Abstract
Aspergillus oryzae has been utilized as a host for heterologous protein production because of its high protein secretory capacity and food-safety properties. However, A. oryzae often produces lower-than-expected yields of target heterologous proteins due to various underlying mechanisms, including degradation processes such as autophagy, which may be a significant bottleneck for protein production. In the present study, we examined the production of heterologous protein in several autophagy (Aoatg) gene disruptants of A. oryzae. We transformed A. oryzae gene disruptants of Aoatg1, Aoatg13, Aoatg4, Aoatg8, or Aoatg15, with a bovine chymosin (CHY) expression construct and found that the production levels of CHY increased up to three fold compared to the control strain. Notably, however, conidia formation by the Aoatg gene disruptants was significantly reduced. As large amounts of conidia are necessary for inoculating large-scale cultures, we also constructed Aoatg gene-conditional expression strains in which the promoter region of the Aoatg gene was replaced with the thiamine-controllable thiA promoter. Conidiation by the resultant transformants was clearly enhanced in the absence of thiamine, while autophagy remained repressed in the presence of thiamine. Moreover, these transformants displayed increased CHY productivity, which was comparable to that of the Aoatg gene disruptants. Consequently, we succeeded in the construction of A. oryzae strains capable of producing high levels of CHY due to defects in autophagy. Our finding suggests that the conditional regulation of autophagy is an effective method for increasing heterologous protein production in A. oryzae.
Introduction
The filamentous fungus Aspergillus oryzae (Ahlburg) Cohn has the ability to secrete large amounts of proteins and it has been safely used in the preparation of fermented foods for over a thousand year in Japan [1]. Due to these merits, A. oryzae is generally considered to be an outstanding host for the production of heterologous proteins [2], [3]. However, when heterologous proteins derived from higher organisms are expressed in A. oryzae, lower-than-desired yields of the target protein are often obtained [4]–[7]. These reduced yields are thought to result from bottlenecks in the transcription, translation, and secretory pathways of A. oryzae. In our previous research, we observed that the expression of a mutant α-amylase protein with defective disulfide bonds in A. oryzae resulted in accumulation of the protein in the endoplasmic reticulum (ER) [8]. Furthermore, we confirmed that autophagy delivers the misfolded secretory proteins from the ER to vacuoles [9]. Based on these observations, autophagy appears to be a significant process adversely affecting the production of heterologous proteins in A. oryzae.
Autophagy, which is a highly conserved intracellular degradation pathway in eukaryotes, functions as a survival mechanism under nutrient starvation conditions by recycling intracellular components [10]. The autophagic process consists of several sequential steps: 1) induction, 2) autophagosome formation, 3) fusion of autophagosomes to lysosomes/vacuoles, and 4) degradation of autophagic bodies [11], [12]. In addition to nutrient recycling, autophagy plays important roles in cell development and differentiation, immune responses, and cell death, and has also been shown to participate in various diseases including cancer [13]. Thus, autophagy is an extremely important system in eukaryotes; however, no studies have examined the mechanisms by which autophagy affects the production of recombinant proteins.
Our group previously demonstrated that decreasing protease activity in A. oryzae through the disruption of ten protease genes led to increased productivity of heterologous proteins [14]. Moreover, disruption of the gene coding for the vacuolar protein sorting (Vps) receptor AoVps10 led to decreased transportation of recombinant proteins to vacuoles via the Vps pathway and increased secretion of recombinant proteins in the culture medium [15]. This finding suggests that repression of vacuolar degradation may be effective in enhancing the productivity of heterologous proteins.
In the present study, the effect of disruption of genes involved in autophagy on heterologous protein production in A. oryzae was examined. A. oryzae disruptants were constructed for five genes related to autophagy: Aoatg1, encoding a kinase involved autophagy induction [16]; Aoatg13, encoding a component of the AoAtg1 complex [17], [18]; Aoatg4 and Aoatg8, which are essential genes for the formation of autophagosomes and membrane fusion [19]; and Aoatg15, encoding a lipase required for the breakdown of autophagic bodies [20]–[22]. Using the A. oryzae gene disruptants, the production of bovine chymosin (CHY) was measured as a reporter heterologous protein. This is the first report demonstrating that the disruption of autophagy-related genes in a filamentous fungus enhances the production levels of heterologous proteins.
Materials and Methods
Strains, Media, and Transformation
A. oryzae wild-type strain RIB40 and strain NSPlD1, which has a highly efficient gene-targeting background (niaD − sC − ΔpyrG ΔligD) [23], were used as a DNA donor and transformation host, respectively. The strains used and generated in this study are listed in Table 1. Potato Dextrose (PD) agar (Nissui Phamaceutical) medium was used for the growth and maintenance of all strains. PD agar containing 10 µM thiamine was used for harvesting conidia from Aoatg gene-conditional expression strains. M medium [0.2% NH4Cl, 0.1% (NH4)2SO4, 0.05% KCl, 0.05% NaCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, and 2% glucose, pH 5.5] supplemented with 0.15% methionine (M+Met) was used as a selective medium for replacing the Aoatg promoter. 5×DPY medium [10% dextrin, 5% polypeptone, 2.5% yeast extract, 0.5% KH2PO4, and 0.05% MgSO4·7H2O, pH 5.5] was used for CHY production. Czapek-Dox (CD) medium (0.3% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, and 2% glucose, pH 5.5) supplemented with 0.0015% methionine was used for niaD-based plasmid integration. Escherichia coli DH5α [supE44 ΔlacU169 (Φ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] was used for DNA manipulation. A. oryzae was transformed according to a previously reported method [1].
Table 1. List of strains used in the study.
| Strain name | Parental strain | Genotype | Reference |
| RIB40 | Wild-type | ||
| NSRKu70-1-1 | NSAR1 | niaD− sC− adeA- ΔargB::adeA− Δku70::argB | [36] |
| NSKu70-AA | NSRKu70-1-1 | niaD− sC− adeA− ΔargB::adeA− Δku70::argB adeA | [37] |
| NSKu70-Aoatg1 | NSRKu70-1-1 | niaD− sC− adeA− ΔargB::adeA− Δku70::argB ΔAoatg1::adeA | [30] |
| NSKu70-ΔAoatg4-2 | NSRKu70-1-1 | niaD− sC− adeA− ΔargB::adeA− Δku70::argB ΔAoatg4::adeA | [29] |
| ΔAoatg8 | NSR13 | niaD− sC− adeA− ΔAoatg8::adeA | [28] |
| NSKu70-ΔAoatg13 | NSRKu70-1-1 | niaD− sC− adeA− ΔargB::adeA- Δku70::argB ΔAoatg13::adeA | [29] |
| NSKu70-ΔAoatg15-10-1 | NSRKu70-1-1 | niaD− sC− adeA− ΔargB::adeA- Δku70::argB ΔAoatg15::adeA | [29] |
| SKu70-AA-AKC1 | NSKu70-AA | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD]sC− adeA− ΔargB::adeA− Δku70::argB ΔAoatg1::adeA | [27] |
| SKu70-ΔAoatg1-AKC2 | NSKu70-ΔAoatg1 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA- Δku70::argB ΔAoatg1::adeA | This study |
| SKu70-ΔAoatg4-AKC1 | NSKu70-ΔAoatg4-2 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC − adeA− ΔargB::adeA− Δku70::argB ΔAoatg4::adeA | This study |
| ΔAoatg8-AKC1 | ΔAoatg8 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔAoatg8::adeA | This study |
| SKu70-ΔAoatg13-AKC1 | NSKu70-ΔAoatg13 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA− Δku70::argB ΔAoatg13::adeA | This study |
| SKu70-ΔAoatg15-AKC2 | NSKu70-ΔAoatg15-10-1 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA− Δku70::argB ΔAoatg15::adeA | This study |
| NSPlD1 | NSR-ΔlD2 | niaD− sC− adeA− ΔargB::adeA− ΔligD::argB pyrG− | [23] |
| NSlD1 | NSPlD1 | niaD− sC− adeA− ΔargB::adeA− ΔligD::argB | [14] |
| SlD-AKC1 | NSlD1 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA- ΔligD::argB pyrG- | [14] |
| NSlD-PtA1 | NSPlD1 | niaD− sC− adeA− ΔargB::adeA− ΔligD::argB pyrG−::[PAoatg1::pyrG-PthiA::Aoatg1] | This study |
| NSlD-PtA4 | NSPlD1 | niaD− sC− adeA− ΔargB::adeA− ΔligD::argB pyrG−::[PAoatg4::pyrG-PthiA::Aoatg4] | This study |
| NSlD-PtA8 | NSPlD1 | niaD− sC− adeA− ΔargB::adeA− ΔligD::argB pyrG−::[PAoatg8::pyrG-PthiA::Aoatg8] | This study |
| NSlD-PtA15 | NSPlD1 | niaD− sC− adeA- ΔargB::adeA- ΔligD::argB pyrG-::[PAoatg15::pyrG-PthiA::Aoatg15] | This study |
| SlD-PtA1-AKC | NSlD-PtA1 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA- ΔligD::argB pyrG−::[Aoatg1::pyrG-PthiA::Aoatg1] | This study |
| SlD-PtA4-AKC | NSlD-PtA4 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC- adeA- ΔargB::adeA- ΔligD::argB pyrG−::[PAoatg4::pyrG-PthiA::Aoatg4] | This study |
| SlD-PtA8-AKC | NSlD-PtA8 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA− ΔligD::argB pyrG-::[PAoatg8::pyrG-PthiA::Aoatg8] | This study |
| SlD-PtA15-AKC | NSlD-PtA15 | niaD−::pgAKCN[PamyB::amyB-kex2-CHY::TamyB::niaD] sC− adeA− ΔargB::adeA− ΔligD::argB pyrG−::[PAoatg15::pyrG-PthiA::Aoatg15] | This study |
Construction of Conditional Expression Strains
Plasmid pgPtA1 was constructed to replace the Aoatg1 promoter with the thiA promoter using the MultiSite Gateway cloning system (Invitrogen). The upstream and N-terminal 1.5-kb regions of the Aoatg1 gene were amplified by PCR using the primer pairs attB4-PAoatg1-F and attB1-PAoatg1-R, and attB2-Aoatg1-F and attB3-Aoatg1-R, respectively (Table 2). The amplified attB-flanked upstream and Aoatg1 fragments were introduced into pDNORTMP4-P1R and pDNORTMP2R-P3, respectively, using the Gateway BP Clonase Reaction Mix (Invitrogen), generating the Entry Clone plasmids pg5′upAoatg1 and pg3′Aoatg1, respectively. The plasmids pg5′upAoatg1 and pg3′Aoatg1, an Entry Clone plasmid containing the A. oryzae pyrG gene as a selective marker and the thiA promoter, and the Destination vector pDESTTMR4-R3 (Invitrogen) were mixed and then subjected to the Gateway LR reaction using the Gateway LR Clonase reaction mix (Invitrogen) to generate pgPtA1. Using plasmid pgPtA1 as a template, the sequence containing the replacement cassette, which consisted of the upstream region of Aoatg1 (1.5 kb), pyrG gene (2.0 kb), thiA promoter (1.3 kb), and N-terminal region of the Aoatg1 gene (1.5 kb), was amplified by PCR with the primers attB4-upAoatg1-F and attB3-Aoatg1-R, and then transformed into A. oryzae NSRKu70-1-1. Recombination was confirmed by Southern blotting using a 1.5-kb fragment of the Aoatg1 gene upstream region as a probe, which was generated by PCR with the primers attB4-upAoatg1-F and attB1-upAoatg1-R (Figure S1).
Table 2. List of primers used in this study.
| Primer name | Sequence (5'-3') |
| attB4-PAoatg1-F | GGGGACAACTTTGTATAGAAAAGTTGCCTTTCTCCTCTCTACCTTG |
| attB1-PAoatg1-R | GGGGACTGCTTTTTTGTACAAACTTGCCTAAGAGGAGCGTCTACAA |
| attB2-Aoatg1-F | GGGGACAGCTTTCTTGTACAAAGTGGATGTCGTCTTCACACCACAG |
| attB3-Aoatg1-R | GGGGACAACTTTGTATAATAAAGTTGCCGTAGCGTTTCGATCTACA |
| attB4-PAoatg4-F | GGGGACAACTTTGTATAGAAAAGTTGTCATCTTCACTAGAGCTGTCACG |
| attB1-PAoatg4-R | GGGGACTGCTTTTTTGTACAAACTTGTATGGTTGGCTGGGTAGTGAA |
| attB2-Aoatg4-F | GGGGACAGCTTTCTTGTACAAAGTGGATGAACAGTGTAGACATAGGGCG |
| attB3-Aoatg4-R | GGGGACAACTTTGTATAATAAAGTTGCCCTTCTGCCTTGGTTGTAAGTA |
| attB4-PAoatg8-F | GGGGACAACTTTGTATAGAAAAGTTGTCTGAAAGTTGCAAGGTGCG |
| attB1-PAoatg8-R | GGGGACTGCTTTTTTGTACAAACTTGATTGATGGATCGAATCAGTTAATGG |
| attB2-Aoatg8-F | GGGGACAGCTTTCTTGTACAAAGTGGATGCGCTCCAAGTTCAAG |
| attB3-Aoatg8-R | GGGGACAACTTTGTATAATAAAGTTGTTTTCACTACTTATTTTCAATTACC |
| attB4-PAoatg15-F | GGGGACAACTTTGTATAGAAAAGTTGCCGACTAGAAGTAATGTGGC |
| attB1-PAoatg15-R | GGGGACTGCTTTTTTGTACAAACTTGATTTGTTGAGAGGTACCTTATACTTC |
| attB2-Aoatg15-F | GGGGACAGCTTTCTTGTACAAAGTGGATGATTATTTCAAATGCTCTTCTGGG |
| attB3-Aoatg15-R | GGGGACAACTTTGTATAATAAAGTTGTCAGGGTGGCGTGGTGAT |
The underlined sequences indicate the MultiSite Gateway attB recombination sites.
The plasmids pgPtA4, pgPtA8, and pgPtA15 for replacement of the Aoatg4, Aoatg8, and Aoatg15 promoters, respectively, were constructed by the identical method used for replacement of the Aoatg1 promoter. The 1.0-kb upstream region of the Aoatg4 gene and a 1.0-kb fragment containing the Aoatg4 gene were amplified by PCR using the primer pairs attB4-PAoatg4-F and attB1-PAoatg4-R, and attB2-Aoatg4-F and attB3-Aoatg4-R, respectively (Table 2). The 1.0-kb upstream region of the Aoatg8 gene and a 0.9-kb region containing the Aoatg8 gene were amplified by PCR using the primer pairs attB4-PAoatg8-F and attB1-PAoatg8-R, and attB2-Aoatg8-F and attB3-Aoatg8-R, respectively (Table 2). The 1.5-kb upstream region of the Aoatg15 gene and a 1.8-kb region containing the Aoatg15 gene were amplified by PCR using the primer pairs attB4-PAoatg15-F and attB1-PAoatg15-R, and attB2-Aoatg15-F and attB3-Aoatg15-R, respectively (Table 2). All primers were based on the sequence in the A. oryzae genome database (http://www.bio.nite.go.jp/dogan/project/view/AO). The PCR reactions were performed using the genomic DNA of A. oryzae RIB40 [24] as a template.
Southern Blot Analysis
The A. oryzae autophagy gene-conditional expression strains and strains expressing CHY were analyzed by Southern blot analysis. Briefly, after electrophoresis, the digested genomic DNAs of each strain were transferred onto a Hybond N+ membrane (GE Healthcare). The Enhanced Chemiluminescence (ECL) Direct Nucleic Acid Labeling and Detection System (GE Healthcare) and a LAS-4000miniEPUV luminescent image analyzer (Fuji Photo Film) were used for detection.
Western Blot Analysis
CHY-expressing transformants were cultured in 20 ml 5×DPY (pH 5.5) medium at 30°C for 4 days. Four microliters of the culture supernatant was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the separated proteins were then transferred onto a cellulose nitrate membrane (Immobilon-NC; Millipore) using a semi-dry blotting system (Nihon Eido). The membrane was immunostained using a polyclonal rabbit serum against CHY (kindly gifted by Dr. Tsuchiya [25]), followed by treatment with antirabbit immunoglobulin G labeled with horseradish peroxidase (Cotalog No. PI-1000; Vector Laboratories), and bands were visualized using the ECL Advance™ Western Blotting Detection kit (GE Healthcare).
Measurement of CHY Production Yield
The number of conidia harvested from PD agar plates counted using a hemocytometer under the microscope. Approximately 2×105 conidia of the CHY-expressing transformant were inoculated into 20 ml 5×DPY medium (pH 5.5) and thiamine-supplemented 5×DPY medium (pH 5.5), and were then cultured at 30°C for 3–6 days. CHY activity in the culture supernatant was measured by a modification of a method of Foltmann [26] as described previously by Yoon et al. [27]. Briefly, culture supernatant (100 µl) was collected every 24 h and then mixed with 1 ml of a 12% skim milk solution containing 10 mM CaCl2. During the milk-clotting reaction, the mixture was shaken (60 strokes per minute) at room temperature. The time point at which the thin film of milk began to form visible particles was designated as the clotting time. The CHY production level (mg/L) in each sample was estimated from a standard curve of clotting time versus CHY protein concentration that was generated using authentic CHY (Sigma).
Statistics
Microsoft Excel was used as a tool for statistics including means comparisons, standard deviation analyses and t-tests for significance.
Results
Effects of A. oryzae Autophagy (Aoatg) Gene Disruption on CHY Production
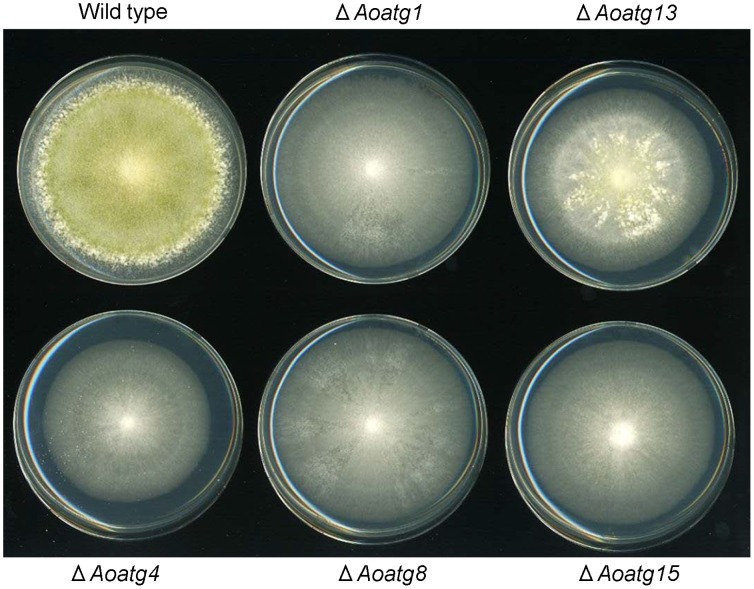
To examine the effect of autophagy impairment on heterologous protein production in A. oryzae, gene disruptants of Aoatg1, Aoatg13, Aoatg4, Aoatg8, and Aoatg15, which were previously constructed in our laboratory [28]–[30], were modified to produce CHY by transformation of a CHY expression plasmid fused with AmyB [27]. The plasmid for CHY expression was introduced into the control strain and the autophagy gene disruptants using the niaD gene as the selectable marker. The transformants possessing a single copy of the plasmid, which was integrated homologously at the niaD locus were identified by Southern blot analysis (data not shown). When the Aoatg1, Aoatg4, Aoatg8, and Aoatg15 disruptants expressing CHY were cultured for 5 days at 30°C on PD agar medium, conidia formation was clearly impaired (Figure 1). Under the same conditions, the Aoatg13 disruptant formed a modest conidia (Figure 1).
Figure 1. Phenotype of the A.oryzae autophagy gene disruptants.
Images of the Aoatg disruptants expressing CHY after growth on PD agar plates for 4 days at 30°C.
The production levels of CHY by the autophagy gene disruptants were estimated by measuring the milk-clotting activity of the culture supernatant (Figure 2). Each disruptant produced the highest level of CHY after 4 days of cultivation in 5×DPY medium (Figure 2). Compared to the control strain (SlD-AKC1), the production of CHY by the Aoatg1, Aoatg4, and Aoatg8 disruptants was increased by 2.3, 3.1, and 2.5 fold, respectively. In contrast, the Aoatg13 disruptant showed only a slight increase (1.4 fold) in CHY production. Additionally, no significant increase in CHY production by the Aoatg15 disruptant was observed.
Figure 2. Extracellular bovine chymosin (CHY) production by A. oryzae autophagy gene disruptants.
Approximately 2×105 conidia of each strain were inoculated into 20 ml 5×DPY medium (pH 5.5) and the CHY activity in the culture supernatant was measured daily after 3–6 days of growth at 30°C. Three experiments were performed, and the values of the average and standard deviations are represented (*p<0.01, Student’s t test).
Construction of Aoatg Gene-conditional Expression Strains
Disruption of autophagy in A. oryzae has almost no influence on growth on a nutrient-rich medium, but markedly reduces conidia formation. Therefore, we constructed four Aoatg gene-conditional expression strains by replacing the Aoatg1, Aoatg4, Aoatg8, and Aoatg15 promoters with the thiA promoter, whose activity can be controlled by the external concentration of thiamine. The integration of the thiA promoter was confirmed by Southern blotting (Figure S1). Genes under control of the thiA promoter are induced in the absence of thiamine, and have reduced expression levels in the presence of thiamine [31].
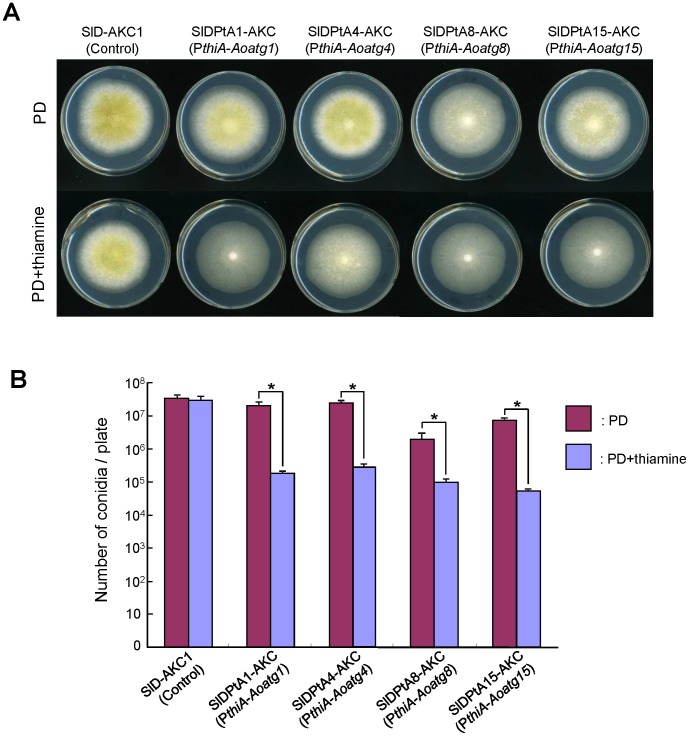
To examine conidia formation by the four Aoatg gene-conditional expression strains, each strain was cultured for 4 days on PD agar supplemented with and without thiamine. Conidia were formed by all transformants on the PD agar without thiamine, and yellowish-green colonies were observed (Figure 3A, upper), whereas few conidia were formed by any strain on the PD agar containing thiamine (Figure 3A, lower). Enumeration of the conidia formed per plate showed that the Aoatg1 and Aoatg4 conditional expression strains had a substantial recovery of conidiation, approaching the level of the control strain. In the case of the Aoatg8 and Aoatg15 conditional expression strains, however, conidiation was relatively poorly recovered on PD agar compared to the control strain, although an increased number of conidia were observed compared to their gene disruptants (Figure 3B). These results indicate that a sufficient amount of conidia for inoculation are successfully harvested from the Aoatg gene-conditional expression strains.
Figure 3. Conidiation in autophagy gene-conditional expression strains.
(A) Images of the Aoatg conditional expression strains after growth on PD agar plates supplemented with or without thiamine for 4 days at 30°C. (B) The number of conidia formed per plate are shown for each strain under the indicated conditions. Three experiments were performed, and the values of the average and standard deviations are represented (*p<0.01, Student’s t test).
Repression of Aoatg Genes Enhances CHY Production
To examine heterologous protein production in the Aoatg1, Aoatg4, Aoatg8, and Aoatg15 conditional expression strains, the CHY expression plasmid was introduced into these strains, and the transformants possessing a single copy of the plasmid, which was integrated homologously at the niaD locus were identified by Southern blot analysis (data not shown). The production levels of CHY were then measured (Figure 4 and Figure S2). For each strain, conidia formed on PD agar were harvested and inoculated into 5×DPY medium supplemented with and without thiamine. The CHY production level after 4 days of cultivation in the 5×DPY medium increased by 1.7 fold (Aoatg1), 1.7 fold (Aoatg4), 2.4 fold (Aoatg8), and 1.6 fold (Aoatg15) compared to that of the control strain (Figure 4A). Notably, a similar amount of CHY was produced by all strains in the presence and absence of externally supplied thiamine, suggesting that sufficient thiamine to repress the Aoatg gene expression was present in the yeast extract component of the 5×DPY medium. Western blotting analysis using an anti-chymosin antibody revealed an increased level of CHY production in the transformants compared to the control strain (Figure 4B). These results suggested that autophagy in the transformants was repressed during submerged culture in the 5×DPY medium.
Figure 4. Extracellular bovine chymosin (CHY) production by A.oryzae autophagy gene-conditional expression strains.
(A) Approximately 2×105 conidia of the control (SlD-AKC1), SlD-PtA1-AKC, SlD-PtA4-AKC, SlD-PtA8-AKC, and SlDPtA15-AKC strains expressing CHY were inoculated into 20 ml 5×DPY medium (pH 5.5) supplemented with and without thiamine. CHY activities in the culture supernatant were measured after 4 days of growth at 30°C. Five experiments were performed, and the values of the average and standard deviations are represented (*p<0.01, Student’s t test). (B) Western blot analysis of the culture supernatant of the CHY-expressing strains. Mature CHY bands of 35.4 kDa were detected using an anti-CHY antibody.
Discussion
Autophagy is an intracellular degradation mechanism that delivers cytoplasmic constituents to vacuoles/lysosomes and has recently been shown to play various complex cytological and physiological roles. However, despite the apparent importance of autophagy in eukaryotes, prior to the present study, no reports have examined modification of the autophagic process for enhancing the industrial production of recombinant heterologous proteins. As we predicted that autophagy is a bottleneck for heterologous protein production by A. oryzae, we transformed several autophagy gene disruptants with a CHY expression construct and confirmed that the production levels of CHY increased up to 3 fold. However, the poor conidiation by autophagy gene disruptants to be used as hosts for heterologous protein production at the industrial scale is problematic, as sufficient numbers of conidia for inoculation are difficult to obtain. Therefore, we also constructed conditional expression strains of each of the four autophagy genes and demonstrated that the transformants formed increased numbers of conidia, in addition to higher CHY production.
As part of protein quality control in the ER, misfolded proteins are degraded in the ER-associated protein degradation (ERAD) pathway [32]. Autophagy has been reported to serve as another route for ERAD and appears to degrade misfolded proteins that accumulate in the ER lumen [32]. Furthermore, it has been demonstrated that ER stress leads to the induction of autophagy in yeast and mammals [33], [34]. Thus, we speculate that the improvement of CHY protein productivity in most of the A. oryzae autophagy gene disruptants was due to impairment of the autophagic-induced degradation of CHY that normally occurs as part of the ER protein quality control process.
In contrast to the Aoatg1, Aoatg4, Aoatg8, Aoatg13 gene disruptants, the productivity of CHY did not markedly increase in the Aoatg15 gene disruptant. However, increased CHY production was detected in the Aoatg15 conditional expression strain. Notably, the Aoatg15 gene disruptant also displayed the greatest deficiency in the development of aerial hyphae and formation of conidia [29]. Thus, we consider that conidia formed by the Aoatg15 gene disruptant did not fully mature, because the harvested conidia were white in color, indicating they lacked pigment. In contrast, the conidia recovered from the Aoatg15 gene-conditional expression strain appeared green in color, and were therefore considered to be fully mature. These results suggest that mature conidia are insufficient to grow of mycelia, which yield an adequate amount of CHY, in the Aoatg15 disruptant.
In addition, the Aoatg13 disruptant formed a modest conidia (Figure 1), indicating that autophagic flux was not completely inhibited. This result was consistent with the reported phenotype of these mutants [29], [30]. The disruptant also showed only a slight increase (1.4 fold) in CHY production. These results suggest that the increase of CHY production was correlated with the repression level of autophagy.
In the fission yeast Schizosaccharomyces pombe, disruption of multiple protease genes, including ppp80/atg4, enhances the productivity of human growth hormone (hGH) [35]. However, the atg4 gene was one of seven protease genes that were disrupted based on a search for such genes in the S. pombe genomic database, but the functional effects of atg4 disruption on hGH production were not examined. In the present study, we revealed that autophagy may have direct effects on heterologous protein production in filamentous fungi by examining CHY in several A. oryzae autophagy gene disruptants, including Aoatg4, which is involved in autophagosome formation, but also Aoatg1, Aoatg8, Aoatg13, and Aoatg15. In addition, the observed correlation between the level of CHY production and repression of autophagy (to the extent that reduction in conidia formation indicates a repression of autophagy) also indicates that autophagy can limit heterologous protein production.
Recently, we identified Aovps10 encoding an A. oryzae vacuolar protein sorting receptor, AoVps10, which is responsible for the recognition and vacuolar sorting of carboxypeptidase Y. Moreover, we demonstrated that the disruption of Aovps10 in A. oryzae leads to enhanced production levels of heterologous proteins [15]. Therefore, it is expected that control of autophagy gene expression, in addition to the disruption of vacuolar protein sorting receptor gene Aovps10, will synergistically improve heterologous protein productivity in A. oryzae.
Despite the successful enhancement of the extracellular production of CHY by the autophagy gene disruption, there still exist proteolytic problems that should be solved. We previously constructed an A. oryzae ten extracellular protease genes disruptant [14]. Thus, another synergy effect of autophagy gene disruptant and the ten protease genes disruptant could be expected. In other words, yields for the heterologous protein production could be efficiently increased when the intracellular (autophagic degradation) and extracellular (proteolysis by proteases) bottlenecks were eliminated.
In conclusion, we successfully constructed A. oryzae strains capable of producing high levels of CHY as a model of heterologous protein production due to the repression of autophagy. In addition, to overcome the poor formation of conidia in these strains, the autophagic genes were expressed under control of an inducible promoter. This is the first report to demonstrate that the manipulation of autophagy mechanisms, which have important functions at a basic cellular level, can be applied towards the enhanced production of heterologous proteins. It may be possible to combine this approach for controlling bulk degradation pathways with the disruption of protease genes, which has been demonstrated to be effective [14], [27], for further improving the productivity of heterologous proteins in A. oryzae.
Supporting Information
Replacement of the Aoatg promoter. Schemes for the integration of the thiA promoter and Southern blotting in Aoatg1 (A), Aoatg4 (B), Aoatg8 (C), and Aoatg15 (D).
(TIFF)
Extracellular bovine chymosin (CHY) production by Aoatg8 conditional expression strains. (A) Approximately 2×105 conidia of the control and three individual Aoatg8 conditional expression strains expressing CHY were inoculated into 20 ml 5×DPY medium (pH 5.5). CHY activities in the culture supernatant were measured after 4 days of growth at 30°C. Three experiments were performed, and the values of the average and standard deviations are represented (*p<0.01, Student’s t test).
(TIFF)
Funding Statement
This study was supported by a Grant-in-Aid for Challenging Exploratory Research to K. Kitamoto from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kitamoto K (2002) Molecular biology of the Koji molds. Adv Appl Microbiol 51: 129–153. [DOI] [PubMed] [Google Scholar]
- 2. Christensen T, Woeldike H, Boel F, Mortensen SB, Hjortshoej K, et al. (1988) High level expression of recombinant genes in Aspergillus oryzae . Bio/Technology 6: 1419–1422. [Google Scholar]
- 3. Punt PJ, van Biezen N, Conesa A, Albers A, Mangnus J, et al. (2002) Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol 20: 200–206. [DOI] [PubMed] [Google Scholar]
- 4. Ito K, Asakura T, Morita Y, Nakajima K, Koizumi A, et al. (2007) Microbial production of sensory-active miraculin. Biochem Biophys Res Commun 360: 407–411. [DOI] [PubMed] [Google Scholar]
- 5. Jin FJ, Watanabe T, Juvvadi PR, Maruyama J, Arioka M, et al. (2007) Double disruption of the proteinase genes, tppA and pepE, increases the production level of human lysozyme by Aspergillus oryzae . Appl Microbiol Biotechnol 76: 1059–1068. [DOI] [PubMed] [Google Scholar]
- 6. Tsuchiya K, Nagashima T, Yamamoto Y, Gomi K, Kitamoto K, et al. (1994) High level secretion of calf chymosin using a glucoamylase prochymosin fusion gene in Aspergillus oryzae . Biosci Biotechnol Biochem 58: 895–899. [DOI] [PubMed] [Google Scholar]
- 7. Tsuchiya K, Tada S, Gomi K, Kitamoto K, Kumagai C, et al. (1992) High level expression of the synthetic human lysozyme gene in Aspergillus oryzae . Appl Microbiol Biotechnol 39: 738–743. [DOI] [PubMed] [Google Scholar]
- 8. Kimura S, Maruyama J, Watanabe T, Ito Y, Arioka M, et al. (2010) In vivo imaging of endoplasmic reticulum and distribution of mutant α-amylase in Aspergillus oryzae . Fungal Genet Biol 47: 1044–54. [DOI] [PubMed] [Google Scholar]
- 9. Kimura S, Maruyama J, Kikuma T, Arioka M, Kitamoto K (2011) Autophagy delivers misfolded secretory proteins accumulated in endoplasmic reticulum to vacuoles in the filamentous fungus Aspergillus oryzae . Biochem Biophys Res Commun 406: 464–470. [DOI] [PubMed] [Google Scholar]
- 10. Reggiori F, Klionsky DJ (2002) Autophagy in the eukaryotic cell. Eukaryot Cell 1: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mizushima N (2007) Autophagy: process and function. Genes Dev 21: 2861–2873. [DOI] [PubMed] [Google Scholar]
- 12. Pollack JK, Harris SD, Marten MR (2009) Autophagy in filamentous fungi. Fungal Genet Biol 46: 1–8. [DOI] [PubMed] [Google Scholar]
- 13. Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306: 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoon J, Maruyama J, Kitamoto K (2011) Disruption of ten protease genes in the filamentous fungus Aspergillus oryzae highly improves production of heterologous proteins. Appl Microbiol Biotechnol 89: 747–59. [DOI] [PubMed] [Google Scholar]
- 15. Yoon J, Aishan T, Maruyama J, Kitamoto K (2010) Enhanced production and secretion of heterologous proteins by the filamentous fungus Aspergillus oryzae via disruption of vacuolar protein sorting receptor gene Aovps10 . Appl Environ Microbiol 76: 5718–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuura A, Tsukada M, Wada Y, Ohsumi Y (1997) Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae . Gene 192: 245–250. [DOI] [PubMed] [Google Scholar]
- 17. Kamada Y (2000) Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol 150: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Funakoshi T, Matsuura A, Noda T, Ohsumi Y (1997) Analyses of APG13 gene involved in autophagy in yeast, Saccharomyces cerevisiae . Gene 192: 207–213. [DOI] [PubMed] [Google Scholar]
- 19. Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y (2009. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10: 458–467. [DOI] [PubMed] [Google Scholar]
- 20. Epple UD, Suriapranata I, Eskelinen EL, Thumm M (2001) Aut5/Cvt17p, a putative lipase essential for disintegration of autophagic bodies inside the vacuole. J Bacteriol 183: 5942–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teter SA, Eggerton KP, Scott SV, Kim J, Fischer AM, et al. (2001) Degradation of lipid vesicles in the yeast vacuole requires function of Cvt17, a putative lipase. J Biol Chem 276: 2083–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Epple UD, Eskelinen EL, Thumm M (2003) Intravacuolar membrane lysis in Saccharomyces cerevisiae. Does vacuolar targeting of Cvt17/Aut5p affect its function? J Biol Chem 278: 7810–7821. [DOI] [PubMed] [Google Scholar]
- 23. Maruyama J, Kitamoto K (2008) Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (ΔligD) in Aspergillus oryzae . Biotechnol Lett 30: 1811–1817. [DOI] [PubMed] [Google Scholar]
- 24. Machida M, Asai K, Sano M, Tanaka T, Kumagai T, et al. (2005) Genome sequencing and analysis of Aspergillus oryzae . Nature 438: 1157–1161. [DOI] [PubMed] [Google Scholar]
- 25. Tsuchiya K, Gomi K, Kitamoto K, Kumagai C, Tamura G (1993) Secretion of calf chymosin from the filamentous fungus Aspergillus oryzae . Appl Microbiol Biotechnol 40: 327–332. [DOI] [PubMed] [Google Scholar]
- 26. Foltmann B (1970) Prochymosin and chymosin (prorennin and rennin). Methods Enzymol 19: 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoon J, Kimura S, Maruyama J, Kitamoto K (2009) Construction of quintuple protease gene disruptant for heterologous protein production in Aspergillus oryzae . Appl Microbiol Biotechnol 82: 691–701. [DOI] [PubMed] [Google Scholar]
- 28. Kikuma T, Ohneda M, Arioka M, Kitamoto K (2006) Functional analysis of the ATG8 homologue Aoatg8 and role of autophagy in differentiation and germination in Aspergillus oryzae . Eukaryot Cell 5: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kikuma T, Kitamoto K (2011) Analysis of autophagy in Aspergillus oryzae by disruption of Aoatg13, Aoatg4, and Aoatg15 genes. FEMS Microbiol Lett 316: 61–69. [DOI] [PubMed] [Google Scholar]
- 30. Yanagisawa S, Kikuma T, Kitamoto K (2011) Functional analysis of Aoatg1 and detection of the Cvt pathway in Aspergillus oryza . FEMS Microbiol Lett 338: 168–176. [DOI] [PubMed] [Google Scholar]
- 31. Shoji JY, Maruyama J, Arioka M, Kitamoto K (2005) Development of Aspergillus oryzae thiA promoter as a tool for molecular biological studies. FEMS Microbial Lett 244: 41–46. [DOI] [PubMed] [Google Scholar]
- 32. Kario E, Amar N, Elazar Z, Navon A (2011) A new autophagy-related checkpoint in the degradation of an ERAD-M target. J Biol Chem 286: 11479–11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response PLoS Biol. 4: e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281: 30299–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Idiris A, Tohda H, Bi KW, Isoai A, Kumagai H, et al. (2006) Enhanced productivity of protease-sensitive heterologous proteins by disruption of multiple protease genes in the fission yeast Schizosaccharomyces pombe . Appl Microbiol Biotechnol 73: 404–420. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi T, Masuda T, Koyama Y (2006) Identification and analysis of Ku70 and Ku80 homologs in the koji molds Aspergillus sojae and Aspergillus oryzae . Biosci Biotechnol Biochem 70: 135–143. [DOI] [PubMed] [Google Scholar]
- 37. Higuchi Y, Shoji JY, Arioka M, Kitamoto K (2009) Endocytic recycling at the tip region in the filamentous fungus Aspergillus oryzae . Eukaryot Cell 8: 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Replacement of the Aoatg promoter. Schemes for the integration of the thiA promoter and Southern blotting in Aoatg1 (A), Aoatg4 (B), Aoatg8 (C), and Aoatg15 (D).
(TIFF)
Extracellular bovine chymosin (CHY) production by Aoatg8 conditional expression strains. (A) Approximately 2×105 conidia of the control and three individual Aoatg8 conditional expression strains expressing CHY were inoculated into 20 ml 5×DPY medium (pH 5.5). CHY activities in the culture supernatant were measured after 4 days of growth at 30°C. Three experiments were performed, and the values of the average and standard deviations are represented (*p<0.01, Student’s t test).
(TIFF)