig-like receptor-mediated control of natural killer cell alloreactivity in haploidentical hematopoietic stem cell transplantation. Blood. 2011;117:764–771. doi: 10.1182/blood-2010-08-264085. [DOI] [PubMed] [Google Scholar]
- 63.Lui KO, Waldmann H, Fairchild PJ. Embryonic stem cells: overcoming the immunological barriers to cell replacement therapy. Curr Stem Cell Res Ther. 2009;4:70–80. doi: 10.2174/157488809787169093. [DOI] [PubMed] [Google Scholar]
- 64.Taylor AL, Negus SL, Negus M, Bolton EM, Bradley JA, Pettigrew GJ. Pathways of helper CD4 T cell allorecognition in generating alloantibody and CD8 T cell alloimmunity. Transplantation. 2007;83:931–937. doi: 10.1097/01.tp.0000257960.07783.e3. [DOI] [PubMed] [Google Scholar]
- 65.Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sayegh MH, Watschinger B, Carpenter CB. Mechanisms of T cell recognition of alloantigen. The role of peptides. Transplantation. 1994;57:1295–1302. doi: 10.1097/00007890-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 67.Fluck N, Witzke O, Morris PJ, Wood KJ. Indirect allorecognition is involved in both acute and chronic allograft rejection. Transplant Proc. 1999;31:842–843. doi: 10.1016/s0041-1345(98)01797-7. [DOI] [PubMed] [Google Scholar]
- 68.Fairchild PJ, Cartland S, Nolan KF, Waldmann H. Embryonic stem cells and the challenge of transplantation tolerance. Trends Immunol. 2004;25:465–470. doi: 10.1016/j.it.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Firner S, Onder L, Nindl V, Ludewig B. Tight control - decision-making during T cell-vascular endothelial cell interaction. Front Immunol. 2012;3:279. doi: 10.3389/fimmu.2012.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 71.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 73.Wakitani S, Takaoka K, Hattori T, Miyazawa N, Iwanaga T, Takeda S, Watanabe TK, Tanigami A. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology (Oxford) 2003;42:162–165. doi: 10.1093/rheumatology/keg024. [DOI] [PubMed] [Google Scholar]
- 74.Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Prokhorova TA, Harkness LM, Frandsen U, Ditzel N, Schroder HD, Burns JS, Kassem M. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009;18:47–54. doi: 10.1089/scd.2007.0266. [DOI] [PubMed] [Google Scholar]
- 76.Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15:254–259. doi: 10.1089/scd.2006.15.254. [DOI] [PubMed] [Google Scholar]
- 77.Drukker M. Immunogenicity of embryonic stem cells and their progeny. Methods Enzymol. 2006;420:391–409. doi: 10.1016/S0076-6879(06)20019-3. [DOI] [PubMed] [Google Scholar]
- 78.Cao F, van der Bogt KE, Sadrzadeh A, Xie X, Sheikh AY, Wang H, Connolly AJ, Robbins RC, Wu JC. Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells Dev. 2007;16:883–891. doi: 10.1089/scd.2007.0160. [DOI] [PubMed] [Google Scholar]
- 79.Koch CA, Jordan CE, Platt JL. Complement-dependent control of teratoma formation by embryonic stem cells. J Immunol. 2006;177:4803–4809. doi: 10.4049/jimmunol.177.7.4803. [DOI] [PubMed] [Google Scholar]
- 80.Tian X, Woll PS, Morris JK, Linehan JL, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 81.Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-transduced embryonic stem cell-derived Lin-c-kit+ and Lin-Sca-1+ hematopoietic progenitors express H60 and are targeted by NK cells. J Immunol. 2009;183:5449–5457. doi: 10.4049/jimmunol.0901807. [DOI] [PubMed] [Google Scholar]
- 82.Pearl JI, Lee AS, Leveson-Gower DB, Sun N, Ghosh Z, Lan F, Ransohoff J, Negrin RS, Davis MM, Wu JC. Short-term immunosuppression promotes engraftment of embryonic and induced pluripotent stem cells. Cell Stem Cell. 2011;8:309–317. doi: 10.1016/j.stem.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kofidis T, deBruin JL, Tanaka M, Zwierzchoniewska M, Weissman I, Fedoseyeva E, Haverich A, Robbins RC. They are not stealthy in the heart: embryonic stem cells trigger cell infiltration, humoral and T-lymphocyte-based host immune response. Eur J Cardiothorac Surg. 2005;28:461–466. doi: 10.1016/j.ejcts.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, Ito R, Ito M, Minegishi M, Minegishi N, Tsuchiya S, Sugamura K. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 85.Andre MC, Erbacher A, Gille C, Schmauke V, Goecke B, Hohberger A, Mang P, Wilhelm A, Mueller I, Herr W, Lang P, Handgretinger R, Hartwig UF. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J Immunol. 2010;185:2710–2720. doi: 10.4049/jimmunol.1000583. [DOI] [PubMed] [Google Scholar]
- 86.Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, Esser S. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212–222. doi: 10.1080/14653240410006031. [DOI] [PubMed] [Google Scholar]
- 87.Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, Connolly AJ, Robbins RC, Wu JC. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–2612. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen YT, Venditti CA, Theiler G, Stevenson BJ, Iseli C, Gure AO, Jongeneel CV, Old LJ, Simpson AJ. Identification of CT46/HORMAD1, an immunogenic cancer/testis antigen encoding a putative meiosis-related protein. Cancer Immun. 2005;5:9. [PubMed] [Google Scholar]
- 89.Zhou YB, Cao JB, Yang HM, Zhu H, Xu ZG, Wang KS, Zhang X, Wang ZQ, Han ZG. hZG16, a novel human secreted protein expressed in liver, was down-regulated in hepatocellular carcinoma. Biochem Biophys Res Commun. 2007;355:679–686. doi: 10.1016/j.bbrc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 90.Ben-David U, Benvenisty N, Mayshar Y. Genetic instability in human induced pluripotent stem cells: classification of causes and possible safeguards. Cell Cycle. 2010;9:4603–4604. doi: 10.4161/cc.9.23.14094. [DOI] [PubMed] [Google Scholar]
- 91.Sykes M. Mixed chimerism and transplant tolerance. Immunity. 2001;14:417–424. doi: 10.1016/s1074-7613(01)00122-4. [DOI] [PubMed] [Google Scholar]
- 92.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–1098. [PubMed] [Google Scholar]
- 94.Slavin S, Strober S, Fuks Z, Kaplan HS. Long-term survival of skin allografts in mice treated with fractionated total lymphoid irradiation. Science. 1976;193:1252–1254. doi: 10.1126/science.785599. [DOI] [PubMed] [Google Scholar]
- 95.Woodley SL, Gurley KE, Hoffmann SL, Nicolls MR, Hagberg R, Clayberger C, Holm B, Wang X, Hall BM, Strober S. Induction of tolerance to heart allografts in rats using posttransplant total lymphoid irradiation and anti-T cell antibodies. Transplantation. 1993;56:1443–1447. doi: 10.1097/00007890-199312000-00032. [DOI] [PubMed] [Google Scholar]
- 96.Levin B, Hoppe RT, Collins G, Miller E, Waer M, Bieber C, Girinsky T, Strober S. Treatment of cadaveric renal transplant recipients with total lymphoid irradiation, antithymocyte globulin, and low-dose prednisone. Lancet. 1985;2:1321–1325. doi: 10.1016/s0140-6736(85)92624-8. [DOI] [PubMed] [Google Scholar]
- 97.Lui KO, Boyd AS, Cobbold SP, Waldmann H, Fairchild PJ. A role for regulatory T cells in acceptance of ESC-derived tissues transplanted across an major histocompatibility complex barrier. Stem Cells. 2010;28:1905–1914. doi: 10.1002/stem.506. [DOI] [PubMed] [Google Scholar]
- 98.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, Zhao G, Sykes M. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 100.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 101.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 102.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pearl JI, Kean LS, Davis MM, Wu JC. Pluripotent stem cells: immune to the immune system? Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3005090. 164ps125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kirk AD, Tadaki DK, Celniker A, Batty DS, Berning JD, Colonna JO, Cruzata F, Elster EA, Gray GS, Kampen RL, Patterson NB, Szklut P, Swanson J, Xu H, Harlan DM. Induction therapy with monoclonal antibodies specific for CD80 and CD86 delays the onset of acute renal allograft rejection in non-human primates. Transplantation. 2001;72:377–384. doi: 10.1097/00007890-200108150-00005. [DOI] [PubMed] [Google Scholar]
- 105.Elster EA, Xu H, Tadaki DK, Montgomery S, Burkly LC, Berning JD, Baumgartner RE, Cruzata F, Marx R, Harlan DM, Kirk AD. Treatment with the humanized CD154-specific monoclonal antibody, hu5C8, prevents acute rejection of primary skin allografts in nonhuman primates. Transplantation. 2001;72:1473–1478. doi: 10.1097/00007890-200111150-00001. [DOI] [PubMed] [Google Scholar]
- 106.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burridge PW, Keller G, Gold JD, Wu JC. Production of De Novo Cardiomyocytes: Human Pluripotent Stem Cell Differentiation and Direct Reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8 T cells. Nat Rev Immunol. 2011;11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grinnemo KH, Genead R, Kumagai-Braesch M, Andersson A, Danielsson C, Mansson-Broberg A, Dellgren G, Stromberg AM, Ekberg H, Hovatta O, Sylven C, Corbascio M. Costimulation blockade induces tolerance to HESC transplanted to the testis and induces regulatory T-cells to HESC transplanted into the heart. Stem Cells. 2008;26:1850–1857. doi: 10.1634/stemcells.2008.0111. [DOI] [PubMed] [Google Scholar]
- 111.Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, Swenson KG, Zhao G, Sykes M. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 112.Taylor PA, Lees CJ, Waldmann H, Noelle RJ, Blazar BR. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood. 2001;98:467–474. doi: 10.1182/blood.v98.2.467. [DOI] [PubMed] [Google Scholar]
- 113.Sykes M, Szot GL, Swenson KA, Pearson DA. Induction of high levels of allogeneic hematopoietic reconstitution and donor-specific tolerance without myelosuppressive conditioning. Nat Med. 1997;3:783–787. doi: 10.1038/nm0797-783. [DOI] [PubMed] [Google Scholar]