Abstract
In light of the growing list of human disorders associated with their dysfunction, primary cilia have recently come to attention as being important regulators of developmental signaling pathways and downstream processes. These organelles, present on nearly every vertebrate cell type, are highly conserved structures allowing for study across a range of species. Zebrafish, in particular, have emerged as useful organisms in which to explore the consequences of ciliary dysfunction and to model human ciliopathies. Here, we present a range of useful techniques that allow for investigation of various aspects of ciliary function. The described assays capitalize on the hallmark gastrulation defects associated with ciliary defects as well as relative ease of visualization of cilia in whole-mount embryos. Further, we describe our recently developed assay for querying functionality of human gene variants in live developing embryos. Finally, a current catalog of known zebrafish ciliary mutant lines is included. The techniques presented here provide a basic toolkit for in vivo investigation of both the biological and genetic mechanisms underlying a growing class of human diseases.
I. Introduction
Primary cilia are highly conserved cellular structures, now understood to be present on the surface of most vertebrate cell types, at least during some point of their life cycle. In contrast to the multiciliated nature of cells bearing motile cilia, cells typically bear a solitary primary cilium that extends from the apical cell surface and is made up of a microtubule-based axoneme, anchored at the base by the basal body. Although the functions of the basal body are not fully understood, one of its roles is to regulate trafficking to the cilium (Jin et al., 2010), where proteins undergo transport along the axoneme. This mechanism, termed intraflagellar transport (IFT), is mediated by complexes of IFT and motor proteins that transport proteins between the basal body and the tip of the cilium (Pedersen and Rosenbaum, 2008).
While motile cilia have long been recognized as being of physiological importance in specialized ciliated cell types, such as those lining the lung epithelium, primary cilia (also known as sensory or immotile cilia) have only recently gained prominence as having an important role in the regulation of reception and transduction of cellular signaling (Berbari et al., 2009; Goetz et al., 2009; Satir et al., 2010). Two pathways with major roles in developmental specification, Sonic hedgehog (Shh) and Wnt, require intact functioning cilia for proper transduction (Corbit et al., 2005, 2008; Gerdes et al., 2007; Huangfu et al., 2003). There is some controversy over whether ciliary dysfunction in zebrafish produces defects in Wnt only, Shh only, or both. Although studies have shown Wnt-related defects in basal body suppressed embryos, including convergent extension phenotypes bearing the hallmark of defects in the noncanonical Wnt planar cell polarity (PCP) pathway (Ross et al., 2005) and a concomitant slight upregulation of canonical Wnt targets (Gerdes et al., 2007), a maternal zygotic ift88 mutant showed no sign of Wnt defects, even in the complete absence of cilia (Huang and Schier, 2009). The latter study showed Shh defects exclusively. A recent study, however, showed no sign of Hedgehog signaling defects in ift57, ift88, and ift172 mutants (Lunt et al., 2009). Although the exact role of cilia in in vivo regulation of these pathways remains elusive, the zebrafish has emerged as a useful model in which to query signaling defects associated with ciliary dysfunction.
A structure that has made this organism particularly useful in the study of cilia is the highly ciliated Kupffer’s vesicle (KV), a transient spherical structure appearing at the 5-somite stage and persisting until Prim-5. KV, which plays a role in patterning akin to the roles of the mammalian node, is comprised of cells bearing cilia that beat to direct fluid flow across the structure and contribute to asymmetrical gene expression. Thus, KV offers a useful tool to investigate the extent of ciliary defects, both in the formation of cilia and in their functionality.
In addition to assays of motile ciliary beat function using KV flow as a model, recent efforts have focused increasingly on the use of this system to assay defects in primary cilia (Jaffe et al., 2010). This has paved the way for the development of the zebrafish as a useful model for many of the disorders associated with ciliary dysfunction, the ciliopathies. The ciliopathies are a group of disorders often characterized by pleiotropic defects as a result of mutations in genes that affect ciliary function. Diseases of cilia range from relatively rare syndromes, such as Bardet–Biedl syndrome, Meckel–Gruber syndrome, and Joubert syndrome, to more common clinical entities, such as polycystic kidney disease. Although these disorders vary by their combinations of phenotypes, hallmark characteristics of ciliary dysfunction are often observed across them. These include renal dysfunction, retinal degeneration, obesity, mental retardation, and left–right patterning defects (Cardenas-Rodriguez and Badano, 2009). Many of these features, particularly kidney cysts, are found in zebrafish models of ciliopathies and have been used to identify ciliary mutants in forward genetic screens (Sullivan-Brown et al., 2008; Sun et al., 2004).
A useful feature in the development of zebrafish models for these disorders is the significant genetic overlap between ciliopathies, in which multiple disorders can be caused by mutations in the same gene or multiple genes can contribute to the same disorder. For example, the Meckel–Gruber syndrome genes, MKS1 and MKS3, can contribute causative and modifying alleles to Bardet–Biedl syndrome, respectively, as does one of the nephronophthisis genes, NPHP6/CEP290 (Leitch et al., 2008). These data are likely representative of the mutual contribution of ciliary genes to similar biological processes that underlie overlapping disease phenotypes. As such, zebrafish ciliary mutants and morphants with defects in various genes often display similar phenotypes, such as the cystic kidneys and body curving defects observed in ift mutants (Sun et al., 2004) or the gastrulation defects observed in BBS and NPHP morphants (Gerdes et al., 2007; Leitch et al., 2008; Zhou et al., 2010); consequently, individual lines may serve as models for multiple ciliopathies.
Zebrafish models of human ciliopathies have been established in lines with null mutations in IFT or basal body genes as well as in wild-type embryos by suppressing protein expression of ciliary genes via injection of translation- or splice-blocking antisense morpholinos. For example, several mutant lines and a morpholino have been developed that target the zebrafish polycystic kidney disease ortholog, polycystin-2 (Sun et al., 2004). Morphant embryos develop pronephric cysts early in development and enlarged pronephric ducts (Obara et al., 2006; Schottenfeld et al., 2007; Sun et al., 2004). Similarly, two lines have been identified through forward genetic screens with mutations in Joubert syndrome genes, arl13b and cc2d2a (Owens et al., 2008; Sun et al., 2004). These lines provide useful developmental systems in which to investigate phenotypes resulting from the loss of these genes. In addition to ciliary mutants, zebrafish ciliopathy models have been generated using targeted gene knockdown via morpholino injection. Each of the 14 Bardet–Biedl syndrome genes, for example, has been investigated in this way with suppression resulting in early developmental phenotypes, including perturbed gastrulation movements (Gerdes et al., 2007; Zaghloul et al., 2010). In addition to providing insight as to the developmental ramifications of perturbing BBS gene expression, we have recently demonstrated the power of this system to model human BBS gene functionality. Using human BBS mRNA to rescue the morphant phenotypes, this system allows for interrogation of the functional consequences of any introduced variant (Leitch et al., 2008; Zaghloul et al., 2010). This provides amethod of “humanizing” the zebrafish to use it as a model for human disease gene function and variability. The ability of human variants to function in zebrafish represents a tremendous advantage of this system due to the possibility of exploring on a large scale the consequences of gene variation on developmental phenotypes in an in vivo vertebrate system with physiological relevance to humans. This method, described below, adds power of the zebrafish model.
II. Methods
A. Visualization of Cilia in Zebrafish Embryos
Visualization of cilia in ciliary morphant or mutant embryos is often a necessary first step in verifying a ciliary defect. Because ciliary structural proteins are highly conserved, reagents developed for use in mammalian systems can be readily applied to zebrafish. This includes common antibodies used for specific labeling of individual cilia.
1. Whole-mount Fluorescence Imaging of Cilia
The following protocol was developed for whole-mount immunostaining of cilia using a mouse anti-acetylated α-tubulin primary antibody (Sigma T6793):
-
Embryos cultured in embryo medium (Westerfield, 2000) should be fixed at the desired embryonic stage. We have found that use of Dent’s fixative (80% MeOH, 20% DMSO) is more effective for labeling of cilia compared to 4% paraformaldehyde. Fix embryos at room temperature for 2 h to overnight. To dehydrate, transfer embryos to 100% MeOH for at least 2 h before proceeding and store up to several weeks at −20 °C.
Rehydrate embryos sequentially for 10 min in each wash: 75% MeOH in PBS, 50% MeOH in PBS, 25% MeOH in PBS, and 100% PBS. Wash once in immunostaining buffer (1× PBS, 0.1% Tween 20, 1% BSA) for 10 min.
Transfer embryos to blocking buffer (10% sheep serum, 1% BSA, 1× PBS) for 1 h at room temperature. Incubate embryos in 1° antibody (1:500 in blocking buffer) solution overnight at 4 °C.
To remove 1° antibody solution, wash embryos in immunostaining buffer twice, 10 min each. Transfer embryos to 2° antibody solution – goat anti-mouse (Invitrogen A-10667) – at 1:1000 in blocking buffer. Incubate for 1 h at room temperature. Wash embryos in immunostaining buffer twice, 10 min each.
Embryos can be incubated in DAPI (1:5000) solution for 10 min, at this point, and then washed twice, 10 min each, in immunostaining buffer.
Following the protocol, embryos are stored at 4 °C in immunostaining buffer or 100% MeOH until imaging.
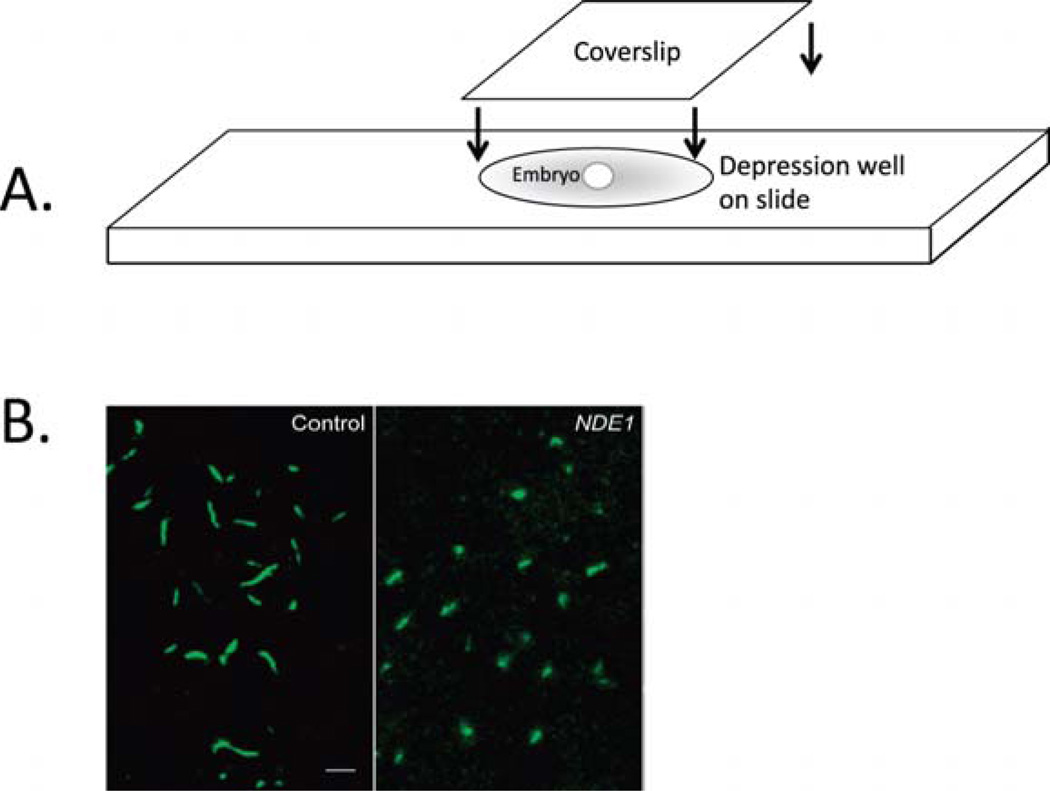
For imaging, place individual embryo in a depression slide, covered with Permount. Quickly, place a coverslip over the depression well and slide the coverslip over embryo until the desired orientation is attained (Fig. 1A). Embryos should be imaged using confocal microscopy (Fig. 1B).
Fig. 1.
Visualization of cilia in whole-mount fixed embryos. (A) After immunostaining for acetylated α-tubulin,whole embryos are mounted in depression well slides and covered with a coverslip. Once placed in the well, the embryo is rotated by gentle rotation of the coverslip until the desired orientation for imaging is achieved. (B) Confocal imaging of cilia in Kupffer’s vesicle in immunostained, mounted embryos. Cilia conformation and length can be easily observed. In this example, control embryos exhibit normal length cilia, whereas NDE1-overexpressing embryos exhibit shorter cilia (modified from Kim et al., 2011). (Permission from Nat. Cell Biol., License #: 2721450638335.) (For color version of this figure, the reader is referred to the web version of this book.)
2. Visualization of Kupffer’s Vesicle
KV can be visualized in segmentation and early pharyngula stage embryos from five somites until prim-5 (24 hpf). This heavily ciliated spherical structure makes it ideal for visualization of the presence/absence of cilia, ciliary length, and ciliary orientation. KV is best observed by dual antibody staining of both cilia (via anti-acetylated α-tubulin antibody) and the apical surface of epithelial KV cells (via anti-atypical protein kinase C antibody; Amack et al., 2007). This is carried out using the immunostaining protocol described above with two minor adjustments:
Include the second, anti-aPKC antibody (Santa Cruz Biotechnology SC-216) in the primary antibody solution for overnight incubation at 4 °C. The final concentration should be 1:100 while the anti-acetylated α-tubulin concentration remains at 1:500.
Include a second, goat anti-rabbit (Invitrogen A-11012) antibody in the secondary antibody solution. Both secondary antibodies should be used at 1:1000.
B. Phenotypic Assays of Ciliary Dysfunction
Though the molecular mechanisms underlying the early developmental steps giving rise to ciliopathy phenotypes have yet to be fully deciphered, assessment of zebrafish embryos early in development provides insight into defects in patterning and specification that may give rise to later organ-specific defects. The most prominent of these early defects are those associated with perturbed PCP signaling and resulting defects in convergent extension movements. Embryos with PCP defects exhibit characteristic phenotypes at the segmentation stages. We have developed a suite of assays to systematically assess these phenotypes to determine the extent of the defect conferred by ciliary dysfunction.
1. Live Embryo Phenotypes
Defective convergent extension, as a result of disrupted PCP signaling, produces aberrant movement of cells during gastrulation. The result is perturbed specification of anatomical structures during segmentation stages, which may give rise to later organogenesis defects. This assay of ciliopathy mutants and morphant phenotypes focuses on phenotypes established in PCP signaling mutants (Sepich et al., 2000).
Inject wild-type embryos at the one- to two-cell stage with a morpholino targeting a ciliary gene of interest or, alternatively, collect embryos from matings of a ciliary mutant line.
Culture embryos in embryo medium for 11–14 h until the 8–10 somite stage.
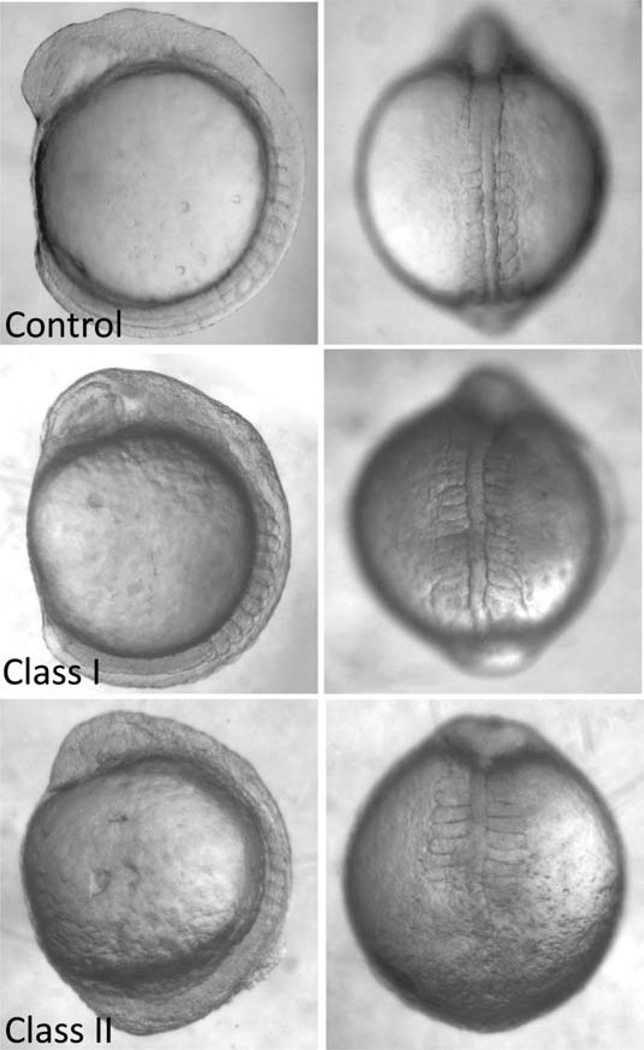
Embryos can be scored directly in the culture dish, without dechorionation. We have devised a system of scoring and categorization into classes based on the extent and number of defects. Observed convergent extension defects are: shortening of the body axis (from lateral view), reduced head size (lateral view), broadening of the notochord (from dorsal view), kinking of the notochord (dorsal view), wide somites (dorsal view), and thin somites (dorsal view). Embryos considered “Class I” or moderately affected are characterized by the presence of one very severe defect or two moderate defects (Fig. 2). Embryos classed as “Class II” or severely affected are characterized by the presence of three or more defects of moderate or extreme severity (Fig. 2). An additional class, “Class Y,” was introduced to denote embryos in which gastrulation epiboly movements were not completed.
Fig. 2.
Gastrulation defect phenotypes associated with ciliary dysfunction. Embryos with disrupted ciliary gene expression (by morpholino suppression in this example) exhibit gastrulation defects that manifest at somitic stages as shortened body axes, widened and kinked notochords, and widened and thinned somites. Embryos can be categorized, based on the extent and severity of the phenotypes, into Class I (moderately affected) and Class II (severely affected).
2. Measurement of Convergent Extension Defects by ISH
To verify and quantify the extent of the convergent extension defect, we have developed a method by which to measure the most prominent convergent extension defect, shortening of the body axis.
Fix morphant or mutant embryos at 8–10 somites in 4% paraformaldehyde overnight at 4 °C. Transfer to 100% MeOH. Store at −20 °C for at least 2 h or as long as several months.
Perform whole-mount in situ hybridization according to standard protocol (Thisse and Thisse, 2008) using a riboprobe cocktail of myoD, krox20, and pax2 to label adaxial mesodermal cells, somites, anterior neural structures, and pronephric mesoderm, respectively. Individual embryos must be flat-mounted and imaged with consistent magnification across all samples.
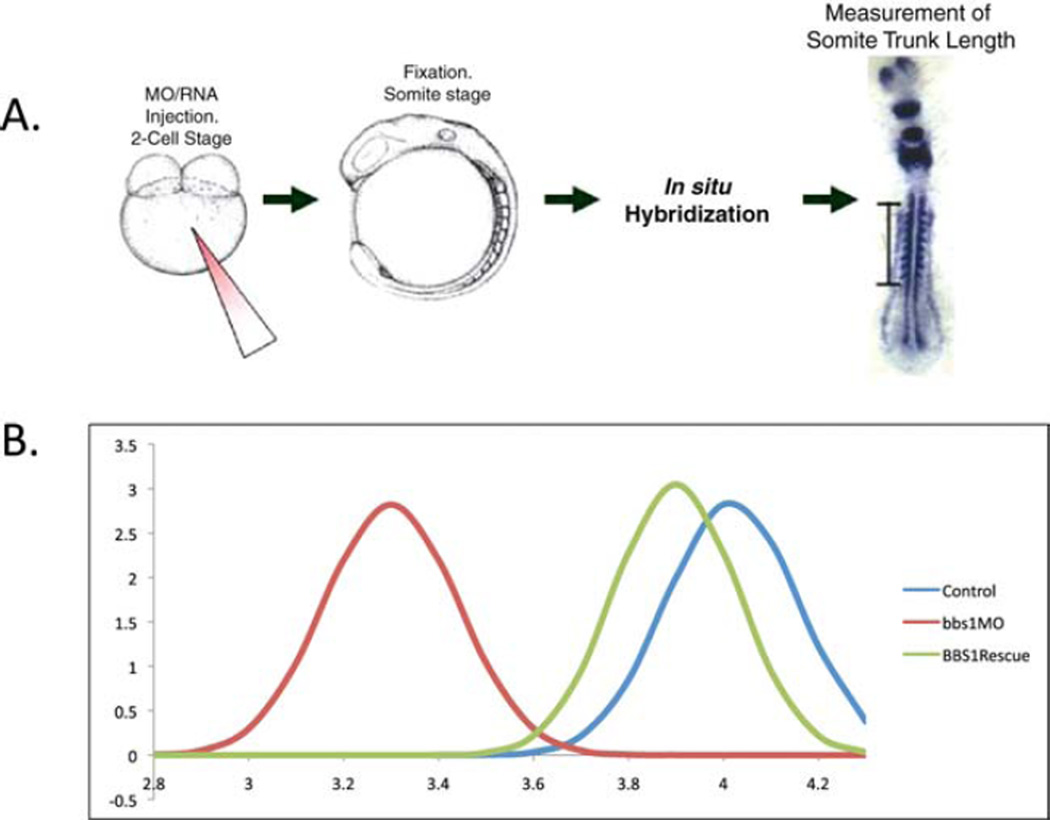
On each embryo image, measure the distance from the first to the last (anterior to posterior) appreciable somite. Measurements for embryos in each injection or mutant treatment, or controls, are normally distributed when plotted. Each curve can then be compared to others as a means of quantification of the average shortened body axis defect for each group (Fig. 3).
Fig. 3.
Measurement of somite-trunk length at somite stages. (A) Shortening of the body axis, the most prominent defect observed in ciliary mutant and morphant embryos at somitic stages, is quantified by labeling of somite stage embryos with markers of somites and notochord, and anterior/posterior markers. Embryo trunk length is measured as the distance from the first to the last somite (bar). (B) BBS1 morpholino knockdown and rescue by wild-type mRNA. For each injection treatment, the trunk lengths of groups of embryos are measured; the observed lengths are normally distributed. Defects in ciliary function, shown here by treatment with bbs1 morpholino, produce shorter body axes compared to controls, and the morphant phenotype can be rescued by co-injection of BBS1 wild-type mRNA. (For color version of this figure, the reader is referred to the web version of this book.)
3. Epiboly Cell Tracking
Convergent extension movements occur during epiboly and are characterized by convergence of cells on the dorsal axis and extension along that axis. We have developed a method to track the movement of cells throughout gastrulation to observe the process of convergence and extension.
After injection of morpholinos into 1- or 2-cell stage embryos or collection of mutant embryos, culture embryos in embryomedium until the 16-cell stage. Inject a single marginal blastomere (see Fig. 4A) with 1 nL of 10,000-MW dextran-conjugated fluorescent lineage tracer (Invitrogen D-22910). Note: At the 16-cell stage, it is impossible to determine the dorsal from the ventral axis of the embryo. Therefore, only 50% of lineage-tracer-injected embryos will express fluorescence on the dorsal part of the embryo. This is easily determined at epiboly stages when the dorsal axis is easily distinguishable due to formation of the shield.
Culture embryos in embryo medium until 30% epiboly (4.67 hpf). Position embryos in embryo medium on an agarose imaging dish (5% agarose with semispherical wells indented in agarose, one per embryo) such that the lateral view of the embryo is facing up.
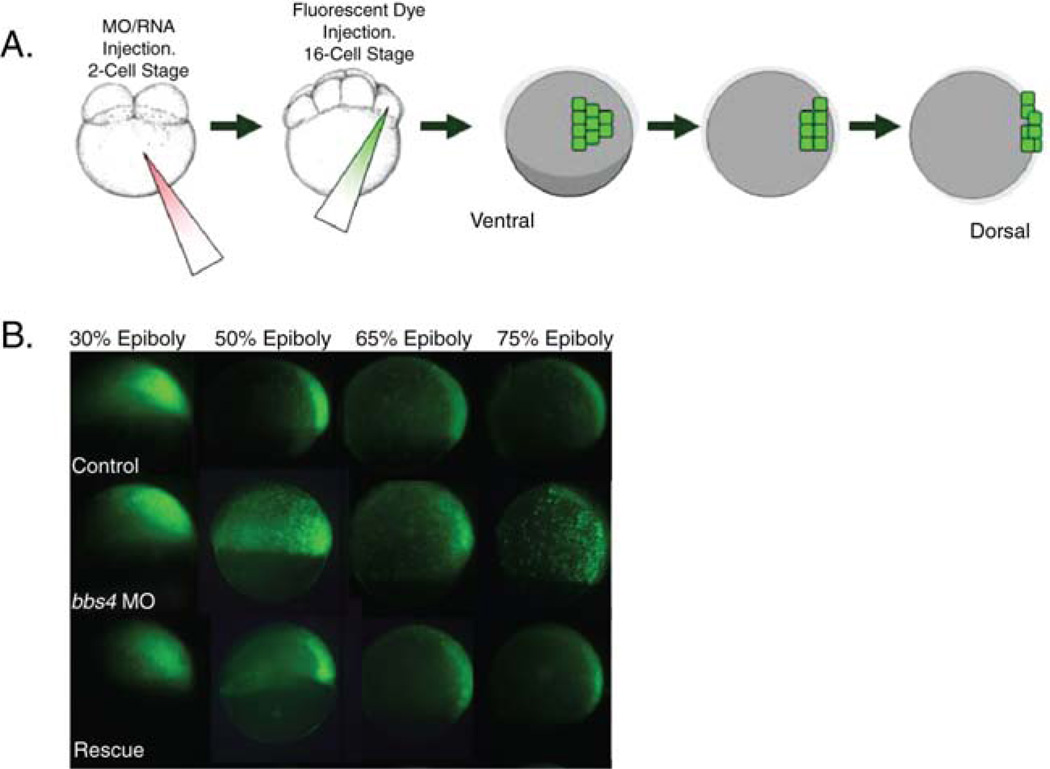
Image embryo using a fluorescence-capable dissecting microscope and camera at 30% epiboly. Without moving the embryo, continue to culture the embryos in the dish until 50% epiboly. Image the embryo again. Repeat at 65 and 75% epiboly (Fig. 4B).
On comparison to each other, control embryos at 30, 50, 65, and 75% epiboly will exhibit a distinct migration of fluorescent cells toward the dorsal axis and an elongation along the axis in a narrowing arc. Ciliary morphants and mutants exhibit a defect in this process characterized by a delay or failure of cells to migrate to and along the dorsal axis. This can be easily visualized by the failure of the distinctive fluorescent arc to form by 75% epiboly.
Fig. 4.
Labeling of cells and tracking of movements through gastrulation. (A) After embryo treatment with morpholino or RNA injection at the 1- to 2-cell stage, fluorescent dye is injected into a single marginal blastomere at the 16-cell stage. At gastrulation stages, fluorescing cells can be tracked to monitor movement. (B) Ciliary morphant embryos (bbs4 shown here) exhibit defects in the characteristic movements whereby cells converge on and extend along the dorsal axis. This defect can be rescued by co-injection of wild-type mRNA (modified from Zaghloul et al., 2010). (Permission from Nat. Cell Biol., License #: 2721450638335). (See color plate.)
4. Assaying Human Gene Variation in Zebrafish
The zebrafish model has many benefits, particularly in the areas of functional genetics and disease. A considerable advantage is the functionality of exogenous human genes in zebrafish embryos. We have developed a protocol for suppression of endogenous embryonic genes and introduction of the homologous human gene to assay for altered functionality conferred by known variants.
Prepare cDNA of the human ciliary gene of interest by subcloning into the pCS2+ vector. For analysis of mutation effect, variants can be introduced by site-directed mutagenesis via PCR (Quick Change, Stratagene). Linearize the cDNA plasmid with the appropriate restriction enzyme and transcribe using SP6 polymerase (mMessage mMachine Kit, Ambion).
Prepare a range of concentrations for a ciliary morpholino of interest with known efficacy in zebrafish. It is imperative for a translation-blocking morpholino that a portion of the sequence is 3′ of the ATG start site. Otherwise, the morpholino may bind to and prevent translation of the co-introduced exogenous mRNA as well.
Starting with a morpholino concentration of moderate effect (i.e., approximately 50–60% of embryos exhibiting phenotypes), co-inject morpholino with three different concentrations of wild-type human mRNA (typically, 50, 100, and 125 pg/nL and 1 nL delivered per injection) at the one- to two-cell stage. Culture embryos until desired stage for phenotyping (8–10 somites for PCP phenotypes) in embryo medium. If scoring PCP phenotypes, the proportion of unaffected, Class I, and Class II embryos should be quantified for each injection combination, as well as morpholino alone and uninjected controls. On introduction of wild-type mRNA, a curve of efficacy for the differing mRNA concentrations can be determined based on the proportion of unaffected embryos. An increase in unaffected embryos with co-injection indicates a rescue of the morphant phenotype. Based on this curve, the optimal rescue concentration can be determined using either one of these concentrations or another concentration within this range.
Once the concentrations for both morpholino and mRNA have been determined for wild-type rescue, the same concentrations are used for subsequent co-injection of morpholino and mutant mRNA. Inject the morpholino/mRNA cocktail at the one- to two-cell stage and culture embryos in embryo medium until the desired stage for phenotyping. Note: This stage must be exactly the same for mutant mRNA rescue embryos and wild-type rescue embryos. Embryos should be categorized by phenotypic class with quantification of the proportion of unaffected, Class I, and Class II embryos.
Compare the quantification of the mutant rescue experiment to the wild-type rescue experiment to determine if mutant mRNA behaves similarly to wild type or causes a perturbed phenotype. Mutant mRNAs that rescue the morphant phenotype in a manner similar to wild type are deemed benign or wild type. Those that do not rescue the morphant phenotype, producing defects similar to morpholino alone, have probably lost all functionality and are, therefore, functionally null. An mRNA that partially rescues the phenotype is likely to be a hypomorphic or partial loss-of-function variant. Finally, those variants that do not rescue the phenotype and produce defects more severe than morpholino alone may be behaving in a dominant negative fashion, a possibility that can be verified by injection of mutant mRNA alone. Categorization of variants in this way can be used to assay a large series of variants and to assign functionality to ambiguous alleles identified in the context of human genetic disease.
III. Zebrafish Ciliary Mutant Lines
Although we lack mutant lines for most ciliary genes, 11 zebrafish lines with mutations in known ciliary genes and 1 transgenic reporter line have been developed. Most of these are readily available via the Zebrafish International Research Center (http://zebrafish.org/zirc/home/guide.php).
oval and MZoval (gene: ift88, allele(s): tz288): Both the mutant line bearing the point mutation (ovl) (Tsujikawa and Malicki, 2004) and the line with the maternal zygotic allele (MZovl) (Huang and Schier, 2009) display a loss of cilia but have intact basal bodies. The transduction of Shh signaling differs, however, in these lines with the former embryos showing no effect on Shh (Lunt et al., 2009), whereas the maternal zygotic mutant embryos have dampened Shh (Huang and Schier, 2009). Embryos do display morphological defects associated with ciliary dysfunction including degeneration of photoreceptor outer segments (Tsujikawa and Malicki, 2004) and disrupted neural and somite patterning.
fleer (ift70, m477): Embryos produced with this point mutation exhibit hallmark pleiotropic features associated with ciliary defects, including kidney cysts, randomized left–right asymmetry, hydrocephalus, and rod outer segment defects (Pathak et al., 2007). Further, this mutant exhibits shortened cilia with reduced beat amplitude, possibly caused by defects in tubulin polyglutamylation. The fleer locus is homologous to the C. elegans DYF1 gene.
seahorse (lrrc6l, hi3308/fa20r/tg238a): Kidney cysts are highly prevalent in these mutants, as well as the characteristic body curvature and left–right patterning defects often seen in ciliary mutants (Kishimoto et al., 2008; Sun et al., 2004). Cilia are present in seahorse embryos, although with reduced motility (Serluca et al., 2009), which suggests that this gene is not necessary for ciliogenesis. However, canonical Wnt signaling is suppressed while noncanonical Wnt is upregulated, indicating an important role of the gene in the regulation of this pathway (Kishimoto et al., 2008).
Elipsa (traf3ip1, m649/tp49d): elipsa embryos exhibit kidney cysts, body axis curving, and defects in retinal development, as well as defects in ciliogenesis (Malicki et al., 1996; Omori et al., 2008). The protein, homologous to the C. elegans DYF-11, localizes to basal bodies and axonemes in otic vesicle and olfactory epithelial cells, whereas in retina the protein is only found in basal bodies. The protein plays an important role in linking the IFT particle to the membrane-associated small GTPase Rab8 via its interaction with Rabaptin5 (Omori et al., 2008).
switch hitter (lrrc50, tm317/hu255): swt mutant embryos develop downward body curving, left–right patterning defects, and severe pronephric cysts (Sullivan-Brown et al., 2008). Although mutants exhibit cilia, lrrc50 is necessary for ciliary motility in KV and thus gives rise to the patterning defect (van Rooijen et al., 2008). This gene is mutated in primary cilia dyskinesia (PCD) patients (Loges et al., 2009), which suggests the potential of this mutant as a model for the disorder.
qilin (qilin, hi3959A): Identified in a forward genetic screen, mutant embryos develop kidney cysts and body curving (Sun et al., 2004). The C. elegans ortholog, DYF-3, undergoes IFT, thereby indicating a role for this gene in promoting formation of sensory cilia (Ou et al., 2005).
curly (ift57, hi3417): This mutation results in loss of cilia (Lunt et al., 2009) and produces renal cysts and body curving (Sun et al., 2004) as well as shortened photoreceptor outer segments with decreased opsin and retinal degeneration by 5 dpf (Krock and Perkins, 2008). Since no defects in Shh signaling were observed in ift57 mutants (Lunt et al., 2009), the mutant phenotype is attributed to the role of ift57 in regulating kinesin-II dissociation from the IFT particle (Krock and Perkins, 2008).
larry (ift81, hi409Tg) and moe (ift172, hi2211Tg): Although embryos exhibit kidney cysts and body curvature, neither cilia formation nor Shh signaling is affected (Lunt et al., 2009; Sun et al., 2004) in these mutants. However, outer segments of photoreceptors do not form, giving rise to a severe retinal defect (Sukumaran and Perkins, 2009).
pkd2/curly up (polycystin-2, hi4166Tg/hu2173/tc321/tg226d/tp85a/ty30): Although these mutants show body curvature and left–right patterning defects, no kidney cysts develop and ciliogenesis does occur (Sun et al., 2004). However, morpholino suppression of the gene produces cysts and hydrocephalus, which suggests that the gene may also be involved in regulation of these phenotypes (Obara et al., 2006; Schottenfeld et al., 2007; Sun et al., 2004).
scorpion (arl13b, hi459Tg): Amodel for Joubert syndrome, cilia do not develop in mutant embryos, resulting in characteristic ciliary phenotypes including kidney cysts, body curvature, and left–right patterning defects (Sun et al., 2004). The protein localizes to the cilium, a feature that is required for its proper function (Duldulao et al., 2009).
sentinel (cc2d2a, w38): Also an ortholog of a Joubert syndrome gene, sentinel mutants exhibit pronephric cysts and body curvature defects (Owens et al., 2008). The mammalian ortholog localizes to the basal body and interacts with another ciliopathy-associated protein, CEP290 (Gorden et al., 2008).
Arl13b–GFP transgenic (Tg(βact::Arl13b–GFP)): This line has been developed for visualization of fluorescent cilia in live embryos. Embryos produce an Arl13b–GFP fusion protein under the control of the β-actin promoter, which allows for strong expression across all tissues. GFP-expressing cilia are observed in the axonemes of motile cilia (KV, floor plate) and primary cilia (ectoderm, notochord, somites, and neural tube) (Borovina et al., 2010).
IV. General Considerations/Future Development
Zebrafish have proven to be an extremely useful model for the study of ciliary biology due to the developmental ramifications of ciliary dysfunction. It is important to note that special consideration must be applied when assessing the defects associated with ciliary knockdown. For example, defective gastrulation movements are a hallmark of disrupted ciliary function and have been observed by morpholino-induced knockdown as well as in various mutant strains. However, this is also a common side effect of morpholino-associated toxicity, making imperative the need for verification of knockdown specificity by rescue. The use of gastrulation defects as a phenotypic readout for ciliary gene knockdown highlights the challenges of morpholinos in comparison with genomic manipulation. As more mutant lines become available, the concerns raised with morpholinos will be alleviated although other factors will remain as issues. Masking of a ciliary phenotype due to other genomic factors is a potential problem that may result in milder phenotypes or none at all. Morpholino injection, a more acute treatment, will therefore still remain a useful technique for knockdown as it can circumvent this problem.
As zebrafish tools for the study of ciliary dysfunction, such as mutant lines, antibodies, and cultured cells, continue to be developed, the advantages of this system for analysis of ciliary biology will expand. For example, zebrafish are not inbred strains and therefore exhibit considerable phenotypic variability. Considering the extent of variability across human ciliopathies and the complexity of those disorders, zebrafish present the opportunity to investigate ciliopathies in the context of genomic variability. Further, the continued development of transgenic reporter lines and transient reporter fusion proteins will allow for direct visualization of ciliary processes in vivo. This type of manipulation is elegantly demonstrated by the recent observation of centrosomal movement during gastrulation in vivo through the use of GFP-labeled centrosomal proteins (Sepich et al., 2011). Similar studies can track ciliogenesis, ciliary orientation, and movement of ciliary proteins through various stages of development. Detailed cellular studies such as these will capitalize on the many advantages of the zebrafish to make it an indispensable tool in the study of ciliary biology.
Acknowledgment
We thank Iain Drummond for generously sharing his protocol for immunostaining of cilia in zebrafish, from which our own protocol has benefited greatly.
References
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer’s vesicle in zebrafish. Dev. Biol. 2007;310:196–210. doi: 10.1016/j.ydbio.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr. Biol. 2009;19:R526–R535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2009;151C:263–280. doi: 10.1002/ajmg.c.30227. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Duldulao NA, Lee S, Sun Z. Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development. 2009;136:4033–4042. doi: 10.1242/dev.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Ocbina PJ, Anderson KV. The primary cilium as a Hedgehog signal transduction machine. Methods Cell Biol. 2009;94:199–222. doi: 10.1016/S0091-679X(08)94010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden NT, Arts HH, Parisi MA, Coene KL, Letteboer SJ, van Beersum SE, Mans DA, Hikida A, Eckert M, Knutzen D, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am. J. Hum. Genet. 2008;83:559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Jaffe KM, Thiberge SY, Bisher ME, Burdine RD. Imaging cilia in zebrafish. Methods Cell Biol. 2010;97:415–435. doi: 10.1016/S0091-679X(10)97022-2. [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The conserved Bardet–Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loges NT, Olbrich H, Becker-Heck A, Häffner K, Heer A, Reinhard C, Schmidts M, Kispert A, Zariwala MA, Leigh MW, Knowles MR, Zentgraf H, Seithe H, Nurnberg G, Nunberg P, Reinhardt R, Omran H. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am. J. Hum. Genet. 2009;85:883–889. doi: 10.1016/j.ajhg.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 2011;13(4):351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev. Cell. 2008;14:954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle–kinesin-II dissociation in vertebrate photoreceptors. J. Cell Sci. 2008;121:1907–1915. doi: 10.1242/jcs.029397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet–Biedl syndrome. Nat. Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- Lunt SC, Haynes T, Perkins BD. Zebrafish ift57, ift88, and ift172 intraflagellar transport mutants disrupt cilia but do not affect hedgehog signaling. Dev. Dyn. 2009;238:1744–1759. doi: 10.1002/dvdy.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J, Neuhauss SC, Schier AF, Solnica-Krezel L, Stemple DL, Stainier DY, Abdelilah S, Zwartkruis F, Rangini Z, Driever W. Mutations affecting development of the zebrafish retina. Development. 1996;123:263–273. doi: 10.1242/dev.123.1.263. [DOI] [PubMed] [Google Scholar]
- Obara T, Mangos S, Liu Y, Zhao J, Wiessner S, Kramer-Zucker AG, Olale F, Schier AF, Drummond IA. Polycystin-2 immunolocalization and function in zebrafish. J. Am. Soc. Nephrol. 2006;17:2706–2718. doi: 10.1681/ASN.2006040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat. Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Ou G, Qin H, Rosenbaum JL, Scholey JM. The PKD protein qilin undergoes intraflagellar transport. Curr. Biol. 2005;15:R410–R411. doi: 10.1016/j.cub.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol. Biol. Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet–Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J. Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development. 2007;134:1605–1615. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Solnica-Krezel L. Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. Genesis. 2000;27:159–173. doi: 10.1002/1526-968x(200008)27:4<159::aid-gene50>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Usmani M, Pawlicki S, Solnica-Krezel L. Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development. 2011;138:543–552. doi: 10.1242/dev.053959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, et al. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left– right patterning. Development. 2009;136:1621–1631. doi: 10.1242/dev.020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran S, Perkins BD. Early defects in photoreceptor outer segment morphogenesis in zebrafish ift57, ift88 and ift172 intraflagellar transport mutants. Vision Res. 2009;49:479–489. doi: 10.1016/j.visres.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter CL, Serluca FC, Thiberge SY, Burdine RD. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev. Biol. 2008;314:261–275. doi: 10.1016/j.ydbio.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. Agenetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- van Rooijen E, Giles RH, Voest EE, van Rooijen C, Schulte-Merker S, van Eeden FJ. LRRC50, a conserved ciliary protein implicated in polycystic kidney disease. J. Am. Soc. Nephrol. 2008;19:1128–1138. doi: 10.1681/ASN.2007080917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th ed. Eugene: University of Oregon Press; 2000. [Google Scholar]
- Zaghloul NA, Liu Y, Gerdes JM, Gascue C, Oh EC, Leitch CC, Bromberg Y, Binkley J, Leibel RL, Sidow A, et al. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet–Biedl syndrome. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10602–10607. doi: 10.1073/pnas.1000219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Dai J, Attanasio M, Hildebrandt F. Nephrocystin-3 is required for ciliary function in zebrafish embryos. Am. J. Physiol. Renal Physiol. 2010;299:F55–F62. doi: 10.1152/ajprenal.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]