Abstract
Mutations in Notch signaling pathway members cause developmental phenotypes that affect the liver, skeleton, heart, eye, face, kidney, and vasculature. Notch associated disorders include the autosomal dominant, multi-system, Alagille syndrome caused by mutations in both a ligand (Jagged1 (JAG1)) and receptor (NOTCH2) and autosomal recessive spondylocostal dysostosis, caused by mutations in a ligand (Delta-like-3 (DLL3)), as well as several other members of the Notch signaling pathway. Mutations in NOTCH2 have also recently been connected to Hajdu-Cheney syndrome, a dominant disorder causing focal bone destruction, osteoporosis, craniofacial morphology and renal cysts. Mutations in the NOTCH1 receptor are associated with several types of cardiac disease and mutations in NOTCH3 cause the dominant adult onset disorder CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), a vascular disorder with onset in the 4th or 5th decades. Studies of these human disorders and their inheritance patterns and types of mutations reveal insights into the mechanisms of Notch signaling.
Keywords: Alagille syndrome, Spondylocostal dysostosis, Hajdu Cheney, Cardiac disease, Notch signaling
1. Introduction
The Notch pathway is one of the basic signaling pathways used repeatedly in development, and it is involved in both cell type specification and organogenesis. The role of the Notch signaling pathway in Drosophila development has been studied since a dominant notched wing phenotype was first reported in 1914, but its role in human development and disease has only been recognized since 1991, when mutations in NOTCH1 were associated with a form of T-cell acute lymphoblastic leukemia (T-ALL) [1]. In 1996, NOTCH3 mutations were found to cause CADASIL, a disorder characterized by stroke and dementia, with onset in the 3rd or 4th decade [2]. CADASIL is caused by mutations in the extracellular domain of NOTCH3, resulting from the gain or loss of cysteine residues in the epidermal growth factor-like repeats (EGFR-like). Pathological studies of tissue from CADASIL patients reveal accumulation of NOTCH3 protein in brain lesions, but a precise mechanism for the disorder is not known. We will not discuss CADASIL further in this review, as it is not a developmental disorder. A year after discovery of the role of NOTCH3 in CADASIL, JAG1 mutations were identified in the multi-system, developmental disorder Alagille syndrome, confirming the pleiotropic functioning of the pathway in mammals that had already been demonstrated in lower organisms [3]. Since then, other developmental disorders associated with Notch signaling have been identified, and animal models have been developed, to determine how Notch pathway members contribute to the development of the numerous organ systems implicated in the disease studies. In this review, we present data on the human phenotypes associated with mutations in Notch signaling pathway members, and in some cases, provide an overview of the model organism studies underway to better understand the mechanisms of abnormal development caused by these mutations.
2. Alagille syndrome
Alagille syndrome (ALGS, OMIM# 118450) was initially described as a case of two siblings with intrahepatic biliary dysgenesis and polycystic kidneys in 1965 and over the following years, the description of the syndrome became more precise, with recognition of cardiac, vertebral, ocular, facial and renal features [4–6]. By 1987, the criteria for the “classic” form of Alagille syndrome was established, which included the presentation of 3/5 clinical features including anomalies of the liver, heart, vertebrae, eye or face, along with bile duct paucity on liver biopsy. In addition, several lower penetrance findings are frequently identified including renal anomalies, growth retardation, bone abnormalities, a high pitched voice, vascular malformations and delayed puberty [7].
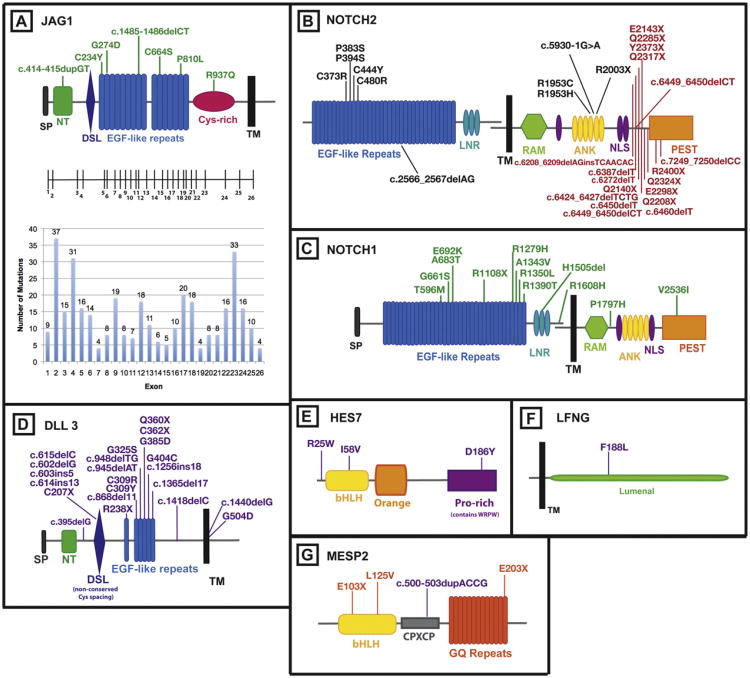
JAG1, one of 5 human NOTCH signaling ligands is the primary disease gene for ALGS [3]. The JAG1 mutations are spread across the entire protein, and there is a high de novo mutation rate (60%) (Fig. 1A) [8]. Protein-truncating mutations (frameshift, nonsense, splice site) account for 80% of the total, whole gene deletions for 7%, and missense mutations for 12%. The finding that whole gene deletions result in a phenotype similar to that seen in patients with truncating and missense mutations suggest that haploinsufficiency is the mechanism of disease causation [8,9]. Analysis of the functional consequences of the missense mutations is also consistent with this interpretation [9– 11].
Fig. 1.
Notch signaling pathway genes with associated mutations in human disease. (A) The protein domains of JAG1 are shown, with the corresponding exon structure for the gene. The number of ALGS associated mutations identified in each exon is represented on the graph below the exon structure. Mutations in patients with isolated cardiac disease are indicated directly on the protein structure diagram (green). (B) The protein domains of NOTCH2 are shown. NOTCH2 mutations are found in Alagille syndrome (black) and Hajdu-Cheney syndrome (red). (C) The protein domains of NOTCH1 are shown. NOTCH1 mutations are found in patients with isolated cardiac defects (green). (D) The protein domains of DLL3 are shown. DLL3 mutations are found in patients with SCD (purple). (E) The HES7 protein domains are shown. HES7 mutations are found in patients with SCD (purple). (F) The LFNG protein domains are shown. A mutation in LFNG was identified in a patient with SCD (purple). (G) The protein domains of MESP2 are shown. Mutations in MESP2 are found in patients with SCD (purple) and STD (orange). [References for mutations are identified in the text.] ANK, ankryrin repeats; bHLH, basic-helix–loop–helix; CPXCP, CPXCP motif; Cys-rich, cysteine rich domain; DSL, Delta/Serrate/lag-2; EGF repeats, epidermal growth factor-like repeats; GQ repeats, glycine-glutamine repeats; LNR, LIN-12/Notch repeats; Lumenal, luminal domain; NLS, nuclear localization signal; NT, N-terminal domain; Orange, orange domain, PEST, proline/glutamic acid/serine/threonine rich domain; Pro-rich, proline rich domain; RAM, RBP-Jк-associated module; SP, signal peptide; TM, transmembrane domain; WRPW, tryptophan-arginine-proline-tryptophan domain.
Mutations in NOTCH2 also cause ALGS in a smaller number of cases, and these include both extracelluar (n = 6) and intracellular (n = 4) mutations [12] (Fig. 1B). The 4 intracellular mutations, all occur in the ankyrin domain, which mediates protein-protein interactions, therefore affecting the ability of the Notch intracellular domain to bind other transcription factors.
The penetrance of JAG1 mutations is about 95% if we consider the presence of any clinical feature but expressivity is highly variable, and many mutation carriers have only subclinical manifestations [13, 14].The penetrance of clinically significant liver disease is estimated at 31%, cardiac disease (including heart murmurs) at less than 75% and facial features at 91% [14].
2.1. ALGS associated liver disease and Notch function in bile duct development
The liver disease in ALGS is the major presenting clinical feature and requires the most consistent medical treatment for the majority of symptomatic patients. Liver phenotypes vary from mild asymptomatic elevations in serum bilirubin, bile acids and liver enzymes to severe cholestasis resulting in disability and requiring liver transplant. The characteristic pathologic alteration is intrahepatic bile duct paucity [15]. Paucity emerges post-natally and is found in 60% of infants younger than 6 months, and in 95% of patients older than 6 months [16].
Studies in mice and zebrafish in which Notch components are eliminated show that these model organisms recapitulate certain aspects of the ALGS phenotype [17]. Unlike humans, mice that are heterozygous for Jag1 or Notch2 mutations do not recapitulate the liver phenotypes seen in ALGS with one exception [18]. Homozygotes for strong loss of function mutations in Jag1 or Notch2 result in lethality before bile duct formation [19,20]. In contrast, mice that are homozygous for a Notch2 hypomorphic allele, or that are double heterozygotes for a Jag1 null allele and a Notch2 hypomorphic allele (Jag1/+, Notch2/+), display bile duct paucity just after birth [21]. Mice that are double heterozygotes for mutations in Jag1 and the O-glucosylation transferase Rumi (Jag1/+, Rumi/+) have severe perinatal bile duct paucity at P0 [22]. In addition, mice that are heterozygous for Jag1 and the glycosylaminoglycase radical fringe (Rfng) also have duct paucity at P0 [23]. These studies reveal that formation of the intrahepatic bile ducts is sensitive to the dosage of Notch family genes and supports the idea that variable expressivity in ALGS is at least partly due to genetic modifiers.
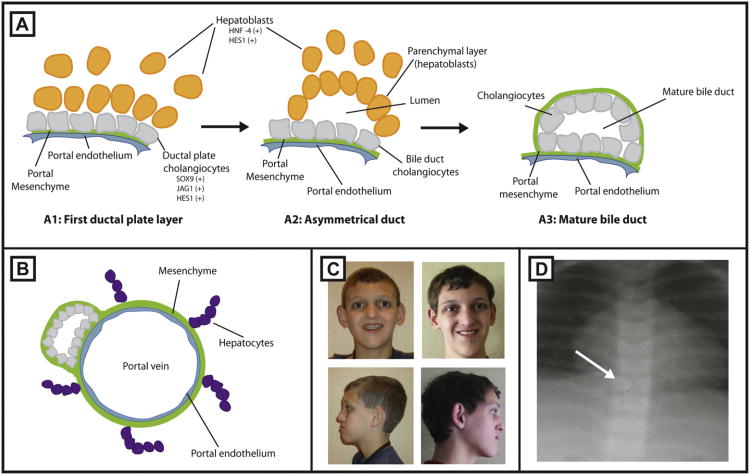
Development of the intrahepatic bile ducts in mice begins at E12.5-E14.5 with the specification of biliary epithelial cells (BECs), also known as cholangiocytes (Fig. 2A and B), and recent studies demonstrate a role for Notch signaling in 2 distinct stages of bile duct formation: (1) induction of biliary cell fate during ductal plate formation and (2) subsequent tubulogenesis of BECs [24,25]. Support for an involvement of Notch signaling in the induction of biliary cell fate comes from experiments in cultured cells which show that an increase in Notch signaling results in the down-regulation of hepatocyte genes such as Hnf1α and Hnf4, and an up-regulation of Hnf1 β which is essential for bile duct development [26]. In vivo experiments show that elimination of Notch signaling by hepatoblast specific deletion of the mammalian Notch pathway transcription factor Rbp-jк, at E15.5 impairs ductal plate specification indicated by the loss or reduction of expression of the Notch target genes Hes1 and Sox9 [25]. In contrast, elimination of Rbp-jк signaling at E16.5 does not alter the specification of BECs but results in an inability of these newly specified cells to undergo tubulogenesis. This suggests that Notch signaling acts early to specify ductal plate cell fate and later to induce their tubulogenesis.
Fig. 2.
Development of intrahepatic bile ducts. (A1) Cholangiocytes (gray) line up along the portal vein forming the ductal plate.Jag1, Hes1 and Sox9 are expressed by cells of this layer. (A2) Subsequently, hepatoblasts expressing Hes1 and HNF4 (yellow) come into contact with the ductal plate forming the outer (parenchymal) layer and move to create an asymmetric tubular structure. Unincorporated hepatoblasts and cholangiocytes are eliminated. (A3) The outer hepatoblasts differentiate into cholangiocytes to complete the formation of the duct. (B) A mature duct aligned along the portal vein, enclosed by a mesenchyme. Mature hepatocytes develop in close proximity to bile ducts (purple). (C) Representative facies of a patient with ALGS are shown in frontal and side view. (D) Anteroposterior X-ray of a patient with butterfly vertebrae.
There is evidence that Jag1 protein from the portal vein mesenchyme activates Notch2 in the adjacent BECs to induce tubulogenesis.Jag1 – hepatoblast specific conditional mouse knockouts, demonstrate no effect on bile duct development but Jag1 expression in portal vein mesenchyme is crucial for tubulogenesis [24,27]. Unlike Jag1, Notch2 is expressed in both hepatoblasts and BECs during embyogensis, and Notch2 hepatoblast specific knockouts using Alb-Cre recombinase do result in bile duct abnormalities [24,28,29].
Although bile duct paucity is initially seen when Notch signaling is attenuated, the presence of abnormal cytokeratin positive BECs or proliferation of ductal like structures is also observed in postnatal mice, consistent with the findings in ALGS patients [23,27,28,30]. This suggests that the intrahepatic bile duct phenotype can change with age when Notch signaling is altered. Because ALGS patients demonstrate an increase in bile duct paucity as they age it will be important to elucidate the postnatal mechanisms for bile duct expansion and or maintenance as well as the embryonic initiation of duct formation [16]. Interestingly, recent data in mice indicate that there is not an increase in bile duct paucity but that communication among ductal structures is disrupted by chronic cholestatsis [31].
2.2. ALGS associated cardiac disease
A high percentage of patients with JAG1 or NOTCH2 mutations have cardiac disease, which includes (in order of frequency) branch pulmonary artery stenosis, peripheral pulmonary stenosis, tetralogy of Fallot (TOF), valvar pulmonic stenosis, ventricular septal defect, atrial septal defect, coarctation of the aorta, and several less common right and left sided lesions [12,14,16,32]. Mice that are attenuated for Notch signaling (Jag1/+; Notch2/+) have multisystem defects that strongly resemble those seen in patients with Alagille syndrome, including pulmonary artery stenosis and ventricular septal defects [21]. Therefore, Notch signaling is directly implicated in development of the heart valves, ventricular septum formation, and cardiac outflow tracts [32,33].
2.3. ALGS facial features
ALGS patients have characteristic facial features consisting of a prominent forehead, deep-set widely spaced eyes, a straight nose with a flattened tip and a prominent pointed chin (Fig. 2C). These features give the impression of an inverted triangular face. The face is distinctive in both childhood and adulthood and changes in characteristic ways over time. It is unclear what the etiology of these features are but it is likely to be a result of reduced Notch signaling rather than arising as a sequelae to the metabolic consequences of metabolic abnormalities [34]. Recent experiments indicate that Notch signaling is essential for mid-face development in mice [35]. Humphreys et al. found that deletion of Jag1, but not Notch1, in cranial neural crest cells resulted in a reduced maxilla, aberrant vascular branching, and decreased proliferation and extracellular matrix formation during embryogenesis. These experiments imply that Jag1 induces maxilla morphogenesis by signaling in a Notch1 independent manner within cranial neural crest cells, or that it signals to a distinct cell population.
2.4. ALGS skeletal and ocular findings
Skeletal and ocular features in ALGS are relatively mild and of little clinical consequence, although useful diagnostically. The most common skeletal finding is the “butterfly vertebrae” or sagittal cleft which is found in 33–87% of patients [16]. The affected vertebral bodies appear to be split into paired hemivertebrae because of a failure of the fusion of the anterior arches of the vertebrae (Fig. 2D). Other skeletal abnormalities that have been observed in patients with ALGS include narrowing of the interpedicular space in the lumbar spine, spina bifida oculta, fusion of the adjacent vertebrae, hemivertebrae, absence of the 12th rib and the presence of a bony connection between the ribs. In addition, short fingers with broad thumbs have been reported [5].
Ocular anomalies in ALGS involve the cornea, iris, retina and optic disk, though the most common is posterior embryotoxon (PE). PE is a prominent, centrally positioned Schwalbe's line at the point at which the corneal endothelium and the uveal trabecular meshwork join. This variant occurs in 56–95% of patients with ALGS, and also occurs in 22% of normal eyes [7,16].
2.5. ALGS renal
A variety of renal anomalies have been reported and in the largest study done so far, renal anomalies were validated in 73 of 187 patients with ALGS, (39%) with the majority of patients having generalized or focal renal dysplasia. Abnormalities included renal tubular acidosis (10%), vesico ureteric reflux (8%) and urinary obstruction (8%) [36]. Several patients were diagnosed with ALGS as adults, following the onset of renal disease, suggesting that ALGS is under-diagnosed outside of the pediatric GI clinic [37]. Notch genes are expressed throughout renal development and the pathway plays a key role in nephron segmentation and glomerular development [38,39].
2.6. ALGS vascular anomalies
While not initially included as one of the diagnostic features of Alagille syndrome, vascular anomalies have been reported in the earliest descriptions of this syndrome. Pulmonary artery abnormalities are well known as one of the hallmark cardiac features of the syndrome, and in addition, intracranial bleeding secondary to vascular abnormalities occurs in 15% of patients, with high mortality [40]. Underlying vessel abnormalities in the central nervous system such as aneurysms of the basilar and middle cerebral arteries that could explain the occurrence of bleeding and stroke in ALGS have been described. Moyamoya disease (progressive intracranial arterial occlusive disease) also has been described in several children with ALGS [41]. A key role for Jagged1 in the formation of normal vasculature is supported by the lethal vascular phenotype of Jag1 knockout mice and a role for Jagged-Notch signaling in the direct assembly of the arterial wall was recently demonstrated [42].
3. Cardiac disease
In addition to the cardiac defects seen in association with ALGS, mutations in JAG1 or NOTCH1 have been found in individuals with non-syndromic right-sided cardiac disease that is similar in type to that seen in ALGS (Fig. 1A and C). JAG1 mutations have been identified in patients with isolated TOF and in fewer cases, NOTCH1 mutations have been associated with TOF as well [10,33,43–45]. JAG1 sequence variants have been identified in 4% of patients presenting with pulmonic stenosis, peripheral pulmonic stenosis or pulmonary artery stenosis who did not meet the diagnostic criteria for Alagille syndrome [10]. We do not currently have a good explanation for why some patients with a JAG1 mutation have the full range of clinical abnormalities associated with ALGS, while others have only cardiac disease. There was initial evidence that the JAG1 missense mutation (G274D) identified in a large family segregating apparently isolated cardiac disease had a “leaky” phenotype, some of the protein appearing on the cell surface, with the ability to signal, but subsequent studies have shown that some cardiac only mutations appear to code for proteins with no activity leading to the hypothesis that there may be genetic modifiers [10,11,33].
NOTCH1 mutations are associated with structural abnormalities of the aortic valve, such as bicusupid aortic valve and have been found in individuals with more serious left ventricular outflow tract abnormalities such as aortic valve stenosis, coarctation of the aorta and hypoplastic left heart syndrome [46,47]. Functional work on the missense mutations associated with left ventricular outflow abnormalities have demonstrated reduced binding of the mutant receptor to the Notch ligands, as well as a reduction of the amount of receptor at the surface, with increased localization to the endoplasmic reticulum [48]. This reduced signaling is associated with defective epithelial-to-mesenchymal transition, which is necessary for proper formation of the aortic and pulmonary valves [49]. For a more in depth analysis of the role of Notch signaling in cardiac development there are several excellent reviews [33,50].
4. Spondylocostal dysostosis (SCD) and spondylothoracic dysostosis (STD)
Notch signaling has emerged as a key regulator of the process of somitogenesis, by which vertebrae are formed. For an in depth review of somitogenesis, see Pourquie et al [51]. In addition to the clinically benign isolated vertebral defects seen in ALGS, mutations in several of the Notch signaling pathway genes cause severe vertebral and costal abnormalities in spondylocostal dysostosis (SCD, OMIM# 277300) and spondylothoracic dysostosis (STD). These latter diseases are grouped into the general category of abnormal vertebral segmentation (AVS) disorders characterized by congenital malformations in which the vertebrae are fused or altered in shape, position, or size [52]. AVS can be caused by genetic or environmental perturbations and can present with additional malformations or as an isolated abnormality.
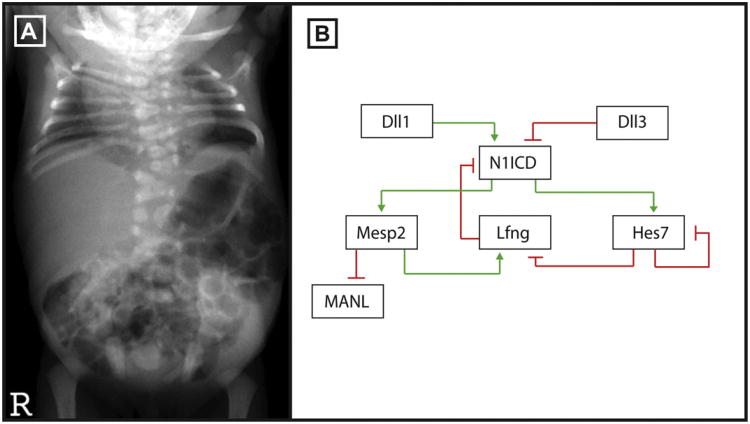
Clinically, a diagnosis of SCD and STD is established by the malformation of 10 or more contiguous vertebrae and mis-alignment and or fusion of some ribs (Fig. 3A) [53]. The two conditions are distinguished by several parameters. SCD patients present with variable alignment of ribs and variable costal fusions and this disorder occurs worldwide with an incidence of 1 in 40,000. Most patients survive. STD patients present with severe truncal shortening along with pronounced respiratory insufficiency that leads to 75% lethality before adulthood [54]. STD is found primarily in Puerto Rican populations and the incidence of the disease is unknown. Radiographic imaging studies reveal that the ribs of STD patients are fused posteriorly at the point where they originate from the vertebrae. SCD and STD can present sporadically, or in multiple family members with both autosomal dominant and autosomal recessive patterns of inheritance reported [53]. Several genes in the Notch signaling pathway have been associated with autosomal recessive SCD and STD including the ligand Delta like 3 (DLL3; OMIM SCD01) the glycosyltransferase Lunatic Fringe (LFNG; OMIM SCD03), and the basic helix–loop–helix Notch target genes, Hairy-and-enhancer-of-split-7 (HES7; OMIM SCD04) and Mesoderm Posterior 2 (MESP2; OMIM SCD02). The functions of these proteins are evolutionarily conserved and these 4 genes demonstrate remarkably similar phenotypes in mice knockouts and in deficient humans [55–58].
Fig. 3.
Notch signaling is required for somitogenesis. (A) Anteroposterior X-ray of a patient with SCD due to a mutation in DLL3 courtesy of Dr. Peter Turnpenny. The thoracic and cervical spine have severe vertebral segmentation defects including hemivertebrae. (B) A negative feedback loop consisting of the Mesp2, Lfng, Hes7 and Dll3 genes regulate Notch signaling activity during somitogenesis.
DLL3 was the first gene linked to SCD and DLL3 mutations are responsible for the SCD phenotype in 20–25% of patients analyzed with more than 24 mutations reported to date (Fig. 1D) [59]. Most occur in the extracellular domain and truncate the protein suggesting that these mutations act as null alleles [56,60]. Structurally DLL3 is predicted to encode a Notch ligand but cell culture experiments and injection assays in Xenopus embryos indicate that Dll3 does not bind to Notch receptors in trans and acts instead as a cis inhibitor [61]. Recent data show that Dll3 interacts with full length NOTCH1 in the late endocytic compartment and that high levels of Dll3 expression are correlated with low levels of Notch1 suggesting that Dll3 targets Notch1 for lysosomal degradation [62].
LFNG is an important regulator of somitogenesis in vertebrates and a homozygous mutation in LFNG was identified in an SCD proband of Lebanese background (Fig. 1F) [63]. Like patients carrying DLL3 mutations, vertebral anomalies were present along the entire length of the spine in this patient. LFNG is an N-acetylglucosaminyltransferase that adds N-acetylglucosamine to fucose residues on Notch receptors and acts to suppress Notch signaling during somitogenesis in mice. The mutant protein substitutes a leucine for a highly conserved phenylalanine, has no enzymatic activity and acts like a null in cell culture assays.
Homozygosity mapping identified a mutation in the basic helix loop helix protein MESP2, in a consanguineous Lebanese family [64]. Mesp2 is essential for rostral-caudal patterning during somitogenesis in mice and knockouts have skeletal defects along the entire spine [58]. The mutation, c.500-503dupACCG, is predicted to interrupt splicing, and to cause a frameshift mutation that truncates the carboxy-terminus after the CPXCP repeat (Fig. 1G). MESP2 mutations were also detected in individuals diagnosed with STD (Fig. 1G) [65]. MESP2 acts in synergy with the NICD to activate expression of the LFNG enhancer in NIH3T3 cells and when tested cDNA constructs of c.500-503dupACCG, E103X, and L125V behaved as null mutations in this assay [65]. It is unclear why c.500-503dupACCG causes a mild SCD phenotype while the other mutations cause STD but it was suggested that c.500-503dupACCG escapes nonsense mediated decay and may act as a hypomorph in vivo [65]. Differences in phenotype could result from allelic heterogeneity or be due to the effects of genetic modifiers.
HES7 belongs to the family of Hairy and Enhancer of split proteins, first described in Drosophila. HES7 functions as a transcriptional repressor, and acts by binding to N box (CACNAG) and class C sites (CACG(C/A)G) in target DNA [66]. Autozygosity mapping in a consanguineous family with SCD led to the identification of a mutation in HES7 (R25W). This mutation occurs in the DNA binding domain of HES7 and alters a conserved amino acid (Fig. 1E) [67]. This mutation was functionally tested in the mouse Hes7 gene and was unable to repress transcription in two assays indicating that the R25W mutation is likely to cause SCD in this patient. Subsequently, two affected individuals were found to be compound heterozygotes for an additional two mutations, I58V and D186L, in the basic helix–loop–helix domain and a proline rich domain of the HES7 gene respectively [68]. Neither mutation was detected in control individuals and D186L showed reduced activity in the functional assay while I58V behaved as wildtype suggesting more work is needed to confirm the significance of these genetic variants.
Interestingly, DLL3, LFNG, MESP2 and HES7 all act as repressors of Notch signaling or of Notch family member expression during somitogenesis (Fig. 3B) [52]. HES7 acts to downregulate LFNG expression. Lfng, Dll3 and Mesp2 mutant embryos display a broadening of Notch signaling activity during somitogenesis, detected by antibodies to NICD [62,69]. Dll3 is predicted to participate in lysosomal degradation of NOTCH1, MESP2 destabilizes MAML, and LFNG down-regulates Notch signaling immediately posterior to the forming somite [62,70]. Not surprisingly, ubiquitous activation of Notch in the presomatic mesoderm results in abnormal somite formation [71].
5. Hajdu Cheney syndrome
Recently, NOTCH2 has been implicated in the pathogenesis of the Hajdu-Cheney and serpentine fibula polycystic kidney syndromes [72–74]. Hajdu-Cheney syndrome (HCS, OMIM# 102500) is a rare condition characterized by focal bone destruction and osteoporosis with other features including craniofacial abnormalities, renal cysts, cleft palate and cardiac defects. Serpentine fibula syndrome has considerable overlap with HCS, with increased prominence of the characteristic fibula findings. NOTCH2 mutations were identified following exome sequencing to identify a pathogenic mutation in HCS patients, and subsequent sequencing of NOTCH2 in patients with serpentine fibula syndrome showed that they also have similar NOTCH2 mutations. The NOTCH2 mutations in both disorders are all localized in the last exon (exon 34) of NOTCH2 (Fig. 1B), and it is now thought that these two disorders represent different manifestations of the same syndrome. The resultant mRNA is predicted to escape nonsense mediated decay, and the mutations are predicted to disrupt the intracellular PEST (proline-glutamateserine-threonine-rich) domain, which is hypothesized to result in decreased turn over of the NOTCH2 protein and therefore increased NOTCH2 signaling [75]. While functional studies have not been carried out for the HCS mutations, similar mutations were identified in the highly related NOTCH1 protein in a patient with T-cell acute lymphoblastic leukemia and in NOTCH2 in patients with large B-cell lymphomas, and in both cases, the mutations were found to show increased activity, secondary to prolonged half life of the Notch intracellular domain [75,76]. Therefore, these mutations have an opposing effect on NOTCH2 as compared to the ALGS-associated mutations. A role for the Notch signaling pathway in bone metabolism has also been suggested by additional findings, including the increased risk of fractures in ALGS syndrome, and the finding, via a genome-wide association study, that JAG1 variants may be associated with bone mineral density regulation in postmenopausal women [77].
6. Conclusions
Data from human studies confirms a key role for the Notch signaling pathway in development of the liver, heart, kidney, vasculature, bone, eye, skeleton and other organ systems. Although Notch family genes were initially identified in Drosophila and Caenorhabditis elegans, the discovery that aberrant Notch signaling results in human disease such as ALGS has led to the development of vertebrate models to unravel the mechanisms by which Notch acts. Conversely vertebrate models led to the discovery of the disease genes in SCD. Together, human and animal studies have provided important advances over the last 20 years. Nevertheless, many questions remain unanswered. Why is the expressivity of JAG1 mutations so variable in ALGS? What causes SCD in the 70% of patients who do not have DLL3, LFNG, MESP2 or HES7 mutations? [62]. What is the mechanism for bone abnormalities in Hajdu-Cheney syndrome? Will genome-sequencing studies of patients with additional phenotypes reveal mutations in other Notch signaling pathway members? Moreover, the mechanisms for developmental abnormalities of the liver, heart, and kidney are still incompletely understood. Finally, translating insights about the mechanisms of Notch signaling into clinically beneficial treatments is a great challenge.
Acknowledgments
We thank the members of the Spinner Laboratory and Drs. Krantz, Piccoli, Loomes and Kamath for continued collaboration and discussion. We are grateful to Dr. Peter Turnpenny for contributing the SCD X-ray in Fig. 3. Work from our laboratory has been funded by the NIDDK (DK53104; NHLBI P50 HL62177; UO1DK062481; 1R01DK081702) and the Fred and Susanne Biesecker Center for Pediatric Liver Disease at the Children's Hospital of Philadelphia.
References
- 1.Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66(4):649–61. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 2.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16(3):243–51. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 4.Alagille D, Borde J, Habib EC, Thomassin N. Surgical attempts in atresia of the intrahepatic bile ducts with permeable extrahepatic bile duct. Study of 14 cases in children. Arch Fr Pediatr. 1969;26(1):51–71. [PubMed] [Google Scholar]
- 5.Riely CA, Cotlier E, Jensen PS, Klatskin G. Arteriohepatic dysplasia: a benign syndrome of intrahepatic cholestasis with multiple organ involvement. Ann Intern Med. 1979;91(4):520–7. doi: 10.7326/0003-4819-91-4-520. [DOI] [PubMed] [Google Scholar]
- 6.Watson GH, Miller V. Arteriohepatic dysplasia: familial pulmonary arterial stenosis with neonatal liver disease. Arch Dis Child. 1973;48(6):459–66. doi: 10.1136/adc.48.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alagille D, Estrada A, Hadchouel M, Gautier M, Odievre M, Dommergues JP. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110(2):195–200. doi: 10.1016/s0022-3476(87)80153-1. [DOI] [PubMed] [Google Scholar]
- 8.Crosnier C, Driancourt C, Raynaud N, Dhorne-Pollet S, Pollet N, Bernard O, et al. Mutations in JAGGED1 gene are predominantly sporadic in Alagille syndrome. Gastroenterology. 1999;116(5):1141–8. doi: 10.1016/s0016-5085(99)70017-x. [DOI] [PubMed] [Google Scholar]
- 9.Morrissette JD, Colliton RP, Spinner NB. Defective intracellular transport and processing of JAG1 missense mutations in Alagille syndrome. Hum Mol Genet. 2001;10(4):405–13. doi: 10.1093/hmg/10.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Bauer RC, Laney AO, Smith R, Gerfen J, Morrissette JJ, Woyciechowski S, et al. Jagged1 (JAG1) mutations in patients with tetralogy of Fallot or pulmonic stenosis. Hum Mutat. 2010;31(5):594–601. doi: 10.1002/humu.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu F, Morrissette JJ, Spinner NB. Conditional JAG1 mutation shows the developing heart is more sensitive than developing liver to JAG1 dosage. Am J Hum Genet. 2003;72(4):1065–70. doi: 10.1086/374386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath BM, Bauer RC, Loomes KM, Chao G, Gerfen J, Hutchinson A, et al. NOTCH2 mutations in Alagille syndrome. J Med Genet. 2011 doi: 10.1136/jmedgenet-2011-100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guegan K, Stals K, Day M, Turnpenny P, Ellard S. JAG1 mutations are found in approximately one third of patients presenting with only one or two clinical features of Alagille syndrome. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01749.x. [DOI] [PubMed] [Google Scholar]
- 14.Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet. 2003;40(12):891–5. doi: 10.1136/jmg.40.12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadchouel M, Hugon RN, Gautier M. Reduced ratio of portal tracts to paucity of intrahepatic bile ducts. Arch Pathol Lab Med. 1978;102(8):402. [PubMed] [Google Scholar]
- 16.Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29(3):822–9. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 17.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, et al. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131(22):5753–66. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 18.Vrijens, Thys K, De Jeu S, Postnov MT, Pfister AA, Cox M, et al. A Jag1 vestibular mouse mutant, displays characteristics of Alagille syndrome. Neurobiol Dis. 2006;24(1):28–40. doi: 10.1016/j.nbd.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 19.McCright B, LozierJ J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44(1):29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8(5):723–30. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 21.McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129(4):1075–82. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Valdivia R, Takeuchi H, Samarghandi A, Lopez M, Leonardi J, Haltiwanger RS, et al. Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development. 2011;138(10):1925–34. doi: 10.1242/dev.060020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan MJ, Bales C, Nelson A, Gonzalez DM, Underkoffler L, Segalov M, et al. Bile duct proliferation in Jag1/fringe heterozygous mice identifies candidate modifiers of the Alagille syndrome hepatic phenotype. Hepatology. 2008;48(6):1989–97. doi: 10.1002/hep.22538. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137(23):4061–72. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136(10):1727–39. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raynaud P, Carpentier R, Antoniou A, Lemaigre FP. Biliary differentiation and bile duct morphogenesis in development and disease. Int J Biochem Cell Bio. 2011;43(2):245–56. doi: 10.1016/j.biocel.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Loomes KM, Russo P, Ryan M, Nelson A, Underkoffler L, Glover C, et al. Bile duct proliferation in liver-specific Jag1 conditional knockout mice: effects of gene dosage. Hepatology. 2007;45(2):323–30. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- 28.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, et al. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48(2):607–16. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 29.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. 2004;127(6):1775–86. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Deutsch GH, Sokol RJ, Stathos TH, Knisely AS. Proliferation to paucity: evolution of bile duct abnormalities in a case of Alagille syndrome. Pediatr Dev Pathol. 2001;4(6):559–63. doi: 10.1007/s10024001-0102-6. [DOI] [PubMed] [Google Scholar]
- 31.Sparks EE, Perrien DS, Huppert KA, Peterson TE, Huppert SS. Defects in hepatic Notch signaling result in disruption of the communicating intrahepatic bile duct network in mice. Dis Model Mech. 2011;4(3):359–67. doi: 10.1242/dmm.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElhinney DB, Krantz ID, Bason L, Piccoli DA, Emerick KM, Spinner NB, et al. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation. 2002;106(20):2567–74. doi: 10.1161/01.cir.0000037221.45902.69. [DOI] [PubMed] [Google Scholar]
- 33.MacGrogan D, Nus M, de la Pompa JL. Notch signaling in cardiac development and disease. Curr Top Dev Biol. 2010;92:333–65. doi: 10.1016/S0070-2153(10)92011-5. [DOI] [PubMed] [Google Scholar]
- 34.Kamath BM, Loomes KM, Oakey RJ, Emerick KE, Conversano T, Spinner NB, et al. Facial features in Alagille syndrome: specific or cholestasis facies? Am J Med Genet. 2002;112(2):163–70. doi: 10.1002/ajmg.10579. [DOI] [PubMed] [Google Scholar]
- 35.Humphreys R, Zheng W, Prince LS, Qu X, Brown C, Loomes K, et al. Cranial neural crest ablation of Jagged1 recapitulates the craniofacial phenotype of Alagille syndrome patients. Hum Mol Genet. 2011:1–10. doi: 10.1093/hmg/ddr575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamath BM, Podkameni G, Hutchinson AL, Leonard LD, Gerfen J, Krantz ID, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A. 2011 doi: 10.1002/ajmg.a.34369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacquet A, Guiochon-Mantel A, Noel LH, Sqalli T, Bedossa P, Hadchouel M, et al. Alagille syndrome in adult patients: it is never too late. Am J Kidney Dis. 2007;49(5):705–9. doi: 10.1053/j.ajkd.2007.02.262. [DOI] [PubMed] [Google Scholar]
- 38.Piscione TD, Wu MY, Quaggin SE. Expression of Hairy/Enhancer of Split genes, Hes1 and Hes5, during murine nephron morphogenesis. Gene Expr Patterns. 2004;4(6):707–11. doi: 10.1016/j.modgep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Cheng HT, Kopan R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 2005;68(5):1951–2. doi: 10.1111/j.1523-1755.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 40.Kamath BM, Spinner NB, Emerick KM, Chudley AE, Booth C, Piccoli DA, et al. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation. 2004;109(11):1354–8. doi: 10.1161/01.CIR.0000121361.01862.A4. [DOI] [PubMed] [Google Scholar]
- 41.Woolfenden AR, Albers GW, Steinberg GK, Hahn JS, Johnston DC, Farrell K. Moyamoya syndrome in children with Alagille syndrome: additional evidence of a vasculopathy. Pediatrics. 1999;103(2):505–8. doi: 10.1542/peds.103.2.505. [DOI] [PubMed] [Google Scholar]
- 42.Manderfield LJ, High FA, Engelka KA, Liu F, Li L, Rentschler S, et al. Notch activation of Jagged1 contributes to the assembly of the arterial wall. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenway SC, Pereira AC, Lin JC, DePalma SR, Israel SJ, Mesquita SM, et al. De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nat Genet. 2009;41(8):931–5. doi: 10.1038/ng.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kola S, Koneti NR, Golla JP, Akka J, Gundimeda SD, Mundluru HP. Mutational analysis of JAG1 gene in non-syndromic tetralogy of Fallot children. Clin Chim Acta. 2011;412(23–24):2232–6. doi: 10.1016/j.cca.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Le Caignec C, Lefevre M, Schott JJ, Chaventre A, Gayet M, Calais C, et al. Familial deafness, congenital heart defects, and posterior embryotoxon caused by cysteine substitution in the first epidermal-growth-factor-like domain of jagged 1. Am J Hum Genet. 2002;71(1):180–6. doi: 10.1086/341327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–4. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 47.McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134(2):290–6. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 48.Riley MF, McBride KL, Cole SE. NOTCH1 missense alleles associated with left ventricular outflow tract defects exhibit impaired receptor processing and defective EMT. Biochim Biophys Acta. 2011;1812(1):121–9. doi: 10.1016/j.bbadis.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, et al. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18(1):99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacGrogan D, Luna-Zurita L, de la Pompa JL. Notch signaling in cardiac valve development and disease. Birth Defects Res A Clin Mol Teratol. 2011;91(6):449–59. doi: 10.1002/bdra.20815. [DOI] [PubMed] [Google Scholar]
- 51.Pourquie O. Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell. 2011;145(5):650–63. doi: 10.1016/j.cell.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunwoodie SL. The role of Notch in patterning the human vertebral column. Curr Opin Genet Dev. 2009;19(4):329–37. doi: 10.1016/j.gde.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Turnpenny PD, Alman B, Cornier AS, Giampietro PF, Offiah A, Tassy O, et al. Abnormal vertebral segmentation and the notch signaling pathway in man. Dev Dyn. 2007;236(6):1456–74. doi: 10.1002/dvdy.21182. [DOI] [PubMed] [Google Scholar]
- 54.Berdon WE, Lampl BS, Cornier AS, Ramirez N, Turnpenny PD, Vitale MG, et al. Clinical and radiological distinction between spondylothoracic dysostosis (Lavy-Moseley syndrome) and spondylocostal dysostosis (Jarcho-Levin syndrome) Pediatr Radiol. 2011;41(3):384–8. doi: 10.1007/s00247-010-1928-8. [DOI] [PubMed] [Google Scholar]
- 55.Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, Kageyama R. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001;15(20):2642–7. doi: 10.1101/gad.930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunwoodie SL, Clements M, Sparrow DB, Sa X, Conlon RA, Beddington RS. Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development. 2002;129(7):1795–806. doi: 10.1242/dev.129.7.1795. [DOI] [PubMed] [Google Scholar]
- 57.Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. Lunatic Fringe is an essential mediator of somite segmentation and patterning. Nature. 1998;394(6691):377–81. doi: 10.1038/28632. [DOI] [PubMed] [Google Scholar]
- 58.Saga Y, Hata N, Koseki H, Taketo MM. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997;11(14):1827–39. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- 59.Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, et al. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000;24(4):438–41. doi: 10.1038/74307. [DOI] [PubMed] [Google Scholar]
- 60.Turnpenny PD, Whittock N, Duncan J, Dunwoodie S, Kusumi K, Ellard S. Novel mutations in DLL3, a somitogenesis gene encoding a ligand for the Notch signalling pathway, cause a consistent pattern of abnormal vertebral segmentation in spondylocostal dysostosis. J Med Genet. 2003;40(5):333–9. doi: 10.1136/jmg.40.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ladi E, Nichols JT, Ge W, Miyamoto A, Yao C, Yang LT, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol. 2005;170(6):983–92. doi: 10.1083/jcb.200503113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet. 2010;20(5):905–16. doi: 10.1093/hmg/ddq529. [DOI] [PubMed] [Google Scholar]
- 63.Sparrow DB, Chapman G, Wouters MA, Whittock NV, Ellard S, Fatkin D, et al. Mutation of the Lunatic Fringe gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am J Hum Genet. 2006;78(1):28–37. doi: 10.1086/498879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whittock NV, Sparrow DB, Wouters MA, Sillence D, Ellard S, Dunwoodie SL, et al. Mutated MESP2 causes spondylocostal dysostosis in humans. Am J Hum Genet. 2004;74(6):1249–54. doi: 10.1086/421053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornier AS, Staehling-Hampton K, Delventhal KM, Saga Y, Caubet JF, Sasaki N, et al. Mutations in the MESP2 gene cause spondylothoracic dysostosis/Jarcho-Levin syndrome. Am J Hum Genet. 2008;82(6):1334–41. doi: 10.1016/j.ajhg.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kageyama R, Niwa Y, Shimojo H, Kobayashi T, Ohtsuka T. Ultradian oscillations in Notch signaling regulate dynamic biological events. Curr Top Dev Biol. 2010;92:311–31. doi: 10.1016/S0070-2153(10)92010-3. [DOI] [PubMed] [Google Scholar]
- 67.Sparrow DB, Guillen-Navarro E, Fatkin D, Dunwoodie SL. Mutation of Hairy-and-Enhancer-of-Split-7 in humans causes spondylocostal dysostosis. Hum Mol Genet. 2008;17(23):3761–6. doi: 10.1093/hmg/ddn272. [DOI] [PubMed] [Google Scholar]
- 68.Sparrow DB, Sillence D, Wouters MA, Turnpenny PD, Dunwoodie SL. Two novel missense mutations in Hairy-and-Enhancer-of-Split-7 in a family with spondylocostal dysostosis. Eur J Hum Genet. 2010;18(6):674–9. doi: 10.1038/ejhg.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morimoto M, Takahashi Y, Endo M, Saga Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature. 2005;435(7040):354–9. doi: 10.1038/nature03591. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki N, Kiso M, Kitagawa M, Saga Y. The repression of Notch signaling occurs via the destabilization of mastermind-like 1 by Mesp2 and is essential for somitogenesis. Development. 2011;138(1):55–64. doi: 10.1242/dev.055533. [DOI] [PubMed] [Google Scholar]
- 71.Feller J, Schneider A, Schuster-Gossler K, Gossler A. Noncyclic Notch activity in the presomitic mesoderm demonstrates uncoupling of somite compartmentalization and boundary formation. Genes Dev. 2008;22(16):2166–71. doi: 10.1101/gad.480408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gray MJ, Kim CA, Bertola DR, Arantes PR, Stewart H, Simpson MA, et al. Serpentine fibula polycystic kidney syndrome is part of the phenotypic spectrum of Hajdu-Cheney syndrome. Eur J Hum Genet. 2011;20(1):122–4. doi: 10.1038/ejhg.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Isidor B, Le Merrer M, Exner GU, Pichon O, Thierry G, Guiochon-Mantel A, et al. Serpentine fibula-polycystic kidney syndrome caused by truncating mutations in NOTCH2. Hum Mutat. 2011;32(11):1239–42. doi: 10.1002/humu.21563. [DOI] [PubMed] [Google Scholar]
- 74.Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011;43(4):303–5. doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- 75.Lee SY, Kumano K, Nakazaki K, Sanada M, Matsumoto A, Yamamoto G, et al. Gain-of-function mutations and copy number increases of Notch2 in diffuse large B-cell lymphoma. Cancer Sci. 2009;100(5):920–6. doi: 10.1111/j.1349-7006.2009.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiang MY, Xu ML, Histen G, Shestova O, Roy M, Nam Y, et al. Identification of a conserved negative regulatory sequence that influences the leukemogenic activity of NOTCH1. Mol Cell Biol. 2006;26(16):6261–71. doi: 10.1128/MCB.02478-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bales CB, Kamath BM, Munoz PS, Nguyen A, Piccoli DA, Spinner NB, et al. Pathologic lower extremity fractures in children with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2010;51(1):66–70. doi: 10.1097/MPG.0b013e3181cb9629. [DOI] [PMC free article] [PubMed] [Google Scholar]