Abstract
Background
Congenital central hypoventilation syndrome (CCHS) patients show brain injury in areas that control chemosensory, autonomic, motor, cognitive, and emotion functions, which are deficient in the condition. Many of these abnormal characteristics are present from the neonatal period; however, it is unclear if tissue injury underlying the characteristics progressively worsens with time. We hypothesized that several brain areas in CCHS subjects would show increased gray matter volume loss over time.
Methods
We collected high-resolution T1-weighted images twice (four years apart) from 7 CCHS (age at first study, 16.1±2.7 years; 4 males) and 3 control subjects (15.9±2.1 years; 3 males) using a 3.0-Tesla MRI scanner, and evaluated regional gray matter volume changes with voxel-based-morphometry procedures.
Results
Multiple brain sites in CCHS, including frontal, prefrontal, insular and cingulate cortices, caudate nuclei and putamen, ventral temporal and parietal cortices, and cerebellar cortices showed significantly reduced gray matter volume over time. Only limited brain areas, including sensory, temporal, and medullary regions emerged with increased gray matter at the later age.
Conclusions
CCHS patients show reduced gray matter volume with age progression in autonomic, respiratory, and cognitive regulatory areas, an outcome that may contribute to deterioration of functions found in the syndrome with increasing age.
INTRODUCTION
Congenital central hypoventilation syndrome (CCHS), a genetic condition associated with PHOX2B mutations,1 is characterized by reduced sensitivity to CO2 and O2, diminished drive to breath during sleep, and multiple abnormal physiological, motor, and neuropsychological characteristics.2–4 The syndrome is accompanied by neural injury in both gray and white matter regions, as assessed by MRI-based T2-relaxometery, manual volumetric, 3D surface morphometry, and diffusion tensor imaging (DTI) procedures,5–8 in regions that are implicated in autonomic, physiological, motor, and neuropsychological regulation. The injuries may have arisen from developmental consequences of the PHOX2B gene, mutations of which appear to underlie the syndrome.1 However, affected children are also exposed to intermittent hypoxia, an outcome developing from inadequate ventilatory support, especially during sleep, but occasionally during the day in periods of elevated temperature or inactivity. Such hypoxic exposure has the potential to induce or aggravate neural injury.9–12 In addition, impaired perfusion resulting from PHOX2B influences on autonomic development may also contribute to injury progression. It is unclear whether such brain injury increases with time in patients with CCHS.
Several quantitative MRI procedures, including T2-relaxometry and DTI methods, can be used to assess tissue changes over time.13 Both procedures require consistent MRI data acquisition parameters to allow comparison of pathological changes, and for longitudinal studies, because of scanner upgrades and other issues, scanning parameters may change with time. However, high-resolution T1-weighted images, together with voxel-based-morphometry (VBM) analytical procedures can be used to assess gray matter changes across the brain over time. Since VBM procedures involve partitioning gray from white matter and cerebrospinal fluid tissue types, and comparing regional gray matter voxel-by-voxel across the brain, slightly-altered data acquisition parameters can be expected not to influence findings for longitudinal assessment or data collected from multiple scanners.14 The techniques have been used to assess gray matter changes in longitudinal and cross-sectional studies,14,15 and may be useful for evaluating gray matter changes with time in CCHS subjects.
Gray matter tissue injury can be reflected as volume loss in both adult and pediatric conditions. Regional gray matter volume increases with development in early stages of life in many brain areas, and significantly reorganizes during adolescence.16 However, gray matter volume declines with time in adulthood due to normal aging processes.17 Disease-related gray matter volume loss can be assessed only after accounting for normal age-related volume changes in CCHS subjects.
Our aim was to examine the progression of gray matter injury across the brain in CCHS patients with VBM procedures using high-resolution T1-weighted images. Because of the substantial potential for hypoxic exposure during daily life of CCHS children, we hypothesized that multiple brain areas would show increased gray matter injury over time.
METHODS
Subjects
We studied 7 CCHS and 3 control subjects twice approximately four years apart. The demographic data and other characteristics of CCHS and control subjects are summarized in the Table 1. The diagnosis of CCHS was based on the American Thoracic Society criteria (1999),2 and CCHS subjects were recruited through the CCHS family network (http://www.cchsnetwork.org). All CCHS subjects were of moderate severity, with requirement of ventilator support only during night; subjects requiring ventilatory support during the day were not included. CCHS subjects with other conditions that may induce brain injury, including cardiovascular or neurological conditions, or with diagnosed Hirschsprung’s disease, which may introduce malnutrition (increased risk for neural injury) through malabsorption issues, were excluded as well. All control subjects were healthy, without any history of neurological or other issues that may induce brain injury, and were recruited through advertisements at the university campus and surrounding area.
Table 1.
Demographic data and biophysical variables of CCHS and control subjects.
| Variables | CCHS (n = 7) | Controls (n = 3) | P values | |||
|---|---|---|---|---|---|---|
| Ist time [A] | IInd time [B] | Ist time [C] | IInd time [D] | [A] vs [C] | [B] vs [D] | |
| Age (years) | 16.1±2.7 | 20.6±2.8 | 15.9±2.1 | 20.4±2.2 | 0.92 | 0.95 |
| Gender (Male: Female) | 4:3 | 4:3 | 3:0 | 3:0 | · | · |
| BMI (kg/m2) | 19.7±4.8 | 23.5±5.8 | 22.6±5.9 | 26.0±9.2 | 0.45 | 0.61 |
| PHOX2B mutations | 2 positive; 1 negative; 4 not tested | · | ||||
Brain imaging studies of CCHS and control subjects were performed without any anesthesia or sedatives, and subjects were provided rest from the scanner if required. The study protocol was approved by the Institutional Review Board of the University of California at Los Angeles, and CCHS and control subjects or their parents/caretakers provided informed written consent before the study.
Magnetic resonance imaging
Brain imaging studies were performed using a 3.0-Tesla MRI scanner (Magnetom Trio; Siemens, Erlangen, Germany), with a receive-only 8-channel phased-array head-coil and a whole-body transmitter coil. Both studies at the different ages were performed in the same MRI scanner. We used foam pads on both sides of the head to minimize head motion. Two high-resolution T1-weighted image series were acquired using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence [repetition-time (TR) = 2200 ms; echo-time (TE) = 3.05 ms; inversion time = 1100 ms; flip angle (FA) = 10°; matrix size = 256×256; field-of-view (FOV) = 220×220 mm; slice thickness = 1.0 mm], and proton-density (PD) and T2-weighted images were collected using a dual-echo turbo spin-echo pulse sequence (TR = 8000 ms; TE1, 2 = 17, 133 ms; FA = 150°; matrix size = 256×256; FOV = 240×240 mm; slice thickness = 5.0 mm; turbo factor = 5). After four years, two high-resolution T1-weighted image series were collected again using a MPRAGE pulse sequence (TR = 2200 ms; TE = 2.34 ms; inversion time = 900 ms; FA = 9°; matrix size = 320×320; FOV = 230×230 mm; slice thickness = 0.9 mm), and PD- and T2-weighted images were acquired using a dual-echo turbo spin-echo pulse sequence (TR = 10,000 ms; TE1, 2 = 12, 119 ms; FA = 130°; matrix size = 256×256; FOV = 230×230 mm; slice thickness = 3.5 mm; turbo factor = 5).
Data analysis
We examined high-resolution T1-weighted, PD-, and T2-weighted images of all CCHS and control subjects for presence of any major brain pathology, including cystic lesions, tumors, or major infarcts. No CCHS or control subjects showed any such abnormality on brain images. High-resolution T1-weighted images were also examined to confirm the absence of any head motion-related or other imaging artifacts.
The statistical parametric mapping package (SPM8, http://www.fil.ion.ucl.ac.uk/spm/), MRIcroN, and MATLAB-based (The MathWorks Inc, Natick, MA) custom software were used to process and analyze data.
Realignment, segmentation, normalization, and smoothing
For each study, both high-resolution T1-weighted image volumes were realigned to remove any potential variation from head-motion, and averaged to increase signal-to-noise ratio. The averaged images were bias-corrected for signal intensity differences, partitioned into gray, white, and cerebrospinal fluid (CSF) tissue types, and normalized to the Montreal Neurological Institute (MNI) space, using the unified segmentation approach. The normalized gray matter maps were modulated (scaled to native space) and smoothed with a Gaussian filter (full width at half maximum, 10 mm).
The averaged and bias-corrected T1-weighted images from individual CCHS and control subjects were also normalized to MNI space. The normalized images of all CCHS and controls were averaged to create background images for structural identification.
Statistical analysis
The normalized and smoothed gray matter maps of control subjects were compared voxel-by-voxel between the two time points using paired t-tests (uncorrected threshold, p = 0.001; minimum extended cluster size, 5 voxels). Similarly, the normalized and smoothed gray matter maps of CCHS subjects were also compared between first and second time points using paired t-tests (uncorrected threshold, p = 0.001; minimum extended cluster size, 5 voxels). The clusters showing significantly reduced gray matter volume across the brain in control subjects were converted into a brain mask, and used to exclude brain sites showing reduced gray matter volume from CCHS subjects for partitioning normal age-related changes. The statistical parametric maps with clusters showing significant differences between two time points in CCHS subjects, corrected for normal age-related changes, were overlaid onto background images for structural identification.
RESULTS
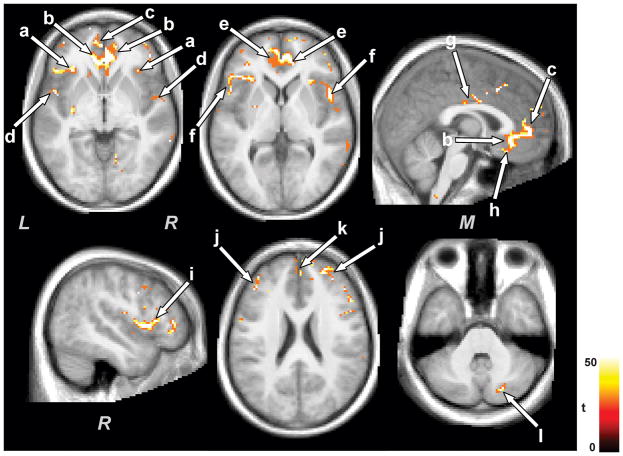
Multiple brain areas in control subjects showed reduced gray matter volume with development. Brain sites that emerged with reduced gray matter volume in controls at the later scans over the first scans included both anterior insulae (Fig. 1a), bilateral genu extending to anterior cingulate (Fig. 1b,e), and ventral medial prefrontal cortices (Fig. 1h), mid cingulate (Fig. 1g), bilateral frontal (Fig. 1i), parietal (Fig. 1f), and temporal operculum (Fig. 1d), prefrontal and fronto-medial cortices (Fig. 1j,k), and right caudal cerebellar cortex (Fig. 1l). A few regions showed increased gray matter volume at the later scan over the earlier scans in control subjects, and included the left rostral cerebellar and right midline occipital cortices.
Figure 1.
Brain sites with decreased gray matter volume in control subjects with age. Decreased gray matter volume with time emerged in the insula (a), cingulate cortices (b, e, g), prefrontal and frontal cortices (h, j, k), frontal (i), parietal (f), and temporal operculum (d), and cerebellum (l). All brain images are displayed in neurological convention (L = Left, M = Middle, R = Right), and color scale represents t-statistic values.
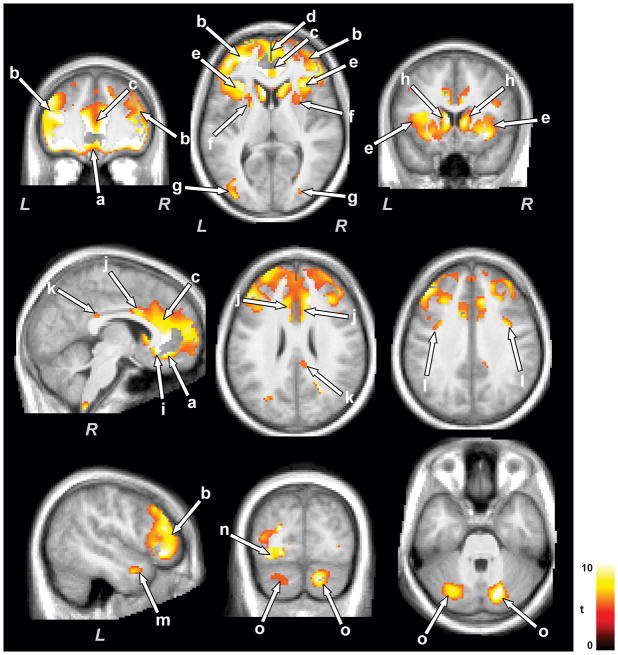
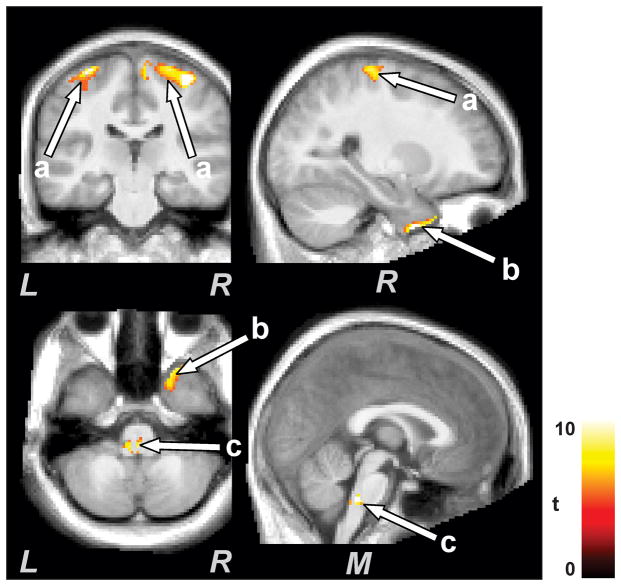
Several brain regions showed reduced gray matter volume in CCHS subjects, corrected for normal age-related changes, at the second time point over the initial scans. Brain regions that showed reduced gray matter in CCHS at the later age included the bilateral ventral medial (Fig. 2a), dorsal prefrontal (Fig. 2b), and fronto-medial (Fig. 2d) cortices, anterior insula (Fig. 2e), caudate nuclei and putamen (Fig. 2f,h), genu (Fig. 2i), anterior (Fig. 2c), mid (Fig. 2j), and posterior cingulate cortices (Fig. 2k), ventral temporal (Fig. 2m), occipital (Fig. 2n), and parietal cortices (Fig. 2l), and bilateral cerebellar cortices (Fig. 2o). Only a few sites in CCHS subjects showed increased gray matter volume over time, and these areas included a bilateral region of the dorsal parietal sensory cortex bordering the motor cortices (Fig. 3a), temporal (Fig. 3b), and dorsal medullary regions (Fig. 3c).
Figure 2.
Brain areas with injury progression with time in CCHS subjects. Brain regions that showed injury progression included the prefrontal and frontal cortices (a, b, d), insular regions (e), caudate nuclei and putamen (f, h), cingulate cortices (c, i-k), temporal (m), occipital (n), and parietal cortices (l), and cerebellar cortex (o). Figure conventions are the same as in Figure 1.
Figure 3.
Brain regions with increased gray matter volume over time in CCHS subjects. Brain structures that showed increased gray matter volume included primary sensory/motor cortex (a), temporal (b), and medullary regions (c). Figure conventions are the same as in Figure 1.
DISCUSSION
Overview
We investigated the progressive gray matter volume changes across the brain in patients with CCHS, controlling for normal developmental-related changes. Multiple brain regions showed reduced gray matter volume in CCHS subjects with time in autonomic, mood, motor, and cognitive regulatory areas, which may contribute to deterioration of those regulatory functions over time in the condition. The pathological processes that contribute to such increased injury are unknown, but may include hypoxic processes frequently encountered in CCHS subjects or sustained perfusion issues resulting from the vascular consequences of the condition.
Gray matter changes with age
In healthy control subjects, gray matter volume increases at an early stage of life, due to increase in neurons and glia, and volume begins to decline from puberty, a consequence of gray matter maturation and increase in neuronal density.16 Such neuro-anatomic changes are region-specific, and appear with variable maturation patterns in pediatric subjects.16 In adult stages of life, with normal aging, neuronal and other cell loss over time leads to gray matter volume reduction with age.15 Other MRI measures, including T2-relaxation values and DTI-based indices, also indicate a similar pattern of tissue changes in gray, as well as white matter regions in adult and pediatric control subjects.18–20
Many sites in CCHS subjects showed reduced gray matter volume, even after controlling for normal age-related volume changes. Such significant reductions in gray matter volume indicate progression of tissue injury over time that may result from hypoxic mechanisms commonly encountered in the condition. A few brain regions in CCHS subjects emerged with increased gray matter volume over time, which possibly results from delayed developmental changes in the condition.
Reduced gray matter volume in autonomic regulatory areas
Brain sites that play significant roles in autonomic regulation include insular, hypothalamic, ventral medullary, and cerebellar regions.21–25 Most of these areas, including bilateral insular and cerebellar sites, showed increased injury with time in CCHS subjects. The augmented damage may contribute to worsening autonomic functions in the syndrome, possibly furthering conditions that lead to the reduced life-span in affected children.
Cerebellar structures, including the cortices, play major roles in blood pressure regulation, especially in coordination of blood pressure changes with body motion or dampening of low and high of blood pressure.21,22 To our knowledge, no evidence yet exists that postural blood pressure issues worsen with development in CCHS, but such an issue could readily be examined.
The autonomic roles of the right insular cortex are principally related to sympathetic regulation, and the left side, parasympathetic activity.26,27 Stimulation of the right anterior insula in humans greatly diminishes the baroreflex, while posterior insular stimulation elicits cardiac arrhythmia.23 Stroke-related injury of the right insula is followed by a high incidence of myocardial infarction,28 possibly from high sympathetic action related to the right insular damage.
CCHS subjects show a range of aberrant cardiovascular issues, including reduced heart rate variability, decreased nocturnal “dipping” of blood pressure, and a propensity for potentially fatal cardiac arrhythmia;29–31 subjects also have a limited life-span, with sudden death common in the condition. At least some of the sudden deaths apparently result from cardiovascular irregularities in the syndrome.32 Positive associations occur between cardiac disturbances and CCHS severity; with some severities, cardiac deficits appear to be progressive as well.32 Altered autonomic tone resulting from progressive injury in insular and other autonomic area may contribute to the worsening of the arrhythmia incidence.
Gray matter volume changes in cognitive, motor, and mood regulatory sites
Multiple brain regions in CCHS subjects showed reduced gray matter volume over time in motor, mood, and cognitive regulatory regions. These sites included caudate, cingulate, prefrontal, frontal, and temporal areas. The caudate nuclei are implicated in motor and cognitive behaviors, including learning, verbal fluency, attention, short- and long-term memory, mental flexibility, and motivation,33–35 and prefrontal and frontal cortices are involved in executive function.36,37 Although prefrontal and frontal cortices also showed increased volume loss in CCHS over time, extensive injury in the caudate nuclei, which project to the frontal and prefrontal cortices, may contribute to the underlying executive dysfunction.38
The progression of injury in regions serving motivation, as well as in frontal cortices for judgment and behaviors based on comprehension of consequences, is a particular concern, since such neural injury likely contributes to the frequent anecdotal reports of CCHS children engaging in high risk behaviors. Some of these behaviors are life-threatening, e.g., underwater breath-holding competitions, alcohol consumption, and some lead to carelessness in nocturnal ventilatory use, which places the individual at grave risk, certainly contributing to the short lifespan in these individuals.
Along with autonomic deficits, CCHS subjects show a variety of motor deficits in addition to the reduced drive to the breathing musculature during sleep.2 These deficits include unilateral smiling following a joke (despite ability to voluntarily smile bilaterally), and eye movement issues.39 Other deficits in CCHS include learning, working memory, attention, and social interaction.3,4 These deficits in motor function and cognition aspects, including learning and memory and executive function in CCHS may result from injury in caudate, frontal, and prefrontal cortices.33,34,36 However, we lack evidence as to whether these functional deficits progressively worsen with age in CCHS.
Potential mechanisms of gray matter injury
Multiple pathological mechanisms in CCHS may have contributed to gray matter volume loss over time. Since the major signs, including failed breathing drive during sleep and severe autonomic symptoms appear early in life, brain injury incurred by PHOX2B mutations in early stages may contribute to these deficient functions. However, the autonomic sequelae in CCHS subjects also alter the cerebral vasculature,40 and those changes may modify perfusion to brain structures, with obvious deleterious consequences, in addition to the repeated hypoxia exposure from loss of ventilatory drive during sleep with failed ventilatory support. We believe that much of the injury to medullary areas in CCHS, especially injury to the raphe system,7 the damage to the locus coeruleus, one structure where PHOX2B is localized, and possibly the hypothalamic damage, due to unique pattern of injury,8 result from initial PHOX2B mutations, with hippocampal, possibly cerebellar, and cortical damage found here resulting from perfusion and hypoxia issues.
Limitations
Some limitations of the study should be acknowledged, including use of different MRI scanning parameters for data acquisition that may influence the findings, and the limited number of subjects, including only male controls. The relative neuroprotection offered by the female sex may significantly reduce hypoxic or other injury in female adolescents. The MRI scanner software was upgraded repeatedly over time, disallowing identical scanning parameters, resulting in slightly different scanning parameters to acquire the high-resolution T1-weighted images. However, the analytical procedures used here, VBM, require partitioning gray matter from other tissue types, and comparison of whole brain regional gray matter changes; small differences in scanning parameters should not drastically influence findings. Such procedures, with variable scanning parameters, have been used reliably to assess tissue changes using data collected from multiple scanners.14 The rare nature of the CCHS condition restricted follow-up to a limited number of CCHS subjects. Moreover, relocation issues limited follow-up of control subjects.
Conclusions
CCHS patients show progressive gray matter volume loss, after partitioning for normal age-related tissue changes, in several brain regions which control autonomic, mood, motor, and cognitive functions. Only a few areas emerge with increased gray matter over time in CCHS subjects. Such progressive gray matter injury in autonomic, motor, and cognitive regulatory regions may contribute to worsening of essential functions found in the condition, including protection against life-threatening risk behaviors and autonomic characteristics that enhance protection against fatal arrhythmia. The pathological mechanisms contributing to progression of injury with age are unknown, but likely include hypoxic processes that accompany the syndrome.
Acknowledgments
Grant support: This study was supported by the National Institute of Child Health and Human Development R01 HD-22695.
We thank Ms. Rebecca Harper, Mr. Edwin Valladares, and Ms. Annaise Magliore for assistance with data collection; parents and children for participating in this research, and the family of Evan Tessier for their support.
Footnotes
Conflicts of interest: No authors have any conflicts of interest.
References
- 1.Dauger S, Pattyn A, Lofaso F, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–42. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic congenital central hypoventilation syndrome: diagnosis and management. Am J Respir Crit Care Med. 1999;160:368–73. doi: 10.1164/ajrccm.160.1.16010. [DOI] [PubMed] [Google Scholar]
- 3.Ruof H, Hammer J, Tillmann B, Ghelfi D, Weber P. Neuropsychological, behavioral, and adaptive functioning of Swiss children with congenital central hypoventilation syndrome. J Child Neurol. 2008;23:1254–9. doi: 10.1177/0883073808318048. [DOI] [PubMed] [Google Scholar]
- 4.Vanderlaan M, Holbrook CR, Wang M, Tuell A, Gozal D. Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol. 2004;37:217–29. doi: 10.1002/ppul.10438. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Lee K, Macey PM, Woo MA, Harper RM. Mammillary body and fornix injury in congenital central hypoventilation syndrome. Pediatr Res. 2009;66:429–34. doi: 10.1203/PDR.0b013e3181b3b363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging. 2006;24:1252–8. doi: 10.1002/jmri.20759. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Macey PM, Woo MA, Alger JR, Harper RM. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr Res. 2008;64:275–80. doi: 10.1203/PDR.0b013e31817da10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Macey PM, Woo MA, Alger JR, Keens TG, Harper RM. Neuroanatomic deficits in congenital central hypoventilation syndrome. J Comp Neurol. 2005;487:361–71. doi: 10.1002/cne.20565. [DOI] [PubMed] [Google Scholar]
- 9.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–50. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veasey SC, Davis CW, Fenik P, et al. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Tuong CM, Zhang Y, et al. Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. J Pathol. 2011 doi: 10.1002/path.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai J, Tuong CM, Gozal D. A neonatal mouse model of intermittent hypoxia associated with features of apnea in premature infants. Respir Physiol Neurobiol. 2011;178:210–7. doi: 10.1016/j.resp.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soria G, Aguilar E, Tudela R, Mullol J, Planas AM, Marin C. In vivo magnetic resonance imaging characterization of bilateral structural changes in experimental Parkinson’s disease: a T2 relaxometry study combined with longitudinal diffusion tensor imaging and manganese-enhanced magnetic resonance imaging in the 6-hydroxydopamine rat model. Eur J Neurosci. 2011;33:1551–60. doi: 10.1111/j.1460-9568.2011.07639.x. [DOI] [PubMed] [Google Scholar]
- 14.Segall JM, Turner JA, van Erp TG, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophr Bull. 2009;35:82–95. doi: 10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 16.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander GE, Chen K, Merkley TL, et al. Regional network of magnetic resonance imaging gray matter volume in healthy aging. Neuroreport. 2006;17:951–6. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Delshad S, Macey PM, Woo MA, Harper RM. Development of T2-relaxation values in regional brain sites during adolescence. Magn Reson Imaging. 2011;29:185–93. doi: 10.1016/j.mri.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Regional brain axial and radial diffusivity changes during development. J Neurosci Res. 2011;90:346–55. doi: 10.1002/jnr.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Delshad S, Woo MA, Macey PM, Harper RM. Age-related regional brain T2-relaxation changes in healthy adults. J Magn Reson Imaging. 2011 doi: 10.1002/jmri.22831. [DOI] [PubMed] [Google Scholar]
- 21.Miura M, Reis DJ. Cerebellum: a pressor response elicited from the fastigial nucleus and its efferent pathway in brainstem. Brain Res. 1969;13:595–9. doi: 10.1016/0006-8993(69)90269-8. [DOI] [PubMed] [Google Scholar]
- 22.Lutherer LO, Lutherer BC, Dormer KJ, Janssen HF, Barnes CD. Bilateral lesions of the fastigial nucleus prevent the recovery of blood pressure following hypotension induced by hemorrhage or administration of endotoxin. Brain Res. 1983;269:251–7. doi: 10.1016/0006-8993(83)90134-8. [DOI] [PubMed] [Google Scholar]
- 23.Oppenheimer SM, Wilson JX, Guiraudon C, Cechetto DF. Insular cortex stimulation produces lethal cardiac arrhythmias: a mechanism of sudden death? Brain Res. 1991;550:115–21. doi: 10.1016/0006-8993(91)90412-o. [DOI] [PubMed] [Google Scholar]
- 24.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–73. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 25.Dampney RA, Coleman MJ, Fontes MA, et al. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–8. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheimer SM, Cechetto DF, Hachinski VC. Cerebrogenic cardiac arrhythmias. Cerebral electrocardiographic influences and their role in sudden death. Arch Neurol. 1990;47:513–9. doi: 10.1001/archneur.1990.00530050029008. [DOI] [PubMed] [Google Scholar]
- 27.Cechetto DF, Chen SJ. Subcortical sites mediating sympathetic responses from insular cortex in rats. Am J Physiol. 1990;258:R245–55. doi: 10.1152/ajpregu.1990.258.1.R245. [DOI] [PubMed] [Google Scholar]
- 28.Oppenheimer SM, Hachinski VC. The cardiac consequences of stroke. Neurol Clin. 1992;10:167–76. [PubMed] [Google Scholar]
- 29.Woo MS, Woo MA, Gozal D, Jansen MT, Keens TG, Harper RM. Heart rate variability in congenital central hypoventilation syndrome. Pediatr Res. 1992;31:291–6. doi: 10.1203/00006450-199203000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Trang H, Boureghda S, Denjoy I, Alia M, Kabaker M. 24-hour BP in children with congenital central hypoventilation syndrome. Chest. 2003;124:1393–9. doi: 10.1378/chest.124.4.1393. [DOI] [PubMed] [Google Scholar]
- 31.Silvestri JM, Hanna BD, Volgman AS, Jones PJ, Barnes SD, Weese-Mayer DE. Cardiac rhythm disturbances among children with idiopathic congenital central hypoventilation syndrome. Pediatr Pulmonol. 2000;29:351–8. doi: 10.1002/(sici)1099-0496(200005)29:5<351::aid-ppul3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE. Congenital central hypoventilation syndrome: PHOX2B genotype determines risk for sudden death. Pediatr Pulmonol. 2008;43:77–86. doi: 10.1002/ppul.20744. [DOI] [PubMed] [Google Scholar]
- 33.Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349–54. doi: 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- 34.Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13:564–74. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- 35.Lombardi WJ, Andreason PJ, Sirocco KY, et al. Wisconsin Card Sorting Test performance following head injury: dorsolateral fronto-striatal circuit activity predicts perseveration. J Clin Exp Neuropsychol. 1999;21:2–16. doi: 10.1076/jcen.21.1.2.940. [DOI] [PubMed] [Google Scholar]
- 36.Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Ma L, Li S, Wang Y, Wang L. A functional MRI evaluation of frontal dysfunction in patients with severe obstructive sleep apnea. Sleep Med. 2011;12:335–40. doi: 10.1016/j.sleep.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Newman LM, Trivedi MA, Bendlin BB, Ries ML, Johnson SC. The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav. 2007;1:3–10. doi: 10.1007/s11682-007-9000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg DS, Ludwig IH. Congenital central hypoventilation syndrome: ocular findings in 37 children. J Pediatr Ophthalmol Strabismus. 1996;33:175–80. doi: 10.3928/0191-3913-19960501-11. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM. Dilated basilar arteries in patients with congenital central hypoventilation syndrome. Neurosci Lett. 2009;467:139–43. doi: 10.1016/j.neulet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]