Abstract
Background
In dislocated proximal tibial fractures, the most frequently used treatment is ORIF with screws and plates. Minimally-invasive techniques using external fixation are an alternative. The aim of this study was to analyse the clinical and radiological results using the Ilizarov technique in both uni- and bicondylar tibial fractures.
Methods
Thirty consecutive patients with isolated fractures of the proximal tibia were treated with the Ilizarov technique, 11 Schatzker I-IV with 2–3 rings and 19 Schatzker V-VI with 3–4 tibial rings and a femoral, hinged, two-ring extension. Unrestricted weight-bearing was allowed. Pre and post-operatively, conventional radiographs, computerized tomography scans, post-operative pain assessments and complications were evaluated. The knee function was evaluated with the EQ-5D, NHP and KOOS scores, as well as self-appraisal.
Results
All the fractures healed. Twenty-five patients achieved a range of motion better than 10-100º. The type I-IV fractures had a shorter operating time and hospital stay, as well as better knee flexion, and the self-appraisal indicated that they tolerated the treatment better. Pin infections occurred in 4% of the pin sites, but only two patients required debridement. Two patients developed compartment syndrome and underwent fasciotomy. No patient complained of functional knee instability. Two patients underwent a total knee arthroplasty because of residual pain. The overall result was judged as satisfactory in twenty-seven patients.
Conclusions
The Ilizarov method produces a good clinical outcome and is a valuable treatment alternative in proximal tibial fractures of all types.
Keywords: Proximal tibial fractures, Ilizarov method, External fixation
Background
The goal of the treatment of tibial plateau fractures is to achieve a stable, well-aligned, mobile, pain-free joint and to minimise the risk of post-traumatic osteoarthritis [1,2].
In non-osteoporotic proximal metaphyseal tibial fractures of the Schatzker I-IV and AO/OTA types B and C1, open reduction and internal fixation (ORIF) using screws and plates is the recommended treatment. Fractures of the Schatzker V -VI and AO C2 and C3 types have also previously been treated in the same manner, but more recently the Ilizarov circular fixator is also considered to be an established treatment alternative [3,4].
Fractures of the articular surface of the tibia, even in fractures with minimal joint extension, are usually the result of a high-energy direct blow [5]. Because of the type of trauma involved and the relatively high frequency of major soft-tissue injuries [6] the complication rate is high, regardless of treatment [7]. The relatively large surgical incisions that are used for internal fixation also add a considerable risk of soft-tissue complications [8].
If the classic Ilizarov technique is used according to the original recommendations [9,10], the reduction and fixation of the fracture fragments can be made with almost no soft-tissue exposure and blood loss. This technique does not leave screws and plates when the fracture has healed. The fixator also allows for the adjustment of the alignment and for compression/distraction both during and after surgery. Another advantage when it comes to using the Ilizarov technique is that the fixation is stable enough to allow early weight-bearing. [11,12], which is the rationale for using the Ilizarov method in unicondylar fractures. In the communited bicondylar high energy fractures the rationale is the same and, in addition, there is no need to use a staged protocol.
Plate fixation and circular external fixation similar to the Ilizarov technique, were compared in a randomised, multicentre study of 83 displaced Schatzker V-VI fractures [13]. Both techniques produced satisfactory fracture reduction, but the number and severity of complications was greater with ORIF. In a review, Mahadeva et al. [14] compared internal and hybrid external fixation in Schatzker type VI tibial plateau fractures. The number of complications were larger in the group treated with ORIF, but, due to the limited number of reports (five), the differences were not statistically significant. To date, the rationale for treating unicondylar fractures is osteosynthesis with screws or plates, percutaneously if possible. The only report we have found in the English literature on Ilizarov applications in unicondylar fractures is by Watson et al. [15]. They included fourteen high-energy fractures treated with a combination of screws/clamps and the Ilizarov techniques (three-ring fixator, half-pins) and reported excellent results.
At our hospital, which is an educational institution, the Ilizarov external fixator was gradually introduced by one experienced trauma surgeon (TR) for displaced proximal tibial fractures in 2002 and, since 2005, it has been the preferred treatment for both unicondylar (Schatzker I-IV) and bicondylar fractures (Schatzker V-VI). The aim of the present study was to compare prospectively the clinical outcome and radiological healing and how the patients experienced the treatment in the two subgroups (unicondylar vs. bicondylar).
Methods
The selection criteria in this study were as follows: Patients aged 18–75 years, with tibial plateau fractures displaced more than 5 mm and/or instability when the knee was stressed in varus or valgus, admitted to the Emergency Department at Skaraborg Central Hospital (Kärnsjukhuset) in Skövde; a referral trauma centre for a population of approximately 280.000 inhabitants. Only patients with isolated fractures and without disorders affecting gait, who were able to understand and follow instructions in Swedish, were enrolled after written informed consent for participation in the study. Between January 2005 and December 2009, 40 patients fulfilled the inclusion criteria. One individual refused to participate and nine patients were treated outside the protocol when the study supervisor was on leave. The remaining 30 patients were included in this prospective follow-up study. Their median age was 51 years (range 18–74), 12 were women and 18 men. Four patients were smokers. The cause of the injury was motor-vehicle accidents in 10 patients, falls in 13, horse riding accidents in 4, work accidents in 2 and a blow (assault) in 1 patient. Two patients were referred from another hospital with a provisional external fixation.
The pre-operative radiographs were supplemented with computerized tomography scans in 26 patients. Eight fractures also had a diaphyseal extension. There were no open fractures. The fractures were classified according to Schatzker [16]. Eleven patients (7 women and 4 men) had type I-IV fractures and 19 (11 women and 8 men) V-VI type. There were no significant demographic differences between the groups. The cause of injury related to the type of fracture is shown in Table 1. The patients were scheduled for early surgery. Sixteen patients were operated on within two days of the accident and the rest between 3 to 11 days. The median time between injury and surgery was 2 days (range 0–11).
Table 1.
Details of all patients treated with the Ilizarov application and fractures types
| Case | Age | Mechanism of injury | Schatzker | AO | Energy type |
|---|---|---|---|---|---|
| 1 |
54 |
Fall |
V |
C3 |
High |
| 2 |
38 |
Traffic |
VI |
C3 |
High |
| 3 |
74 |
Fall |
VI |
C3 |
Low |
| 4 |
18 |
Riding |
II |
B3 |
High |
| 5 |
44 |
Traffic |
III |
B2 |
High |
| 6 |
50 |
Work |
I |
B1 |
High |
| 7 |
57 |
Fall |
II |
B3 |
Low |
| 8 |
54 |
Fall |
VI |
C3 |
Low |
| 9 |
60 |
Fall |
VI |
C3 |
High |
| 10 |
34 |
Assault |
II |
B3 |
Low |
| 11 |
59 |
Fall |
VI |
B3 |
Low |
| 12 |
60 |
Fall |
VI |
C3 |
Low |
| 13 |
35 |
Traffic |
V |
C3 |
High |
| 14 |
58 |
Fall |
II |
B3 |
Low |
| 15 |
62 |
Fall |
II |
B3 |
Low |
| 16 |
70 |
Traffic |
II |
B3 |
High |
| 17 |
43 |
Traffic |
VI |
C3 |
High |
| 18 |
64 |
Fall |
VI |
C3 |
Low |
| 19 |
63 |
Riding |
II |
B3 |
Low |
| 20 |
53 |
Traffic |
VI |
C3 |
High |
| 21 |
34 |
Traffic |
VI |
C3 |
High |
| 22 |
27 |
Work |
VI |
C2 |
High |
| 23 |
20 |
Traffic |
VI |
C1 |
High |
| 24 |
62 |
Fall |
VI |
C3 |
Low |
| 25 |
44 |
Riding |
VI |
C1 |
Low |
| 26 |
44 |
Fall |
IV |
B1 |
Low |
| 27 |
24 |
Riding |
II |
B3 |
Low |
| 28 |
65 |
Fall |
VI |
C3 |
Low |
| 29 |
43 |
Traffic |
VI |
C3 |
High |
| 30 | 50 | Traffic | VI | C3 | Low |
The surgery was performed without a tourniquet on a traction table with the foot fixed in a shoe. An arthrocentesis was made to reduce the intra-articular pression. Biplane fluoroscopy was used during reduction, pin insertion and assembly of the frame. The axial reduction was achieved with traction. The joint surface was reconstructed if necessary, using closed pressure with percutaneously inserted elevators, reduction forceps or/and wires with olives. Arthrotomy or arthroscopy was not used. Subchondral defects were packed with calcium sulphate bone pellets (Osteoset® or β-tri calcium phosphate ChronOs®) in 18 patients. The proximal ring was placed at the level of the fibular head. Additional stability was achieved using 1.8 mm wires parallel to the articular surface with posts secured on the rings (drop-wire techniques). The wires were tensioned to at least 110 Kg. Depending on the complexity of the fracture, another one to three rings were fixed with two to three wires in the tibia, and they were then connected with steel rods (Smith & Nephew, Memphis, Tennessee, USA). In Schatzker type V-VI fractures, two rings in the distal femur were added to the construction in 16 patients with hinged rods over the knee. No additional osteosynthesis was used. Nineteen operations were performed by TR who also supervised residents in the remaining 11. No post-operative corrections were needed.
Cloxacillin (2 g) was used as infection prophylaxis starting pre-operatively. Low-molecular heparin prophylaxis was given from the day of admission until 10 days after leaving the hospital. During the first 24 hours after surgery, all patients had a post-operative continuous i.v. analgesia (PCA) pump with morphine/ketobemidon.
The “Kurgan protocol” [17] was used for postoperative pin site dressings and the Checketts-Otterburns classification [18] was used to describe pin infections.
Physiotherapy was started immediately after the operation to maintain knee and ankle motion and the patients were allowed to start unrestricted weight-bearing.
The femoral extension was used in 16 of the 19 type V-VI fractures and was removed at six weeks. The fractures were regarded as being healed when antero-posterior and lateral radiographs showed a bridging callus of three of four cortices and/or the fracture was stable when stressed manually and the patients were able to walk without pain after the connecting rods had been removed. The fixators could then be removed without anaesthesia, for type I-IV fractures after 11 weeks (range 6–16) and for type V-VI at 12 weeks (range 8–20) post-operatively.
All the patients were followed up clinically after two, four, eight and 12 weeks and finally at one year. Radiography was performed at the same intervals. Additional clinical and radiographic assessments were made when necessary to evaluate fracture healing.
Pain and patient satisfaction were registered using a visual analogue scale (VAS) at four and 12 weeks and at the one year follow-up. The Swedish versions of the EuroQol (EQ-5D) [19] and the Nottingham Health Profile (NHP) [20,21] were used for patient self-appraisal at the same time intervals. The clinical one-year post-operative outcome, including the ROM and manual testing of stability in varus and valgus, was assessed by an independent physiotherapist. The KOOS questionnaire [22,23] was added to the follow-up between one and five years post-operatively and in those patients in whom the observation period exceeded one year. Pain (VAS), EQ-5D and NHP questionnaires were repeated.
The post-operative radiographs were evaluated by one of the authors (TR) and separately by an independent surgeon according to the criteria formulated by Rasmussen [24].
1. The articular step-off – the maximal depression or displacement of the articular surface in an axial direction on antero-posterior and lateral projections.
2. The condylar widening – measured in comparison with the ipsislateral femoral condyles.
3. The plateau tilt – the angle in the varus or valgus direction as measured on the antero-posterior projections perpendicular to the long axis of the tibia.
Statistical analysis
The median values and 95% confidence interval (CI) or range are given. Further statistical comparisons between the groups are not meaningful as the number of patients is small and they would only reflect differences that could be anticipated.
The study was approved by the regional ethical review board at Sahlgrenska University Hospital in Gothenburg (ID. 400–04).
Results
The comparison of the two subgroups, Schatzker I-IV and Schatzker V-VI, is shown in Table 2.
Table 2.
Treatment timing in the two subgroups
| |
Schatzker I-IV (n = 11) |
Schatzker V-VI (n = 19) |
||
|---|---|---|---|---|
| Median | CI | Median | CI | |
| Surgery delay (days) |
3 |
(1–11) |
2 |
(1–9) |
| Operation time (min) |
130 |
(100–165) |
223 |
(164–240) |
| Hospital stay (days) |
4 |
(3–6) |
9 |
(7–11) |
| External fixation (weeks) | 11 | (7–16) | 12 | (10–15) |
The median surgical time, including the time for assembling the frame peri-operatively, was lower for the Schatzker I-IV (130 minutes, range 92–117) than for the Schatzker V-VI fractures (223 minutes, range 97–275).
The total amount of morphine/ketobemidon (PCA pump) varied between 7 and 77 mg (median 46 mg). The demand for additional analgesics was low.
All the patients were allowed full, unrestricted weight-bearing from the first post-operative day and were discharged directly to their homes when they managed to walk using crutches and independently climb stairs. The Schatzker I-IV group had a shorter hospital stay, 4 days (range 3–9), than the Schatzker V-VI; 9 days (range 3–13).
The observed complications are shown in Table 3. Two patients with Schatzker VI fractures developed compartment syndromes (case 2 Schatzker VI/AO C3 and case 28 VI/C2). In the first patient, the compartment syndrome was masked by an over consumption of opiates and the patient did not undergo fasciotomy until one day after the initial operation. He had a muscle necrosis of the lateral compartment of the leg and permanent peroneal nerve palsy. The other patient underwent fasciotomy immediately after the application of the fixator. He developed a fistula in the fasciotomy wound, which required excision, but healed without sequelae.
Table 3.
Complications in all fractures
| Complications | n = 30 |
|---|---|
| Compartment syndrome |
2 |
| Deep vein thrombosis |
1 |
| Secondary dislocation |
2 |
| Pin-site infections |
16 |
| Pin-track infection |
2 |
| Osteomyelitis |
0 |
| Nerve injury | 0 |
A total of 113 rings and 321 wires were used, constituting 642 potential pin-infection sites. Sixteen patients had 25 minor pin site infections, Checketts-Otterburns grades 1–3, all of which subsided with short-term oral antibiotics and two had pin tract infections grade 4 that healed after soft-tissue debridement. There were no clinical or radiological signs of osteomyelitis or septic arthritis in any patient.
One patient developed a distal DVT with the fixator still in place two months after the injury.
At the one-year follow-up, 27 patients had an extension deficit of less than 10°. The patients with Schatzker I-IV fractures had better knee flexion (140°, range 86–156) than those with Schatzker V-VI fractures (120°, range 83–148), see Table 4. Three patients were able to flex their knee less than 90° and they also had extension deficits of more than 10°. Four of the five patients with reduced knee flexion had Schatzker type V or VI fractures. Ankle motion was not affected. Two knees were mobilized under epidural anesthesia postoperatively at five months (case 30) and seven months (case 29).
Table 4.
Range of motion at one year, median (range) in the two subgroups
| |
Schatzker I-IV (n = 11) |
Schatzker V-VI (n = 19) |
||||||
|---|---|---|---|---|---|---|---|---|
| Uninjured | Injured | Uninjured | Injured | |||||
| Knee flexion |
146° |
(124-160°) |
140° |
(86-156°) |
140° |
(120-154°) |
120° |
(83-148°) |
| Knee extension | 0° | (0-8°) | 3° | (0-17°) | 0° | (0-8°) | 0° | (0-20°) |
Residual knee laxity was observed in three patients (cases 19, 20 and 28), but no patient complained of functional instability of the knee. The radiological results at the one-year follow-up were good in 27 patients according to the criteria formulated by Rasmussen [24]. The bone substitutes were all at least partially integrated and there were no signs of adverse reactions. Detailed results for the patients who had instability or significant radiological deformity (residual deformity of 10 mm articular depression and/or condylar widening of more than 10 mm and/or valgus and varus more than 10°) are summarised in Table 5 and compared with the KOOS values and patient satisfaction. Two patients with increasingly severe pain (case 1 and case 24) underwent total knee arthroplasty 1.5 and two years after the initial fracture treatment.
Table 5.
Outcome at one year in patients with instability and/or significant residual radiological deformity
| Case | Stable/Unstable | Tilt degrees | Articular depression mm | KOOS pain | KOOS symptom | KOOS ADL | KOOS Sport | KOOS QoL | VAS Satis-faction mm | VAS Pain mm |
|---|---|---|---|---|---|---|---|---|---|---|
| 8 |
S |
13 varus |
10 |
38.8 |
64.2 |
42.6 |
0 |
25 |
69 |
23 |
| 16 |
S |
8 valgus |
10 |
91.6 |
71.4 |
82.3 |
35 |
68.8 |
57 |
9 |
| 19 |
US |
3 valgus |
7 |
- |
- |
- |
- |
- |
13 |
20 |
| 20 |
US |
1 varus |
2 |
86.1 |
64.2 |
88.2 |
15 |
62.5 |
6 |
8 |
| 26 |
S |
12 varus |
0 |
88.9 |
92.8 |
97.0 |
80 |
62.5 |
14 |
13 |
| 28 | US | 5 valgus | 0 | 91.6 | 46.4 | 61.7 | 25 | 50 | 27 | - |
The pain (VAS), patient satisfaction (VAS), EQ5D and the NHP total score outcomes at different time intervals are shown in Table 6. The differences between the Pain (VAS), EQ-5D, NHP values at one year and the KOOS questionnaire were not significant. The EQ-5D values and NHP total scores show that the overall function was severely affected at four weeks. However, there were no differences between the subgroups. The knee function improved more rapidly in patients with Schatzker I-IV fractures in those with the Schatzker V-VI. Good knee function was registered first at the one-year follow-up when there were no differences between the groups.
Table 6.
Outcome according to patients’ self-appraisal controls in the two subgroups
| Median with 95% CI | Time of assessment | Schatzker I-IV | Schatzker V-VI | ||
|---|---|---|---|---|---|
|
Pain (VAS) |
4 weeks |
20 |
(0–50) |
28 |
(24–47) |
| 12 weeks |
7 |
(0–45) |
25 |
(9–29) |
|
| 1 year |
9 |
(0–21) |
16 |
(1–23) |
|
|
Patient satisfaction (VAS) |
4 weeks |
9 |
(3–28) |
28 |
(7–42) |
| 12 weeks |
8 |
(5–48) |
14 |
(6–30) |
|
| 1 year |
13 |
(0–22) |
13 |
(6–23) |
|
|
NHP total |
4 weeks |
5.3 |
(3.5-36.8) |
37.3 |
(15.7-46.8) |
| 12 weeks |
1.8 |
(0–17.4) |
20.2 |
(11.2-34.5) |
|
| 1 year |
1.8 |
(0–11.4) |
7.4 |
(1.8-19.3) |
|
|
EQ5D |
4 weeks |
0.66 |
(0.29-0.81) |
0.59 |
(0.29-0.62) |
| 12 weeks |
0.76 |
(0.62-1.0) |
0.62 |
(0.29-0.69) |
|
| 1 year | 0.89 | (0.69-1.0) | 0.80 | (0.69-0.85) | |
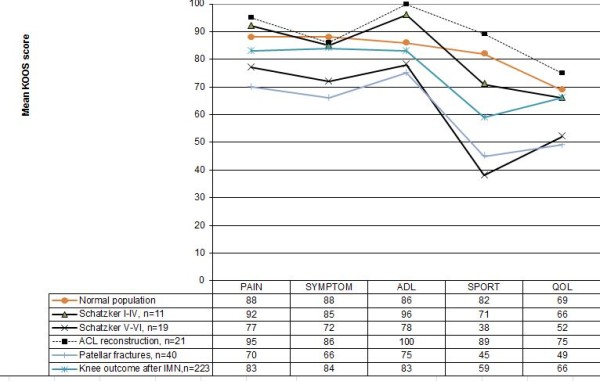
The KOOS values are shown in Figure 1 and compared with the results from the literature.
Figure 1.
Comparison with other studies regarding complications and outcome is given in Tables 7 and 8.
Table 7.
The trial with the cohort subgroup with Schatzker I-IV type of fractures compared with the literature
| Present study | Sament et al. 2012 | Allam et al. 2011 | Watson et al. 1998 | Keogh et al. 1992 | Koval et al. 1992 | |
|---|---|---|---|---|---|---|
|
Study design |
P |
P |
P |
P |
P |
P |
|
Number of patients |
11 |
36+(20 S-V) |
29 |
14 (S I-II) |
13 (1SV) |
20 (S-V) |
|
High-energy |
4 |
50 |
NM |
14 |
NM |
20 |
|
Time to definitive surgery (days) |
2 |
2 |
NM |
12 |
NM |
4 |
|
Intervention |
CEF |
IF+cast |
IF+cast or brace |
ORIF+CEF |
IF+cast or brace |
IF |
|
Full weight-bearing (weeks) |
immediate |
12 |
8-12 |
12 |
8-12 |
12 |
|
Follow-up (months) |
12 |
34 |
30 |
19 |
17 |
16 |
|
Knee ROM injured side |
0-140 |
90% > 90 |
NM |
0-108 |
NM |
0-128 |
|
Healing (weeks) |
11 |
12 |
9 |
29 |
NM |
12 |
|
Compartment Syndrome |
0 |
0 |
0 |
0 |
0 |
0 |
|
Deep Vein Thrombosis |
0 |
0 |
0 |
0 |
1 |
0 |
|
Deep Infection |
0 |
0 |
0 |
0 |
0 |
0 |
|
Reoperations |
Arthroscopy:1 |
0 |
0 |
Staged protocol in 8 patients |
Implant removal:7 |
NM |
| TKR:0 | ||||||
| Skin graft:1 | ||||||
| Delayed wound closure:1 | ||||||
| Average 3 procedures (exclusive the frame removal) | ||||||
|
Functional outcome |
VAS Pain |
Rasmussen score |
|
Knee Society score |
Rasmussen score |
Motion |
| VAS Satisfaction |
Pain |
|||||
| Deformity | ||||||
| NHP |
Ambulation |
|||||
| EQ5-D |
Return to work |
|||||
| KOOS | ||||||
P = Prospective, R = Retrospective, NM = Not mentioned, CEF = Circular External Fixator, IF = Internal Fixation.
Unilat = Unilateral Extern Fixation, TKR = Total Knee Replacement, S = Schatzker fracture type, ROM = Range Of Motion.
Table 8.
The trial with the cohort subgroup with Schatzker V-VI type of fractures compared with the literature
| Present study | Yu et al. 2009 | Catagni et al. 2007 | Lee et al. 2007 | Phisitkul et al. 2007 | Boldin et al. 2006 | Canadian Orthopaedic Trauma Society 2006 | Oh et al. 2006 | Egol et al. 2005 | El-Barbary et al. 2005 | Cole et al. 2004 | Ricci et al. 2004 | Dendrinos et al. 1996 | Zecher et al. 1996 | Yang et al. 1995 | Marsh et al. 1995 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Study design |
P |
P |
R |
R |
R |
P |
Rand |
P |
P |
R |
R |
P |
P |
P |
P/R |
P |
|
Number of patients |
19 |
54 |
59 |
35 |
37 |
25 |
82 |
23 |
53 |
29 |
87 |
28 |
24 |
21 |
22/22 |
20 |
|
High-Energy |
14 |
54 |
59 |
35 |
37 |
18 |
NM |
NM |
53 |
29 |
75 |
NM |
24 |
21 |
40 |
20 |
|
Time to definitive surgery (days) |
2 |
10 |
NM |
12 |
13 |
6 |
4 |
11 |
9 |
NM |
7 |
NM |
2 |
NM |
NM |
2 |
|
Intervention |
CEF |
ORIF |
CEF + IF |
ORIF |
ORIF |
ORIF |
40 ORIF/42 CEF |
ORIF |
ORIF |
CEF + IF |
ORIF |
ORIF |
CEF + IF |
CEF + IF |
ORIF + cast/CEF + IF |
Unilat + IF |
|
Full weight-bearing (weeks) |
immediate |
19 |
immediate in 40 patients with femoral extension |
12 |
16 patients: 11 |
NM |
NM |
14 |
12 |
6 |
13 |
Extra-articular fractures: 6–8 |
14 |
NM |
12/08/12 |
4-8 |
| 19 patients: 15 | ||||||||||||||||
| Intra-articular fractures: 8-12 | ||||||||||||||||
|
Follow-up (months) |
12 |
24 |
21 |
3-26 |
49 |
36 |
24 |
25 |
16 |
27 |
14 |
23 |
37 |
14 |
21–208 |
38 |
|
Knee ROM ° injured side |
0-120 |
0-108 |
0-119 |
0-135 |
0-112 |
0-117 |
0-120 No differences |
0-123 |
0-106 |
0-112 |
0-120 |
NM |
90% >110 |
>90 |
|
0-130 |
|
Healing (weeks) |
12 |
15 |
29 |
16 |
12 |
11 |
NM |
19 |
NM |
NM |
13 |
NM |
14 |
0 |
16/20 |
15 |
|
Compartment syndrome |
2 |
0 |
0 |
0 |
1 |
1 |
3 |
0 |
10 |
0 |
0 |
4 |
3 |
7 |
0 |
0 |
|
Deep Vein Thrombosis |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
1 |
0 |
0 |
|
Deep infection |
0 |
2 |
0 |
2 |
8 |
0 |
18% ORIF |
0 |
2 |
0 |
2 |
0 |
0 |
0 |
5/1 |
9 |
|
Reoperations |
TKR: 2 |
TKR: 2 |
0 |
Irrigation + drainage: 2 |
Staged protocol in 28 fractures with cast, EF and later ORIF |
TKR: 2 |
Removal of EF: 27 |
|
Staged protocol for all patients with knee spanning fixation and later ORIF |
NM |
Quadricepsplasty: 2 |
Stage protocol with EF before ORIF: 2 |
Fasciotomy: 3 |
Revision with ORIF: 2 |
Soft-tissue flap: 8/1 |
Arthrotomy + drainage: 2 |
| Knee manipulation: 2 |
Plate removal: 2 |
Soft tissue flap: 2 |
Irrigation + drainage: 8 |
Fasciotomy: 1 |
Total 37/16 |
Soft tissue flaps: 4 |
|
Loss of fixation: 2 |
TKR:1 |
Revision of fixator: 3 |
||||||
| Fasciotomy: 2 Arthroscopy: 1 |
Plate removal: 6 |
Bone graft: 1 |
TKR: 2/1 |
|
Plate removal: 4 |
|
Irrigation + drainage: 5 |
Fasciotomy: 2 |
||||||||
| Amputation: 1 |
|
Knee manipula- tion: 3/2 |
|
Incision + drainage: 1 |
|
Bone graft: 1 |
Plate removal: 1 |
|||||||||
| Fasciotomy: 3 |
|
Incision + drainage: 8/2 |
|
Bone graft: 2 |
|
Amputation: 1 |
||||||||||
| Skin graft: 1 |
Skin graft: 5/2 |
|
Quadricepsplasty: 1 |
|
Change of implant: 3 |
|||||||||||
| Screw removal: 0/6 |
|
Knee mani- pulation + arthroscopy: 2 |
|
Plate removal: 10 |
||||||||||||
| Plate removal: 8/0 |
|
|
|
|
||||||||||||
| Amputation: 1/0 | ||||||||||||||||
| Soft-tissue flap: 4/0 | ||||||||||||||||
| Other: 6/3 | ||||||||||||||||
|
Functional outcome |
VAS Pain |
HSS score |
ASAMI score |
0 |
0 |
HSS score |
SF-36 WOMAC HSS knees |
Rasmussen score |
WOMAC |
Knee Society score |
0 |
Lower Extremity Measure |
Honkonen score |
|
0 |
SF-36 |
| VAS Satisfaction |
Lysholm score |
Knee Society score |
Iowa |
|||||||||||||
| NHP |
Knee Society scoer |
Knee score |
||||||||||||||
| EQ5-D |
||||||||||||||||
| KOOS | ||||||||||||||||
P = Prospective, R = Retrospective, Rand = Randomiserade, NM = Not mentioned, CEF = Circular External Fixator, IF = Internal Fixation,
ORIF = Open Reduction and Internal Fixation, Unilat = Unilateral Extern Fixation, TKR = Total Knee Replacement, ROM = Range of Motion.
Discussion
The most important finding in this study was that, in both unicondylar (Schatzker I-IV) and bicondylar fractures (Schatzker V-VI), the Ilizarov fixation allowed early weight-bearing without jeopardising the fracture stability and healing.
Maripuri et al. [25] claimed that the Schatzker classification was superior to the AO [26] and the Hohl and Moore [27] classification in terms of both inter-observer reliability and intra-observer reproducibility. However, they also concluded that none of the classifications was able fully to describe all fracture types. In the present study, the Schatzker classification was used to differentiate between two biomechanically different fracture subsets, one with continuity between a part of the articular surfaces and the diaphysis (I-IV types) and one without such continuity (V-VI types). Most unicondylar tibial fractures are caused by a forced varus or valgus load. In bicondylar tibial fractures, there is also an axial load resulting in a combination of depression of the articular surface, metaphyseal crush and shearing of one or both condyles. Vertical displacement is possible because there is no shaft below the fragment, which creates a shear vector. With the “olive wires” in the Ilizarov ring fixator; these forces are counteracted, holding the condyles together, which creates a relatively stable joint surface configuration that can be fixed to the tibia distally of the fracture. The distinction between uni- and bicondylar fracture is important, because, in fracture types I-IV, there is a risk of dislocation of the fractured part of the articular surface relative to the diaphysis when loaded. Because of the discontinuity between the articular fragments and the diaphysis in the V-VI fractures, compressive forces will not normally increase the risk of displacement of the articular surfaces.
As expected, the operating time was longer for the more complex fractures. In spite of this, the operating time in the present study compares favourably with that of Lee et al. [28] who operated on thirty-six tibial plateau fractures using the less invasive stabilisation system (LISS); their mean operation time was 150 minutes. Pre-assembling the frame could reduce the time in the operating room but one important advantage of the Ilizarov technique is that it is an essentially closed method and if the surgical time is extended, the risk of wound contamination is low when compared with open plating of the tibial plateau [29].
The pain subsided rapidly and did not constitute a problem after the first 24 hours post-operatively. We have not found any report of a need for post-operative analgesia in these types of fractures, but the amount of analgesics in the PCA pump corresponds to that in patients with total knee arthroplasties in our hospital data base.
The reported incidence of joint capsule, ligament and meniscal injuries is high. Colletti et al. [6] analysed MRI findings in 29 tibial plateau fractures and found associated collateral ligament injuries in 55%, lateral meniscal tears in 45%, anterior cruciate ligament injuries in 41%, posterior cruciate ligament injuries in 28%, and medial meniscal tears in 21%. Gardner et al. [30] found that only 1% of tibial plateau fractures showed a complete absence of soft-tissue injuries, evaluated by MRI. These injuries can also be diagnosed arthroscopically [31]. However, even if recommended by some authors [32-35] there is no support for this in randomised trials [36]. The percutaneous treatment of fractures of the tibial plateau can be performed using arthroscopy or fluoroscopy to control the reduction of the joint surface. Lobenhoffer et al. [37] were not able to demonstrate any significant benefit from arthroscopy compared with fluoroscopic reduction in 168 patients with tibial plateau fractures. Ohdera et al. [38] found no significant difference between arthroscopic management of tibial plateau fractures compared with the open reduction method in terms of duration of operation, post-operative flexion, and clinical results in 28 patients. The arthroscopic procedure was only recommended in selected tibial plateau fractures. In the present series, it was possible to achieve an acceptable reduction according to the criteria formulated by Rasmussen [39] in most patients without the use of arthroscopy.
In a retrospective study, Park et al. [40] found a low rate (1.6%) of compartment syndromes requiring fasciotomy for proximal tibia fractures. However, in more complex fractures, the risk of compartment syndrome is considerably higher. For Schatzker type VI fractures, Stark et al. [41] found an overall risk of 27%, as well as a difference depending on whether or not the medial plateau was dislocated, 53 and 18% respectively. The incidence of compartment syndrome in the severe fractures (V and VI) in the present series was comparatively low; 2/19 patients; however the observed compartment syndromes were interpreted as a direct result of the fracture and the soft-tissue injury and not of the operation. In spite of the Ilizarov technique is beneficial with respect to soft-tissue injury, minimizing the risk of developing compartment syndrome; the frame should not prevent this salvage procedure when necessary.
Some studies support the staged protocol for proximal tibial fractures, especially if high energy fractures are present [42-45]. The Ilizarov method gives the advantage, independently of fracture pattern, to operate on all patients without delay. In this way, we were able to avoid disturbing the healing process with other further interventions to the soft-tissues which may delay rehabilitation.
Most treatment methods do not allow full weight-bearing in intra-articular proximal tibial fractures [46]. The mobilisation and degree of weight-bearing that is allowed is determined by the fracture displacement, method of treatment, and quality of aftercare [47,48]. In the present study, all the patients were allowed unrestricted weight-bearing without any signs of the reduction being compromised.
In earlier series, the infection rate after treating tibial plateau fractures with ORIF, varies from 6% to 87.5% [49-51]. The use of bilateral incisions and the reduction of the size of the implants have reduced this rate to 3 –8.4% [52-54]. Despite using a generally recommended staged protocol, Egol et al. [43] reported a deep wound infection rate of 5%. When comparing external devices in different locations, Parameswaran et al. [55] reported that ring fixators had the lowest incidence of infection. Using the Ilizarov technique, Catagni et al. [56] did not observe any deep infections in a series of 59 patients with Schatzker V-VI fractures. In the present series, the majority of observed infections were easy-to-treat superficial “pin-site” infections. Only two patients had “pin-tract” infections, and they could be treated without compromising the fixation or fracture healing.
The use of autogenous iliac crest bone grafts is associated with risk of increased morbidity from the donor site [57,58]. Good results have been reported in previous studies using bone graft substitutes in terms of the prevention of redislocation of the articular surface in tibial plateau fractures [59,60]. Beuerlein and McKee [61] found several studies reporting that calcium sulphate is an effective safe void-filler in bone defects after impacted fractures have been reduced. There is also evidence that the bioresorbable calcium phosphate is a better choice than autogeneous iliac bone grafts for the treatment of subarticular defects associated with unstable tibial plateau fractures [62,63]. At the one-year control, we were unable to detect any subsidence of the graft, which can be regarded as being at least partly integrated in all patients.
Conventional radiographs alone are not able to define union in internally fixed fractures with sufficient accuracy to enable their use as end-points of fracture healing. Generally, deciding when a fracture can be regarded as “healed” is difficult. In a recent study, Corrales et al. [64] reported a lack of consensus with regard to the definition of fracture healing. The surgeon’s ability to judge fracture union using chronological radiographs following internal fixation is estimated to be correct in approximately 70% [65]. The use of traditional external fixation methods, such as manual testing of fracture stability and/or pain response to weight-loading with the frame disassembled, can be added to the evaluation of the radiological healing. These tests could therefore be used to assess whether the fracture has healed sufficiently to allow the safe removal of the fixator and full, unprotected weight-bearing. Using these criteria, we had no refractures or increased deformities.
Several authors have discussed the degree of dislocation that can be accepted with remaining good knee function. The long-term results reported by Rasmussen [39] and Lansinger et al. [66] showed that a residual depression of up to 10 mm could be accepted if the knee was stable. In a 5-year follow-up on 109 fractures, Lucht and Pilgaard [67] reported that the functional outcome with a depression of <10 mm was acceptable. In terms of articular depression the recommended “acceptable” dislocation varies between 2 and 10 mm [68]. Marsh et al. [69] pointed out that the scientific basis for the different recommendations is generally weak. Giannoudis et al. [70] found that, in tibial plateau fractures, articular incongruities appear to be well tolerated. In addition to the articular depression, Rasmussen also found that instability and residual joint malalignment with varus and valgus angulations over 10° affected the outcome adversely. The residual displacements observed in the present series are within these limits in all but three patients. No one of the three patients with asymptomatic knee laxity had a valgus plateau tilt exceeding five degrees.
Knee stiffness is a common problem after tibial plateau fracture surgery [7] Gaston et al. [71] reported that, at one year, patients with tibial plateau fractures still ran a risk of 20% risk of knee stiffness, defined as flexion of less than 100° and an extension deficit of less than 5°. However, good results have been achieved with hybrid or ring fixators [72,73] and the results in the present study compare favorably with these. Even in the complex fractures requiring a hinged extension to the femur, only four of 15 patients had knee flexion of 90° or less.
It has been shown that individuals with proximal tibial fractures have substantial residual limb-specific and general health deficits even at two years of follow-up [13]. When we started the present study self-appraisal scores were rarely used in fracture patients. In recent years there has been an increasing interest in the patients opinion about the outcome. However there is no consensus of which score to use. As showed in Tables 7 and 8 different self-appraisal scores are being used or scores that are a mixture of the patients and the surgeons opinion. A previous evaluation of NHP scores in a prospective trial designed to study the effect of Ilizarov reconstruction of post-traumatic lower-limb deformities on general health status showed improvements equal to or better than the improvements reported for other orthopaedic procedures, including total joint arthroplasty [74]. The patients self-appraisals used in the present series (NHP, EQ5D, Pain-VAS, Satisfaction-VAS, KOOS) showed that the Ilizarov fixator was well tolerated and the overall restoration of function was good. Some residual pain was still present at the one-year control, which most probably reflects the severe nature of these fractures more than treatment failure.
Despite successful treatment and improvement in their outcomes, the KOOS subscores showed the lowest values for Sports and QOL activities, which is probably due to the fact that patients studied earlier with this score are commonly younger and more active than the patients enrolled in the present study. In two recently published studies KOOS have been used in the follow-up after intramedullary nailing of tibial shaft fractures and operated patellar fractures showing comparable results as in the present study [75,76]. Apart from this, patients with fractures type I-IV had results similar to patients after ACL reconstruction [22] and also the patients with type V-VI had acceptable results.
Conclusions
The study confirms that the Ilizarov technique is a safe and effective method with a relatively low complication rate. It produces good results in both Schatzker type I-IV and Schatzker type V-VI fractures. The results are comparable to those previously reported with other techniques. Early and definite fixation is achieved with the Ilizarov technique, allowing immediate full weight-bearing, and the compliance is good. From a long-term perspective, the residual fracture displacements were within the range at which the risk of post-traumatic arthritis is low. The results from the present series indicate that the Ilizarov method is a valuable alternative in the treatment of both Schatzker I-IV and V-VI fracture types.
Abbreviations
AO: Arbeitsgemeinschaft für Osteosynthesefragen; COTS: The Canadian Orthopaedic Trauma Society; ORIF: Open Reduction Internal fixation; ROM: Range of Movement
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TR conducted the study and wrote the manuscript. JK and LN participated in the design of the study, which LN supervised. They both helped to analyse the results and revised the manuscript, together with CE and BE. All the authors agreed on the final content of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Telmo Ramos, Email: telmo.ramos@vgregion.se.
Carl Ekholm, Email: carl.ekholm@vgregion.se.
Bengt I Eriksson, Email: bengt.eriksson@gu.se.
Jón Karlsson, Email: jon.karlsson@vgregion.se.
Lars Nistor, Email: lars.nistor@vgregion.se.
Acknowledgements
We thank to biostatistician Salmir Nasic of the Research Fund at Skaraborg Hospital, Sweden, for help with the statistical analysis of the data.
This study was supported by the Research Fund at Skaraborg Hospital, Sweden.
The funding agency was not involved in the design or the conduct of the study; manuscript preparation or in the decision to submit the manuscript for publication.
References
- Gustilo RB. Fractures and dislocations. St. Louis: CV Mosby; 1993. Fractures of the tibial plateau; p. 945. [Google Scholar]
- Schatzker J. In: Skeletal trauma. Browner BD, Jupiter BB, Levine AM, editor. Philadelphia: WB Saunders; 1993. Tibial plateau fractures; p. 1745. [Google Scholar]
- Campbell’s. Operative orthopaedics. 11. Philadelphia: Mosby Elsevier; 2007. [Google Scholar]
- Rockwood and Green’s. Fractures in adults. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Baratz M, Watson AD, Imbriglia JE. Orthopaedic surgery: the essentials. New York: Thieme Medical Publishers; 1999. p. 517. [Google Scholar]
- Colletti P, Greenberg H, Terk MR. MR findings in patients with acute tibial plateau fractures. Comput Med Imaging Graph. 1996;20–5:389–394. doi: 10.1016/s0895-6111(96)00054-7. [DOI] [PubMed] [Google Scholar]
- Papagelopoulos PJ, Partisinevelos AA, Themitocleous GS, Mvrogenis AF, Korres DS, Soucacos PN. Complications after tibial plateau fracture surgery. Injury. 2006;6:475–484. doi: 10.1016/j.injury.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Tscherne H, Lobenhoffer P. Tibial plateau fractures: management and expected results. Clin Orthop. 1993;292:87–100. [PubMed] [Google Scholar]
- Ilizarov GA. In: Collected Scientific Works of the Kurgan Regional Scientific Medical Society. Ilizarov GA, editor. Kurgan: Union of Soviet Socialists Republic; 1954. A New Principle of Osteosynthesis with the Use of Crossing Pins and Rings; pp. 145–160. [Google Scholar]
- Ilizarov GA. Transosseous osteosynthesis. 1. Berlin Heidelberger New York: Springer Verlag; 1992. [Google Scholar]
- Fleming B, Paley D, Kristiansen T, Pope M. A biomechanical analysis of the Ilizarov external fixator. Clin Orthop Relat Res. 1989;241:95–105. [PubMed] [Google Scholar]
- Ylmaz E, Belhan O, Karakurt L, Arslan N, Serin E. Mechanical performance of hybrid Ilizarov external fixator in comparison with the Ilizarov circular external fixator. Clin Biomech. 2003;18–6:518–522. doi: 10.1016/s0268-0033(03)00073-1. [DOI] [PubMed] [Google Scholar]
- The Canadian Orthopaedic Trauma Society. Open reduction and internal fixation compared with the circular fixator application for bicondylar tibial plateau fractures. Results of a multicenter, prospective, randomised clinical trial. J Bone Joint Surg Am. 2006;88–12:2613–2623. doi: 10.2106/JBJS.E.01416. [DOI] [PubMed] [Google Scholar]
- Mahadeva D, Costa ML, Gaffey A. Open reduction and internal fixation versus hybrid fixation for bicondylar/severe tibial plateau fractures: a systematic review of the literature. Arch Orthop Trauma Surg. 2008;128–10:1169–1175. doi: 10.1007/s00402-007-0520-7. [DOI] [PubMed] [Google Scholar]
- Watson JT, Coufal C. Treatment of complex lateral plateau fractures using Ilizarov techniques. Clin Orthop Relat Res. 1998;353:97–106. doi: 10.1097/00003086-199808000-00012. [DOI] [PubMed] [Google Scholar]
- Schatzker J, McBroom R, Bruce D. The tibial plateau fracture. The Toronto experience 1968–1975. Clin Orthop Relat Res. 1979;138:94–104. [PubMed] [Google Scholar]
- Davies R, Holt N, Nayagam S. The care of pin sites with external fixation. J Bone Joint Surg Br. 2005;87–5:716–719. doi: 10.1302/0301-620X.87B5.15623. [DOI] [PubMed] [Google Scholar]
- Checketts RG, Otterburn M, Mac Eachern AG. Pin track infection; definition, incidence and prevention. Int J OrthopTrauma. 1993;3(Suppl 3):16–18. [Google Scholar]
- Brooks R. EuroQol: the current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- Hunt SM, McEwan T. The development of a subjective health indicator. Soc Health Illness. 1980;2:231–246. doi: 10.1111/1467-9566.ep11340686. [DOI] [PubMed] [Google Scholar]
- Wiklund I, Romanus B, Hunt SM. Self-assessed disability in patients with arthrosis of the hip joint. Reliability of the Swedish version of the nottingham health profile. Int Disabil Studies. 1998;10:159–163. doi: 10.3109/09638288809164068. [DOI] [PubMed] [Google Scholar]
- Roos E, Roos H, Lohmander LS, Ekdahl C, Benynnon B. Knee injury and osteoarthritis outcome score (KOOS)-development of a self-administered outcome measure. J Orthop Sports Phsysical Therapy. 1998;78–2:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- Roos E, Roos H, Ekdahl C, Lohmander LS. Knee injury and osteoarthritis outcome score (KOOS)-validation of a Swedish version. Scand J Med Sci Sports. 1998;8:439–448. doi: 10.1111/j.1600-0838.1998.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen PS. Tibia condylar fractures: Impairment of knee joint stability as an indication for surgical treatment. J Bone Joint Surg Am. 1973;55-A:1331–1334. [PubMed] [Google Scholar]
- Maripuri SN, Rao P, Manoj-Thomas A, Mohanthy K. The classification systems for tibial plateau fractures: how reliable are they? Injury. 2008;39–10:1216–1221. doi: 10.1016/j.injury.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Müller ME, Nazarian S, Koch P, Schatzker J. The comprehensive classification of fractures of long bones. New York: Springer; 1990. [Google Scholar]
- Hohl M, Luck JV. Fractures of the tibial condyle; a clinical and experimental study. J Bone Joint Surg Am. 1956;38A5:1001–1018. [PubMed] [Google Scholar]
- Lee JA, Papadakis SA, Moon C, Zalavras CG. Tibial plateau fractures treated with less invasive stabilisation system. Int Orthop. 2007;31–3:415–418. doi: 10.1007/s00264-006-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman M, Wright A, Gruen G, Siska P, Pape HC, Tarkin I. Prolonged operative time increases infection rate in tibial plateau fractures. Injury. 2012. [DOI] [PMC free article] [PubMed]
- Gardner MJ, Yacoubian S, Geller D, Suk M, Mintz D, Potter H, Helfet DL, Lorich DG. The incidence of soft tissue injury in operative tibial plateau fractures: a magnetic ressonance imaging analysis of 103 patients. J Orthop Trauma. 2005;19–2:79–84. doi: 10.1097/00005131-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Abdel-Hamid MZ, Chang CH, Chan YS, Lo YP, Huang JW, Hsu KY, Wang CJ. Arthroscopic evaluation of soft tissues injuries in tibial fractures: retrospective analysis of 98 cases. Arthroscopy. 2006;22–6:669–675. doi: 10.1016/j.arthro.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Attmanspacher W, Dittrich, Staiger M, Stedtfeld HW. Arthroscopic management of tibial plateau fractures. Zentralbl Chir. 2002;127–10:828–36. doi: 10.1055/s-2002-35126. [DOI] [PubMed] [Google Scholar]
- Chan YS, Yuan LJ, Hung SS, Wang CJ, Yu SW, Chen CY, Chao EK, Lee MS. Arthroscopic-assisted reduction with bilateral buttress plate fixation of complex tibial plateau fractures. Arthroscopy. 2003;19–9:974–84. doi: 10.1016/j.arthro.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Lubowitz JH, Elson WS, Guttmann D. Arthroscopic management of tibial plateau fractures. Arthroscopy. 2004;20–10:1063–70. doi: 10.1016/j.arthro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou Z. Arthroscopic percutaneous osteosynthesis of low-energy tibial plateau fractures. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23–11:1316–8. [PubMed] [Google Scholar]
- Levy BA, Herrera DA, Macdonald P, Cole PA. The medial approach for arthroscopy-assisted fixation of lateral tibial fractures: patient selection and mid- to long-term results. J Orthop Trauma. 2008;22–3:201–5. doi: 10.1097/BOT.0b013e31815b35bf. [DOI] [PubMed] [Google Scholar]
- Lobenhoffer P, Schulze M, Gerich T, Tscherne H, Lattermann C. Closed reduction/percutaneous fixation of tibial plateau fractures: arthroscopic versus fluoroscopic control of reduction. J Orthop Trauma. 1999;13–6:426–431. doi: 10.1097/00005131-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Ohdera T, Tokunaga M, Hiroshima S, Yoshimoto E, Tokunaga J, Kobbayashi A. Arthroscopic management of tibial plateau fractures – comparison with open reduction method. Arch Orthop Trauma Surg. 2003;123–9:489–93. doi: 10.1007/s00402-003-0510-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen PS. A functional approach to evaluation and treatment of tibial condylar fractures. PhD thesis. Gothenburg: Gothenburg University Elanders Boktryckeri Aktiebolag; 1971. [Google Scholar]
- Park S, Ahn J, Gee AO, Kuntz AF, Esterhai JL. Compartment syndrome in tibial fractures. J Orthop Trauma. 2009;23–7:514–8. doi: 10.1097/BOT.0b013e3181a2815a. [DOI] [PubMed] [Google Scholar]
- Stark E, Stucken C, Trainer G, Tornetta P 3rd. Compartment syndrome in Schatzker type VI plateau fractures and medial condylar fracture-dislocations treated with temporar external fixation. J Orthop Trauma. 2009;23–7:502–6. doi: 10.1097/BOT.0b013e3181a18235. [DOI] [PubMed] [Google Scholar]
- Tejwani NC, Achan. Staged management of high-energy proximal tibia fractures. Bull Hosp Jt Dis. 2004;62:62–66. [PubMed] [Google Scholar]
- Egol KA, Tejwani NC, Capla EL, Wolinsky PL, Koval KJ. Staged management of high-energy proximal tibia fractures (OTA types 41): the results of a prospective, standardized protocol. J Orthop Trauma. 2005;19–7:448–55. doi: 10.1097/01.bot.0000171881.11205.80. [DOI] [PubMed] [Google Scholar]
- Dirschl DR, Del Gaizo D. Staged management of tibial plateau fractures. Am J Orthop. 2007;36–4:12–7. [PubMed] [Google Scholar]
- Ma CH, Wu CH, Yu SW, Yen CY, Tu YK. Staged external and internal less-invasive stabilization system plating for open proximal tibia fractures. Injury. 2010;41–2:190–196. doi: 10.1016/j.injury.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Ali AM, Burton M, Hashmi M, Saleh M. Outcome of complex fractures of the tibial plateau treated with a beam-loading ring fixation system. J Bone Joint Surg Br. 2003;85–5:691–9. [PubMed] [Google Scholar]
- Gausewitz S, Hohl M. The significance of early motion in treatment of tibial plateau fractures. Clin Orthop Relat Res. 1986;202:135–8. [PubMed] [Google Scholar]
- Segal D, Mallik AR, Wetzler MJ, Franchi AV, Whitelaw GP. Early weight-bearing of lateral tibial plateau fractures. Clin Orthop Relat Res. 1993;294:232–7. [PubMed] [Google Scholar]
- Boszotta H, Helperstorfer W, Kölndorfer G, Prunner K. Long-term results of surgical management of displaced tibial head fractures. Aktuelle Traumatol. 1993;23–4:178–82. [PubMed] [Google Scholar]
- Moore TM, Patzakis MJ, Harvey JP. Tibial fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1–2:97–119. [PubMed] [Google Scholar]
- Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23–2:149–54. [PubMed] [Google Scholar]
- Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18–10:649–57. doi: 10.1097/00005131-200411000-00001. [DOI] [PubMed] [Google Scholar]
- Eggli S, Hartel MJ, Kohl S, Haupt U, Exadaktylos AK, Röder C. Unstable bicondylar tibial plateau fractures: a clinical investigation. J Orthop Trauma. 2008;22–10:673–9. doi: 10.1097/BOT.0b013e31818b1452. [DOI] [PubMed] [Google Scholar]
- Rademakers MV, Kerkhoffs GM, Sirevelt IN, Raaymakers EL, Marti RK. Operative treatment of 109 tibial plateau fractures: five- to 27-year follow-up results. J Orthop Trauma. 2007;21–1:5–10. doi: 10.1097/BOT.0b013e31802c5b51. [DOI] [PubMed] [Google Scholar]
- Parameswaran AD, Roberts CS, Seligson D, Voor M. Pin tract infection with contemporary external fixation: How much of a problem? J Orthop Trauma. 2003;17–7:503–7. doi: 10.1097/00005131-200308000-00005. [DOI] [PubMed] [Google Scholar]
- Catagni M, Ottaviani G, Maggioni M. Treatment strategies for complex fractures of the tibial plateau with external circular fixation and limited internal fixation. J Trauma. 2007;63–5:1043–53. doi: 10.1097/TA.0b013e3181238d88. [DOI] [PubMed] [Google Scholar]
- Fowler BL, Dall BE, Rowe DE. Complications associated with harvesting autogenous iliac bone graft. Am J Orthop. 1995;24:895–903. [PubMed] [Google Scholar]
- Seiler JG 3rd, Johnson J. Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc. 2000;9:91–7. [PubMed] [Google Scholar]
- Bucholz RW, Carlton A, Holmes R. Interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures. Clin Orthop. 1989;240:53–62. [PubMed] [Google Scholar]
- Itokazu M, Matsunaga T. Arthroscopic restoration of depressed tibial plateau fractures using bone and hydroxyapatite grafts. Arthroscopy. 1993;9:103–108. doi: 10.1016/s0749-8063(05)80353-6. [DOI] [PubMed] [Google Scholar]
- Beuerlein MJ, McKee MD. Calcium sulfates: what is the evidence? J Orthop Trauma. 2010;24(Suppl 1):46–51. doi: 10.1097/BOT.0b013e3181cec48e. [DOI] [PubMed] [Google Scholar]
- Bajammal SS, Zlowodski M, Lelwica A, Tornetta P 3rd, Einhorn TA, Buckley R, Leighton R, Russel TA, Larsson S, Bhandari M. The use of calcium phosphate bone cement in fracture treatment. A meta-analysis of randomized trials. J Bone Joint Surg Am. 2008;90–6:1186–96. doi: 10.2106/JBJS.G.00241. [DOI] [PubMed] [Google Scholar]
- Russel TA, Leighton RK. Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study. J Bone Surg Am. 2008;90–10:2057–61. doi: 10.2106/JBJS.G.01191. [DOI] [PubMed] [Google Scholar]
- Corrales LA, Morshed S, Bhandari M, Miclau T 3rd. Variability in the assessment of fracture-healing in orthopaedic trauma studies. J Bone Joint Surg Am. 2008;90–9:1862–8. doi: 10.2106/JBJS.G.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Roberts PJ, Moorcroft CI, Brown MF, Thomas PB, Wade RH. Reliability of radiographs in defining union of internal fixed fractures. Injury. 2004;35–6:557–61. doi: 10.1016/S0020-1383(03)00262-6. [DOI] [PubMed] [Google Scholar]
- Lansinger O, Bergman B, Körner L, Andersson GB. Tibial condylar fractures. A twenty-year follow-up. J Bone Joint Surg Am. 1986;68–1:13–9. [PubMed] [Google Scholar]
- Lucht U, Pilgaard S. Fractures of the tibial condyles. Acta Orthop Scand. 1971;42:366–76. doi: 10.3109/17453677108989057. [DOI] [PubMed] [Google Scholar]
- Ali AM, El-Shafie M, Willet KM. Failure of fixation of tibial fractures. J Orthop Trauma. 2002;16–5:323–9. doi: 10.1097/00005131-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, Llinias A. Articular fractures: does an anatomical reduction really change the result? J Bone Joint Surg Am. 2002;84A–7:1259–71. [PubMed] [Google Scholar]
- Giannoudis PV, Tzioupis C, Patathanassopoulos A, Obakponovwe O, Roberts C. Articular step-off and risk of post-traumatic osteoarthritis. Evidence today. Injury. 2010;41:986–995. doi: 10.1016/j.injury.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Gaston P, Will EM, Keating JF. Recovery of knee function following fracture of the tibial plateau. J Bone Joint Surg Br. 2005;87–9:1233–6. doi: 10.1302/0301-620X.87B9.16276. [DOI] [PubMed] [Google Scholar]
- Dendrinos GK, Kontos S, Katsenis D, Dalas A. Treatment of high-energy tibial plateau fractures by the Ilizarov circular fixator. J Bone Joint Surg Br. 1996;78–5:710–7. [PubMed] [Google Scholar]
- Mikulak SA, Gold SM, Zinar DM. Small wire external fixation of high energy tibial plateau fractures. Clin Orthop Relat Res. 1998;356:230–8. doi: 10.1097/00003086-199811000-00031. [DOI] [PubMed] [Google Scholar]
- McKee MD, Yoo D, Schemetisch EH. Health status after Ilizarov reconstruction of post-traumatic lower-limb deformity. J Bone Joint Surg Br. 1998;80–2:360–4. doi: 10.1302/0301-620x.80b2.8192. [DOI] [PubMed] [Google Scholar]
- Hayashi A. Poor outcomes for surgically treated patella fractures. AAOS Now. January 2010 Issue [ http://www.aaos.org/news/aaosnow/jan10/clinical7.asp]
- Larsen P, Lund H, Laessoe U, Graven-Nielsen T, Petruskevicius J, Rasmussen S. Anterior knee pain and limitations in activity and participation after intramedullary nailing of tibial shaft fracture [abstract] DOS Kongressen. 2012. p. 126. http://www.ortopaedi.dk/fileadmin/abstracts/2012/index.html#/126/zoomed.


