Abstract
Although the effect of niacin on the glucose levels in subjects with diabetes mellitus has been investigated, niacin’s effects on the glucose levels and atherosclerosis in subjects with normal glucose levels have not been well established. We examined the effect of niacin on the glucose levels, coronary stenosis progression using quantitative coronary angiography, and clinical events in 407 subjects who had a baseline glucose level <100 mg/dl and were enrolled in the Familial Atherosclerosis Treatment Study (FATS), HDL-Atherosclerosis Treatment Study (HATS), Armed Forces Regression Study (AFREGS), or Carotid Plaque Composition by MRI during lipid-lowering (CPC) study testing active niacin therapy. Although the fasting glucose levels increased significantly within 3 years in both subjects treated with niacin (from 85.6 – 9.5 to 95.5 – 19.7 mg/dl, p <0.001) and without niacin (from 85.2 – 9.6 to 90 – 17.9 mg/dl, p =0.009), those treated with niacin had a significantly larger increase in glucose levels than those not taking niacin (9.88 vs 4.05 mg/dl, p =0.002). Overall, 29% of subjects developed impaired fasting glucose within 3 years. Incident impaired fasting glucose was significantly more likely to be observed in subjects treated with niacin than in those who were not. However, the frequency of new-onset diabetes mellitus did not differ significantly between the 2 groups (5.6% vs 4.8%, p = 0.5). Niacin-treated subjects compared to untreated subjects had significantly less change in mean coronary stenosis (0.1 – 0.3% vs 2 – 12%, p <0.0001) and less major cardiovascular events (8% vs 21%, p = 0.001). In conclusion, the use of niacin for 3 years in subjects with normal baseline glucose levels was associated with an increase in blood glucose levels and the risk of developing impaired fasting glucose, but not diabetes mellitus, and was associated with a significantly reduced incidence of coronary stenosis progression and major cardiovascular events.
Niacin has been widely used to treat dyslipidemia with elevated triglycerides and low levels of high-density lipoprotein (HDL) cholesterol.1 Multiple randomized, placebo-controlled trials, including the Coronary Drug Project (CDP), have demonstrated the ability of niacin to reduce the progression of atherosclerosis and lower the incidence of cardiovascular events in patients with established vascular disease.2–4 However, a noted side effect of niacin therapy has been an observed increase in serum glucose levels.5,6 Although several randomized control trials have evaluated the glucose effects of niacin in those with diabetes mellitus (DM), insufficient data are available to clearly establish the effect of niacin on glucose levels, incident DM, and cardiovascular disease in a population of subjects with baseline normal glucose levels.5,6 We, therefore, performed the present combined analysis to examine the effects of niacin therapy on glucose levels, coronary stenosis progression, and clinical events in subjects with normal baseline fasting glucose levels using combined data from 4 randomized lipid trials testing active niacin treatment, including the Familial Atherosclerosis Treatment Study (FATS), HDL-Atherosclerosis Treatment Study (HATS), Armed Forces Regression Study (AFREGS), and Carotid Plaque Composition by MRI [magnetic resonance imaging] during lipid-lowering (CPC) study.
Methods
A total of 407 subjects with established vascular disease without a diagnosis of DM who had participated in the FATS, HATS, AFREGS, and CPC study and had been treated with or without niacin and had a baseline fasting glucose level <100 mg/dl were included in the present analysis. The full protocols of these studies have been previously published. In brief, the FATS enrolled 146 men from 1984 to 1987 who had elevated apolipoprotein B levels ≥125 mg/dl, a family history of coronary heart disease, and documented coronary atherosclerosis on an angiogram.3 The subjects were randomized to lovastatin (20 mg twice daily) and colestipol (10 g 3 times daily), immediate release niacin (1 g 4 times daily) and colestipol (10 g 3 times daily), or placebo. The HATS enrolled 160 subjects with a history of coronary artery disease, documented coronary atherosclerosis on the angiogram, and low HDL cholesterol levels (<35 mg/dl in men, <40 mg/dl in women).7 The subjects were randomized to slow-release niacin (2 to 4 g/day) plus simvastatin (10 to 20 mg/day); antioxidants; slow-release niacin (2 to 4 g/day), simvastatin (10 to 20 mg/day) plus antioxidants; or all placebo. The AFREGS enrolled 143 military retirees with documented coronary atherosclerosis by angiogram and HDL cholesterol <40 mg/dl and randomized them to immediate-release niacin (3 g/day), cholestyramine (16 g/day) plus gemfibrozil (600 mg twice daily), or placebo.8 The CPC study randomized 123 subjects with coronary or carotid artery disease, apolipoprotein B >120 mg/dl, and <1 year of lipid therapy to atorvastatin (10 to 80 mg/day); atorvastatin plus extended-release niacin (2 g/day); or atorvastatin and extended release niacin plus colesevelam (3.8 g/day).9
The fasting glucose levels were measured at baseline and throughout each study for 3 years per protocol. A diagnosis of new-onset impaired fasting glucose (IFG) was defined as a glucose level of 100 to 125 mg/dl on ≥2 measurements during treatment, and new-onset DM was defined as fasting glucose levels of ≥126 mg/dl on ≥2 measurements during treatment. The on-treatment glucose levels and frequencies of new-onset IFG and DM were compared between the subjects treated with and without niacin. Of the 4 studies, the FATS, HATS, and AFREGS had the same clinical end points, including the percentage of change in mean proximal coronary stenosis, as measured by quantitative coronary angiography, and major cardiovascular events, including death, myocardial infarction, stroke, and revascularization because of worsening ischemia. A total of 342 subjects in these 3 trials had a baseline glucose level <100 mg/dl and were included in the comparison. Continuous variables are reported as the mean ± SD. Comparisons were made using 2-sided t tests, chi-square tests, and Wilcoxon-Mann-Whitney tests, as appropriate. p Values <0.05 were considered significant.
Results
Of the 407 subjects in the 4 studies with a baseline glucose level <100 mg/dl, 197 received active treatment with niacin and 210 were not treated with niacin. The subjects’ mean age was 58.7 ± 9.0 years, their body mass index was 27.4 ± 4.1, and 90.5% were men (Table 1). The percentage of men in the niacin group was lower than in the group without niacin (85% vs 96%, p = 0.001).
Table 1.
Characteristics of subjects with baseline glucose <100 mg/dl
| Variable | Niacin Treatment
|
p Value* | |
|---|---|---|---|
| No (n = 210) | Yes (n = 197) | ||
| Age (yrs) | 59.1 ± 8.7 | 58.3 ± 9.2 | 0.39 |
| Men | 201 (96%) | 168 (85%) | 0.001 |
| Body mass index (kg/m2) | 27.2 ± 4.0 | 27.6 ± 4.1 | 0.44 |
| Metabolic syndrome | 85 (40%) | 79 (40%) | 0.85 |
| Baseline high-density lipoprotein cholesterol (mg/dl) | 35.8 ± 8.4 | 35.6 ± 7.8 | 0.77 |
| Baseline low-density lipoprotein cholesterol (mg/dl) | 150.6 ± 51.9 | 144.6 ± 45.8 | 0.21 |
| Baseline triglycerides (mg/dl) | 190.2 ±115.2 | 194.3 ± 126.6 | 0.73 |
p Values for continuous data were obtained by t tests (or Mann-Whitney U tests when appropriate); for categorical data, chi-square tests were used.
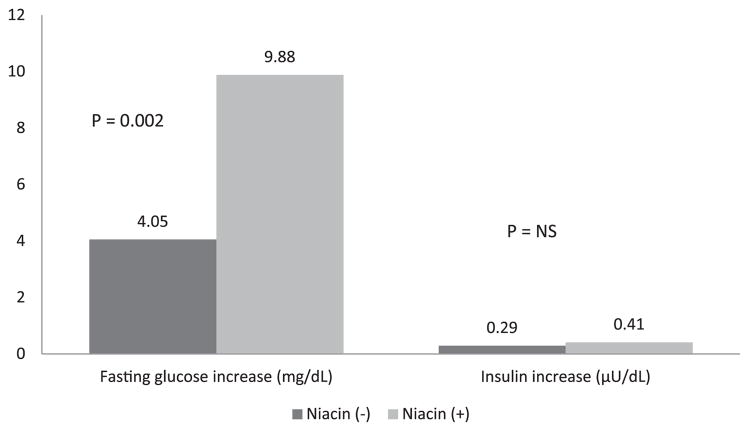
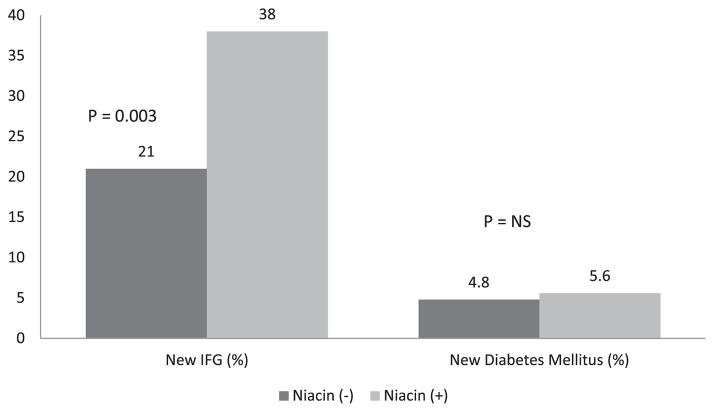
The fasting glucose levels increased significantly during the 3-year period in the subjects treated with niacin (from 85.6 ± 9.5 to 95.5 ± 19.7 mg/dl, p <0.001) and those treated without niacin (from 85.2 ± 9.6 to 90.0 ± 17.9 mg/dl, p = 0.009; Figure 1). The subjects treated with niacin had a significantly larger increase in glucose levels than those not taking niacin (9.88 vs 4.05 mg/dl, p = 0.002). Although a difference was seen in the amount of change in the glucose levels, the insulin levels increased nonsignificantly in the subjects treated without niacin (from 17.2 ± 13.2 to 17.6 ± 13.3 μU/dl, p = 0.77) and in those treated with niacin (from 19.9 ± 16.4 to 21.3 ± 16.0 μU/dl, p = 0.86). Furthermore, no significant difference was seen in the change in insulin levels between the niacin-treated and untreated groups (0.41 vs 0.29 μU/dl, p = 0.36). The glucose increase was not associated with the type or dosage of niacin used. Overall, 122 of 407 (29%) of subjects developed IFG during the 3-year period. IFG was significantly more likely to be seen in subjects treated with niacin than in those without niacin treatment (38% [78 of 197] vs 21% [44 of 210], p = 0.003; Figure 2). A nonsignificant greater number of incident DM was found in the niacin group (5.6% [11 of 197] vs 4.8% [10 of 210]; p = 0.5).
Figure 1.
Change in serum glucose and insulin levels in subjects with and without niacin therapy.
Figure 2.
New diagnosis of IFG and DM in subjects with and without niacin therapy.
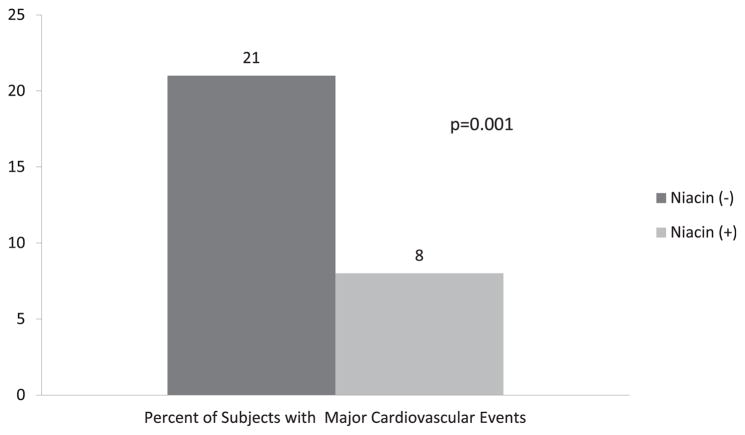
At baseline, the mean percentage of coronary stenosis using quantitative coronary angiography was similar in subjects randomized to treatment with and without niacin (33 ± 8% vs 35 ± 1%, p = 0.29). After 3 years of therapy, the niacin-treated subjects had a mean change in the percentage of stenosis that was significantly less than that in the untreated subjects (0.1 ± 0.3% vs 2 ± 12%, p <0.0001; Figure 3). Of the niacin-treated patient, 8% had major cardiovascular events during follow-up, significantly less than the 21% of untreated patients experiencing major cardiac events (p = 0.0001; Figure 4).
Figure 3.
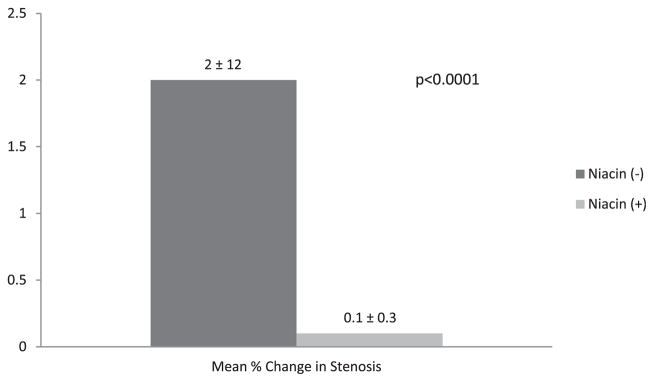
Percentage of change in coronary stenosis as assessed by quantitative coronary angiography in subjects with and without niacin therapy.
Figure 4.
Percentage of subjects with major cardiovascular events stratified by niacin therapy.
Discussion
Our study has demonstrated that niacin therapy for 3 years in subjects with normal baseline glucose levels is associated with an increase in blood glucose levels and the risk of the development of IFG, but not DM, with a significant reduction in coronary stenosis progression and clinical cardiac events. In the absence of DM, aging has been shown to increase blood glucose levels.10 Studies have shown that age-related changes in serum glucose levels are related predominantly to a worsening of insulin resistance.11 We found that after 3 years of follow-up, the fasting glucose levels increased in both groups, although the change in glucose levels in those subjects treated with niacin therapy was significantly greater.
Although the specific mechanism of niacin-induced hyperglycemia has not been clearly established, data have shown that the observed increase in serum glucose might be secondary to a niacin-induced decrease in insulin sensitivity.12 The administration of continuous infusion of niacin in animals has demonstrated an initial lowering of free fatty acids followed by a rebound in free fatty acids that is thought to impair glucose metabolism and promote insulin resistance.13 In a study of 12 healthy subjects without DM, treatment with immediate-release niacin for 2 weeks of ≤2 g of niacin resulted in an 18% reduction in insulin sensitivity that correlated with the serum fatty acids levels.14
Previous randomized control trials have documented that in subjects with established DM, niacin therapy can worsen glycemic control. In the Arterial Disease Multiple Intervention Trial (ADMIT), treatment with ≤3 g/d of immediate release niacin in subjects with DM was associated with a modest increase in blood glucose of 8.7 mg/dl.5 This increase in glucose was transient, returning to baseline after 24 weeks of therapy, and required no significant changes in the doses of anti-DM oral agents or insulin. Treatment with extended-release niacin of 1.5 g/d in the Assessment of Diabetes Control and Evaluation of the Efficacy of Niaspan Trial (ADVENT) showed no on treatment differences in glucose.6
The vascular and clinical benefits observed with niacin therapy appear to be preserved in subjects who experienced worsening of glycemic control. Considering subjects with metabolic syndrome in HATS, Vittone et al15 showed an increase in glucose and insulin levels and a decrease in insulin sensitivity in subjects treated with a niacin–simvastatin combination compared to placebo. Despite this change in glycemic control, those with the metabolic syndrome continued to see a vascular benefit with combination lipid therapy, with significant less coronary artery stenosis change compared to the placebo subjects (0.3% vs 3.0%, p = 0.003). Also, those with metabolic syndrome observed a significant 47% reduction in clinical cardiovascular events compared to the incidence in placebo patients. A similar finding was observed in the Coronary Drug Project (CDP), which randomized men with previous myocardial infarction to several lipid-altering therapies, including ≤3 g of immediate-release niacin.16 In the CDP, the reduction in recurrent myocardial infarction seen after 6 years of follow-up was similar in subjects with fasting glucose levels >100 mg/dl compared to subjects with a baseline glucose level of <100 mg/dl. In our study, despite a greater worsening of glycemic control and more progression to IFG, we observed a significant 95% reduction in coronary stenosis progression and 62% reduction in major cardiovascular events in subjects with normal glucose control who were treated with niacin therapy compared to niacin-untreated subjects.
Our analysis combined data from 5 clinical trials with varying populations. Within each trial, the risk factors for the development of IFG and DM in those subjects with normal baseline glucose levels might differ according to their clinical characteristics, independent of the effects of niacin. These findings need to be confirmed in large, prospective, randomized trials. Future subgroup analysis from the much larger completed Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes (AIM-HIGH) study and soon to be completed Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) will provide more insight into the effects of niacin in patients with normal and abnormal baseline glucose levels.17
Footnotes
Disclosures
Binh An P. Phan reports receiving honoraria for being a part of the speakers’ bureau for Abbott.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 3.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 5.Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, Ganda OP, Rosenson RS, Buse JB, Robertson DD, Sheehan JP. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med. 2002;162:1568–1576. doi: 10.1001/archinte.162.14.1568. [DOI] [PubMed] [Google Scholar]
- 7.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 8.Whitney EJ, Krasuski RA, Personius BE, Michalek JE, Maranian AM, Kolasa MW, Monick E, Brown BG, Gotto AM., Jr A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med. 2005;142:95–104. doi: 10.7326/0003-4819-142-2-200501180-00008. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X-Q, Dong L, Hatsukami T, Phan BA, Chu B, Moore A, Lane T, Neradilek MB, Polissar N, Monick D, Lee C, Underhill H, Yuan C. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging. 2011;4:977–986. doi: 10.1016/j.jcmg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4:493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- 11.Fink RI, Kolterman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71:1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol. 2008;48:79–106. doi: 10.1146/annurev.pharmtox.48.113006.094746. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch DK, Kahn SE, Schwartz MW, Koerker DJ, Palmer JP. Effect of nicotinic acid-induced insulin resistance on pancreatic B cell function in normal and streptozocin-treated baboons. J Clin Invest. 1991;87:1395–1401. doi: 10.1172/JCI115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarsson M, Grill V. Impact of nicotinic acid treatment on insulin secretion and insulin sensitivity in low and high insulin responders. Scand J Clin Lab Invest. 1996;56:563–570. doi: 10.3109/00365519609088812. [DOI] [PubMed] [Google Scholar]
- 15.Vittone F, Chait A, Morse JS, Fish B, Brown BG, Zhao X-Q. Niacin plus simvastatin reduces coronary stenosis progression among patients with metabolic syndrome despite a modest increase in insulin resistance: a subgroup analysis of the HDL-Atherosclerosis Treatment Study (HATS) J Clin Lipidol. 2007;1:203–210. doi: 10.1016/j.jacl.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project) Am J Cardiol. 2005;95:254–257. doi: 10.1016/j.amjcard.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]