Abstract
Background
Cymbidium sinense belongs to the Orchidaceae, which is one of the most abundant angiosperm families. C. sinense, a high-grade traditional potted flower, is most prevalent in China and some Southeast Asian countries. The control of flowering time is a major bottleneck in the industrialized development of C. sinense. Little is known about the mechanisms responsible for floral development in this orchid. Moreover, genome references for entire transcriptome sequences do not currently exist for C. sinense. Thus, transcriptome and expression profiling data for this species are needed as an important resource to identify genes and to better understand the biological mechanisms of floral development in C. sinense.
Results
In this study, de novo transcriptome assembly and gene expression analysis using Illumina sequencing technology were performed. Transcriptome analysis assembles gene-related information related to vegetative and reproductive growth of C. sinense. Illumina sequencing generated 54,248,006 high quality reads that were assembled into 83,580 unigenes with an average sequence length of 612 base pairs, including 13,315 clusters and 70,265 singletons. A total of 41,687 (49.88%) unique sequences were annotated, 23,092 of which were assigned to specific metabolic pathways by the Kyoto Encyclopedia of Genes and Genomes (KEGG). Gene Ontology (GO) analysis of the annotated unigenes revealed that the majority of sequenced genes were associated with metabolic and cellular processes, cell and cell parts, catalytic activity and binding. Furthermore, 120 flowering-associated unigenes, 73 MADS-box unigenes and 28 CONSTANS-LIKE (COL) unigenes were identified from our collection. In addition, three digital gene expression (DGE) libraries were constructed for the vegetative phase (VP), floral differentiation phase (FDP) and reproductive phase (RP). The specific expression of many genes in the three development phases was also identified. 32 genes among three sub-libraries with high differential expression were selected as candidates connected with flower development.
Conclusion
RNA-seq and DGE profiling data provided comprehensive gene expression information at the transcriptional level that could facilitate our understanding of the molecular mechanisms of floral development at three development phases of C. sinense. This data could be used as an important resource for investigating the genetics of the flowering pathway and various biological mechanisms in this orchid.
Keywords: Floral development, Flowering time, Digital gene expression, Transcriptome, Cymbidium sinense
Background
The Orchidaceae is one of the largest and most widespread families of flowering plants, with more than 250000 species [1]. Cymbidium, a very important economically flowering genus with the Orchidaceae, has nearly 55 species throughout the world and is widespread in tropical and subtropical Asia, reaching as far south as Papua New Guinea and Australia [2]. Cymbidium sinense is a perennial terrestrial species native to China and has more than a thousand years of cultural history. It holds a strong position in traditional flower markets in China, Japan, Korea, and Southeast Asia. It is of great horticultural value as an ornamental plant because of its elegance, its upright leaves, and beautiful and fragrant flowers. C. sinense blooms in winter from January to March and is usually regarded as a Spring Festival flower. Although C. sinense is a valuable orchid, its flowering pathway is not clearly understood. Variation in environmental conditions, including light, temperature and hormones, is likely to regulate flowering time and flower quality. Flowering regulation technology for C. sinense is currently scarce on the market, which seriously hinders the development of the orchid industry and also reduces the economic value of C. sinense.
The transition from vegetative growth to flowering is very importance because flowering is the first step of sexual reproduction [3]. In Arabidopsis thaliana, flower initiation takes place via four (gibberellin, autonomous, vernalization, light-dependent) genetic pathways. These processes are integrated by the function of the FLOWERING LOCUS D (FD), FLOWERING LOCUS E (FE), FLOWERING WAGENINGEN (FWA), PROTODERMAL FACTOR2 (PDF2), SUPPRESSOR OF OVEREXPRESSION OF CO 1(SOC1), and FLOWERING LOCUS T (FT) genes. The integrated signal of floral induction is transmitted to the floral meristem identity genes LEATY (LFY) and APETALA1 (AP1), after which floral morphogenesis takes place [4]. Currently only a few flowering genes have been cloned from orchids. DOH1 and DOMADS1 were isolated and identified from Dendrobium Madame. DOH1 plays a negative regulatory role in floral formation while DOMADS1 is a marker gene specifically expressed in the shoot apical meristem during floral transition. DOH1 is a possible upstream regulator of DOMADS1 and inhibits its expression [5,6]. Some MADS-box function genes were also isolated from Dendrobium crumenatum. They include APETALA2 (AP2), PISTILLATA (PI)/GLOBOSA (GLO), APETALA3 (AP3) /DEFIENCE (DEF)-like, AGAMOUS (AG) and SEPALLATA (SEP) genes, which play an important role in the early regulation of the conversion to flowers [7]. OMADS3 was isolated and characterized from Oncidium Gower Ramsey with a function similar to a functional gene regulating flower formation as well as floral initiation [8]. OMADS1 from O. Gower Ramsey promoted flower transition and formation by acting as an activator for FT and SOC1 in Arabidopsis. OMADS1 was able to strongly interact with OMADS3, which influenced flower formation and floral initiation [9].
Compared with other orchids, very little research exists on the role of flowering genes in the regulation of the vegetative-to-flowering transition and flower initiation in Cymbidium. Few reports investigating the functions of flowering time genes of Cymbidium exist. Genomic resources available for the species are also scarce. Together with the nucleotide sequences obtained by NCBI searches, 60 Cymbidium expressed sequence tags (ESTs) are, however, available. Nevertheless, this genetic data is insufficient for elucidating the molecular mechanism of floral regulation in C. sinense.
In recent years, Illumina sequencing techniques have provided fascinating opportunities in life sciences and dramatically improved the efficiency of gene discovery. However, the entire transcriptome of C. sinense has not been sequenced. In this study, RNA-seq and digital gene expression (DGE) were performed using Illumina technology. Illumina sequencing provided comprehensive information about gene expression at the transcriptional level that could facilitate our understanding of the molecular mechanisms of C. sinense floral development. Such data for C. sinense could also be used as an important resource to investigate the flowering pathway and various other biological mechanisms in other orchid species.
Results
Illumina sequencing and sequence assembly
To obtain an overview of the C. sinense transcriptome, a cDNA library was generated from an equal mixture of RNA isolated from all organs, and pair end sequenced using the Illumina Hiseq™2000 platform. After cleaning and quality checks, 54 million 90-bp reads were assembled into 147162 contigs with a mean length of 326 bp (Table 1). Using paired-end reads, these contigs were further assembled into 83580 unigenes by Trinity, including 13315 clusters and 70265 singletons, with a mean length of 612 bp. The size distribution of these contigs and unigenes are shown in Figures 1 and 2. The assembly produced a substantial number of large contigs: 11852 contigs were >1,000 bp in length and 26698 contigs were >500 bp, although most contigs were between 200 and 300 bp in length (Figure 1). 17644 unigenes were >1,000 bp in length (Figure 2).
Table 1.
Summary for C. sinense transcriptome
| Total number of raw reads |
59,512,598 |
| Total number of clean reads |
54,248,006 |
| Total clean nucleotides (nt) |
4,882,320,540 |
| Average read length |
90 |
| Total number of contigs |
147,162 |
| Mean length of contigs |
326 |
| Total number of unigenes |
83,580 |
| Mean length of unigenes | 612 |
Figure 1.
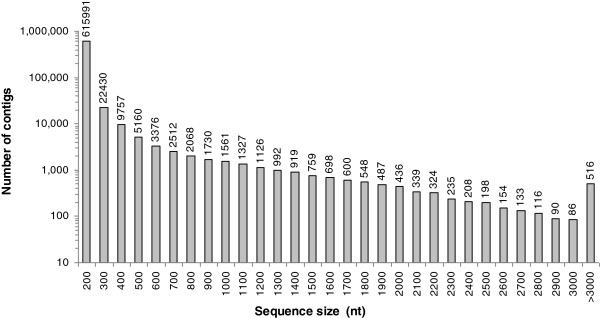
The length distribution of assembled C. sinense contigs.
Figure 2.
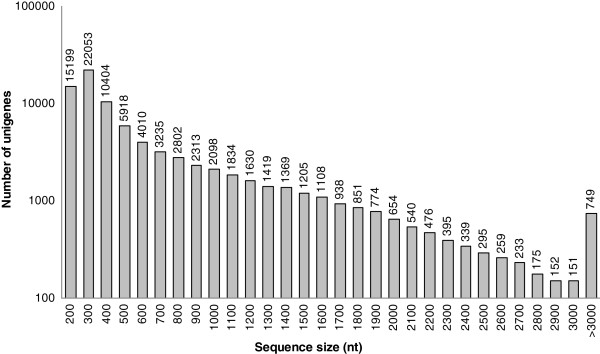
The length distribution of assembled C. sinense unigenes.
Annotation of predicted proteins
After searching the reference sequences using BLASTX against nr, SwissPort, COG and KEGG, we found a total of 41687 (49.88% of all unigenes) unigenes providing a significant BLAST result (Table 2).
Table 2.
Statistics of annotation results for C. sinense unigenes
| Sequence file | All | NR | SwissPort | KEGG | COG | GO |
|---|---|---|---|---|---|---|
| JX-Unigene | 41,687 | 41,161 | 30,606 | 23,092 | 15,041 | 16,565 |
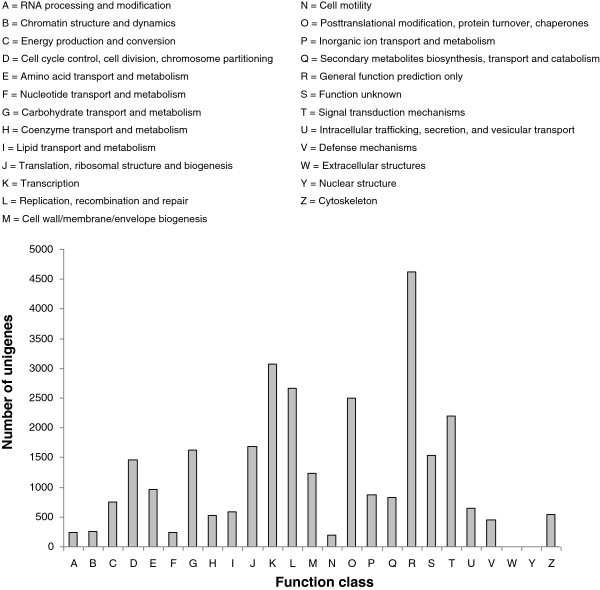
Among the 41687 unigenes, approximately 36.1% could be annotated in COG based on sequence homologies. In the COG classification, 15041 unigenes were classified into 25 function classifications (Figure 3). ‘General function prediction’ was dominant. ‘Translation’, ‘replication, recombination and repair’ and ‘posttranslational’ also shared a high-percentage of genes among the categories, and only a few genes matched the terms ‘nuclear structure’ and ‘extracellular structures’. 2199 unigenes were annotated as the ‘signal transduction mechanisms’ category, which suggests that our study may allow for the identification of novel genes involved in signal transduction pathways. The COG analysis showed that the identified genes are involved in various biological processes.
Figure 3.
COG function classification of C. sinense unigenes.
GO classification for unigenes
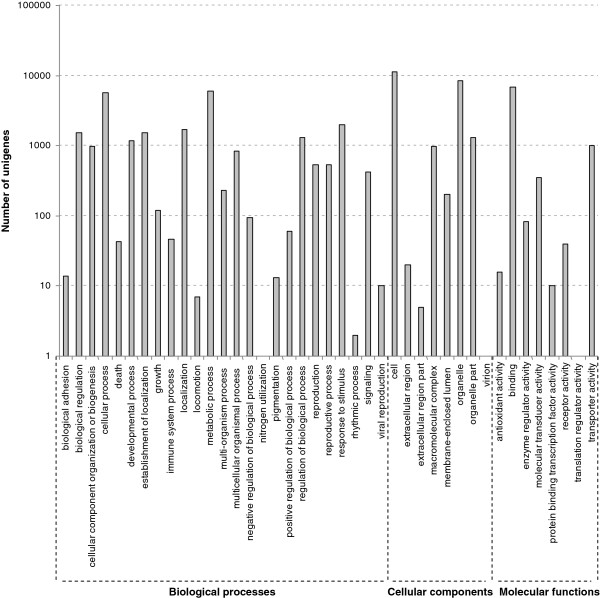
We used GO assignments to classify the functions of the predicted C. sinense unigenes. 16565 annotated unigenes were further categorized into 44 functional groups (Figure 4). Metabolic and cellular processes were the most highly represented groups in the biological process category. 5986 unigenes were annotated as the ‘metabolic process’ category, which suggests that our study may allow for the identification of novel genes involved in secondary metabolite synthesis pathways. Cell and cell parts were dominant groups in the “cellular component function” category. In the “molecular function” category, a high percentage of genes came from the ‘binding’ (41.1%) and ‘catalytic activity’ (44.6%) groups. In the ‘nitrogen utilization’, ‘virion’ and ‘translation regulator activity’ groups, only a single unigene was annotated for each (Figure 4).
Figure 4.
GO classification of C. sinense unigenes.
Metabolic pathway assignment by KEGG
In our study, 41687 annotated sequences were mapped to the reference canonical pathways in KEGG. In total, 23092 sequences were assigned to 126 KEGG pathways (Additional file 1). The pathways with most representation by the unigenes were metabolic pathways (5179 members, 22.43%) and biosynthesis of secondary metabolites (2161 members, 9.36%). These pathways provide a valuable resource for investigating specific processes, functions and pathways during C. sinense research. Interestingly, 1397 unigenes involved in plant hormone signal transduction were found, which contained 9 pathways (Table 3) controlling the signal transduction of several plant growth regulators, for example, auxin, cytokinin, gibberellin, abscisic acid, ethylene, brassinosteroid, jasmonic acid and salicylic acid. These hormones regulate seed dormancy, plant vegetative and reproductive growth, fruit ripening senescence and stress response.
Table 3.
The pathways and the products involved in the pathway of plant hormone signal transduction
| Pathway | Product | Pathway ID |
|---|---|---|
| Brassinosteroid biosynthesis |
Brassinosteroid |
ko00905 |
| Carotenoid biosynthesis |
Abscisic acid |
Ko00906 |
| Cysteine and methionine metabolism |
Ethylene |
Ko00270 |
| Diterpenoid biosynthesis |
Gibberellin |
Ko00904 |
| Indole alkaloid biosynthesis |
Indole-acetic acid |
Ko00901 |
| α-Linolenic acid metabolism |
Jasmonic acid |
Ko00591 |
| Phenylalanine metabolism |
Salicylic acid |
Ko00360 |
| Tryptophan metabolism |
Auxin |
Ko00380 |
| Zeatin biosynthesis | Cytokinin | ko00908 |
The circadian clock is an important factor controlling plant physiology, and is also an important part of the photoperiod pathway. It regulates physiological activities by controlling the circadian rhythm [10]. A circadian rhythm was found in the KEGG pathway involving 222 (0.97%) unigenes. The detailed metabolic pathway for the circadian rhythm is shown in Figure 5. Every gene in the pathway was associated with several unigenes. The pathway will be useful for further studies on the effect of the photoperiod pathway on C. sinense flowering-related processes.
Figure 5.
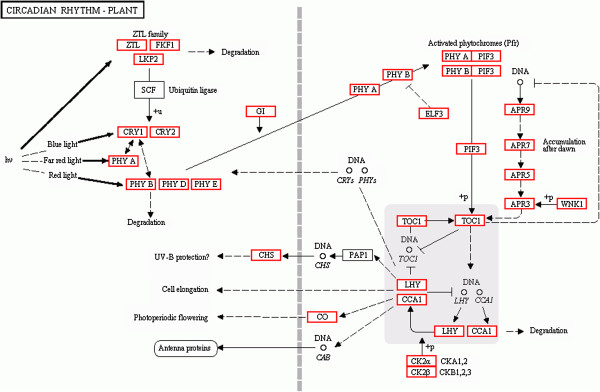
Metabolic pathway of the circadian rhythm for unigenes by KEGG annotation.
Identifying C. sinense flowering time-associated genes and MADS-box genes
According to the annotation of unigenes, we obtained 120 genes associated with flowering time. Some genes are shown in Table 4. These include photoperiod pathway genes such as GIGANTEA (GI), EARLY FLOWERING 3 (EIF3), PHYTOCHROME INTERACTING FACTOR 3 (PIF3), LATE ELONGATED HYPOCOTYL (LHY), CHALCONE SYNTHASE (CHS), CIRCADIAN CLOCK ASSOCIATED 1 (CCA1), and CO; vernalization pathway genes related to VERNALIZATION (VRN); floral integrator pathway genes related to CAULOFLOWER (CAL), AP2, FT, SOC1 and TERMINAL FLOWER 1(TFL); and floral meristem identity genes LFY and AP1 were all identified in our C. sinense transcriptome database. Additionally, 73 MADS-box unigenes were also discovered (Additional file 2). All these unigenes are important resources for the study of floral development and flower organ formation in the future.
Table 4.
C. sinenseunigenes that share homology with flowering time genes
| Category | Gene ID | Homologous gene | Nr-ID |
|---|---|---|---|
| Photoperiod pathway |
CL636.Contig1_JX |
GI |
ADP92454.1 |
| |
CL8490.Contig1_JX |
ADP92454.1 |
|
| |
Unigene61105_JX |
ELF3 |
ABL11477.1 |
| |
Unigene61030_JX |
PIF3 |
NP_001063455.1 |
| |
Unigene31120_JX |
LHY |
ACF60466.1 |
| |
Unigene14045_JX |
CHS |
NP_001064831.1 |
| |
CL2407.Contig1_JX |
O23729.1 |
|
| |
Unigene68739_JX |
CCA1 |
ABW87009.1 |
| |
CL1617.Contig2_JX |
CO |
NP_001047975.1 |
| |
CL1617.Contig1_JX |
NP_001057441.1 |
|
| |
CL10349.Contig1_JX |
NP_001062363.2 |
|
| |
CL10838.Contig1_JX |
ADL36678.1 |
|
| Vernalization pathway |
Unigene49191_JX |
VRN2 |
ABD85301.1 |
| |
Unigene49192_JX |
ABD85301.1 |
|
| |
Unigene50102_JX |
ABD85301.1 |
|
| Floral integrator pathway |
Unigene4182_JX |
FT/HD3a |
ADI58462.1 |
| |
Unigene43540_JX |
ABX11019.1 |
|
| |
Unigene7817_JX |
CBY25182.1 |
|
| |
CL743.Contig1_JX |
ADP89470.1 |
|
| |
CL743.Contig2_JX |
ADP89470.1 |
|
| |
Unigene35813_JX |
TFL |
ACX53295.1 |
| |
Unigene36169_JX |
BAD02372.1 |
|
| |
CL11326.Contig1_JX |
AP2 |
ABB90554.1 |
| |
CL12341.Contig1_JX |
NP_001147356.1 |
|
| |
CL1488.Contig1_JX |
AAZ95247.1 |
|
| |
CL1700.Contig1_JX |
AAZ66389.1 |
|
| |
CL2122.Contig1_JX |
XP_002304138.1 |
|
| |
CL260.Contig1_JX |
XP_002274890.1 |
|
| |
CL260.Contig2_JX |
XP_002274890.1 |
|
| |
CL260.Contig3_JX |
XP_002274890.1 |
|
| |
CL260.Contig4_JX |
XP_002274890.1 |
|
| |
CL3589.Contig1_JX |
XP_002325111.1 |
|
| |
Unigene32766_JX |
SOC1 |
ADP06386.1 |
| |
Unigene59851_JX |
ADJ67241.1 |
|
| |
Unigene32196_JX |
BAC55080.1 |
|
| |
Unigene10614_JX |
ADJ67238.1 |
|
| |
Unigene59851_JX |
CAL |
ADJ67241.1 |
| |
Unigene10812_JX |
ADI58464.1 |
|
| Floral meristem identity |
Unigene32196_JX |
LFY |
BAC55080.1 |
| |
Unigene630_JX |
AAY40170.1 |
|
| |
Unigene2289_JX |
AP1 |
ADP00515.1 |
| |
CL162.Contig1_JX |
ADJ67240.1 |
|
| CL162.Contig2_JX | ADJ67240.1 |
Identifying C. sinense CONSTANS-like gene family
CO plays a central regulatory role in the photoperiod pathway, and is regulated by the circadian clock and light [11,12]. To determine the COL gene family of C. sinense, we analyzed the transcriptome database generated by this study and found 28 unigenes annotated as zinc finger protein CONSTANS. As shown in Additional file 3, they showed homology with 12 Arabidopsis COL genes.
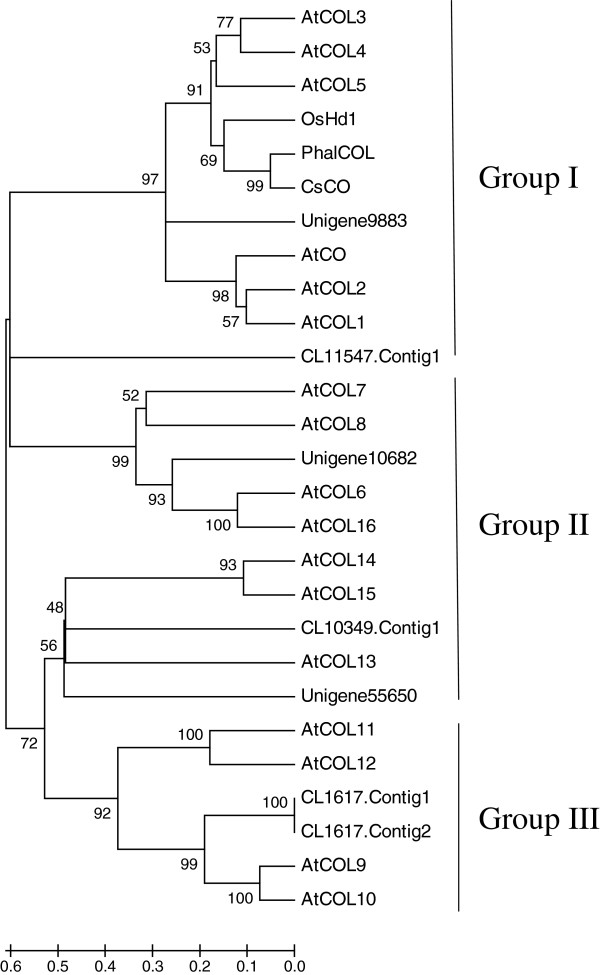
We selected seven unigenes which have two conserved B-box domains and a CCT domain to further examine their relationship with other COL genes from Arabidopsis, rice, Phalaenopsis and C. sinense. The seven unigenes are shown in Table 5. Their amino acid sequences were used to construct a phylogenetic tree using MEGA4 [13] to determine genetic distances. The phylogenetic tree was showed that all members could be divided into three divergent groups (Figure 6). Unigene9883 and CL11547.Contig1 were clustered with CsCO, PhalCOL as well as OsHd1 in group I. They are closely related to Arabidopsis AtCO/AtCOL1/AtCOL2. CL10349.Contig1, Unigene55660, CL1617.Contig1 and CL1617.Contig2 were clustered in group II. CL1617.Contig1 and CL1617.Contig2 are closely related to AtCOL9/AtCOL10. Unigene55650 is closely related to AtCOL13, and CL10349.Contig1 is closely related to AtCOL14/AtCOL15. Unigene10682 was assigned to group III and is closely related to AtCOL6/AtCOL16. These results are consistent with the data shown in Table 5.
Table 5.
C. sinense unigenes that share homology with CONSTANS-like genes of Arabidopsis
| Gene ID | Length of gene (bp) | Swissport annotation | Swissport-ID |
|---|---|---|---|
| CL11547.Contig1_JX |
955 |
Arabidopsis thaliana COL1 |
O50055 |
| Unigene9883_JX |
1023 |
Arabidopsis thaliana COL2 |
Q96502 |
| Unigene10682_JX |
1636 |
Arabidopsis thaliana COL6 |
Q8LG76 |
| CL1617.Contig1_JX |
1957 |
Arabidopsis thaliana COL9 |
Q9SSE5 |
| CL1617.Contig2_JX |
2043 |
Arabidopsis thaliana COL9 |
Q9SSE5 |
| Unigene55650_JX |
1392 |
Arabidopsis thaliana COL13 |
O82256 |
| CL10349.Contig1_JX | 784 | Arabidopsis thaliana COL14 | O22800 |
Figure 6.
Phylogenetic analysis of the CONSTANS or CONSTANS-like proteins from different plant species. The tree is displayed as a phylogram in which branch lengths are proportional to distance. Bootstrap values for 1,000 replicates were used to assess the robustness of the trees. The proteins are as follows: AtCO (accession no. NP_197088), AtCOL1 (accession no. NP_197089), AtCOL2 (accession no. NP_186887), AtCOL3 (accession no. Q9SK53), AtCOL4 (accession no. Q940T9.2), AtCOL5 (accession no. Q9FHH8), AtCOL6 (accession no. Q8LG76), AtCOL7 (accession no. Q9C9A9), AtCOL8 (accession no. Q9M9B3), AtCOL9 (accession no. NP_001118599), AtCOL10 (accession no. Q9LUA9), AtCOL11 (accession no. O23379), AtCOL12 (accession no. Q9LJ44), AtCOL13 (accession no. O82256), AtCOL14 (accession no. O22800), AtCOL15 (accession no. Q9C7E8), AtCOL16 (accession no. Q8RWD0), OsHd1 (accession no. ABB17664), PhalCOL (accession no. FJ469986), CsCO (accession no. GU168786).
DGE library sequencing and evaluation
The materials from VP, FDP, and RP were chosen to construct three DGE libraries, then which were sequenced. Three DGE sequencing quality evaluation and alignment statistics were shown in Table 6. The percent of all low quality reads including only adaptor and containing N reads was less than 0.5% in three libraries. 99.5% of raw tags in each library were clean tags. After filtering the low quality tags, the total number of clean tags in each library was 11.98, 12.65 and 12.16 million, respectively. Among the clean tags, the number of sequences that could be mapped to transcriptome sequences amounted to 10.79, 11.85 and 10.71 million, corresponding to 90.10, 93.67 and 88.07% in the three libraries, respectively.
Table 6.
DGE sequencing quality evaluation and alignment statistics
| Summary | VP | FDP | RP |
|---|---|---|---|
| Only adaptor reads |
25804 |
19835 |
37799 |
| Only adaptor reads% |
0.21% |
0.16% |
0.31% |
| Containing N reads |
37 |
40 |
43 |
| Containing N reads% |
0.00% |
0.00% |
0.00% |
| Low quality reads |
25686 |
32107 |
16577 |
| Low quality reads% |
0.21% |
0.25% |
0.14% |
| Total clean reads |
11981512 |
12647899 |
12164959 |
| Total clean reads% |
99.57% |
99.59% |
99.55% |
| Total base pairs |
587094088 |
619747051 |
596082991 |
| Total mapped reads to gene |
10795616 |
11847282 |
10713910 |
| Total% of mapped reads |
90.10% |
93.67% |
88.07% |
| Perfect match |
7558927 |
10028451 |
8649669 |
| Perfect match% |
63.09% |
79.29% |
71.10% |
| ≤2 bp mismatch |
3236689 |
1818831 |
2064241 |
| ≤2 bp mismatch% |
27.01% |
14.38% |
16.97% |
| Unique match |
9389031 |
10763499 |
8207609 |
| Unique match% |
78.36% |
85.10% |
67.47% |
| Multi-position match |
1406585 |
1083783 |
2506301 |
| Multi-position match% |
11.74% |
8.57% |
20.60% |
| Total unmapped reads |
1185896 |
800617 |
1451049 |
| Total unmapped reads% | 9.90% | 6.33% | 11.93% |
Variation in gene expression among three developmental stages
To identify the differentially expressed genes during three developmental stages, the number of clean tags for each gene was calculated, and the genes that were differentially expressed between the two samples were identified according to the method described by Audic and Claverie [14].
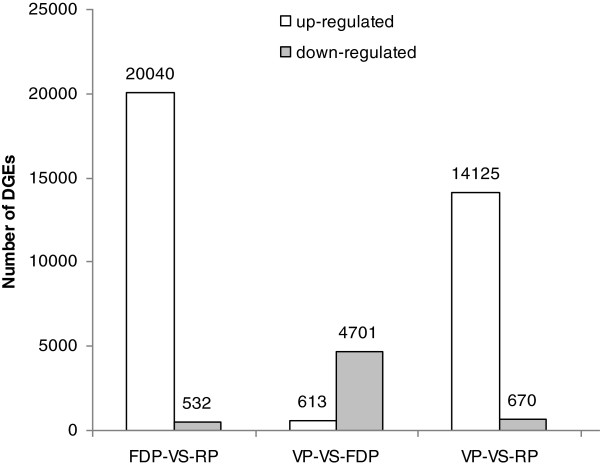
The variations in gene expression were analyzed by comparing VP and FDP, VP and RP, and FDP and RP. The comparison between VP and FDP revealed significant variations in expression. A total of 5314 genes, including 613 up-regulated and 4701 down-regulated genes, were identified in FDP compared to VP (Figure 7). 34 genes showed specific expression in FDP and 107 genes showed specific expression in VP. These specific expression genes were listed in Additional file 4.
Figure 7.
Numbers of DGE unigenes in each comparison.
Based on the GO functional classification, most of the differential expression gene sets demonstrated down-regulated expression in the FDP library, and these genes were mainly correlated to membrane, vesicle and cellular component organization (Additional file 5). In the KEGG classification, 6 gene sets were significantly enriched, and most of these genes were mainly down-regulated in the FDP library and correlated to plant hormone signal transduction (Additional file 5).
Meanwhile, we compared the variations in gene expression between VP and RP. A total of 14795 genes, including 14125 up-regulated and 761 down-regulated genes, were identified in RP compared to VP (Figure 7). 1408 genes showed specific expression in RP and 38 genes showed specific expression in VP. Those genes that showed specific expression were listed in Additional file 6.
Based on the GO functional classification, most of the gene sets demonstrated up-regulated expression in the RP library, and these genes were mainly correlated to hydrolase activity, binding and organelle parts (Additional file 7). In the KEGG classification, 13 gene sets were significantly enriched, and most of these genes were mainly up-regulated in the FDP library and correlated to the spliceosome and ribosome (Additional file 7).
A comparison between FDP and RP revealed more variations in gene expression. The results revealed 20572 genes with significant differential expression levels between FDP and RP. Among them, 20040 and 532 genes were up-regulated and down-regulated, respectively (Figure 7). 2535 genes showed specific expression in RP and 25 genes showed specific expression in FDP. Those genes that showed specific expression are listed in Additional file 8.
Based on the GO functional classification, most of the gene sets demonstrated up-regulated expression in the RP library, and these genes were mainly correlated to binding, membrane-bounded organelles and organelle parts (Additional file 9). In the KEGG classification, 12 gene sets were significantly enriched, and most of these genes were mainly up-regulated in the RP library and correlated to the spliceosome and RNA transport (Additional file 9).
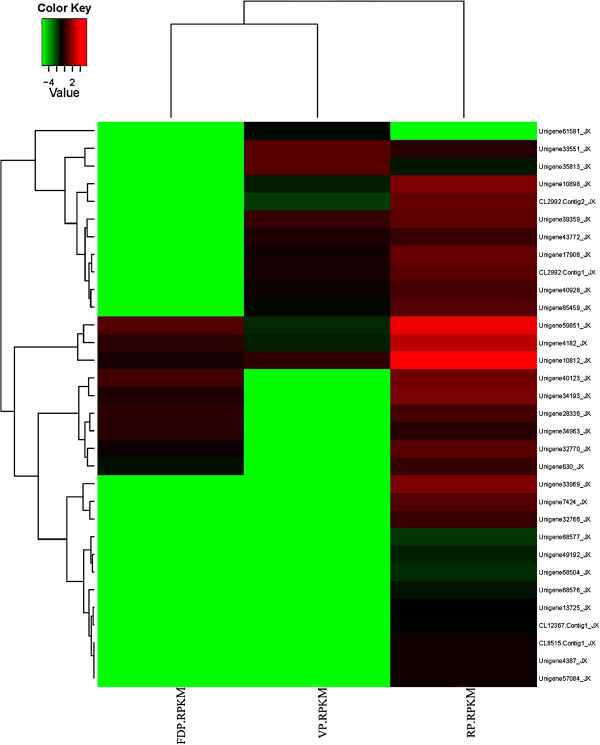
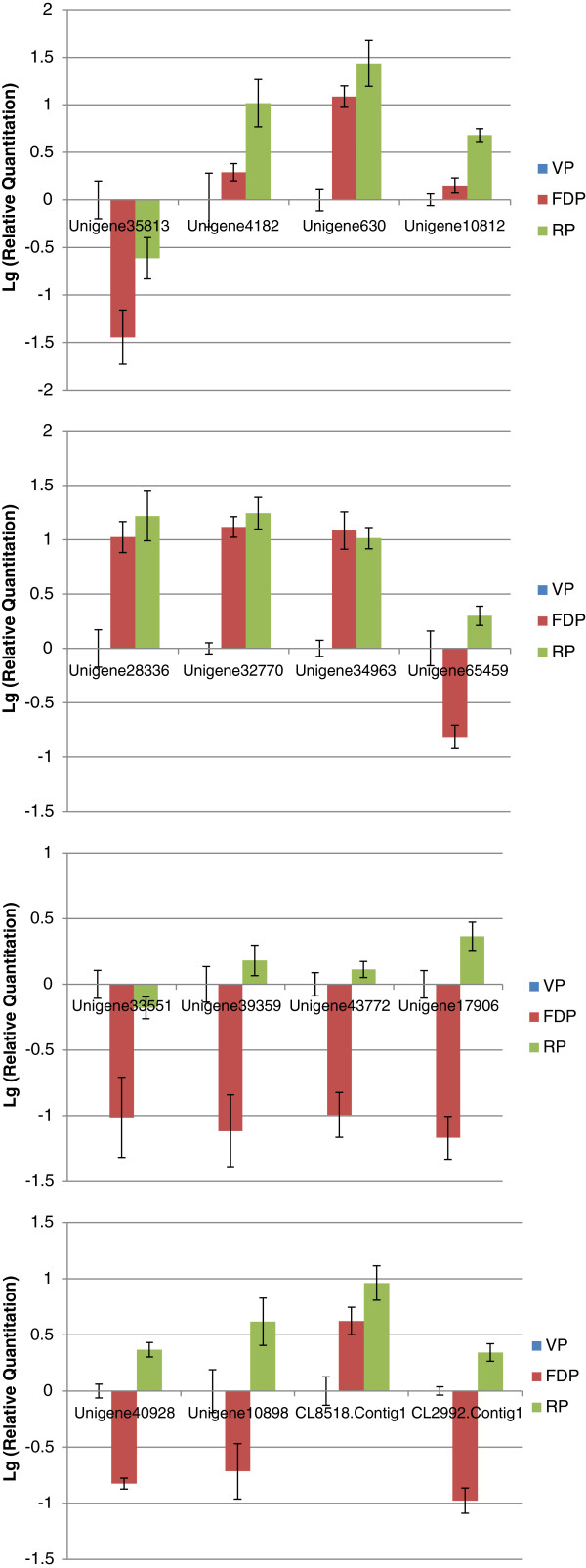
We also focused on the genes related to flower development and chose some genes that showed significantly different expression among three developmental phases (Figure 8). The functional annotation for these unigenes was listed in Additional file 10. For example, Unigene40123 demonstrated higher expression levels in FDP and RP than in VP, Unigene33969 showed higher expression levels in RP than in VP and FDP, and Unigene65459 showed higher expression levels in VP and RP than in FDP, which were all homologous with Arabidopsis thaliana auxin response factor 2 (ARF2) and related to auxin metabolism. Other significant expression of different genes among the three developmental phases might be associated with circadian rhythm (Unigene4387, 33551, 35813, homolog with FD), the vernalization pathway (Unigene49193 and CL2992.Contig2, homologous with EMBRYONIC FLOWER2 (EMF2) and VERNALIZATION INSENSITIVE 3 (VIN3), respectively and so on. To validate DGE-tag profiling, 16 genes were selected randomly to examine the expression using RT-qPCR (Figure 9). The results were exhibited differential expression among three libraries and were identical to those obtained by DGE expression profiling. Thus, the data generated in this study is sufficient to be used as a tool to investigate some specific flowering genes which show comparative expression levels among different developmental phases.
Figure 8.
Differential expression genes related with flower development in C. sinense DGE. Each column represents an experimental sample (e.g. VP, FDP and RP) and each row represents a gene. Expression differences are shown in different colors. Red means high expression and green means low expression.
Figure 9.
The qRT-PCR analysis of gene expression in VP (blue bars), FDP (red bars) and RP (green bars). The y-axis indicates fold change in expression among the samples VP, FDP, and RP using the results from RT-qPCR. The Lg(Relative Quantitation) of 16 genes in the VP was calibrated as zero.
Discussion
Illumina sequencing and sequence annotation
C. sinense is a very popular and traditional orchid in China. However, little is known about the mechanisms responsible for floral development and genomic information for C. sinense is currently unavailable. The aims of this project were to generate a large amount of cDNA sequence data that would facilitate more detailed studies in C. sinense, and to identify the genes controlling flowering time. The availability of transcriptome data for C. sinense will meet the initial information needs for functional studies of this species and its relatives. In this study, a combination of RNA-seq and three DGE analyses were performed using Illumina sequencing, which generated 54,248,006 high quality reads that were assembled into 83580 unigenes with an average sequence length of 612 base pairs. These unigenes were used for BLASTX and annotation against protein databases like nr, SwissPort, COG, KEGG and GO. In total, 41687 sequences were identified through BLAST searches and 50.1% unigenes had no homologues in the NCBI database. This may indicate that C. sinense vegetative and reproductive growth contains many unique processes and pathways.
Identifying C. sinense flowering time-associated genes
The photoperiod and vernalization pathways are two important genetic networks of flowering control [4]. In our study, the sequences associated with both pathways could be identified (Table 4). The photoperiod pathway comprises three parts: photoreceptors, a circadian clock and an output pathway from the clock specific to flowering [15]. Light signals are first received by two photoreceptors, phytochromes (PhyA, PhyB, PhyC, PhyD, PhyE) and cryptochromes (Cryl, Cry2) [16-20], which process the physical signals and produce a circadian clock [21-23]. The circadian clock provides a seasonal signal that adjusts plant development and regulates flowering time. It is controlled by a group of gene complexes that are composed of TOC1, CCA1 and LHY and regulate transcriptional–translational negative feedback loops [24-26]. ELF3 affects light input to the oscillator [27,28]. The processed signal is transmitted to the GI gene and the resultant signal activates the CO gene [11]. GI is another clock-associated protein known to regulate circadian oscillation and flowering time of Arabidopsis[29,30]. The gi mutants are defective for the expression of CCA1 and LHY genes. The circadian clock controls the expression rhythm of CO through GI[31]. CO is a transcription factor that promotes flowering by inducing the expression of the direct downstream genes FT[32] and SOC1[33]. COL genes have been identified in some plant species, each of which seems to have a large family of these genes [34]. In Arabidopsis, there are 17 COL genes [35]. There are at least 16 COL genes in the rice genome [34]. These COL proteins contain two B-box domains at the N-terminus and a CCT domain at the C-terminus [35,36]. In this study, 28 C. sinense COL unigenes containing the conserved CCT domain were identified (Additional file 3). Seven unigenes were used to perform the phylogenetic analysis. They were classified into three conserved COL subgroups as defined previously [35]. COL proteins belonging to different subgroups are expected to perform distinct biological roles, but only several COL genes controlling flowering time have been studied in detail [34]. We presume that seven unigenes in C. sinense are expected to perform distinct biological roles. Sequence homologues for other genes involved in regulation of the circadian clock described above (GI, EIF3, PIF3, LHY, CHS, CCA1) could be found in our database (Table 4). Furthermore, detailed metabolic pathways for the circadian rhythm and 222 related unigenes, such as phytochromes (PhyA, PhyB, PhyC, PhyD, PhyE) and cryptochromes (Cryl, Cry2) were found (Figure 5). Thus, these photoperiod pathways may be concerned with the regulation of flowering time in C. sinense.
VRN2 is a key gene involved in the vernalization pathway [37]. It promotes flowering by repressing the expression of FLOWERING LOCUS C (FLC)[38]. VRN2 codes for a protein with homology to PcG proteins [39]. Thus, VRN2 may function to keep the FLC-chromatin state for down-regulation. Sequence homologs for VRN2 could be found in our database (Table 4). Furthermore, temperature obviously affects the flowering time of C. sinense. We speculate that the vernalization pathway is related to the regulation of flowering time in C. sinense.
Identifying C. sinense flowering integration genes and floral meristem identity genes
Flowering integration genes accept the signal from the genetic pathway, and then induce floral meristem identity (FMI) genes for flowering as a whole [4]. At present, three integration genes, including SOC1/AGL20, FT and TFL, have been identified. SOC1 and FT are the direct target genes for CO. The expression of FT and SOC1 is controlled positively not only by the light pathway, but also by the autonomous pathway acting negatively through FLC[40]. The vernalization signal increases SOC1 expression, presumably by reducing FLC levels, and SOC1 can also be up-regulated by a gibberellin pathway. Accordingly, SOC1 and FT act as the convergence of all four pathways [41]. TFL1 codes for a protein with homology to FT[42]. The mutations in TFL1 are semidominant and early flowering with a determinate inflorescence [43]. Thus, TFL1 codes for a repressor of flowering. TFL2 functions as a negative repressor of FT expression. Sequence homologs for the floral integrator pathway genes related to FT, SOC1 and TFL were all identified in our C. sinense transcriptome database (Table 4).
The floral meristem is initiated by a set of FMI genes that include LFY, AP1, CAL, AP2, and UNUSUAL FLORAL ORGANS (UFO)[44-47]. Among them, LFY and AP1 play a central role in flower meristem identity [48-50]. LFY regulates inflorescence shape and controls flowering time [51,52]. AP1 is the downriver target gene of LFY. LFY induces expression of AP1. LFY protein, combined with the AP1 promoter activates the transcription of AP1[53]. The phenotype of the loss-of-function-type mutations in LFY or AP1 gene is as follows: Flowers either have vegetative characteristics or have been replaced by vegetative shoots [54]. The phenotype of double mutations in lfy/ap1 is a scarce floral structure [49,55], which elucidated the functional redundancies in AP1-LFY. Functional redundancies were detected in AP1-CAL, AP2-AP1 and AP2-LFY genes [56,57]. AP1 and CAL belong to the MADS domain genes and UFO codes for an F-box protein which degrades other proteins through a ubiquitation pathway [46,58,59]. LFY is also thought to promote CAL and UFO expression [60,61]. Therefore, these FMI genes cooperate together to promote the transition from vegetative growth to reproductive growth. Sequence homologs for FMI genes CAL, AP2, LFY and AP1 were all identified in our C. sinense transcriptome database. These information of flowering integration genes and FMI genes would facilitate more detailed studies on the mechanism of floral differentiation for C. sinense.
Differential expression of genes among three developmental phases
We have identified the putative homologs of COL and other key genes involved in controlling flowering time in C. sinense. More studies on their expression patterns and on their interactive relationships in the future could be used to elucidate the molecular mechanisms that regulate the floral transition and the flowering genetic pathway in C. sinense. To better understand the information related to gene expression obtained for C. sinense, we created three DEG libraries to analyze the gene expression patterns under three developmental phases. In the comparison between FDP with VP, most of the expressed genes were down-regulated and parts were up-regulated. In contrast, when RP was compared to VP and FDP, most of the expressed genes were up-regulated (Figure 7). Furthermore, a large amount of genes which showed specific expression in the three phases were likely involved in floral development.
When FDP was compared to VP, TFL1 showed specific expression in VP. TFL1 codes for a flowering repressor that can function to control inflorescence architecture and extend the phases of shoot meristems in Arabidopsis[40]. Our sequence was consistent with this theory. Among all specific expression of annotated genes in RP compared to VP or FDP, auxin response factors (ARF2) were identified in RP. Auxin signaling is important in the regulation of developmental transitions such as seed germination, induction of flowering, leaf senescence and shedding of senescent organs. ARFs are transcription factors that mediate responses to the plant hormone auxin [62]. For example, ARF2 promotes the transition between multiple stages of Arabidopsis development, including initiation of flowering, rosette leaf senescence, floral organ abscission and silique ripening. In contrast, ARF1 is a transcriptional repressor and the arf1 mutation increases the transcription of Aux/IAA genes in Arabidopsis flowers [63]. Moreover, most of the specific gene expression was not annotated as a relative function, which could assist in the search for some new genes associated with floral development.
Although the molecular functions of C. sinense genes and the associated floral genetic pathways remain unknown, the present transcriptome analysis provides valuable information regarding C. sinense development, which could facilitate further investigations of the detailed floral development mechanisms of this culturally important orchid.
Conclusion
The combination of RNA-seq and DGE analysis based on Illumina sequencing technology provided comprehensive information on gene expression. Candidate genes on flowering time genes, MADS-box genes and COL genes were rapidly identified by this approach. This data could be used as a tool to investigate the flowering pathway and various other biological pathways in C. sinense.
Methods
Plant materials and growth conditions
C. sinense ‘Qi Jian Bai Mo’ plants used in this study were grown and maintained in pots in a greenhouse of the South China Botanical Garden at a day/night temperature of 28/25°C with a 12-h period. The cDNA libraries were prepared from the entire plant of C. sinense at vegetative and reproductive stages. The vegetative phase (VP) samples were collected from a non-pseudobulb shoot (Figure 10A). The floral differentiation phase (FDP) samples were collected from the entire adult plant which had developed floral buds at the base of the pseudobulb (Figure 10B). The reproductive phase (RP) samples were collected from entire adult plants which had developed a peduncle with floral organs at the base of the pseudobulb (Figure 10C).
Figure 10.
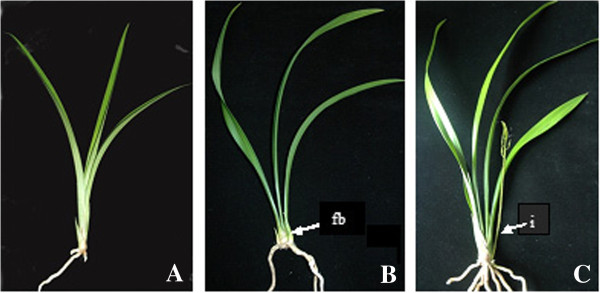
Organs from C. sinense used to prepare DGE libraries for Illumine sequencing. (A) A young C. sinense plant in the vegetative phase (VP); (B) A mature C. sinense plant with floral bud (fb) in the floral differentiation phase (FDP); (C) A mature C. sinense plant with inflorescence (i) in the reproductive phase (RP).
Fresh samples were used to extract total RNA immediately.
cDNA library preparation and Illumina sequencing for transcriptome analysis
Total RNA was extracted using Column Plant RNAout2.0 (Tiandz, Inc. Beijing, China) according to the manufacturer’s protocol. To obtain complete gene expression information, a pooled RNA sample including roots, leaves, pseudobulbs, flower buds, young and mature inflorescences was used for transcriptome sequencing analysis. According to the Illumina manufacturer’s instructions, poly(A)+ RNA was purified from 20 mg of pooled total RNA using oligo(dT) magnetic beads. Fragmentation buffer was added to interrupt mRNA to short fragments. Using these short fragments as templates, a random hexamer-primer was used to synthesize the first-strand cDNA. Second-strand cDNA was synthesized using 10×buffer, 25 mM dNTPs, 20-60 U/μl RNaseH and 5 U/μl DNA polymerase I. Short fragments were purified with the QiaQuick PCR extraction kit and resolved with EB buffer for end reparation and for adding poly(A). Thereafter, the short fragments were connected with sequencing adapters. After agarose gel electrophoresis, suitable fragments were selected for PCR amplification as templates. Finally, the library was sequenced using Illumina HiSeq™ 2000. All raw transcriptome data were deposited in the GeneBank Short Read Archive (Accession SRA058042).
Analysis of Illumina sequencing results
Raw reads from the image data output from the sequencing machine were generated by base calling. After filtering raw reads, de novo assembly of the transcriptome was carried out with a short reads assembling program – Trinity [64]. Trinity connects the contigs and obtains sequences defined as unigenes.
The generated unigenes were used for BLASTX and annotation against protein databases, including non-redundant (nr), SwissPort, COG, KEGG, and GO protein database, with a cut-off E-value of 0.00001. GO (http://www.geneontology.org) has three ontologies: molecular function, cellular component and biological process. With nr annotation, we used the Blast2GO program [65] to obtain the GO annotation of unigenes. After obtaining the GO annotation for every unigene, we used WEGO software [66] to perform GO functional classification for all unigenes and to understand the distribution of gene functions. KEGG is a major public pathway-related database [67] that is able to analyze a gene product during a metabolic process and related gene function in cellular processes. With the help of the KEGG database, we can further study genes’ biological complex behaviors, and by KEGG annotation we can obtain pathway annotation for unigenes. The KEGG pathways annotation was performed using Blastall software against the KEGG database.
Digital gene expression (DGE) library preparation and sequencing
Total RNA was extracted from VP, FDP and RP using Column Plant RNAout2.0. mRNA was enriched by using oligo(dT) magnetic beads. After adding the fragmentation buffer, the mRNA was interrupted to short fragments (about 200 bp). Then the first strand cDNA was synthesized by a random hexamer-primer using the mRNA fragments as templates. 10×buffer, 25 mM dNTPs, 20-60 U/μl RNaseH and 5 U/μl DNA polymerase I were added to synthesize the second strand. The double-stranded cDNA was purified with a QiaQuick PCR extraction kit and washed with EB buffer for end repair and single nucleotide A (adenine) addition. Finally, sequencing adaptors were ligated to the fragments. The required fragments were purified by agarose gel electrophoresis and enriched by PCR amplification. The library products were ready for sequencing analysis via Illumina HiSeq™ 2000. Three raw DGE data (VP, FDP and RP) were deposited in the GeneBank Short Read Archive (accession SRA058974, SRA058977, SRA058978, respectively).
Analysis and mapping of DGE tags
To map the DGE tags, the sequenced raw data were filtered to remove low quality tags (tags with an unknown nucleotide “N”), empty tags (no tag sequence between the adaptors) and tags with only one copy number (which might result from sequencing errors). For the annotation of tags, clean tags containing CATG and 21-bp tag sequences were mapped to our transcriptome reference database using SOAPaligner/soap2 [68]. Mismatches of no more than two bases were allowed in the alignment.
Screening of differentially expressed genes (DEGs)
To compare the differences in gene expression at different developmental stages, the tag frequency in the different DGE libraries was statistically analyzed according to the method described by Audic and Claverie [14]. The false discovery rate (FDR) was used to determine the threshold P-value in multiple tests. We used FDR < 0.001 and an absolute value of the log2 ratio >1 as the threshold to determine the significant difference in gene expression. The differentially expressed genes were used for GO and KEGG enrichment analyses according to a method similar to that described by Xue [69]. GO terms, which take the corrected P-value ≤ 0.05 as a threshold, are significantly enriched in DEGs. KEGG pathways with a Q-value ≤ 0.05 are significantly enriched in DEGs.
Genes with similar expression patterns usually mean functional correlation. We perform cluster analysis of gene expression patterns with cluster [70] software and Java Treeview [71] software. In Figure 8, each column represents an experimental sample (e.g. VP, FDP and RP), each row represents a gene. Expression differences are shown in different colors. Red means high expression and green means low expression.
Quantitative real-time PCR (qRT-PCR) validation
Total RNA was extracted as described for the DGE library preparation and sequencing. Each RNA sample was treated with RNase-free DNase (Promega) following the manufacturer’s protocol in an effort to remove any residual genomic DNA (gDNA). DNase-treated RNA (2 mg) was subjected to reverse transcriptase reactions using oligo-dT primer and PrimeScript™ Reverse Transcriptase (Takara) according to the manufacturer’s protocol.
The sequences of the specific primer sets are listed in Additional file 11. The actin gene of C. sinense (ACC NO. GU181353) was used as an internal gene. qRT-PCR was performed using the SYBR Premix Ex Taq Kit (TaKaRa) according to the manufacturer’s protocol. The results were normalized to the expression level of the constitutive actin gene. A relative quantitative method (DDCt) was used to evaluate the quantitative variation.
Abbreviations
DGE: Digital gene expression; GO: Gene ontology; KEGG: Kyoto encyclopedia of genes and genomes; AP1: Apetala1; AP2: Apetala2; AP3: Apetala3; AG: Agamous; CAL: Cauloflower; CCA1: Circadian clock associated 1; CHS: Chalcone synthase; CO: Constans; COL: Constans-like; DEF: Deficiens; ELF3: Early flowering 3; EST: Expressed sequence tag; EMF2: Embryonic flower 2; FD: Flowering locus D; FE: Flowering locus E; FT: Flowering locus T; FLC: Flowering locus C; FWA: Flowering wageningen; GI: Gigantea; GLO: Globosa; LHY: Late elongated hypocotyl; LFY: Leafy; PDF2: Protodermal factor2; PIF3: Phytochrome interacting factor 3; PI: Pistillata; SEP: Sepallata; SOC1: Suppressor of overexpression of co 1; TFL1: Terminal flower 1; TOC1: Timing of cab expression 1; VIN3: Vernalization insensitive 3; VRN1: Vernalization 1; UFO: Unusual floral organs.
Competing interests
The authors declare that they have no competing interest.
Authors’ contributions
JZ carried out the experiments, performed the bioinformatics analyses, and drafted the manuscript. KW cultured and provided the experimental material. SZ participated in the qRT-PCR experiment. JATdS critically evaluated the protocol, the data and interpretation and revised the manuscript. HX performed the bioinformatics analyses. JD designed the study and revised the manuscript. XZ and CT participated in the design and coordination. All authors read and approved the final manuscript.
Supplementary Material
Metabolic pathway analysis result for unigenes by KEGG annotation.
The unigenes that share homology with MADS-box genes.
The unigenes that share homology to CONSTANS-like genes.
Specific expression of genes in the comparison between FDP with VP.
Gene set enrichment analysis in the comparison between FDP with VP by GO and KEGG.
Specific expression of genes in the comparison between RP and VP.
Gene set enrichment analysis in the comparison between RP with VP by GO and KEGG.
Specific expression of genes in the comparison between RP and FDP.
Gene set enrichment analysis in the comparison between RP and FDP by GO and KEGG.
The functional annotation for the unigenes related with flower development in C. sinense DGE.
Primer sequences for qRT-PCR.
Contributor Information
Jianxia Zhang, Email: zhangjianxia@scib.ac.cn.
Kunlin Wu, Email: wu_kunlin@163.com.
Songjun Zeng, Email: zengsongjun@scbg.ac.cn.
Jaime A Teixeira da Silva, Email: jaimetex@yahoo.com.
Xiaolan Zhao, Email: xiaolanpeng@scau.edu.cn.
Chang-En Tian, Email: changentian@yahoo.com.cn.
Haoqiang Xia, Email: hqxia@genedenovo.com.
Jun Duan, Email: duanj@scbg.ac.cn.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (CNSF31101572 and CNSF31170654), the Science and Technology Project Foundation of Guangdong Province (2011A020201001 and 2012B091100255), Knowledge Innovation Program of the Chinese Academy Sciences (Y021121001) and the Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences (201222ZS).
References
- Leitch IJ, Kahandawala I, Suda J, Hanson L, Ingrouille MJ, Chase MW, Fay MF. Genome size diversity in orchids: consequences and evolution. Ann Bot. 2009;104(3):469–481. doi: 10.1093/aob/mcp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZY, Raven PH, Hong DY. Flora of China. Volume 25 (Orchidaceae) Beijing, St. Louis: Science Press and Missouri Botanical Garden Press; 2009. p. 260. [Google Scholar]
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. Physiological signals that induce flowering. Plant Cell. 1993;5:1147–1155. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeda Y. Genetic regulation of time to flower in Arabidopsis thaliana. Annu Rev Plant Biol. 2004;55:521–535. doi: 10.1146/annurev.arplant.55.031903.141644. [DOI] [PubMed] [Google Scholar]
- Yu H, Yang SH, Goh CJ. DOH1, a class 1 knox gene, is required for maintenance of the basic plant architecture and floral transition in orchid. Plant Cell. 2000;12(11):2143–2159. doi: 10.1105/tpc.12.11.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Yang SH, Goh CJ. Spatial and temporal expression of the orchid floral homeotic gene DOMADS1 is mediated by its upstream regulatory regions. Plant Mol Biol. 2002;49(2):225–237. doi: 10.1023/A:1014958118852. [DOI] [PubMed] [Google Scholar]
- Xu Y, Teo LL, Zhou J, Kumar PP, Yu H. Floral organ identity genes in the orchid Dendrobium crumenatum. Plant J. 2006;46:54–68. doi: 10.1111/j.1365-313X.2006.02669.x. [DOI] [PubMed] [Google Scholar]
- Hsu HF, Yang CH. An orchid (Oncidium Gower Ramsey) AP3-like MADS gene regulates floral formation and initiation. Plant Cell Physiol. 2002;43(10):1198–1209. doi: 10.1093/pcp/pcf143. [DOI] [PubMed] [Google Scholar]
- Hsu HF, Huang CH, Chou LT, Yang CH. Ectopic expression of an orchid (Oncidium Gower Ramsey) AGL6-like gene promotes flowering by activating flowering time genes in Arabidopsis thaliana. Plant Cell Physiol. 2003;44(8):783–794. doi: 10.1093/pcp/pcg099. [DOI] [PubMed] [Google Scholar]
- Niwa Y, Yamashino T, Mizuno T. The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol. 2009;50(4):838–854. doi: 10.1093/pcp/pcp028. [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7(10):986–95. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- Simpson GG. Evolution of flowering in response to day length flipping the CONSTANS switch. Bioessays. 2003;25:829–832. doi: 10.1002/bies.10330. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A, Okada K, Salomon M, Rüdiger, Sakai T, Takano M, Wada M, Watson JC. The phototropin family of photoreceptors. Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yang H, Guo H, Mockler T, Chen J, Cashmore AR. Enhancement of the blue-light sensitivity of Arabidopsis young seedlings by a blue-light receptor cry2. Proc Natl Acad Sci USA. 1998;95:2686–2690. doi: 10.1073/pnas.95.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM. Quail PH: hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. GIGANTEA: a circadian clock controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane spanning domains. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks KA, Albertson TM, Wagner DR. EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell. 2001;13:1281–1292. doi: 10.1105/tpc.13.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Somers DE. The physiology and molecular bases of the plant circadian clock. Plant Physiol. 1999;121:9–20. doi: 10.1104/pp.121.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabadid D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science. 2001;293:880–883. doi: 10.1126/science.1061320. [DOI] [PubMed] [Google Scholar]
- Millar AJ. Input signals to the plant circadian clock. J Exp Bot. 2004;55:277–283. doi: 10.1093/jxb/erh034. [DOI] [PubMed] [Google Scholar]
- McWatters HG, Bastow RM, Hall A, Millar AJ. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408:716–720. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- Dixon LE, Knox K, Kozma-Bognar L, Southern MM, Pokhilko A, Millar AJ. Temporal repression of core circadian genes is mediated through EARLY FLOWERING 3 in Arabidopsis. Curr Biol. 2011;21:120–125. doi: 10.1016/j.cub.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, Coupland G. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell. 2005;17:2255–2270. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JCW, Kozma-Bognar L, Gould PD, Fehér B, Kevei E, Nagy F, Turner MS, Hall A, Millar AJ. Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol Syst Biol. 2006;2:59. doi: 10.1038/msb4100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Davis SJ, Bastow RM, McWatters HG, Kozma-Bognar L, Nagy E, Millar AJ, Amasino RM. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature. 2002;419:74–77. doi: 10.1038/nature00954. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Griffths S, Dunford RP, Coupland G, Laurie DA. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003;131:1855–1867. doi: 10.1104/pp.102.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson F, Costa MMR, Hepworth SR, Vizir I, Pineiro M, Reeves PH, Putterill J, Coupland G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001;28:619–631. doi: 10.1046/j.1365-313x.2001.01163.x. [DOI] [PubMed] [Google Scholar]
- Ledger S, Strayer C, Ashton F, Kay SA, Putterill J. Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J. 2001;26:15–22. doi: 10.1046/j.1365-313x.2001.01003.x. [DOI] [PubMed] [Google Scholar]
- Chandler J, Wilson A, Dean C. Arabidopsis mutants showing an altered response to vernalization. Plant J. 1996;10:637–644. doi: 10.1046/j.1365-313X.1996.10040637.x. [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC) Proc Natl Acad Sci USA. 2000;97:3753–3758. doi: 10.1073/pnas.97.7.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall AR, Levy YY, Wilson A, Dean C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell. 2001;107:525–535. doi: 10.1016/S0092-8674(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Amador V, Monte E, Garcia-Martinez JL, Prat S. Gibberellins signal nuclear import of PHOR1, a photoperiod-responsive protein with homology to Drosophila armadillo. Cell. 2001;106(3):343–354. doi: 10.1016/S0092-8674(01)00445-7. [DOI] [PubMed] [Google Scholar]
- Hepworth SR, Valverde F, Ravenscroft D, Mouradov A, Coupland G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 2002;21:4327–4337. doi: 10.1093/emboj/cdf432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. Inflorescence commitment and architecture in Arabidopsis. Science. 1997;275:80–83. doi: 10.1126/science.275.5296.80. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu XH, Smyth DR. Terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992;2:103–116. doi: 10.1111/j.1365-313X.1992.00103.x. [DOI] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature. 1992;360:273–277. doi: 10.1038/360273a0. [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF. Molecular basis of the cauliflower phenotype of Arabidopsis. Science. 1994;267:522–525. doi: 10.1126/science.7824951. [DOI] [PubMed] [Google Scholar]
- Yanofsky MF. Floral meristems to floral organs: genes controlling early events in Arabidopsis flower development. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:167–188. doi: 10.1146/annurev.pp.46.060195.001123. [DOI] [Google Scholar]
- Schultz EA, Haughn GW. LEAFY, a homeotic gene that regulates inflorescence development in Arabidopsis. Plant Cell. 1991;3:771–781. doi: 10.1105/tpc.3.8.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992;69(5):843–859. doi: 10.1016/0092-8674(92)90295-N. [DOI] [PubMed] [Google Scholar]
- Pidkowich MS, Klenz JE, Haughn GW. The making of a flower: control of floral meristem identity in Arabidopsis. Plant Sci. 1999;4:64–70. doi: 10.1016/S1360-1385(98)01369-7. [DOI] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliott R, Murphy G, Carpenter R. floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell. 1990;63:1311–1322. doi: 10.1016/0092-8674(90)90426-F. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Lee Y, Blazquez MA, Weigel D. Flowering time genes modulate the response to LEAFY activity. Genetics. 1998;150:403–410. doi: 10.1093/genetics/150.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D. A genetic framework for floral patterning. Nature. 1998;395:561–566. doi: 10.1038/26903. [DOI] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. Function of the APETALA1 gene during Arabidopsis floral development. Plant Cell. 1990;2:741–753. doi: 10.1105/tpc.2.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Sussex IM. LEAFY interacts with floral homeotic genes to regulate Arabidopsis floral development. Plant Cell. 1992;4:901–913. doi: 10.1105/tpc.4.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Alvarez J, Weigel D, Meyerowitz EM, Smyth DR. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development. 1993;119:721–743. [Google Scholar]
- Shannon S, Meeks-Wagner DR. Genetic interactions that regulate inflorescence development in Arabidopsis. Plant Cell. 1993;5:639–655. doi: 10.1105/tpc.5.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Yanofsky MF. A gene triggering flower formation in Arabidopsis. Nature. 1995;377:522–524. doi: 10.1038/377522a0. [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/S0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Ferrandiz C, Gu Q, Martienssen R, Yanofsky MF. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development. 2000;127:725–734. doi: 10.1242/dev.127.4.725. [DOI] [PubMed] [Google Scholar]
- Lee I, Wolfe DS, Nilsson O, Weigel D. A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol. 1997;7:95–104. doi: 10.1016/S0960-9822(06)00053-4. [DOI] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle TJ. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol. 2002;49:373–385. doi: 10.1023/A:1015207114117. [DOI] [PubMed] [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development. 2005;132:4563–4574. doi: 10.1242/dev.02012. [DOI] [PubMed] [Google Scholar]
- Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis Z, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L, Wang J. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34(Web Server issue):W293–297. doi: 10.1093/nar/gkl031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36(Database issue):D480–484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25(15):1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- Xue J, Bao YY, Li BL, Cheng YB, Peng ZY, Liu H, Xu HJ, Zhu ZR, Lou YG, Cheng JA, Zhang CX. Transcriptome analysis of the brown planthopper Nilaparvata lugens. PLoS One. 2010;5(12):e14233. doi: 10.1371/journal.pone.0014233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon MJL, Imoto S, Nolan J, Miyano S. Open Source Clustering Software. Bioinformatics. 2004;20(9):1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview-extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metabolic pathway analysis result for unigenes by KEGG annotation.
The unigenes that share homology with MADS-box genes.
The unigenes that share homology to CONSTANS-like genes.
Specific expression of genes in the comparison between FDP with VP.
Gene set enrichment analysis in the comparison between FDP with VP by GO and KEGG.
Specific expression of genes in the comparison between RP and VP.
Gene set enrichment analysis in the comparison between RP with VP by GO and KEGG.
Specific expression of genes in the comparison between RP and FDP.
Gene set enrichment analysis in the comparison between RP and FDP by GO and KEGG.
The functional annotation for the unigenes related with flower development in C. sinense DGE.
Primer sequences for qRT-PCR.