Abstract
Because of limitations in the oculomotor range, many gaze shifts must be accomplished using coordinated movements of the eyes and head. Stimulation and recording data have implicated the primate superior colliculus (SC) in the control of these gaze shifts. The precise role of this structure in head movement control, however, is not known. The present study uses reversible inactivation to gain insight into the role of this structure in the control of head movements, including those that accompany gaze shifts and those that occur in the absence of a change in gaze. Forty-five lidocaine injections were made in two monkeys that had been trained on a series of behavioral tasks that dissociate movements of the eyes and head. Reversible inactivation resulted in clear impairments in the animals’ ability to perform gaze shifts, manifested by increased reaction times, lower peak velocities, and increased durations. In contrast, comparable effects were not found for head movements (with or without gaze shifts) with the exception of a very small increase in reaction times of head movements associated with gaze shifts. Eye-head coordination was clearly affected by the injections with gaze onset occurring relatively later with respect to head onset. Following the injections, the head contributed slightly more to the gaze shift. These results suggest that head movements (with and without gaze shifts) can be controlled by pathways that do not involve SC.
INTRODUCTION
It has long been known that the superior colliculus (SC) plays an important role in the control of saccadic eye movements. In recent years, neurophysiological data from a number of species have led to the view that this is part of a more general role in the control of orienting movements. When the head is free to move, microstimulation of SC in primates (Freedman et al. 1996; Klier et al. 2001) and cats (Guillaume and Pelisson 2001; Paré et al. 1994) evokes coordinated eye-head gaze shifts. The discharge of single neurons in the intermediate and deep layers is better correlated with gaze than either eye or head (Freedman and Sparks 1997a; Munoz et al. 1991). Microstimulation of SC evokes movements of the pinnae in both cats (Stein and Clamann 1981) and monkeys (Cowie and Robinson 1994). In bats, stimulation evokes not only pinnae movements but also sonar vocalizations (Valentine et al. 2002). Various other movements have been evoked by collicular stimulation, including eye and tail movements in goldfish (Herrero et al. 1998) and circling behavior in rodents (Tehovnik and Yeomans 1986). In primates, several studies have attributed a collicular role for arm movements (Courjon et al. 2004; Nagy et al. 2006; Stuphorn et al. 2000; Werner et al. 1997).
A natural extension of this view of SC is the hypothesis that this structure is involved in the control of head movements independent of gaze. Several lines of evidence exist that are at least consistent with such a possibility. First, at some SC sites, microstimulation can evoke head movements in the absence of gaze shifts (Cowie and Robinson 1994; Pélisson et al. 2001; Tehovnik 1989), particularly if the current is decreased to levels below the threshold for producing gaze shifts (Corneil et al. 2002). Signals from SC could reach the neck muscles through any of several different pathways. In monkeys, direct projections exist from the SC to the spinal cord, presumably to neck muscle motoneurons, although anatomical evidence shows that this tectospinal pathway is weak (May and Porter 1992). Signals can also reach neck muscle motoneurons via an indirect, tectoreticulospinal pathway (Cowie et al. 1994; Huerta and Harting 1982; May and Porter 1992; Robinson et al. 1994). Recently we have isolated neurons in the intermediate and deep layers of the SC that modulate their discharge in association with head movements in the absence of gaze shifts as well as coordinated eye-head gaze shifts (Walton et al. 2007).
Reversible inactivation experiments have revealed much about the role of the SC in the control of head-restrained saccades. Injection of lidocaine or muscimol into SC has been repeatedly shown to result in increased saccade reaction times, decreased peak velocity, longer duration (e.g., Hikosaka and Wurtz 1985, 1986; Lee et al. 1988; Quaia et al. 1998), and impaired target selection (McPeek and Keller 2004). Inactivation of the SC has also been demonstrated to produce deficits in nonsaccadic eye movements, such as smooth pursuit (Basso et al. 2000) but, to our knowledge, there are no detailed published data regarding the effects of reversible SC inactivation on gaze shifts in head-unrestrained monkeys. It is known, however, that inactivation of structures downstream of the SC, particularly regions of the mesencephalic reticular formation (Farshadmanesh et al. 2007; Klier et al. 2002; Waitzman et al. 2000), produce severe deficits in head posture. In the present study, reversible inactivation was used to investigate the role of the primate SC in the control of head movements, including not only those that assist in redirecting gaze but also those that occur in the absence of a change in gaze (i.e., vestibuloocular reflex gain equals 1). Preliminary results of this study have been published previously (Walton and Gandhi 2006).
METHODS
Two macaque monkeys (Macaca mulatta) were used in this study. Approval was granted by the Institutional Animal Care and Use Committee for the University of Pittsburgh, and all procedures were in compliance with guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals. A detailed description of the surgical preparation, training procedure, experimental setup, behavioral paradigms, and data analysis has been published previously (Walton et al. 2007). Briefly, each monkey was surgically fitted with a Teflon-coated wire around the eye and in the skull cap to monitor, respectively, gaze and head positions using the magnetic search coil technique (Collewijn 1977). A stainless steel head post was affixed to the skull to permit head restraint and for placement of a miniature micro-laser module that, when turned on, provided visual feedback regarding head position during the behavioral tasks (Edmund Scientific, Tonawanda, NY) (Gandhi and Sparks 2001). Also a stainless steel chamber, slanted posteriorly at an angle of 38° in the sagittal plane, was placed stereotactically on the skull to permit electrode penetrations orthogonal to both colliculi.
Behavioral tasks and visual display
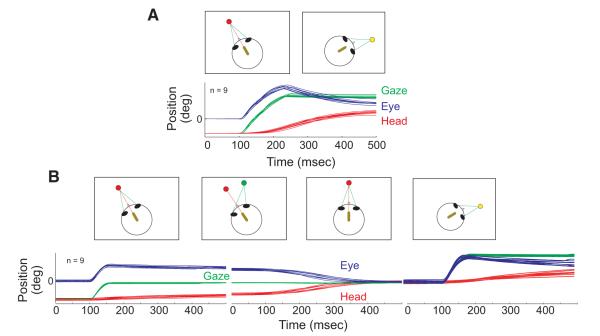
Targets were presented on a cylindrical, tri-state light-emitting diode (LED) board spanning 96° horizontally and 80° vertically. Monkeys sat ~70 cm away from the board; at this distance, the LEDs were spaced 2° apart. The monkeys were tested on the two behavioral tasks schematized in Fig. 1. A red LED and the head-mounted laser were illuminated at the onset of each trial. The animal first aligned the laser with a red LED and then maintained this alignment for a variable interval (800–1,200 ms). For the gaze shift task (A), the next step was to extinguish the red LED and the laser at the same time that a yellow LED was illuminated at a new location. The monkey then had 500 ms to look to the location of the yellow target, which remained lit for the duration of the trial. No constraints were placed on head position for this part of the task. Note that this ensured that the eyes were approximately centered in the orbits at the time that the gaze shift occurred.
FIG. 1.
Schematic representation of the gaze (A) and dissociation (B) tasks. A miniature laser (yellow oval), mounted on the head, produces a red spot on the light-emitting diode (LED) board. Green lines indicate the animal’s line of sight (gaze). Below the schematic representations of the tasks, eye (blue), head (red), and gaze (green) position traces are shown as a function of time. In each trace, data are aligned on movement onset and 100 ms are shown before the onset of the movement.
For the dissociation task (B), alignment of the laser spot with the first red target was followed by the appearance of a green LED at a different location. The monkey was required to make a saccade to this new target without allowing the head to leave a circular imaginary window (generally 5–7°) around the initial red LED. Animals were then required to maintain these eye and head positions for a variable interval (800–1,200 ms). Next the initial red LED was extinguished and the other LED changed from green to red. The animal was required to align the laser spot with the red LED. Because the monkey was already looking at this target, this required a head movement without a change in gaze (hereafter referred to as a “head-only” movement). This was followed by another fixation period of 800–1,200 ms, requiring maintenance of eye and head positions within the imaginary window. The next step was to illuminate a yellow target at a new location at the same time that the red target and laser were switched off. The animal was then required to make a gaze shift to the location of the yellow target within 500 ms and then to maintain fixation for 800–1,200 ms. For this step, no constraints were placed on the animal’s head position. Only the first movement was studied. Corrective saccades and head movements occurred ~100–200 ms after the end of the primary movement and were not analyzed.
For ~50% of dissociation task trials, the laser was extinguished during head-only movements. This was done to address the concern that monkeys may be able to use visual feedback from the laser to perform an on-line correction of head-only movements. Our analyses revealed no effect of blanking the laser spot during head-only movements. Therefore data were pooled for all such movements regardless of whether or not the laser was extinguished during the movement.
Injections
Lidocaine and muscimol are standard pharmacological agents used to reversibly inactivate neural tissue in awake, behaving animals. Comparable deficits in saccadic eye movements have been reported after microinjection of either agent in the SC (Hanes and Wurtz 2001; Hikosaka and Wurtz 1985, 1986; McPeek and Keller 2004). Lidocaine inactivates both cell bodies and axons by blocking sodium channels. Muscimol is a γ-aminobutyric acid (GABA) agonist that inhibits cell bodies without affecting axon propagation (Andrews and Johnston 1979). Another distinction between the two is that lidocaine has a quick onset and is short lasting (~30 min), whereas muscimol is slower to take effect and lasts for hours (cf. Tehovnik and Sommer 1997). In the present study, it was expected that SC inactivation would result in longer reaction times and lower velocities for head movements. Because fatigue can cause these same effects, it was deemed important to choose a pharmacological agent that permitted repeated cycles of injection and recovery to avoid this potential confound. Thus we opted to use lidocaine as our inactivation agent.
Each inactivation experiment was preceded by preparation of an injection cannula. A 32G cannula was prepared with a beveled tip at one end. Its blunt end was glued to a 29G Teflon tube (~3 ft in length) and flushed with either lidocaine or saline. It was verified that gravity did not cause the liquid to leak out of the cannula or at its connection point with the tubing by dangling the apparatus in air.
At the onset of the experiment, a microelectrode was lowered through a 26G guide tube to the SC. The penetration was stopped when the electrode tip was judged to be within the intermediate/deep layers as assessed by strong gaze shift related visuomotor or premotor activity heard on the audio monitor. On occasion, we also isolated neurons and qualitatively mapped their movement fields. To establish the rostral-caudal and medial-lateral positions of the electrode within the SC map (Ottes et al. 1986; Robinson 1972), microstimulation (100–200 ms, 300 Hz, 50 μA) was delivered, with the head unrestrained, during either spontaneous behavior or gap trials (see Gandhi and Sparks 2007 for a description of this trial type) to evoke the site-specific maximal gaze shift. Next, control (preinjection) data were collected. Target locations were set based on the vector of the stimulation-evoked gaze shifts. In most cases, 9–11 target locations were used for eye-only and gaze movements. Two or three of these were placed in the ipsilateral hemifield. Another five were set such that the gaze shifts should involve the part of SC that was expected to be inactivated by the lidocaine. The remaining two to four locations were selected to yield contraversive movements that were ≥12° away from the vector evoked by stimulation in regions that were expected to not (or minimally) overlap with the to-be-inactivated region. For the dissociation task, target locations were set with the goal of yielding head-only movements across a wide range of amplitudes (maximum amplitude 30°, 75% contraversive). The vertical component was limited to ±10°. The gaze shift and dissociation tasks, as well as the target locations, were randomly interleaved in equal proportions.
Next the head was restrained, and the electrode’s depth was recorded before it was withdrawn. The injection cannula was inserted through the same guide tube that had held the electrode. Its shaft was clamped to a hydraulic drive, and its tip was lowered to a depth of 1 mm below the stimulation site. After a waiting period of ~30 min, the open end of the Teflon tube was fit snugly over the tip of a 10 μl Hamilton microsyringe, and a pressure injection (1–4 μl) of either 2% lidocaine hydrochloride or 2% saline was made at a rate of ~400 nanoliters per minute. Note that the volumes injected in the present study were much greater than the 50–200 nl used by Lee et al. (1988) to test the vector averaging hypothesis using head-restrained saccades. The volumes used in the present study likely inactivated 100% of neurons ≥0.5–1.0 mm away from the injection site (Sandkühler et al. 1987; Tehovnik and Sommer 1997). Because of the lack of an inactivation effect on head movements (see results), it was necessary to use relatively large injection volumes to reduce the risk of effects being overlooked. Therefore no attempt was made to perform a head-unrestrained test of the vector averaging hypothesis.
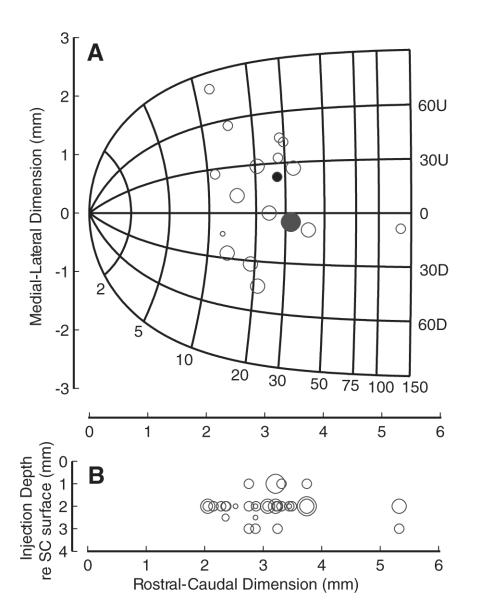
The first injection was always at a depth of 1 mm below the stimulation site. Doing so helps to counteract the tendency of the fluid to migrate upward along the shaft of the cannula after the injection (M. Sommer, personal communication). In addition, the inserted end of the cannula was beveled, which means that the lidocaine exited <1 mm below the stimulation site. Following the injection, the tubing was disconnected from the microsyringe and taped to the animal’s chair with enough slack to ensure that it did not interfere with head movements. The head was unrestrained, and data were collected for 40 min using the same target locations and trial types used to collect preinjection data. Gaze latency, defined in the following text, was monitored on-line to verify that the inactivation effect had diminished (usually 20–30 min). Cycles of injection and recovery were repeated as long as the animals were willing to continue working (usually 2–4 injections/day at either the same site or at a different depth along the same track). Typically the injections at different depths were deeper within the SC, and they were most likely confined within the SC because the observed gaze shift impairments were comparable to previously reported head-restrained saccade deficits (e.g., Lee et al. 1988). Also inspection of the animal from a camera revealed no visually detectable signs of tilt in head posture, which has been reported to occur after inactivation of the oculomotor regions of the underlying mesencephalic reticular formation (Farshadmanesh et al. 2007; Klier et al. 2002; Waitzman et al. 2000). In all, 45 lidocaine injections were made in 18 electrode penetrations. The locations of the electrode penetrations and the depths of the injections sites, relative to the SC surface, are plotted in Fig. 2. Histology is still not available on the animals as they are being used for other projects.
FIG. 2.
Distribution of lidocaine injections within the superior colliculus (SC). A) The location of each each electrode penetration (n = 18) on the collicular map is shown as a circle, and its diameter indicates the number of lidocaine injections (1–4) made on each track. The map was derived by the formula provided by Ottes et al. (1986). The gaze shift amplitude increases logarithmically along the rostral-caudal extent, and the movement direction changes linearly along the medial (upward) to lateral (downward) dimension. The large, filled, gray circle shows the location of the injections for the example data shown in Figs. 3–6. The smaller, filled, black circle shows the location of the injections for the example data shown in Figs. 7–8. B: the depth of each lidocaine injection (n = 45) relative to the SC surface is plotted as a function of its rostral-caudal location. The diameter of the circle indicates the volume of injection (1–4 μl).
Data analysis
Gaze and head position were measured using a phase-angle detection system (CNC Engineering, Seattle, WA). This system is insensitive to translation and does not measure torsion. Measurement of rotation, however, was linear to within 2% over 360° azimuth. Eye position was computed by subtracting head from gaze position. Saccade/gaze onsets and offsets were determined by threshold-based velocity criteria of 50°/s for onset and 30°/s for offset. Accurate measurement of head movement onset and offset are complicated by the fact that the head is a high inertia system with sluggish acceleration. Using a simple velocity threshold criterion often does not reliably distinguish between brief, small (<2°) head movements and a larger head movement that occurs later in the trial. Manual correction of this problem has the drawback of permitting possible experimenter bias. For these reasons, a sliding window algorithm, as described elsewhere (Chen and Walton 2004; Walton et al. 2007), was used to define head movement onsets and offsets.
For head movements associated with gaze shifts, the head often contributes to the gaze shift. Thus “head contribution to gaze” was computed as the difference between the amplitudes of the gaze shift and the eye saccade. It must be emphasized that this is different from total head amplitude because the head movement normally continues well after the end of the gaze shift. Eye, head, and gaze latencies were defined as the number of milliseconds between the cue to initiate the movement and the movement onset. For the dissociation task (Fig. 1B), gaze latency was measured with respect to the appearance of a green target, and the head-only reaction time was relative to when the green target switched its color to red. For the gaze shift task (Fig. 1A), both gaze and head latencies were referenced to the appearance of a yellow target.
Our data-acquisition system (Bryant and Gandhi 2005) is capable of measuring numerous behavioral parameters (latency, amplitude, peak velocity, etc.) on-line. Gaze latency was monitored on-line to verify that the effect of inactivation had diminished. Off-line, comparisons were made between control (preinjection) data and postinjection trials before gaze latency recovered to preinjection levels. Recovery was operationally defined as the trial number at which the average gaze latency, plotted as a function of trial number in bins of 50 trials, fell within 1 SD of the preinjection mean. For gaze signal parameters, this analysis was normally restricted to the five target locations that were designed to involve the inactivated region of SC.
For head movements, two separate analyses were performed. The first involved comparing control and postinjection data for each injection. For head movements associated with gaze shifts, this analysis was carried out on the same trials used for the gaze analysis. For head-only movements, data from all contralateral trials were included as there was no a priori reason to assume a topographic organization for these movements (Walton et al. 2007). For analyses involving peak velocity or duration, however, the potentially confounding effects of amplitude were circumvented by testing for changes in the slopes of the velocity-amplitude and duration-amplitude relationships.
Because little is known about the neural circuitry that controls head movements, it is possible that effects may be overlooked in the preceding analyses. For head-only movements, it is possible that a subset of amplitudes might be affected. If this is the case, pooling data from all contraversive movements might obscure effects. In the case of head movements associated with gaze shifts, one should not exclude the possibility that the affected trials might be different from the trials that showed effects on gaze. Conceivably, this could happen if eye and head movements are encoded separately in SC. For these reasons, an additional analysis was conducted on all contraversive head-only and head-with-gaze movements ≤35°. These data were binned in 5° amplitude increments (i.e., 0–5°, 5–10°,…30–35°). Head movements larger than 35° could not be consistently elicited (due to the nature of the behavioral tasks and the size of the LED board) and were, therefore excluded from these analyses. T-tests (P < 0.05) were used to compare each postinjection bin with the corresponding control bin. T-tests were only performed on a given bin if there was a minimum of five trials in both the control and postinjection groups.
Monkeys often made small-amplitude, low-velocity head drifts during and after “eye-only” movements. It is possible that SC inactivation might affect these drifts, for example by interfering with the animals’ ability to keep the head still. To assess this possibility, these head drifts were compared before and after the injections. To do this, trials were grouped in 5° increments according to eye-only amplitude. Within each group, a dynamic ms-by-ms comparison (t-test, P < 0.05) of head velocity was performed for control and postinjection data.
RESULTS
Gaze shifts
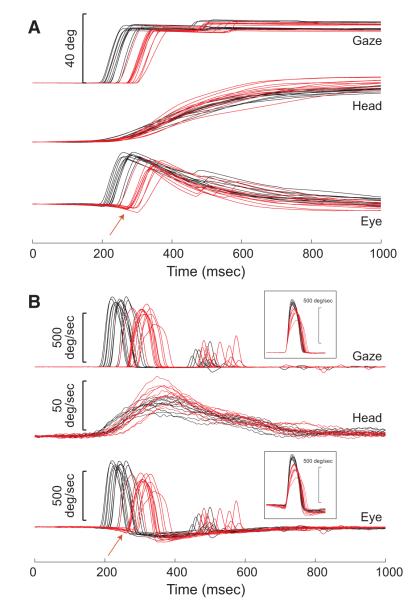
The effectiveness of the injections was first assessed by examining the effects of reversible SC inactivation on the amplitude, peak velocity, latency, and duration of gaze shifts. These analyses were restricted to the five target locations that were expected to involve the inactivated regions of SC. Figure 3 shows amplitude (A) and velocity (B) traces recorded before (black) and after (red) one example injection. Data are aligned on target onset. Reversible inactivation caused a clear increase in the latencies of the eye movements, a fact that can be appreciated from examination of the gaze and eye traces. In contrast, the head movements that accompanied these gaze shifts were only slightly delayed. Thus the gaze shift began much later, relative to the start of the head movement. In the eye traces, the eyes can be seen to counter-rotate before saccade onset in the postinjection data but not in the preinjection data (see red arrows). The injection also caused a clear decrease in peak gaze velocity (B). These (and other) effects are explored in more detail below. The injection site for these data are depicted in Fig. 2A as a large, gray circle.
FIG. 3.
Example position (A) and velocity (B) traces for coordinated eye-head gaze shifts. Each panel shows data for gaze, head, and eye aligned on target onset. Trials before and after injection of 2 μl lidocaine are shown in black and red, respectively. Gaze/eye reaction time is clearly increased following the injection. In contrast, there is little effect on the head movement that accompanies the gaze shift. As a result of this altered eye-head timing, the eyes begin to counter-rotate in the orbits before the gaze shift begins (red arrows). B: the effects on gaze and eye peak velocities are apparent. Insets: velocity traces for gaze and eye aligned on movement onset.
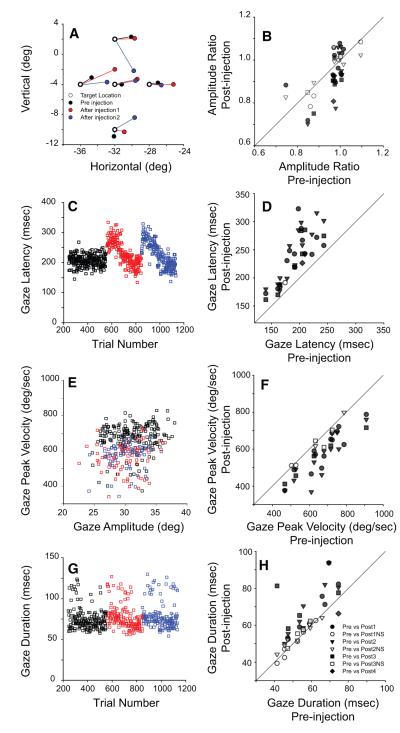
Figure 4A shows, for one electrode penetration, the mean endpoints of gaze shifts to each of the five target locations that were expected to involve the inactivated region of SC (based on the vector of the stimulation-evoked movements). Initial gaze position was (0,0). Open circles depict target locations; filled black, red, and blue circles show endpoints for control data, injection 1, and injection 2, respectively for this electrode track. Both injections were at the same site, ~2 mm below the surface of SC. This is also the same site shown in Fig. 3. In this example, gaze amplitudes were clearly smaller following lidocaine injection. To quantify the effect of inactivation on gaze amplitude, desired amplitude was first computed as the difference between target location and initial gaze position. The ratio of actual to desired amplitude was then computed for each trial. Figure 4B shows summary data for all injections in both monkeys. Each data point represents the mean value of the amplitude ratio for a single injection. Significant changes (filled symbols) were seen for 31/45 injections, with seven being increases and 24 decreases. Circles, triangles, squares, and diamonds show comparisons between control data and data following the first, second, third, and fourth injections, respectively, for a given electrode track. It must be emphasized that these amplitude changes were small, with most being under 10% (i.e., 1°–2°). Across all injections, there was no significant difference (mean ± SD: pre, 0.959 ± 0.08; post, 0.956 ± 0.08).
FIG. 4.
Effects of reversible inactivation on gaze shifts. Left: example data from 2 injections from monkey WL. The same 2 injections are shown in all panels in the left column. Control data are shown in black; data from the 1st and 2nd injection on this track are shown in red and blue, respectively. Right: summary data. Each individual data point compares postinjection values of the parameter of interest with the associated control values. Circles, triangles, squares, and diamonds show data from the 1st, and, if available, 2nd, 3rd, and 4th injections of each track. Filled symbols indicate that significant changes occurred for the postinjection data. A and B: gaze amplitude; C and D: gaze latency; E and F: peak gaze velocity; G and H: gaze duration. Reversible inactivation of SC clearly impaired monkeys’ ability to perform head-unrestrained gaze shifts, as indicated by decreased amplitude, longer reaction times, decreased peak velocity, and longer durations. See text for more details.
Gaze latency was consistently longer following injections of lidocaine. Figure 4C plots latency as a function of trial number for two injections that showed robust increases in latency following injections of 2 μl lidocaine. All data are from the same injections showed in A. Reaction times increased immediately following the injections, and then returned to baseline over the next 10–30 min. Figure 4D plots mean postinjection latency as a function of mean control latency for all injections in both monkeys. Significant increases (filled symbols) in reaction time occurred for 45 injections. With one exception (shown in D as an open circle), nonsignificant results were consistently associated with identifiable problems with the injection system (i.e., blocked cannula or incorrect injection depth) and are not included in the analyses reported in this manuscript. Across all injections, SC inactivation resulted in a significant increase in gaze reaction time (pre: 191.2 ± 27.5 ms; post: 235.4 ± 44.7 ms).
For most sites, peak gaze velocities were lower following inactivation of the SC (Fig. 4, E and F). E plots peak velocity as a function of amplitude for two injections for control (black) and postinjection (red and blue) data. F compares averaged peak velocity for control and postinjection data. Significant reductions in peak gaze velocity were found for 35/45 injections. Across all injections, gaze peak velocity was significantly reduced for the postinjection data (pre: 652.9 ± 115.0°/s; post: 578.3 ± 113.6°/s). Note that this analysis was restricted to targets within 10° of the stimulation-evoked gaze shift and the amplitudes were highly similar in the control and postinjection data (discussed in the following text). Thus this analysis is unlikely to have been significantly affected by amplitude differences.
Significant increases in the duration of gaze shifts were observed for 21/45 injections (Fig. 4H). Across all injections, gaze duration was significantly longer for the postinjection data (pre: 58.1 ± 11.7 ms; post: 64.6 ± 15.0 ms). G shows example data from the same two injections shown in A and C. At the top of this figure, some trials can be seen with unusually long durations. This phenomenon is attributable to blinks that sometimes occurred during the gaze shifts (Evinger et al. 1994; Gandhi 2007). Summary data are shown in H.
Head movements associated with gaze shifts
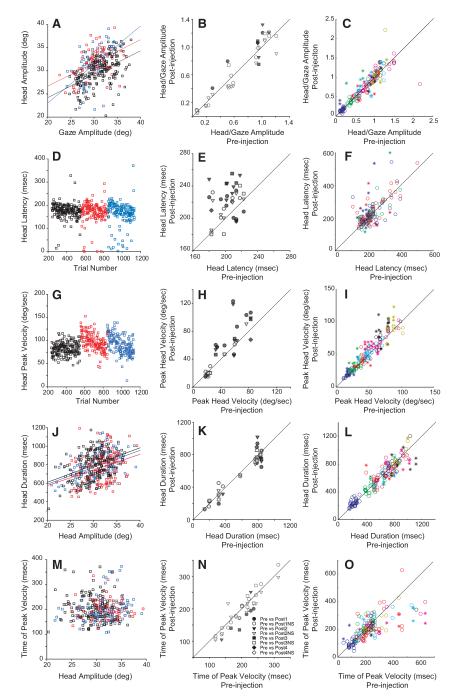
Figure 5 compares the amplitude, latency, peak velocity, duration, and time of peak velocity for head movements associated with gaze shifts. The left column shows example data for two injections (red and blue data points) and preinjection control data (black). The same two injections are shown throughout the left column of this figure, and these are the same data in the left column of Fig. 4. The middle column shows summary data for all injections, following the same format used for the right column of Fig. 4.
FIG. 5.
Effects of reversible inactivation on head movements accompanying gaze shifts. Left and middle: example data and summary data, respectively, using the same format as Fig. 3. Right: data have been binned according to head movement amplitude (i.e., blue, 0–5°; green, 5–10°; red, 10–15°; light blue, 15–20°; cyan, 20–25°; black, 25–30°; yellow, 30–35°). *, bins with significant changes in the parameter of interest; ○, bins with nonsignificant effects are shown. A–C: head amplitude; D–F: head latency; G–I: peak head velocity.; J–L: head duration; M–O: time of peak head velocity. In contrast with the clear effects on gaze in Fig. 3, the effects on head movements that accompany gaze shifts were minimal. See text for more details.
For relatively rostral sites (n = 5), the stimulation-evoked gaze vector was typically associated with a negligible head movement. Amplitude-matched gaze shifts generated after the lidocaine inactivation at these sites also did not yield measurable head movements. Data from these sites were not included in the head movement analyses represented in the middle column of Fig. 5. In the right column, data from all contralateral movements have been binned according to head amplitude and each data point represents a comparison between amplitude-matched control and postinjection bins (see methods). Asterisks show bins with significant changes in the parameter of interest, and colors indicate the amplitude of each bin (see legend).
The first step was to assess the effect of inactivation on the amplitudes of head movements associated with gaze shifts. Figure 5A plots head amplitude as a function of gaze amplitude for individual preinjection trials as well as trials after the first (red) and second injections (blue). To facilitate comparison between injection sites, head amplitude was divided by gaze amplitude for each trial. The mean value of this ratio was then computed for all gaze shifts to the five target locations that were expected to involve the inactivated region of SC. B shows the resulting values for pre- and postinjection data for all injection sites. Significant increases were seen for only 8/40 sites and significant decreases were seen for 2/40 sites. Across all injections, there was no significant difference (pre: 0.64 ± 0.39; post: 0.66 ± 0.42). The mean value of this parameter was then computed for every head amplitude bin for all injections and all control data. C plots these means for postinjection data as a function of the preinjection mean. Significant increases (denoted by asterisks) were found for 31/182 bins and significant decreases for 12/182 bins. Overall, however, there was no significant difference (pre: 0.728 ± 0.380; post: 0.759 ± 0.388). It is worth noting that in Fig. 5A, the head movements were relatively large for the gaze shifts that they were associated with. This animal often oriented itself in the chair at an angle (i.e., with the right shoulder forward). The relatively large head movements are, therefore attributable to the position of the head on the body at the time of gaze onset (McCluskey and Cullen 2007).
Because the monkeys were permitted to make any combination of eye and head movements to acquire the gaze targets, head movements associated with gaze shifts were not required to achieve a desired final position. Due to the lack of constraint on head position, a direct comparison of head amplitude in the control and postinjection conditions, like that performed for gaze amplitude, is likely inappropriate. For example, a change in head amplitude could just mean that the gaze amplitude changed and that the head movement remained appropriate for the gaze shift. Hence we chose to compare the mean ratio of head to gaze amplitude before and after the injection. Given the small and inconsistent effects of lidocaine inactivation on the head component of gaze shifts, we expect to reach the same conclusion with either approach.
Next we examined the latencies of head movements associated with gaze shifts. Figure 5D plots the latencies of head movements associated with gaze shifts for the two example injections. Immediately following the injections, head reaction time increased slightly for both injections and then slowly returned to baseline. Significant increases were seen for 16/40 injections and significant decreases for 5/40 injections (E). Across all injections, head latency was significantly longer for postinjection data (pre: 207.8 ± 24.4 ms; post: 222.4 ± 24.1 ms). When data were binned according to head amplitude, head latency was significantly longer for 26/182 bins and significantly shorter for 11/182 bins (Fig. 5F). Across all bins, head latency was significantly longer (pre: 221.4 ± 67.0 ms; post: 234.5 ± 90.4 ms). Thus both approaches revealed a small, but significant, increase in the latencies of head movements accompanying gaze shifts. It should be emphasized, however, that these effects were quite small compared with the effects on gaze latency (Fig. 4, C and D).
In Fig. 5D, a few head movements can be seen at very short latencies. To obtain a sufficient number of trials for robust statistical analyses, it was necessary that ~75% of target locations be contralateral. For this reason, it is possible that the animals may have come to expect contraversive movements. Thus occasionally this expectation may have led the animal to begin to move the head earlier than usual. Nonetheless such trials were relatively uncommon, and, overall, this phenomenon did not vary between pre- and postinjection conditions.
Next we examined the effects of SC inactivation on the peak velocities of head movements associated with gaze shifts. For some injections, peak head velocity was clearly enhanced following inactivation of SC. This can be appreciated from the examples shown in Fig. 5G. Peak velocity is clearly greater following both injections, and the effect recovers following a time course that is similar to that for gaze latency for the same injections (Fig. 4C). Overall, however, this effect was small and was present only for injections involving the right SC of monkey WL. H shows mean peak velocities for head movements associated with gaze shifts for all injections. Postinjection peak velocity was significantly higher for 19/40 injections and significantly lower for 5/40 injections. Across all injections a small, but significant, increase in peak velocity was observed (pre: 47.2 ± 24.6°/s; post: 56.2 ± 32.5°/s). I shows summary data for all bins from all injections. Significant increases in peak velocity were found for 47/182 bins and significant decreases for 14/182 bins. Across all bins there was a significant, but very small, increase in peak velocity for postinjection data (pre: 48.1 ± 23.0°/s; post: 52.2 ± 25.8°/s).
It is worth considering whether there were any underlying commonalities among the significant bins. The small but significant increase in peak head velocity occurred only for leftward movements in monkey WL. Although the reason for the facilitation is not clear, as noted in the preceding text, this animal often oriented itself in the chair at an angle (i.e., with the right shoulder forward). However, note that the difference in mean peak velocity, thought statistically significant, is very small and not convincing enough to argue for physiological significance. The most important result, across both monkeys and both directions, is that reversible inactivation did not produce deficits in the kinematics of the head component of gaze shifts.
For a few sites, increases in the velocity of head movements were associated with decreases in duration. In most cases, however, there was very little effect. This can be seen in the examples shown in Fig. 5J. Significant increases were found for 3/40 injections and significant decreases for 9/40 injections (pre: 572. 2 ± 273.9 ms; post: 561.0 ± 271.3 ms; Fig. 5K). In K, two “clusters” of data points can be seen, one with durations between ~150 and 400 ms and a second one with durations of >700 ms. The former are from rostral SC sites for which gaze shifts were accompanied by small head movements. The latter are from sites with much larger head movements. Across all injections, there was no significant difference. Significant decreases in head duration were found for only 23/182 bins (L). Significant increases in duration were found for 13/182 bins. Overall, there was no significant difference (pre: 618.8 ± 246.6 ms; post: 612.4 ± 238.5 ms).
The preceding results seem to indicate that reversible inactivation of SC does not consistently lead to measurable impairments in head movements associated with gaze shifts. It is possible, however, that deficits may be masked by compensatory mechanisms. If so, then the dynamics of the head movements should be perturbed, even if the amplitude, peak velocity, duration, and latency are unaffected. For example, if the postinjection head movements are abnormally slow during the early part of the movement, an additional command may be issued that causes a reacceleration. In this case, the peak velocity may occur later in the movement, although the example traces shown in Fig. 3B do not support this possibility. To test this quantitatively, for each trial, the time of peak velocity was measured with respect to head movement onset. In the example data shown in Fig. 5M, time of peak velocity is plotted as a function of head amplitude. In this example, there was no significant effect for either injection. Across all 40 injections, peak velocity occurred earlier in six cases and later in one case (N). Across all injections, peak head velocity occurred slightly, but significantly, earlier (pre: 201.2 ± 47.8 ms; post: 193.4 ± 54.2 ms). When the data were binned according to head amplitude, peak head velocity was significantly later in 5/182 bins and significantly earlier in 16/182 bins (O). Overall, peak head velocity occurred slightly, but significantly, earlier in the postinjection data (pre: 228.7 ± 119.3 ms; post: 204.8 ± 85.9 ms). Thus reversible inactivation had relatively little effect on the time of peak velocity.
It is also possible that rapid motor learning may mask deficits. If so, then impairments may exist for a short time after the injection but may rapidly disappear as the animal learns to overcome the deficit. If this is the case, then pooling data across all trials in which gaze shifts were affected may obscure the effect. To test for rapid compensatory behavior, the postinjection data were binned by trial number with 10 trials in each bin. For example, the first bin would include the first 10 postinjection trials that involved the five target locations that should have been affected by the injection, the second bin would include the next 10 such trials, and so on. Trends in amplitude, latency, peak velocity, and duration of the head movement were sought for each injection. The head amplitude was normalized by gaze amplitude, as done for Fig. 5, to account for the different targets used to pool the data. Similarly, the peak velocity and duration parameters were normalized by taking advantage of the highly linear main sequence properties of head movements (Freedman and Sparks 1997b). A linear regression was applied to the preinjection data to correlate head amplitude to peak velocity and to duration. The parameters (slope and intercept of each fit) were then used to predict the peak velocity and duration for each postinjection trial. The difference between the observed and predicted values represents the residual of each postinjection trial. A similar residual was also computed for the preinjection trials and compared with the mean residuals of the postinjections trials within each bin. For each of the four parameters, the means were usually <1 SD away from the preinjection mean. In fact, <5% of the bins showed a deviation of >1 SD in the direction that would be considered a deficit. Moreover, the rare bins that did show evidence of a deficit were fairly uniformly distributed among the first 10 bins. This suggests that these bins were merely attributable to random variability. Across the injections, there was no evidence of early deficits that were corrected by rapid adaptive processes. In an additional analysis, the data for each bin were combined across all injections, such that the first bin, for example, contained the first 10 postinjection trials from all injections. T-tests were used to compare these pooled bins to the preinjection data. Overall, the results were similar to other analyses, and there was no sign of any early deficits that were overcome by adaptive processes (data not shown). As an additional test, data for the first postinjection trial were pooled across all injections to determine if initial deficits were immediately compensated for within the first several trials. When this was done, no significant differences were found between the first postinjection trial and preinjection data for any of the analyses considered in the preceding text (amplitude, latency, duration, and peak velocity). As was the case for the analyses involving the first 10 postinjection trials, the (nonsignificant) differences were usually in the opposite direction from what might be considered a deficit.
It is also possible that head movement may become more variable following SC inactivation. To test this possibility, a two sample F test (using the vartest2 command in Matlab) was used to compare the variability of post- and preinjection head movements. The analysis was performed with respect to amplitude, latency, duration, and peak velocity. For latency, variance increased significantly for 8/40 injections and decreased significantly for 8/40 injections. The variability of the amplitude ratio increased for 7/40 injections and decreased for 15/40 injections. Movement duration was more variable for 8/40 injections and less variable for 3/40 injections. Finally peak head velocity was more variable for 13/40 injections and less variable for 6/40 injections. Although changes in variability may occur for a variety of reasons (fatigue, lapses of attention, etc), there was no evidence of any consistent effects on the variability of movements.
Eye-head coupling
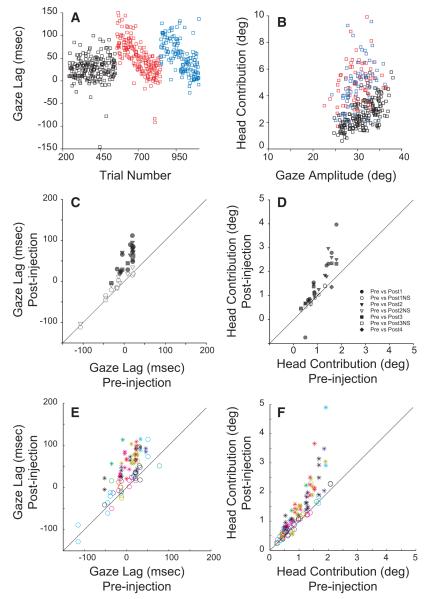
Although some injections resulted in significant increases in head latency, the small size of the effect meant that the gaze shift was generally delayed more than the head movement that accompanied it. To more fully investigate these perturbations in eye-head timing, we computed gaze lag by subtracting head reaction time from gaze reaction time. Thus positive values of this parameter indicate that the head started to move first. Figure 6A plots gaze lag as a function of trial number for the same two injections shown in Figs. 4 and 5. If the saccade is delayed more than the head movement, then the head will probably be moving faster during the time that the gaze shift is occurring. Along with the tendency for postinjection gaze shifts to increase in duration, this means that the head should contribute more to the gaze shift following the injections. This is confirmed in B, which plots head contribution (Freedman et al. 1996), i.e., the head displacement spanning the duration of the gaze shift (see methods), as a function of gaze amplitude for preinjection data (black) and prerecovery trials (red and blue) from the two injections shown in A. C shows the mean gaze lag for each injection. The gaze lag was significantly greater for 23/40 injections. Following the injections, gaze lag significantly increased (pre: −6.1 ± 31.7 ms; post: 25.3 ± 50.8 ms). D shows the mean head contribution to gaze for each injection. After reversible SC inactivation, the head contribution to gaze was significantly greater for 25/40 injections, and significantly smaller for 3/40 injections. Overall, the head contribution was slightly, but significantly, greater for the postinjection data (pre: 0.99 ± 0.45°; post: 1.34 ± 0.90°). Similar results were found when the data were binned according to head amplitude. Because this issue is of interest only for target locations for which gaze latency was increased, this analysis was restricted to the five target locations that were expected to involve the inactivated region of the SC. For 44/86 bins, gaze onset was significantly delayed with respect to head onset time (E). Gaze onset was never significantly earlier with respect to head onset time. Following lidocaine injection, gaze lag significantly increased (pre: −2.4 ± 39.7 ms; post: 33.7 ± 53.6 ms). Postinjection, the head contribution to gaze was significantly greater for 55/108 bins and significantly less for 2/108 bins (F). Overall the head contribution to gaze was significantly greater (pre: 0.96 ± 0.52°; post: 1.30 ± 0.85°). Trials with no head movement were eliminated from analyses involving gaze lag because it is impossible to compute this parameter if there is no head movement. However, when there is no head movement, the head contribution can legitimately be computed and is, of course, zero. As a result, the number of trials differed for these two analyses, and this explains the difference in the number of bins.
FIG. 6.
Effects of reversible inactivation on eye-head coordination. A: gaze lag is plotted as a function of trial number for control and the 2 injections illustrated in Fig. 4. Gaze lag is computed as gaze latency minus head latency, so that positive values indicate that the gaze shift began after head onset. B: head contribution is plotted as a function of the amplitude of gaze shifts to the 5 target locations that were expected to involve the inactivated region of SC. Control trials are shown in black and postinjection data are shown in red. C and D: a comparison of gaze lag and head contribution between control and postinjection conditions. Each symbol corresponds to 1 injection. E and F: same comparison but the data were binned according to head amplitude. The format is the same as that used in the right columns of Figs. 3 and 4. From this figure, it is apparent that reversible inactivation caused the gaze shifts to begin later, relative to the time of head onset. The head clearly contributes more to the gaze shifts after the injections than in the preinjection data.
To summarize, the present data demonstrate that head movements associated with gaze shifts are not appreciably impaired by reversible inactivation of SC. With the exception of latency, when significant results were found, they were usually in the direction that would be considered facilitation rather than a deficit (i.e., higher peak velocity, shorter duration, etc). When data were binned according to head amplitude, over half of the injections produced no bins with significant changes. This was the case for 24/45 injections for amplitude, 24/45 for latency, 19/45 for peak velocity, 24/45 for duration, and 30/45 for time of peak velocity. Instead, bins with significant changes tended to cluster in a minority of injections for which small effects were seen (see middle column of Fig. 5).
Head-only movements
In general, reversible SC inactivation had little effect on head-only movements. This can be seen in the example movements shown in Fig. 7. The top traces show head position before (black) and after (red) an injection of 3 μl lidocaine. Bottom traces show velocity profiles. It is clear in these examples that head-only movements were not impaired even by this relatively large injection. The injection site is depicted in Fig. 2 as a small, black circle.
FIG. 7.
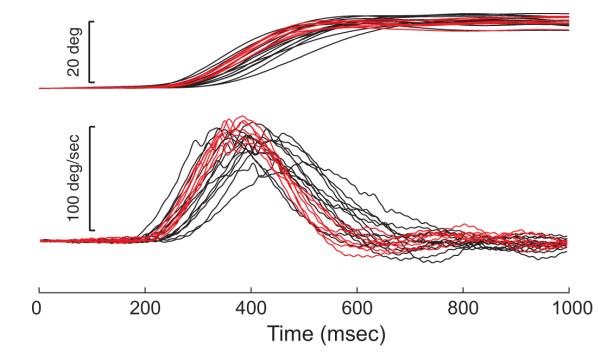
Example position and velocity traces for head-only movements. Data are aligned on the onset of the cue to make the head-only movement. Head only movements were highly similar before (black) and after (red) reversible inactivation.
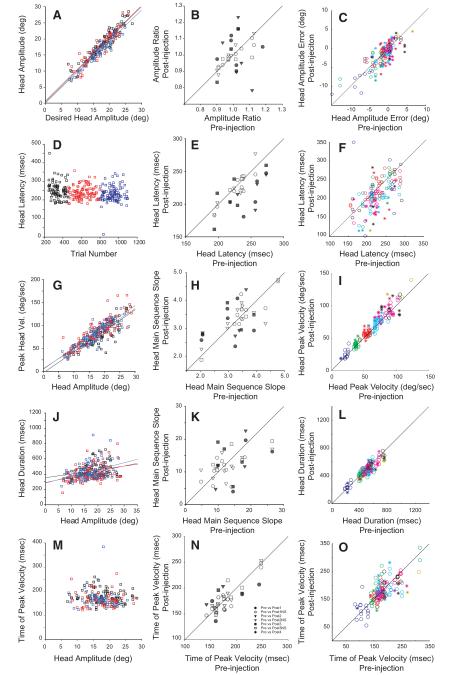
As a first step toward quantitatively assessing the effects of reversible inactivation on head-only movements, desired head amplitude was computed as the difference between target position and initial head position. Figure 8A plots head-only amplitude as a function of desired head amplitude for two injections and for the associated control data. For the analysis shown in B, the ratio of actual/desired amplitude was computed for each trial. This parameter was used because head movement data were collected across a wide range of amplitudes for each injection. A ratio is likely to be more constant across different amplitudes than a difference. B plots the mean ratio for each injection as a function of the mean ratio for preinjection data from the same track. Significant amplitude increases were found for 8/36 injections and significant decreases were found for 7/36 injections. Overall there was no difference between pre- and postinjection head only amplitude ratios (pre: 1.00 ± 0.07; post: 1.00 ± 0.10). When data were binned according to head amplitude, the aforementioned issue was not a problem because each bin contained head movements from a small range of amplitudes. Therefore for the analysis shown in C, the amplitude error was computed for each trial as actual-desired amplitude. Significant increases were found for 21/159 bins, and significant decreases were found for 39/159 bins. Although there were a number of significant results, the actual changes in error were quite small, never exceeding ~5°. Postinjection, the mean value of this difference was slightly more negative, indicating that head-only movements were slightly, but significantly, more hypometric (pre: −1.1 ± 3.0°; post: −1.5 ± 3.1°). Note, however, that the difference was <0.5°.
FIG. 8.
Effects of reversible inactivation on head-only movements. The format is the same as that in Fig. 5. As was the case for head movements associated with gaze shifts, reversible inactivation had only minimal effects on head-only movements.
The effect of lidocaine injection on head latency can be assessed in panels D–F. D shows example data from the same two injections shown in A. In this case, the injections had no effect on the latencies of head-only movements. Significant increases in latency were found for only 2/36 injections and significant decreases in latency were found for 13/36 injections (E). Overall, head latency was slightly, but significantly, reduced (pre: 233.2 ± 22.5 ms; post: 221.3 ± 24.5 ms). When data were binned by head amplitude, significant increases in latency were found for 3/159 bins and significant decreases for 28/159 bins (D). Overall there was a slight, but significant, decrease in head-only reaction time (pre: 230.7 ± 45.5 ms; post: 214.2 ± 53.5 ms). This result was significant for both monkeys.
During the dissociation period of our tasks, the monkey already knows the vector of the head movement that he will be asked to make. For this reason, the monkeys occasionally made early, anticipatory, head movements. These trials appeared as outliers when latency was plotted as a function of trial number. A latency criterion of 130 ms was empirically set to consistently separate these outliers from the main cluster of data points. To minimize these anticipatory responses, the head window was made as small as possible without allowing the head to routinely leave the window during the dissociation period due to drifts and/or small head movements. Anticipatory head movements were found to occur in 5.6% of control trials and 7.5% of postinjection trials. The difference in the percentage of anticipatory responses was largely due to a greater percentage of postinjection latencies between 50 and 90 ms; there was little difference for latencies of <50 ms (2.4% for preinjection data vs. 2.8% for postinjection data). Anticipatory trials were excluded from all other analyses.
We also examined the effects of lidocaine on the peak velocity of head-only movements. Figure 8G shows the main sequence (peak velocity – head amplitude) relationship before and after inactivation for the same two injections shown in A and D. H shows summary data, with each data point representing the slope of this relationship for an individual injection and its associated control. The postinjection slope was significantly greater for 9/36 injections and significantly smaller for 6/36 injections. Overall, however, there was no significant difference (pre: 3.30 ± 0.71 s−1; post: 3.37 ± 0.64 s−1). Significant increases in peak velocity were seen for 33/159 bins and significant decreases were observed for 8/159 bins (I). Overall, there was a very small, but significant, increase in peak velocity (pre: 62.5 ± 23.6°/s; post: 65.8 ± 25.1°/s). This effect was significant for both monkeys.
Reversible inactivation had little effect on the duration of head only movements (J–L). J plots head duration as a function of amplitude for control (black) and post injection (red and blue) data for the example injections. K compares the slope of this relationship for each individual injection to the slope for the preinjection slope for the same track. The postinjection slope was significantly greater for 6/36 injections and significantly smaller for 6/36 injections. Across all injections, there was no significant difference (pre: 13.3 ± 5.4 ms/°; post: 12.2 ± 4.6 ms/°). L shows summary data for all bins for all injections. Significant increases in duration occurred for 6/159 bins and significant decreases for 22/159 bins. Overall there was a small, but significant, decrease in head duration (pre: 495.5 ± 124.7 ms; post: 477.0 ± 114.1 ms).
The time of peak velocity (with respect to head movement onset) was also analyzed. No consistent effects were detected for the two example injections (M). The mean time of peak velocity was significantly later for 4/36 injections and significantly earlier for 5/36 injections (N). Across all injections, there was no significant difference (pre: 179.7 ± 29.2 ms; post: 178.9 ± 27.4 ms). Postinjection peak velocity was significantly later in 7/159 bins and significantly earlier in 12/159 bins. Overall, there was no significant difference (pre: 178.1 ± 41.1 ms; post: 175.9 ± 41.8 ms).
As is the case for head movements associated with gaze shifts, it is necessary to consider the possibility that rapid motor learning may mask deficits in the control of head-only movements. To address this, the amplitude, latency, peak velocity, and duration data were binned by trial number as described in the preceding text. Again, <5% of the bins showed a deviation of >1 SD in the direction that would be considered a deficit. Pooling the data for each bin across injections showed that significant changes, when they occurred, were always in the direction that might be considered “facilitation”—shorter latency, increased peak velocity, shorter duration, etc. As was the case for head movements associated with gaze shifts, data from the first postinjection trial were then pooled across injections. Results were not significantly different from the preinjection data for amplitude, latency, duration, or peak velocity. The (nonsignificant) differences in means were always in the opposite direction from what might be considered a deficit. Thus there was no evidence that any transient deficits were being masked by motor learning.
To summarize, the present data provide no evidence that reversible SC inactivation consistently impairs monkeys’ ability to make head only movements. When significant differences were found, they were usually in the opposite direction from what would be considered an impairment. When data were binned according to amplitude, over half of the injections produced no bins with significant changes (this was the case for 11/36 for amplitude, 19/36 for latency, 16/36 for peak velocity, 19/36 for duration, and 22/36 for time of peak velocity). Instead significant bins tended to be clustered in a minority of injections for which small effects were found (see Fig. 8, middle). Thus there was no evidence that any subset of amplitudes were differentially affected by the injections.
Returning to Fig. 7, the temporal traces give the impression that the postinjection traces exhibited far less variability than the preinjection data. To test for changes in the variability of head only movements, the variability of head-only movements was analyzed using the same approach used to assess the variability of head movements associated with gaze shifts. For latency, variance increased significantly for 7/36 injections and decreased significantly for 12/36 injections. The variability of the amplitude ratio increased for 2/36 injections and decreased for 6/36 injections. Movement duration was more variable for 1/36 injections and less variable for 1/36 injections. Finally, peak head velocity was more variable for 6/36 injections and less variable for 3/36 injections. Thus as was the case for head movements associated with gaze shifts, there was no evidence of consistent changes in the variability of head only movements following lidocaine injection.
Head drifts
We have isolated neurons in SC that tend to fire tonically when the head is stationary, and pause for head movements (Walton et al. 2007). Conceivably, such cells could be important during actions that require the head to remain stationary during gaze shifts that normally would be associated with significant head movement. If this is the case, inactivation of SC might disrupt the animals’ ability to keep the head stationary during the performance of these tasks. Additionally, one must consider the possibility that reversible inactivation impairs the monkeys’ ability to maintain the eyes at eccentric locations without moving the head. If, for example, the injections cause the head to drift faster before our algorithm detects head movement onset, then our measurements of duration, amplitude, and latency would be affected. To test these possibilities, we performed a dynamic, ms-by-ms analysis of the head drifts that occurred in the 500-ms period prior to the detected head onset. This analysis was performed for 12 injections across 6 days. Trials were grouped according to initial head position and dissociation distance, yielding a total of 44 comparisons. Of these, 18 were significantly different during the first 100–200 ms. Even when the pre- and postinjection drifts were significantly different, they were quite small, and the drift velocity was well below the 6°/s velocity threshold for head onset. This was in contrast to the relatively rapid accelerations that characterized the onsets of the head-only movements. The average horizontal drift was 1.16 and 1.06° for the pre- and postinjection trials, respectively. The average vertical drift was 0.61 and 0.63° for the pre- and postinjection trials, respectively. Overall, neither of these was significantly different for the comparison between pre and post. Note that these drifts occurred over a 500-ms period and were, therefore quite small before and after inactivation. Thus reversible inactivation did not impair the animals’ ability to maintain the eyes at eccentric orbital positions without moving the head.
Saline injections
Five saline injections were made using a protocol identical to that used for the lidocaine injections. The same data analysis procedures were also used. No significant differences were found for any parameter for either the gaze shifts or head-only movements.
DISCUSSION
Gaze shifts
The present data show that reversible inactivation of SC in head-unrestrained primates affects gaze in ways similar to previous reports based on head-restrained preparations (Hikosaka and Wurtz 1985, 1986; Lee et al. 1988; McPeek and Keller 2004; Quaia et al. 1998). Consistent with these previous studies, injection of lidocaine into the intermediate and deep layers of SC caused decreases in peak velocity as well as increases in gaze reaction time and duration. Given the large body of research showing that the SC issues commands related to coordinated eye-head gaze shifts (Bergeron and Guitton 2000; Choi and Guitton 2006; Corneil et al. 2002, 2007; Freedman and Sparks 1997a; Freedman et al. 1996; Guillaume and Pelisson 2006; Klier et al. 2001; Matsuo et al. 2004; Pelisson et al. 2001), the natural expectation was that head movements associated with these gaze shifts would be similarly affected. Surprisingly, reversible inactivation never substantially impaired the head component of gaze shifts. Some injections resulted in slight increases in the reaction times of head movements associated with gaze shifts, but even this effect was small when it occurred (i.e., Fig. 5, D–F).
Because the effect on head latency was generally weaker than the effect on gaze reaction time, the usual timing relationship of the eye and head components was perturbed. Postinjection, saccades tended to start later in the head movement than they normally would—at a time when the head was moving at a faster velocity. For this reason, and possibly also because the gaze shifts were of longer duration, the head made a larger contribution to the gaze shift. At first, this result might seem inconsistent with the hypothesis that single neurons in SC encode gaze. However, the present results may simply reflect the fact that weaker SC activity takes longer to turn off pontine omnipause neurons (OPNs). If a portion of the collicular gaze command drives the neck motoneurons without being subjected to the OPN inhibition, then the reduced collicular activity could simply take longer to trigger the saccade than the head movement (Corneil et al. 2002; Gandhi and Sparks 2007; Pélisson et al. 2001).
Although the latency effect can be readily accounted for, the lack of effects on the kinematics and dynamics of head movements is more difficult to explain. According to the dual coding hypothesis (Sparks and Mays 1990), the spatial locus of activity on the SC map dictates the metrics of the movement and the firing rate encodes its kinematics and dynamics. A logical extension of this thinking to the head-unrestrained condition would lead one to expect an analysis of the kinematics of head movements associated with gaze shifts to reveal evidence of measurable impairment. The fact that no such deficits, not even signs of rapid motor adaptation, were found suggests that the brain stem circuitry that controls head movements is not as dependent on the SC output as is the oculomotor burst generator that produces saccadic eye movements. When the SC outputs a robust signal, it contributes to head movement generation as can be appreciated by head-only movements generated by low-frequency stimulation (Corneil et al. 2002). When the SC input is weakened, altered, or absent, such as after inactivation, head movements can be adequately controlled by pathways that bypass SC.
Anatomical and neurophysiological evidence supports the possibility that the necessary redundancy exists to drive head movements even after SC has been inactivated. The necessary signals could originate from cortical projections that reach the nucleus reticularis gigantocellularis (NRG), which, in turn, projects to the spinal cord (Alstermark et al. 1985; Iwamoto and Sasaki 1990; Iwamoto et al. 1990; Peterson et al. 1975, 1978; Shinoda et al. 2005) and participates in the control of head movements (e.g., Cowie and Robinson 1994; Quessy and Freedman 2004). The frontal eye field (FEF) is one potential cortical source because head movements, with or without accompanying eye movements, can be evoked by stimulation (Chen 2006; Elsley et al. 2007; Knight and Fuchs 2007; Tu and Keating 2000). The slightly longer time required for the FEF signal to reach the neck muscles (Elsley et al. 2007) could account for the small increase in head latency observed following SC inactivation. Permanent lesion of a FEF, however, does not attenuate head movements (van der Steen et al. 1986). Following a recovery period of ~1 wk, orienting responses to the contralateral hemifield were performed largely by the head, and the range of saccadic eye-in-head movements decreased. Furthermore, the head movements exhibited higher peak velocity and shorter duration than amplitude-matched control movements. In other words, the compensated behavior after a permanent FEF insult bears some resemblance to the acute behavior following a reversible SC inactivation. Such changes in head movement strategy have also been observed after adaptation to a limited visual field of view (Ceylan et al. 2000; Constantin et al. 2004). The supplementary eye field (SEF) is another likely source as stimulation evokes coordinated eye-head movements (Chen and Walton 2005; Martinez-Trujillo et al. 2003); in fact, head movements appear to be generated more readily by stimulation of the SEF compared with the FEF (Chen 2006; Chen and Walton 2005). To our knowledge, a systematic investigation of the effects of SEF inactivation on eye-head movements has yet to be reported. Another noncortical source of head movement command may be the oculomotor region of the fastigial nucleus, the inactivation of which results in differential deficits in the eye and head component of gaze shifts (Quinet and Goffart 2005, 2007).
In summary, microstimulation studies indicate that higher-order elements in the oculomotor neuraxis play a role in the control of head movements associated with gaze shifts. Yet inactivation of any one region does not appear to exhibit an appreciable deficit in head movements in contrast to the effects on eye movements. Intuitively, an effect is expected because an attenuated command is trying to move a mass with heavy inertial load. The lack of a pronounced effect, however, implies that the head movement is driven by parallel and perhaps redundant commands and a complex biomechanical system, such that weakening or removal of a subset of the inputs does not reduce the effective drive needed to innervate the neck muscles. These parallel pathways likely merge in the mesencephalic and pontine reticular formation since inactivation of the former produces gross deficits in head movements and posture (Farshadmanesh et al. 2007; Klier et al. 2002; Waitzman et al. 2000). Another nonexclusive possibility is that the animals were able to compensate rapidly after the inactivation, although no signs of deficits, not even transient, were observed. Yet another viable explanation is that the throughput of cortical activity to their motoneurons can change rapidly to enhance the functional connectivity from motor cortex to muscles (Davidson et al. 2007). Under normal circumstance, the cortex probably delegates to the SC a greater responsibility for head movement control. As soon as the SC is inactivated, the cortex recognizes that the SC function is compromised and immediately changes its functional connectivity. It may not even be necessary to produce gaze shifts to implement the rapid switch in the cortical throughput—perhaps proprioceptive or postural signals from the neck muscles are sufficient.
One potential limitation of the present study involves the fact that pharmacological inactivation does not affect the entire SC. In previous studies involving reversible inactivation of SC (e.g., Hanes and Wurtz 2001; Lee et al. 1988), regions surrounding the affected area remain functional. It is possible that unaffected cells in the active population of SC may have differential effects on the gaze and head movement systems. The present study attempted to address this concern by using relatively large injection volumes (1–4 μl of 2% lidocaine). Larger volumes would be likely to inactivate portions of the underlying reticular formation in addition to SC. Based on previous studies, these injections should have effectively inactivated neurons ≥0.5 mm away from the injection site (Sandkühler et al. 1987; Tehovnik and Sommer 1997). Nonetheless, this clearly would not involve the entire SC, so the possibility remains that unaffected areas of this structure might have differential effects on the eye and head components. Indeed in cats, cryogenic inactivation of both superficial and intermediate layers of the SC abolishes both gaze saccades and head movements (Lomber et al. 2001). On the other hand, although eye-head-body orienting movements were grossly impaired following unilateral cooling of SC, they were virtually normal following bilateral deactivation of this structure (Lomber and Payne 1996). Taken together, these data seem consistent with the suggestion that head movements can be adequately controlled by pathways that bypass SC.
Head-only movements
Next we discuss the lack of an inactivation effect on head-only movements in light of our recordings of cells in SC that modulate their discharge in association with head movements (Walton et al. 2007). There are several possible reasons for this. First, it might be that SC simply plays little or no functional role in the control of head movements not accompanying gaze shifts. For example, the cells we have recently isolated might be an evolutionary hold-over that no longer perform any useful function. A second possibility is that these cells might play some role other than the actual generation of the head movements themselves. They might, for example, relay information regarding ongoing head movements to other structures that are not directly involved in the control of head movements. A third possibility is that SC plays only a relatively minor role in the control of head-only movements and that our injections, although large in volume, simply didn’t inactivate a large enough region of SC to detect a consistent effect. We have found no evidence that head cells in SC are topographically organized in the way that gaze signals are. Thus if head cells from across the entire SC contribute to every head-only movement, then it may be difficult to detect an effect without inactivating most of the structure. However, it must be emphasized that the small effects that were sometimes detected were usually in the opposite direction from what would be expected if SC inactivation impairs head movement control (i.e., shorter reaction times for head-only movements and higher peak velocities, shorter durations, and earlier times of peak velocity for both types of head movement). Fourth, as discussed in the preceding text for head movements that contribute to gaze shifts, it is possible that redundancy exists in the head-only movement pathways such that, when SC is inactivated, an alternative pathway can produce the required movement. Finally, we cannot rule out the possibility that the animals might have adopted compensatory strategies that mask impaired control of head movements. Potentially, the small facilitatory effects that were sometimes found could be explained as a slight overcompensation when the animals attempted to mitigate the effects of SC inactivation.
With regard to the issue of compensatory strategies, one must consider whether or not the animals were using visual feedback to perform on-line corrections of head movements to compensate for lidocaine-induced deficits. To address this concern, we blanked the laser during head-only movements for many of our injections. With the laser turned off, the animals had no visual feedback regarding ongoing head movements. This had no effect on the results. Proprioceptive feedback is, of course, still a possibility. However, it should be pointed out that where the injections altered the timing of peak velocity, the effect was quite small and in the opposite direction from what would be expected if the animals were performing an on-line, feedback mediated correction.
It is worth noting that the slight effects on latency were in opposite directions for head movements associated with gaze shifts and head only movements. However, because the effects were quite small in both cases, it is unclear whether or not this has any physiological significance, and one should be hesitant to draw firm conclusions.
Additional considerations
In the present study, most of the injections were at sites encoding gaze shifts of <40°. Because the head contribution to such movements is small, one may wonder whether more caudal injections would have revealed deficits in head movements. However, several points should be made here. First, five injections were made in two electrode tracks (with 2 different depths for each of these tracks) at sites encoding gaze shifts of over 40°, and the results were highly similar to injections made at more rostral sites. A second important point is that a major goal of the present study was to test the functional importance of the head cells reported in Walton, Bechara, and Gandhi (2007). These cells were found throughout the rostrocaudal and mediolateral extent of the SC, and most of the neurons in our sample were recorded at sites encoding movements of <40°. It makes sense to inactivate the same parts of SC where head cells have been recorded. Third, although head contribution to gaze may be small for 20–30° gaze shifts, total head amplitude is not (see, for example, Fig. 6, A and B, in Freedman and Sparks 1997b and Fig. 5, A–C, in the present report). Thus most of the gaze shifts analyzed for this manuscript were accompanied by significant head movement. Finally, it must be remembered that injections of 1–4 μl 2% lidocaine should effectively inactivate neurons ≥0.5 mm away from the injection site (Sandkühler et al. 1987; Tehovnik and Sommer 1997). Thus injection at a 30° site should affect 40° gaze shifts (see Fig. 2).
In our opinion, little should be made of the small facilitatory effects that were sometimes found for head movements. Although sometimes significant, these effects were so small that it is difficult to argue that they are physiologically relevant. In addition, for most injections, the small size of the changes in timing and kinematics makes it difficult to convincingly establish the familiar pattern of effect and recovery that is characteristic of lidocaine-induced effects. Of much greater importance is the fact that head movements (with or without gaze shifts) are clearly not impaired, a result that seems to suggest that these movements can be adequately controlled by pathways that do not involve the SC.
ACKNOWLEDGMENTS
The authors are grateful to C. Bryant and K. Pearson for computer programming, Dr. Marc Sommer for valuable advice on technical aspects of inactivation experiments, B. Hughes and T. Wheeler for machining services, J. Buhrman and Dr. Charles Scudder for electronics assistance, and G. Duffy for general assistance.
GRANTS This study was supported by National Institutes of Health Grants EY-015485, EY-015060, and DC-05205 and by the University of Pittsburgh Eye and Ear Foundation.
REFERENCES
- Alstermark B, Pinter MJ, Sasaki S. Pyramidal effects in dorsal neck motoneurones of the cat. J Physiol. 1985;363:287–302. doi: 10.1113/jphysiol.1985.sp015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PR, Johnston GA. GABA agonists and antagonists. Biochem Pharmacol. 1979;28:2697–2702. doi: 10.1016/0006-2952(79)90549-5. [DOI] [PubMed] [Google Scholar]
- Basso MA, Krauzlis RJ, Wurtz RH. Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol. 2000;84:892–908. doi: 10.1152/jn.2000.84.2.892. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Guitton D. Fixation neurons in the superior colliculus encode distance between current and desired gaze positions. Nat Neurosci. 2000;3:932–939. doi: 10.1038/78847. [DOI] [PubMed] [Google Scholar]
- Bryant CL, Gandhi NJ. Real-time data acquisition and control system for the measurement of motor and neural data. J Neurosci Methods. 2005;142:193–200. doi: 10.1016/j.jneumeth.2004.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan M, Henriques DY, Tweed DB, Crawford JD. Task-dependent constraints in motor control: pinhole goggles make the head move like an eye. J Neurosci. 2000;20:2719–2730. doi: 10.1523/JNEUROSCI.20-07-02719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL. Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol. 2006;95:3528–3542. doi: 10.1152/jn.01320.2005. [DOI] [PubMed] [Google Scholar]
- Chen LL, Walton MM. Use of a central difference differential algorithm in combination with a flexible, sliding window for defining head movement onset and offset thresholds (Abstract) Neural Control Movement. 2004 [Google Scholar]
- Chen LL, Walton MM. Head movement evoked by electrical stimulation in the supplementary eye field of the rhesus monkey. J Neurophysiol. 2005;94:4502–4519. doi: 10.1152/jn.00510.2005. [DOI] [PubMed] [Google Scholar]
- Choi WY, Guitton D. Responses of collicular fixation neurons to gaze shift perturbations in head-unrestrained monkey reveal gaze feedback control. Neuron. 2006;50:491–505. doi: 10.1016/j.neuron.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Collewijn H. Eye- and head movements in freely moving rabbits. J Physiol. 1977;266:471–498. doi: 10.1113/jphysiol.1977.sp011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin AG, Wang H, Crawford JD. Role of superior colliculus in adaptive eye-head coordination during gaze shifts. J Neurophysiol. 2004;92:2168–2184. doi: 10.1152/jn.00103.2004. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Olivier E. Priming of head premotor circuits during oculomotor preparation. J Neurophysiol. 2007;97:701–714. doi: 10.1152/jn.00670.2006. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle activity evoked by stimulation of the monkey superior colliculus. II. Relationships with gaze shift initiation and comparison to volitional head movements. J Neurophysiol. 2002;88:2000–2018. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Olivier E, Pelisson D. Direct evidence for the contribution of the superior colliculus in the control of visually guided reaching movements in the cat. J Physiol. 2004;556:675–681. doi: 10.1113/jphysiol.2004.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol. 1994;72:2648–2664. doi: 10.1152/jn.1994.72.6.2648. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Smith MK, Robinson DL. Subcortical contributions to head movements in macaques. II. Connections of a medial pontomedullary head-movement region. J Neurophysiol. 1994;72:2665–2682. doi: 10.1152/jn.1994.72.6.2665. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Chan V, O’Dell R, Schieber MH. Rapid changes in through-put from single motor cortex neurons to muscle activity. Science. 2007;318:1934–1937. doi: 10.1126/science.1149774. [DOI] [PubMed] [Google Scholar]
- Elsley JK, Nagy B, Cushing SL, Corneil BD. Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. J Neurophysiol. 2007;98:1333–1354. doi: 10.1152/jn.00386.2007. [DOI] [PubMed] [Google Scholar]
- Evinger C, Manning KA, Pellegrini JJ, Basso MA, Powers AS, Sibony PA. Not looking while leaping: the linkage of blinking and saccadic gaze shifts. Exp Brain Res. 1994;100:337–344. doi: 10.1007/BF00227203. [DOI] [PubMed] [Google Scholar]
- Farshadmanesh F, Klier EM, Chang P, Wang H, Crawford JD. Three-dimensional eye-head coordination after injection of muscimol into the interstitial nucleus of Cajal (INC) J Neurophysiol. 2007;97:2322–2338. doi: 10.1152/jn.00752.2006. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol. 1997a;78:1669–1690. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol. 1997b;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol. 1996;76:927–952. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ. Consequence of blinks on interactions between the eye and head components of gaze shifts. Soc Neurosci Abstr. 2007;178:2. [Google Scholar]
- Gandhi NJ, Sparks DL. Experimental control of eye and head positions prior to head-unrestrained gaze shifts in monkey. Vision Res. 2001;41:3243–3254. doi: 10.1016/s0042-6989(01)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. J Neurophysiol. 2007;98:360–373. doi: 10.1152/jn.00252.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume A, Pelisson D. Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. I. Effect of the locus and the parameters of stimulation. Eur J Neurosci. 2001;14:1331–1344. doi: 10.1046/j.0953-816x.2001.01744.x. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Pelisson D. Kinematics and eye-head coordination of gaze shifts evoked from different sites in the superior colliculus of the cat. J Physiol. 2006;577:779–794. doi: 10.1113/jphysiol.2006.113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol. 2001;85:804–815. doi: 10.1152/jn.2001.85.2.804. [DOI] [PubMed] [Google Scholar]
- Herrero L, Rodriguez F, Salas C, Torres B. Tail and eye movements evoked by electrical microstimulation of the optic tectum in goldfish. Exp Brain Res. 1998;120:291–305. doi: 10.1007/s002210050403. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA- related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Saccadic eye movements following injection lidocaine into the superior colliculus. Exp Brain Res. 1986;61:531–539. doi: 10.1007/BF00237578. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Projections of the superior colliculus to the supraspinal nucleus and the cervical spinal cord gray of the cat. Brain Res. 1982;242:326–331. doi: 10.1016/0006-8993(82)90317-1. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Sasaki S. Monosynaptic excitatory connexions of reticulospinal neurons in the nucleus reticularis pontis caudalis with dorsal neck motoneurons in the cat. Exp Brain Res. 1990;80:277–289. doi: 10.1007/BF00228155. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Sasaki S, Suzuki I. Input-output organization of reticulospinal neurones, with special reference to connexions with dorsal neck motoneurones in the cat. Exp Brain Res. 1990;80:260–276. doi: 10.1007/BF00228154. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Constantin AG, Crawford JD. Midbrain control three-dimensional head orientation. Science. 2002;295:1314–1316. doi: 10.1126/science.1067300. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci. 2001;4:627–632. doi: 10.1038/88450. [DOI] [PubMed] [Google Scholar]
- Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts the head-unrestrained monkey: effects of microstimulation. J Neurophysiol. 2007;97:618–634. doi: 10.1152/jn.00256.2006. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature. 1988;332:357–360. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. Removal of two halves restores the whole: reversal of visual hemineglect during bilateral cortical or collicular inactivation the cat. Vis Neurosci. 1996;13:1143–1156. doi: 10.1017/s0952523800007781. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P. Role of the superior colliculus analyses of space: superficial and intermediate layer contributions to visual orienting, auditory orienting, and visuospatial discriminations during unilateral and bilateral deactivations. J Comp Neurol. 2001;441:44–57. doi: 10.1002/cne.1396. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Wang H, Crawford DJ. Electrical stimulation of the supplementary eye fields in the head-free macaque evokes kinematically normal gaze shifts. J Neurophysiol. 2003;89:2961–2974. doi: 10.1152/jn.01065.2002. [DOI] [PubMed] [Google Scholar]
- Matsuo S, Bergeron A, Guitton D. Evidence for gaze feedback to the cat superior colliculus: discharges reflect gaze trajectory perturbations. J Neurosci. 2004;24:2760–2773. doi: 10.1523/JNEUROSCI.5120-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Vis Neurosci. 1992;8:257–276. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- McCluskey MK, Cullen KE. Eye, head, and body coordination during large gaze shifts in rhesus monkeys: movement kinematics and the influence posture. J Neurophysiol. 2007;97:2976–2991. doi: 10.1152/jn.00822.2006. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Guitton D, Pélisson D. Control of orienting gaze shifts by the tectoreticulospinal system in the head-free cat. III. Spatiotemporal characteristics of phasic motor discharges. J Neurophysiol. 1991;66:1642–1666. doi: 10.1152/jn.1991.66.5.1642. [DOI] [PubMed] [Google Scholar]
- Nagy A, Kruse W, Rottmann S, Dannenberg S, Hoffmann KP. Somatosensory-motor neuronal activity in the superior colliculus of the primate. Neuron. 2006;52:525–534. doi: 10.1016/j.neuron.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JA, Eggermont JJ. Visuomotor fields of the superior colliculus: a quantitative model. Vision Res. 1986;26:857–873. doi: 10.1016/0042-6989(86)90144-6. [DOI] [PubMed] [Google Scholar]
- Paré M, Crommelinck M, Guitton D. Gaze shifts evoked by stimulation of the superior colliculus in the head-free cat conform to the motor map but also depend on stimulus strength and fixation activity. Exp Brain Res. 1994;101:123–139. doi: 10.1007/BF00243222. [DOI] [PubMed] [Google Scholar]
- Pélisson D, Goffart L, Guillaume A, Catz N, Raboyeau G. Early head movements elicited by visual stimuli or collicular electrical stimulation in the cat. Vision Res. 2001;41:3283–3294. doi: 10.1016/s0042-6989(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and branching of reticulospinal neurons. Exp Brain Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K, Mackel R. Reticulospinal excitation and inhibition of neck motoneurons. Exp Brain Res. 1978;32:471–489. doi: 10.1007/BF00239548. [DOI] [PubMed] [Google Scholar]
- Quaia C, Aizawa H, Optican LM, Wurtz RH. Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. J Neurophysiol. 1998;79:2097–2110. doi: 10.1152/jn.1998.79.4.2097. [DOI] [PubMed] [Google Scholar]
- Quessy S, Freedman EG. Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. I. Characteristics of evoked head movements. Exp Brain Res. 2004;156:342–356. doi: 10.1007/s00221-003-1787-8. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Saccade dysmetria in head-unrestrained gaze shifts after muscimol inactivation of the caudal fastigial nucleus in the monkey. J Neurophysiol. 2005;93:2343–2349. doi: 10.1152/jn.00705.2004. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Head unrestrained gaze shifts after muscimol injection in the caudal fastigial nucleus of the monkey. J Neurophysiol. 2007;98:3269–3283. doi: 10.1152/jn.00741.2007. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brain stem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Sandkühler J, Maisch B, Zimmermann M. The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res. 1987;68:168–178. doi: 10.1007/BF00255242. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Izawa Y, Hata Y. Long descending motor tract axons and their control of neck and axial muscles. Prog Brain Res. 2005;151:527–563. doi: 10.1016/S0079-6123(05)51017-3. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Mays LE. Signal transformations required for the generation of saccadic eye movements. Annu Rev Neurosci. 1990;13:309–336. doi: 10.1146/annurev.ne.13.030190.001521. [DOI] [PubMed] [Google Scholar]
- Stein BE, Clamann HP. Control of pinna movements and sensorimotor register in cat superior colliculus. Brain Behav Evol. 1981;19:180–192. doi: 10.1159/000121641. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Bauswein E, Hoffmann KP. Neurons in the primate superior colliculus coding for arm movements in gaze-related coordinates. J Neurophysiol. 2000;83:1283–1299. doi: 10.1152/jn.2000.83.3.1283. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ. Head and body movements evoked electrically from the caudal superior colliculus of rats: pulse frequency effects. Behav Brain Res. 1989;34:71–78. doi: 10.1016/s0166-4328(89)80091-9. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA. Effective spread and timecourse of neural inactivation caused by lidocaine injection in monkey cerebral cortex. J Neurosci Methods. 1997;74:17–26. doi: 10.1016/s0165-0270(97)02229-2. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Yeomans JS. Two converging brainstem pathways mediating circling behavior. Brain Res. 1986;385:329–342. doi: 10.1016/0006-8993(86)91080-2. [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol. 2000;84:1103–1106. doi: 10.1152/jn.2000.84.2.1103. [DOI] [PubMed] [Google Scholar]
- Valentine DE, Sinha SR, Moss CF. Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol A Sens Neural Behav Physiol. 2002;188:89–108. doi: 10.1007/s00359-001-0275-5. [DOI] [PubMed] [Google Scholar]
- van der Steen J, Russell IS, James GO. Effects of unilateral frontal eye-field lesions on eye-head coordination in monkey. J Neurophysiol. 1986;55:696–714. doi: 10.1152/jn.1986.55.4.696. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Silakov VL, DePalma-Bowles S, Ayers AS. Effects of reversible inactivation of the primate mesencephalic reticular formation. I. Hypermetric goal-directed saccades. J Neurophysiol. 2000;83:2260–2284. doi: 10.1152/jn.2000.83.4.2260. [DOI] [PubMed] [Google Scholar]
- Walton MM, Gandhi NJ. The role of superior colliculus in the control of head movements: effects of reversible inactivation. Soc Neurosci Abstr. 2006;211:3. [Google Scholar]
- Walton MMG, Bechara BP, Gandhi NJ. The role of the primate superior colliculus in the control of head movements. J Neurophysiol. 2007;98:2022–2037. doi: 10.1152/jn.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]