Abstract
Astrocytes are electrically non-excitable cells that, on a slow time scale of seconds, integrate synaptic transmission by dynamic increases in cytosolic Ca2+. A number of groups have recently shown that astrocytic Ca2+ signaling regulates vascular tones and that astrocytes play a central role in functional hyperemia by Ca2+-dependent release of Prostaglandin E2 (PGE2). Astrocytes are, however, not simple detectors of excitatory transmission, since a number of neuromodulator and hormones trigger elevations in astrocytic Ca2+ independently of synaptic transmission. Furthermore, astrocytes exhibit ex vivo intrinsic Ca2+ excitability, or spontaneous increases in Ca2+ that are not triggered by receptor activation. The notion that astrocytes can regulate vascular tone independently of synaptic transmission challenges the notion that changes in the blood oxygenation level dependent (BOLD) signal is directly proportional to neuronal activity and may thus require a reevaluation of the large body of data accumulated using functional magnetic resonance imaging (fMRI).
Keywords: Photolysis, 2-photon imaging, functional brain imaging
Until about two decades ago, astrocytes were regarded as non-excitable supporting cells in the brain, lacking membrane properties required to generate action potentials. The view of astrocytes as simple supporting cells was questioned in the early 1990s, with the demonstration that glutamate can trigger Ca2+ oscillations among cultured glial cells often resulting in propagating intercellular Ca2+ waves (1). The notion that astrocytes can actively propagate Ca2+ waves among each other led to the studies of Ca2+ signaling between astrocytes and neurons in mixed cultures. These studies revealed that astrocytic Ca2+ waves modulate neuronal cytosolic Ca2+, large spike-like increases in neuronal Ca2+ levels, suggesting that astrocytes participate more directly in neurotransmission than previously recognized (2, 3). Bidirectional communication between neurons and astrocytes was later demonstrated in acute brain slices (4–7) and the isolated retina preparation (8). Although bidirectional communication has been studied for more than a decade, it was first recently demonstrated that astrocytes are activated during sensory stimulation in vivo (9). This was an important step, because activation of astrocytes in previous ex vivo experiments often required excessive stimulation, including high intensity electrical stimulation (10), mechanical distortion (11), traumatic injury (12), agonist application (13) and laser light (14). Understanding the role of astrocytic Ca2+ signaling in the intact brain has direct implication for functional brain imaging, since new lines of evidence suggest that astrocytes play a key role in functional hyperemia (reviewed in (15)). The basis of blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI), and positron emission tomography (PET) functional imaging, as well as intrinsic signal optical imaging, is that neural activity is coupled to increases in local blood flow (16). However, the mechanisms linking neuronal activity to increases in local perfusion remain poorly understood. Since the first description by Golgi at the end of 1800s that astrocytic endfeet contact arterioles and capillaries in the brain, it has been speculated that astrocytes have the potential to modulate the cerebral blood flow. Indeed, several in vitro studies highlighted the importance of neuron-to-glia signaling in vessel diameter (10, 17–20). Although different mechanisms have been proposed for the function of astrocytes in control of microcirculation, a common conclusion is that elevations in astrocytic cytosolic Ca2+ levels are necessary and sufficient to cause changes in the diameter of arterioles. Yet, an essential question to be addressed is whether these astrocytic Ca2+ transients occur in vivo during physiological neural activation.
We have, in recent work, addressed the signaling pathway from neurons to astrocytes in live adult mice using 2-photon laser scanning microscopy. We analyzed changes in neuronal local field potentials (LFPs) and astrocytic Ca2+ signaling evoked by whisker stimulation in barrel cortex layer 2 of adult mice. We found that whisker stimulation promptly evoked astrocytic Ca2+ responses in vivo. The slow onset (~3 s) and long time course (~20 s) of the astrocytic Ca2+ signaling compared with the properties of LFPs suggested that temporal summation of electrical events with a long time constant determined the development of the astrocytic Ca2+ elevation. We found that the changes of astrocytic Ca2+ signaling were a direct function of the summated LFPs amplitude, which was commonly used to predict the amplitude of increases in cerebral blood flow changes during sensory stimulation (21, 22). Our recent observation on astrocytic Ca2+ signaling will here be discussed in the context of functional brain imaging.
2. Astrocytes Can Sense and Modulate Synaptic Activity
2.1. Astrocytes Increase Ca2+ in Response to Receptors Activation
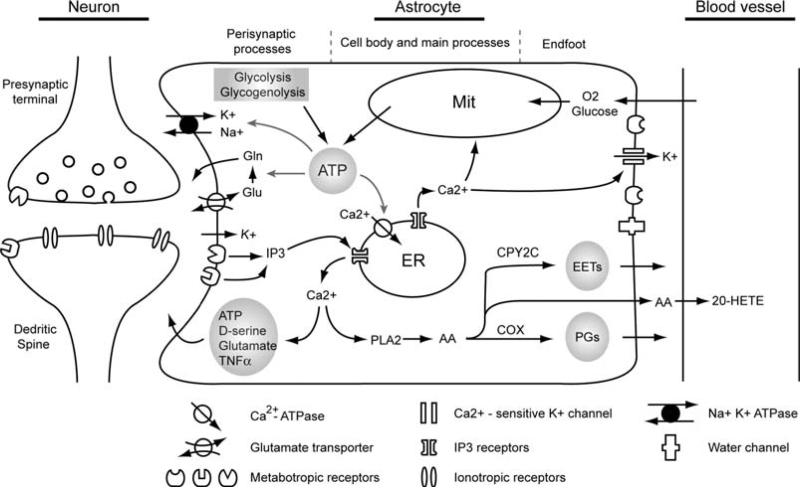
Astrocytes make contact with most synapses in the CNS and respond to synaptic activity with increases in cytosolic Ca2+ ex vivo(4, 23). Although astrocytes are electrically non-excitable, they express receptors for most neurotransmitters (24), including glutamate (1), GABA (4), norepinephrine (25) and acetyl-choline (26). Retinal Müller cells and astrocytes also respond to synaptic release of ATP by activation of purinergic receptors (14). The types of neurotransmitter receptors found on astrocytes in situ and in vivo are mainly metabotropic receptors, which link to the second messenger system including, activation of phospholipase C, adenyl cyclase and production of IP3, Ca2+ and cAMP (27,28) (Fig. 5.1).
Fig. 5.1.
Proposed mechanisms that link synaptic activity, cerebral vasculature regulation and astrocytic energy metabolism with astrocytic Ca2+signaling. Synaptic released neurotransmitters activate metabotropic receptors in astrocytic perisynaptic processes, causing elevation of intracellular Ca2+ through a phospholipase C-dependent pathway. Increases in astrocytic Ca2+ are associated with release of gliotransmitters which feed back to the synaptic activity. Astrocytic Ca2+ signaling may also propagate to the astrocytic endfoot, releasing vasoactive agents through phospholipase A2(PLA2)-dependent pathway as well as activation of Ca2+-sensitive K+ channels. Furthermore, Ca2+ is a well established stimulator of oxidative-phosphorylation which accelerates ATP production from mitochondria (Mit). Due to the small diameter of perisynaptic processes and the absence of mitochondria in these structures, ATP can also be generated through glycolysis and glycogenolysis pathways particularly in astrocyte perisynaptic processes. The major energy consuming processes in astrocyte include converting glutamate (Glu) to glutamine (Gln) after taken up by glutamate transporters, uptake of K+ from extracellular space through Na+-K+ ATPase and restoring astrocytic cytosolic Ca2+ concentration by activating both plasma membrane and endoplasmic reticulum (ER) Ca2+ -ATPase.
Several lines of evidence indicate that metabotropic glutamate receptors (mGluR) play a key role in the mobilization of intracellular Ca2+ stores in response to synaptic release of glutamate (5,6). In culture, astrocytes respond to glutamate with oscillatory increases in Ca2+ and intercellular Ca2+ waves propagation (1). Glutamate released during neuronal activity may reach astrocytic receptors through synaptic “spillover” (29, 30) or ectopic release (31). In addition, neurotransmitters may directly activate astrocytes through volume transmission (32).
2.2. Modulatory Role of Gliotransmitters Released by Astrocytes
Although we know that astrocytes sense the neurotransmitters in extracellular space, we know very little about the consequences of astrocytic receptors activation. In vitro preparations have shown that astrocytic Ca2+ signaling is associated with release of gliotransmitters. Several of the best studied gliotransmitters include glutamate, ATP, D-serine, TNF-alpha, and arachidonic acid metabolites (3,10,33–35) (Fig. 5.1). Thus, by sensing the extracellular neurotransmitter concentration and increasing intracellular Ca2+, astrocytes may actively participate in the information processing in the normal brain.
3. Interface Between Astrocytic Endfeet and the Vasculatures
Astrocytes send processes to contact not only synapses but also the vasculature forming specialized endfoot structures in the astrocyte-vasculature interface (AVI). A variety of channels and receptors required for interaction between astrocytes and vasculatures are clustered in the astrocytic endfoot processes. For instance, purinergic receptors P2Y2 and P2Y4 as well as gap junctions composed of Cx43 have been shown to be preferentially expressed in the astrocytic endfeet (36). Accordingly, direct application of ATP, electrical stimulation and uncaging evoked astrocytic Ca2+ waves can propagate along the AVI (17,36). Increases of astrocytic Ca2+ in the endfeet can activate Ca2+ – sensitive K+ channels, which is also abundantly expressed in the endfeet (37), and subsequently dilate intracerebral arterioles by increasing extracellular K+ concentration in the AVI (19,38) (Fig. 5.1).
4. In Vivo Imaging of Astrocytic Ca2+ Signaling by 2-Photon Laser Scanning Microscopy (2PLSM)
Due to the technical challenges, imaging astrocytic Ca2+ signaling in vivo was only possible after the introduction of 2PLSM (39) and the in vivo Ca2+ indicator loading techniques (40,41).
The invention of 2PLSM by Webb's group in 1990 (39) has tremendously improved our understanding of neurobiological phenomena in both normal and diseased brain. With two-photon excitation (2PE), it is possible to image deep brain tissue in the live animals with single cell spatial resolution (42). We have used 2PLSM because 2PE provides several key advantages compared with single photon excitation (1PE). First, because of the localized excitation in 2PE, all emission fluorescent photons contribute to the useful signals. In contrast, a pinhole has to be used to block the emission light from the unfocused plan with 1PE. Therefore, 2PE has much higher efficiency than 1PE. Second, since only the fluophores in the focused point are activated by 2PE, whereas the entire light pathway is activated during 1PE, phototoxicity and photobleaching are greatly reduced in 2PE. This is especially important for time-lapse imaging of live tissue. Moreover, the excitation wavelength used in 2PE (740~900 nm) penetrates tissue better than 1PE (350~550 nm), which is essential for in vivo imaging of live animals.
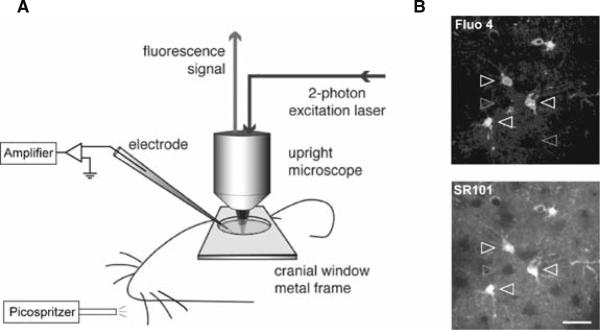
Although blind intracellular recordings (43) have been widely used for loading Ca2+ indicators into neuronal structures under 2PLSM, delivering Ca2+ indicators into single astrocyte through recording pipette in live animals is still challenging. However, recent studies demonstrated that astrocyte population can be labeled by either local dye injection (40) or pial surface dye application (41). Together with the discovery of specific astrocyte fluorescent indicator sulforhodamine 101 (44), these pioneer studies enable, for the first time, the observation of astrocytic Ca2+ activity in response to physiological stimulation in the live animals (9) (Fig. 5.2).
Fig. 5.2.
Diagrams of experimental setup. (A) A cranial window was opened on the primary somatosensory cortex. A metal frame was glued to the skull to prevent the movement of the brain. Whisker stimulation was performed in the contra lateral side of the animal's snout by air puffs which were generated from a high pressure nitrogen tank and control by picospritzer. A plastic tube connected to the picospritzer was placed ~2 cm away from the C6 whisker in a rostral-caudal direction to deliver the high pressure air. Electrical field potential recording and 2-photon Ca2+ imaging were performed simultaneously during whisker stimulation. Agarose (1%) was poured onto the pial surface before the coverglass was mounted to the metal frame to minimize the brain pulsation. (B) Dual labeling of barrel cortex with Ca2+ indicator fluo-4 AM (top) and astrocyte specific marker SR101 (bottom). Only SR101 positive astrocytes were labeled with fluo-4 AM (white arrowheads). Neurons appear as dark, round areas without either fluo-4 AM or SR101 labeling (gray arrowheads). The image shown was located 120 μm below the pial surface. Scale bar, 30 μm.
We found that astrocytic Ca2+ signals can be clearly detected from pial surface to ~300 μm deep in adult mouse primary somatosensory cortex (barrel cortex) following surface loading with fluorescent Ca2+ indicators. This penetration depth correlates with the layer1 and part of the layer 2/3 of mouse barrel cortex.
4.1. Animal Preparation
Adult FVB or C57BL/6 mice (~8 weeks old) were used for in vivo imaging primarily because application of fluorescent Ca2+ indicators on mouse pial surface produced satisfactory astrocyte loading in vivo. Same surface loading procedure only works on immature rats up to ~3 weeks old (unpublished observations). Animals were anesthetized with intraperitoneal injection of ketamine (0.12 mg/g) and xylazine (0.01 mg/g). Depth of anesthesia was monitored by field potential recording and hind limb pinch withdrawal reflexes and kept constant at stage III/3 (45) with supplemental doses of anesthetics. Tracheotomy was performed and the mice were intubated and artificially ventilated with a small animal ventilator (SAAR-830, CWE). The respiration rate was set at ~100/min; inspiration time, 0.3 s; and the tidal volume, ~0.2 ml. Lung pressure was carefully monitored and maintained at lower than 10 cm H2O to prevent lung injury. Blood gas was tested by taking arterial blood through a femoral artery catheter and pCO2, pO2 and pH were analyzed in microsamples (Rapidlab 248, Bayer, sample size 40 μl). Blood samples were taken every 1–2 h and totally 3–4 times from each animal. Experiments were completed only if physiological variables remained within the normal limits. The normal limits for pCO2 were set at 30–45 mm Hg; pO2, 80–115 mm Hg; and pH, 7.35–7.45. To image astrocytic Ca2+ signals in mouse barrel cortex, a cranial window (2–3 mm in diameter) was prepared centered at 0.5 mm posterior to the bregma and 3.5 mm lateral from midline. To stabilize the brain during imaging, a custom-made metal plate was glued to the skull with dental acrylic cement. Dura matter was removed for surface loading of fluorescent indicators. Body temperature was monitored by a rectal probe and maintained at 37°C by a heating blanket (BS4, Harvard Apparatus).
4.2. Ca2+ Indicators Loading
The major difference comparing surface bulk loading and local dye injection is that the former loading procedure only labels astrocyte structures due to the uptake by astrocyte limiting membranes that completely cover the pial surface (41), whereas the latter protocol loads both astrocytes and neurons (40,46). For the surface bulk loading, Fluo-4 AM (0.5–1 mM) was dissolved in dimethylsulphoxide (DMSO) with 20% pluronic acid and mixed in artificial cerebrospinal fluid (aCSF) containing 126 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgCl2, 2 mM CaCl2, 10 mM glucose and 26 mM NaHCO3 (pH 7.4), gassed with 95% O2 and 5% CO2 at 37°C (47). aCSF containing the dye (10–15 μl) was dropped on the pial surface, then the cranial window was covered by a small piece of parafilm to prevent the dye from drying. After a 45-min incubation, the exposed brain was washed for 15 min with aCSF without dye. In selected experiments, the exposed cortex was also loaded with the astrocyte-specific fluorescent indicator sulforhdamine 101 (SR101, 10 μM) (44) for 10 min after fluo-4 AM loading (Fig. 5.2). To minimize brain pulsation, 0.9% NaCl containing 1% agarose (37°C) was poured on the cranial window and the glass coverslip was glued to the metal plate with dental acrylic cement. A small opening between coverglass and metal plate was left for inserting recording electrode into the cortex (Fig. 5.2).
Serial images from pial surface to barrel cortex layer2/3 (~300 μm deep) revealed that fluo-4 AM signals strictly co-localized with SR101 staining (Fig. 5.2). Astrocytes appear as bright fluorescent cells with multiple processes. Astrocyte end-feet are clearly labeled which outline the vasculatures. In contrast, neuronal cell bodies appear as dark, round shaped areas which can be detected at ~90 μm deep in the barrel cortex, owing to their lack of uptake of both fluo-4 AM and SR101(Fig. 5.2).
4.3. Two-Photon Imaging
A custom-built microscope attached to Tsunami/Millinium laser (10W, SpectraPhysics) and a scanning box (FV300, Olympus) using Fluoview software and a 20X (0.9 NA, Olympus) objective was used for in vivo imaging. Excitation wavelength was in the range of 820~840 nm for both fluo-4 AM and SR101 imaging simultaneously. Two-channel detection of emission wavelength was achieved by using a 565 nm dichroic mirror (Chroma) and two external photomultiplier tubes. A 525/40 bandpass filter (Chroma) was used to detect fluo-4 AM emission light, and a 620/60 bandpass filter (Chroma) was used to detect SR101 signals (Fig. 5.2). For image collection, ~1 s was needed to record a single frame of image at 512×512 pixels resolution. Time-lapse images of astrocytic Ca2+ signaling were collected every 1~3 s. Normally, a sampling interval of 3 s was found sufficient to detect whisker stimulation evoked astrocytic Ca2+ elevation, and this low sampling rate was used to avoid the photodamage. The two-photon laser power was carefully adjusted according to the depth of imaging in the brain, since the photodamage induced by high power laser can cause astrocytic Ca2+ oscillation in vivo (9). The average laser power that was used for detecting astrocytic Ca2+ signaling in barrel cortex layer 2 was less than 30 mV under the objective.
4.4. Whisker Stimulation
Whiskers were stimulated by air puffs (48). Briefly, whiskers on one side of the snout were trimmed to a 10-mm length. Air puffs consisted of pulses of compressed air, which were generated from a high pressure nitrogen tank and controlled by picospritzer (General Valve) at a pressure of 10 psi and delivered by a thick wall plastic tubing (4.5 mm outer-diameter, 2.5 mm inner-diameter). The tube was placed parallel to the left side of the mouse snout and 10–20 mm away from the C6 whisker. The direction of air flow was from rostral to caudal along the whisker row. This protocol will stimulate most of the vibrissae on one side of the whisker pad (at least three rows of whiskers were consistently activated by air puffs) (Fig. 5.2). The frequency of air puffs was set to 1, 3, 5, 7 or 10 Hz and controlled by Master-8 (A.M.P.I). Pulse width in all experiments was 10 ms. The duration of stimulation was adjusted from 1 to 60 s in different experiments. Single whisker stimulation was not attempted at this point, but in future studies, it will be intriguing to deflect a principle whisker using mechanic stimulation (49) and image the astrocyte Ca2+ responses in its related barrel column and the nearby columns.
4.5. Electrophysiological Recording
LFP recordings were obtained from layer 2 of barrel cortex (100–150 μm below the pial surface) by a patch pipette (TW100F-4, WPI; outer diameter, 1.0 mm; inner diameter, 0.75 mm; tip diameter, ~3 μm; tip resistance 3–5 MΩ), containing 0.2% Texas red–dextran (77,000 molecular weight, Sigma) in aCSF. LFP signals were amplified using Axopatch 700B and pCLAMP 8.2 program with DigiData 1332A interface (Axon Instruments). The signals were bandpass filtered at (1–100 Hz) and digitized at 10 kHz.
5. Properties of Astrocytic Ca2+ Signaling Evoked by Whisker Stimulation
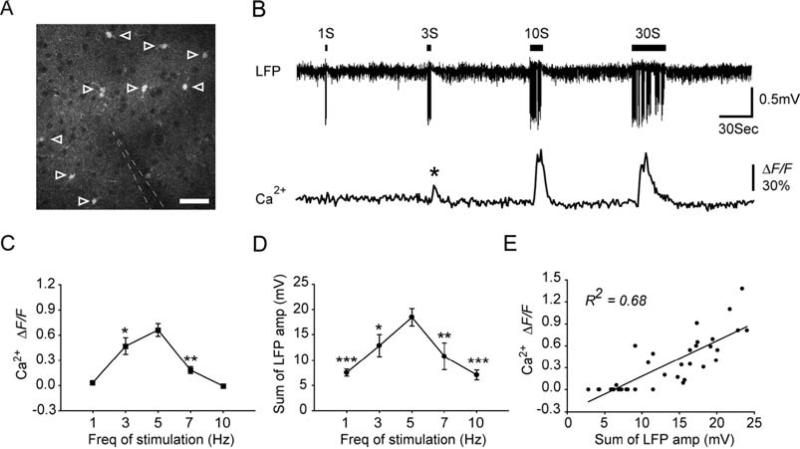
Astrocytes in barrel cortex layer 2/3 of adult mouse were labeled with Ca2+ indicator fluo4-AM. Recording electrode was inserted into the field of imaging to detect LFP activity (Fig. 5.3A). Although increased neuronal activity in layer 2/3 of barrel cortex can be detected as early as 10–12 ms after whisker deflection (50), increases in astrocyte somatic Ca2+ were delayed by ~3 s after the onset of whisker stimulation (9). The onset of the increase in astrocytic Ca2+ was defined as the earliest time point at which the changes of somatic fluorescent signal reached 3 standard deviation of baseline fluorescent activity. Notably, 1-s stimulation failed to evoke astrocytic Ca2+ signaling, whereas 3-s stimulation was sufficient to induce astrocytic Ca2+ elevation (asterisk) (Fig. 5.3B).
Fig. 5.3.
Whisker stimulation evokes astrocytic Ca2+ increases in mouse barrel cortex. (A) Two-photon fluorescence image taken from layer 2/3 of mouse barrel cortex (~150 μm below the pial surface), showing that astrocytes were labeled with Ca2+ indicator fluo-4 AM (open arrowheads). Neuronal cell bodies appear as dark, round area, due to their lack of uptake of fluo-4 AM. The location of recording electrode is indicated by white dashed line. Scale bar, 30 μm. (B) 5 Hz whisker stimulation induced astrocytic Ca2+ increases as a function of the stimulation duration. Upper trace, LFP recorded from recording electrode shown in panel A. Lower trace, averaged relative fluorescent change ΔF/F in the astrocytic cell bodies indicated in panel A. (C) Mean increase in fluo-4 emission after 9 s of different frequencies of stimulation. (D) Summed LFP amplitude within the first 9 s of whisker stimulation as a function of the frequency of stimulation. Data are presented as mean ± s.e.m. * P<0.05, ** P<0.01 and *** P< 0.001 compared with 5 Hz whisker stimulation induced responses. One-way analysis of variance with Dunnett's test. (E) Correlation between the summed LFP amplitude within the first 9 s and the mean increase in fluo-4 emission 9 s after the onset of stimulation (R2=0.68, P<0.001).
In addition, the amplitude of astrocytic Ca2+ increases was similar in response to 10-s and 30-s stimulation, while the duration of astrocytic Ca2+ elevation was longer during 30-s stimulation. This evidence indicates that the amplitude of astrocytic Ca2+ elevation was mainly determined by the neuronal activity during the first ~10 s of stimulation (Fig. 5.3B). Most astrocytes showed a single increase in Ca2+ in response to 1 min of whisker stimulation (72 cells from 13 mice), whereas others showed a second Ca2+ increase (11 cells from 13 mice).
In addition to the effects of the duration of stimulation on astrocytic Ca2+ elevations, astrocytic Ca2+ responses are also a function of the frequency of stimulation. During 1 Hz whisker stimulation, only 3 of 37 astrocytes tested in 9 mice showed an increase in Ca2+ (9). Rapid sensory adaptation, or a decrease in LFP amplitude within 1 s, occurred at 10 Hz stimulation. Accordingly, 10 Hz whisker stimulation did not trigger astrocytic Ca2+ responses (9). Additional experiments using 3 and 7 Hz whisker stimulation confirmed that astrocytic Ca2+ responses peaked at 5 Hz and decreased at both lower and higher frequencies (Fig. 5.3C). Since astrocytic Ca2+ signaling was delayed by ~3 s compared with neuronal field potential signals, there must be an accumulating effect of neuronal activity on the astrocytic Ca2+ elevations. To test the idea that astrocytic Ca2+ increases are a function of the intensity of local synaptic input, we calculated the summed LFP amplitude during the first 9 s of stimulation. As an index of total neuronal activity within a given time window, the sum of LFPs has also been widely used to positively correlate neuronal activity with cerebral blood flow (CBF) (21). Notably, the summed LFP amplitudes peaked at 5 Hz during the first 9 s of stimulation (Fig. 5.3D) and had a strong correlation with astrocyte somatic Ca2+ signaling (R2 = 0.68, P < 0.001; Fig. 5.3E)
6. Implications for BOLD Signaling
6.1. Astrocytic Ca2+ Elevations Contribute to Cerebral Blood Flow Regulation
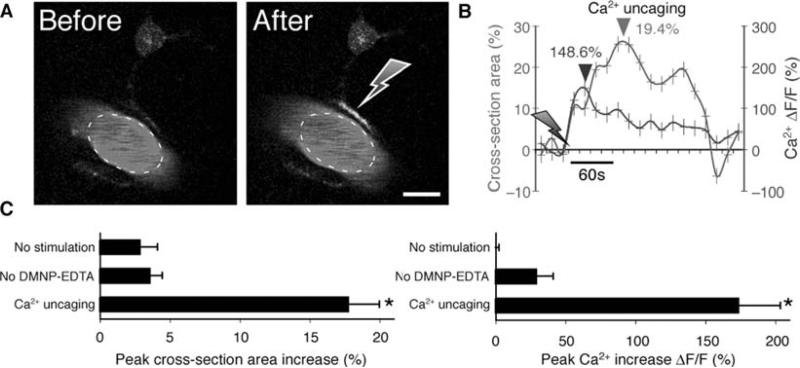
The basis for a positive BOLD signal is the increase in blood flow and volume that occur during neural activity. As discussed above, new development has shown that astrocytes can mediate functional hyperemia by release of vasoactive agents, indicating that astrocytic Ca2+ signaling may be both necessary and sufficient for changes in the BOLD signal (10, 17–19, 51). The initial studies on astrocytic control of the microvasculature were performed in slice preparations. One line of work showed that astrocytic Ca2+ increases are linked to activation of Ca2+ - sensitive phospholipase A2 (PLA2), which, in turn, stimulate the production of arachidonic acid (AA). Several AA metabolites are powerful vasoactive compounds. For example, the products of cyclooxygenase (COX) induced vessel dilation in both brain slice and in vivo preparations (10). Furthermore, the cytochrome P450 epoxygenase pathway produces epoxyeicosatrienoic acids (EETs), which mediated vessel dilation in retina (18), whereas astrocytic Ca2+ signaling triggered vessel dilation through activation of Ca2+ -sensitive K+ channels in the astrocyte endfeet in cortical slices (19). In addition, two lines of work have suggested that astrocytic Ca2+ signaling in both retina and acute hippocampal slices is linked to production of the vasoconstrictor 20-hydroxyeicosatetraenoic acid, 20-HETE (17,18) (Fig. 5.1). However, a major drawback of studying blood flow regulation in ex vivo preparation is the loss of pressure-induced vasculature tone. Our in vivo experiments, employing targeted photolysis of caged Ca2+ in astrocytic endfeet, indicated that the primary mechanism by which astrocytes mediated vessel dilation is through release of PGE2 in a COX-1-dependent pathway (51) (Fig. 5.4).
Fig. 5.4.
Vasodilation triggered by uncaging (A) Upper panel: Two-photon images of a vascular astrocyte, which displayed Ca2+ increases in the endfoot following photolysis of caged Ca2+. Astrocytes were loaded with the Ca2+ indicator dye, rhod-2/am and DMNP-EDTA/am, whereas the vasculature was stained with FITC-dextran. Gray arrow indicates the position of photostimulation. Scale bar, 10 μm. (B) Time-course tracings show that photostimulation caused a rapid Ca2+ increase and arterial vasodilation. Arrows indicate time of photolysis stimulation. Dark arrowhead indicates the peak of Ca2+ elevation, and light arrowhead indicates peak dilation. (C) Summary histograms of maximum arterial cross-section area (left panel) and maximum Ca2+ increase (right panel) without photostimulation, with photostimulation but omitting DMNP-EDTA/am loading, and with DMNP-EDTA loading and photolysis. Maximum increase within 1 min after photostimulation was measured relative to baseline before stimulation. Mean ± s.e.m. * P < 0.01 compared to no-stimulation, Tukey-Kramer.
Interestingly, several lines of work have documented that the changes in blood flow and BOLD signal detected during brain activation have a stronger correlation with synaptic inputs compared with spiking activity (52, 53). Indeed, local spiking activity does not appear to play a central role in functional hyperemia (53). Thus, activity-dependent vascular signals reflect excitatory synaptic activity or synaptic release of glutamate (54, 55). Similarly, astrocytes respond to the synaptic inputs, or glutamate released from synaptic terminal during sensory stimulation, and local spiking activity has no effect upon astrocytic Ca2+ elevation (9). Thus, astrocytic Ca2+ signaling may represent a signaling pathway that locally integrates synaptic inputs and controls the microvasculature. Astrocytes detect presynaptic neurotransmitters release and display increases in intracellular Ca2+. In turn, astrocytic Ca2+ signaling activates PLA2 resulting in release of vasoactive COX-1 metabolic products. Other pathways, such as NO, adenosine and pH may in parallel contribute to functional hyperemia. Several of these pathways may be similar to glutamatergic pathways which require astrocytes as a necessary intermediary linking synaptic activity to vasodilatation.
6.2. Astrocytic Ca2+ Signaling May Increase Oxidative Metabolism
Glutamate transporters (GluTs), which are mainly expressed in the perisynaptic processes of astrocytes, play an important role in removing the glutamate released during synaptic transmission preventing the extracellular glutamate concentration from reaching excitotoxic concentrations (56). Glutamate uptake is an energy requiring process and stimulates the glycolytic metabolism in cultured astrocytes (57, 58). The main energy consuming processes associated with glutamate transport into astrocytes include extrusion of Na+ and conversion of glutamate to glutamine (59). In the model proposed by Pellerin and Magistretti, astrocytes utilize ATP produced from glycolytic glucose metabolism and lactate is transported to neurons for oxidative metabolism (57, 58). Because astrocytes express the full machinery for oxidativephosphorylation (60), it is somewhat surprising that glutamate uptake increases glycolysis rather than oxidative metabolism (57). A possible explanation is that the perisynaptic processes of astrocytes are essential devoid of most organelles (Fig. 5.1). The fine processes of astrocyte covering synapses have a small diameter (~0.3 μm) and are almost devoid of mitochondria (61). Therefore, the glycolytic metabolism of glucose, most likely occur in these fine structures of astrocytes which are heavily engaged in glutamate uptake (59, 62). Does oxidative metabolism in the astrocytic cell bodies contribute to increased ATP production during periods of intense glutamate uptake? Astrocytes contain a large number of mitochondria and the mitochondria volume fraction in the somatic areas of neurons and astrocytes are directly comparable (60,61).
It is in this regard important to note that mGluRs are expressed in the astrocyte perisynaptic processes in both rodent and primate (63, 64). Ex vivo experiments have shown that electrical stimulation of Schaffer collaterals elicits mGluRs-dependent Ca2+ elevation in hippocampal astrocytes (5, 6). Depending on the intensity of electrical stimulation, astrocytic Ca2+ signaling can be detected either in the perisynaptic microdomains or propagate intracellularly into the soma. Furthermore, activation of astrocytic mGluRs contributes to increases of astrocytic Ca2+ signaling during sensory stimulation in vivo (9). Astrocytic Ca2+ increases are first initiated in the perisynaptic processes and propagate within a time frame of 0.5–2 s to the soma triggering a 50–300% fold increase in cytosolic Ca2+ (9). Although little is known about the role of Ca2+ signaling as a modulator of glycolytic metabolism, it has been established that elevations in mitochondria Ca2+ are strong stimulator of oxidative-phosphorylation and accelerate ATP production (65). Therefore, we proposed that astrocytic Ca2+ signaling initiated in the perisynaptic processes following propagation into mitochondria rich somatic regions may stimulate the oxidative-phosphorylation. ATP produced by mitochondria in the soma may in turn diffuse back into the perisynaptic processes and supplement ATP produced by local glycolysis during periods of intense glutamate uptake. Thus, a possible function of astrocytic Ca2+ signaling evoked during synaptic activity may be to link energy metabolism in processes and cell body by activating oxidative-phosphorylation in mitochondria located distant to the perisynaptic processes.
6.3. Energy Consumption by Astrocytes During Neural Activation
Several reviews have proposed that astrocytes only account for 5% of the total energy budget consumed in rodents and about 6% in humans (66). These estimations were based on calculation of known properties of channels and synapses in neurons and astrocytes (66). In contrast, studies using in vivo nuclear magnetic resonance spectroscopy indicate that astrocytes contribute to 15% to 30% of total oxidative metabolism in the brain (67, 68). The prior calculations may neglect many of the energy requiring functions of protoplasmic astrocytes (66). In addition to glutamate uptake, multiple ATP consuming processes are activated in astrocytes during neural activation. For instance, restoring astrocytic Ca2+ concentrations following mGluRs mediated Ca2+ mobilization requires activation of both plasma membrane Ca2+-ATPase and ER Ca2+-ATPase (69). Furthermore, astrocytes buffer at least, in part, extracellular K+ by active uptake of K+ through Na+-K+-ATPase. Astrocytes are the principle cell type responsible for water homeostasis and clearance of tissue swelling during episodes of intense synaptic activity likely requires increased metabolism (70). Moreover, Ca2+ dependent activation of PLA2 results in release of arachidonic acid, which in turn requires new lipid synthesis. In addition to this energy requiring supportive function of astrocytes, astrocytic Ca2+ signaling likely evokes downstream effects that increase energy demands of both neurons and other non-neuronal cells. Intercellular astrocytic Ca2+ signaling is mediated by ATP release from astrocytes (71). ATP is, in addition to its function as an energy metabolite, an important gliotransmitter that triggers Ca2+ signaling in surrounding astrocytes (33), neurons (72), microglia (73) and smooth muscle cells (19). Normalization of Ca2+ in all of these cell types occurs through activation of Ca2+-ATPases. Conversely, hydrolysis of extracellular ATP results in accumulation of adenosine, which reduces release of glutamate and thereby dampens energy demands. The notion that astrocytic Ca2+ signaling is an energy requiring process initiated during synaptic activity finds support in the observation that inhibition of postsynaptic activity by application of CNQX/APV and TBOA failed to block changes in NADH signaling evoked during intense electrical stimulation in brain slice preparation (74). Glycolytic metabolism was shown not to play an essential role in the CNQX/APV resistant NADH changes (74), indicating that astrocytic Ca2+ increases most significantly affect oxidative- phosphorylation.
Of interest is the observation that the various tasks of astrocytes may preferentially activate oxidative or glycolytic metabolism. In a recent study using 2-photon NADH imaging, we found that cortical spreading depression was associated with a sharp decrease in NADH signaling reflecting increased oxidation in perivascular endfeet. The decrease in NADH occurred during the repolarization phase during which plasma membrane pumps are engaged in normalization of ion gradients (75).
7. Concluding Remarks
Deciphering the metabolic responses of astrocytes in response to neural activation is likely to add a new dimension to our understanding of BOLD signal changes during fMRI. A question for future research is whether astrocytic Ca2+ signaling is necessary and sufficient for increases in local blood flow similar to what have been demonstrated in mice using targeted photolysis of caged Ca2+ (51).
Acknowledgments
This work was supported by NINDS/NIH NS030007, NS038073, NS50315.
References
- 1.Cornell-Bell AH, et al. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247(4941):470–3. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263(5154):1768–71. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- 3.Parpura V, et al. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–7. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 4.Kang J, et al. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1(8):683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 5.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16(16):5073–81. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasti L, et al. Intracellular calcium oscillations in astrocytes: A highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17(20):7817–30. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual O, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–6. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 8.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci. 1998;18(11):4022–8. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, et al. Astrocytic Ca(2+) signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9(6):816–23. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 10.Zonta M, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 11.Araque A, et al. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998;18(17):6822–9. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rzigalinski BA, et al. Intracellular free calcium dynamics in stretch-injured astrocytes. J Neurochem. 1998;70(6):2377–85. doi: 10.1046/j.1471-4159.1998.70062377.x. [DOI] [PubMed] [Google Scholar]
- 13.Fellin T, et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43(5):729–43. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25(23):5502–10. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86(3):1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 16.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83(4):1140–4. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–9. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 18.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J Neurosci. 2006;26(11):2862–70. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filosa JA, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 20.Peppiatt CM, et al. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443(7112):700–4. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathiesen C, Caesar K, Lauritzen M. Temporal coupling between neuronal activity and blood flow in rat cerebellar cortex as indicated by field potential analysis. J Physiol. 2000;523(Pt 1):235–46. doi: 10.1111/j.1469-7793.2000.t01-1-00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngai AC, et al. Frequency-dependent changes in cerebral blood flow and evoked potentials during somatosensory stimulation in the rat. Brain Res. 1999;837(1–2):221–8. doi: 10.1016/s0006-8993(99)01649-2. [DOI] [PubMed] [Google Scholar]
- 23.Charles AC. Glia-neuron intercellular calcium signaling. Dev Neurosci. 1994;16(3–4):196–206. doi: 10.1159/000112107. [DOI] [PubMed] [Google Scholar]
- 24.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol. 1997;51(4):439–55. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 25.Duffy S, MacVicar BA. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J Neurosci. 1995;15(8):5535–50. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perea G, Araque A. Properties of synaptically evoked astrocyte calcium signal reveal synaptic information processing by astrocytes. J Neurosci. 2005;25(9):2192–203. doi: 10.1523/JNEUROSCI.3965-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: Homeostasis and signaling function. Physiol Rev. 1998;78(1):99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 28.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat Rev Neurosci. 2005;6(8):626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 29.Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19(6):1297–308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 30.Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. 1997;51:455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 31.Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40(6):1173–83. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- 32.Zoli M, et al. The emergence of the volume transmission concept. Brain Res Brain Res Rev. 1998;26(2–3):136–47. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 33.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18(21):8794–804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: Localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92(9):3948–52. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beattie EC, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295(5563):2282–5. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 36.Simard M, et al. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price DL, et al. Distribution of rSlo Ca2+-activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956(2):183–93. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- 38.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95(10):e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 39.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–6. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 40.Stosiek C, et al. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100(12):7319–24. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirase H, et al. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2(4):E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50(6):823–39. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Svoboda K, et al. Spread of dendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex in vivo. Nat Neurosci. 1999;2(1):65–73. doi: 10.1038/4569. [DOI] [PubMed] [Google Scholar]
- 44.Nimmerjahn A, et al. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1(1):31–7. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- 45.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anesthesia. J Neurophysiol. 1999;81(5):2243–52. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- 46.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci U S A. 2005;102(39):14063–8. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang J, et al. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1(8):683–92. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 48.Sosnik R, Haidarliu S, Ahissar E. Temporal frequency of whisker movement. I. Representations in brain stem and thalamus. J Neurophysiol. 2001;86(1):339–53. doi: 10.1152/jn.2001.86.1.339. [DOI] [PubMed] [Google Scholar]
- 49.Pinto DJ, Brumberg JC, Simons DJ. Circuit dynamics and coding strategies in rodent somatosensory cortex. J Neurophysiol. 2000;83(3):1158–66. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- 50.Petersen CC, Grinvald A, Sakmann B. Spatiotemporal dynamics of sensory responses in layer 2/3 of rat barrel cortex measured in vivo by voltage-sensitive dye imaging combined with whole-cell voltage recordings and neuron reconstructions. J Neurosci. 2003;23(4):1298–309. doi: 10.1523/JNEUROSCI.23-04-01298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takano T, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–7. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 52.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–69. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 53.Mathiesen C, et al. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512(Pt 2):555–66. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peppiatt C, Attwell D. Neurobiology: Feeding the brain. Nature. 2004;431(7005):137–8. doi: 10.1038/431137a. [DOI] [PubMed] [Google Scholar]
- 55.Lauritzen M. Reading vascular changes in brain imaging: Is dendritic calcium the key? Nat Rev Neurosci. 2005;6(1):77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- 56.Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol. 1999;9(3):293–8. doi: 10.1016/s0959-4388(99)80043-9. [DOI] [PubMed] [Google Scholar]
- 57.Pellerin L, Magistretti PJ. Food for thought: Challenging the dogmas. J Cereb Blood Flow Metab. 2003;23(11):1282–6. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 58.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91(22):10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dienel GA, Cruz NF. Nutrition during brain activation: Does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int. 2004;45(2–3):321–51. doi: 10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Pysh Y, Khan T. Variation in mitochondrial structure and content of neurons and neuroglia in rat brain: An electron microscopic study. Brain Research. 1972;36(1):1–18. doi: 10.1016/0006-8993(72)90762-7. [DOI] [PubMed] [Google Scholar]
- 61.Peters A, Sandford LP, Webster HD. Fine structure of the Nervous System: Neurons and Their Supporting Cells. 3rd edition ed. Oxford University Press; Oxford: 1991. [Google Scholar]
- 62.Hertz L, Peng L, Dienel G. Energy metabolism in astrocytes: High rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. Journal of Cerebral Blood Flow & Metabolism. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 63.van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol. 1995;362(1):134–50. doi: 10.1002/cne.903620108. [DOI] [PubMed] [Google Scholar]
- 64.Munoz A, Liu XB, Jones EG. Development of metabotropic glutamate receptors from trigeminal nuclei to barrel cortex in postnatal mouse. J Comp Neurol. 1999;409(4):549–66. [PubMed] [Google Scholar]
- 65.Brookes PS, et al. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 66.Attwell D, Laughlin S. An Energy Budget for Signaling in the Grey Matter of the Brain. Journal of Cerebral Blood Flow and Metabolism. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Lebon V, et al. Astroglial Contribution to Brain Energy Metabolism in Humans Revealed by 13C Nuclear Magnetic Resonance Spectroscopy: Elucidation of the Dominant Pathway for Neurotransmitter Glutamate Repletion and Measurement of Astrocytic Oxidative Metabolism. J Neurosci. 2002;22(5):1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oz G, et al. Neuroglial Metabolism in the Awake Rat Brain: CO2 Fixation Increases with Brain Activity. The Journal of Neuroscience. 2004;22(50):11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kahlert S, Reiser G. Requirement of Glycolytic and Mitochondrial Energy Supply for Loading of Ca2+ Stores and InsP3-Mediated Ca2+ Signaling in Rat Hippocampus Astrocytes. J Neurosci Res. 2000;61:409–420. doi: 10.1002/1097-4547(20000815)61:4<409::AID-JNR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 70.Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. 2004;35:1168. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- 71.Arcuino G, et al. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99(15):9840–5. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang JM, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40(5):971–82. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 73.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 74.Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescent transients after synaptic activity in brain slices: Predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006;26(11):1389–406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- 75.Takano T, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10(6):754–62. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]