Changes in Ca2+ homeostasis render oocytes competent to undergo [Ca2+]i oscillations and activation. During mouse oocyte maturation Ca2+ influx and SOCE are down-regulated, whereas [Ca2+]ER content increases. Bypassing the down-regulation of Ca2+ influx disturbs oocyte maturation.
Abstract
In preparation for fertilization, mammalian oocytes undergo optimization of the mechanisms that regulate calcium homeostasis. Among these changes is the increase in the content of the Ca2+ stores ([Ca2+]ER), a process that requires Ca2+ influx. Nevertheless, the mechanism(s) that mediates this influx remains obscure, although is known that [Ca2+]ER can regulate Ca2+ influx via store-operated Ca2+ entry (SOCE). We find that during maturation, as [Ca2+]ER increases, Ca2+ influx decreases. We demonstrate that mouse oocytes/eggs express the two molecular components of SOCE—stromal interaction molecule 1 (Stim1) and Orai1—and expression of human (h) Stim1 increases Ca2+ influx in a manner that recapitulates endogenous SOCE. We observe that the cellular distribution of hStim1 and hOrai1 during maturation undergoes sweeping changes that curtail their colocalization during the later stages of maturation. Coexpression of hStim1 and hOrai1 enhances influx throughout maturation but increases basal Ca2+ levels only in GV oocytes. Further, expression of a constitutive active form of hStim1 plus Orai1, which increases basal Ca2+ throughout maturation, disturbs resumption of meiosis. Taken together, our results demonstrate that Ca2+ influx and SOCE are regulated during maturation and that alteration of Ca2+ homeostasis undermines maturation in mouse oocytes.
INTRODUCTION
Changes in the intracellular concentration of free calcium ([Ca2+]i) represent an important signaling mechanism involved in a wide range of cellular events, including muscle contraction, secretion, neurotransmission, and cell death (Berridge et al., 2000). [Ca2+]i signaling also plays a dominant role during fertilization in all species examined (Stricker, 1999; Runft et al., 2002; Malcuit et al., 2006; Swann et al., 2006). In mammals, the sperm-induced [Ca2+]i signal adopts a pattern of brief [Ca2+]i rises interspersed among long intervals of basal concentration, which are referred to as [Ca2+]i oscillations. The oscillations are believed to be initiated by a sperm-specific phospholipase C (PLC), PLC zeta1 (ζ), after fusion of the gametes (Saunders et al., 2002). PLCζ is believed to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2), resulting in the production of 1,4,5-inositol-trisphosphate (IP3), the ligand for IP3R1, the Ca2+ channel located in the endoplasmic reticulum (ER), the egg's main Ca2+ store (Miyazaki et al., 1992; Miyazaki, 2006; Ducibella and Fissore, 2008). Activation of this pathway causes the initial intracellular Ca2+ release, but persistence of the oscillations requires Ca2+ influx, as without external Ca2+ ([Ca2+]e) only a few rises occur after sperm entry (Kline and Kline, 1992). Notwithstanding the importance of Ca2+ influx, the mechanisms that underlie it during mammalian fertilization are unknown.
Fertilization in most vertebrate species happens at the metaphase II stage of meiosis (MII), although changes in Ca2+ homeostasis that occur before this stage during maturation enable eggs to mount [Ca2+]i oscillations. Fully grown mammalian oocytes are arrested at the germinal vesicle stage (GV) and are endowed with Ca2+ stores ([Ca2+]ER) low in Ca2+ reserves. As maturation ensues after the luteinizing hormone surge, [Ca2+]ER increases steadily until the MII stage (Tombes et al., 1992; Jones et al., 1995), enhancing IP3R1-mediated Ca2+ release and promoting the acquisition of fertilization-like oscillations (Miyazaki et al., 1993; Mehlmann and Kline, 1994). The mechanism(s) that underlie this increase in [Ca2+]ER and the plasma membrane (PM) channels that underpin the Ca2+ influx are unknown, although insights may be gleaned from the spontaneous [Ca2+]i oscillations displayed by GV oocytes (Carroll and Swann, 1992; Carroll et al., 1994). These oscillations require Ca2+ influx and end approximately as the resumption of meiosis begins (Carroll and Swann, 1992), which is when the first increase in [Ca2+]ER content is noted (Jones et al., 1995). These results both predict an association between [Ca2+]ER content and Ca2+ influx, which is reminiscent of the mechanism believed to underlie store-operated Ca2+ entry (SOCE; Putney, 1986; Hoth and Penner, 1993) and active regulation of Ca2+ influx during maturation.
SOCE was first proposed as a means for cells to refill Ca2+ stores after Ca2+ release induced by agonist stimulation (Putney, 1986). Subsequent studies showed that depletion of [Ca2+]ER caused by inhibitors of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps such as thapsigargin (TG) also triggered Ca2+ influx, demonstrating the prevalence of SOCE in somatic cells (Takemura et al., 1989; Hoth and Penner, 1993; Zweifach and Lewis, 1993; Hsu et al., 2001). Subsequent electrophysiological studies revealed unique properties of this current, which was named ICRAC (Hoth and Penner, 1993; Zweifach and Lewis, 1993; Parekh et al., 1997; Putney, 2009). Although unknown for many years, the molecular effectors of SOCE have been identified, and two components, stromal interaction molecule 1 (Stim1), which acts as the ER Ca2+ sensor (Liou et al., 2005; Roos et al., 2005; Collins and Meyer, 2011), and Orai1, the PM channel that mediates Ca2+ influx upon Stim1-induced oligomerization (Feske et al., 2006; Vig et al., 2006), are believed to coordinate Ca2+ influx after Ca2+ release. The presence of SOCE has been well characterized during the early stages of maturation in Xenopus oocytes (Yu et al., 2009), and Ca2+ influx has been described in mammalian eggs during fertilization and after addition of TG (Kline and Kline, 1992; McGuinness et al., 1996; Mohri et al., 2001), although the mechanism(s) and molecules underlying this Ca2+ entry remain to be identified. Consistent with this, detection of transcripts for Stim1 and Orai1 has been reported in mammalian oocytes, although protein expression and cellular distribution during maturation require additional characterization (Gomez-Fernandez et al., 2009, 2012; Koh et al., 2009; Wang et al., 2012).
Although Ca2+ influx is required for [Ca2+]i oscillations at the GV stage, its contributions to the filling of [Ca2+]ER have not been carefully examined. Further, the role of [Ca2+]i changes during oocyte maturation is unclear. For instance, in mouse GV oocytes suppression of spontaneous oscillations with the Ca2+ chelator BAPTA-AM did not affect resumption of meiosis, although it caused cell cycle arrest at metaphase I (MI) of meiosis (Tombes et al., 1992). In porcine and bovine oocytes, addition of BAPTA-AM prevented resumption of meiosis, and removal of [Ca2+]e precluded progression of meiosis beyond the MI stage (Homa, 1991; Kaufman and Homa, 1993). Remarkably, in Xenopus oocytes, in which SOCE is inactivated during maturation (Machaca and Haun, 2000), increased Ca2+ influx by elevation of [Ca2+]e and persistent increase in [Ca2+]i delayed resumption of meiosis and caused spindle abnormalities (Sun and Machaca, 2004). Whether enhanced Ca2+ influx and persistent elevation of basal [Ca2+]i affect the initiation or progression of maturation in mammalian oocytes has not been examined.
In this study in mouse oocytes we investigate whether Ca2+ influx contributes to the filling of [Ca2+]ER during maturation and whether it is differentially regulated during this process. We also study whether SOCE contributes to this influx, as well as the expression of the molecular components of SOCE and their regulation during maturation. Finally, we evaluate the impact of persistently elevated [Ca2+]i on resumption and progression of meiosis.
RESULTS
Spontaneous Ca2+ influx is suppressed during maturation in parallel with the increase of ER Ca2+ store
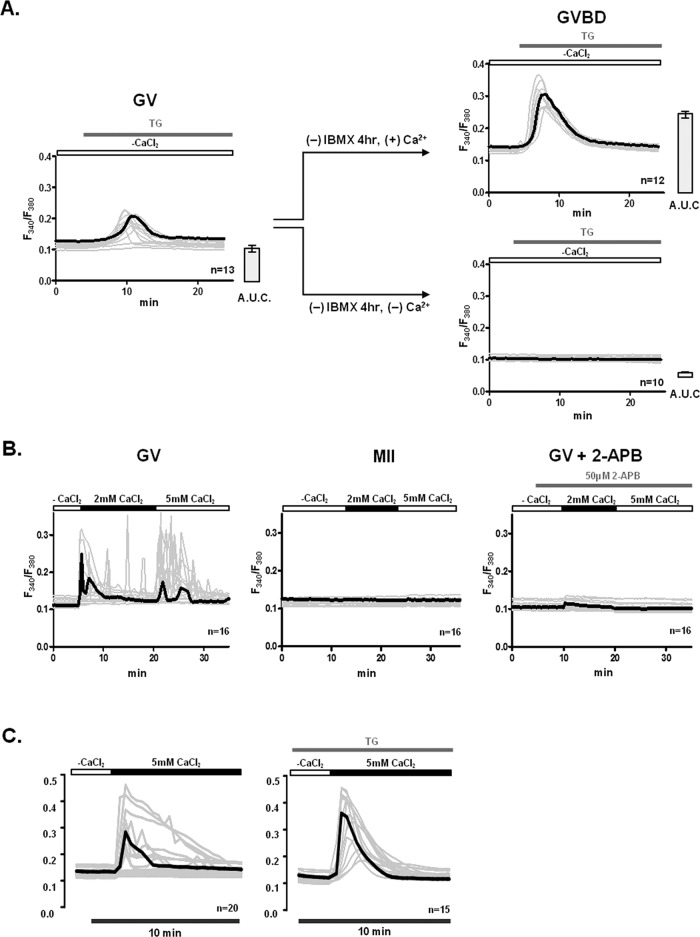
To gain insight into the mechanism and molecular effectors that mediate Ca2+ influx in mouse oocytes and eggs, we ascertained whether Ca2+ influx across the PM was required for the increase in [Ca2+]ER that occurs during the transition from the GV to the germinal vesicle breakdown (GVBD) stage (Jones et al., 1995). To accomplish this, GV oocytes were allowed to transition to the GVBD stage in the presence/absence of extracellular Ca2+ (1.7 mM). We estimated [Ca2+]ER levels after addition of TG 4 h after the removal of 3-isobutyl-1-methylxanthine (IBMX). In the presence of extracellular Ca2+, [Ca2+]ER increased significantly in GVBD oocytes (Figure 1A, top, left and right), although this was not the case when maturation was initiated in nominal Ca2+-free medium (Figure 1A, bottom, right). These results therefore demonstrate that [Ca2+]e is required for [Ca2+]ER increase during maturation.
FIGURE 1:
[Ca2+]e and Ca2+ influx are required to fill [Ca2+]ER in oocytes. The underlying Ca2+ influx mechanism(s) are inactivated during maturation and are sensitive to 2-APB and TG at the GV stage. (A) The contribution of extracellular Ca2+ to [Ca2+]ER content was estimated in GVBD-stage oocytes after culturing GV oocytes for 4 h in media supplemented with 1.7 mM CaCl2 or without supplementation, nominal Ca2+-free medium. Release of [Ca2+]ER was induced by addition of 10 μM TG. All [Ca2+]i responses are shown in the graphs, and the bold trace in each graph represents the mean response; bar graphs to the right of each Ca2+ panel denote mean ± SEM of [Ca2+]ER content estimated as area under the curve. (B) Spontaneous Ca2+ influx was measured in GV oocytes and MII eggs. Oocytes and eggs were placed in Ca2+-free conditions, after which 2 and 5 mM CaCl2 were successively added. Given that only GV oocytes showed Ca2+ influx, they were pretreated with 50 μM 2-APB for 5 min before addition of CaCl2 to prevent influx. (C) Ca2+ influx was promoted by addition of CaCl2 into GV oocytes with and without prior treatment with TG. GV oocytes were placed in nominal Ca2+-free media or exposed to 10 μM TG for 30 min in nominal Ca2+-free medium to deplete [Ca2+]ER, after which 5 mM CaCl2 was added. Representative traces are shown, and bold trace represents mean response.
We next examined whether the Ca2+ entry pathway(s) that mediate Ca2+ influx in GV oocytes are also functional in MII eggs, as both of these cellular stages are known to support oscillations. We incubated GV oocytes and MII eggs in nominal Ca2+-free media and soon thereafter sequentially added 2 and 5 mM CaCl2 while we monitored [Ca2+]i responses. Although most GV oocytes responded to the addition of CaCl2 by displaying a noticeable [Ca2+]i rise and in some cases oscillations (Figure 1B, left), MII eggs displayed no such changes (Figure 1B, middle). We next examined whether the Ca2+ entry at the GV stage could be abrogated by pretreatment with 50 μM 2-APB, a pharmacological agent that has been shown to regulate SOCE (DeHaven et al., 2008); addition of 2-APB prevented the increase in [Ca2+]i after the addition of either concentration of CaCl2 (Figure 1B, right).
The inhibitory effect of 2-APB and the low [Ca2+]ER levels in GV oocytes suggested that SOCE may be involved, at least in part, in mediating Ca2+ entry in GV oocytes. Accordingly, we investigated whether Ca2+ influx at the GV stage could be modified by exposure to TG before CaCl2 add back, a method commonly used to test SOCE in somatic cells (Bird et al., 2008; DeHaven et al., 2008). Although 3 of 20 untreated oocytes showed large and prolonged [Ca2+]i responses after addition of CaCl2, the majority of oocytes, 9 and 8 of 20, respectively, showed moderate or minor responses (Figure 1C, left). Conversely, TG-exposed oocytes uniformly responded to the addition of CaCl2 with robust [Ca2+]i responses (Figure 1C, right). Taken together, these results show that Ca2+ entry is functionally regulated during mouse oocyte maturation and that SOCE is one of the underlying mechanisms of Ca2+ influx at the GV stage.
[Ca2+]ER content and Ca2+ influx undergo distinct regulation during mouse oocyte maturation
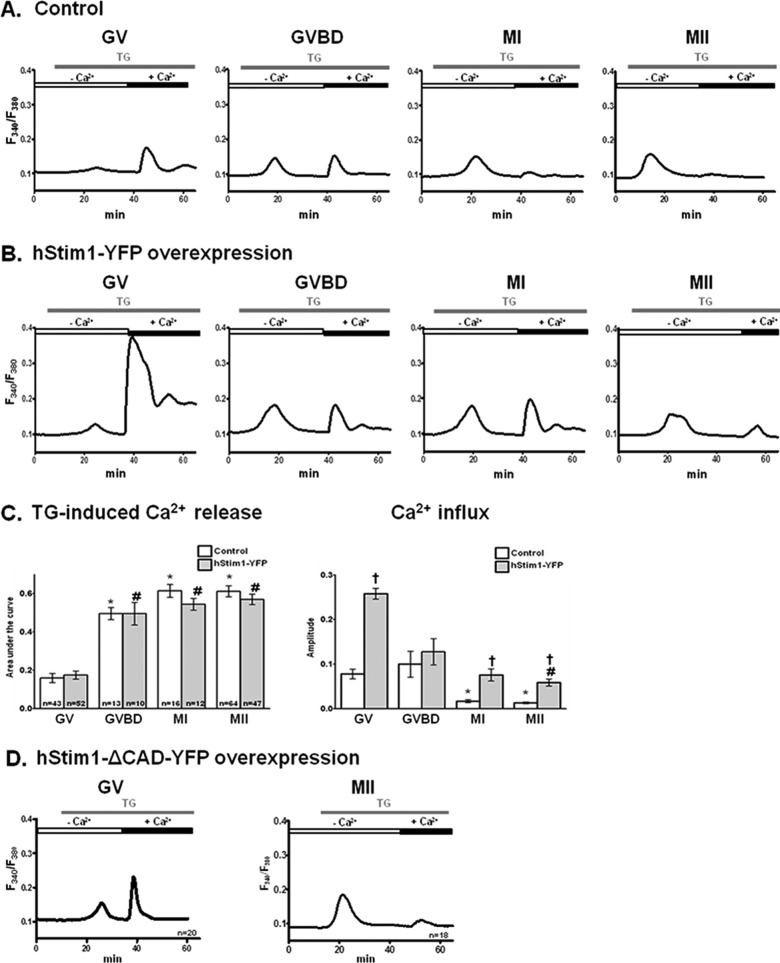
The previous findings led us to investigate the function of SOCE throughout oocyte maturation, using the TG and CaCl2 add-back method. Addition of TG, as expected, caused an increase in [Ca2+]i that became gradually higher as maturation progressed (Figure 2, A and C; p < 0.05), which is in agreement with previous reports (Kline and Kline, 1992; Jones et al., 1995) and suggests increasing [Ca2+]ER content with progression of maturation. In contrast, after CaCl2 addition, Ca2+ influx progressively decreased during maturation, with GV- and GVBD-stage oocytes displaying greater Ca2+ influx than MI and MII eggs (Figure 2, A and C; p < 0.05), demonstrating gradual inactivation of Ca2+ entry with progression of maturation.
FIGURE 2:
[Ca2+]ER content increases, whereas Ca2+ influx induced by TG, SOCE, decreases during oocyte maturation. [Ca2+]ER content was estimated from the [Ca2+]i responses caused by addition of 10 μM TG in oocytes incubated in Ca2+-free medium, and Ca2+ influx was estimated by the [Ca2+]i rise generated by the addition of 5 mM CaCl2 soon after the TG-induced [Ca2+]i rise had subsided. Representative [Ca2+]i traces are shown for control oocytes (A) or for oocytes expressing hStim1-YFP (B). (C) TG- and CaCl2-induced [Ca2+]i changes were quantified, and data are presented in bar graphs as mean ± SEM. Control and hStim1-YFP–expressing oocytes are displayed in open and gray columns, respectively, and the number of oocytes evaluated is shown within each bar. *,#Stages significantly different from the GV-stage values within treatment group. †Significant differences within the same meiotic stage but between treatments (p < 0.05). (D) TG-induced [Ca2+]i responses and subsequent Ca2+ influx in oocytes and eggs expressing hStim1-ΔCAD-YFP, an Stim1 variant incapable of interacting with Orai1.
The inverse relationship between [Ca2+]ER content and Ca2+ entry during maturation indicates the participation of SOCE as one of the mechanisms involved in Ca2+ homeostasis in mouse oocytes. Remarkably, our results suggest that SOCE is progressively disabled during maturation. To extend these observations, we injected GV-stage oocytes with hStim1–yellow fluorescent protein (YFP) cRNA, the Ca2+ sensor component of SOCE, and examined the effects of such injections on [Ca2+]ER and Ca2+ influx during different stages of maturation. hStim1 and mStim1 are highly homologous, sharing ∼97% of the amino acids at the whole-protein level and 99% of the amino acids in the CRAC-activating domain (CAD), which is the domain that directly interacts with Orai1 (Park et al., 2009). Therefore hStim1-YFP overexpression was expected to mimic mStim1 function. Expression of hStim1-YFP did not affect [Ca2+]ER content throughout maturation (Figure 2, B and C, left), at least as estimated by our approach, but clearly enhanced Ca2+ influx in all stages of maturation, especially in GV oocytes (Figure 2, B and C, right; p < 0.05). To demonstrate that the enhanced influx was due to interaction of hStim1 with endogenous Orai1, we injected oocytes with hStim1-ΔCAD-YFP mRNA, which cannot activate Orai1 (Park et al., 2009); overexpression of hStim1-ΔCAD-YFP failed to enhance Ca2+ influx at any stage of maturation (Figure 2D). Collectively the data suggest that hStim1-YFP overexpression broadly recapitulates the regulation of [Ca2+]ER and Ca2+ influx observed in mouse oocytes, suggesting that SOCE is operational during mouse oocyte maturation, although it is seemingly inactivated at the most advanced stages of meiosis.
Stim1 and Orai1 are expressed in mouse oocytes
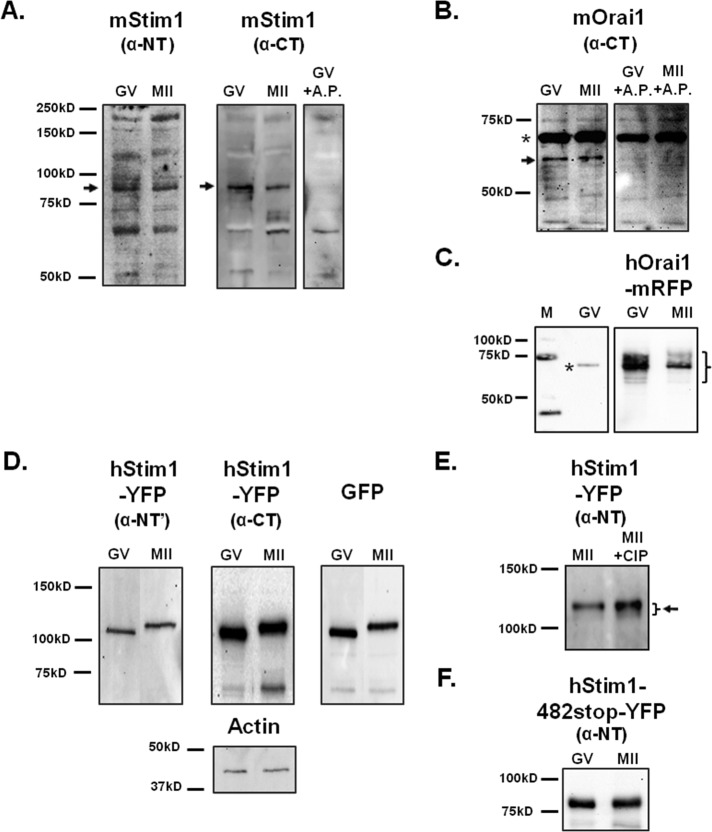
We next examined in mouse oocytes/eggs the expression of the two molecular effectors of SOCE—Stim1 and Orai1. Two different anti-Stim1 antibodies were used for Western blotting, and both antibodies detected a band of ∼90 kDa in GV and MII stages (Figure 3A, left and center), which is the reported molecular weight (MW) of Stim1 (Darbellay et al., 2011); the observed reactivity was likely Stim1’s, as preincubation of the anti–C-terminus antibody with an antigenic peptide specifically obliterated recognition of this band (Figure 3A, right). Expression of Orai1 was also detected in mouse eggs (Figure 3B, left) with an approximate MW of 56 kDa, which, although higher than predicted for the native protein, is consistent with the MW of the protein in some mammalian tissues (Balghi et al., 2011); Orai1 has been shown to be glycosylated in somatic cells (Prakriya et al., 2006; Gwack et al., 2007). Preincubation of the antibody with its antigenic peptide abrogated the reactivity of the 56 kDa band but not that of a prominent higher, nonspecific band (Figure 3B, right; asterisk), confirming the specificity of the antibody. Further, the same antibody detected expression of hOrai1–monomeric red fluorescent protein (mRFP) in oocytes and eggs, where it detected several polypeptides ranging in MW from ∼52 to 82 kDa, likely the reflection of various degrees of glycosylation (Figure 3C).
FIGURE 3:
Mouse oocytes express Stim1 and Orai1. (A, B) The expression of Stim1 and Orai1 molecules was probed using lysates of GV oocytes (n = 100 and 200, respectively) and MII (n = 100 and 200, respectively) eggs and specific antibodies. (A) Left and middle, black arrows point to the band corresponding to Stim1. An antigenic peptide was used to confirm the specificity of the anti–C-terminus Stim1 antibody. (B) Right, black arrow points to the band that represents Orai1. The upper band of ∼68 kDa marked with an asterisk is believed to be nonspecific reactivity, as it was not abolished by pretreatment with the antigenic peptide. (C) hOrai1-mRFP expression (n = 16 GV oocytes/MII eggs) was analyzed in overexpressing cells using the same antibody. (D) Heterologous expression of hStim1-YFP was demonstrated in lysates of oocytes/eggs (n = 45) using the anti–N-terminus and anti–C-terminus Stim1 antibodies and an anti-GFP antibody. The actin protein was probed as a loading control (bottom, middle). (E) To examine the possible phosphorylation of hStim1-YFP in mouse oocytes, lysates of hStim1-YFP–expressing MII oocytes (n = 18) were incubated with or without calf intestine phosphatase and then immunoblotted with anti-Stim1 antibody (right). The presence of hStim1 is denoted by an arrow, and the bracket denotes the presence of polypeptides of different MWs. (F) hStim1–482-stop-YFP–overexpressing GV and MII cells (n = 18) were probed with the anti–N-terminus Stim1 antibody, and blots are shown.
To confirm the functional results obtained by injection of hStim1-YFP cRNA, we examined the expression of hStim1-YFP in GV and MII oocytes. Both anti-Stim1 antibodies recognized a band at ∼105 kDa, which is consistent with the MW of hStim1 and the added MW of YFP (Figure 3D, left and middle); an anti-YFP antibody recognized the same bands (Figure 3D, right). Actin was used as loading control, and Western blotting against it revealed approximate equal loading of the samples (Figure 3D, bottom, middle). In all cases, hStim1-YFP migration was more retarded in MII eggs than in GV oocytes (Figure 3D), suggesting phosphorylation of hStim1-YFP, as reportedly occurs in Xenopus oocytes and in mammalian somatic cells (Smyth et al., 2009; Yu et al., 2009). To ascertain whether this was also the case in our system, we injected hStim1-YPF cRNAs into GV oocytes; these cells matured to the MII stage, at which time lysates were prepared and either left untreated or treated with alkaline phosphatase (AP) to induce widespread dephosphorylation. Compared to untreated controls, the AP-treated hStim1-YPF displayed a smeared migration, which suggests different degrees of phosphorylation, and higher reactivity (Figure 3E), which is possibly due to better antibody recognition, as the antibody's epitope falls within this domain. To confirm this observation, we injected hStim1-482stop-YFP cRNA, which encodes for a protein that lacks all the C-term M-phase kinase phosphorylation sites (Smyth et al., 2009); hStim1-482stop did not undergo a mobility shift during maturation (Figure 3F). Collectively our data show that the components of SOCE are expressed in mouse oocytes/eggs and that during maturation hStim1 undergoes phosphorylation.
hStim1-YFP and hOrai1-mRFP undergo changes in distribution during oocyte maturation
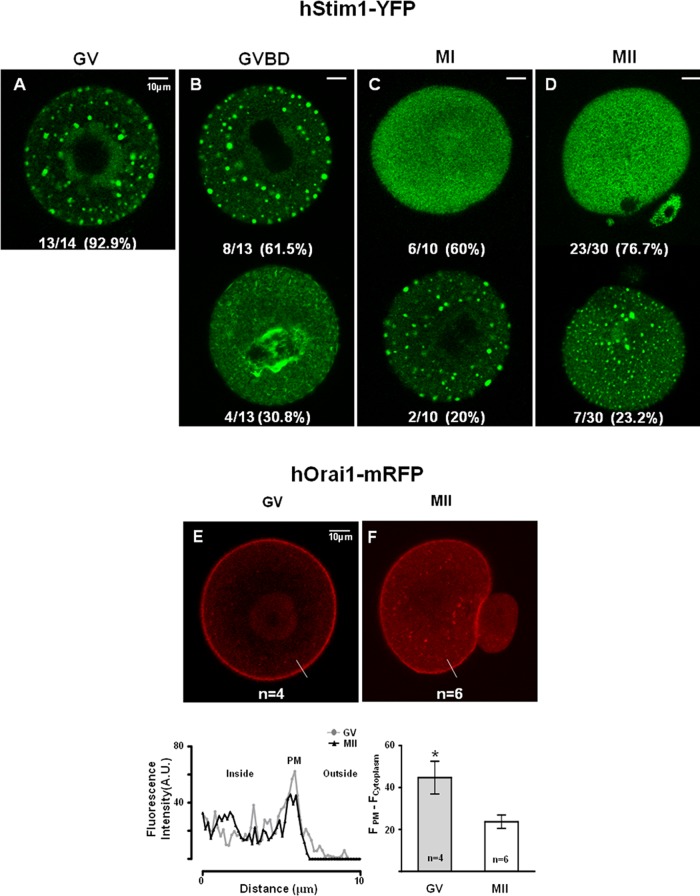
Such findings led us to examine whether the decline in Ca2+ influx during maturation coincided with changes in the cellular distribution of Stim1 and Orai1. To follow their distribution, we injected oocytes with hStim1-YFP or hOrai1-mRFP mRNAs. All oocytes were injected at the GV stage and remained at this stage in media supplemented with IBMX for variable times, so that by 20 h postinjection all stages of maturation could be simultaneously examined. hStim1-YFP underwent marked changes in distribution with progression of meiosis. For example, at the GV stage, hStim1-YFP displayed a “patched” distribution, with these patches spread throughout the cell (Figure 4A). In GVBD oocytes, ∼4 h after removal of IBMX, large internal patches were still observed in most oocytes (Figure 4B, top), but in ∼30% of the cells the distribution of hStim1 became more diffuse, although some patches remained around the spindle area (Figure 4B, bottom). As maturation progressed, the distribution of hStim1 became more disperse and acquired a pattern consistent with its ER localization (Figure 4, C and D, top), although a small number of MI and MII oocytes still showed internal patches of smaller size (Figure 4, C and D, bottom); ER distribution was confirmed by injection of ds-Red ER cRNA (data not shown).
FIGURE 4:
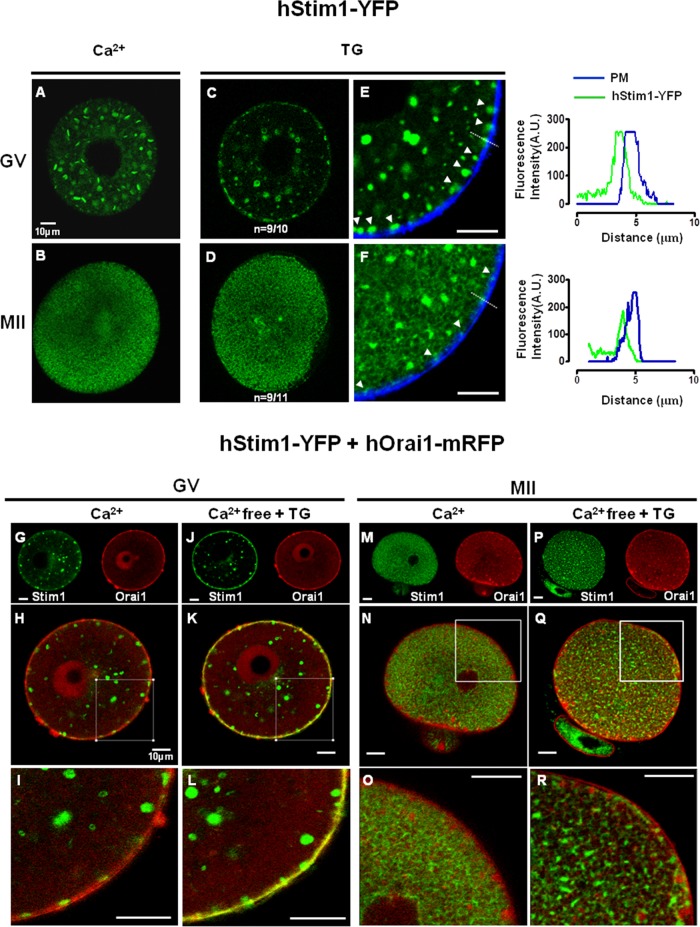
The distribution and organization of hStim1-YFP and hOrai1-mRFP change during oocyte maturation. (A–D) The distribution of hStim1-YFP was examined using confocal microscopy from images taken at the equatorial plane of live hStim1-YFP–expressing oocytes. The number of oocytes examined at each stage is shown at the bottom of each representative image. hStim1-YFP displayed two distinct patterns of organization in all stages of maturation except the GV stage; the most representative configurations are shown, along with the proportion of oocytes/eggs exhibiting the particular pattern. (E, F) The distribution of hOrai1 was examined as in the foregoing in GV and MII oocytes expressing hOrai1-mRFP. Intensity profiles of the line scans drawn in oocytes and eggs are shown below E, and a bar graph displaying the relative intensity of Orai1 signal between PM and cytoplasm is shown below F. Scale bar, 10 μm.
The distribution of hOrai1-mRFP also changed during maturation (Figure 4, E and F). For example, at the GV stage, hOrai1-mRFP was highly enriched at the PM, where it formed a near-perfect ring around the cell (Figure 4E), whereas at the MII stage, even though hOrai1-mRFP was still present there, its presence was weaker (Figure 4F); this apparent reduction of hOrai1-mRFP at the PM was accompanied by increased fluorescence in the subcortical area (Figure 4F; line and bar graphs below figures), suggesting recycling of the protein, as reported in Xenopus oocytes (Yu et al., 2009, 2010). Collectively these results suggest that the molecular components of SOCE undergo cellular redistribution during maturation.
hStim1 puncta formation and colocalization with hOrai1 decrease during oocyte maturation
After depletion of [Ca2+]ER, Stim1 undergoes oligomerization and migration to the cell cortex, nearly reaching the PM, where these aggregates, also known as “puncta,” recruit and gate Orai1 (Penna et al., 2008; Park et al., 2009). To assess whether the ability of Stim1 to undergo puncta formation and migration to the cortex changed during oocyte maturation, we treated hStim1-YFP–expressing GV and MII oocytes with TG and observed hStim1-YFP reorganization by confocal microscopy. To estimate the proximity of hStim1-YFP puncta to the PM, we used a diffusible dye, wheat germ agglutinin–Alexa 633, to stain the PM. After treatment with TG, hStim1 in GV oocytes readily formed distinct puncta that aligned along the PM (Figure 5, C and E), whereas this ability was severely reduced at the MII stage, along with the size of the puncta (Figure 5, D and F); the line graph to the right of Figure 5, E and F, shows the reduced intensity of the puncta in MII eggs. It is worth noting that even after TG, hStim1-YFP in MII eggs displayed a reticular organization, which was not observed in GV oocytes.
FIGURE 5:
hStim1 puncta formation and colocalization with hOrai1 decreases during oocyte maturation. (A, B) Under control conditions, hStim1-YFP forms internal aggregates in GV oocytes, whereas in MII eggs it acquires a more homogenous, ER-like organization. After depletion of [Ca2+]ER, hStim1-YFP undergoes “puncta” formation in GV oocytes (C), whereas hStim1-YFP distribution is hardly changed in MII oocytes (D). Arrowheads point to hStim1-YPF puncta (E, F), and line graphs depicting the fluorescence intensity of these puncta, marked with a broken line, are shown to the right of E and F. (E, F) The proximity of the hStim1-YFP puncta/aggregates to the PM was estimated by staining the PM with wheat germ agglutinin–Alexa 664. hStim1-YPF puncta are bigger and more numerous in GV oocytes than in MII eggs. (G–R) Confocal images before and after TG treatment of GV oocytes (G–L) and MII eggs (M–R) expressing hStim1-YFP+hOrai1-mRFP. The same oocytes/eggs were imaged before and after TG. Top, separate fluorescent channels; middle, merged images; bottom, amplified regions of these are. hStim1 and hOrai1 display extensive overlap after the addition of TG in GV oocytes (K, L), but the overlap is negligible at the MII stage (Q, R). Scale bar, 10 μm.
Research in somatic cells shows that Stim1 and Orai1 directly interact at the PM (Penna et al., 2008; Park et al., 2009). Using simultaneous expression of hStim1-YFP and hOrai1-mRFP mRNAs and confocal microscopy, we examined whether the distribution of these molecules overlapped and, if so, whether this property changed during maturation. Under resting conditions, hStim1 and hOrai1 showed their expected distributions in GV oocytes and MII eggs (Figure 5, G–I and M–O, respectively), and in GV oocytes some overlap between the molecules was noticeable (Figure 5, H and I). After addition of TG, hStim1 and hOrai1 showed extensive colocalization at the GV stage (Figure 5, K and L), although this was not evident in MII eggs (Figure 5, Q and R). Collectively the data show that the organization of hStim1 and hOrai1 follows distinct but parallel redistribution during oocyte maturation, which temporally coincides with the decline in Ca2+ influx during this process.
Coexpression of SOCE components enhances TG-induced Ca2+ influx at all stages of oocyte maturation
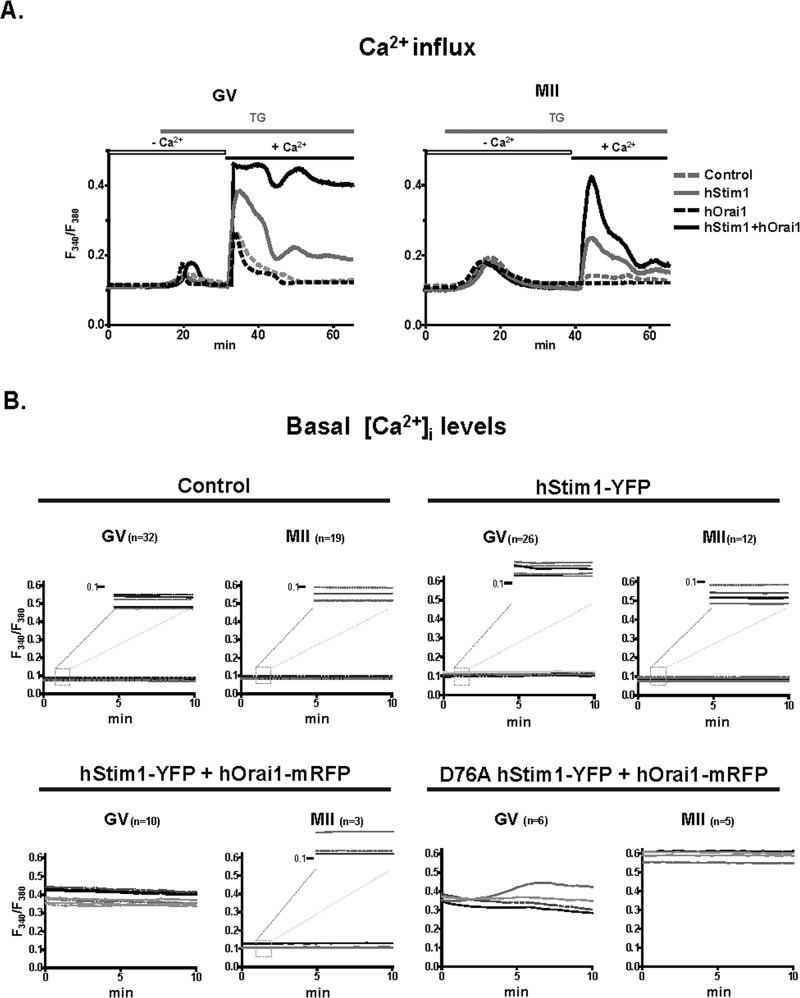
The diminished colocalization of hStim1 and hOrai1 in MII eggs after depletion of Ca2+ stores led us to examine whether Ca2+ influx was reduced at this stage. We expressed either component of SOCE alone or in combination and compared [Ca2+]i responses in GV and MII stages using the TG and CaCl2 add-back method. As shown in Figure 6A, expression of hStim1 alone enhanced influx in both stages of maturation but to a greater extent in GV than in MII, which is consistent with our previous results. Expression of hOrai1, on the other hand, failed to modify influx at either stage, whereas hStim1 and hOrai1 coexpression increased Ca2+ influx in both stages, although the [Ca2+]i rise was considerably smaller in MII eggs, consistent with the reduced colocalization of hStim1 and hOrai1 at this stage.
FIGURE 6:
Overexpression of SOCE components alters Ca2+ homeostasis. (A) Expression of hStim1-YFP+hOrai1-mRFP increased Ca2+ influx in both GV and MII cells after TG and CaCl2 add-back, although the increase was greater and more prolonged in GV oocytes. Expression of hStim1-YFP also increased Ca2+ influx in both GV and MII stages but to a lesser extent, whereas hOrai1 had no effect. (B) Expression of hStim1 or hStim1+hOrai1 differentially increased basal [Ca2+]i during maturation. Baseline [Ca2+]i traces in control (top, left), hStim1-YFP–expressing (top, right), hStim1-YFP+hOrai1-mRFP–expressing (bottom, left), and D76A hStim1-YFP+ hOrai1-mRFP–expressing (bottom, right) oocytes. Insets in each treatment depict a magnified version of the y-axis at the 0.1 mark so that minor differences in basal [Ca2+]i between GV and MII stages can be appreciated for some of the treatments.
Expression of SOCE components alters basal Ca2+ homeostasis during oocyte maturation
We next examined whether expression/coexpression of the SOCE components changed basal [Ca2+]i levels under natural regular conditions, that is, without emptying the stores and under normal external Ca2+ concentration, ∼1.7 mM CaCl2. In control GV oocytes, basal [Ca2+]i remained steady, as the fluorescence ratio of ∼0.1 remained unchanged until the MII stage (Figure 6B, top, left). Expression of hStim1 increased baseline [Ca2+]i in GV oocytes (p < 0.05), although by the MII stage basal [Ca2+]i had returned to levels that were indistinguishable from those of noninjected controls (Figure 6B, top, right). Coexpression of hStim1 and hOrai1 dramatically increased basal [Ca2+]i at the GV stage (p < 0.05), and although basal [Ca2+]i levels were reduced by the MII stage, they remained higher than for controls (Figure 6B, bottom, left). Taken together, these results demonstrate that spontaneous Ca2+ influx is differentially regulated during mouse oocyte maturation and is greatest at the GV stage, consistent with the presence of spontaneous oscillations at this stage (Carroll and Swann, 1992).
To ascertain whether the downturn of Ca2+ influx was likely due to increasing levels of [Ca2+]ER, we expressed an EF-hStim1 mutant, D76A-hStim1, which is insensitive to [Ca2+]ER levels (Liou et al., 2005). Coexpression of D76A-hStim1+hOrai1 increased basal [Ca2+]i in GV oocytes, but, unlike the case for previous treatments, basal [Ca2+]i did not decrease, but effectively increased, as oocytes progressed to the MII stage (p < 0.05; Figure 6B, bottom, right).
Regulation of Ca2+ influx is required to complete oocyte maturation
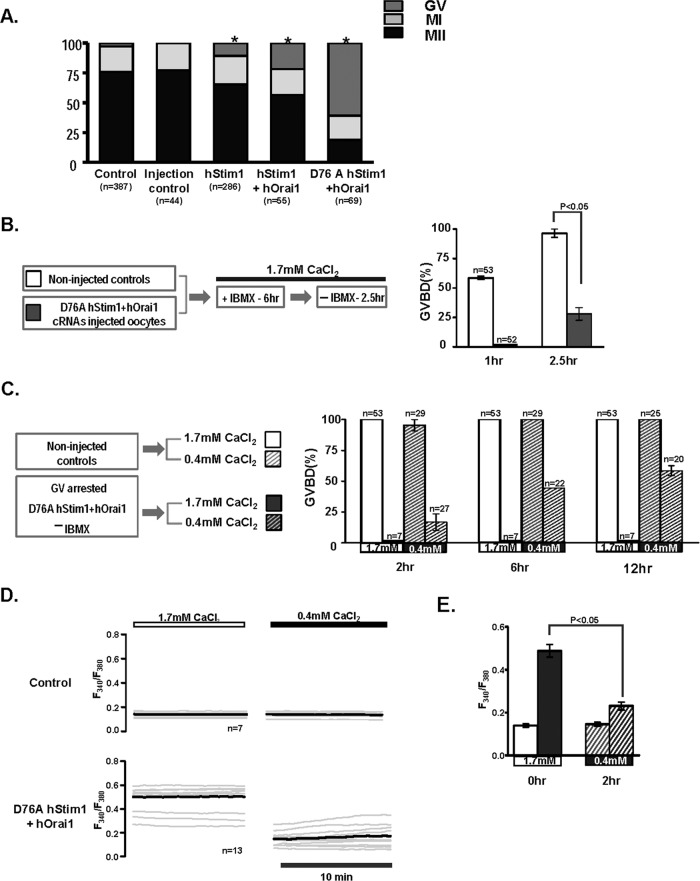
We then examined whether the distinct basal [Ca2+]i profiles generated by expression of one or both of the SOCE components had differential effects on the rates of in vitro maturation. To accomplish this, we momentarily maintained control GV oocytes or oocytes injected with the selected cRNAs at the GV stage to allow for protein translation, after which in vitro maturation proceeded for 14–16 h. The majority of control oocytes, ∼80%, resumed meiosis and reached the MII stage (Figure 7A), although oocytes expressing hStim1 or hStim1+hOrai1, which displayed elevated basal [Ca2+]i at the GV stage, showed reduced rates of maturation, with more oocytes remaining arrested at the GV stage (p < 0.05). Remarkably, oocytes expressing D76A hStim1+hOrai1, which displayed persistent elevation of basal [Ca2+]i, failed to consistently resume meiosis (Figure 7A).
FIGURE 7:
Changes in Ca2+ homeostasis affect resumption of meiosis and oocyte maturation. (A) Control, sham-injected, and oocytes expressing hStim1-YFP, hStim1-YFP+hOrai1-mRFP, or D76A Stim1-YFP+hOrai1-mRFP were in vitro matured and their maturation rates assessed. Expression of any mRNAs that increased basal [Ca2+]i even transiently caused a reduction in maturation rates, although expression D76A hStim1-YFP+hOrai1-mRFP nearly completely prevented resumption of meiosis. Asterisks above bars represent treatments that significantly reduced GVBD rates (p < 0.05). (B) The effect of coexpression of D76A hStim1-YFP + hOrai1-mRFP on meiotic resumption was investigated as depicted in the flow chart (left), and data are summarized in the bar graph (right). Expression of D76A hStim1-YFP+hOrai1-mRFP in GV oocytes delayed and mostly prevented GVBD under normal [Ca2+]e (p < 0.05). (C) As before, but selected GV-arrested oocytes were transferred to low-[Ca2+]e medium (0.4 mM), which partly rescued the arrest caused by expression of D76A hStim1-YFP+hOrai1-mRFP. (D) Ca2+ traces depicting the effect of lowering [Ca2+]e on basal [Ca2+]i in control and GV-arrested D76A hStim1+hOrai1–expressing oocytes monitored before and 2 h after lowering [Ca2+]e from 1.7 to 0.4 mM; the bold trace represents the mean of the responses. (E) A bar graph was used to summarize in the same group of oocytes the mean ± SEM change in basal [Ca2+]i caused by lowering external [Ca2+]e; p < 0.05.
To examine whether the excessive Ca2+ influx caused by coexpression of D76A hStim1+ hOrai1 was responsible for the GV arrest, we repeated the foregoing experiment but 2.5 h after release from IBMX oocytes were transferred to medium containing 0.4 mM CaCl2. Under normal [Ca2+]e, as expected, all control oocytes reached the GVBD stage by 2.5 h, although only ∼25% of oocytes expressing D76AhStim1+hOrai1 did (Figure 7B; p < 0.05). Subsequently, all D76A-hStim1+hOrai1–expressing oocytes maintained in normal [Ca2+]e-containing media remained arrested at the GV stage, whereas cohort oocytes transferred to 0.4 mM CaCl2-containing media progressively underwent GVBD, and by 12 h, ∼50% had undergone GVBD (Figure 7C); progression of maturation in control oocytes was not affected by 0.4 mM CaCl2 media (Figure 7C). We then examined whether moving oocytes to 0.4 mM CaCl2 media altered basal [Ca2+]i. As shown in Figure 7, D and E, 2 h after changing media oocytes expressing D76A hStim1+hOrai1 displayed a significantly lower basal [Ca2+]i (p < 0.05), whereas control oocytes were not affected by the switch. Taken together, these results suggest that regulation of Ca2+ influx and Ca2+ homeostasis play a role during normal progression of meiosis in mouse oocytes.
DISCUSSION
In this study we examined how [Ca2+]e and Ca2+ influx contribute to [Ca2+]ER content during mouse oocyte maturation. We also investigated the presence and function of SOCE and the effect of Ca2+ influx misregulation on oocyte maturation. We found that whereas [Ca2+]ER levels increase during maturation, Ca2+ entry declines. We detected expression of Stim1 and Orai1 in oocytes and eggs, as well as a change in their distribution such that hStim1-YFP and hOrai1-mRFP only extensively overlapped in GV oocytes after addition of TG. Finally, expression of hStim1+hOrai1 increased basal [Ca2+]i in GV oocytes but not in MII eggs and persistently elevated basal [Ca2+]i compromised oocyte maturation. In total, our studies demonstrate that Ca2+ influx is closely regulated during oocyte maturation and that alteration of Ca2+ homeostasis undermines the completion of maturation.
[Ca2+]ER, [Ca2+]i influx, and SOCE during mouse oocyte maturation
Although [Ca2+]ER levels have been known to increase during mouse oocyte maturation, the source of Ca2+ and the Ca2+ influx mechanism(s) underlying this increase have not been established. Here we show that [Ca2+]e is required for the increase in [Ca2+]ER and that as [Ca2+]ER increases during maturation, spontaneous or TG-induced Ca2+ influx decreases; Ca2+ influx is lowest at the MII stage, which is when [Ca2+]ER is greatest. This relationship between [Ca2+]ER and Ca2+ influx, which is especially evident at the GV and GVBD stages, suggests participation of SOCE and is reminiscent of data for Xenopus, in which SOCE-mediated Ca2+ entry is abruptly inactivated at the GVBD stage (Machaca and Haun, 2000, 2002). Our results, unlike those for Xenopus, show that SOCE is only partly inactivated by the GVBD stage and can contribute to the increase in [Ca2+]ER during this period. Our results differ from other studies in the mouse showing progressively enhanced SOCE activity during maturation (Gomez-Fernandez et al., 2009, 2012).
Several additional findings in our study suggest a role for SOCE during mouse oocyte maturation. For example, addition of 2-APB, a broad-spectrum SOCE inhibitor (DeHaven et al., 2008; Putney, 2010), abrogated Ca2+ influx in GV oocytes. Moreover, both Stim1 and Orai1 were produced by oocytes throughout maturation, and expression of functional hStim1 caused enhanced Ca2+ influx, whereas an inactive form, hStim1-ΔCAD-YFP, failed to promote Ca2+ influx. In spite of such findings indicating a role of SOCE during oocyte maturation, the progressive inactivation of SOCE during maturation raises questions regarding its contribution to Ca2+ influx during fertilization. Recent research in mouse eggs suggests a minor role for SOCE during fertilization, for, although it was functional in MII eggs, abrogating its function by pharmacological and molecular means failed to modify sperm-initiated oscillations (Miao et al., 2012; Takahashi et al., 2013). Of importance, in porcine oocytes, inactivation of SOCE by small interfering RNA against Stim1 and Orai1 inhibited persistent fertilization-associated oscillations (Lee et al., 2012; Wang et al., 2012). Taken together, these results suggest that in mammals different Ca2+ influx mechanisms regulate [Ca2+]ER during maturation and Ca2+ influx during fertilization, and future studies should identify these channels and their regulation.
Reorganization of hStim1 and hOrai1 during oocyte maturation
The inactivation of spontaneous and SOCE-mediated Ca2+ influx led us to examine whether changes in the distribution of Stim1 and Orai1 could be undermining Ca2+ influx. Given the inconsistent results obtained using immunofluorescence to localize endogenous Stim1 (data not shown), we used corresponding mRNAs also encoding fluorescent proteins. Marked changes in the organization of these proteins began around GVBD. For example, in GV oocytes internal “patches” and peri-GV accumulation highlighted the widespread distribution of hStim1, but after GVBD and for the rest of maturation, hStim1 distribution became diffuse and acquired a reticular, ER-like pattern consistent with its localization. Further, addition of TG, which in GV oocytes caused hStim1 to diffuse to the cortex and form distinct puncta, hardly changed the distribution of hStim1 in eggs. The distribution of Orai1 also changed, as in GV oocytes it was primarily present on the PM, but by MII its PM presence was reduced and its signal in the ooplasm increased. In agreement with these changes, after addition of TG, hStim1 and hOrai1 only colocalized on the PM of GV oocytes. Our results are in agreement with those in Xenopus oocytes, where inactivation of SOCE during maturation was associated with inhibition of Stim1 oligomerization/puncta formation and Orai1 internalization (Yu et al., 2009, 2010). Nevertheless, our results show that hStim1 retained certain clustering capacity and Orai1 internalization was incomplete in MII eggs, as even at this stage expression of hStim1+hOrai1 enhanced SOCE activity. Our results also concur with data in somatic cells, where SOCE is down-regulated at mitosis (Russa et al., 2008; Smyth et al., 2009) and this change was associated with reduced ability of Stim1 to form puncta (Smyth et al., 2009). Data from studies in mammalian oocytes also support our findings. For example, after depletion of [Ca2+]ER Stim1 displayed some degree of oligomerization in porcine MII eggs (Koh et al., 2009), and in mouse eggs Stim1 showed conspicuous clusters/puncta organization, although without emptying of the stores, and Orai1 distribution was mostly concentrated at the PM (Gomez-Fernandez et al., 2012).
We did not investigate the mechanisms that regulate Stim1 reorganization in oocytes. Nevertheless, Stim1 was originally described as a phosphoprotein (Manji et al., 2000), and phosphorylation of its S/T-P consensus sites by M-phase kinases was demonstrated in somatic cells (Manji et al., 2000; Smyth et al., 2009; Pozo-Guisado et al., 2010) and Xenopus oocytes (Yu et al., 2009). We found that exogenous hStim1 was phosphorylated in MII eggs, as it experienced a mobility shift that was reduced by phosphatase treatment. We did not observe similar changes in the endogenous protein, although the comparable temporal inactivation of TG-induced Ca2+ influx between uninjected and hStim1 mRNA–injected oocytes suggest common regulatory mechanisms.
Ca2+ influx and progression of oocyte maturation
To gain further insight into the mechanisms underlying the brief presence of [Ca2+]i oscillations in GV stage mouse oocytes (Carroll and Swann, 1992; Carroll et al., 1994), we expressed, singly or together, hStim1 and hOrai1 mRNAs and monitored their effects on basal [Ca2+]i in oocytes and eggs. We found that their combined expression markedly and persistently increased basal [Ca2+]i in GV oocytes, although by the MII stage, basal [Ca2+]i levels had returned to near-normal levels. These results suggest close regulation of Ca2+ homeostasis/influx during the early stages of maturation.
To better understand the role of basal [Ca2+]i and Ca2+ influx on oocyte maturation, we coexpressed D76A hStim1+hOrai1 mRNAs, which increased basal [Ca2+]i throughout maturation. Under these conditions, progression of maturation was greatly reduced, with most oocytes remaining at the GV stage. These effects were due to the inability to inactivate Ca2+ influx and decrease basal [Ca2+]i, as lowering external [Ca2+]e reduced [Ca2+]i and rescued the ability of these oocytes to undergo GVBD. Similar detrimental effects of elevated basal [Ca2+]i were observed in Xenopus oocytes, where enhanced Ca2+ influx promoted throughout maturation hindered the progression of meiosis (Sun and Machaca, 2004). Therefore it appears that regulation of Ca2+ influx is required for normal progression of oocyte maturation in the mouse, although we did not explore the functional mechanism(s) positively affected by the progressive inactivation of Ca2+ entry.
We propose a model in which mouse GV oocytes exhibit a low but persistent Ca2+ influx that contributes to the GV's overall cellular metabolism. On resumption of meiosis, Ca2+ influx is progressively inactivated to allow for normal progression of meiosis, spindle organization, and MII arrest. Future studies in mammalian oocytes should elucidate the mechanism(s) responsible for the inactivation of Ca2+ influx and the identity of the channel(s) that mediate Ca2+ entry during maturation and fertilization.
MATERIALS AND METHODS
Collection and preparation of mouse oocytes
Fully grown GV-stage oocytes were collected from the ovaries of 6- to 10-wk-old CD-1 female mice 44–46 h after injection of 5 IU of pregnant mare serum gonadotropin (PMSG; Sigma-Aldrich, St. Louis, MO; all chemicals are from Sigma-Aldrich unless otherwise indicated), as we previously described (Kurokawa et al., 2007). Cumulus intact GVs were recovered into a 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)–buffered Tyrode's lactate solution (TL-HEPES) containing 5% fetal calf serum (Invitrogen, Carlsbad, CA) and supplemented with 100 μM IBMX to block spontaneous progression of meiosis. When necessary, cumulus cells were removed by repetitive pipetting using a fine capillary glass. Oocytes were matured in vitro for 12–14 h in IBMX-free Chatot, Ziomek, and Bavister medium (CZB; Chatot et al., 1989) supplemented with 3 mg/ml bovine serum albumin (BSA) or 0.02% polyvinyl alcohol (average molecular weight 30,000–70,000) under paraffin oil at 36.5°C in a humidified atmosphere containing 6% CO2. In vivo–matured MII oocytes were collected from the oviducts 12–14 h after administration of 5 IU of human chorionic gonadotropin, which was injected 46–48 h after PMSG. All procedures were performed according to research animal protocols approved by the University of Massachusetts Institutional Animal Care and Use Committee.
Generation of constructs and mRNA preparation
hStim1-YFP and hOrai1 were generously provided by T. Meyer (Stanford University, Stanford, CA) and M. Trebak (Albany Medical College, Albany, NY), respectively. hStim1-YFP was subcloned into a pcDNA6/Myc-His B vector (Invitrogen) between the restrictions sites AgeI and XbaI. The hOrai1 insert was amplified by PCR and ligated to the N-terminus of the mRFP-bearing pcDNA6/Myc-His B vector (D. Alfandari, University of Massachusetts, Amherst, MA) between EcoRI and XhoI restriction sites. Constitutively active hStim1, D76A hStim1-YFP, and hStim1-482 stop were generated either by substituting D76 to A or introducing the stop codon after amino acid 481 using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA), as previously reported (Liou et al., 2005; Smyth et al., 2009). Before performing in vitro transcription reactions, we verified the sequences of all new constructs and presence of targeted mutations by DNA sequencing. Constructs were linearized outside of the coding region with PmeI and in vitro transcribed using T7 mMESSAGE mMACHINE Kit (Ambion, Austin, TX). A poly(A) tail was added to the mRNAs using a Tailing Kit (Ambion). All mRNAs were prepared to final concentrations of 1.5 μg/μl, aliquoted, and frozen at −80°C until use.
Microinjection of mRNAs
Microinjections were performed as described previously by our laboratory (Kurokawa et al., 2007). Before injection, cRNAs were heat denatured and centrifuged, and the top 1.2 μl was used to prepare microdrops from which glass micropipettes were loaded by aspiration. cRNAs were delivered into oocytes using pneumatic pressure (PLI-100 picoinjector; Harvard Apparatus, Holliston, MA). When cRNAs were injected simultaneously, as in the case of hOrai1–mRFP+hStim1-YFP or hOrai1-mRFP+D76A hStim1-YFP, cRNAs were mixed immediately before the injection procedure in 1–3 M ratios, respectively, to allow similar protein expression, which was estimated by comparing fluorescence intensities. When injections were performed in GV oocytes and to allow for maximal translation, oocytes were kept in CZB plus IBMX for 6 h, after which IBMX was removed to allow the commencement of maturation. GVBD- and MI-stage oocytes were maturated for 4 and 8 h, respectively, whereas MII oocytes were matured for 12–14 h in CZB.
[Ca2+]i measurements and Ca2+ reagents
[Ca2+]i monitoring was performed as previously reported by our laboratory (Kurokawa et al., 2007). In brief, Fura-2 acetoxymethyl ester (Fura-2AM) was loaded by incubating oocytes in a HEPES-buffered CZB solution (HCZB) containing 1.25 μM Fura-2AM for 20 min at room temperature. Oocytes were then immobilized on glass-bottom dishes (MatTek Corp., Ashland, MA) and placed on the stage of an inverted microscope (Nikon, Melville, NY). Fura-2 fluorescence was excited with 340- and 380-nm wavelengths every 20 s, and emitted light was collected at wavelengths >510 nm by a cooled Photometrics SenSys charge-coupled device camera (Roper Scientific, Tucson, AZ). Acquisition of fluorescence ratios and rotation of the filter wheel were controlled by Simple PCI software (C-Imaging System Software, Cranberry Township, PA).
To examine the role of Ca2+ influx on refilling of [Ca2+]ER, we placed IBMX-treated GV oocytes in CZB medium either with or without 1.7 mM CaCl2 and allowed them to mature for 4 h, after which we placed them in nominal Ca2+-free HCZB and after a 5-min interval assessed interval [Ca2+]ER levels by adding TG (Calbiochem, San Diego, CA), an inhibitor of the SERCA pump, which induced Ca2+ leak by an unknown mechanism. TG-induced Ca2+ rises were regarded as [Ca2+]ER content that could be estimated from the area under the curve of the [Ca2+]i rise using Prism software (GraphPad Software, La Jolla, CA). To estimate SOCE and assess the effect of [Ca2+]ER on hStim1-YFP distribution, we followed oocytes by the method in Bird et al. (2008). Before adding 10 μM TG, we placed oocytes in Ca2+-free HCZB supplemented with 1 mM ethylene glycol tetraacetic acid (EGTA). When [Ca2+]i returned to near baseline values, ∼35 min after TG addition, 5 mM CaCl2 was added to the medium, and the amplitude of the [Ca2+]i rise caused by the addition was used to estimate SOCE.
Western blotting procedures
To detect endogenous/exogenous Stim1 and Orai1, we prepared protein lysates from 200 or 20–45, respectively, GV or MII oocytes. Oocytes were washed in Dulbecco's phosphate-buffered saline (DPBS), lysed in 2× sample buffer, and stored at −20°C until use. Heat-denatured proteins (95°C for 3 min) were separated by 7.5 or 10% SDS–PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). Membranes were blocked with 6% skim milk dissolved in PBS plus 0.1% Tween-20 for 2 h at 4°C. Two different antibodies were used against Stim1, one raised to recognize the N-terminus (1:100; BD Biosciences, San Jose, CA) and the other to identify the C-terminus end of the molecule (1:500; ProSci, Poway, CA), for which a blocking antigenic peptide (AP) was available. Orai1 was detected using an anti-Orai1 antibody (1:300; ProSci) raised against the C-terminus of the molecule, and an AP was also available for this antibody. For these experiments, equal volumes of AP (ProSci) and of the specific antibody were incubated for 2 h at 4°C, after which this mixture was used to complete the Western blotting procedure. In all samples, an anti-actin antibody was used to detect actin reactivity, which was used as a loading control (1:500; Millipore). Blots were incubated overnight at 4°C with primary antibodies, and goat anti-mouse or anti-rabbit antibodies conjugated with horseradish peroxidase were used as secondary antibodies (1:2000; Bio-Rad, Hercules, CA) and incubated for 1 h at room temperature. The membranes were then exposed to chemiluminescence reagents (NEN Life Science Products, Boston, MA) and the signal assessed using a Kodak 440 Image Station (Rochester, NY). The same anti-Stim1 antibodies were used to detect exogenously expressed hStim1-YFP (n = 45), which were also detected using an anti-GFP antibody (1:1000; MBL, Woburn, MA).
Phosphatase treatment was carried out on wild-type hStim1-YFP–expressing GV and MII oocytes/eggs. Samples were washed in DPBS and placed in phosphatase buffer (50 mM Tris-HCl, 100 mM NaCl, 10 mM MgCl2, and 1 mM dithiothreitol) supplemented with a protease inhibitor cocktail (Roche, Indianapolis, IN); control samples were also supplemented with 50 mM β-glycerophosphate to inhibit endogenous phosphatases. All samples were lysed by repeated cycles of freezing and thawing using liquid nitrogen, and 0.5 U of calf intestine phosphatase (New England BioLabs, Ipswich, MA) was added to the indicated groups; all samples were incubated at 37°C for 30 min. The reaction was stopped by addition of 2× sample buffer, after which Western blotting was performed as described.
Plasma membrane staining
To estimate the proximity of hStim1-YFP puncta to the PM during the different stages of maturation, we stained the PM of oocytes/eggs using 10 μg/ml wheat germ agglutinin conjugated with Alexa Fluor 633 (Invitrogen) according to manufacturer's instructions. Before staining, the zona pellucida was removed using Tyrode's acidic solution, pH 2.5, to facilitate the diffusion of the stain. ImageJ (National Institutes of Health, Bethesda, MD) software was used to quantify the distances of the hStim1 puncta to the PM in micrometers and the diameters of hStim1 aggregates and to draw the line scan to compare hOrai1 PM distribution.
Live imaging of oocytes using confocal microscope
Oocytes/eggs expressing proteins tagged with fluorescent proteins were collected at variable times of maturation and attached to glass-bottom dishes while incubated in BSA-free HCZB medium. Fluorescence was examined using an LSM 510 META confocal microscope (Carl Zeiss Microimaging, Jena, Germany) outfitted with a 63×/1.4 numerical aperture oil immersion lens. Images were taken at the equatorial and cortical regions of oocytes/eggs.
Statistical analysis
Statistical analysis was performed using Prism software (GraphPad, La, Jolla, CA). All data are presented as mean ± SEM. Mean data were compared using unpaired t test or analysis of variance, as appropriate. Categorical values such as those generated by maturation rates were analyzed using the chi-square test. p <0.05 was considered significant.
Acknowledgments
These studies were supported in part by Grant HD051872 from the National Institutes of Health to R.A.F. We thank Nan Zhang for useful discussions and Changli He for overall technical support.
Abbreviations used:
- AP
antigenic peptide
- BSA
bovine serum albumin
- CAD
CRAC-activating domain
- [Ca2+]e
external Ca2+
- [Ca2+]ER
Ca2+ in endoplasmic reticulum
- [Ca2+]i
intracellular concentration of free calcium
- CIP
calf intestine phosphatase
- CRAC
Ca2+ release-activated Ca2+
- cRNA
complementary RNA
- CZB
Chatot, Ziomek, and Bavister medium
- EGTA
ethylene glycol tetraacetic acid
- ER
endoplasmic reticulum
- Fura-2AM
Fura-2 acetoxymethyl ester
- GV
germinal vesicle
- GVBD
germinal vesicle breakdown
- HCZB
HEPES-buffered CZB
- IBMX
3-isobutyl-1-methylxanthine
- IP3
1,4,5-inositol-trisphosphate
- IP3R1
1,4,5-inositol-trisphosphate receptor 1
- MI
metaphase I
- MII
metaphase II
- mRFP
modified red fluorescent protein
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PM
plasma membrane
- PMSG
pregnant mare serum gonadotropin
- PVA
polyvinyl alcohol
- SEM
standard error of mean
- SERCA
sarcoendoplasmic reticulum Ca2+-ATPase
- SOCE
store-operated Ca2+ entry
- Stim1
stromal interaction molecule1
- TG
thapsigargin
- TL-HEPES
Tyrode's lactate solution
- WGA
wheat germ agglutinin
- YFP
yellow fluorescent protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-01-0065) on March 6, 2013.
REFERENCES
- Balghi H, et al. Enhanced Ca2+ entry due to Orai1 plasma membrane insertion increases IL-8 secretion by cystic fibrosis airways. FASEB J. 2011;25:4274–4291. doi: 10.1096/fj.11-187682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bird GS, DeHaven WI, Smyth JT, Putney JW., Jr Methods for studying store-operated calcium entry. Methods. 2008;46:204–212. doi: 10.1016/j.ymeth.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J, Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J Biol Chem. 1992;267:11196–11201. [PubMed] [Google Scholar]
- Carroll J, Swann K, Whittingham D, Whitaker M. Spatiotemporal dynamics of intracellular [Ca2+]i oscillations during the growth and meiotic maturation of mouse oocytes. Development. 1994;120:3507–3517. doi: 10.1242/dev.120.12.3507. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Collins SR, Meyer T. Evolutionary origins of STIM1 and STIM2 within ancient Ca2+ signaling systems. Trends Cell Biol. 2011;21:202–211. doi: 10.1016/j.tcb.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbellay B, Arnaudeau S, Bader CR, Konig S, Bernheim L. STIM1L is a new actin-binding splice variant involved in fast repetitive Ca2+ release. J Cell Biol. 2011;194:335–346. doi: 10.1083/jcb.201012157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW., Jr Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;283:19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol. 2008;315:257–279. doi: 10.1016/j.ydbio.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez C, Lopez-Guerrero AM, Pozo-Guisado E, Alvarez IS, Martin-Romero FJ. Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Mol Hum Reprod. 2012;18:194–203. doi: 10.1093/molehr/gar071. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez C, Pozo-Guisado E, Ganan-Parra M, Perianes MJ, Alvarez IS, Martin-Romero FJ. Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction. 2009;138:211–221. doi: 10.1530/REP-09-0126. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- Homa ST. Neomycin, an inhibitor of phosphoinositide hydrolysis, inhibits the resumption of bovine oocyte spontaneous meiotic maturation. J Exp Zoology. 1991;258:95–103. doi: 10.1002/jez.1402580111. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S, O'Connell PJ, Klyachko VA, Badminton MN, Thomson AW, Jackson MB, Clapham DE, Ahern GP. Fundamental Ca2+ signaling mechanisms in mouse dendritic cells: CRAC is the major Ca2+ entry pathway. J Immunol. 2001;166:6126–6133. doi: 10.4049/jimmunol.166.10.6126. [DOI] [PubMed] [Google Scholar]
- Jones KT, Carroll J, Whittingham DG. Ionomycin, thapsigargin, ryanodine, and sperm induced Ca2+ release increase during meiotic maturation of mouse oocytes. J Biol Chem. 1995;270:6671–6677. doi: 10.1074/jbc.270.12.6671. [DOI] [PubMed] [Google Scholar]
- Kaufman ML, Homa ST. Defining a role for calcium in the resumption and progression of meiosis in the pig oocyte. J Exp Zool. 1993;265:69–76. doi: 10.1002/jez.1402650110. [DOI] [PubMed] [Google Scholar]
- Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–17630. [PubMed] [Google Scholar]
- Koh S, Lee K, Wang C, Cabot RA, Machaty Z. STIM1 regulates store-operated Ca2+ entry in oocytes. Dev Biol. 2009;330:368–376. doi: 10.1016/j.ydbio.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Yoon SY, Alfandari D, Fukami K, Sato K, Fissore RA. Proteolytic processing of phospholipase Czeta and [Ca2+]i oscillations during mammalian fertilization. Dev Biol. 2007;312:407–418. doi: 10.1016/j.ydbio.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Wang C, Machaty Z. STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev Biol. 2012;367:154–162. doi: 10.1016/j.ydbio.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K, Haun S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. J Biol Chem. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaca K, Haun S. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca2+ store depletion from store-operated Ca2+ entry. J Cell Biol. 2002;156:75–85. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206:565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- McGuinness OM, Moreton RB, Johnson MH, Berridge MJ. A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development. 1996;122:2199–2206. doi: 10.1242/dev.122.7.2199. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kline D. Regulation of intracellular calcium in the mouse egg: calcium release in response to sperm or inositol trisphosphate is enhanced after meiotic maturation. Biol Reprod. 1994;51:1088–1098. doi: 10.1095/biolreprod51.6.1088. [DOI] [PubMed] [Google Scholar]
- Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. Thirty years of calcium signals at fertilization. Semin Cell Dev Biol. 2006;17:233–243. doi: 10.1016/j.semcdb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158:62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–255. doi: 10.1126/science.1321497. [DOI] [PubMed] [Google Scholar]
- Mohri T, Shirakawa H, Oda S, Sato MS, Mikoshiba K, Miyazaki S. Analysis of Mn2+/Ca2+ influx and release during Ca2+ oscillations in mouse eggs injected with sperm extract. Cell Calcium. 2001;29:311–325. doi: 10.1054/ceca.2000.0196. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci. 2010;123:3084–3093. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry: from concept to molecules. Immunol Rev. 2009;231:10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- Putney JW. Pharmacology of store-operated calcium channels. Mol Interv. 2010;10:209–218. doi: 10.1124/mi.10.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Roos J, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Russa AD, Ishikita N, Masu K, Akutsu H, Saino T, Satoh Y. Microtubule remodeling mediates the inhibition of store-operated calcium entry (SOCE) during mitosis in COS-7 cells. Arch Histol Cytol. 2008;71:249–263. doi: 10.1679/aohc.71.249. [DOI] [PubMed] [Google Scholar]
- Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development. 2002;129:3533–3544. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–1472. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- Sun L, Machaca K. Ca2+cyt negatively regulates the initiation of oocyte maturation. J Cell Biol. 2004;165:63–75. doi: 10.1083/jcb.200309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, Saunders CM, Rogers NT, Lai FA. PLCζ(zeta): a sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17:264–273. doi: 10.1016/j.semcdb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H. Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem Biophys Res Commun. 2013;430:60–65. doi: 10.1016/j.bbrc.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Takemura H, Hughes AR, Thastrup O, Putney JW., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- Tombes RM, Simerly C, Borisy GG, Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J Cell Biol. 1992;117:799–811. doi: 10.1083/jcb.117.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lee K, Gajdocsi E, Papp AB, Machaty Z. Orai1 mediates store-operated Ca2+ entry during fertilization in mammalian oocytes. Dev Biol. 2012;365:414–423. doi: 10.1016/j.ydbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci USA. 2009;106:17401–17406. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Sun L, Machaca K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J Cell Biol. 2010;191:523–535. doi: 10.1083/jcb.201006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]