Abstract
Objective
To identify specific ECG and clinical predictors that separate atherosclerotic sudden cardiac death (SCD) from incident coronary heart disease (CHD) (non-fatal events and non-sudden death) in the combined cohorts of the Atherosclerosis Risk in Communities study and the Cardiovascular Health Study.
Methods
This analysis included 18 497 participants (58% females, 24% black individuals, mean age 58 years) who were initially free of clinical CHD. A competing risk analysis was conducted to examine the prognostic significance of baseline clinical characteristics and an extensive electronic database of ECG measurements for prediction of 229 cases of SCD as a first event versus 2297 incident CHD cases (2122 non-fatal events and 175 non-sudden death) that occurred during a median follow-up time of 13 years in the Cardiovascular Health Study and 14 years in the Atherosclerosis Risk in Communities study.
Results
After adjusting for common CHD risk factors, a number of clinical characteristics and ECG measurements were independently predictive of SCD and CHD. However, the risk of SCD versus incident CHD was significantly different for race/ethnicity, hypertension, body mass index (BMI), heart rate, QTc, abnormally inverted T wave in any ECG lead group and level of ST elevation in V2. Black race/ethnicity (compared to non-black) was predictive of high SCD risk but less risk of incident CHD (p value for differences in the risk (HR) for SCD versus CHD <0.0001). Hypertension, increased heart rate, prolongation of QTc and abnormally inverted T wave were stronger predictors of high SCD risk compared to CHD (p value=0.0460, 0.0398, 0.0158 and 0.0265, respectively). BMI was not predictive of incident CHD but was predictive of high SCD risk in a quadratic fashion (p value=0.0220). On the other hand, elevated ST height as measured at the J point and that measured at 60 ms after the J point in V2 were not predictive of SCD but were predictive of high incident CHD risk (p value=0.0251 and 0.0155, respectively).
Conclusions
SCD and CHD have many risk factors in common. Hypertension, race/ethnicity, BMI, heart rate, QTc, abnormally inverted T wave in any ECG lead group and level of ST elevation in V2 have the potential to separate between the risks of SCD and CHD. These results need to be validated in another cohort.
INTRODUCTION
Sudden cardiac death (SCD) is a major health problem that affects 236 000–325 000 people in the USA and 350 000–700 000 people in Europe each year.1, 2 Most of these SCD cases occur outside the hospital with few or no early warning signs.3 Therefore, it is important to identify those at risk of SCD if preventive measures are to be implemented.
Atherosclerotic coronary heart disease (CHD) is considered the main cause of SCD.4 Nearly half of all CHD deaths are sudden, and approximately 15% of these deaths are the first clinical manifestation of disease.5 SCD could also be caused by non-atherosclerotic CHD and non-CHD causes.6–10 However, these SCD causes are much less common compared to atherosclerotic CHD causes.
A number of studies have attempted to predict SCD in patients with acute myocardial infarction (MI) and in other high-risk patient-based populations.11–13 Thus, a number of ECG and clinical predictors of SCD have already been identified. Nevertheless, atherosclerotic SCD shares many of the same risk factors of CHD, whether non-fatal or non-sudden fatal. This creates a challenge in identifying those at risk of SCD while considering the competing risk of future CHD, especially in the general population. In this analysis from the combined cohorts of the Atherosclerosis Risk in Communities (ARIC) study and the Cardiovascular Health Study (CHS), we conducted a competing risk analysis aiming to identify specific ECG and clinical predictors that separate the risk of SCD from that of incident CHD.
METHODS
Study population
The ARIC study is a population-based cohort study designed to investigate the natural history and aetiology of atherosclerotic and cardiovascular disease events in four US communities in Maryland, Minnesota, Mississippi and North Carolina. The design, objectives and recruitment of study participants are described in detail elsewhere.14 Briefly, at baseline (1987–1989), the cohort was composed of 15 792 men and women 45–64 years of age who were selected by list of area probability sampling. Eligible participants were interviewed at home and invited to a baseline clinical examination where various cardiovascular risk factors and conditions were measured. Three triennial study visits occurred subsequently, with the last visit from 1996 to 1998. Additionally, participants or their proxies were contacted annually by telephone to ascertain hospitalisations and death.
The CHS is an observational cohort study of risk factors for CHD and stroke in older people. The design, objectives and recruitment of study participants are described in detail elsewhere.15 Briefly, between 1989 and 1990, four field centres recruited a total of 5201 people 65 years or older from Medicare eligibility lists in four communities in the USA (North Carolina, California, Maryland, Pennsylvania). To enhance minority representation, during 1992–1993, 687 African–American participants were recruited in three of the four field centres. Participants had annual examinations through 1999.
The CHS and ARIC study protocols were approved by the institutional review board of each participating centre, and informed consent was obtained from each study participant.
There were 21 671 participants in the original combined cohorts with comparable data. After excluding 629 participants with poor-quality ECG recordings, 1815 with prevalent CHD at baseline, 10 participants with missing medical records and 57 cases of SCD classified as ‘possible’ and not definite, the data of 19 160 participants remained. We then excluded 663 participants with major conduction defects defined as QRS duration ≥120 ms (eg, complete left bundle branch block) to allow for an appropriate examination of the ECG variables that are better interpreted/measured in the absence of major conduction defects (eg, level of ST elevation, QTc, spatial QRST angle, spatial QRS axis, T axis and ECG-left ventricular hypertrophy/mass).
ECG variables
Identical electrocardiographs (MAC PC, Marquette Electronics, currently GE Healthcare, Milwaukee, Wisconsin, USA) were used in all clinic centres for ARIC and CHS, and standard 12-lead ECGs were recorded in all participants by strictly standardised procedures. A 10-second segment of simultaneous ECG leads was sampled at a rate of 250 samples per second. The electronic ECGs stored in the ECG machines were transmitted regularly over analogue phone lines to a central ECG core laboratory for reading. The ECGs were initially processed with the Dalhousie ECG program and then were later processed with the 2001 version of the GE Marquette 12-SL program (GE Healthcare). The Marquette measurement matrix contained several ECG measures of different parts of the ECG waveform. In this analysis, we included the ECG variables that have the potential to be predictive of SCD and/or CHD. This included level of ST height as measured at the J point (STJ) and at 60 ms after the J point (ST60), the amplitude and duration of P, P′, R, R′, Q, S and S′ waves, the R intrinsicoid deflection, and the amplitude, duration and area of T and T′ waves in each of the 12 ECG leads. Also included were total QRS duration, axis and area, QT duration (and QTc by Bazett), PR duration, P wave axis, T wave axis and two time domain short-term heart rate variability indices: standard deviation of normal RR intervals (SDNN) and root mean square of successive differences between RR intervals (RMSSD). In addition, the following indices were created: the frontal plane and spatial QRST angle, spatial T wave and QRS axis16 and left ventricular mass using a multivariate ECG model.17 In addition to these continuous ECG variables, a number of categorical variables were derived as well. Minnesota ECG classification was used to define significant ST depression (Minnesota Code 4), significant ST elevation (Minnesota Code 9.2) and T wave abnormalities (Minnesota Code 5).18 Left ventricular hypertrophy was defined by the Cornell voltage index (RaVL+SV3 ≥2200 μV for women, ≥2600 μV for men).19
Ascertainment of SCD and CHD events
The ARIC study and CHS have detailed information on cardiovascular disease deaths including fatal and non-fatal CHD. CHD events included clinical MI, silent MI (made from the study scheduled ECGs), CHD and coronary artery revascularisation. To standardise the case definition of SCD in this analysis of the combined cohorts, all cases of fatal CHD that occurred by July 31, 2002, in CHS (13 years of median follow-up) and December 31, 2002, in ARIC (14 years of median follow-up) were reviewed and adjudicated by a committee of physicians. Definite SCD was defined as a sudden pulseless condition of cardiac origin in a previously stable individual. Possible SCD was defined as death occurring <24 h from a stable condition for unwitnessed events without other evidence indicating instantaneous death. Only definite sudden death cases were used in this analysis.
Statistical methods
Cox proportional hazards regression models were used to determine which demographic and clinical characteristics were associated with the risk of definite SCD and incident CHD events (excluding definite or possible SCD) and to assess the effects of ECG variables on these risks after adjustment for the demographic and clinical characteristics and study cohort (ARIC and CHS). Age was used as the timescale, and birth cohort (<1920, 1920–1929, 1930–1939 and 1940+) was used as a stratification factor in all analyses.20 For each outcome, a backward stepwise algorithm determined the demographic and clinical characteristics significantly associated with that outcome. From those, a set of covariates (shown in table 1) that were associated with either SCD or CHD was selected. A proportional hazards competing risk analysis21 was then done to determine if the ECG predictors for the risk of incident CHD differed from those for the risk of definite SCD. Two additional strata were specified, one for each event type (CHD vs SCD), with all participants appearing in each stratum. The clinical and demographic characteristics that were significantly associated with the risk of either CHD or SCD were included in the models. The ECG variables were then included one at a time in the multivariable models which included the clinical and demographic covariates. The interaction between each ECG variables and event type was assessed to determine if the effect of the ECG variable differed by outcome, adjusting for common covariates. Whenever possible, ECG variables were included in the models as continuous measurements, and HRs were calculated for 1 SD increase. All analyses were performed with the SAS system for Windows, V.9.1. (SAS Institute, Inc.).
Table 1.
Characteristics of the combined ARIC and CHS cohorts
| Characteristic* | N | Mean±SD or N (%) |
|---|---|---|
| Total | 18497 | 18497 (100) |
| Study | 18497 | |
| ARIC | 14322 (77) | |
| CHS | 4175 (23) | |
| Age | 18497 | 58.1±9.6 |
| Sex | 18497 | |
| Female | 10764 (58) | |
| Male | 7733 (42) | |
| Race/ethnicity | 18497 | |
| Black | 4491 (24) | |
| Non-black | 14006 (76) | |
| Body mass index (kg/m2) | 18470 | 27.4±5.3 |
| Smoking status | 18471 | |
| Never | 7776 (42) | |
| Former | 6108 (33) | |
| Current | 4587 (25) | |
| Diabetes | 18336 | 2159 (12) |
| Hypertension | 18433 | 7145 (39) |
| LDL-cholesterol (mg/dl) | 17950 | 135.5±37.9 |
| HDL-cholesterol (mg/dl) | 18208 | 53.0±16.7 |
| Triglycerides (mg/dl) | 18008 | 125.5±62.9 |
| White blood cell count (×103m/μl) | 18080 | 6.01.7 |
Clinical variables not included because they were not predictive of CHD or SCD include education, family income, family history of cardiovascular disease, alcohol, asthma, cancer, intermittent claudication, physical activity, forced expiratory volume in 1 s, haematocrit value, haemoglobin, total calories, ankle brachial index, fasting glucose, insulin, creatinine, fibrinogen and uric acid.
ARIC, Atherosclerosis Risk in Communities; CHS, Cardiovascular Health Study; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
RESULTS
After exclusions, 18 497 participants (14 322 from ARIC and 4175 from CHS) were included in this analysis. The age of the combined cohort ranged from 44 to 95 years (median 57 years), with 58% females and 24% black individuals. Demographics and clinical characteristics of the combined cohort that were predictive of the outcomes in the preliminary analysis and were used later in the competing risk analysis are shown in table 1. Additional characteristics assessed in the preliminary analyses but not included in the competing risk analyses are included in the footnote to table 1.
Most participants (N=13 471; 72.8%) had no event during the follow-up period and were censored in the analysis at their date of last contact. Non-CHD deaths occurred in 2500 (13.5%) participants and were censored at their date of death. As their first event, 229 (1.2%) participants experienced a definite SCD compared to 2297 participants who experienced a CHD event (2122 (11.5%) non-fatal CHD events and 175 (0.9%) non-sudden CHD deaths).
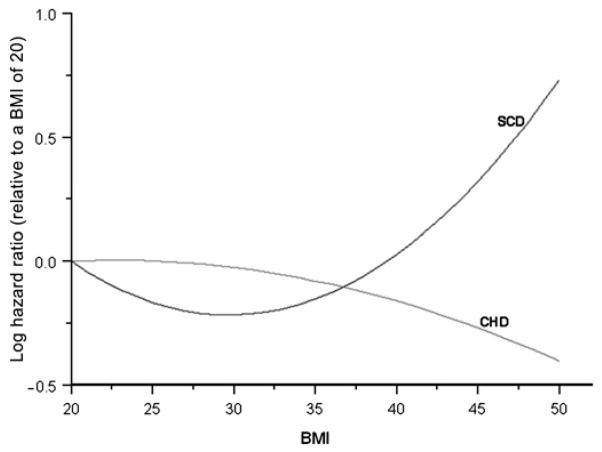
Table 2 shows the results of the multivariable competing risk analysis for the risk of SCD and incident CHD associated with different demographic and clinical variables. The p values in the table assess the difference in the HRs for SCD versus incident CHD. As shown, among the list of CHD risk factors listed in the table, only race/ethnicity, body mass index (BMI) and hypertension discriminated between the risk of SCD and incident CHD. Black race/ethnicity (compared to non-black race) was predictive of high SCD risk (HR (95% CI): 1.81 (1.31 to 2.49)) but less risk of incident CHD (0.76 (0.67 to 0.86)); p value for differences in the HRs <0.0001. Hypertension was a stronger predictor of high SCD risk compared to CHD (2.17 (1.60 to 2.93) and 1.57 (1.43 to 1.72)); p value for differences in the HRs=0.0460. BMI was not significantly predictive of incident CHD but was predictive of high SCD risk in a quadratic fashion; p value for differences in the HRs=0.0220. Figure 1 shows the log(HR) for BMI values ranging from 20 to 50 kg/m2 relative to a BMI of 20 kg/m2. As shown, the risk of SCD decreases as BMI increases from 20 to 29 and then begins to increase. On the other hand, the risk of CHD is not greatly related to BMI but decreases slightly as BMI increases.
Table 2.
Multivariable competing risk analysis for the risk of SCD and incident CHD associated with different demographic and clinical variables
| Characteristic | Definite SCD HR (95% CI)* | Incident CHD HR (95% CI)* | p Value† |
|---|---|---|---|
| ARIC study (vs CHS) | 1.09 (0.62 to 1.89) | 0.84 (0.70 to 1.00) | 0.3889 |
| Age (per decade)‡ | 2.61 (1.71 to 3.97) | 1.75 (1.53 to 2.01) | 0.0788 |
| Male sex (vs female) | 2.42 (1.77 to 3.30) | 2.03 (1.84 to 2.24) | 0.2930 |
| Black race/ethnicity (vs non-black) | 1.81 (1.31 to 2.49) | 0.76 (0.67 to 0.86) | <0.0001 |
| BMI | 0.0220 | ||
| BMI (kg/m2) | 0.49 (0.23 to 1.03) | 1.14 (0.79 to 1.63) | |
| BMI2 (kg/m2) | 1.07 (1.01 to 1.13) | 0.99 (0.96 to 1.02) | |
| Smoking status (vs current) | 0.7477 | ||
| Never | 0.59 (0.40 to 0.86) | 0.69 (0.61 to 0.78) | |
| Former | 0.70 (0.48 to 1.01) | 0.76 (0.68 to 0.85) | |
| Diabetes | 2.54 (1.84 to 3.50) | 2.08 (1.86 to 2.32) | 0.2470 |
| Hypertension | 2.17 (1.60 to 2.93) | 1.57 (1.43 to 1.72) | 0.0460 |
| LDL-cholesterol (mg/dl) | 1.12 (0.98 to 1.29) | 1.27 (1.22 to 1.33) | 0.0869 |
| HDL-cholesterol (mg/dl) | 0.92 (0.76 to 1.11) | 0.80 (0.75 to 0.86) | 0.1880 |
| Triglycerides (mg/dl) | 0.99 (0.85 to 1.15) | 1.06 (1.02 to 1.11) | 0.3517 |
| White blood cell count (×103/μl) | 1.27 (1.11 to 1.46) | 1.15 (1.11 to 1.21) | 0.1759 |
Unless described otherwise, HRs for continuous variables are per 1 SD increase and for the abnormal value for the categorical variables adjusted for the rest of the variables in the table.
Assessing equivalence of HRs for the two end points.
For age, timescale is time since study entry; for all other variables, timescale is age.
BMI, body mass index; CHD, coronary heart disease; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SCD, sudden cardiac death.
Figure 1.
Body mass index and risk of SCD and incident CHD. SCD, sudden cardiac death; CHD, coronary heart disease.
Among the large number of ECG variables we used in this analysis, only heart rate; QTc; abnormally inverted T wave in any ECG lead group and STJ amplitude in leads V2, V3, II, and aVF, and ST60 in V2 were associated with HRs for SCD that are significantly different from those for incident CHD, as follows (table 3): Increased heart rate, prolongation of QTc and presence of abnormally inverted T wave in any ECG lead group were stronger predictors of high SCD compared to incident CHD (p value for differences in HRs=0.0398 for heart rate, 0.0158 for QTc and 0.0265 for T wave). The ability of the abnormally inverted Twave in any ECG lead group to discriminate between SCD and CHD was not driven by a specific ECG lead group (results not shown). Elevated STJ amplitude in leads II, aVF and V3 was predictive of less SCD risk but was not significantly predictive of incident CHD. On the other hand, STJ and ST60 in V2 were predictive of high incident CHD risk but were not significantly predictive of SCD.
Table 3.
Multivariable competing risk analysis for the risk of SCD and incident CHD associated with different ECG variables
| ECG variable* | Definite SCD HR (95% CI)† | Incident CHD HR (95% CI)† | p Value‡ |
|---|---|---|---|
| ECG-LVH | 1.74 (1.03 to 2.93) | 1.59 (1.30 to 1.94) | 0.7522 |
| ECG-LV mass | 1.31 (1.06 to 1.61) | 1.14 (1.06 to 1.23) | 0.2116 |
| Spatial QRST angle | 1.30 (1.15 to 1.47) | 1.19 (1.14 to 1.24) | 0.1835 |
| Spatial T axis | 1.14 (1.03 to 1.27) | 1.10 (1.06 to 1.15) | 0.5540 |
| Frontal QRST angle | 1.17 (1.06 to 1.28) | 1.13 (1.09 to 1.17) | 0.4737 |
| Heart rate | 1.21 (1.07 to 1.37) | 1.05 (1.01 to 1.10) | 0.0398 |
| QTc duration (ms) | 1.19 (1.08 to 1.31) | 1.05 (1.01 to 1.09) | 0.0158 |
| T wave inversion§ | 2.03 (1.49 to 2.75) | 1.41 (1.27 to 1.56) | 0.0265 |
| ST depression§ | 2.44 (1.69 to 3.51) | 1.66 (1.45 to 1.91) | 0.0532 |
| STJ in I (μv) | 0.84 (0.74 to 0.95) | 0.91 (0.87 to 0.95) | 0.2310 |
| STJ in II (μv) | 0.78 (0.69 to 0.89) | 0.96 (0.92 to 1.00) | 0.0029 |
| STJ in aVF (μv) | 0.83 (0.73 to 0.94) | 1.00 (0.96 to 1.04) | 0.0068 |
| STJ in aVL (μv) | 0.98 (0.86 to 1.12) | 0.93 (0.89 to 0.97) | 0.4318 |
| ST60 in I (μv) | 0.77 (0.68 to 0.87) | 0.86 (0.82 to 0.90) | 0.0971 |
| ST60 in II (μv) | 0.83 (0.73 to 0.94) | 0.92 (0.88 to 0.96) | 0.1334 |
| ST60 in III (μv) | 1.05 (0.92 to 1.19) | 1.05 (1.01 to 1.10) | 0.9766 |
| ST60 in aVL (μv) | 0.83 (0.73 to 0.94) | 0.88 (0.85 to 0.92) | 0.3241 |
| STJ in V1 (μv) | 1.07 (0.95 to 1.21) | 1.10 (1.05 to 1.15) | 0.6951 |
| STJ in V2 (μv) | 0.93 (0.81 to 1.07) | 1.10 (1.05 to 1.15) | 0.0251 |
| STJ in V3 (μv) | 0.86 (0.75 to 0.99) | 1.04 (1.00 to 1.09) | 0.0105 |
| STJ in V4 (μv) | 0.85 (0.75 to 0.96) | 0.96 (0.92 to 1.00) | 0.0774 |
| STJ in V5 (μv) | 0.83 (0.74 to 0.93) | 0.93 (0.89 to 0.97) | 0.0841 |
| STJ in V6 (μv) | 0.82 (0.73 to 0.92) | 0.91 (0.87 to 0.95) | 0.1542 |
| ST60 in V1 (μv) | 1.10 (0.98 to 1.25) | 1.14 (1.09 to 1.18) | 0.6588 |
| ST60 in V2 (μv) | 0.91 (0.78 to 1.05) | 1.10 (1.05 to 1.15) | 0.0155 |
| ST60 in V4 (μv) | 0.84 (0.73 to 0.97) | 0.93 (0.89 to 0.98) | 0.1722 |
| ST60 in V5 (μv) | 0.81 (0.72 to 0.92) | 0.88 (0.84 to 0.92) | 0.2114 |
| ST60 in V6 (μv) | 0.82 (0.72 to 0.92) | 0.87 (0.84 to 0.91) | 0.2668 |
Other ECG variables tested for predictive value but not found significant for either outcomes in the preliminary analysis or the competing analysis were excluded from the table. This included QRS duration, spatial QRS axis, ST60 in V3, STJ in III, ST60 in aVF, the amplitude and duration of P, P′, R, R′, Q, S and S′ waves, the R intrinsicoid deflection, T and T′ amplitude, duration and area in each of the 12 ECG leads as well as total QRS duration, axis and area, QT (and QTc by Bazett) duration, PR duration, P wave axis, T wave axis, and time domain heart rate variability indices of SDNN and RMSSD and ST elevation by Minnesota Code.
Unless described otherwise, HRs for continuous variables are per 1 SD increase and for the abnormal value for categorical variables adjusted for the variables in table 2.
Assessing equivalence of HRs for the two end points.
Abnormally inverted T wave and significant ST depression were defined by Minnesota Code as abnormality in any of the ECG lead groups: anterior, lateral or inferior.
SCD, sudden cardiac death; CHD, coronary heart disease; STJ, ST height as measured at the J point; ST60, ST height as measured at 60 ms after the J point.
Presence of significant ST depression by Minnesota Code in any of the lead groups was predictive of SCD and incident CHD, but the difference in the HRs did not reach statistical significance (p=0.532). Similar results were obtained when ST depression was examined in each of the ECG lead groups separately (results not shown).
Including participants with prevalent CHD at baseline in the cohort showed that the greatest risk of SCD was a prior CHD event. The risk of SCD was approximately four times higher for those with prevalent CHD compared to those with no evidence of CHD (95% CI: 3.18 to 5.10), adjusted for age, race/ethnicity and sex. Similarly, including participants with major conduction defects showed that QRS duration ≥120 ms is significantly predictive of SCD (1.80 (1.13 to 2.87)) and incident CHD (1.22 (1.02 to 1.46)) but could not distinguish between the two outcomes (p values for differences in the HRs=0.1230). The results of the non-ECG variables in table 2 were similar regardless of including or excluding those participants with major conduction defects.
DISCUSSION
We identified a number of specific predictors separating the risk of SCD from incident CHD in a cohort of men and women initially free of CHD who were enrolled in two of the largest community-based studies in the USA: ARIC and CHS. These predictors included race/ethnicity, hypertension, BMI, heart rate, QTc, abnormally inverted T wave in any ECG lead group and level of ST elevation (STJ and ST60) in V2. Elevated STJ amplitude in leads V3, II and aVF was ‘protective’ from SCD but was not associated with incident CHD and, hence, can be used as a ‘favourable’ marker for less risk of SCD. As expected and given that the SCD cases in our study are mostly atherosclerotic, we also showed that many demographic, clinical and ECG predictors/risk factors are shared between SCD and incident CHD and, subsequently, could not separate between these two outcomes.
The previously reported association between high resting heart rate and marker of ventricular arrhythmogenesis independently from myocardial ischaemia22 as well as being predictive of SCD in post-MI patients and in the general population23–25 may explain why heart rate was more predictive of SCD in our study. Similarly, prolonged QTc has been frequently linked to SCD,26 and minor T wave abnormalities have been linked to fatal CHD but not non-fatal MI, suggesting that T wave abnormalities are harbingers of arrhythmic sudden death.27 On the other hand, there is no clear explanation why race/ethnicity, hypertension and BMI were more predictive of SCD than incident CHD. Genetic predisposition (for race/ethnicity) or indirect effect on the electrophysiology of the heart (hypertension and BMI) may be part of the explanation—a hypothesis that requires further investigation.
ST segment elevation has been reported in many conditions and disease states including acute coronary syndrome, acute pericarditis, early repolarization and Brugada syndrome. The latter two conditions have been looked at as two extreme examples of conditions associated with non-atherosclerotic ST elevation. Early repolarization has always been considered benign, while Brugada syndrome has been associated with sudden death. Nevertheless, recent reports showed that early repolarization (albeit with an idiosyncratic definition of early repolarization) could also be associated with sudden death.28 In our study, ST elevation (STJ and ST60) was mostly protective from SCD. This accords with what is always believed as a benign nature of early repolarization, in which ST elevation is a key feature. Differences in the outcomes associated with ST elevation (or early repolarization) could be explained by differences in the pathophysiological basis of SCD (non-atherosclerotic vs. atherosclerotic as in our study) or differences in the populations studied (patient-based vs community-based).
Although presence of STelevation (STJ or ST60) was generally protective from SCD, it was predictive of high incident CHD risk. This may explain the recently reported increased cardiovascular and all-cause mortality associated with early repolarization as defined mainly on the basis of STelevation.29, 30 The mechanism by which ST elevation could be predictive of high CHD risk is unclear.
Our results should be interpreted in the context of some limitations. We have conducted many statistical tests without adjusting for multiple comparisons. Thus, some of the positive results could be due to chance. However, since this analysis is the first attempt to get a general idea on what could be potentially SCD-specific predictors, adjusting for multiple comparisons and using a more stringent p value may lead to missing some potentially important predictors. This is the same reason for using most of the predictor variables in our analysis as continuous variables in the statistical models (ie, reporting HRs per 1 SD increase) rather than using arbitrary cut points that can miss important associations which may exist with other cut points. For example, using ST elevation, whether in any lead group or separately, as a categorical variable (defined by Minnesota Code) was not predictive of either SCD or CHD (results not shown), but using ST elevation (STJ and ST60) as a continuous variable highlighted the potential importance of ST elevation in V2. Noteworthy, STJ and ST60 in the same lead or in different leads in the same lead group are correlated to some degree, and subsequently, the tests may not be fully independent. However, we preferred to test STJ and ST60 in individual leads because different ion channels may be active at these times or clearly manifested in some leads compared to others. Validation of the SCD distinguishing predictors which we identified and searching for the optimal cut points for these predictors are needed. Another limitation of our study is that we depended solely on resting standard 12-lead ECG without utilisation of other recent prognostically important ECG predictors of SCD such as heart rate turbulence, T wave alternans, late potentials detected by signal-averaged ECG, etc. Nevertheless, lack of widespread availability of these new ECG predictors would be an obstacle to make practical use of them even if they proved useful in distinguishing between SCD from CHD.
The strengths of this study are the very large size of the cohort studied and that our classification of SCD is much more precise and rigorous than that of other investigations. Also, most of the previous studies on predictors of SCD have ignored the competitive risk of CHD, which does not provide meaningful risk stratification of patients if definitive preventive strategies are to be implemented.
In conclusion, SCD and incident CHD have many risk factors in common. However, we identified specific predictors that have the potential to separate between the risks of SCD and CHD. These predictors include race/ethnicity, hypertension, BMI, heart rate, QTc, abnormally inverted T wave in any ECG lead group and level of ST elevation in V2. These results need to be validated in another cohort.
Acknowledgments
The authors thank the staff and participants of the ARIC study and CHS for their important contributions.
Funding This work was supported by the Donald W. Reynolds Cardiovascular Clinical Research Center at the Johns Hopkins University School of Medicine. The Atherosclerosis Risk in Communities (ARIC) study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C and HHSN268201100012C. The Cardiovascular Heart Study is supported by contracts. This study was supported by contracts NHLBI N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01-HC-15103, N01-HC-55222, N01-HC-75150, N01-HC-45133, U01-HL-080295, R01-HL-087652 and R01-HL-088456. The funding source had no involvement in the design, analysis and interpretation of the data presented in this paper.
Footnotes
Competing interests None.
Ethics approval The CHS and ARIC study protocols were approved by the institutional review board of each participating centre, and informed consent was obtained from each study participant.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sans S, Kesteloot H, Kromhout D. The burden of cardiovascular diseases mortality in Europe. Task Force of the European Society of Cardiology on Cardiovascular Mortality and Morbidity Statistics in Europe. Eur Heart J. 1997;18:1231–48. [PubMed] [Google Scholar]
- 3.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 2010;121:709–29. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 4.Lippert FK, Violetta R, Georgiou M, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 10. The ethics of resuscitation and end-of-life decisions. Resuscitation. 2010;81:1445–51. doi: 10.1016/j.resuscitation.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Hill SF, Sheppard MN. Non-atherosclerotic coronary artery disease associated with sudden death. Heart. 2010;96:1119–25. doi: 10.1136/hrt.2009.185157. [DOI] [PubMed] [Google Scholar]
- 6.Myerburg RJ, Castellanos A. Emerging paradigms of the epidemiology and demographics of sudden cardiac arrest. Heart Rhythm. 2006;3:235–9. doi: 10.1016/j.hrthm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 7.De Giorgio F, Abbate A, Vertrugno G, et al. Non-atherosclerotic coronary pathology causing sudden death. J Clin Pathol. 2007;60:94–7. doi: 10.1136/jcp.2005.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bille K, Figueiras D, Schamasch P, et al. Sudden cardiac death in athletes: the Lausanne recommendations. Eur J Cardiovasc Prev Rehabil. 2006;13:859–75. doi: 10.1097/01.hjr.0000238397.50341.4a. [DOI] [PubMed] [Google Scholar]
- 9.Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischemic causes of sudden cardiac death. Heart. 2006;92:316–20. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiene G, Basso C. Sudden coronary deathdnot always atherosclerotic. Heart. 2010;96:1084–5. doi: 10.1136/hrt.2009.188953. [DOI] [PubMed] [Google Scholar]
- 11.Liew R. Prediction of sudden arrhythmic death following acute myocardial infarction. Heart. 2010;96:1086–94. doi: 10.1136/hrt.2010.194407. [DOI] [PubMed] [Google Scholar]
- 12.Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm. 2009;6:836–47. doi: 10.1016/j.hrthm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Shiga T, Hagiwara N, Ogawa H, et al. Sudden cardiac death and left ventricular ejection fraction during long-term follow-up after acute myocardial infarction in the primary percutaneous coronary intervention era: results from the HIJAMI-II registry. Heart. 2009;95:216–20. doi: 10.1136/hrt.2008.145243. [DOI] [PubMed] [Google Scholar]
- 14.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.de Torbal A, Kors JA, van Herpen G, et al. The electrical T-axis and the spatial QRS-T angle are independent predictors of long-term mortality in patients admitted with acute ischemic chest pain. Cardiology. 2004;101:199–207. doi: 10.1159/000076697. [DOI] [PubMed] [Google Scholar]
- 17.Rautaharju PM, Park LP, Gottdiener JS. Race- and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African–Americans. J Electrocardiol. 2000;33:205–18. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
- 18.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Boston, MA: John Wright PSG, Inc; 1982. [Google Scholar]
- 19.Casale PN, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–80. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 20.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 21.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. Cary. Chapter 6. North Carolina: SAS Institute Inc; 1995. pp. 185–210. [Google Scholar]
- 22.Soliman EZ, Elsalam MA, Li Y. The relationship between high resting heart rate and ventricular arrhythmogenesis in patients referred to ambulatory 24-hour electrocardiographic recording. Europace. 2010;12:261–5. doi: 10.1093/europace/eup344. [DOI] [PubMed] [Google Scholar]
- 23.Jouven X, Empana J, Schwartz PJ, et al. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–8. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- 24.Shaper AG, Wannamethee G, Macfarlane PW, et al. Heart rate, ischaemic heart disease, and sudden cardiac death in middle-aged British men. Br Heart J. 1993;70:49–55. doi: 10.1136/hrt.70.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouven X, Schwartz PJ, Escolano S, et al. Excessive heart rate increase during mild mental stress in preparation for exercise predicts sudden death in the general population. Eur Heart J. 2009;30:1703–10. doi: 10.1093/eurheartj/ehp160. [DOI] [PubMed] [Google Scholar]
- 26.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 27.Kumar A, Prineas RJ, Arnold AM, et al. Prevalence, prognosis, and implications of isolated minor nonspecific ST-segment and T-wave abnormalities in older adults: cardiovascular health study. Circulation. 2008;118:2790–6. doi: 10.1161/CIRCULATIONAHA.108.772541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haïssaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–23. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 29.Sinner MF, Reinhard W, Müller M, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7 :e1000314. doi: 10.1371/journal.pmed.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–37. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]