Abstract
HIV+ substance-dependent individuals (SDIs) show emotional distress and executive impairment, but in isolation these poorly predict sexual risk. We hypothesized that an executive measure sensitive to emotional aspects of judgment (Iowa Gambling Task; IGT) would identify HIV+ SDIs whose sexual risks were influenced by emotional distress. We assessed emotional distress and performance on several executive tasks in 190 HIV+ SDIs. IGT performance interacted significantly with emotional distress, such that only in better performers were distress and risk related. Our results are interpreted using the somatic marker hypothesis and indicate that the IGT identifies HIV+ SDIs for whom psychological distress influences HIV risk.
Keywords: Substance dependence, HIV, AIDS, Executive functioning, Emotion, Sexual risk
Although learning of an HIV diagnosis tends to decrease risky sexual behavior, a subset of HIV-infected individuals continue to engage in such behavior after diagnosis (Marks, Crepaz, Senterfitt, & Janssen, 2005). This puts their partners at risk for contracting HIV and increases their own risk for coinfection with other sexually transmitted diseases or reinfection with drug-resistant HIV. Substance-dependent HIV+ individuals may be particularly prone to continued sexual risk taking, making this a key population for study (Kalichman, 2008; Kalichman, Rompa, & Cage, 2000). Examining risk taking in HIV+ substance-dependent individuals (SDIs) may also be uniquely informative to theories linking emotion and executive functioning to risk. HIV+ SDIs have higher rates of compromised neurocognitive functioning and of emotional distress, thus giving the opportunity to study a population with wide variations in those factors. To date, literature examining the separate and individual contributions of emotional distress and neurocognitive functioning to sexual risk has produced mixed results. In this paper we use the somatic marker hypothesis, which posits that affective (somatic) signals indicating reward and punishment play a crucial role in decision making, to suggest that examining the interaction between neurocognitive performance and emotional distress may produce better prediction of sexual risk taking in this key population.
HIV results in neurocognitive impairment in a variety of areas, including deficits in learning and retrieval of information, slowed processing speed, and executive deficits (Reger, Welsh, Razani, Martin, & Boone, 2002). Substance abuse impairs many of the same abilities, particularly executive abilities (Verdejo-Garcia, Lopez-Torrecillas, Gimenez, & Perez-Garcia, 2004). Studies comparing HIV+ and HIV− SDIs have found additional burden from HIV infection on inhibition (Martin et al., 2004a; Martin et al., 1992), working memory (Farinpour et al., 2000; Martin et al., 2003), and decision making (Martin et al., 2004b), suggesting that HIV+ SDIs are at “double jeopardy” for neurocognitive impairments (Gonzalez & Cherner, 2008). Deficits in executive abilities are of particular interest, as difficulties inhibiting prepotent responses or overfocus on immediate rewards at the expense of future consequences may lead to risky hedonic behaviors. However, it has not been established that measures of executive functioning, in isolation, predict reports of risky sexual activity.
Indeed, the one study to date using neuropsychological measures to predict sexual risk in this population (Gonzalez et al., 2005) did not find direct associations between several measures of executive functioning and sexual risk in HIV+ SDIs. However, in the Gonzalez and colleagues study, performance on one executive functioning task, the Iowa Gambling Task (IGT), was observed to mediate the relationship between sensation seeking (a personality factor known to be related to risky sex in substance users; Kalichman, Heckman, & Kelly, 1996) and sexual risk in HIV+ SDIs. Specifically, sensation seeking predicted sexual risk only in HIV+ individuals with better decision-making performance on the Iowa Gambling Task.
The Iowa Gambling Task (Bechara, Damasio, Damasio, & Anderson, 1994) was developed to quantify impairments of the type displayed by patients with lesions to the ventromedial prefrontal cortex (VMPFC), an area of neural circuitry linked to the generation of anticipatory affective signals of potential reward and punishment. Because the reward schedule of the IGT is comparatively opaque, good performance is theorized to require reliance on quickly developing anticipatory emotional responses to guide advantageous choices (Bechara, Damasio, & Damasio, 2000 ; Bechara, Damasio, Tranel, & Damasio, 2005; but cf. Dunn, Dalgleish, & Lawrence, 2006). This research is closely tied to the development of the “somatic marker hypothesis,” which states that the decision-making process is critically guided by these anticipatory affective signals (somatic states).
The IGT is a complex task, and impaired IGT performance can be produced by multiple cognitive and affective deficits (e.g., impaired working memory, hypersensitivity to reward and/or hyposensitivity to punishment; Bechara, 2005). However, one possible explanation for the moderating effect of the IGT in the Gonzalez et al. (2005) study is that better IGT performance in this sample represents better functioning of the affective systems involved in decision making. Sensation seeking is strongly linked to affective brain systems and may motivate risky sexual practices by making those risky practices feel exciting and enjoyable (Zuckerman & Kuhlman, 2000). Only individuals with “more intact” feedback from the affective part of the decision-making process would then be influenced by sensation seeking in their risk taking. Thus, the somatic marker hypothesis combined with the findings of Gonzalez and colleagues (2005) suggests that performance on the IGT may discriminate HIV+ SDIs whose risks will be influenced by emotional states from those whose risks are determined by other factors.
Although the Gonzalez et al. (2005) study suggested an influence of positive affect in risk taking, basic research has proposed that negative affective states may also increase hedonic risk taking (Leith & Baumeister, 1996; Tice, Bratslavsky, & Baumeister, 2001). This theory has been extended to HIV research, with some researchers proposing that negative affect motivates HIV-risk behaviors (McKirnan, Ostrow, & Hope, 1996). As with cognitive impairment, HIV+ SDIs are at greater risk for emotional distress, so much that the combination of HIV, substance abuse, and mental illness has been described as a “syndemic” (Walkup et al., 2008). Thus, symptoms of depression and anxiety could be risk factors for negative sexual behavior in HIV+ SDIs.
However, as with neurocognitive impairment, the relationship between emotional distress and sexual risk is not quite as clear empirically as it is theoretically. At least one meta-analytic review (Crepaz & Marks, 2001) examining depression, anxiety, and anger concluded that none of those emotional states significantly contributed to sexual risk in either HIV− or HIV+ individuals. Similarly, some have posited that posttraumatic stress disorder (PTSD) symptoms are a mediating pathway between childhood abuse and sexual risk (Gore-Felton & Koopman, 2002; Plotzker, Metzger, & Holmes, 2007). But other reviews note that the association between reports of childhood sexual abuse and risky sexual behavior appears stronger and more consistent than that of PTSD symptoms and risky sex (O’Cleirigh, Hart, & James, 2008). Taken together, although theory and basic laboratory research suggest that emotional distress should relate to sexual risk, findings regarding reports of “real-world” risk are mixed.
Some authors have suggested that this failure to find clear links between distress and sexual risk is due to unmeasured moderating variables (Crepaz & Marks, 2001). One such unmeasured variable in HIV+ SDIs may be the capacity to make affective judgments and weigh the pros and cons of a given choice based on their emotional and affective values. Therefore, we examined whether performance on the IGT moderated the relationship of depression, anxiety, and PTSD symptoms to reported sexual risk taking in a sample of HIV+ polysubstance users. We predicted that for better performers on the IGT, greater emotional distress would be related to greater reports of risk, while for those with worse performances on the IGT, emotional distress and risk would be unrelated. For comparison purposes we included two measures of executive functioning without a prominent affective component—the Stroop task (which measures inhibition) and the delayed nonmatching to sample (DNM) task (which measures working memory)—to rule out a moderation of the relationship between distress and risk by executive functioning in general. In addition, we examined the contribution of global premorbid cognitive functioning to the relationship between the IGT and emotional distress. If a significant interaction between IGT performance and emotional distress is found, this would suggest that failure to take both neurocognitive functioning and emotional distress into account simultaneously might be responsible for some of the mixed findings in the literature. It would also emphasize the need for comprehensive assessment of both neurocognitive and emotional variables when attempting to predict or influence sexual risks in this key population.
METHOD
Participants
Participants were 190 HIV+ polydrug users recruited from the Jesse Brown Veterans Association Healthcare–West Side Division, the HIV Early Intervention Clinic at Mile Square Health Center, community addiction and HIV treatment programs, and “word-of-mouth” referrals, all from the Chicago metro area. Participation was voluntary and not a requirement for any substance use treatment program. The University of Illinois at Chicago Institutional Review Board approved the study procedures, and all participants provided written informed consent prior to study procedures.
Inclusion/exclusion criteria and potential covariates
Substance use
All participants met DSM–IV (Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition; American Psychiatric Association, 1994) criteria for a history of substance dependence (96%) or abuse (4%) for at least one substance, with most meeting criteria for abuse or dependence for more than one substance (96%) in their lifetime (excluding nicotine and caffeine). Participants meeting criteria for alcohol dependence or abuse exclusively were not included, but those with a comorbid diagnosis of alcohol abuse or dependence were retained. Substance dependence history was determined using the Structured Clinical Interview for DSM–IV–Substance Abuse Module (SCID–SAM; First, Spitzer, Gibbon, & Williams, 1996). Drug of choice was most typically cocaine (57%), heroin (23%), or both (13%). Further self-reported drug use parameters, including years of use and length of abstinence were collected through an additional history questionnaire (see Table 1). Recent abstinence from drug use (for at least one week) was required, with no participants showing signs of acute intoxication or withdrawal and all participants undergoing Breathalyzer testing and rapid urine toxicology screening at testing.
TABLE 1.
Means of potential covariates and correlations or F values with RAB sexual risk scores
| Possible covariates | Correlation or F value |
p value | Mean | % of sample | |
|---|---|---|---|---|---|
| Demographic | Age | r = −.32 | <.001* | 43.53 (6.63) | |
| Sex (male) | F = 1.75 | .18 | 79 | ||
| Ethnicity | F = 1.33 | .27 | |||
| Black | 93 | ||||
| Hispanic | 3 | ||||
| White | 3 | ||||
| Other | 1 | ||||
| Education | r = .05 | .50 | 12.43 (1.76) | ||
| Medical | Hepatitis C | F = 5.46 | .02* | ||
| Coinfected | 34 | ||||
| CD4 count | r = −.03 | .68 | 462.57 (314.18) | ||
| RNA load | r = −.03 | .71 | 28,763.76 (90,588.44) | ||
| Protease inhibitors | F = 0.14 | .71 | |||
| On drug | 31 | ||||
| Reverse transcriptase inhibitors | F = 0.43 | .51 | |||
| On drug | 78 | ||||
| HAART | |||||
| On drug | F = 0.14 | .71 | 33 | ||
| Drug use | Years of use | r = −.17 | .02* | 20.66 (9.08) | |
| Days since last use | r = −.23 | .002* | 522.15 (1,147.90) | ||
| Hx. of injection drug use | F = 10.51 | .001* | |||
| Had used | 52 | ||||
| Methadone use | F = 12.37 | <.001* | |||
| Not using | 90 | ||||
| Drug of choice | F = 2.43 | .05* | |||
| Cocaine | 57 | ||||
| Heroin | 23 | ||||
| Cocaine and heroin | 13 | ||||
| Alcohol | 6 | ||||
| Marijuana | 1 | ||||
| DSM–IV dx. for alcohol | F = 0.01 | .94 | |||
| Meeting criteria | 87 | ||||
| DSM–IV dx. for cocaine | F = 2.07 | .15 | |||
| Meeting criteria | 96 | ||||
| DSM–IV dx. for heroin | F = 11.33 | <.001* | |||
| Meeting criteria | 58 | ||||
| DSM–IV dx. for cannabis | F = 0.02 | .90 | |||
| Meeting criteria | 77 | ||||
| DSM–IV dx. for other drugs | F = 0.64 | .42 | |||
| Meeting criteria | 46 | ||||
| Personality and intellectual | Sensation seeking | r = .39 | <.001* | 15.43 (6.05) | |
| AmNART IQ | F = 0.001 | .99 | 102.16 (8.87) | ||
| WRAT Reading | F = 0.0004 | .99 | 41.73 (7.15) |
Note. Scores transformed. Standard deviations in parentheses. RAB = Risk Assessment Battery. HAART = highly active antiretroviral therapy. AmNART = North American Adult Reading Test. WRAT = Wide Range Achievement Test. DSM–IV = Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition. Hx. = history; dx. = diagnosis.
p < .10, was entered into backward stepwise regression to produce final set of covariates.
HIV status and health history
All participants were HIV+ with serostatus verified by ELISA (enzyme-linked immunosorbent assay) with Western Blot confirmation. Additional health history was collected through a history questionnaire. The majority of participants (79%) reported taking antiretroviral medications. Further information about HIV medication types and rates can be seen in Table 1. Participants demonstrated affected immune functioning based on HIV RNA levels in plasma and CD4 T-lymphocyte counts. A total of 19% had an AIDS-defining CD4 count (see Table 1 for sample means). Sero-verified hepatitis C coinfection was present in 34% of the sample. Further medical inclusion and exclusion criteria were: no evidence of clinical dementias (including AIDS-associated dementia), no history of any neurologic disorders (including AIDS-defining disorders, stroke, and epilepsy), no history of open head injury or closed head injury with loss of consciousness longer than 30 minutes, no history of schizophrenia, untreated bipolar disorder, or current psychosis, and not taking neuroleptic medications.
Demographic, intellectual, and personality factors
To be included in the study, participants had to report at least nine years of education and be able to speak and read in English. The final sample was primarily high-school educated, African American, and male, in the fourth decade of life. In addition to these basic demographics, estimates of verbal IQ were taken using the North American Adult Reading Test (AmNART; Grober & Sliwinski, 1991). This test provides an estimate of premorbid general cognitive functioning based on word reading and is considered relatively insensitive to neuropsychological impairments resulting from disease or degenerative processes (Franzen, Burgess, & Smith-Seemiller, 1997). Reading ability was further measured using the Oral Reading subtest of the Wide Range Achievement Test–Third Edition (WRAT–3; Wilkinson, 1993). Given previous evidence that sensation seeking relates to sexual risk in this population (Gonzalez et al., 2005), scores on the Sensation Seeking Scale–Version V (SSS-V; Zuckerman, 1996) were also considered. Sample means for these demographic, intellectual, and personality factors may be seen in Table 1.
Emotional distress
Symptoms of depression, anxiety, and PTSD were measured in the current study using self-report. Depression was measured with the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961), a standard 21-item self-report of depressive symptoms with an extensive history of use (Beck, Steer, & Carbin, 1988). The average level of depression was “minimal” based on clinical cutoffs (M = 11.23, SD = 8.78), although we had participants across all ranges from “minimal” to “severe” (observed scores from 0 to 37). Anxiety was measured using the State form of the State–Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacob, 1983), another frequently used measure with 20 items. There is no single cutoff point for the STAI, as scores are compared to gender- and age-matched normative samples, but the average level of anxiety in our sample (M = 37.87, SD = 11.46) would similarly be considered “normal” for most individuals (near 50th percentile), but with participants ranging up into the 99th percentile (observed scores from 20 to 73). The PTSD Checklist–Civilian Version (PCL-C), also a commonly used inventory (Brewin, 2005), measured PTSD symptoms with 17 items representing the DSM–IV criteria for PTSD (Weathers, Litz, Huska, & Keane, 1994). Again, the mean for our participants (M = 38.46, SD = 13.86) fell below the cut-score of 50 established to predict PTSD in HIV+ SDIs (Bollinger, Cuevas, Vielhauer, Morgan, & Keane, 2008), but as with the other emotional distress variables, the distribution ranged well above this cut-point (observed scores from 17 to 83).
Iowa Gambling Task
This task presents participants with a computerized display of four decks of cards, from which the participants must repeatedly choose. Each of the decks is associated with a different amount of reward upon each choice, with two smaller reward decks ($50) and two larger reward decks ($100). Each deck is also associated with periodic losses, with lower reward decks resulting in smaller losses, while large reward decks result in larger penalties. These penalties are allocated periodically so that the participant is unable to predict when a penalty will occur. Participants are instructed to choose cards so as to maximize their “winnings,” and the computer screen continuously shows the participant the total amount of money won and the amount won and lost each time a card is drawn. The most advantageous strategy is to choose from the “good” small reward/small penalty decks, and avoid the “bad” large reward/large penalty decks, with most normal control groups generally adopting this strategy over the course of the task. In the current paper, performance was quantified as the number of choices from good decks minus the number of choices from bad decks (M = −7.65, SD = 24.75; Bechara & Martin, 2004).
Stroop Task: Reaction time version
In the Stroop paradigm, participants are shown a colored word and are asked to name the display color and ignore the word. Trials may be color congruent (e.g., “blue” printed in the color blue), neutral (an animal name printed in color), or color incongruent (e.g., “green” printed in the color red). This task measures ability to inhibit prepotent responses. A computerized version, which has previously been employed in this population, was used in this current study (Martin et al., 2004a; Martin et al., 1992). Of the 192 trials presented, 25% were color congruent, 25% color incongruent, and 50% neutral, with performance quantified by calculating the median reaction time for each type of stimulus. Reaction times for the neutral condition were subtracted from reaction times for the incongruent condition to provide an estimate of ability to inhibit prepotent responses while removing simple reaction time (M = 136.47, SD = 87.29; Martin et al., 1992). In addition to reaction times, the main variable of interest, the Stroop also provides a measure of accuracy (i.e., correctly identifying the color of the word). As is typical even in impaired populations, most participants were near ceiling on accuracy in non-conflict trials. However, 7 individuals were excluded from all analyses based on their overall error rate (+3 SDs from the mean, with all observations above this point visibly separated from the rest of the distribution). These individuals were removed on the basis that they were not adequately attending to the tasks.
Delayed nonmatching to sample
In the computerized delayed nonmatching to sample (DNM) task, participants are shown a red or a black card on a computer screen for 2 s, followed by an array of four cards (two red and two black). Participants much select two of the four cards using the initial card as a guide. After an initial training period, 45 trials are given, with delays of 10, 30, or 60 s between presentation of the sample card and the four-card array. Delay times occur in random order, with a total of 15 trials administered with each time delay. During the delay, participants read aloud from a written narrative to minimize rehearsal of the correct response. Bechara and colleagues (Bechara, Damasio, Tranel, & Anderson, 1998) developed this computerized task based on the traditional delayed nonmatching to sample paradigm, and it has previously been used with this population (Gonzalez et al., 2005; Martin et al., 2003). This task measures working memory, or the ability to hold and manipulate information in memory over a short time delay. Following these previously published studies, performance on the DNM was quantified in this study as the percentage of correct responses averaged across the 30- and 60-s delay conditions (M = 85.17, SD = 11.90).
Assessment of sexual risk
Sexual risk was assessed using a subscale of the Risk Assessment Battery (RAB). The RAB is a self-report questionnaire containing questions regarding risky injection drug use and sexual practices (Metzger, 1993; Navaline et al., 1994) that has been used previously with HIV+ substance users (Avants, Warburton, Hawkins, & Margolin, 2000; Woody et al., 2003). The “Sexual Practices” (RAB–SP) subscale was the dependent variable in the current study. This subscale contains multiple-choice items reflecting frequency or quantity of specific sexual behaviors in the past six months (e.g., “In the past 6 months, how often did you use condoms when you had sex?; In the past 6 months, how often have you had sex so you could get drugs?”). The total possible score of the Sexual Practices section ranges from 0 to 18 points, and the full range of scores from 0 to 18 was seen in the current study. The mean level of risky sexual practices was on the lower end of the scale (M = 4.06, SD = 3.51), but this does not necessarily indicate innocuous sexual behavior in our sample. For example, an HIV+ respondent with 2–3 sexual partners in the last 6 months using condoms “some of the time” would score a 4 on the RAB–SP scale.
We did not examine the Drug Use subscale of the RAB due to its reliance on injection drug use behavior and the relative lack of recent injection drug use in our sample. Although approximately half of our participants reported previous use of injection drugs, only 21 out of 183 participants scored anything above a 0 on the Drug Use subscale (indicating risky injection drug practices like sharing needles in the last six months) providing insufficient variability to support a multiple regression with several predictors.
Data analyses
Multiple linear regression was used to examine the effect of emotional distress and executive functioning on self-reported risky sexual practices while controlling for the effects of possible confounding variables. Potential confounding variables were identified on the basis of a significant relationship to reported sexual risk. Hierarchical multiple regression was used, with the covariates entered in Block 1, the main effects of the neurocognitive measures and emotional distress in Block 2, and the interactions between the neurocognitive measures and emotional distress in Block 3. This blocking approach ensures that potential confounding variables enter the model before the primary variables of interest and thus allows an examination of the incremental predictive ability of the main effects and interactions of interest above a model containing only the covariates.
RESULTS
Selection and transformation of variables
First, we examined potential covariates to determine whether they needed to be included in the models examined for our primary analyses of interest. These potential confounds included all of the demographic, substance use, medical, neurocognitive, and personality variables described above. As comparatively few participants met criteria for current abuse or dependence, we collapsed across current and lifetime abuse and dependence and considered “DSM–IV diagnosis for abuse or dependence at some time in life” versus “never any DSM–IV diagnosis for this drug.” Although all variables were evaluated as covariates, it is important to note that several variables (ethnicity, drug of choice, methadone use, and cocaine diagnosis) had a very small number of cases in certain categories (for example, only 5 individuals identified themselves as “White”). This reduces the probability of finding a statistically significant association of these variables with sexual risk (Tabachnick & Fidell, 2001), so a lack of association in the current sample does not indicate that these variables do not impact sexual risk in the greater population. Continuous potential covariates were evaluated for correlation with RAB–SP scores, while categorical variables were used as independent variables in one-way analyses of variance (ANOVAs) with RAB–SP scores as the dependent variable. To initially select potential covariates for evaluation, we used a liberal value of p < .10 (see Table 1 for significance tests and descriptive statistics for the potential covariates). As some of the variables selected in this way were largely redundant with one another (for example, only 15 of 78 injection drug users were not also heroin dependent), a backward stepwise multiple linear regression (using the stepAIC function from the MASS package in the R environment; R Development Core Team, 2009; Venables & Ripley, 2002) was then conducted on all univariate-significant predictors. At each step, the variable that predicted the least unique variance in sexual risk taking (i.e., the variable with the lowest t-value) was removed, and Akaike’s information criterion (AIC) for this compact model was compared to that for the previous model. The AIC statistic weighs the improvement in prediction produced by a more complex model against a penalty term representing the number of variables included (Venables & Ripley, 2002). This process continued until the minimum AIC value was reached (i.e., no more variables could be removed without significantly degrading the prediction of sexual risk). This resulted in maximum prediction of sexual risk while eliminating variables that largely overlapped with other covariates, allowing a balance between potential “left out variables error” (Mauro, 1990), in which a missing covariate’s absence affects results, and “left in variables error” (see Clogg & Haritou, 1997, for a discussion) in which modeling extra variables may lead to spurious associations between the variables of interest. While this process may capitalize on chance associations between the covariates and sexual risk, thus inflating the R2 of our final total model, we note that the covariate model is not central to our questions of interest. We judged that there was greater scientific risk in failing to control for a potential confounding variable than there was in including a theoretically irrelevant variable in our covariates. On this basis, age, number of days since last drug use, use of methadone, and sensation seeking were retained for use as covariates in our analyses of primary interest. The correlations between the chosen covariates, scores on the predictor variables, and sexual risk scores are presented in Table 2.
TABLE 2.
Intercorrelations between selected covariates, predictor variables, and dependent variables
| RAB sexual risk |
Age | Methadone use |
Days since last use |
Sensation seeking |
BDI | STAI | PTSD | Distress | IGT | Stroop | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Age | −.32** | ||||||||||
| Meth. use | −.25** | .19** | ||||||||||
| Days since last use | −.23** | .09 | −.17* | |||||||||
| Sensation seeking | .39** | −.16* | −.11 | −.12 | ||||||||
| Emotional distress | BDI | .01 | .07 | .22** | −.14* | −.01 | ||||||
| STAI | .07 | −.05 | .23** | −.17* | .03 | .71** | ||||||
| PTSD | .14 | −.13 | −.01 | −.07 | .06 | .52** | .49** | |||||
| Emotional distress composite | .08 | −.04 | .18* | −.15* | .03 | .88** | .87** | .79** | ||||
| Executive functioning | IGT | −.03 | −.05 | −.10 | .07 | .13 | .07 | .02 | .02 | .04 | ||
| Stroop | −.07 | .09 | .09 | −.01 | −.03 | −.04 | .01 | .01 | −.01 | .12 | ||
| DNM | −.01 | .08 | .02 | −.09 | −.04 | .19* | .21** | .12 | .20** | −.11 | .04 |
Note. After transformations as needed. RAB = Risk Assessment Battery. BDI = Beck Depression Inventory. STAI = State–Trait Anxiety Inventory. PTSD = posttraumatic stress disorder. IGT = Iowa Gambling Task. DNM = delayed nonmatching to sample. Meth. = methadone.
p < .05.
p < .01.
As can also be seen in Table 2, our measures of emotional distress were all moderately to highly correlated (between r = .49 and .71). This is not surprising, given known overlaps in symptoms of anxiety and depression (Sartorius, Ustun, Lecrubier, & Wittchen, 1996) and the fact that the scales used were not specifically constructed to avoid these overlaps (see Beck, Epstein, Brown, & Steer, 1988). Thus, in order to minimize risk of multicollinearity and reduce the total number of analyses to control for Type I error, the decision was made to construct a composite “emotional distress” variable by standardizing scores on the BDI, STAI, and PTSD scales and taking an equally weighted average of the scores. This composite emotional distress variable had acceptable reliability (Cronbach’s α = .76), suggesting that these three emotional distress scales can be argued empirically to be measuring largely the same construct despite their varied intended uses. This also suggests that a piecemeal analysis (analyzing distress variables separately) would be suboptimal, given the highly overlapping variance accounted for by these scales.
All variables were examined for normality, and, when necessary, variables were transformed to ensure that assumptions of normality were met. RAB–SP scores, Stroop reaction times, and days since last use all demonstrated positive skews that were adequately corrected with square root or log transformations. DNM scores had a negative skew, which was adequately corrected by reflection and square root transformation. Analyses for univariate and multivariate outliers were conducted using the Mahalanobis distance and Cook’s D statistic, with no univariate or multivariate outliers detected after the noted transformations.1 Any participant missing data for any of the variables was removed list-wise, leaving a total of 180 complete participants available for analysis. All variables were mean-centered.
First-order correlations
Examining the correlations between the covariates and sexual risk (presented in Table 2), methadone usage and greater age were related to lower reports of sexual risk, while fewer days since last drug usage and higher sensation seeking predicted higher reports of sexual risk. Age and likelihood of methadone usage were positively related, while age and sensation seeking were negatively related, and fewer days since last drug usage predicted greater likelihood of methadone usage (although, as noted above, despite these relations each of these variables uniquely contributed to predicting sexual risk). Methadone usage predicted greater emotional distress, and fewer days since last drug usage also predicted greater emotional distress. Emotional distress did not impact any of the executive functioning variables aside from DNM, where greater emotional distress predicted worse DNM performance (represented as a positive correlation due to the transformation of DNM scores). Neither emotional distress nor the executive functioning variables significantly correlated with RAB–SP scores. Finally the IGT, Stroop, and DNM were also not significantly related to each other, further indicating that they tap comparatively distinct aspects of executive functioning.
Primary regression analyses
Examining the overall regression equation, the model was statistically significant, accounting for 26% of the variance in reported sexual risk (see Table 3). However, as noted above, the overall estimate of variance accounted for may be inflated by the method in which the covariates were selected and does not solely reflect the predictive value of the variables of interest. As would be expected from the preliminary regression, all covariates significantly predicted self-reported sexual risk. The second step of the regression revealed that no direct relationships between emotional distress, IGT performance, and reported sexual risk reached significance when controlling for the covariates (also Table 3). However, the third step produced a small but significant improvement in prediction over Step 2, R2Change = .02, F(3, 168) = 3.22, p = .02. In the third step, we observed a significant IGT × Distress interaction (β = .15, p = .02) but no significant Stroop × Distress interaction (β = −.10, p = .11), or DNM × Distress interaction (β = −.08, p = .25).
TABLE 3.
Multiple regression results
| R2 | R2Change | F Change | Standardized beta | p value | |||
|---|---|---|---|---|---|---|---|
| Step 1 | Covariates | .25 | .25 | F(4, 175) = 16.21 | <.001* | ||
| Age | −.22 | .001* | |||||
| Methadone use | −.20 | .003* | |||||
| Days since last use | −.14 | .04* | |||||
| Sensation seeking | .32 | <.001* | |||||
| Step 2 | Main effects | .24 | .00 | F(4, 171) = 0.99 | .42 | ||
| Emotional distress | .09 | .20 | |||||
| IGT | −.09 | .17 | |||||
| Stroop | −.03 | .69 | |||||
| DNM | .01 | .90 | |||||
| Step 3 | Interactions | .26 | .02 | F(3, 168) = 3.22 | .02* | ||
| IGT × Emotional Distress | .15 | .02* | |||||
| Stroop × Emotional Distress | −.10 | .11 | |||||
| DNM × Emotional Distress | −.08 | .25 | |||||
| Total model | .26 | .26 | F(11, 168) = 7.37 | <.001* |
Note. IGT = Iowa Gambling Task. DNM = delayed nonmatching to sample.
Significant at p < .05.
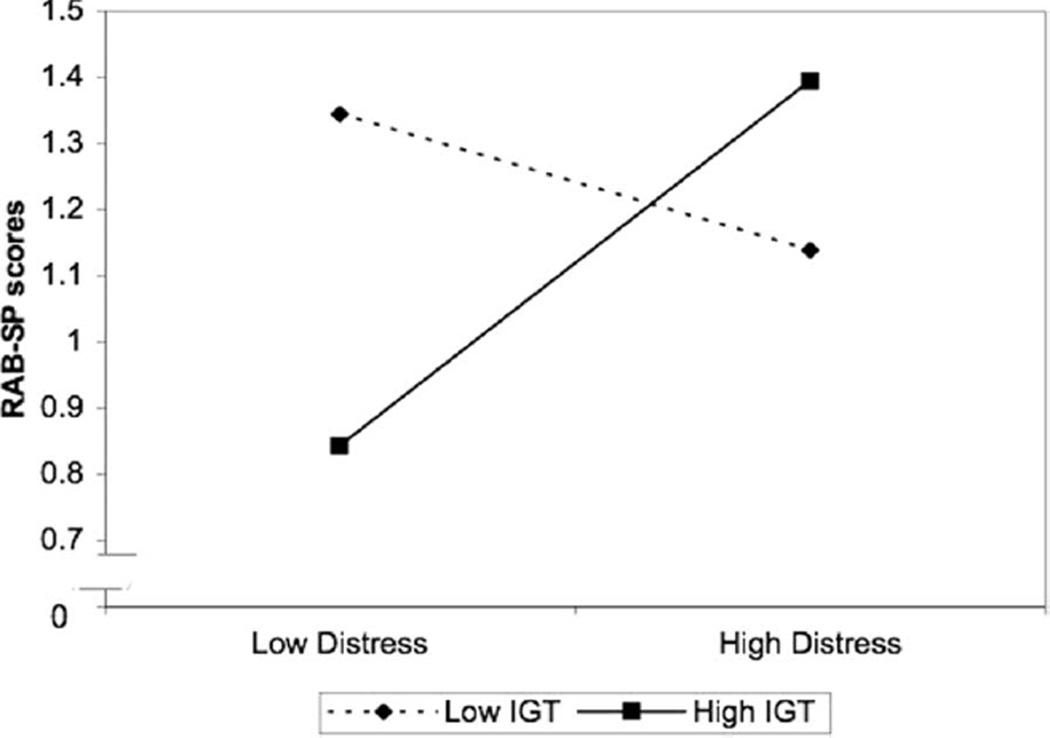
To understand this significant interaction between the IGT and distress, we examined the simple slope of distress on sexual risk at +1 and −1 standard deviations above and below mean performance on the IGT with all other covariates and effects controlled for (as recommended by Aiken & West, 1991; and Cohen, Cohen, West, & Aiken, 2003).2 At +1 standard deviations from the IGT mean, a significant positive simple slope for distress scores was seen (β = .28, p = .01), indicating that higher distress predicted higher reports of sexual risk for individuals with better performance on the IGT. At −1 standard deviation from the IGT mean, the simple slope of distress on reported sexual risk was not significant (β = −.10, p = .33), indicating no relationship between distress and sexual risk for those with worse performance on the IGT (see Figure 1).
Figure 1.
Interaction between Iowa Gambling Task (IGT) and distress on sexual risk plotted at +1 and −1 standard deviations from mean IGT performance and mean distress scores.
Although the lack of direct relationships between the IGT, Stroop, and DNM makes multicollinearity between these predictors unlikely, we also wished to rule out the possibility that significant Stroop × Distress or DNM × Distress interactions existed, but were not observed in our full model due to shared variance with the IGT × Distress interaction (as might be the case if general executive impairments were driving the results, with IGT being simply the strongest indicator of general impairment). Therefore, we repeated the third step of the regression model three times, first including only the IGT × Distress interaction, second only the Stroop × Distress interaction, and third only the DNM × Distress interaction. Doing so produced results parallel to the previous analysis, with the IGT × Distress interaction remaining significant (β = .14, p = .03), while the Stroop × Distress (β = −.10, p = .12) and DNM × Distress (β = −.10, p = .14) were not.
Supplementary regression analyses
Our hypothesis predicted (and our primary analyses provided support for) the IGT as uniquely moderating the relationship between emotional distress and risk. The lack of interaction between other measures of executive functioning and distress supports the idea that generalized executive dysfunction is not responsible for this relationship. However, we had available one additional measure of general cognitive functioning—estimated IQ scores from the AmNART. This measure is distinct from the measures used in the primary analyses in that it is not directly related to executive functioning, and it is considered a “hold” test, or one relatively unlikely to be affected by disease processes (unlike the measures used in the primary analyses). Thus, it could be used to test whether differences in overall premorbid cognitive functioning account for the observed interaction between IGT scores and distress. Examining the first-order correlations, estimated IQ did not correlate significantly with IGT scores (r = .12, p = .10), emotional distress (r = −.06, p = .39), or sexual risk (r = .001, p = .98). Using estimated IQ scores in a hierarchical multiple regression we entered the same covariates as those above in Block 1, main effects of IGT, distress, and estimated IQ in Block 2, and interactions between IQ × Distress and IGT × Distress in Block 3. Examining the hierarchical regression, neither the main effect of estimated IQ (β = .01, p = .97) nor the interaction of IQ × Distress (β = −.08, p = .19) was significant. Thus, even when the main effects of estimated IQ and its interaction with distress were controlled for, the IGT × Distress interaction continued to be significant (β = .15, p = .02).
DISCUSSION
Understanding the factors motivating risky sex in HIV+ SDIs is important to preventing the spread of HIV, as SDIs may be particularly prone to continued sexual risk taking after diagnosis with HIV. HIV+ SDIs are at greater risk for executive impairment and emotional distress, two factors theoretically likely to relate to increased sexual risk taking. However, in previous research, neither executive functioning nor emotional distress alone predicted sexual risk in this key population. In this study, we hypothesized that performance on one measure of executive functioning, the Iowa Gambling Task, might help to discriminate individuals who are motivated by negative emotion in their sexual risks. This proposal was guided by the somatic marker hypothesis, which posits that affective signals of reward and punishment crucially guide judgment, and that IGT performance is sensitive to the disruption of this system. Congruent with this hypothesis, we found that decision-making performance on the IGT moderated the relationship between emotional distress and reported sexual risk. In individuals with better performance on the IGT, greater distress predicted greater sexual risk taking. In contrast, individuals with worse performance on the IGT did not show a significant relationship between distress and reported sexual risk. Importantly, there was no confounding association between the IGT and distress, or between the IGT and sexual risk, so it was not that ceiling or floor effects obscured links between distress and risk in one group. Rather, it would seem that risks in better versus worse performers are simply motivated differently. We interpret this to mean that a neural circuitry that is critical for affective decision making needs to be intact at first, as reflected by good IGT performance, in order for emotional distress to have an influence on the functioning of this neural circuitry, thereby increasing risk-taking behavior. This finding is important because it suggests that in this subgroup of patients with intact decision-making circuitry, reducing their emotional distress can help ameliorate their risky behavior. In contrast, in patients who seem to have an already dysfunctional decision-making circuitry, as reflected by poor IGT performance, emotional distress has little influence on their decision-making capacity.
Furthermore, our results appeared to be specific to the IGT, not other measures of executive functioning or global neurocognitive functioning. Estimated global neurocognitive functioning (measured with the AmNART) did not account for the relationship between the IGT, distress, and sexual risk. Further, performance on other executive functioning measures—namely, a reaction time version of the Stroop task, which measures inhibition— or on the DNM, a working memory task, did not predict sexual risk taking and did not interact with the emotional distress variable to predict risk. Further, the DNM measures one of the cognitive skills previously shown to be crucial to IGT performance (Bechara & Martin, 2004), but working memory impairment as measured by the DNM did not explain the moderation of distress and risk taking by the IGT. One relevant difference between these tasks and the IGT is that both of these tasks lack a known emotional aspect. Thus, we suggest that that the greater predictive ability of the IGT for distress-related risk taking stems from its unique affective characteristics. However, we cannot rule out that other differences between these tasks and the IGT may account for our findings. The IGT is a complex task, and there are areas of active debate regarding the skills necessary for adequate IGT performance and about the relationship between the IGT and emotion (Bechara et al., 2005; Dunn et al., 2006; Maia & McClelland, 2005). Thus, examining the moderating ability of other measures tapping multiple different areas of executive functioning is warranted in future studies.
The Iowa Gambling Task was developed to detect the type of decision-making deficits often observed among patients with ventromedial prefrontal (VMP) lesions. The orbital frontal cortex serves as a region of convergence/divergence for structures that integrate information on bodily and affective states with perceptions of objects of judgment. These structures include the amygdala, the insular and somatosensory cortices, the basal ganglia, and the anterior cingluate (Bechara & Damasio, 2005). The somatic marker hypothesis proposes that this neural network is critical to the generation of affective signals marking the potential for reward or punishment, particularly when the affective value of the stimulus depends on the integration of memories, hypothetical thoughts, or imagination. Making a card choice in the IGT is theorized to depend on this ability to project the affective outcome likely to be associated with each deck by drawing on previous affective experiences with those decks (Bechara et al., 2000). As noted in the introduction, both drug dependence and HIV infection are known to cause dysfunction in several of these critical neural areas, but particularly in the prefrontal and basal ganglia loops involved in executive functioning (Gonzalez & Cherner, 2008). Given that the IGT has been successful in identifying VMP patients with poor judgment, it is somewhat surprising that poor IGT performance alone did not predict increased sexual risk. Indeed, one interpretation of our findings is that emotional distress may affect risk taking only when IGT performance is good. Thus, our results would seem to suggest that IGT performance in isolation may be insufficient to influence sexual risk, at least in this population. We would hypothesize that while risk taking in better performers may be normally motivated by emotional aspects of judgment, as we elaborate below, risk in poorer performers may be more motivated by different factors, perhaps such as environmental cues or situational opportunity, as is often observed among patients with VMP lesions who also perform poorly on the IGT.
Little is known about how chronic, but possibly subacute, emotional distress (of the type measured in this study) interacts with the somatic marker mechanism described above. It has been suggested that in general affective/emotional signals (or somatic states) that are integral to the decision task at hand (for example, the punishment produced by loss on the IGT itself) are beneficial to the decision-making process, whereas moods and emotions that are unrelated to the task (for example, receiving sad news or experiencing fear while making a decision) are in fact disruptive to the operation of the somatic marker circuitry and tend to interfere with advantageous decision making (Bechara & Damasio, 2005). In this case, the distress experienced by patients would be considered as emotion that is unrelated to the decision task at hand, such as engaging in a risky sexual activity. There are a few mechanisms that might account for the interference of emotional distress in the decision making of individuals with better IGT performance. One is that emotional distress produces a contrast effect, in which a negative emotional background increases the salience and appeal of possible rewards indicated by the somatic marker system (Abele & Gendolla, 1999). Or it may be that negative mood reduces regulatory resources, leading to difficulty inhibiting approach to rewards that are highlighted by the somatic marker system (Tice et al., 2001). If any of these mechanisms are operating, individuals with poor performance on the IGT (indicating a dysfunction of this affective valuation system) would not be as strongly influenced by emotional distress in their risk taking. It should be noted that we do not hypothesize our “better” performers as being hypersensitive to emotional distress. Rather, we believe that the somatic marker system must be intact in order for the contribution of chronic emotional distress to sexual risk to be evident.
Our findings highlight the importance of examining a combination of neurocognitive and affective variables, as neither the IGT nor distress directly related to risky sexual practices when examined in isolation. However, when considered together, both factors shed light on mechanisms involved in the risky sexual practices. Additionally, although this was not of central interest to the study, only DNM performance (among all the measures of executive functioning) was impacted by emotional distress, with distress degrading DNM performance. This in combination with the specificity of our findings relative to the IGT suggests that the relationship between emotional distress and executive functioning and the combined effects of emotional distress and executive functioning on important criterion variables such as sexual behavior may be dependent on which specific executive skills are examined. Further, although for practical reasons we examined a composite measure of emotional distress, it is likely that finer grained analyses that examine a variety of emotional states and neurocognitive skills may reveal even more nuanced interplay between affect and neurocognition. Different negative emotions have previously been shown to differently impact decision making in laboratory studies (Lerner & Keltner, 2000, 2001). Further, Crepaz and Marks (2001) in their meta-analysis found that sexual risks in HIV+ individuals may be more closely related to anger than other negative emotions, perhaps due to different appraisals of sexual risk (i.e., HIV+ individuals may view risky sex as predominantly harmful to others and be less attentive to that harm when experiencing other-directed anger; Crepaz & Marks, 2001). Additionally, although many theorists conceive of negative emotion as increasing risk (as would be suggested by the findings of this study and Tice et al., 2001), some theorists have proposed that anxiety may actually reduce risk by serving as a “stop signal,” indicating the need for greater caution (Pham, 2007). Further clarification of the mechanisms linking different specific negative emotions to risky sexual activity in this population could thus inform basic questions of which negative emotions influence risk in which ways.
Sexual risk is admittedly a complex and multideter-mined process in which neurocognitive and emotional variables appear to account for a small but significant amount of variance. Nonetheless, this line of research could eventually lead to additional tailoring of interventions to reduce HIV risk by increasing control over emotional distress. For example, our findings suggest that emotional distress, even at a subclinical level (at +1 SD, our participants were still just at the cutoff for moderate depression on the BDI), may still be influential in risk taking for those with better IGT performance. Our findings thus suggest that a group that might not normally be identified for emotional interventions could in fact benefit from such interventions to reduce HIV risk. It also provides some explanation for why emotional distress and risk may be only weakly related in population level studies that do not consider neurocognitive moderating variables.
Some alternative explanations for our findings are worth considering. First, it is possible that more cognitively intact individuals with high distress are more inclined to self-report risk than those with low distress. Perhaps for these better functioning individuals, emotional distress functions as a cue to examine their own behavior more carefully (congruent with the “stop signal” hypothesis about anxiety noted above). In contrast, those impaired on the IGT might not perceive emotional distress as a cue to examine their own behavior for risk, even though they may actually be engaging in risky behaviors. Another possibility is that better performers on the IGT are actually distressed by taking greater sexual risks, while worse performers are not distressed by sexual risk taking. Although it is difficult to eliminate use of self-report when examining intimate nonpublic acts, future studies could attempt to address both of these alternative explanations by utilizing prospective designs incorporating ecological momentary assessment techniques to reduce retrospective bias and elucidate the temporal order of risk and emotional distress.
In the current study we confined our investigation to HIV+ individuals with a history of substance dependence: a sample that provided an opportunity to examine a greater range of emotional and neurocognitive functioning than healthy normal adults. However, this may limit our ability to generalize these results to populations with different characteristics. For example, Gonzalez and colleagues (2005) only found a significant interaction between sensation seeking and risky sexual practices among HIV+, but not HIV−, individuals. It is also important to note that our sample consisted mainly of African-American men, and factors such as gender or ethnicity might impact the relationship between emotions and judgment (Fessler, Pillsworth, & Flamson, 2004). Indeed, there is emerging evidence of gender-related brain asymmetry in decision making (Tranel, Damasio, Denburg, & Bechara, 2005). Although our sample did not have sufficient numbers of women to examine gender as a moderating factor, this and other possible moderators should be considered in future studies.
In attempting to determine why HIV+ SDIs are particularly prone to continued sexual risk taking, most investigations to date have examined emotional distress and executive functioning in isolation and have produced few positive results. Our findings suggest that the relationship between executive functioning, emotional distress, and sexual risk in this population is more complex. In particular, measures sensitive to the emotional aspects of judgment, such as the IGT, may discriminate which HIV+ SDIs will be influenced by emotional distress in their sexual risk taking. If this finding is confirmed in other samples, it could help programs aimed at reducing HIV risk to decide whether to include interventions aimed at increasing emotional coping skills with any given individual and perhaps even indicate a value to attempting these interventions with individuals that show only mild levels of emotional distress.
Acknowledgments
Supported by HHS R01DA12828 to Eileen Martin-Thormeyer from the National Institute on Drug Abuse.
Footnotes
Removal of the Stroop error outliers and these transformations somewhat improved the fit of the model, and thus these changes were retained. However, analyses run with and without these alterations showed a largely unchanged significance pattern for all effects.
This method is preferred to dichotomizing one of the predictors, as dichotomization reduces power and may under certain specific circumstances result in the detection of spurious effects (Maxwell & Delaney, 1993).
REFERENCES
- Abele AE, Gendolla GH. Satisfaction judgments in positive and negative moods: Effects of concurrent assimilation and contrast producing processes. Personality and Social Psychology Bulletin. 1999;25:883–995. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Avants SK, Warburton LA, Hawkins KA, Margolin A. Continuation of high-risk behavior by HIV-positive drug users: Treatment implications. Journal of Substance Abuse Treatment. 2000;19:15–22. doi: 10.1016/s0740-5472(99)00092-6. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. The Journal of Neuroscience. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: Some questions and answers. Trends in Cognitive Sciences. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer R, Carbin M. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bollinger AR, Cuevas CA, Vielhauer MJ, Morgan EE, Keane TM. The operating characteristics of the PTSD Checklist in detecting PTSD in HIV+ substance abusers. Journal of Psychological Trauma. 2008;7:213–234. [Google Scholar]
- Brewin C. Systematic review of screening instruments for adults at risk of PTSD. Journal of Traumatic Stress. 2005;18:53–62. doi: 10.1002/jts.20007. [DOI] [PubMed] [Google Scholar]
- Clogg CC, Haritou A. The regression method of causal inference and a dilemma confronting this method. In: McKim VR, Turner SP, editors. Causality in crisis? Statistical methods and the search for causal knowledge in the social sciences. Notre Dame, IN: University of Notre Dame Press; 1997. pp. 113–162. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Crepaz N, Marks G. Are negative affective states associated with HIV sexual risk behaviors? A meta-analytic review. Health Psychology. 2001;20:291–299. doi: 10.1037//0278-6133.20.4.291. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: A critical evaluation. Neuroscience & Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, et al. Verbal working memory in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- Fessler DM, Pillsworth EG, Flamson TJ. Angry men and disgusted women: An evolutionary approach to the influence of emotions on risk taking. Organizational Behavior and Human Decision Processes. 2004;95:107–123. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM–IV Axis I Disorders. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- Franzen MD, Burgess EJ, Smith-Seemiller L. Methods of estimating premorbid functioning. Archives of Clinical Neuropsychology. 1997;12:711–738. [PubMed] [Google Scholar]
- Gonzalez R, Cherner M. Co-factors in HIV neu-robehavioural disturbances: Substance abuse, hepatitis C and aging. International Review of Psychiatry. 2008;20:49–60. doi: 10.1080/09540260701872028. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, et al. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Gore-Felton C, Koopman C. Traumatic experiences: Harbinger of risk behavior among HIV-positive adults. Journal of Trauma & Dissociation. 2002;3:121–135. [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Kalichman S. Co-occurrence of treatment nonadherence and continued HIV transmission risk behaviors: Implications for positive prevention interventions. Psychosomatic Medicine. 2008;70:593. doi: 10.1097/PSY.0b013e3181773bce. [DOI] [PubMed] [Google Scholar]
- Kalichman S, Heckman T, Kelly J. Sensation seeking as an explanation for the association between substance use and HIV-related risky sexual behavior. Archives of Sexual Behavior. 1996;25:141–154. doi: 10.1007/BF02437933. [DOI] [PubMed] [Google Scholar]
- Kalichman S, Rompa D, Cage M. Sexually transmitted infections among HIV seropositive men and women. Sexually Transmitted Infections. 2000;76:350–354. doi: 10.1136/sti.76.5.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith KP, Baumeister RF. Why do bad moods increase self-defeating behavior? Emotion, risk tasking, and self-regulation. Journal of Personality and Social Psychology. 1996;71:1250–1267. doi: 10.1037//0022-3514.71.6.1250. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Beyond valence: Toward a model of emotion-specific influences on judgement and choice. Cognition & Emotion. 2000;14:473–493. [Google Scholar]
- Lerner JS, Keltner D. Fear, anger, and risk. Journal of Personality and Social Psychology. 2001;81:146–159. doi: 10.1037//0022-3514.81.1.146. [DOI] [PubMed] [Google Scholar]
- Maia TV, McClelland JL. The somatic marker hypothesis: Still many questions but no answers. Trends in Cognitive Sciences. 2005;9:162–164. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: Implications for HIV prevention programs. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- Martin EM, Novak RM, Fendrich M, Vassileva J, Gonzalez R, Grbesic S, et al. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. Journal of the International Neuropsychological Society. 2004a;10:298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Rains N, Grbesic S, Pursell K, Nunnally G, et al. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology. 2003;17:283–288. doi: 10.1037/0894-4105.17.2.283. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, et al. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004b;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martin EM, Robertson LC, Edelstein HE, Jagust WJ, Sorenson DJ, San Giovanni D, et al. Performance of patients with early HIV-1 infection on the Stroop task. Journal of Clinical and Experimental Neuropsychology. 1992;14:857–868. doi: 10.1080/01688639208402867. [DOI] [PubMed] [Google Scholar]
- Mauro R. Understanding L.O.V.E. (left out variables error): A method for estimating the effects of omitted variables. Psychological Bulletin. 1990;108:314–329. [Google Scholar]
- Maxwell SE, Delaney HD. Bivariate median splits and spurious statistical significance. Psychological Bulletin. 1993;113:181–190. [Google Scholar]
- McKirnan DJ, Ostrow DG, Hope B. Sex, drugs and escape: A psychological model of HIV-risk sexual behaviours. AIDS Care. 1996;8:655–669. doi: 10.1080/09540129650125371. [DOI] [PubMed] [Google Scholar]
- Metzger DS. The Risk Assessment Battery; Paper presented at the Sixth Annual Meeting of the National Cooperative Vaccine Development Groups for AIDS; Alexandria, VA. 1993. [Google Scholar]
- Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger DS, Alterman AI, et al. Preparations for AIDS vaccine trials. An automated version of the Risk Assessment Battery: Enhancing the assessment of risk behaviors. AIDS Research and Human Retroviruses. 1994;10:S281–S283. [PubMed] [Google Scholar]
- O’Cleirigh C, Hart TA, James CA. HIV and anxiety. In: Zvolensky MJ, J. Smits AJ, editors. Anxiety in health behaviors and physical illness. New York: Springer; 2008. pp. 317–340. [Google Scholar]
- Pham MT. Emotion and rationality: A critical review and interpretation of empirical evidence. Review of General Psychology. 2007;11:155–178. [Google Scholar]
- Plotzker RE, Metzger DS, Holmes WC. Childhood sexual and physical abuse histories, PTSD, depression, and HIV risk outcomes in women injection drug users: A potential mediating pathway. The American Journal on Addictions. 2007;16:431–438. doi: 10.1080/10550490701643161. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing [Computer software] Vienna, Austria: R Foundation for Statistical Computing. 2009 http://www.R-project.org
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Sartorius N, Ustun TB, Lecrubier Y, Wittchen HU. Depression comorbid with anxiety: Results from the WHO study on psychological disorders in primary health care. British Journal of Psychiatry: Supplement. 1996;168:38–43. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacob GA. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4th ed. Needham Heights, MA: Allyn and Bacon; 2001. [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: If you feel bad, do it! Journal of Personality and Social Psychology. 2001;80:53–67. [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain: A Journal of Neurology. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York: Springer; 2002. [Google Scholar]
- Verdejo-Garcia A, Lopez-Torrecillas F, Gimenez CO, Perez-Garcia M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychology Review. 2004;14:1–41. doi: 10.1023/b:nerv.0000026647.71528.83. [DOI] [PubMed] [Google Scholar]
- Walkup J, Blank MB, Gonzalez JS, Safren S, Schwarz R, Brown LK, et al. The impact of mental health and substance abuse factors on HIV prevention and treatment. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;47:S15–S19. doi: 10.1097/QAI.0b013e3181605b26. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Huska JA, Keane TM. PCL-C for DSM-IV. Boston, MA: National Center for PTSD, Behavioral Science Division; 1994. [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. 3rd ed. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Woody GE, Gallop R, Luborsky L, Blaine J, Frank A, Salloum IM, et al. HIV risk reduction in the National Institute on Drug Abuse Cocaine Collaborative Treatment Study. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2003;33:82–87. doi: 10.1097/00126334-200305010-00012. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. The psychobiological model for impulsive unsocialized sensation seeking: A comparative approach. Neuropsychobiology. 1996;34:125–129. doi: 10.1159/000119303. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: Common biosocial factors. Journal of Personality. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]