Abstract
Cells of the oligodendrocyte lineage, which form the myelinating glia of the vertebrate central nervous system, undergo a stepwise developmental progression entailing specification from neuroepithelial precursors, proliferation, migration to expand and distribute the population, and differentiation to ensheath axons with myelin. Understanding the genetic mechanisms that regulate each of these steps during development is important, because this might lead to therapies to promote remyelination following neural injury or disease. Genetic studies in mice indicated that the Sox10 transcription factor is required during the differentiation stage to promote myelin gene expression. However, whether Sox10 also promotes other features of oligodendroctye differentiation remained unknown. In this study, we used time-lapse imaging to investigate the behavior and fates of oligodendrocyte lineage cells in zebrafish embryos and larvae that lacked Sox10 function. This revealed that the myelinating subset of oligodendrocyte progenitor cells (OPCs) migrates, divides, and wraps axons normally, but then dies. Nonmyelinating oligodendrocyte progenitors divided more frequently, maintaining a normal population size. New oligodendrocytes produced by these progenitors wrapped axons and survived, but did not express myelin genes at high levels. We conclude that, in addition to promoting myelin gene expression, Sox10 function is necessary for the survival of myelinating oligodedrocytes subsequent to axon wrapping but is not required for the survival of nonmyelinating OPCs.
Keywords: Glia, myelin, CNS development, zebrafish, glia-neuron interaction, glia differentiation, Nkx2.2, Olig2
INTRODUCTION
Oligodendrocytes, the myelinating glial cells of vertebrate central nervous systems, are produced by specific subsets of neural precursors during development. In the spinal cord, the majority of oligodendrocytes arise from ventral precursors called pMN cells, which express the Olig2 bHLH transcription factor (Lu et al., 2000; Masahira et al., 2006; Park et al., 2004; Takebayashi et al., 2000; Zhou et al., 2000), although a small subset of oligodendrocytes arises from dorsal spinal cord (Cai et al., 2005; Fogarty et al., 2005; Vallstedt et al., 2005). Initially, oligodendrocyte lineage cells pass through a progenitor phase. These cells, called oligodendrocyte progenitor cells (OPCs), divide to expand the OPC population and migrate to become dispersed throughout the spinal cord (Baumann and Pham-Dinh, 2001; Miller, 2002; Pfeiffer et al., 1993). Near the end of embryogenesis and during early postnatal stage, many OPCs stop dividing and extend long-membrane processes that wrap and myelinate axons. The stepwise progression of oligodendrocytes development implies the action of mechanisms that regulate their specification from neural precursors, migration from their origins to their target axons, proliferation, axon recognition, and wrapping and formation of myelin.
Among factors necessary for the development of oligodendrocyte are similar HMG-domain proteins of the SoxE family, Sox8, Sox9, and Sox10 (Wegner, 2008). Although genetic analyses in mice have revealed that these proteins have partially overlapping or redundant functions, they also play distinct roles in the oligodendrocyte cell lineage. For example, in the absence of Sox9 function, mouse embryos initially have a severe deficit of OPCs (Stolt et al., 2003). Therefore, Sox9 appears to be particularly important for OPC specification. On the other hand, Sox10-deficient embryos have normal number of OPCs, but deficits of myelin gene-expressing cells, indicating that Sox10 is required for oligodendrocyte differentiation (Stolt et al., 2002).
Analysis of mutant mice left unresolved whether Sox10 function is required early during oligodendrocyte differentiation to promote axon interaction or subsequent to axon wrapping to promote myelin formation. To address this, we investigated sox10 function and oligodendrocyte development in zebrafish. Similar to mice, sox10 mutant zebrafish embryos have normal numbers of OPCs, but a deficit of cells that expressed myelin genes. Using in vivo time-lapse imaging, we then learned that the myelinating subpopulation of oligodendrocytes initiate axon wrapping, but die soon after in sox10 mutant embryos. Notably, mutant OPCs from a nonmyelinating subpopulation divide more frequently and wrap axons following oligodendrocyte death, but do not express myelin genes. These data therefore reveal a specific requirement for Sox10 in promoting survival of cells fated to form myelinating oligodendrocytes subsequent to axon wrapping.
METHODS
Animals
Embryos were obtained by pair-wise matings and kept at 28.5°C in egg water or embryo medium (15 mM NaCl, 0.5 mM KCl, 1 mM CaCl2, 1 mM MgSO4, 0.15 mM KH2PO4, 0.05 mM NH2PO4, and 0.7 mM NaHCO3). Embryos were staged to hours postfertilization (hpf) or days postfertilization (dpf) according to Kimmel et al. (1995). To prevent pigmentation, embryos were raised in egg water containing 0.003% phenylthiourea from 2 dpf. The following zebrafish lines were used: Tg(olig2:egfp) (Shin et al., 2003), Tg(nkx2.2a:megfp) (Kirby et al., 2006), Tg(sox10(7.2):mrfp) (Kucenas et al., 2008), and colorlesstw11 (clstw11) (Dutton et al., 2001).
Whole-Mount RNA In Situ Hybridization, Immunocytochemistry, and BrdU Labeling
Embryos and larvae were fixed in 4% paraformaldehyde in 1× PBS overnight at 4°C and stored in methanol at −20°C at least for 1 day. In situ hybridization was performed essentially as described previously (Hauptmann and Gerster, 2000) using DIG-labeling RNA probes with the following modification: 4 dpf larvae were permeabilized with 10 mg/ml Proteinase K for 20 min at room temperature and additionally washed with 0.2× SSC twice for 15 min and then 0.1× SSC twice for 15 min following hybridization. cldnk (http://zfin.org/; NCBI accession no. NM_001003464) was identified in a microarray screen for oligodendrocyte-specific genes (N. Takada, unpublished). Other RNA probes used for this study were plp1a (Park et al., 2002) and mbp (Brosamle and Halpern, 2002). Images were collected using an Olympus AX70 or Zeiss Axio Observer microscope equipped with DIC optics and a Retiga Exi 1300 color digital camera. Once captured, images were imported into Adobe Photoshop, and adjustments were limited to contrast, levels, color-matching settings, and cropping.
For immunocytochemistry, embryos and larvae were fixed in 4% AB fix (4% paraformaldehyde, 8% sucrose, and 1× PBS) overnight at 4°C. Embryos and larvae for sectioning were embedded in 1.5% agar/5% sucrose and placed in 30% sucrose/PBS solution to equilibrate. The blocks were frozen in 2-methyl-butane chilled by immersion in liquid nitrogen. The sections (12 μm) were obtained by using a cryostat microtome. A rabbit antibody against a MBP peptide (CSRSRSPPKRWSTIF) was raised commercially (Open Biosystems). Other primary antibodies used were rabbit anti-Sox10 (1:500) (Park et al., 2005), rabbit anti-cleaved Caspase 3 (PC697, Calbiochem, 1:100), mouse anti-acetylated Tubulin (6-11B-1, Sigma Aldrich, 1:100), and mouse anti-BrdU (G3G4, Developmental Studies Hybridoma Bank, 1:1,000). For fluorescent detection of antibody labeling, we used Alexa Fluor 568 and Alexa Fluor 647 goat anti-mouse or goat anti-rabbit conjugates (1:200, Invitrogen). Images were collected using a Zeiss LSM 510 laser scanning confocal microscope and exported and analyzed using Volocity software (Improvision) and Adobe Photoshop. Image adjustments were limited to level settings, contrast, and cropping.
For BrdU labeling, larvae were incubated in 0.5% solution of BrdU (Roche) in embryo medium for 24 h at the indicated stages. The embryos were then fixed using 4% pfa in PBS and sectioned as described earlier. Before anti-BrdU immunocytochemistry, the sections were treated for 30 min with 2 M HCl.
In Vivo Time-Lapse Imaging
Embryos and larvae were anesthetized with 3-aminobenzonic acid ethyl ester (Tricaine), mounted laterally in 0.8% low-melting temperature agarose on 35-mm glass-bottomed dishes, and incubated in embryo medium. Confocal time-lapse movies were obtained by using a 40× oil immersion objective mounted on a Zeiss Axiovert 200 microscope equipped with a PerkinElmer spinning disk confocal system. Z-stack images were obtained and complied into a QuickTime movie using Volocity software (Improvision). During time lapse, embryos and larvae were maintained at 28.5°C using a heated stage chamber.
RESULTS
Sox10 Is Required for Oligodendrocyte Differentiation
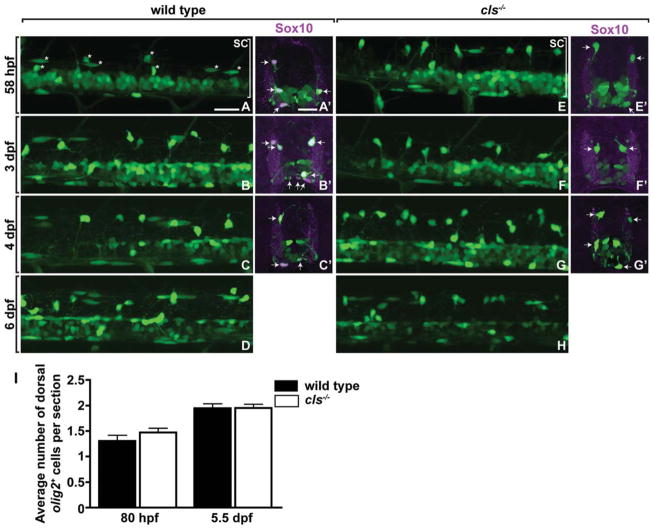
We began our investigation of zebrafish Sox10 by analyzing the number and distribution of OPCs in colourlesstw11 (cls) mutant embryos, which carry a nonsense mutation that terminates translation immediately before the Sox10 transcription activation domain (Dutton et al., 2001). Embryos and larvae heterozygous for the tw11 allele appear identical to wild type; consequently, this allele produces no appreciable dominant effect. To visualize OPCs, we used the transgenic reporter Tg(olig2: egfp), which expresses EGFP in pMN precursors, motor neurons, and OPCs under control of olig2 regulatory DNA (Shin et al., 2003). OPCs migrated normally in cls−/−;Tg(olig2:egfp) embryos and were indistinguishable in number from wild type through 6 dpf (Fig. 1A–H). To quantify OPCs, we cut transverse sections and counted EGFP+ cells that occupied the dorsal spinal cord. Consistent with our observations of living embryos, we found no difference in the number of dorsal OPCs at 80 hpf or 5.5 dpf (Fig. 1A′–H′,I). To confirm identification of OPCs, we examined expression of Sox10, which specifically marks oligodendrocyte lineage cells in zebrafish (Park et al., 2002), using immunocytochemistry. Both dorsal and ventral EGFP+ cells expressed Sox10 in wild-type embryos and larvae (Fig. 1A′–D′). However, cls mutant embryos and larvae were not labeled by anti-Sox10 antibody (Fig. 1E′–H′), because the clstw11 allele eliminates the peptide sequence used for immunization. Nevertheless, both ventral and dorsal EGFP+ cells had morphologies and occupied positions characteristic of OPCs.
Fig. 1.
Loss of sox10 function does not alter oligodendrocyte lineage cell number or distribution. A–H: Lateral views of Tg(olig2:egfp) reporter expression at the level of the trunk spinal cord in wild-type and cls−/− embryos and larvae. Dorsal is up. Asterisks in (A) indicate examples of dorsally migrated OPCs. Bracket marks the spinal cord (SC). A′ –G′ : Transverse sections, dorsal up, showing Tg(olig2:egfp) reporter expression (green) and Sox10 protein expression (red). Arrows indicate olig2+ Sox10+ OPCs in wild-type embryos and larvae (A′ –C′) and olig2+ Sox10− OPCs in cls−/− embryos and larvae (E′ –G′). I: Quantification of dorsally migrated olig2+ OPCs reveals no difference between wild type and mutant. Error bars represent SEM. Scale bars, 24 μM.
We next used gene expression to assess oligodendrocyte differentiation. At 4.5 dpf, oligodendrocytes of wild-type larvae expressed RNA encoded by plp1a, mbp, and cldnk (Fig. 2A–C). On the other hand, very few cells expressed the same genes in similarly staged cls−/− larvae (Fig. 2D–F,I). We also examined MBP expression using immunocytochemistry. By 6 dpf, some MBP accumulation became evident in wild-type larvae, particularly surrounding the large Mauthner axons in ventral spinal cord (Fig. 2G), whereas cls−/− larvae had very little MBP expression (Fig. 2H). Together, these data show that cls mutant larvae have normal numbers of oligodendrocyte lineage cells, but a deficit of cells that express myelin genes. Therefore, Sox10 function is necessary for oligodendrocyte differentiation but not specification, indicating that Sox10 has similar functions in zebrafish and mice.
Fig. 2.
sox10 function is required for oligodendrocyte differentiation. A–F: Lateral views of spinal cords, dorsal up, showing plp1a, cldnk, and mbp RNA expression in 4.5-dpf larvae. Brackets mark spinal cord (SC) and notochord (NC). G, H: Transverse sections showing MBP expression (red) and anti-acetylated tubulin labeling (blue) at 6 dpf detected by immunocytochemistry. Arrows indicate prominent MBP deposition surrounding Mauthner axons in wild-type larva. I: Quantification of plp1a+ and cldnk+ cells. Graphs show dorsal, ventral, and total spinal cord cells that express these genes. Error bars represent the SEM. Scale bar, 24 μM.
Sox10 Function Is Required for Oligodendrocyte Survival Following Axon Wrapping
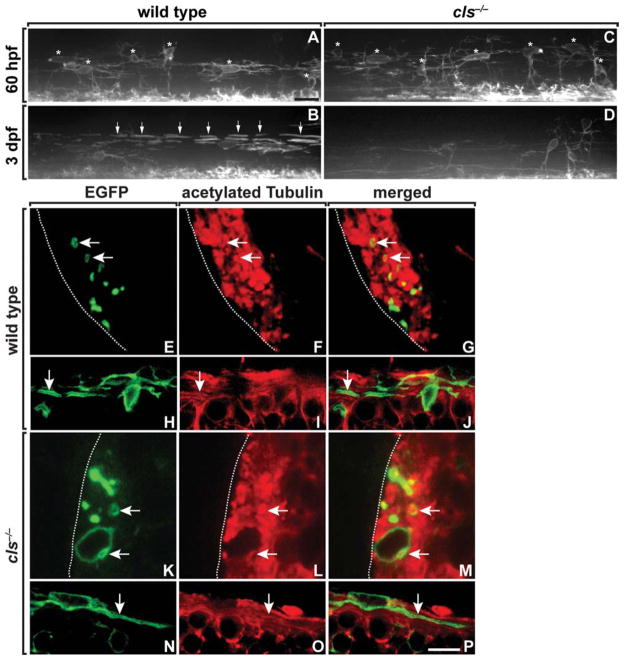
Oligodendrocyte differentiation proceeds through a series of steps including membrane extension, axon contact, and wrapping, formation of paranodal and juxtaparanodal specializations, and expression of myelin genes and myelin compaction. To further define the requirement for Sox10 function in oligodendrocyte differentiation, we used the Tg(nkx2.2a:megfp) transgenic reporter, which expresses membrane-tethered EGFP in a myelinating subset of oligodendroctye lineage cells (Kirby et al., 2006; Kucenas et al., 2008). As we have shown previously (Kirby et al., 2006), premyelinating OPCs extended long-membrane processes in wild-type embryos (Fig. 3A). By 3 dpf, oligodendrocytes began to wrap axons, forming longitudinally arrayed internodes (Fig. 3B). At 60 hpf, OPC morphology in cls−/− embryos was similar to that of OPCs in wild type (Fig. 3C). However, at 3 dpf, cls−/− larvae had a deficit of nkx2.2a+ cells and showed little evidence of axon wrapping (Fig. 3D).
Fig. 3.
OPCs initiate axon wrapping in cls mutant embryos. A–D: Lateral views of spinal cords, dorsal up, of wild-type and cls−/− embryos and larvae carrying the Tg(nkx2.2a:megfp) reporter to mark the myelinating subset of oligodendrocyte lineage cells. Asterisks mark dorsally migrated OPCs and arrows indicate axon wrapping. OPC number and morphology are similar in wild-type and cls−/− embryos at 60 hpf (A, C), but mutants have a deficit of oligodendrocytes at 3 dpf (B, D). Transverse (E–G, K–M) and sagital (H–J, N–P) sections of 64 hpf wild-type and cls−/− spinal cords expressing the Tg(nkx2.2a:megfp) reporter (green) and labeled with anti-acetylated Tubulin (red). OPC processes ensheath axons in both wild-type (E–J) and mutant (K–P) embryos. Scale bars, 24 μM (A–D), 4.5 μM (E–G, K–M), and 9 μM (H–J, N–P).
To learn if OPCs can initiate axon wrapping in the absence of Sox10 function, we examined sectioned material obtained from 64 hpf embryos and labeled with anti-acetylated tubulin to mark axons. In transverse sections of wild-type Tg(nkx2.2a:megfp) embryos, green OPC processes encircle axons, indicative of wrapping (Fig. 3E–G). Wrapped axons were also evident in cls−/−:Tg(nkx2.2a: megfp) embryos, although at lower frequency (Fig. 3K–M). Sagital sections also revealed axon wrapping by OPCs in both wild-type and cls−/− embryos (Fig. 3H–J,N–P). These data raise the possibility that Sox10 function is not necessary to initiate axon wrapping by OPCs, but is required for the maintenance of wrapping.
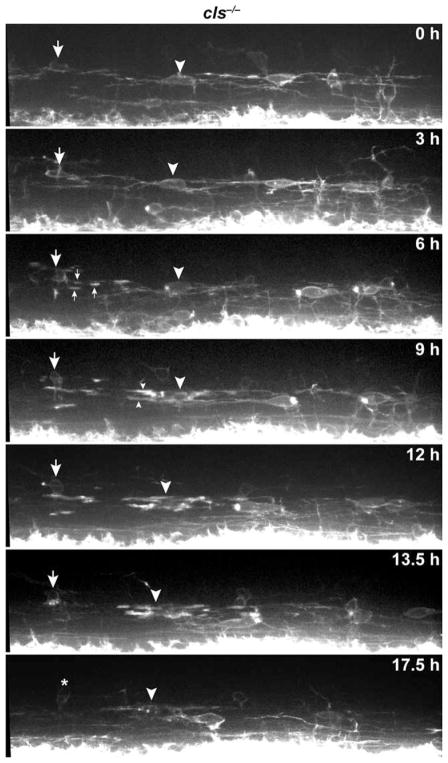
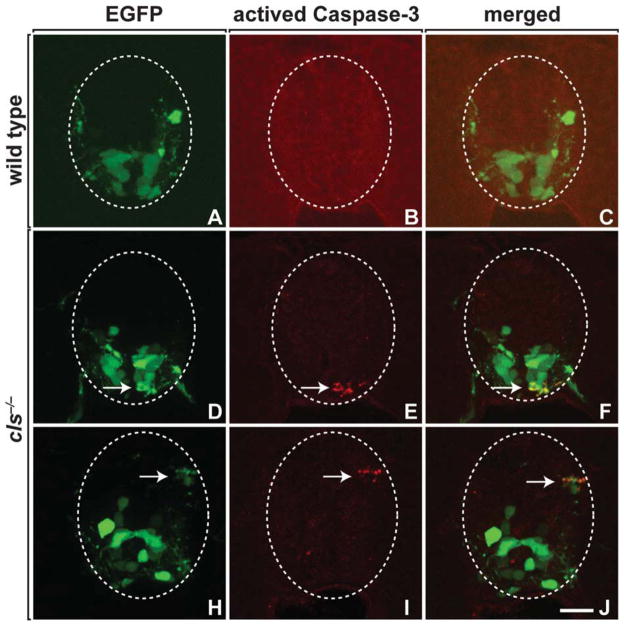
To learn the fate of nkx2.2a+ OPCs, we performed in vivo time-lapse imaging. This confirmed our observations made by examining embryos and larvae at fixed time points that OPC migration and membrane process activity were normal (Fig. 4; Supp. Info. Movie 1). However, soon after the initiation of axon wrapping, oligodendrocytes fragmented and died (Fig. 4; Supp. Info. Movie 1). We also labeled wild-type and cls−/− larvae, carrying the Tg(olig2:egfp) reporter with antibody specific to activated Caspase-3, which serves as a marker of cells undergoing apoptotic death. olig2+ cells were rarely labeled by activated Caspase-3 antibody (Fig. 5A–C), whereas mutant larvae had numerous olig2+ oligodendrocyte lineage cells that expressed activated Caspase-3 (Fig. 5D–J). Therefore, Sox10 is required for the survival of myelinating oligodendrocytes following initiation of axon wrapping.
Fig. 4.
Sox10 is required for oligodendrocyte survival following axon wrapping. Frames captured from time-lapse movie of a cls−/−; Tg(nkx2.2a:megfp) embryo beginning at about 60 hpf. Panels show lateral images of the spinal cord, with dorsal up. Numbers indicate time elapsed since the beginning of the sequence. Large arrow and arrowhead point to the soma of oligodendrocytes, and small arrows and arrowheads indicate initiation of axon wrapping. Oligodendrocytes begin to fragment and die following axon wrapping. Asterisk marks an OPC that migrated into the field of view following oligodendrocyte death. Scale bar, 24 μM.
Fig. 5.
Oligodendrocytes undergo apoptotic death in cls mutant embryos. Transverse sections of 64-hpf wild-type (A–C) and cls mutant (D–J) spinal cords expressing the Tg(olig2:egfp) reporter (green) and labeled with antiactivated Caspase-3 antibody (red). Arrows indicate olig2+ activated Caspase-3+ cells with fragmented appearance. Scale bar, 15 μM.
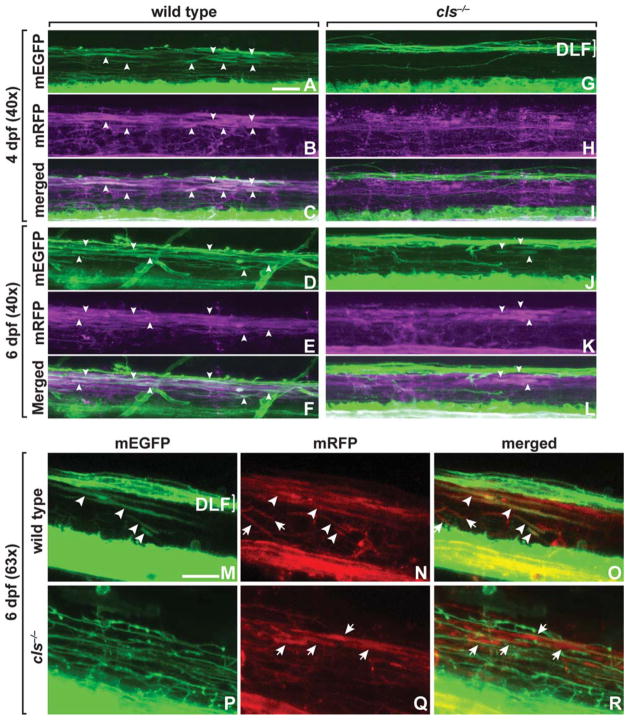
Because OPC number, marked by olig2 reporter gene expression, was normal in cls−/− larvae, the deficit of nkx2.2a+ oligodendrocytes at 3 dpf from cell death was unexpected. We therefore examined the nkx2.2a− subpopulation of OPCs. To do so, we used Tg(sox10(7.2):mrfp), which expresses a membrane-tethered RFP in all oligodendrocyte lineage cells under the control of sox10 regulatory DNA (Kirby et al., 2006; Kucenas et al., 2008). At 4 and 6 dpf, the majority of oligodendrocytes with wrapping morphologies in wild-type Tg(nkx2.2a:megfp); Tg(sox10(7.2):mrfp) larvae were nkx2.2a+ sox10+ (Fig. 6A–F,M–O). A smaller number of wrapping oligodendrocytes and all nonmyelinating OPCs were nkx2.2a− sox10+ (Fig. 6A–F and data not shown) (Kucenas et al., 2008). On the other hand, the majority of oligodendrocyte lineage cells in cls−/−; Tg(nkx2.2a:megfp);Tg (sox10(7.2):mrfp) larvae were nkx2.2a− sox10+, and, as shown earlier, there were fewer wrapping oligodendrocytes (Fig. 6G–L,P–R). Furthermore, cls−/− larvae appeared to have more nkx2.2a− sox10+ OPCs than wild type. Therefore, loss of Sox10 function results in a deficit of myelinating oligodendrocytes and an apparent expansion in the nonmyelinating OPC population.
Fig. 6.
nkx2.2a− oligodendrocytes wrap axons in cls−/− larvae. All images show lateral views of spinal cords, dorsal up, of wild-type and cls−/− larvae carrying Tg(sox10(7.2):mrfp) and Tg(nkx2.2a:megfp) reporters. In wild type (A–F), most myelinating oligodendrocytes express both nkx2.2 and sox10 reporters (arrowheads). In cls mutants (G–L), some axon wrapping by cells marked by sox10 reporter expression is evident, but these cells appear to express the nkx2.2 reporter at low or nonexistent levels (arrowheads). (M–R) Higher magnification views showing axon wrapping by sox10+ nkx2.2a+ oligodendrocytes in wild type (M–O; arrowheads) but that in cls mutants oligodendrocytes that wrap axons express only the sox10 reporter (P–R; arrows). Some axons in wild type are also wrapped by oligodendrocytes that express only the sox10 reporter (N,O; arrows). Brackets mark nkx2.2a+ axons that descend from the hindbrain through the dorsal longitudinal fasciculus (DLF). Scale bars, 24 μM.
To examine directly the behaviors of myelinating and nonmyelinating subpopulations of oligodendrocyte lineage cells in the absence of Sox10 function, we performed time-lapse imaging of cls−/−;Tg(nkx2.2a: megfp);Tg(sox10(7.2):mrfp) larvae. Notably, we observed frequent divisions of both nkx2.2a+ and nkx2.2a− OPCs (Fig. 7A and Supp. Info. Movie 2). OPC divisions during similar periods in wild-type larvae are more rare (Kirby et al., 2006). Both nkx2.2a+ and nkx2.2a− OPCs initiated axon wrapping. However, nkx2.2a+ oligodendrocytes died within a few hours after wrapping, whereas nkx2.2a− OPCs persisted (Table 1). To further investigate OPC division, we exposed wild-type and mutant larvae to BrdU, a thymidine analog that is incorporated into the DNA of S-phase cells. cls−/− larvae had significantly more BrdU+ olig2+ OPCs than wild type, whereas the number of BrdU+ olig2+ ventral spinal cord precursors was unchanged (Fig. 7B). We conclude that, in the absence of sox10 function, non-myelinating OPCs divide more frequently, consequently maintaining normal numbers of oligodendendrocyte lineage cells, but are unable to generate myelinating oligodendrocytes.
Fig. 7.
OPCs divide and wrap axons following oligodendrocyte death in cls−/− larvae. A: Frames captured from a time-lapse movie of a cls−/−; Tg(nkx2.2a:megfp);Tg(sox10(7.2):mrfp) larva beginning at 4.5 dpf. Numbers indicate time elapsed since the beginning of the sequence. Panels show lateral views of the spinal cord, with dorsal up. Arrowheads and arrows indicate OPCs. Most OPCs within the field of view divided during the 14-h period of the time-lapse imaging. Dotted box outlines axon wrapping by an oligodendrocyte expressing only the sox10 transgenic reporter. B: Quantification of OPC division. Wild-type and cls−/− larvae carrying the Tg(olig2:egfp) transgenic reporter were incubated in a BrdU solution for 24 h from 5 to 6 dpf. Graphs show number of BrdU+ olig2+ dorsal OPCs and BrdU+ olig2+ pMN precursors. Error bars indicate the SEM. Statistical significance was determined using the unpaired t-test.
TABLE 1.
Survival Time of Oligodendrocytes After Initiating the Axon Wrapping in cls−/−
| Nkx2.2a+ | Nkx2.2a− | ||
|---|---|---|---|
| Case 1 | 9 h | Case 1 | >14 |
| Case 2 | 11 h | Case 2 | >14 |
| Case 3 | 8 h | Case 3 | >20 |
| Case 4 | 11 h | ||
| Case 5 | 3 h | ||
| Case 6 | 4 h | ||
| Case 7 | 12 h | ||
DISCUSSION
In the zebrafish spinal cord, OPCs intitiate expression of sox10 as they are formed by ventral neural precursors (Kucenas et al., 2008; Park et al., 2002). Many OPCs migrate dorsally as they constantly extend and retract long-membrane processes until, at early larval stage, a subset begins to wrap and myelinate axons (Kirby et al., 2006). Most myelinating oligodendrocytes express nkx2.2a, whereas nonmyelinating OPCs do not (Kucenas et al., 2008). Very few spinal cord oligodendrocyte lineage cells die during normal development in zebrafish, but cells ablated with a laser are rapidly replaced by nearby OPCs (Kirby et al., 2006). Therefore, zebrafish oligodendrocyte lineage cells consist of dynamic and interacting populations of myelinating and nonmyelinating cells.
The structurally similar SoxE transcription factors Sox8, Sox9, and Sox10 appear to contribute both comparable and distinct functions in the development of oligodendrocyte lineage cells in mice (Wegner, 2008). Sox9 is expressed widely in neuroepithelial precursors, including pMN cells, during neurogenic and gliogenic periods (Stolt et al., 2003). Sox8 is initiated later and apparently restricted to pMN precursors and their descendent oligodendrocyte lineage cells, whereas Sox10 expression is limited to OPCs and oligodendrocytes (Stolt et al., 2002, 2005). Therefore, although expression of each Sox protein is initiated at a different time in development, there is a substantial overlap of expression in cells of the oligodendrocyte lineage. The results of various gene inactivation tests are consistent with the possibility that coexpressed Sox proteins provide at least partially overlapping functions. Sox9 mutant embryos initially have substantial deficits of OPCs, but these are later restored to near normal number (Stolt et al., 2003). Loss of Sox8 function, which, on its own, has little impact on OPC formation, in combination with Sox9 mutation, eliminates nearly all OPCs (Stolt et al., 2005). In Sox10 mutant mice, OPCs are formed in apparently normal number, but fail to differentiate into myelinating oligodendrocytes (Stolt et al., 2002). Oligodendrocyte differentiation is only slightly and transiently delayed in Sox8 homozygous mutant mice, but the additional inactivation of one copy of Sox10 greatly delays differentiation (Stolt et al., 2004), indicating that both Sox8 and Sox10 can promote oligodendrocyte maturation and myelination. The combined loss of Sox9 and Sox10 functions following OPC formation causes a reduction of oligodendrocyte lineage cells by apoptosis, which results, at least in part, from reduced expression of the PDGF receptor alpha (PDGFRα) in OPCs (Finzsch et al., 2008). Therefore, Sox8, Sox9, and Sox10 together promote formation, differentiation, and survival of oligodendrocyte lineage cells.
Thus far, published evidence indicates that Sox10 promotes oligodendrocyte differentiation by promoting expression of a subset of myelin genes. Sox10 binds to MBP genomic DNA in vitro and in vivo and can promote reporter gene expression via MBP sequence in cell culture (Li et al., 2007; Stolt et al., 2002; Wei et al., 2004). Sox10 also binds to genomic DNA from the Myelin Protein Zero gene to promote its expression in Schwann cells (Peirano and Wegner, 2000; Peirano et al., 2000). The reduction of myelin gene expression, we observed in cls/sox10 mutant zebrafish embryos, is consistent with the direct action of Sox10 at regulatory sequences associated with myelin genes. However, Sox10 alone or in combination with Olig1 did not promote expression of zebrafish plp1b or mpz, consistent with an apparent absence of Sox10-binding sites within genomic sequence upstream of the plp1b and mpz transcription start sites (Li et al., 2007). Nevertheless, Plp gene expression is reduced in both mouse and zebrafish Sox10 mutants (Stolt et al., 2002) (this study). Therefore, reduced Plp gene expression might be an indirect consequence of Sox10 deficiency.
Our time-lapse imaging now adds an additional level of insight to the role of Sox10 in oligodendrocyte development. These studies revealed that in embryos and larvae homozygous for the clstw11 allele, the myelinating subset of OPCs, which expresses olig2, sox10, and nkx2.2a, migrates, extends membrane processes, and wraps axons normally, but then rapidly dies. Therefore, Sox10 is required for the survival of myelinating oligodendrocytes, and the absence of myelin gene expression reflects loss of this cell population. This observation raises the possibility that Sox10 also promotes expression of genes that encode factors that mediate interactions between axons and oligodendrocytes necessary for oligodendrocyte survival, although we cannot rule out the possibility that oligodendrocyte death is an indirect consequence of their failure to fully differentiate. A potential direct requirement is probably distinct from the requirement of Sox10 in combination with Sox8 to maintain high levels of PDGFRα expression in OPCs, because oligodendrocytes downregulate PDGFRα expression as they differentiate (Butt et al., 1997; Chittajallu et al., 2005; Ellison and de Vellis, 1994; Oumesmar et al., 1997).
Several previous observations have implicated the importance of axons for oligodendrocyte survival. For example, transection of rat optic nerves resulted in loss of oligodendrocytes by programmed cell death and oligodendrocytes survived longer in culture in the presence of neurons (Barres et al., 1993). In the rodent brain, many oligodendrocyte lineage cells that were not in apparent contact with axons died, whereas oligodendrocytes that wrapped axons were spared (Trapp et al., 1997). One class of molecule implicated in mediating axon-dependent oligodendrocyte survival and is therefore a candidate target for Sox10 regulation is the integrins, which are receptors for extracellular matrix components such as laminin. Neurons express laminins (Liesi et al., 2001), and some axons are surrounded by a laminin-rich extracellular matrix at the time of myelination (Colognato et al., 2002; Farwell and Dubord-Tomasetti, 1999). Laminin-2 and the laminin receptor α6β1 increase oligodendrocyte survival in response to growth factors in culture (Frost et al., 1999). Consistent with this, mice lacking the α6 integrin subunit have an elevated number of dying oligodendrocytes (Colognato et al., 2002). Mice lacking the β1 subunit similarly had elevated levels of apoptotic oligodendrocytes, although myelination appeared normal (Benninger et al., 2006). Therefore, expression of genes encoding the α6 and β1 subunits is conceivably controlled by Sox10.
Despite the loss of mylenating oligodendrocytes from cls/sox10 mutants, the overall number of oligodendrocyte lineage cells was normal. Similarly, Sox10 mutant mice have normal numbers of oligodendrocyte lineage cells but a deficit of cells that express myelin genes (Stolt et al., 2002). Our time-lapse imaging and BrdU studies provide evidence that oligodendrocyte death is accompanied by more frequent divisions of nonmyelinating OPCs. Previously, we showed that following ablation of oligodendrocytes and OPCs using a laser, neighboring OPCs could divide more frequently, migrate into the ablated region, and wrap axons (Kirby et al., 2006). OPCs continuously extend long-membrane processes, and we interpreted such protrusive activity as a surveillance mechanism that helps determine oligodendrocyte spacing. We speculate that the replacement of dying oligodendrocytes in cls/sox10 mutant larvae also results from the surveillance of oligodendrocyte distribution by nonmyelinating OPCs, which express the transgenic sox10 reporter (but are genetically deficient for sox10 function) but do not express nkx2.2a. Notably, these newly produced OPCs wrapped axons, but did not express myelin genes at high levels. Consequently, neither sox10 nor nkx2.2a functions are necessary for axon-wrapping behavior by oligodendrocyte lineage cells. Additionally, these cells persisted until the end of our imaging experiments, longer than the first wave of myelinating oligodendrocytes. Although we do not know the fate of these secondary wrapping OPCs, because cls mutant larvae die by about 6 dpf, this observation suggests that nonmyelinating OPCs do not become dependent on axonal signals for survival, even when they come in physical contact with axons. During normal development and homeostasis, different survival mechanisms for OPCs and oligodendrocytes likely help match the number of myelinating cells to target axons while maintaining a large population of OPCs. Coordinate action of Sox10 and Nkx2.2 transcription factors therefore might couple expression of myelin genes with the expression of factors that mediate axon-dependent survival.
Supplementary Material
Acknowledgments
The anti-BrdU antibody, developed by S. J. Kaufman, was obtained from the Developmental Studies Hybridoma Bank developed under the aupices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. Confocal microscopy was performed using equipment provided by the Vanderbilt Academic Venture Capital Fund, the Gates Frontiers Fund, the Vanderbilt University Medical Center Cell Imaging Shared Resource and the Light Microscopy Core Facility at the University of Colorado Denver Anschutz Medical Campus.
Grant sponsor: National Mutliple Sclerosis Society; Grant number: 3420B.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Barres BA, Jacobson MD, Schmid R, Sendtner M, Raff MC. Does oligodendrocyte survival depend on axons? Curr Biol. 1993;3:489–497. doi: 10.1016/0960-9822(93)90039-q. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Benninger Y, Colognato H, Thurnherr T, Franklin RJ, Leone DP, Atanasoski S, Nave KA, Ffrench-Constant C, Suter U, Relvas JB. β1-Integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26:7665–7673. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosamle C, Halpern ME. Characterization of myelination in the developing zebrafish. Glia. 2002;39:47–57. doi: 10.1002/glia.10088. [DOI] [PubMed] [Google Scholar]
- Butt AM, Hornby MF, Ibrahim M, Kirvell S, Graham A, Berry M. PDGF-α receptor and myelin basic protein mRNAs are not coexpressed by oligodendrocytes in vivo: A double in situ hybridization study in the anterior medullary velum of the neonatal rat. Mol Cell Neurosci. 1997;8:311–322. doi: 10.1006/mcne.1996.0590. [DOI] [PubMed] [Google Scholar]
- Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M, Qiu M. Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of nkx6 regulation and shh signaling. Neuron. 2005;45:41–53. doi: 10.1016/j.neuron.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre AA, Gallo V. Downregulation of platelet-derived growth factor-α receptor-mediated tyrosine kinase activity as a cellular mechanism for K+-channel regulation during oligodendrocyte development in situ. J Neurosci. 2005;25:8601–8610. doi: 10.1523/JNEUROSCI.2122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development. 2001;128:4113–4125. doi: 10.1242/dev.128.21.4113. [DOI] [PubMed] [Google Scholar]
- Ellison JA, de Vellis J. Platelet-derived growth factor receptor is expressed by cells in the early oligodendrocyte lineage. J Neurosci Res. 1994;37:116–128. doi: 10.1002/jnr.490370116. [DOI] [PubMed] [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA. Thyroid hormone regulates the expression of laminin in the developing rat cerebellum. Endocrinology. 1999;140:4221–4227. doi: 10.1210/endo.140.9.7007. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Stolt CC, Lommes P, Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development. 2008;135:637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Richardson WD, Kessaris N. A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development. 2005;132:1951–1959. doi: 10.1242/dev.01777. [DOI] [PubMed] [Google Scholar]
- Frost EE, Buttery PC, Milner R, Ffrench-Constant C. Integrins mediate a neuronal survival signal for oligodendrocytes. Curr Biol. 1999;9:1251–1254. doi: 10.1016/s0960-9822(99)80506-5. [DOI] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Multicolor whole-mount in situ hybridization. Methods Mol Biol. 2000;137:139–148. doi: 10.1385/1-59259-066-7:139. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucenas S, Snell H, Appel B. nkx2.2a promotes specification and differentiation of a myelinating subset of oligodendrocyte lineage cells. Neuron Glia Biol. 2008;4:71–81. doi: 10.1017/S1740925X09990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lu Y, Smith HK, Richardson WD. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J Neurosci. 2007;27:14375–14382. doi: 10.1523/JNEUROSCI.4456-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Fried G, Stewart RR. Neurons and glial cells of the embryonic human brain and spinal cord express multiple and distinct isoforms of laminin. J Neurosci Res. 2001;64:144–167. doi: 10.1002/jnr.1061. [DOI] [PubMed] [Google Scholar]
- Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky I, Chan J, McMahon AP, Stiles CD, Rowitch DH. Sonic hedgehog-regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Masahira N, Takebayashi H, Ono K, Watanabe K, Ding L, Furusho M, Ogawa Y, Nabeshima Y, Alvarez-Buylla A, Shimizu K, Ikenaka K. Olig2-positive progenitors in the embryonic spinal cord give rise not only to motoneurons and oligodendrocytes, but also to a subset of astrocytes and ependymal cells. Dev Biol. 2006;293:358–369. doi: 10.1016/j.ydbio.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Oumesmar BN, Vignais L, Baron-Van Evercooren A. Developmental expression of platelet-derived growth factor α-receptor in neurons and glial cells of the mouse CNS. J Neurosci. 1997;17:125–139. doi: 10.1523/JNEUROSCI.17-01-00125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Boyce J, Shin J, Appel B. Oligodendrocyte specification in zebrafish requires notch-regulated cyclin-dependent kinase inhibitor function. J Neurosci. 2005;25:6836–6844. doi: 10.1523/JNEUROSCI.0981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Mehta A, Richardson JS, Appel B. olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev Biol. 2002;248:356–368. doi: 10.1006/dbio.2002.0738. [DOI] [PubMed] [Google Scholar]
- Park H, Shin J, Appel B. Spatial and temporal regulation of ventral spinal cord precursor specification by Hedgehog signaling. Development. 2004;131:5959–5969. doi: 10.1242/dev.01456. [DOI] [PubMed] [Google Scholar]
- Peirano RI, Goerich DE, Riethmacher D, Wegner M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol. 2000;20:3198–3209. doi: 10.1128/mcb.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3:191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Shin J, Park HC, Topczewska JM, Mawdsley DJ, Appel B. Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci. 2003;25:7–14. doi: 10.1023/B:MICS.0000006847.09037.3a. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Friedrich RP, Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. 2004;131:2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Schmitt S, Lommes P, Sock E, Wegner M. Impact of transcription factor Sox8 on oligodendrocyte specification in the mouse embryonic spinal cord. Dev Biol. 2005;281:309–317. doi: 10.1016/j.ydbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Takebayashi H, Yoshida S, Sugimori M, Kosako H, Kominami R, Nakafuku M, Nabeshima Y. Dynamic expression of basic helix-loop-helix Olig family members: Implication of Olig2 in neuron and oligodendrocyte differentiation and identification of a new member, Olig3. Mech Dev. 2000;99:143–148. doi: 10.1016/s0925-4773(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallstedt A, Klos JM, Ericson J. Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron. 2005;45:55–67. doi: 10.1016/j.neuron.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Wegner M. A matter of identity: Transcriptional control in oligodendrocytes. J Mol Neurosci. 2008;35:3–12. doi: 10.1007/s12031-007-9008-8. [DOI] [PubMed] [Google Scholar]
- Wei Q, Miskimins WK, Miskimins R. Sox10 acts as a tissue-specific transcription factor enhancing activation of the myelin basic protein gene promoter by p27Kip1 and Sp1. J Neurosci Res. 2004;78:796–802. doi: 10.1002/jnr.20342. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.