Abstract
A dual role of B cells in experimental autoimmune encephalomyelitis (EAE), the animal model of the human autoimmune disease multiple sclerosis (MS), has been established. In the first role, B cells contribute to the pathogenesis of EAE through the production of anti-myelin antibodies that contribute to demyelination. On the contrary, B cells have also been shown to have protective functions in that they play an essential role in the spontaneous recovery from EAE. In this review, we summarize studies conducted in a number of species demonstrating the conditions under which B cells are pathogenic in EAE. We also discuss the phenotype and anti-inflammatory mechanisms of regulatory B cells.
Keywords: B cell, experimental autoimmune encephalomyelitis, immune regulation, immunoglobulin, multiple sclerosis
Introduction
One key outcome from autoimmunity is a disruption in the balance between pro- and anti-inflammatory immune responses, ultimately leading to potentially irreparable tissue damage. It has become clear that B lymphocytes can contribute both to the pathogenesis and regulation of autoimmunity [1]. The exact mechanisms of these dual functions have been difficult to unravel, given that B cells perform a spectrum of functions during an inflammatory immune response. Also, a complicating factor is that are a number of B cell subsets each with unique functions and anatomical locations. Broadly, B cells are divided into the B1 and B2 subpopulations.
B1 cells primarily reside in the peritoneal and pleural cavities, but are also found in the spleen in low numbers and through production of natural IgM, which is often germline encoded and polyreactive contribute to the early innate defense against pathogens. Also contributing to innate immunity are marginal zone B cells that reside in the spleen and due to their preactivated phenotype can quickly differentiate into antibody secreting plasmablast and plasma cells in the absence of T cell help. The majority of peripheral B2 cells are called follicular B cells and are the most prominent B cells in the spleen and lymph nodes. Follicular B cells help form the germinal center where they generate high affinity IgG antibodies in response to T-dependent antigens. Although each subclass of B cells is highly efficient in the generation of antibodies, they all also have the capacity to present antigen and produce a spectrum of pro- and anti-inflammatory cytokines.
The elucidation of the role of B cells in multiple sclerosis (MS) was greatly facilitated by the generation of an animal model now referred to as experimental autoimmune encephalomyelitis (EAE). Although no animal model completely recapitulates a complex human disease, such as MS, the ability to induce EAE in a number of species by a variety of mechanisms allows for the testing of specific hypotheses in the most relevant model. EAE can be induced in many species including rabbit, guinea pig, rat, mouse and monkeys.
Although early studies were conducted in rabbit, guinea pig and rat most current studies are conducted in mouse due to their smaller size and the copious reagents and genetically altered mouse strains available. EAE can be induced by both active and passive approaches. For active induction, animals are immunized with self-antigen in the form of spinal cord homogenate, purified self-protein or as a self-peptide emulsified in adjuvant, typically complete Freund's adjuvant (CFA). Active EAE induction in mice also includes the injection of two doses of pertussis toxin.
Passive EAE induction involves the adoptive transfer of encephalitogenic T cells with specificity to self-antigens. The recipient animals are often irradiated to achieve a more consistent and predictable disease course [2]. The encephalitogenic T cells are derived from self-antigen immunized animals or from TCR-transgenic (tg) mice with self-reactivity. EAE onset occurs 7–10 days later and depending on the animal species and strain the disease course can vary from acute and monophasic, acute and chronic, progressive or relapsing and remitting.
Induction of EAE is due to an immune response to self-antigen presented by MHC class II molecules. In mice the three most prominent myelin antigens used are myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte protein (MOG). The antigen used for EAE induction is dictated by the MHC haplotype of the experimental animal. Table I outlines the strain, antigen usage and typical EAE disease course of the most commonly used rat and mouse models. The evolution of EAE models over the last century has led us to a better understanding of the complex immune interactions that result in disease and has also allowed the discovery of regulatory mechanisms that prevent and control the extent of disease. This review will focus on the role of the B lymphocyte outlining our current understanding of their pathogenic and regulatory functions in EAE.
Table I.
Common models of rat and mouse EAE
| Species | Strain | Antigen | EAE induction | Disease course | B cell role | Referencesa |
|---|---|---|---|---|---|---|
| Rat | Lewis | MBP protein | Active immunization | Acute Monophasic Recovery | EAE onset, Antibody? | [9,10,14] |
| Mouse | C57BL/6 | MOG35-55 peptide | Active immunization | Acute Monophasic Recovery or chronic | Regulatory role in recovery | [28,43,50,61] |
| Mouse | C57BL/6 | rhMOG protein | Active immunization | Acute Monophasic Chronic | EAE onset, Antibody | [41–43,45] |
| Mouse | SJL/J | PLP139-151 peptide | Active immunization | Relapsing and remitting Chronic | None known | [49] |
| Mouse | B10.PL | MBPAc1-11 peptide | Active immunization | Acute Monophasic Recovery | Regulatory role in recovery | [39] |
| Mouse | B10.PL | MBPAc1-11 peptide | Passive transfer of MBP Ac1-11-specific T cells | Acute Monophasic Recovery | Regulatory role in recovery | [2, 56] |
References are selected from this review and are not necessarily the first historical report.
Early evidence for a pathogenic role for B cells and immunoglobulin in EAE
The experimentally induced animal disease now known as EAE was first investigated in rabbits and monkeys [3]. It was in 1949 that EAE induction in mice was successful [4]. This achievement literally opened the floodgate regarding EAE research with thousands of studies having been published using the mouse model. However, most of the early studies defining the role of immune cells in EAE were conducted in the rat. In 1953 the transfer of EAE from a donor rat immunized with guinea pig spinal cord homogenate in adjuvant to another rat was first demonstrated by parabiosis, suggesting that the disease was caused by either soluble and/or cellular components within the blood [5].
It then took until 1960 to demonstrate that transfer of lymph node cells alone from a rat with EAE was sufficient to lead to EAE in the recipient [6]. These early insights into the immunological nature of EAE were remarkable given that T and B lymphocytes weren't definitively identified until the mid 1960s [7]. The identification of T cells alone as being sufficient for EAE induction came from studies conducted in 1981, whereby the adoptive transfer of MBP-specific T cell lines resulted in disease in syngeneic rats [8]. Although this later experiment implicated T cells in EAE induction, it did not rule out the possibility that the transferred T cells interacted with B cells that then in turn played a pathogenic role in EAE.
The first hard evidence that B cells were involved in EAE pathogenesis came from studies in rats administered anti-IgM resulting in a depletion of B lymphocytes as well as serum IgM that was accompanied by low levels of IgG. These depleted rats failed to both respond to LPS and make antibodies to sheep red blood cells [9]. These same rats failed to exhibit signs of EAE following immunization with either guinea pig spinal cord homogenate or purified MBP [9]. In a follow-up study, the transfer of serum containing anti-MBP into the anti-IgM-treated resistant rats resulted in EAE [10].
Although this experiment suggested a role for Ig in EAE induction and/or pathogenesis, the adoptive transfer of activated lymphocytes from MBP immunized anti-IgM treated rats into either WT or anti-IgM-treated rats also lead to EAE [10]. Thus, the final conclusion was that passively induced EAE was not dependent upon the presence of B cells and antibody production [10]. Of interest in terms of antibody-mediated pathogenesis is that the studies conducted by Willenborg and colleagues used MBP as the immunogen. Thus, even if anti-MBP antibodies were generated, how they would invoke pathogenic mechanisms is not clear due to the intracellular location of MBP.
The answer as to whether or not antibodies play a pathogenic role likely resides in the specificity of the antibodies. When whole spinal cord homogenate is used, antibody responses to multiple myelin antigens is possible, with myelin oligodendrocyte glycoprotein (MOG) being of particular interest because of its cell surface expression making it accessible to antibody recognition. Indeed, serum containing high levels of immunoglobulins specific to MOG, but not MBP, when infused into the subarachnoid space of rats resulted in demyelination [11]. Further support for anti-MOG in EAE pathogenesis came from studies using MOG monoclonal antibodies (mAb), which accelerated EAE in Lewis rats and exacerbated passively induced EAE in SJL mice and Lewis rats [12–14].
Similar findings were reported in a primate EAE model, where immunization with whole white matter or rat rMOG (aa 1-125) led to inflammation and extensive demyelination [15]. In contrast, immunization with the intracellular proteins MBP or proteolipid protein (PLP) resulted in inflammation, but no demyelination [15]. As with rodents, the transfer of anti-MOG into MBP-immunized nonhuman primates lead to fully demyelinated lesions [15]. Thus collectively, these early studies indicated that T cells alone could induce and drive EAE disease, but that myelin-specific antibodies could augment EAE pathogenesis if they recognize a protein expressed on the cell surface of oligodendrocytes. In other words, the studies fell short of demonstrating a requirement for B cells or their production of immunoglobulin in EAE induction.
Potential mechanisms of antibody augmentation of EAE
Demyelination accompanying the transfer of an anti-MOG mAb provided solid evidence of a pathogenic role for B cells via production of Ig in EAE. However, of interest is that all of these early studies used the same antibody clone (8.18.C5) [11–16], which is a mouse IgG1. Thus, it is not surprising that all of the studies obtained consistent results with the induction of demyelination being a critical factor. The mechanism by which Ig could induce demyelination includes complement and Fc receptor (FcR) engagement.
The ability of anti-MOG antibodies to fix complement was directly related to their pathogenesis with IgG2a mAbs being the most potent [17]. Interestingly, the 8.18.C5 mAb is an IgG1 and consistent with characteristics of this subclass of Ig its ability to fix complement and induce pathogenesis was lower that for the IgG2a mAb [17]. As with the 8.18.C5 mAb, the IgG2a Z12 mAb, also likely mediated demyelination via a complement-dependent mechanism that did not occur in MOG-deficient animals [18].
Further evidence for a role for complement in antibody-induced demyelination was the blockage of pathogenesis by the administration of a soluble recombinant form of human complement receptor 1, which also led to a reduction in the deposition of C1, C3 and C9 within the CNS [19]. The issue of a role for complement was definitively resolved by the use of knockout mice deficient in FcRγ and C1q, which showed that the demyelinating capacity of mAb 8.18.C5 relies on complement activation but not FcR that contain the γ chain (FcγR1, FcγRIII, Fc1εRI and FcαRI) [20,21].
Further evidence that complement plays a role in antibody-induced demyelination was the finding that rats deficient in C6, which prevents the formation of the membrane attack complex, were not susceptible to exacerbated disease following anti-MOG administration [22]. However, the above experiments did not resolve the finding that FcRγ knockout mice are resistant to EAE induction by rat MOG immunization [23]. Because the FcRγ signaling subunit is also utilized by proteins other than FcR, the exact role of FcR in EAE induction remains unresolved.
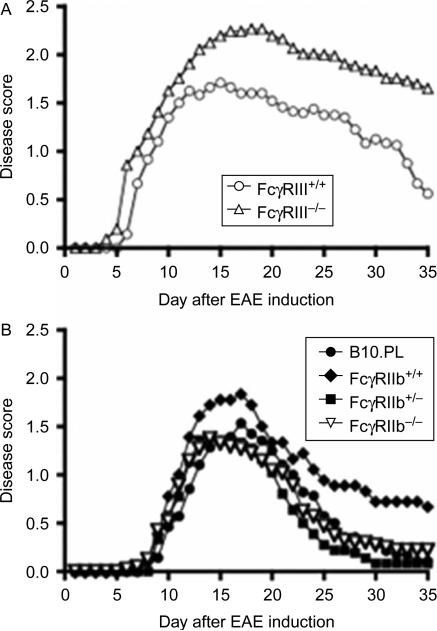
Because the FcRγ subunit is utilized by a number of FcR, we determined whether FcγRIII played a role in EAE pathogenesis by inducing EAE in mice deficient its ligand binding a chain [24]. FcγRIII is a low affinity FcR and plays a role in activating immune cells such as macrophages and mast cells through binding of Ig. Mice deficient in FcγRIII had deficiencies in mast cell degranulation, IgG-dependent anaphylaxis, and Arthus reactions [24]. Using a passive transfer model of EAE [2], we found that FcγRIII-deficient mice were similar to their WT littermates in day of disease onset, but exhibited a significantly more severe disease course, indicating that FcγRIII plays a protective or inhibitory role during EAE progression presumably through the activation of an immune cell (Figure. 1A). However, neither the target cell nor the mechanism of this regulation is known.
Figure 1.
B6.129P2-Fcgr3tm1Sjv/J and B6;129S-Fcgr2btm1tk/J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and backcrossed to B10.PL mice for three generations to generate H-2uxu mice that were then intercrossed to generate WT, heterozygous and knockout mice. EAE was induced in littermates by adoptive transfer of 1 × 106 encephalitogenic T cells generated in vitro from the spleen of B10.PL mice bearing a TCR transgene specific for MBP. Mice were scored for signs of disease daily starting on day 4 using a 5-point scale: 0- no disease; 1- tail weakness or wobbly walk; 1.5-tail weakness and wobbly walk; 2-hind limb paresis; 2.5- paralysis in one hind limb; 3- hind limb paralysis; 4-hind and forelimb paralysis and 5- death. A) Data are the mean daily scores of 24 FcγRIII+/+ and 43 FcγRIII–/ – mice from 5 separate experiments with 4-13 mice in each experiment. The FcγRIII–/ – group contains two mice that died on day 13 and 16 and were scored as 5 for the remaining timepoints. p = 0.0001. B) Data are the mean daily scores of 14 B10.PL, 9 FcγRIIb+/+, 18 FcγRIIb+/ – and 20 FcγRIIb–/ – mice from 4 separate experiments with 1-8 mice in each experiment. Mice that did not receive a score of at least 1.5 were not included in the disease curves.
Since FcγRIIb is an inhibitory receptor [25], we also determined whether deletion of this receptor would result in more severe EAE. However, as shown in Figure 1B, there was no difference in the EAE disease curves between B10.PL control mice and FcγRIIb+/- and FcγRIIb-/- mice. The FcγRIIb+/+ littermate controls did not recover to the same extent as the other groups, but this was likely due to this group containing the lowest number of experimental mice.
A similar study in DBA/1 mice immunized with rat MOG FcγRIIb-/- mice were similar to their WT littermates in disease onset, but then the text stated that they exhibited a trend toward worsened disease [23]. However, no statistics were provided and one caveat to the report was a potential error in the figure legend that indicated that the WT mice exhibited the more severe disease [23]. Nevertheless, the difference in disease severity was not substantial and when combined with our data indicates that FcγRIIB plays a minimal to no role in EAE. In our study, using passively induced EAE, in retrospect the lack of a role for FcγRIIB is not surprising since we now know that this model of EAE is antibody-independent.
Evidence that complement is not required for EAE induction are studies demonstrating that mice deficient in C3, C3aR or C5 or rats deficient in C6 succumb to MOG-peptide EAE similar to WT mice, although the trend was a less severe EAE disease course [26–30]. One caveat to the above studies is that they were all performed using an EAE model induced by antigen immunization in CFA. This experimental design does not resolve the issue of whether components of the complement system play a role in T cell priming, with reduced priming leading to reduced EAE severity. This issue was addressed using two approaches. In the first, cobra venom factor, which depletes C3 and C5, was administered to Lewis rats on days 9 and 12 post-immunization leading to attenuation of EAE disease [31].
In the second approach, EAE was induced by adoptive transfer of WT encephalitogenic T cells into WT or C3-/- recipients, thus avoiding T cell priming in a C3-deficient environment, which resulted in attenuated disease in the C3-/- mice. However, the variability in disease scores were large and no statistics were provided [32]. Thus while good evidence support a role for complement in antibody-induced demyelination, its impact on progression of EAE disease is likely marginal and it may even play a positive role in remyelination and axonal survival [29].
Another function of anti-myelin antibodies is the clearance of myelin debris by opsonization of myelin facilitating its phagocytosis by microglial cells and macrophages [33–35]. This clearance of myelin could be beneficial or it could be pathogenic. In support of the former possibility, macrophages that had phagocytized myelin were shown to reduce antigen-specific T cell proliferation in a mechanism that involved macrophage production of nitric oxide [36]. However, in this same study, the onset and severity of EAE were higher in mice treated with myelin [36].
Nevertheless, the clearance of myelin debris is likely essential if the lesion is to recover. Although the phagocytosis of myelin is a sign of demyelination, it is not necessarily indicative of the pathogenic mechanism that led to the demise of the oligodendrocyte. In addition, reparative properties of natural IgM with specificity for myelin have been implicated by the Rodriguez laboratory in promoting remyelination [37].
Current state of our understanding of B cell pathogenesis in EAE
Although earlier experiments demonstrated mechanisms for antibody-dependent roles in EAE, we now appreciate more about B cell function, not only in characterization of subtypes and phenotypic markers, but also with respect to activation requirements, antigen presentation, and production of cytokines that are unique to the B cells in immune responses. Thus in the late 1990s several groups re-examined the question of whether B cells were required for the induction of EAE using a mouse deficient in B cells by the disruption of the IgM heavy chain (μMT) [38].
In the first report, we demonstrated that μMT mice on the B10.PL background were susceptible to EAE following immunization with MBP peptide [39]. A second study conducted by Ruddle and colleagues confirmed this result using C57BL/6μMT mice immunized with MOG peptide [40]. A third study confirmed the Ruddle result, but added additional contradictory data in human rMOG-immunized μMT mice, which did not succumb to EAE onset (41). In a follow-up study, the Cross group demonstrated a requirement for MOG-specific antibody in EAE pathogenesis in the human rMOG group [42].
Finally, the Ruddle group demonstrated that the species source of the rMOG was critical for determining whether antibody was required for EAE. They confirmed that EAE induction by human rMOG was B cell-dependent, while rat rMOG was not [43]. Interestingly, the anti-MOG titers were not different in the two groups, thus small differences in the sequence of rat and human MOG dictates as to whether or not pathogenic antibodies are formed. These cumulative studies demonstrate that anti-myelin antibodies do play a role in EAE pathogenesis and by extrapolation, MS.
Recently, the above studies have been revisited using a new model of B cell depletion via anti-CD20. The depletion of B cells prior to EAE onset with human rMOG prevented EAE onset in one study [44] and attenuated EAE severity in a second study [45]. Both studies reported diminished T cell responses [45,46], which suggests that B cells may function as antigen presenting cells (APC). Evidence for this possibility comes from an elegant study whereby MOG-TCR transgenic mice on the SJL/J background developed spontaneous disease accompanied by the generation of anti-MOG antibodies mainly of the IgG1 and IgG2a/b isotypes, which were able to activate complement [47].
Anti-CD20 depletion of B cells largely prevented spontaneous EAE and the production of anti-MOG antibodies [47]. Even when MOG-TCR transgenic mice were crossed with an anti-MOG BCR transgenic, anti-MOG antibody was generated from the endogenous B cell pool [47]. This result indicated that T cell:B cell interactions are occurring with self-antigen specific B cells serving as APC facilitating the activation of the autoreactive T cells and their ultimate production of autoantibodies. Interestingly, while the MOG-TCR transgenic succumbs to spontaneous EAE by expanding endogenous B cells, MOG-BCR transgenics cannot expand endogenous autoreactive T cells to drive spontaneous EAE [48].
Thus, autoreactive T cells are the driving force for the induction of EAE and while autoreactive B cells can potentially serve as APC, they on their own, even in the presence of high titer myelin-reactive antibody, are not sufficient to induce EAE. Rather B cells seem only to augment disease caused by T cells.
Evidence for a regulatory role for B cells in EAE
Our study whereby we demonstrated that B cells were not required for the onset of EAE, revealed the unexpected finding that B10.PL mice deficient in B cells (μMT) failed to spontaneously recover from EAE [39]. This was the first indication in animal autoimmune models that B cells play a regulatory role in downregulating inflammation. In contrast, similar studies in C57BL/6 and (B10.PL × SJL/J)F1 mice did not suggest this novel B cell regulatory mechanism [40,42,49].
When an EAE induction protocol that allowed for recovery from EAE was used, C57BL/6μMT mice also were not able to recover from EAE [50]. This later study also presented data indicating that B cell production of IL-10 and expression of CD40 were requirements for their regulatory activity [50]. The Tedder laboratory then demonstrated that signaling was important for regulatory B cell functions since genetic disruption of CD19 exacerbated MOG-peptide EAE [51].
Interestingly, the transfer of CD19-/- B cells from MOG peptide-primed mice also exacerbated disease [51]. The signal transduction pathways affected in CD19-/- B cells in this study is unclear since CD19 can affect CD40 (discussed above) and TLR signaling in addition to its well-established affects on the BCR [52]. In regards to TLR signaling, Myd88 expression by B cells was required for recovery from EAE in MOG-peptide EAE [53]. More specifically, the regulation required TLR2/4. TLR2/4 ligands are present in the CFA adjuvant used to induce EAE and they also induce the production of IL-10 by B cells [53]. Thus, it is highly possible that the CFA used to induce EAE is facilitating and/or driving B cell regulation by inducing them to produce IL-10.
The μMT mouse, although a powerful tool, has many caveats that include alterations in T cell function that could affect the interpretations of EAE studies, given that conventional EAE is mediated by CD4 T cells. However, this concern was recently alleviated by the recapitulation of both the pathogenic and regulatory roles for B cells in EAE using an anti-CD20 depletion strategy. The depletion of B cells prior to immunization with human rMOG (B cell-dependent), prevented EAE onset in the mouse [45]. In the marmoset, anti-CD20 depletion of B cells three weeks after EAE induction with human rMOG prevented the development of neurological deficits [54]. Consistent with a pathogenic role for B cells in the human rMOG model. The regulatory role of B cells was confirmed in two separate studies, whereby depletion of B cells prior to EAE induction in the mouse prevented recovery [45,55].
Because all of the above studies utilized EAE models induced by immunization using CFA and pertussis toxin, we sought to determine whether regulatory B cells functions could be revealed in their absence. To address this question, we used a passive EAE model whereby in vitro activated encephalitogenic T cells from MBP-TCR transgenic mice were adoptively transferred into either WT B10.PL or B10.PLμMT mice [2].
The μMT mice did not recover from EAE, indicating that regulatory B cell functions were not dependent upon the presence of CFA [56]. In this same study, we showed that B cell expression of B7 was a requirement for their regulatory activity [56]. The requirement for B cell expression of both CD40 and B7 is highly suggestive that cognate interactions between B cells and T cells required [50,56]. To begin to address this question, we investigated whether B cells regulated the presence of CD4+Foxp3+T regulatory cells (Treg) in the CNS and found that in the absence of B cells or B cell expressed B7 the emergence of Treg in the CNS was delayed [50].
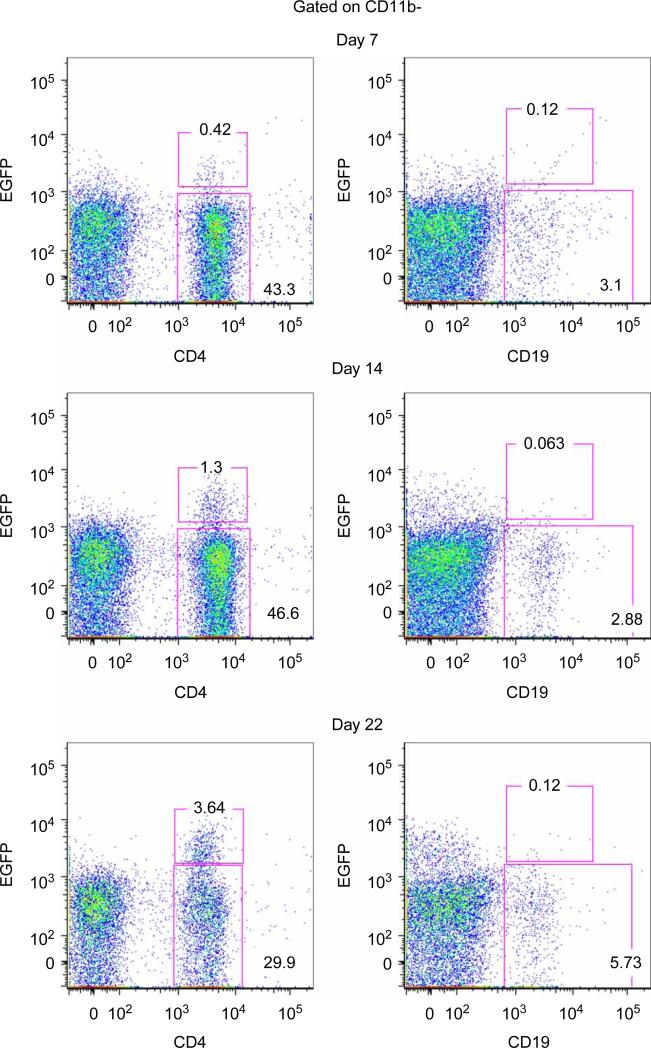
Because B cell production of IL-10 was previously implicated in their regulatory activity, we also examined IL-10 within the CNS during EAE and found that it was also delayed [50]. Using an IL-10 reporter mouse [57], we found that CD4+T cells were the most prominent IL-10 producing cells in the CNS (Figure 2). Of the few B cells that were in the CNS, they were not producing IL-10 (Figure. 2). Our data are consistent with a study by Anderton and colleagues that also reported few B cells in the CNS during EAE and CD4+CD25+ cells being the primary IL-10 producing cells [58].
Figure 2.
CD4+ cells produce IL-10 in the CNS during EAE. EAE was induced in IL-10–IRES–eGFP reporter mice by adoptive transfer of 1 × 106 encephalitogenic T cells per mouse. Clinical signs of EAE were scored daily. At onset (day 7), peak (day 14) and resolution (day 22) of EAE disease the percentages of CD11b–-gated CNS infiltrating CD4+ and CD19+ that were EGFP– and EGFP+ were determined by flow cytometry and the percentage of each population is indicated on the graphs. Representative data from one of three mice at each time point is shown.
IL-10 producing B cell subsets and potential mechanisms of B cell-derived IL-10 suppression during EAE
Although all B cell subsets have the capacity to produce IL-10, especially upon binding of TLR ligands, of particular interest is the phenotype of the IL-10 producing cell during EAE. A clue came from the Tedder laboratories’ analysis of CD19-/- and human CD19 transgenic (hCD19tg) mice, with the later harboring hyperactive B cells. CD19/- mice exhibit enhanced T cell-mediated inflammation (contact hypersensitivity (CHS)), and inflammation in hCD19tg mice was reduced [59].
When B cell populations were compared in the two mice, it was found that CD1dhiCD5+B cells were absent from CD19-/- mice and increased in hCD10tg mice [59]. It was subsequently found that these B cells produce IL-10 and suppress inflammation upon transfer [59]. It should be noted that IL-10 production was observed after stimulation with LPS, PMA and ionomycin while in the presence of monensin. Using an IL-10 reporter mouse, the population of splenic B cells with the greatest percentage of IL-10 producers at baseline was shown to be CD19+B220low/-(35%) [57]. Upon LPS administration, this percentage increased to 57%. IL-10 production in the conventional CD19+B220+ subset increased from 1 to 10% following LPS administration [57]. The CD19+CD138+ population exhibited the most dramatic change with LPS increasing the percentage of IL-10 producers from 7.1 to 65% [57]. These studies confirm that TLR4 signaling is a potent inducer of IL-10 production by B cells in the absence of any other inflammatory stimuli. However, using these same mice, we were unable to detect such an increase in IL-10 producing B cell in the spleen using an EAE adoptive transfer model that is independent of exogenously added TLR agonists (CFA) (data not shown).
In the CHS model used in the above study, the TLR signal likely occurred through TLR2 as this receptor has been shown to be essential for oxazolone induced CHS [60]. However, it is not clear how physiologically relevant the above vitro B stimulation is to EAE or MS since it is essentially equivalent to a strong BCR signal occurring simultaneously with a TLR signal. In EAE induced by immunization the TLR signal likely comes from the CFA. However, the BCR signal is more difficult to explain in models of EAE induced with peptides or by adoptive transfer in which antibodies are not generated.
Nevertheless, the Tedder laboratory showed that the adoptive transfer of CD1dhiCD5+B cells from CD19-/- mice or from MOG-sensitized animals prior to EAE induction by MOG-peptide attenuated EAE disease severity [61]. In the later group, B cells from IL-10-/- mice did not reduce disease severity, indicating an IL-10-dependent mechanism [61].
An important question regarding the mechanism of B cell regulation during EAE is whether antigen specificity is required. For CD1dhiCD5+B cells described by the Tedder laboratory the absence of MHC class I/II hindered their development. In regards to BCR diversity, the CD1dhiCD5+B cells developed in BCR transgenic mice bearing a transgene specific for hen egg lysozyme (HEL) [62], but failed to produce IL-10 when stimulated with a combination of LPS, ionomycin and PMA [63].
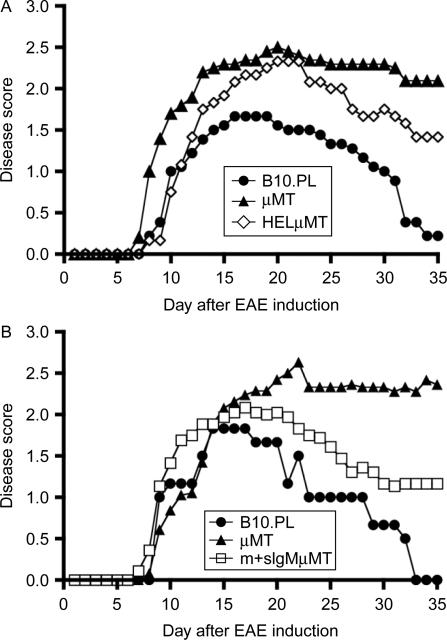
Although it was not addressed whether MHC molecules and BCR diversity are required for the regulatory activity of CD1dhiCD5+B cells. We investigated whether BCR diversity is required for B cells to promote recovery from EAE. To address this question, we bred the HEL BCR transgenic [62] to B10.PLμMT mice generating mice bearing a single IgM BCR. When EAE was induced by adoptive transfer, we found that HELμMT mice exhibited a cumulative disease score intermediate between WT and μMT mice (Figure. 3A). As these data suggested that BCR diversity did play a role in EAE recovery, we performed the same experiment using an IgM heavy chain BCR transgenic mouse that we also bred to B10.PLμMT mice (Vh186.2μMT). This mouse has some limited BCR diversity because the heavy chain can pair with any light chain [64].
Figure 3.
C57BL/6-Tg(IghelMD4)4Ccg/J (Jackson Laboratories, Bar Harbor, ME) and Vh186.2 BCR transgenic mice, which were kindly provided by Dr. M. Shlomchik, Yale University, New Haven, CT, were bred to B10.PLμMT mice to generate transgenic positive H-2uxu mice that were then intercrossed with transgenic negative mice to generate transgene positive μMT mice. EAE was induced as for Figure 1. A) Data are the mean daily scores of 9 B10.PL, 13 HEL-negative μMT (littermate control) and 15 HELμMT mice from two experiments for the B10.PL and three experiments for the μMT and HELμMT mice with 3-9 mice per group. B) Data are the mean daily scores of 3 B10.PL, 19 μMT and 18 Vh186.2 BCR mice from one experiment for the B10.PL and two experiments for the μMT and Vh186.2 BCR mice and each experiment contained 3–15 mice per group.
Interestingly, we obtained the same result with the Vh186.2μMT mice that also exhibited a partial ability to recovery (Figure. 3B). From these cumulative data, we concluded that the mere presence of B cells is not sufficient for their regulatory activity in EAE. However, the biological context in which B cells require BCR specificity in antibody-independent EAE has not been explored.
IL-10 is a pleiotropic cytokine that exhibits very potent anti-inflammatory activity [65]. The location, mechanism and cellular targets of B cell-derived IL-10 regulation in EAE are not known. Precedence for a role for IL-10 in controlling EAE are studies showing that IL-10 transcripts reach maximum at the peak of EAE disease just before the animals begin to recover [56,66,67], IL-10-/- mice exhibit a severe EAE disease course [68], IL-10 transgenic mice are resistant to EAE [69] and intracranial injection of IL-10 producing fibroblasts reduced EAE severity [70].
However, the regulation of EAE by B cell-derived IL-10 production does not likely occur in the CNS because low numbers of B cells enter the CNS during disease and we have not been able to detect their IL-10 secretion using an IL-10 reporter mouse (Figure. 2) [57]. Interestingly, CD4+T cells were the most prevalent population of IL-10 producers in the CNS (Figure. 2), which are likely Foxp3+ Treg. CD1dhiCD5+B cells are typically classified as B1 cells and reside in the peritoneal cavity; however, these cells do recirculate and are also found in the spleen and likely gut tissues.
Regardless of the site of regulation, based on the known functions of IL-10 the cellular target is not likely B cells themselves, because IL-10 enhances B cell functions [65]. Because IL-10 effectively downregulates the antigen presentation capacity of dendritic cells [65], this cell type is the likely target in MOG-peptide induced EAE with CFA. Evidence for this are two studies demonstrating that TLR-stimulated B cells producing IL-10 have been shown limit the T cell priming/activation capacity of dendritic cells [53,71]. Interestingly, the study by Lo-Man and colleagues detected IL-10 production from CD5+, but not CD5- B cells, which was recapitulated in later studies by the Tedder group [63].
IL-10-independent mechanisms of regulatory B cells
The cumulative studies to date convincingly demonstrate that B cell production of IL-10 can limit the severity of EAE. However, B cell regulation in an IL-10-independent manner has also been described. Previously, helminth infection was shown to greatly decrease EAE severity in SJL/J mice [72]. Skewing of the immune response away from a Th1 and towards a Th2 response was suggested as the mechanism [72,73]. The transfer of splenic B cells from helminth infected, but not from naïve mice, prior to EAE induction reduced both the incidence and severity of EAE [74].
B cells from the mesenteric lymph node from infected mice were not enriched for CD5, but did express high levels of CD23 (marker of follicular B cells) and increased production of IL-6 and IL-10 upon TLR9 signaling [74]. However, the B cell regulation was found to be independent of IL-10, leaving the mechanism of suppression unknown [74]. Since B cells have the capacity to produce Th cell polarizing cytokines [75], the sensitized B cells could have skewed encephalitogenic T cell priming towards a Th2 phenotype that would result in less severe EAE because Th2 cells are not encephalitogenic.
Another possible mechanism whereby B cells regulate EAE in an IL-10-independent manner is via the induction or regulation of Treg. In B cell deficient mice, we reported that the emergence of Treg into the CNS was delayed as compared to WT mice and was dependent upon B cell expression of B7 [56]. Since B7 is important for Treg development and function [76,77], these studies suggested that B cell:Treg interactions occur during EAE. The nature of this interaction is unknown, but data from others have shown the B cells can drive the generation of inducible (i) Treg in vitro in a B7-dependent manner [78]. Also in vitro, B cells were shown to expand Treg numbers via a TGF-β-dependent mechanism suggesting the generation of iTreg [79], although the later wasn't definitely demonstrated [80].
An in vivo role for B cells in Treg induction/expansion is supported by several groups that have reported that μMT have reduced percentages of Treg [80,81]. A similar observation was observed during EAE in anti-CD20-depleted mice [45]. Additional studies have reported a role for B cells in promoting Treg induction/expansion in an allogeneic manner [82], in oral tolerance [81], during antigen presentation of self-antigen [83] and in arthritis autoimmune models [84]. An importance for Treg in controlling EAE was demonstrated by a reduction in EAE severity following the adoptive transfer of Treg and augmented disease following the depletion of Treg during EAE [58, 61, 85]. Although the exact location and mechanism of Treg suppression during EAE is not known, Treg are found within the CNS during EAE [45, 56, 58, 61, 86, 87].
Conclusions
Although B cells were first discovered as the producers of immunoglobulin our understanding of B cells rapidly expanded to include antigen presentation and cytokine production. We now know that each of the three prominent B cell functions can promote immunity, including to self-antigens, as well as contribute to the downregulation of inflammation. However, much still needs to be learned regarding how each B cell function can harnessed to either prevent or induce the recovery from MS. B cell depletion therapy in early MS clinical trials with anti-CD20 showed remarkable efficacy in preventing disease progression [88,89]. Although the animal studies in EAE have shed some light on the possible immunological mechanisms leading to this encouraging result, the exact role(s) of B cells in MS remains elusive.
Acknowledgements
The authors thank Dr. Mark Shlomchik, Yale University, New Haven, CT for providing the Vh186.2 mice. We also thank Dr. Eugene Ponomarev for technical assistance and Shelley Morris for assistance with the mice. This work was supported by grants from the National Multiple Sclerosis Society (RG 3299-A-2) and from the NIH, NIAID (R01 AI069358).
Footnotes
Declaration of interest: The authors report no conflicts of interests. The authors alone are responsible for the content and writing of the paper.
References
- 1.Ray A, Mann MK, Basu S, Dittel BN. A case for regulatory B cells in controlling the severity of autoimmune-mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroimmunol. 2010;230:1–9. doi: 10.1016/j.jneuroim.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittel BN, Merchant RM, Janeway CA., Jr. Evidence for Fas-dependent and Fas-independent mechanisms in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6392–6400. [PubMed] [Google Scholar]
- 3.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 4.Olitsky PK, Yager RH. Experimental disseminated encephalomyelitis in white mice. J Exp Med. 1949;0:213–224. doi: 10.1084/jem.90.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipton MM, Freund J. The transfer of experimental allergic encephalomyelitis in the rat by means of parabiosis. J Immunol. 1953;71:380–384. [PubMed] [Google Scholar]
- 6.Paterson PY. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Nun A, Wekerle H, Cohen IR. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol. 1981;11:195–199. doi: 10.1002/eji.1830110307. [DOI] [PubMed] [Google Scholar]
- 9.Willenborg DO, Prowse SJ. Immunoglobulin-deficient rats fail to develop experimental allergic encephalomyelitis. J Neuroimmunol. 1983;5:99–109. doi: 10.1016/0165-5728(83)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Willenborg DO, Sjollema P, Danta G. Immunoglobulin deficient rats as donors and recipients of effector cells of allergic encephalomyelitis. J Neuroimmunol. 1986;11:93–103. doi: 10.1016/0165-5728(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 11.Linington C, Lassmann H. Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol. 1987;17:61–69. doi: 10.1016/0165-5728(87)90031-2. [DOI] [PubMed] [Google Scholar]
- 12.Schluesener HJ, Sobel RA, Linington C, Weiner HL. Monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987;139:4016–4021. [PubMed] [Google Scholar]
- 13.Lassmann H, Brunner C, Bradl M, Linington C. Experimental allergic encephalomyelitis: the balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol. 1988;75:566–576. doi: 10.1007/BF00686201. [DOI] [PubMed] [Google Scholar]
- 14.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 15.Genain CP, Nguyen MH, Letvin NL, Pearl R, Davis RL, Adelman M, Lees MB, Linington C, Hauser SL. Antibody facilitation of multiple sclerosis-like lesions in a nonhuman primate. J Clin Invest. 1995;96:2966–2974. doi: 10.1172/JCI118368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linnington C, Webb M, Woodhams PL. A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol. 1984;6:387–396. doi: 10.1016/0165-5728(84)90064-x. [DOI] [PubMed] [Google Scholar]
- 17.Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol. 1993;143:555–564. [PMC free article] [PubMed] [Google Scholar]
- 18.Morris-Downes MM, Smith PA, Rundle JL, Piddlesden SJ, Baker D, Pham-Dinh D, Heijmans N, Amor S. Pathological and regulatory effects of anti-myelin antibodies in experimental allergic encephalomyelitis in mice. J Neuroimmunol. 2002;125:114–124. doi: 10.1016/s0165-5728(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 19.Piddlesden SJ, Storch MK, Hibbs M, Freeman AM, Lassmann H, Morgan BP. Soluble recombinant complement receptor 1 inhibits inflammation and demyelination in antibody-mediated demyelinating experimental allergic encephalomyelitis. J Immunol. 1994;152:5477–5484. [PubMed] [Google Scholar]
- 20.Urich E, Gutcher I, Prinz M, Becher B. Autoantibody-mediated demyelination depends on complement activation but not activatory Fc-receptors. Proc Natl Acad Sci USA. 2006;103:18697–18702. doi: 10.1073/pnas.0607283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takai T. Roles of Fc receptors in autoimmunity. Nat Rev Immunol. 2002;2:580–592. doi: 10.1038/nri856. [DOI] [PubMed] [Google Scholar]
- 22.Mead RJ, Singhrao SK, Neal JW, Lassmann H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168:458–465. doi: 10.4049/jimmunol.168.1.458. [DOI] [PubMed] [Google Scholar]
- 23.Abdul-Majid KB, Stefferl A, Bourquin C, Lassmann H, Linington C, Olsson T, Kleinau S, Harris RA. Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis. Scand J Immunol. 2002;55:70–81. doi: 10.1046/j.1365-3083.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- 24.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, Schmidt RE, Sandor M, Capel PJ, Daeron M, van de Winkel JG, Verbeek JS. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity. 1996;5:181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 25.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 26.Nataf S, Carroll SL, Wetsel RA, Szalai AJ, Barnum SR. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J Immunol. 2000;165:5867–5873. doi: 10.4049/jimmunol.165.10.5867. [DOI] [PubMed] [Google Scholar]
- 27.Calida DM, Constantinescu C, Purev E, Zhang GX, Ventura ES, Lavi E, Rostami A. Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol. 2001;166:723–726. doi: 10.4049/jimmunol.166.2.723. [DOI] [PubMed] [Google Scholar]
- 28.Boos L, Campbell IL, Ames R, Wetsel RA, Barnum SR. Deletion of the complement anaphylatoxin C3a receptor attenuates, whereas ectopic expression of C3a in the brain exacerbates, experimental autoimmune encephalomyelitis. J Immunol. 2004;173:4708–4714. doi: 10.4049/jimmunol.173.7.4708. [DOI] [PubMed] [Google Scholar]
- 29.Weerth SH, Rus H, Shin ML, Raine CS. Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am J Pathol. 2003;163:1069–1080. doi: 10.1016/S0002-9440(10)63466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tran GT, Hodgkinson SJ, Carter N, Killingsworth M, Spicer ST, Hall BM. Attenuation of experimental allergic encephalomyelitis in complement component 6-deficient rats is associated with reduced complement C9 deposition, P-selectin expression, and cellular infiltrate in spinal cords. J Immunol. 2002;168:4293–4300. doi: 10.4049/jimmunol.168.9.4293. [DOI] [PubMed] [Google Scholar]
- 31.Piddlesden S, Lassmann H, Laffafian I, Morgan BP, Linington C. Antibody-mediated demyelination in experimental allergic encephalomyelitis is independent of complement membrane attack complex formation. Clin Exp Immunol. 1991;83:245–250. doi: 10.1111/j.1365-2249.1991.tb05622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szalai AJ, Hu X, Adams JE, Barnum SR. Complement in experimental autoimmune encephalomyelitis revisited: C3 is required for development of maximal disease. Mol Immunol. 2007;44:3132–3136. doi: 10.1016/j.molimm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenberg PZ, Kwon EE, Benjamins JA, Whitaker JN, Quarles RH, Prineas JW. Opsonization of normal myelin by anti-myelin antibodies and normal serum. J Neuroimmunol. 1989;23:157–166. doi: 10.1016/0165-5728(89)90035-0. [DOI] [PubMed] [Google Scholar]
- 34.Trotter J, De Jong LJ, Smith ME. Opsonization with antimyelin antibody increases the uptake and intracellular metabolism of myelin in inflammatory macrophages. J Neurochem. 1986;47:779–789. doi: 10.1111/j.1471-4159.1986.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 35.Van der Goes A, Kortekaas M, Hoekstra K, Dijkstra CD, Amor S. The role of anti-myelin (auto)-antibodies in the phagocytosis of myelin by macrophages. J Neuroimmunol. 1999;101:61–67. doi: 10.1016/s0165-5728(99)00133-2. [DOI] [PubMed] [Google Scholar]
- 36.Bogie JF, Stinissen P, Hellings N, Hendriks JJ. Myelinphagocytosing macrophages modulate autoreactive T cell proliferation. J Neuroinflam. 2011;8:85. doi: 10.1186/1742-2094-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright BR, Warrington AE, Edberg DD, Rodriguez M. Cellular mechanisms of central nervous system repair by natural autoreactive monoclonal antibodies. Arch Neurol. 2009;66:1456–1459. doi: 10.1001/archneurol.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 39.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- 41.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Lyons JA, Ramsbottom MJ, Cross AH. Critical role of antigen-specific antibody in experimental autoimmune encephalomyelitis induced by recombinant myelin oligodendrocyte glycoprotein. Eur J Immunol. 2002;32:1905–1913. doi: 10.1002/1521-4141(200207)32:7<1905::AID-IMMU1905>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 43.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental auto-immune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 44.Monson NL, Cravens P, Hussain R, Harp CT, Cummings M, de Pilar Martin M, Ben LH, Do J, Lyons JA, Lovette-Racke A, Cross AH, Racke MK, Stuve O, Shlomchik M, Eagar TN. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e17103. doi: 10.1371/journal.pone.0017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber MS, Prod'homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, Bernard CC, Martin F, Zamvil SS. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monson NL, Cravens PD, Frohman EM, Hawker K, Racke MK. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol. 2005;62:258–264. doi: 10.1001/archneur.62.2.258. [DOI] [PubMed] [Google Scholar]
- 47.Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, Wekerle H. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Litzenburger T, Fassler R, Bauer J, Lassmann H, Linington C, Wekerle H, Iglesias A. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med. 1998;188:169–180. doi: 10.1084/jem.188.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dittel BN, Urbania TH, Janeway CA., Jr. Relapsing and remitting experimental autoimmune encephalomyelitis in B cell deficient mice. J Autoimmun. 1998;14:311–318. doi: 10.1006/jaut.2000.0371. [DOI] [PubMed] [Google Scholar]
- 50.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 51.Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujimoto M, Poe JC, Inaoki M, Tedder TF. CD19 regulates B lymphocyte responses to transmembrane signals. Semin Immunol. 1998;10:267–277. doi: 10.1006/smim.1998.9999. [DOI] [PubMed] [Google Scholar]
- 53.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 54.Kap YS, van Driel N, Blezer E, Parren PW, Bleeker WK, Laman JD, Craigen JL, Hart BA. Late B cell depletion with a human anti-human CD20 IgG1k monoclonal antibody halts the development of experimental autoimmune encephalomyelitis in marmosets. J Immunol. 2010;185:3990–4003. doi: 10.4049/jimmunol.1001393. [DOI] [PubMed] [Google Scholar]
- 55.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 57.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: Contribution of CD4+CD25+regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 59.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Jin H, Kumar L, Mathias C, Zurakowski D, Oettgen H, Gorelik L, Geha R. Toll-like receptor 2 is important for the T(H)1 response to cutaneous sensitization. J Allergy Clin Immunol. 2009;123:875–882. e871. doi: 10.1016/j.jaci.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 Cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mason DY, Jones M, Goodnow CC. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/IgD transgenic mice. Int Immunol. 1992;4:163–175. doi: 10.1093/intimm/4.2.163. [DOI] [PubMed] [Google Scholar]
- 63.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 67.Jander S, Pohl J, D'Urso D, Gillen C, Stoll G. Time course and cellular localization of interleukin-10 mRNA and protein expression in autoimmune inflammation of the rat central nervous system. Am J Pathol. 1998;152:975–982. [PMC free article] [PubMed] [Google Scholar]
- 68.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 69.Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Croxford JL, Feldmann M, Chernajovsky Y, Baker D. Different therapeutic outcomes in experimental allergic encephalomyelitis dependent upon the mode of delivery of IL-10: a comparison of the effects of protein, adenoviral or retroviral IL-10 delivery into the central nervous system. J Immunol. 2001;166:4124–4130. doi: 10.4049/jimmunol.166.6.4124. [DOI] [PubMed] [Google Scholar]
- 71.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity. 2005;22:467–477. doi: 10.1016/j.immuni.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 73.Gruden-Movsesijan A, Ilic N, Mostarica-Stojkovic M, Stosic-Grujicic S, Milic M, Sofronic-Milosavljevic L. Mechanisms of modulation of experimental autoimmune encephalomyelitis by chronic Trichinella spiralis infection in Dark Agouti rats. Parasite Immunol. 2010;32:450–459. doi: 10.1111/j.1365-3024.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 74.Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, Maizels RM. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson L, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 76.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 77.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37:2400–2404. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 79.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah S, Qiao L. Resting B cells expand a CD4+CD25+-Foxp3+ Treg population via TGF-β3. Eur J Immunol. 2008;38:2488–2498. doi: 10.1002/eji.200838201. [DOI] [PubMed] [Google Scholar]
- 81.Sun JB, Flach CF, Czerkinsky C, Holmgren J. B lymphocytes promote expansion of regulatory T cells in oral tolerance: powerful induction by antigen coupled to cholera toxin B subunit. J Immuno. 2008;181:8278–8287. doi: 10.4049/jimmunol.181.12.8278. [DOI] [PubMed] [Google Scholar]
- 82.Chen X, Jensen PE. Cutting edge: primary B lymphocytes preferentially expand allogeneic FoxP3+ CD4 T cells. J Immunol. 2007;179:2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 83.Morlacchi S, Soldani C, Viola A, Sarukhan A. Self-antigen presentation by mouse B cells results in regulatory T-cell induction rather than anergy or clonal deletion. Blood. 2011;118:984–991. doi: 10.1182/blood-2011-02-336115. [DOI] [PubMed] [Google Scholar]
- 84.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 85.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 86.O'Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 87.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsingremitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 89.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, Sarkar N, Agarwal S, Smith CH. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]