Abstract
Objective
To retrospectively evaluate the imaging findings of triple-negative [TN] breast cancer (estrogen receptor [ER], progesterone receptor [PR], and human epidermal growth factor receptor 2 [HER2] negative). In addition, to compare the mammography findings and clinical characteristics of TN to non-triple-negative [NTN] cancers (ER+, PR+ and/or HER2+).
Method and Materials
IRB approval was obtained. TN (n= 207) mammogram and ultrasound findings were reviewed. Mammogram findings and various clinical characteristics were documented for TN and NTN (n=967) cancers.
Results
Mammography findings: mass without calcifications (TN= 121/207 (58%) vs. NTN= 435/967 (45%)), mass with calcifications (TN= 60/207 (30%) vs. NTN= 311/967 (32%)) or calcifications alone (TN= 15/207 (7%) vs. NTN= 112/967 (12%)), p=0.0001. TN margins on mammography were most commonly ill-defined (63/133 (47%)) or spiculated (27/133 (20%)) and mass shape was most commonly irregular (65/133 (49%)). TN ultrasound findings: hypoechoic or complex masses with an irregular shape (78/120 (65%)) and ill-defined (57/120 (48%)) or microlobulated (38/120 (32%)) margins. Significant clinical differences: TN cancer was found in younger women (51.2 years (17–95) vs. NTN 55.9 years (24–92), p <0.0001), commonly detected clinically (TN= 68% vs. NTN= 48%, p <0.0001), and a higher pathologic grade (Grade 3: TN =71%, NTN= 20%, p <.0001).
Conclusion
Triple negative cancer was most commonly an irregular non-calcified mass with ill-defined or spiculated margins on mammography and a hypoechoic or complex mass with an irregular shape and noncircumscribed margins on ultrasound. The majority of TN cancers were discovered on physical exam, found in younger women and a higher pathologic grade.
Introduction
Triple-negative breast cancer is a subtype of breast cancer which has a more aggressive clinical course than other forms of breast cancer, and is associated with aggressive histology and poor clinical outcomes [1]. TN cancers have a higher rate of distant metastatic disease with a shorter mean time to distant metastatic disease when compared to NTN cancers [1]. Patients with TN breast cancer also have a high early incidence of brain metastases [2]. TN tumors are unresponsive to the usual endocrine therapies, contributing to a poor survival rate [1]. Although African American women have a lower risk of developing breast cancer compared to Caucasian women in general, premenopausal African American women have a higher risk of developing TN cancer, which is a contributing factor to the poor prognosis of young African American women with breast cancer [3]. In addition, women with BRCA1 mutation are reported to develop breast cancer at early age with a high prevalence of TN cancers [3, 4].
There are numerous prior studies addressing the aggressive clinical characteristics of TN breast cancer, however only a few studies with relatively fewer patients report the imaging findings [5,6,7,]. In the prior studies documenting imaging characteristics of TN breast cancers, these cancers were most commonly masses without calcifications with circumscribed margins in up to 30% on mammogram [6]. On ultrasound, TN cancers were most commonly hypoechoic, irregular masses with circumscribed margins in up to 57% of cases [7].
Method and Materials
Institutional review board approval was obtained (IRB number: 080325). Using our cancer center registry database, we studied a cohort of women with invasive breast cancer treated at our institution (MRS Cancer Registry; Impac Medical Systems, Sunnyvale, CA). The study group consisted of 1322 women diagnosed with invasive breast cancer between 1997 and 2006. There were 236 women diagnosed with triple negative breast cancer (TN) (cancer with the following biological markers: estrogen receptor [ER] negative, progesterone receptor [PR] negative, and human epidermal growth factor receptor 2 [HER2] negative) and 1086 patients diagnosed with non-triple negative breast cancer (NTN) (having at least one of the biological markers positive). The date that the patients were last seen at our institution was retrieved from our clinical data base records using EMERSE (a medical record text searchtool). The length of follow-up (in months) was calculated. Patients with less than 12 months follow-up after diagnosis of TN breast cancer were removed from our study group (29 TN and 119 NTN). Therefore, a total of 207 women diagnosed with TN cancer and 967 patients with NTN cancer were the subjects of our investigation. Our study group consisted of Caucasian (TN =84%, NTN=87%), African-American (TN=8%, NTN=5%), Asian (TN=3%, NTN=3%) and Hispanic women (TN=2%, NTN=1%).
Data was extracted from our institutional cancer center database that contained imaging and clinical information. Imaging information had been entered into this database from the breast imaging reports, in which 1 of 12 MQSA-certified radiologists (11 of 12 of which are breast fellowship trained) at our institution had dictated the breast imaging findings on each of the patients. The data we extracted from this database for both TN and NTN cancers included: age at diagnosis, date of diagnosis, method of detection (pain/discomfort, nipple discharge, mass detected on mammogram or self/clinical breast exam), tumor grade at diagnosis (using Bloom-Richardson grading system), receptor status (ER/PgR/HER2) and date of prior mammogram. The mammogram findings for both TN and NTN cancers were also acquired from the database, classified as mass without calcifications, mass with calcifications, calcifications alone, or occult. Breast density and BIRADS assessment (categorized according to ACR BI-RADS [8]) were also acquired from the database entries for TN and NTN cancers. Regional lymph node status at the time of diagnosis for TN cancers was obtained from this database, as well as size for the TN tumors using both mammogram and ultrasound reports. The overall TN tumor size at diagnosis was determined on the basis of the size indicated in the mammography and ultrasound reports, averaged. Pathologic size was not used due to a large proportion of the patients receiving neo-adjuvant chemotherapy prior to mass resection. Mammographic and ultrasound images of TN cancers were additionally retrospectively reviewed by two MQSA certified, dedicated breast imagers with 16 years (SP) and 30 years (CP) of experience. Both mammogram and ultrasound images for TN cases were reviewed if available (a total of 172 cases were available for review, some of which contained only mammographic, only sonographic or both mammographic and sonographic images.) If a mass was identified by both reviewers on mammography (n=133), mass margin and shape were categorized using the BIRADS lexicon [8]. (In the other 39 cases available for review, the mammogram portion was either unavailable (28 cases), negative (3 cases) or calcifications only (8 cases)). If a mass was identified by both reviewers on ultrasound (n=120), the shape, margin, echo pattern, orientation in relation to the skin surface, mass boundary and posterior acoustic features were classified according to the ACR BI-RADS ultrasound descriptors [8]. (In the other 52 cases available for review, the ultrasound portion was either unavailable (49 cases) or negative (3 cases)). The images were reviewed independently by the 2 reviewers and individual data was recorded. The overall data for the TN imaging section is the average of these two independent data sets (i.e. for mass shape on ultrasound, reviewer 1 classified 75 masses as irregular and reviewer 2 classified 80 masses as irregular, and thus 77.5 masses were documented as irregular in the overall results displayed in the tables and throughout the remaining text (rounded to the nearest whole number for simplicity)). For TN mass margin on both mammogram and ultrasound, when one reader called the margins circumscribed and the other called non-circumscribed, the third reader (MR; an MQSA certified, dedicated breast imager with 20 years experience) determined the consensus result on margins for these cases (21 mammogram cases and 20 ultrasound cases). Given the interobserver variability inherent in interpreting these images according to the BIRADS lexicon [9], the other categories were kept as an average of the first 2 readers, and a consensus read was not sought; however, given that the results of our study concerning the imaging margins of TN cancer is different from prior studies, we felt a consensus read in this category would provide more validation for this point. Of the cases available for review, 3 were post core biopsy (with clip markers in place), and none were post surgical biopsy. All of the ultrasounds were performed by an MQSA certified, dedicated breast imager at our institution.
Statistical Analysis
The data was summarized using means and standard deviations for continuous data and counts and percentages for categorical data. Comparisons were made between the groups using a 2 sample t-test for the continuous data and chi-square test for the categorical data. Distribution assumptions were reviewed and found to be satisfied, and non-parametric tests gave the same results as the t-tests. The variables associated with type of cancer were evaluated using a logistic regression model and found to be independent predictors. A significance level of p<0.05 was used to determine significance. No corrections were made for multiple tests. All analyses were conducted using SAS® v 9.2.
Results
Mammographic findings of TN cancers are summarized in Tables 1 and 2. TN cancers were most commonly a mass without calcifications (121/207, 58%) and less commonly a mass with calcifications (60/207, 30%) or suspicious calcifications alone (15/207, 7%). Upon review of the mass margins on mammography for TN cancer, the majority had either ill-defined (63/133, 47%), spiculated (27/133, 20%) or obscured (25/133, 19%) margins, with the remaining categories far less common including circumscribed (11/133, 8%) and microlobulated (7/133, 5%) margins (see figures 1a, 2 and 3). Mass shape on mammography for TN cancer was most commonly irregular (65/133, 49%), with the remaining categories less common. Mammography was negative in 5 TN cases (2%) and not available in 6 TN cases (3%) of the original TN cases (see Table 1), and similarly, mammography was negative in 2% of TN cases available for review by both readers (3/144; 133 cases with masses, 8 with calcifications alone and 3 negative) (see Table 2). NTN cancers were more equally divided between masses without calcifications (435/967, 45%), masses with calcifications (311/967, 32%) and suspicious calcifications alone (109/967, 11%). Breast density and BIRADS category assignment were not significantly different in TN cancer in comparison to NTN cancer (Table 1). BIRADS category was most commonly Category IV (Suspicious) in both TN (122/207, 58%) and NTN (522/967, 54%) cancers, and less commonly Category V (Highly suggestive) (71/207 (34%) for TN and 291/967 (31%) for NTN cancers) (p= 0.25). For the TN cancers, the “other/NA” category on table 1 is broken down as follows: BIRADS Category 1 (5 cases), Category 3 (1 case), Category 6 (5 cases) and not available (3 cases). The one TN cancer that was a Category 3 on mammogram demonstrated a hypoechoic, irregular mass with noncircumscribed (microlobulated) margins on ultrasound, and therefore a biopsy was indicated. There were not any Category 2 TN lesions.
Table 1.
Mammography Findings: TN vs. NTN (n=207 for TN and 967 for NTN)
| TN | NTN | Chi Square | |
|---|---|---|---|
|
| |||
| Findings | 0.0001 | ||
| Mass without calcifications | 121 (58%) | 435 (45%) | |
| Mass with calcifications | 60 (30%) | 311 (32%) | |
| Suspicious calcifications alone | 15 (7%) | 109 (11%) | |
| Other:
|
11 (5%) | 112 (12%) | |
| Negative
|
5 | 90 | |
| N/A | 6 | 22 | |
|
| |||
| Breast Density:* | 0.72 | ||
| Fatty | 10 (5%) | 39 (5%) | |
| Scattered densities | 68 (34%) | 233 (30%) | |
| Heterogeneously dense | 106 (52%) | 439 (57%) | |
| Dense | 19 (9%) | 66 (8%) | |
|
| |||
| BIRADS Category: | 0.25 | ||
| Suspicious (IV) | 122 (58%) | 522 (54%) | |
| Highly suggestive (V) | 71 (34%) | 291 (31%) | |
| Other/NA: | 14 (8%) | 154 (16%) | |
| Negative (I) | 5 | 90 | |
| Benign (II) | 0 | 0 | |
| Probably Benign (III) | 1 | 15 | |
| Known Cancer (IV) | 5 | 19 | |
| Not available | 3 | 30 | |
Note: 4 TN and 190 NTN cases N/A
Table 2.
Triple Negative Breast Cancers Mammogram Characteristics (in cases with masses, n=133*)
| Mass Margin | |
|---|---|
| Noncircumscribed: | 122 (92%) |
| - Ill-defined | 63 (47%) |
| - Spiculated | 27 (21%) |
| - Obscured | 25 (19%) |
| - Microlobulated | 7 (5%) |
| Circumscribed | 11 (8%) |
| Mass Shape | |
|---|---|
| Irregular | 65 (49%) |
| Lobulated | 27 (20%) |
| Oval | 23 (17%) |
| Round | 18 (14%) |
Of the cases available for review, 3 were negative on mammography (2%) and 8 had calcifications only (6%)
Figure 1.
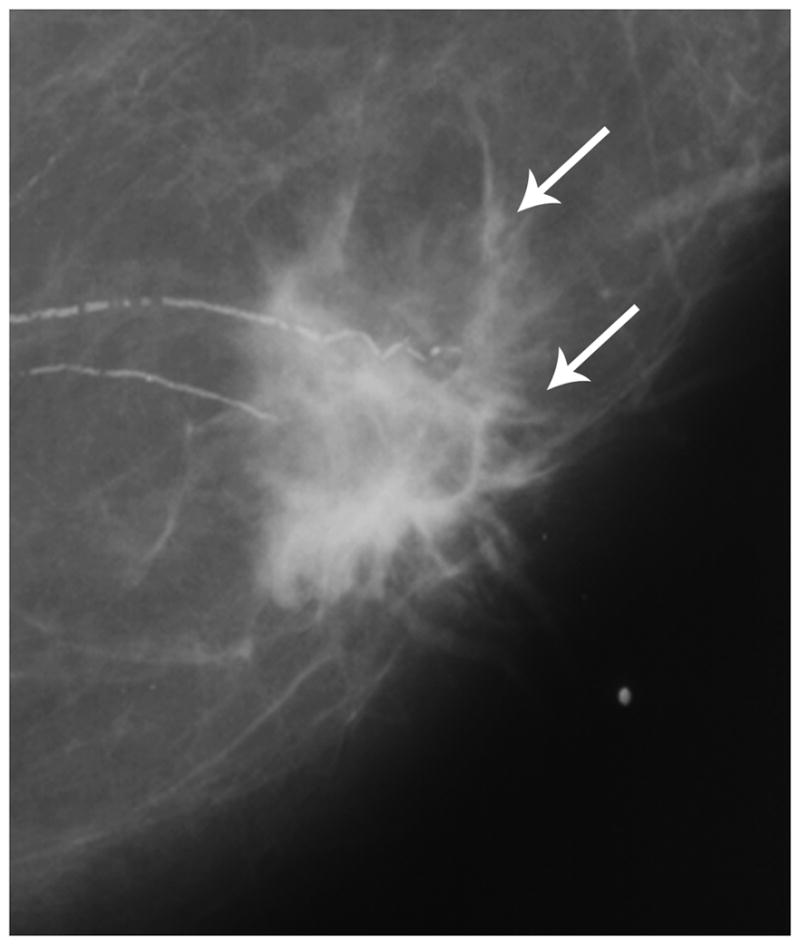
Figure 1a. Post-menopausal female presenting with a palpable mass, found to be TN breast cancer. Mammography demonstrates an irregular mass with spiculated margins without calcifications (arrows).
Figure 1b. (Same TN patient as in Fig. 1a.) Ultrasound demonsrates an irregular, hypoechoic mass with spiculated margins (arrows).
Figure 2.
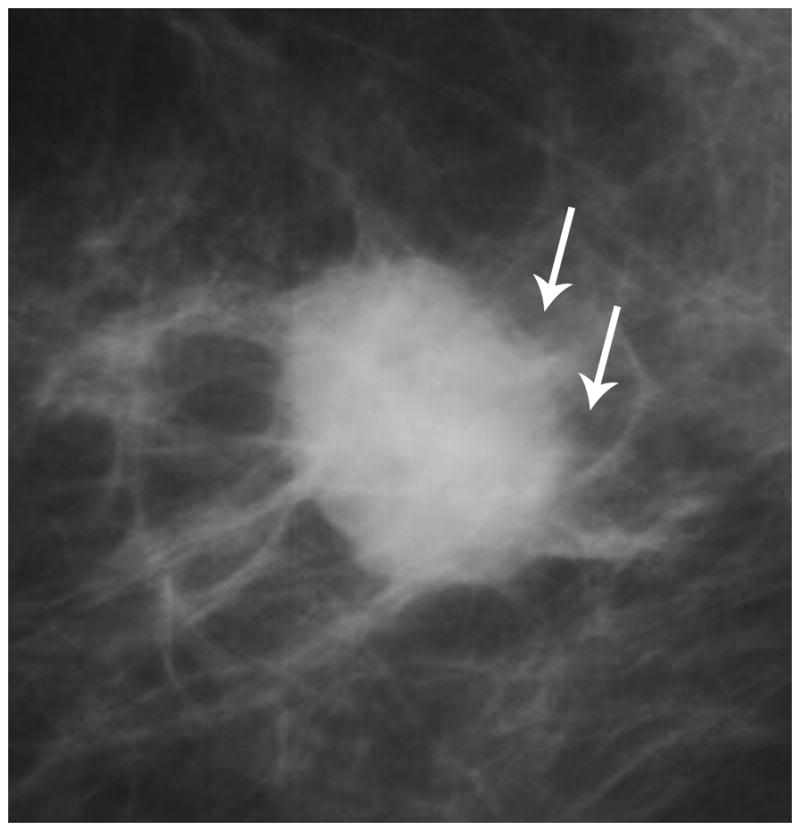
Mammography in a TN case demonstrates an irregular retroareolar mass with ill-defined margins (arrows).
Figure 3.
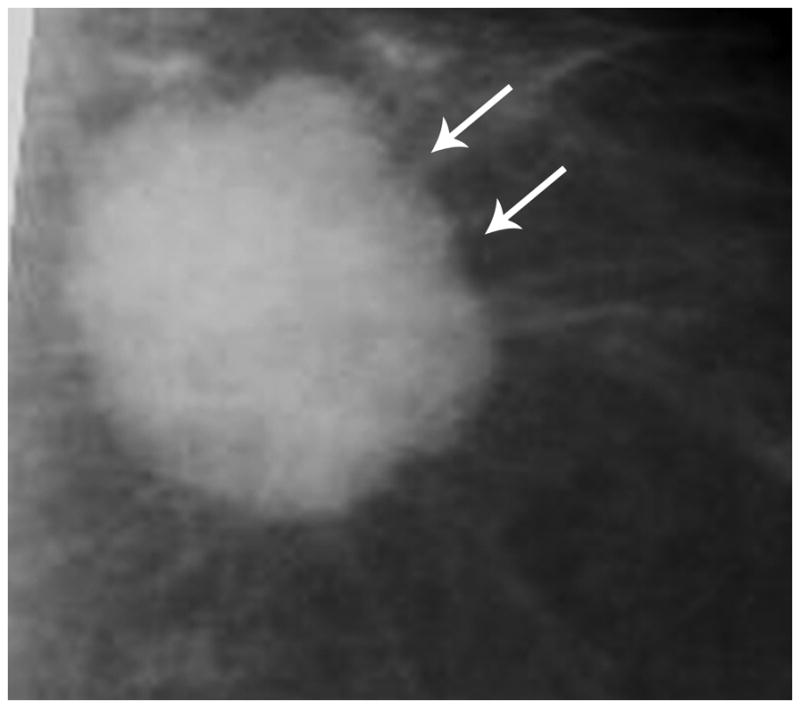
Mammography in a TN case demonstrates a round mass with microlobulated margins (arrows).
As summarized in Table 3, on ultrasound, the echo pattern of the majority of TN cancers was hypoechoic (92/120, 76%) or complex (mixed echogenicity containing both echogenic and hypoechoic areas) (22/120, 18%). The shape of TN cancers on ultrasound was most commonly irregular (78/120, 65%), and less commonly oval (27/120, 23%) or round (15/120, 13%). The margins of TN cancers on ultrasound were most commonly noncircumscribed (104/120, 87%) in comparison to circumscribed (16/120, 13%). The noncircumscribed masses were ill-defined in the majority of cases (57/120, 48%) with the remainder microlobulated (38/120, 32%) and spiculated (8/120, 7%) (see figures 1b, 4 and 5). The TN mass orientation on ultrasound was parallel to the skin surface in 58% (70/12) and non-parallel in 42% (50/120). The TN mass boundary most commonly demonstrated an abrupt surface (86/120, 71%), and less commonly an echogenic halo (34/120, 28%). The TN mass posterior acoustic features on ultrasound were none (51/120, 43%), followed by mixed (both echogenic and shadowing components) (28/120, 23%) and enhancement (28/120, 23%). Of the cases available for review by both readers, 3 TN cases were negative by ultrasound (3/123, 2%) (see Table 3). The 3 ultrasound negative TN cases were detected by mammography (all were masses with non-circumscribed margins ( 2 ill-defined and 1 spiculated) on mammography), and the 3 mammography negative TN cases available for review were detected by ultrasound (all were masses with non-circumscribed (all ill-defined) margins on ultrasound), with one exception that was negative on both modalities and was diagnosed by a surgical biopsy of a palpable finding (note that this case was not available for review by both reviewers)..
Table 3.
Triple Negative Breast Cancers Ultrasound Characteristics (in cases with masses, n=120*)
| Echo Pattern | |
| Anechoic | 2 (2%) |
| Hypoechoic | 92 (76%) |
| Isoechoic | 4 (3%) |
| Hyperechoic | 0 |
| Complex | 22 (18%) |
| Shape | |
| Irregular | 78 (65%) |
| Oval | 27 (23%) |
| Round | 15 (13%) |
| Margin | |
| Noncircumscribed: | 104 (87%) |
| - Ill-defined | 57 (48%) |
| - Microlobulated | 38 (32%) |
| - Spiculated | 8 (7%) |
| - Obscured | 1 (<1%) |
| Circumscribed | 16 (13%) |
| Mass Orientation | |
| Parallel | 70 (58%) |
| Non-parallel | 50 (42%) |
| Mass Boundary | |
| Abrupt Surface | 86 (71%) |
| Echogenic Halo | 34 (28%) |
| Posterior Acoustic Features | |
| None | 51 (43%) |
| Mixed | 28 (23%) |
| Enhancement | 28 (23%) |
| Shadowing | 13 (11%) |
Of the cases available for review, 3 were negative on ultrasound (3/123, 2%)
Figure 4.
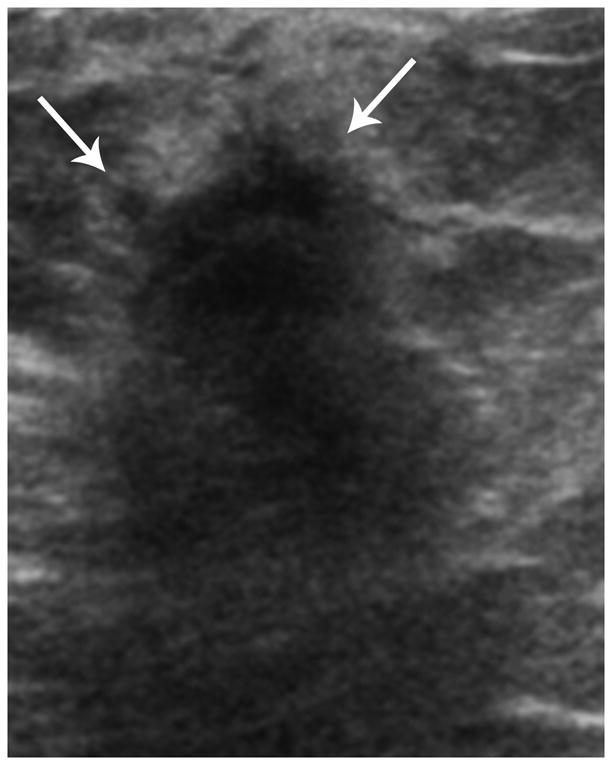
Ultrasound in a TN case demonstrates a round mass with microlobulated margins (arrows) and a complex echo pattern.
Figure 5.
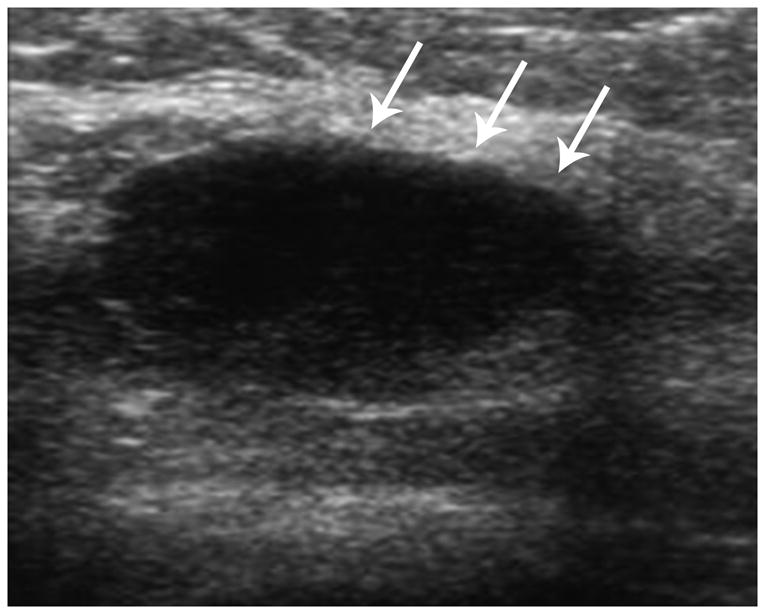
Ultrasound in a TN case demonstrates an irregular mass with ill-defined margins and a complex echo pattern.
There were significant clinical differences between TN and NTN cancers (summarized in Table 4). TN cancer in our series was significantly more likely to be detected clinically, either on self breast exam, clinician-performed breast exam, or by clinical symptoms such as breast pain or nipple discharge in comparison to NTN cancers (141/207 (68%) vs. 468/967 (48%) for NTN, p= <.0001). The remaining cancers were found on screening mammogram (66/207 (32%) for TN vs. 499/967 (52%) for NTN). In the TN cancers detected clinically, 35% (48 cases) had no prior mammogram. In addition, in both the clinically detected and imaging detected TN cancers with prior mammograms, many of the cancers were found within 12 months of a prior mammogram (70/207, 34%). TN breast cancer was more commonly found in younger women (mean age 51.1 years (range 17–95) vs. 55.9 years (range 24–92) for NTN, p= <0.0001). The TN tumors were a higher grade (138/195 (71%) were Grade 3 vs. 180/891 (20%) for NTN, p= <.0001). In TN cancers, the masses were categorized by size at diagnosis and regional lymph node status was documented. The mean size of TN tumors was 2.4 cm on mammography (range 0.5–9.5), 2.1 cm on ultrasound (range 0.6–9.0) and 2.3 cm mean size when mammogram and ultrasound values were averaged (range 0.6–9.0). With average tumor sizes from 0–0.9 cm, 1.0–1.9 cm and 2.0–2.9 cm, the majority of cases had negative regional nodes at diagnosis (20/24 (83%), 44/74 (59%) and 38/54 (70%) respectively); with average tumor sizes from 3.0–3.9 cm and 4.0–4.9 cm, the majority had positive regional lymph nodes at diagnosis (18/26 (69%) and 10/14 (71%) respectively). Masses >5.0 cm had positive nodes in 6/14 (43%).
Table 4.
Clinical Characteristics: TN vs. NTN (n=207 for TN and 967 for NTN)
| TN | NTN | Chi-square | ||
|---|---|---|---|---|
|
| ||||
| Age | Mean (SD): 51.2 (12.7) yrs. Range: 17–95 yrs. |
Mean (SD): 55.9 (12.9) yrs. Range: 24–92 yrs. |
< .0001 | |
|
| ||||
| Grade* | I | 6 (3%) | 220 (25%) | < .0001 |
| II | 51 (26%) | 490 (55%) | ||
| III | 138 (71%) | 180 (20%) | ||
|
| ||||
| Mode of Detection | Mammogram | 66 (32%) | 499 (52%) | <0.0001 |
| Clinical detection** | 141 (68%) | 468 (48%) | ||
Note: 12 TN and 76 NTN were unknown.
By self and clinician performed breast exam, breast pain or nipple discharge
Discussion
Our study addresses the imaging characteristics and clinical features of triple negative breast cancer. Features have been compared to breast cancers that are non-triple-negative [NTN] which have at least one of the 3 receptors positive. Although there are numerous prior studies reporting the clinical characteristics of TN breast cancer, only some studies, with fewer cases, report the imaging characteristics of these cancers. Triple-negative [TN] breast cancer is defined as cancer with the following biological markers: estrogen receptor [ER] negative, progesterone receptor [PR] negative, and human epidermal growth factor receptor 2 [HER2] negative. The mammographic findings among TN cancers were most commonly a mass without calcifications (58%) with ill-defined (47%) or spiculated (21%) margins and an irregular shape (49%) (see figures 1a, 2 and 3). Mammography was negative in 2% (5/207) and not available in 3% (6/207) of original TN cases (see Table 1), and similarly in the cases available for review by both readers, was negative in 2% (3/144) of cases (see Table 2). This false negative rate may be lower than for NTN breast cancers in part because of the nature of our TN patients, often presenting for a second opinion or specialty-level care with advanced disease, and such cases were often diagnostic rather than screening mammograms. Breast density and BIRADS category assignment were not significantly different in TN cancer compared to NTN cancer. Yang et al. [5] reported the mammographic findings of TN cancer in 2007 in 38 cases, finding that TN cancer was most likely a mass without calcifications (85%) and less likely a mass with calcifications (15%). The most common mass margins on mammography were indistinct (45%), less commonly circumscribed (24%) or spiculated (18%). In 2008, Wang et al. [6] compared the mammographic findings of ER-negative/HER2-negative cancers (96% of which were also PR-negative, and thus TN (n=32)) with those of ER-negative/HER2-positive cancer, and concluded that ER-negative/HER2-negative cancers were most often masses without calcifications (48%), and less likely occult (18%) or masses with calcifications (12%). The margins on mammogram were indistinct in 45%, circumscribed in 30% and spiculated in 15%. In 2009, Ko et al. [7] found that TN cancers (n=87) were most commonly masses without calcifications (49%) and less likely focal asymmetries (22%), masses with calcifications (21%) or calcifications alone (7%). In 2009, Dogan et al. [10] found that the majority of TN cancers (n=44) were masses without calcifications (54%), and 32% had circumscribed margins. Our study confirms findings from the prior studies that TN was most commonly an irregular mass without calcifications with ill-defined margins by mammography, although in comparison, few of our cases demonstrated circumscribed margins. We feel that the higher percentage of circumscribed margins in prior studies may be in part due to the strictness of a reviewer’s definition of circumscribed masses, as well as smaller sample sizes on prior studies.
In our study, ultrasound features among TN cancers were most commonly hypoechoic or complex masses with an irregular shape and noncircumscribed margins (see figures 1b, 4 and 5). Other documented features include a parallel mass orientation with an abrupt surface boundary and no posterior acoustic features. In 2008, Wang et al. [6] found that TN cancers (n=20) on ultrasound were likely to be hypoechoic (80%) masses with an irregular (54%) or lobulated shape (20%) and either indistinct (40%), microlobulated (33%) or circumscribed/smooth (27%) margins. Ultrasound was negative in 21% of their cases. In 2009, Ko et al. [7] found that TN cancers (n=87) on ultrasound were likely to be hypoechoic or markedly hypoechoic (89%) masses with an irregular (83%) or oval (16%) shape and either circumscribed (57%), angular (16%), indistinct (12%), microlobulated (9%) or spiculated (5%) margins. Ultrasound demonstrated a non-mass lesion in 14% of their cases. Dogan et al. [10] found that 21% of TN cancers (n=44) had circumscribed margins on ultrasound. In general, our study results agree with the findings of the two prior studies, except for the mass margin wherein we found that 89% of the masses had noncircumscribed margins, again felt to be in part due to smaller sample sizes previously, as well as possibly secondary to a dedicated breast imager performing all of our ultrasounds.
We found differences in the mode of detection between TN and NTN cancer. The majority of TN cancers were detected clinically either by self breast exam, physician-performed breast exam, or clinical symptoms such as breast pain or nipple discharge. In addition, of the TN cancers detected clinically, more than one-third had no prior mammogram; and in both the clinically detected and imaging detected TN cancers with prior mammograms, one-third of the cancers were found within 12 months of a prior mammogram. We feel that this may be due to the rapid growth rate and aggressive nature of triple negative cancers. These results support prior studies; for example, Dent et al.[1] found that TN cancer had a much lower proportion of breast cancer detected by mammography or ultrasound than NTN cancer, and were more likely to be detected clinically. In their study, 71% of TN cancers (n=92) were detected clinically (vs. 53% for NTN) and only 19% were detected first by mammography (vs. 36% for NTN). Collett et al. [11] evaluated cancers diagnosed in a screening program and found that TN cancer was more likely to present in the interval between regular mammograms than NTN cancer. They postulated that this difference may be due to differences in breast density. However, our study showed no difference in breast density between TN and NTN cancers.
TN cancer has other clinical features that were significantly different from NTN cancer in our study. TN cancer was more commonly found in younger patients and associated with a higher pathologic grade. This suggests that TN cancer is a biologically more aggressive disease with a poorer prognosis. Similarly, Dent et al. [1] also found that TN cancer (n=180) when compared to NTN cancer, was found in younger women (53 vs. 57.7 yrs. for NTN) with a higher pathologic grade (66% Grade 3 vs. 28% for NTN) at diagnosis. Carey et al. [3] also found that TN cancers are associated with a higher pathologic grade in comparison to NTN cancers (84% Grade 3 vs. 46% for NTN cancers). In a large California Cancer Registry study in 2007, Bauer et al. [12] found that TN cancers (n=6,370) were significantly more likely to be found in women under 40 years of age (12% vs. 6% for NTN) and have a significantly higher grade (76% were poorly differentiated or undifferentiated vs. 28% for NTN). Dent et al. [1] found that in NTN cancers, the rate of regional lymph node positivity increases as tumor size increases, however this traditional relationship was less consistently evident among the TN group in their study (positive lymph nodes in tumors <2 cm= 58% from 2–5 cm = 49%). Foulkes et al. [13] noted that the phenomenon of no proportional relationship of tumor size to lymph node positivity was also observed in BRCA-associated cancers. In our study, when TN masses were categorized by average imaging size at diagnosis, a relationship between increasing mass size and regional lymph node positivity was also not evident (this may be due in part by the lower number of TN cases in the larger tumor subgroups). Even with very small tumors under 2 cm, there was a relatively high percentage of regional lymph node positivity. This disproportionate relationship may be in part due to the aggressive nature and higher pathologic grade of the TN tumors.
Limitations of our study include a referral bias, inasmuch as our data was obtained from a large cancer center. Patients who were more complex or with more advanced disease than the average breast cancer patient may have presented to us for a second opinion or specialty-level care. Another potential weakness was unavoidable selection bias in this retrospective study. For our study, only patients that had available ER, PR and HER2 biological marker data were included. In addition, there were data pieces that were missing or unavailable for review such as images and histopathological specimen from outside institutions. Like other data base studies, our study depended on accuracy of the data entries.
In conclusion, using standard BIRADS assessment, TN cancer was most commonly an irregular mass without calcifications with ill-defined or spiculated margins on mammogram and a hypoechoic or complex mass with an irregular shape and noncircumscribed margins on ultrasound. The majority of TN cancer was detected clinically, among younger women, and often without a prior mammogram. In addition, TN cancer was more frequently associated with a higher pathologic grade and higher rate of regional lymph node positivity at diagnosis even with very small tumor sizes, reflecting its aggressive nature.
Acknowledgments
Statistics supported by NIH UL1RR024986
References
- 1.Dent R, Trudeau M, Pritchard KI, et al. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clinical Cancer Research. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Dawood S, Broglio K, Esteva FJ, et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Annals of Oncology. 2009;20:621–627. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, et al. Race, Breast Cancer Subtypes, and Survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.Kandel MJ, Stadler Z, Masciari S, et al. Prevalence of BRCA1 mutations in triple negative breast cancer (BC) J Clin Oncol (Meeting Abstracts) 2006;24:508. [Google Scholar]
- 5.Yang W-T, Dryden M, Broglio K, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Research & Treatment. 2008;111:405–410. doi: 10.1007/s10549-007-9810-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Ikeda DM, Narasimhan B, et al. Estrogen Receptor-Negative Invasive Breast Cancer: Imaging Features of Tumors with and without Human Epidermal Growth Factor Receptor Type 2 Overexpression 1. Radiology. 2008;246:367–375. doi: 10.1148/radiol.2462070169. [DOI] [PubMed] [Google Scholar]
- 7.Ko E, Lee B, Kim H-A, Noh W-C, Kim M, Lee S-A. Triple-negative breast cancer: correlation between imaging and pathological findings. European Radiology. 2010;20:1111–1117. doi: 10.1007/s00330-009-1656-3. [DOI] [PubMed] [Google Scholar]
- 8.American College of Radiology. Breast Imaging Reporting and Data System Atlas (BI-RADS Atlas. Reston, Virginia: 2003. [Google Scholar]
- 9.Lazarus E, Mainiero M, Schepps B, et al. BI-RADS Lexicon for US and Mammography: Interobserver Variability and Positive Predictive Value. Radiology. 2006;239:385–391. doi: 10.1148/radiol.2392042127. [DOI] [PubMed] [Google Scholar]
- 10.Dogan BE, Gonzalez-Angulo AM, Gilcrease M, et al. Multimodality Imaging of Triple Receptor-Negative Tumors with Mammography, Ultrasound and MRI. AJR. 2010;194:1160–1166. doi: 10.2214/AJR.09.2355. [DOI] [PubMed] [Google Scholar]
- 11.Collett K, Stefansson IM, Eide J, et al. A Basal Epithelial Phenotype Is More Frequent in Interval Breast Cancers Compared with Screen Detected Tumors. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1108–1112. doi: 10.1158/1055-9965.EPI-04-0394. [DOI] [PubMed] [Google Scholar]
- 12.Bauer K, Brown M, Cress R, Parise C, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 13.Foulkes W, Metcalfe K, Hanna W, et al. Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer. 2003;98:1569–1577. doi: 10.1002/cncr.11688. [DOI] [PubMed] [Google Scholar]


