Abstract
Intracellular polyamines are absolutely required for cell proliferation and many tumors have abnormal requirements for polyamines. Therefore, the polyamine metabolic pathway represents a rational target for antineoplastic intervention. A number of polyamine analogues act as potent modulators of cellular polyamine metabolism and exhibit encouraging effects against tumor growth in both cell culture and animal studies. In this study we demonstrate that specific polyamine analogues exhibit differential inhibitory action against growth of human breast cancer MCF-7 cells. Treatment of MCF-7 cells with oligoamine analogues and the symmetrically substituted bis(alkyl)-substituted analogue, BENSpm, produced a G1 cell cycle arrest, while the unsymmetrically substituted bis(alkyl)-substituted analogue, CHENSpm, induced a G2/M cell cycle arrest. All four compounds significantly upregulated p53 and p21 expression in MCF-7 cells. Stable transfection of small interfering RNA (siRNA) targeting p53 blocked the expression of p21 induced by the polyamine analogues and significantly reduced polyamine analogue-induced growth inhibition and apoptosis, suggesting that polyamine analogue-induced p21 expression occurs through p53-dependent mechanisms. The effects of analogue exposure on cyclins and cyclin dependent kinases varied with the specific agent used. Expression of p53 siRNA reversed only BENSpm-modulated the cell cycle arrest, suggesting that regulation of cell cycle arrest by p53/p21 induced by polyamine analogues occurs through agent-specific mechanisms. Understanding the mechanism of p53-mediated cellular responses to polyamine analogue may help to improve the therapeutic efficacy of polyamine analogues in human breast cancer.
Keywords: polyamine analogues, breast cancer cells, cell cycle arrest, cell death, p53/p21waf1/Cip1
INTRODUCTION
Polyamines are naturally occurring polycations that are required for cell growth, and manipulation of cellular polyamine levels can lead to decreased proliferation, and, in some cases, increased cell death. Natural polyamine biosynthesis is regulated by the rate-limiting enzymes ornithine decarboxylase (ODC) and S-Adenosylmethionine decarboxylase (SAMDC), while polyamine catabolism is driven by spermidine/spermine N1-acetyltransferase/ polyamine oxidase (SSAT/PAO) and spermine oxidase SMO(PAOh1).1–3 The requirement for polyamine action in cell proliferation and tumorigenesis has underscored the rationale of targeting polyamine metabolism as a therapeutic strategy.4,5 Polyamine analogues have been synthesized as metabolic modulators that deplete natural intracellular polyamine pools, or polyamine mimetics that displace the natural polyamines from binding sites, but do not substitute for their growth promoting function.2,6 Symmetrically substituted bis(alkyl)polyamine analogues represent the first generation of these analogues, some of which downregulate polyamine biosynthesis and increase SSAT activity in certain tumor cell types like non-small cell lung cancer cells, melanoma and human breast cancer cells.7–9 A second generation of polyamine analogues are unsymmetrically substituted compounds that display structure-dependent and cell type-specific effects on regulation of polyamine metabolism.10 Recently, a series of new polyamine analogues designated conformationally restricted, cyclic and oligoamine analogues have been developed.11–13 Some of these agents incorporate alterations that limit the free rotation of the single bonds in otherwise flexible molecules such as spermine or its analogues, thus restricting the molecular conformation that they may assume. Oligoamine analogues consist of synthetic octa-, deca-, dodeca- and tetradecamines with longer chains than natural mammalian polyamine molecules, with or without conformational restriction. Some of these novel analogues have shown significant activity against multiple human tumors both in vitro and in vivo.6,12,13
Although it is clear that polyamine analogues can induce cell death, the mechanisms remain elusive. Interaction with DNA, displacement of natural polyamines from their binding sites, induction of polyamine catabolic enzyme activity and depletion of mitochondrial DNA have all been proposed as possible mechanisms underlying the anti-tumor action of polyamine analogues. Polyamines are required at different stages of cell cycle progression, and use of polyamine biosynthesis inhibitors or analogues to target the polyamine biosynthetic and catabolic machinery can disrupt normal cell cycle regulation and subsequently lead to the cessation of tumor cell growth. For example, treatment of human breast cancer MCF-7 cells with the specific ODC inhibitor, DFMO, led to an increased level of cyclin B1 in early G1 phase.14 A symmetrically substituted analogue, BENSpm, was demonstrated to upregulate the expression of the tumor suppressor, p53, and the CDK inhibitor, p21, in a human melanoma cell line.15 Recent studies indicate that apoptotic cell death might be a common characteristic for polyamine analogue-induced cytotoxicity in cancer cells. Although the ‘superinduction’ of SSAT and H2O2 produced by polyamine catabolism has been implicated in the cytotoxic response of specific solid tumor phenotypes to specific analogues such as BENSpm and CPENSpm,15–17 other analogues like oligoamines do not highly induce polyamine catabolic enzymes but can still inhibit tumor cell growth and induce apoptosis.18 We have recently demonstrated that multiple apoptotic mechanisms were associated with the oligoamine CGC-11144-induced cytotoxic effects in specific breast cancer cell lines.19 These findings indicate that polyamine analogue-induced cell growth inhibition and death may occur through multiple structure-specific and cell type-specific mechanisms.
To elucidate the molecular mechanisms of polyamine analogue cytotoxicity as well as the structural determinants for cytotoxicity in human breast cancer cells, several well-characterized polyamine analogues with different structures were analyzed for their effects on cell cycle and apoptotic regulatory pathways in MCF-7 cells. Our findings indicate that the oligoamine analogues (CGC-11144 and CGC-11172) and the symmetrically substituted alkylpolyamine analogue, BENSpm, arrested MCF-7 cells at G1 phase, while the unsymmetrically substituted alkylpolyamine analogue, CHENSpm, led to G2/M arrest. All four analogues significantly upregulated p53/p21 levels in MCF-7 cells, and suppression of p53 activation by siRNA abrogated p21 activation by polyamine analogues and significantly reduced polyamine analogue-induced growth inhibition and apoptosis. However, inhibition of p53/p21 activation reversed only BENSpm induced cell cycle arrest, suggesting that effects of p53/p21 on polyamine analogue-induced cell cycle arrest occur through agent-specific mechanisms.
METHODS
Compounds and culture conditions
N1, N11-bis(ethyl)norspermine (BENSpm), N1-ethyl-N11-(cycloheptyl)methyl-4,8, diazaundecane (CHENSpm), CGC-11144 and CGC-11172 (previously named SL-11144 and SL-11172) were synthesized as previously reported.11,13,20 Stock solutions (10 mM in ddH2O) of each analogue were diluted with medium to the desired concentrations for specific experiments. The human breast cancer MCF-7 cells were maintained in DMEM supplemented with 5% fetal bovine serum, 2 mM glutamine and incubated at 37°C in a 5% CO2 atmosphere.
MTT growth inhibition assays
Growth inhibition was assessed by MTT assays as described previously.18 Briefly, 2,000–5,000 cells were plated in 96-well dishes and treated with increasing concentrations of compounds for the indicated times. All of the experiments were plated in quadruplicate and were carried out at least three times. The results of assays are presented as means ± S.D. of all determinants.
Western blotting
After treatment, cells were harvested, and proteins (50 μg/lane) were fractionated on 12% SDS-PAGE gels, transferred to PVDF membranes and immunoblotted with antibodies as indicated. Primary antibodies against p53, p21, p27, cyclin D1, cyclin B1, CDK2, CDK4 and CDK6 were purchased from Santa Cruz Biotechnology Company (Santa Cruz, CA). Antibodies against p15 and cyclin E1 were from Oncogene Science (Cambridge, MA). To detect pRb protein, one hundred micrograms of total protein per sample was separated by 6% SDS-PAGE, transferred to nitrocellulose membrane and immunoblotted with monoclonal antibody RB-PMG3-245 (Pharmingen, UK) as described previously.21 Actin was used to normalize for protein loading. All experiments were performed at least twice with similar results.
Plasmid construction and stable transfection
Small interfering RNA (siRNA) oligonucleotides targeting p53 (sense, 5′-GACTCCAG-TGGTAATCTAC-3′; anti-sense, 5′-GTAGATTACCAC-TGGAGTC-3′) were designed and synthesized based on previously published sequences.22 The complementary oligonucleotides were ligated into pSilencer siRNA expression vectors (Ambion, Austin, Texas). Transfections were performed with LipofectAMINE PLUS™ reagent (Invitrogen, Carlsbad, CA) as recommended by the manufacturer. Stable transfectants were selected by incubating the cells in medium containing 500 μg/ml geneticin (G418). Cells from individual colonies were examined for p53 expression by Western blot.
Determination of internucleosomal DNA cleavage
After treatment, cells were harvested, counted, and washed with phosphate-buffered saline (PBS) at 4°C. Cells were then suspended in lysis buffer (5 mM Tris-HCL, 20 mM EDTA and 0.5% Triton X-100) and incubated for 20 min on ice. Detection of DNA fragmentation was performed as described previously.18 DNA samples were analyzed by electrophoresis in a 1.2% agarose slab gel containing 0.2 mg/ml ethidium bromide, and visualized under UV illumination. The experiments were performed at least twice with similar results.
Immunoprecipitation and kinase assays
The immunoprecipitation and kinase assays were performed as described previously.23 Cells were harvested by trypsinization and washed with PBS buffer. The cell pellet was resuspended in 1 ml of immunoprecipitation lysis buffer (20 mM Tris-HCl, pH 7.4, 137 mM NaCl, 1% Nonidet P-40, 50 mM EDTA, 1× protease inhibitor) and was rotated in a rotary shaker for 30 min at 4°C. Kinase complexes were immunoprecipitated by incubation for 2h at 4°C with the indicated rabbit polyclonal or monoclonal antibodies (Santa Cruz Biotechnology) bound to protein-A or G Sepharose (Amersham Biosciences). The immunoprecipitates were washed three times with lysis buffer and twice with kinase buffer (50 mM HEPES, pH 7.4, 10 mM-glycerophosphate, 10 mM MgCl2, 1 mM dithiothreitol, 0.1 mM sodium orthovanadate). The kinase assays were initiated by the addition of 2 μg of substrate protein, 20 μM cold ATP and 10 μCi [γ-32P] ATP. Reaction mixtures were incubated for 30 min at 30°C and stopped by the addition of 2×SDS-PAGE sample buffer. The phosphorylation of the substrate proteins was analyzed by SDS-polyacrylamide gel electrophoresis followed by autoradiography. The experiments were performed at least twice with similar results.
Flow cytometry analysis
Cells were harvested, counted, washed with phosphate-buffered saline (PBS) at 4°C, fixed with formaldehyde, and stained with Hoechst 33258(Sigma). BD-LSR was used to perform FACS and the cell cycle was analyzed using Cell-Quest software.
Statistical analysis
The Student’s t test was used to determine the statistical differences between various experimental and control groups. p ≤ 0.05 was considered significant.
RESULTS
Growth inhibition and cell cycle arrest by polyamine analogues
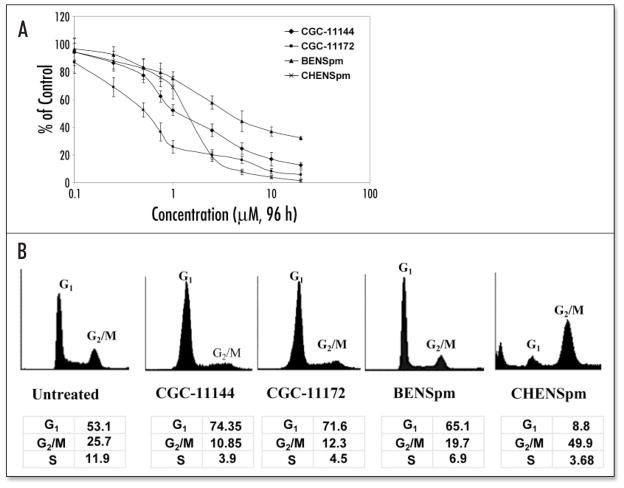
BENSpm is a symmetrical bisalkylated analogue with structural similarity to natural polyamines, while CHENSpm, an unsymmetrically substituted analogue, is structurally different from BENSpm with one terminal ethyl group being substituted by a cycloheptylmethyl group. The oligoamines, CGC-11144 and CGC-11172, are alkylamines with longer chains than natural polyamines and each NH2+ residue is separated by four CH2 residues with central cis double bonds that restrict the analogue conformation (Fig. 1). The sensitivity of estrogen receptor α (ER) positive, p53 wild type human breast cancer MCF-7 cells to these polyamine analogues was assessed by MTT growth inhibiton assay. The IC50 values for oligoamines are about 0.75–1.5 μM for 96 h treatment. The bisalkylated analogue, CHENSpm, exhibited a lower IC50 value (~2.5 μM) than that of another bisalkylated analogue BENSpm (~5–7.5 μM) (Fig. 2A). Flow cytometric analysis indicated an increase in the proportion of cells in the G1 phase of the cell cycle after treatment with CGC-11144, CGC-11172 or BENSpm for 72 h, whereas CHENSpm arrested MCF-7 cells at the G2/M phase (Fig. 2B). These results suggest that modifications in analogue structure may affect the drug cytotoxicity and cell cycle regulation in MCF-7 cells.
Figure 1.
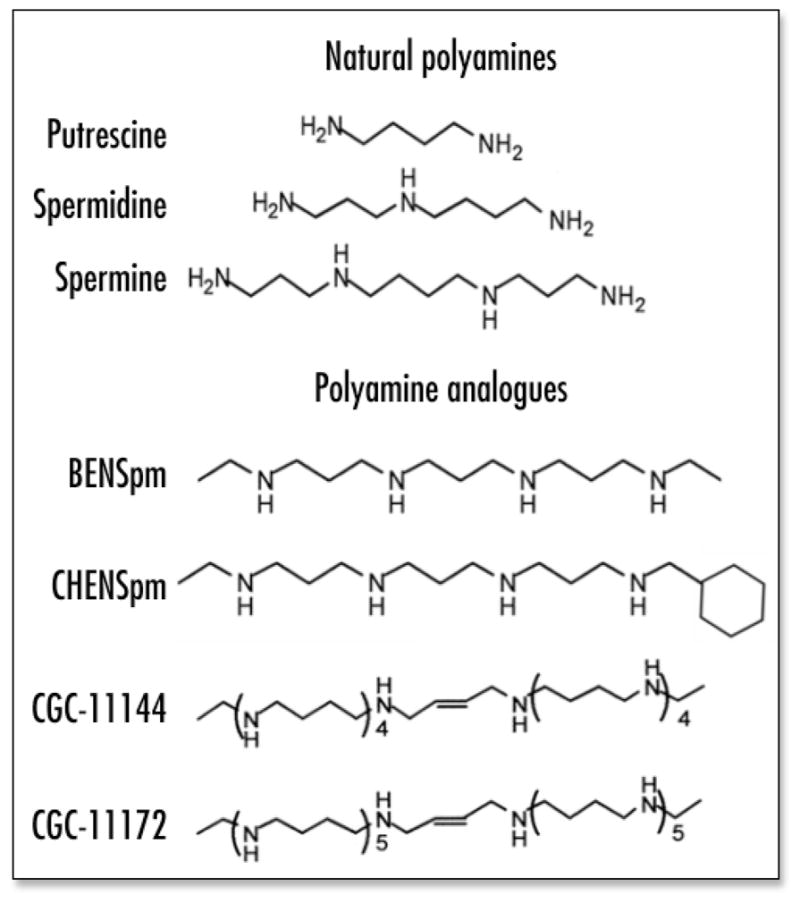
Structures of natural polyamines and polyamine analogues used in this study.
Figure 2.
Polyamine analogues inhibit growth and induce cell cycle arrest in MCF-7 cells. (A) Cells were treated with increasing concentrations of the indicated polyamine analogues and analyzed by MTT assays. Shown are means ± S.D of three independent experiments performed in quadruplicate. (B) Cells were incubated with 10 μM of polyamine analogue for 72 h, harvested, and stained for DNA with Hoechst 33258 for flow cytometry. The peaks corresponding to G1 and G2/M phases of the cell cycle are indicated. Each experiment was performed twice with similar results.
Effects of polyamine analogues on cell cycle regulatory proteins
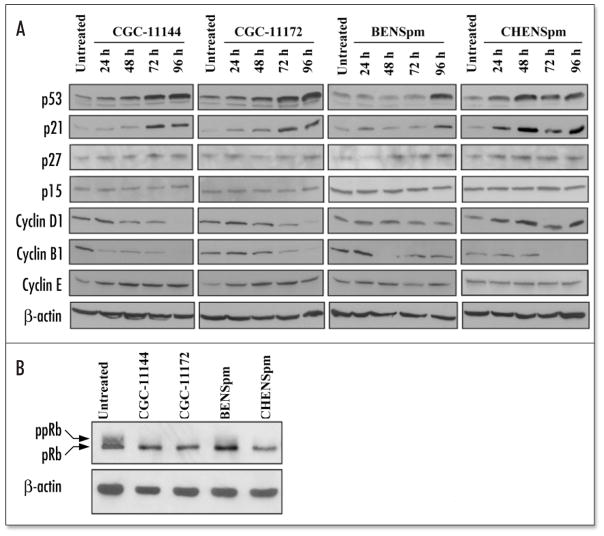
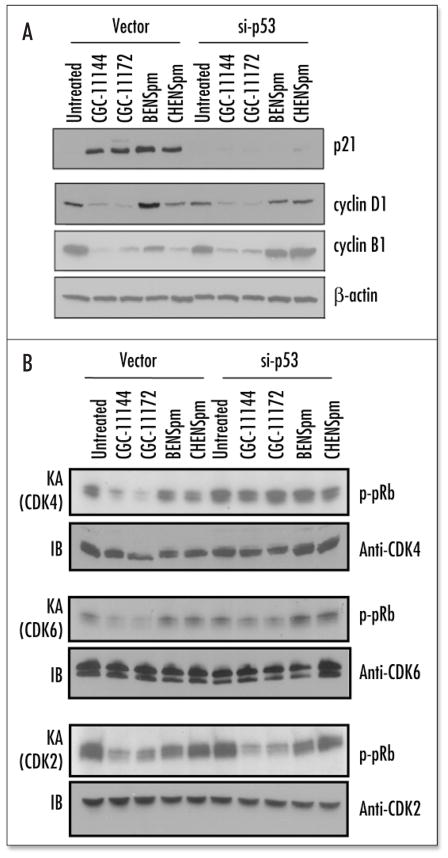
A number of cell cycle regulatory genes have been demonstrated to play important roles in controlling cell growth and death. These genes include the tumor suppressors p53 and pRb, cyclins, CDKs, CDK inhibitors (CDKi) of the Cip/Kip family p21Waf1/Cip1 and p27, and the INK4 family of CDKi, p15 and p16.24 To determine whether these proteins are involved in the mediation of polyamine analogue-induced cell cycle arrest and death in human breast cancer cells, their expression in MCF-7 cells after treatment with 10 μM polyamine analogue for 24 h to 96 h was examined. All analogues studied induced p53 and p21 protein expression, suggesting that p53/p21 pathway activation is a common cellular response of MCF-7 cells to different polyamine analogue treatment (Fig. 3A). The expression of another member of the Cip/Kip family, p27kip1, was unaffected by each of the analogue. No analogue altered the p15 protein levels and p16 expression was essentially undetectable in MCF-7 cells under the conditions examined (data not shown). Cyclin D1, which is complexed with either CDK4 or CDK6, controls cell cycle progression in the G1 phase by enabling the enzymes to phosphorylate retinoblastoma protein (pRb). Protein levels of cyclin D1 were downregulated by oligoamines after 72 h treatment, but were virtually unchanged by BENSpm and somewhat increased by CHENSpm. Cyclin B1 interacts with Cdk1 (CDC2) to form an active complex that drives cellular progression from G2- to M-phase. All four analogues downregulated cyclin B1 but at different time points. Cyclin E forms a complex with CDK2 to target components of the DNA synthesis machinery, leading to the assembly of complexes necessary for DNA replication at the G1/S-phase. Oligoamines but not bis(alkyl)-substituted analogues increased cyclin E expression.
Figure 3.
Effects of polyamine analogues on cell cycle regulatory proteins. (A) MCF-7 cells were treated with 10 μM polyamine analogue for 24–96 h (A) or 96 h (B). Equal amounts of protein (50 μg/lane) were fractionated on 12% (A) or 6% (B) SDS-PAGE gels (6% gel for pRb) and transferred to PVDF or nitrocellulose membranes followed by immunoblotting with different monoclonal or polyclonal antibodies. Actin protein was blotted as a control. Each experiment was performed twice with similar results.
pRb functions as a master switch of the cell cycle and tumor suppressor whose hyperphosphorylation can be prevented by CDK inhibitors (p21, p27, etc) and blocks cell cycle progression by interacting with transcription factors such as E2F. Interestingly, complete dephosphorylation of pRb was observed in MCF-7 treated by oligoamines and CHENSpm, while BENSpm induced the dephosphorylated form of pRb (lower band) and reduced the hyperphosphorylated form (upper band) (Fig. 3B). These results indicated that maintenance of pRb in the hypophosphorylated state is a common growth inhibitory mechanism shared by each of the analogues.
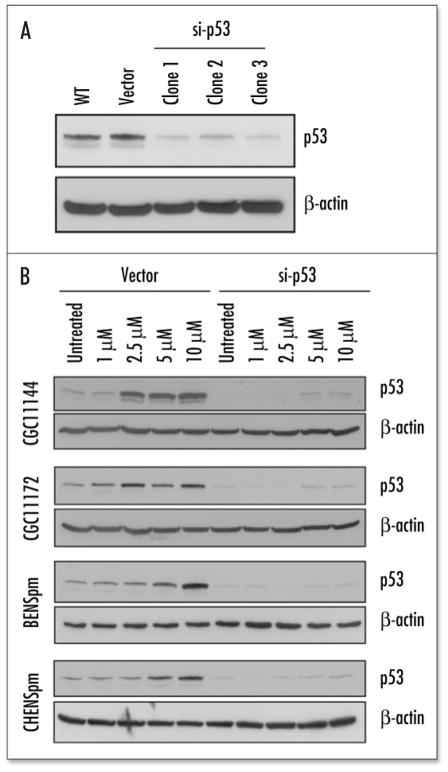
Suppression of p53 expression by p53 siRNA. To study the specific role of p53 activation in polyamine analogue induced cell cycle arrest and growth inhibition, siRNA was used to knock down the expression of p53 in MCF-7 cells. Stable transfection of a p53 pSilencer-vector suppressed endogenous p53 expression by approximately 80–90% in three clones (Fig. 4A), while the empty vector transfection showed no change in p53 expression. These three colonies were selected for the next series of experiments. Analytic data from all these three pairs of transfectants showed similar results. Here, analysis of clone #3 is presented. The vector control and p53 siRNA transfectants were treated with increasing concentrations (1–10 μM) of each polyamine analogue for 96 hour53 siRNA significantly inhibited the dose dependent induction of p53 (Fig. 4B).
Figure 4.
Suppression of p53 by p53 siRNA. (A) MCF-7 cells were stably transfected with empty pSilencer or pSilencer-p53 siRNA vector. Proteins isolated from parental, empty vector transfectants and p53-siRNA transfected single colonies were subjected to immunoblotting with an antibody to p53. Three geneticin-resistant clones were shown to have suppressed p53 level by si RNA. (B) Vector and p53 siRNA transfected MCF-7 cells were treated with 1–10 μM polyamine analogue for 96 h and subjected to immunoblotting with an antibody to p53.
p53 siRNA reduces sensitivity of MCF-7 cells to polyamine analogue-induced growth inhibition and apoptosis
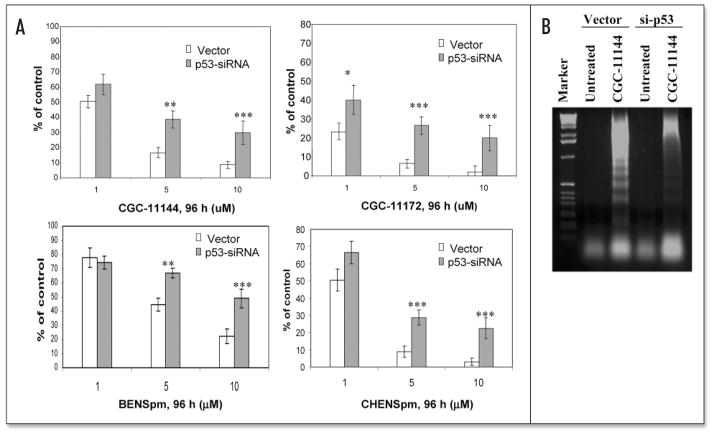
MCF-7 cells transfected with empty vector or p53 siRNA were treated with increasing concentrations of each analogue (1–10 μM) for 96 h followed by MTT growth inhibition assays to determine if the expression of the p53 siRNA could alter the sensitivity of tumor cells to polyamine analogue cytotoxicity. MCF-7 cells transfected with p53 siRNA exhibited more resistance to growth inhibition by each analogue at concentrations above 1 μM (Fig. 5A). This was associated with the inhibition of apoptotic internucleosomal DNA fragmentation by p53 siRNA in CGC-11144 treated MCF-7 cells (Fig. 5B). These data suggests that activation of p53 is a common mechanism that may contribute to growth inhibition and apoptotic cell death induced by these structurally distinct analogues.
Figure 5.
Effects of p53 siRNA on polyamine analogue-induced growth inhibition and apoptosis. (A). Vector and p53 siRNA transfected MCF-7 cells were treated with 1–10 μM polyamine analogue for 96 h and analyzed by MTT assays. Shown are means ± S.D. of three independent experiments performed in quadruplicate. There were statistically significant differences between transfected cells and vector transfected cells treated by >5 μM CGC-11144, BENSpm, CHENSpm and >1 μM CGC-11172. (*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test). (B) Vector and p53 siRNA transfected MCF-7 cells were treated with 10 μM CGC-11144 for 96 h. Fragmented DNA was analyzed by electrophoresis in 1.2% agarose gel containing 0.1% ethidium bromide. Lane 1 is 1-kilobase DNA markers. The experiment was performed twice with similar results.
Effects of p53 siRNA on cell cycle regulatory proteins
p53 siRNA significantly inhibited the dose dependent induction of p21 (Fig. 6A) by each analogue, demonstrating that activation of p21 by polyamine analogues occurs through p53 dependent mechanisms in MCF-7 cells. Downregulation of cyclin D1 and cyclin B1 by oligoamines was not blocked by p53 siRNA transfection (Fig. 6A). However, p53 siRNA prevented the decrease in cyclin B1 levels induced by BENSpm and CHENSpm. CDK activity was assessed by kinase assays following immunoprecipitation from cell extracts harvested from vector or p53 siRNA-transfected MCF-7 cells treated with specific polyamine analogues at 10 μM for 96 h. The oligoamines, CGC-11144 and CGC-11172, significantly inhibit CDK4, CDK6 and CDK2 kinase activities in vector-transfected control cells (Fig. 6B), whereas BENSpm decreased only CDK2 activity and CHENSpm did not modulate the activities of any of the examined kinases. Expression of p53 siRNA completely blocked the downregulation of CDK4 activity and partially blocked the decreased CDK6 activity by oligoamines. However, the inhibition of CDK2 activity by CGC-11144, CGC-11172 and BENSpm was not affected by the knockdown of p53 expression. In addition, the CDC2 (CDK1) activity was not changed by any of the polyamine analogues in either vector or p53 siRNA transfectants (data not shown).
Figure 6.
Effects of p53 siRNA on cell cycle regulatory proteins. (A) Vector and p53 siRNA transfected cells were treated with 10 μM of polyamine analogue for 96 h and subjected to immunoblotting with an antibody to p21, cyclin D1 or cyclin B1. Actin protein was blotted as a control. Each experiment was performed twice with similar results. (B) CDKs complexes were immunoprecipitated with anti-CDKs antibody and then subjected to an in vitro kinase assay (KA) using pRb as the substrate. CDK protein expression was analyzed by Western blotting as a control (IB).
Effects of p53 siRNA on polyamine analogue induced cell cycle arrest
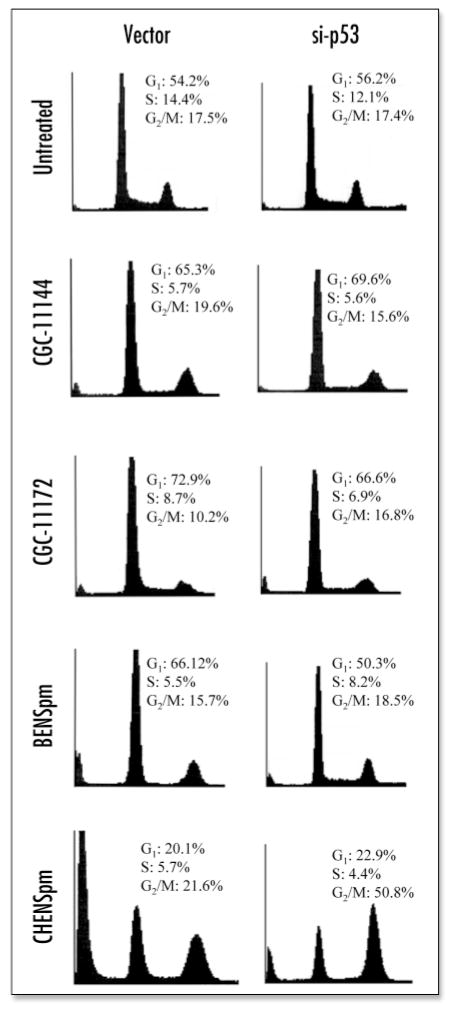
To assess the effects of siRNA-mediated downregulation of the p53/p21 pathway on polyamine analogue-induced cell cycle arrest, vector and p53 siRNA transfectants were treated with 10 μM of each analogue for 96 h followed by flow cytometric analysis. Figure 7 shows that suppression of analogue-mediated p53/p21 induction by siRNA has little effect on oligoamine-induced G1 arrest. However, the BENSpm-induced G1 arrest was almost completely reversed by p53 siRNA transfection. CHENSpm treatment of vector transfectants resulted in significant G2/M arrest and induction of a large fraction of cells detected as a sub-G1 peak, which is believed to represent an apoptotic cell population. Expression of p53 siRNA considerably reduced the fraction of apoptotic cells induced by CHENSpm without altering the proportion of cells at G1. However, an increase of cells in G2/M was observed in p53 siRNA transfectants treated by CHENSpm, suggesting that p53 siRNA suppresses CHENSpm-induced apoptosis without affecting CHENSpm’s effect on cell cycle arrest at G2/M phase. Together, these data indicate that the p53/p21 signaling pathway may mediate polyamine analogue-induced cell cycle arrest through agent-specific mechanisms.
Figure 7.
Effects of p53 siRNA on polyamine analogue induced cell cycle arrest. Vector and p53 siRNA transfected MCF-7 cells were treated with 10 μM of polyamine analogue for 96 h. After treatment, cells were harvested and stained for DNA with Hoechst 33258 for flow cytometric analysis. The fractions corresponding to G1, S and G2/M phases of the cell cycle are indicated. Each experiment was performed twice with similar results.
DISCUSSION
Polyamine analogues chosen for this study represent three major classes of analogues. All four analogues inhibited the in vitro growth of MCF-7 cells at micromolar concentrations. Previous studies have demonstrated that natural polyamines are required for cell cycle checkpoint regulation.25 Polyamines are essential for cells to enter S-phase and depletion of intracellular polyamines may lead to the 26 In this study, oligoamines and inhibition of G1-S phase transition. BENSpm blocked the cell cycle at G1 phase, while CHENSpm produced a significant G2/M arrest in MCF-7 cells (Fig. 2B). Interestingly, all three polyamine analogues capable of G1 arrest are symmetrical, which is consistent with previous reports in lung cancer and melanoma cells.27,28 The mechanism underlying this observation is not clear. During the cell cycle, ODC activity increases during G1 phase and reaches a second peak at 29 G2 phase prior to mitosis.29 Studies by several other groups have demonstrated that inhibition of ODC by DFMO led to G1 arrest in a variety of cell lines including tumor cells.30–32 Both oligoamines and BENSpm profoundly decrease ODC activity within 24 h in MCF-7 cells, potentially through the activation of the ODC inhibitor, antizyme (AZ),18,33 implying that downregulation of ODC could be one mechanism leading to G1 arrest. In contrast, CHENSpm differs from BENSpm only at the terminally modified cycloheptylmethyl group, but leads to a G2/M arrest. A previous study found that CHENSpm altered microtubule density and polymerization in lung cancer cells.27 The mechanism underlying this effect is still under investigation. However, these findings suggest that small changes in analogue structure may lead to profound difference in cellular effects and the ability of analogues to alter cell cycle transition and tubulin polymerization.
That all four polyamine analogues tested induce p53/p21 in MCF-7 cells indicates that activation of p53/p21 is a common response of MCF-7 cells to polyamine analogues with different structures. The p53 tumor suppressor gene is a central mediator of apoptotic and cell cycle response to a variety of stimuli such as DNA damage, hypoxia, genotoxic stress, and ribonucleoside triphosphate deficiency.34 BENSpm-induced p53/p21 activation and G1 arrest has been reported in human melanoma cells,15 indicating that p53/p21 might play an active role in the mediation of polyamine analogue-induced cell cycle arrest and growth inhibition in multiple types of cancer. Specific siRNA targeting p53 mRNA significantly reduced the sensitivity of MCF-7 cells to polyamine analogue-induced growth inhibition and apoptosis. These results support our preliminary hypothesis that activation of the p53/p21 signaling pathway may contribute to polyamine analogue cytotoxicity in human breast cancer cells.
Our data also demonstrated that the effect of p53/p21 on polyamine analogue-induced cell cycle arrest exhibits a complicated pattern that is agent-dependent. Suppression of p53/p21 was not sufficient to reverse the G1 cell cycle arrest induced by oligoamines. This finding implies that p53 induced growth inhibition and apoptosis may occur through cell cycle arrest-independent pathway(s). Activation of the cyclin-dependent kinase inhibitor, p21, plays a critical role in the induction of cell cycle arrest by p53, but p21 does not appear to be a p53-targeted apoptotic gene, like Bax, Fas, Noxa, DR5, etc.35 Also, although oligoamine-inhibited CDK4 and CDK6 activities were restored by p53 knockdown, expression of p53 specific siRNA did not alter the inhibition of CDK2 activity or the down-regulation of cyclin D1 and cyclin B1 protein expression by oligoamines (Fig. 6), suggesting that p53-independent mechanisms exist in oligoamine-induced cell cycle arrest. That oligoamines can also cause the G1 arrest in p53 mutant breast cancer cells like MDA-MB231 (data not shown) further suggests that oligoamines may induce cell cycle arrest via some p53-independent mechanisms. Perhaps, the strong interaction between oligoamine and DNA molecules may cause more severe damage than that seen with other analogues and G1 arrest due to such profound DNA damage may not be overcome by inhibition of the CDK inhibitor activities only. Conversely, BENS pm-induced G1 arrest was reversed by p53 siRNA, indicating that this effect is more dependent on the p53/p21 pathway than other analogues. It is possible that BENSpm may exert cytotoxicity largely by superinduction of SSAT activity, rather than direct interaction with DNA. Suppression of p53/p21 activation significantly inhibited CHENSpm-induced apoptosis but did not affect the accompanying G2/M arrest (Fig. 7). A previous study has demonstrated that CHENSpm induces G2/M arrest by altering tubulin dynamics.27 The effect of CHENSpm on microtubules could trigger the activation of downstream apoptotic pathways that are regulated by p53/p21 signaling pathway. Suppression of p53 activation by siRNA could selectively inhibit the apoptotic pathway without affecting the ability of CHENSpm to change tubulin dynamics and induce G2/M arrest. In aggregate, these results suggest that the role of the p53/p21 pathway in polyamine analogue-induced cell cycle arrest varies by agent and greatly depends on the balance between the extent of DNA damage and the response of repair/apoptotic mechanisms.
Acknowledgments
This work is supported by the NIH Grants P50CA88843 (to N.E.D.), CA98454, CA51085 (to R.A.C.), Breast Cancer Research Foundation (to N.E.D.), DOD Grants DAMD 17-03-1-0376 (to Y.H.), W81XWH-04-1-0457 (to A.P.), and CA85509 (to PMW).
ABBREVIATIONS
- ODC
ornithine decarboxylase
- SAMDC
S-Adenosylmethionine decarboxylase
- PAO
polyamine oxidase
- SMO/PAOh1
Spermine Oxidase
- DFMO
α-difluoromethylornithine
- BENSpm
N1, N11-bis(ethyl)norspermine
- CHEMSpm
N1-(cycloheptylmethyl)-N11-ethyl-4,8-diazaundecane
- MTT
3-(4,5,-dimethyl-2-yl)-2,5,-diphenyl-tetrazolium
- DMSO
Dimethyl Sulfoxide
- PBS
phosphate-buffered saline
- PAGE
polyacrylamide gel electrophoresis
- SSAT
spermidine/spermine N1-acetyltrans-ferase
References
- 1.Vujcic S, Diegelman P, Bacchi CJ, Kramer DL, Porter CW. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem J. 2002;367:665–75. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–3. [PubMed] [Google Scholar]
- 3.Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759–74. [PubMed] [Google Scholar]
- 4.Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmaco Toxicol. 1995;35:55–91. doi: 10.1146/annurev.pa.35.040195.000415. [DOI] [PubMed] [Google Scholar]
- 5.Casero RA, Jr, Pegg AE. Spermidine/spermine N1-acetyltransferase-the turning point in polyamine metabolism. FASEB J. 1993;7:653–61. [PubMed] [Google Scholar]
- 6.Huang Y, Pledgie AM, Casero RA, Davidson NE. Molecular mechanisms of polyamine analogues in cancer cells. Anti-Cancer Drugs. 2005;16:229–41. doi: 10.1097/00001813-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron RJ, Neims AH, McManis JS, Hawthorne TR, Vinson JR, Bortell R, Ingeno MJ. Synthetic polyamine analogues as antineoplastics. J Med Chem. 1998;31:1183–90. doi: 10.1021/jm00401a019. [DOI] [PubMed] [Google Scholar]
- 8.Porter CW, Bergeron RJ. Regulation of polyamine biosynthetic activity by spermidine and spermine analogs—a novel antiproliferative strategy. Adv Exp Med Biol. 1998;250:677–90. doi: 10.1007/978-1-4684-5637-0_60. [DOI] [PubMed] [Google Scholar]
- 9.Casero RA, Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–33. [PubMed] [Google Scholar]
- 10.Casero RA, Jr, Woster PM. Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem. 2001;44:1–26. doi: 10.1021/jm000084m. [DOI] [PubMed] [Google Scholar]
- 11.Bacchi CJ, Weiss LM, Lane S, Frydman B, Valasinas A, Reddy V, Sun JS, Marton LJ, Khan IA, Moretto M, Yarlett N, Wittner M. Novel synthetic polyamines are effective in the treatment of experimental microsporidiosis, an opportunistic AIDS-associated infection. Antimicrob Agents Chemother. 2002;46:55–61. doi: 10.1128/AAC.46.1.55-61.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frydman B, Porter CW, Maxuitenko Y, Sarkar A, Bhattacharya S, Valasinas A, Reddy VK, Kisiel N, Marton LJ, Basu HS. A novel polyamine analog (SL-11093) inhibits growth of human prostate tumor xenografts in nude mice. Cancer Chemother Pharmacol. 2003;51:488–92. doi: 10.1007/s00280-003-0598-8. [DOI] [PubMed] [Google Scholar]
- 13.Valasinas A, Reddy VK, Blokhin AV, Basu HS, Bhattacharya S, Sarkar A, Marton LJ, Frydman B. Long-chain polyamines (oligoamines) exhibit strong cytotoxicities against human prostate cancer cells. Bioorg Med Chem. 2003;11:4121–31. doi: 10.1016/s0968-0896(03)00453-x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas T, Thomas TJ. Regulation of cyclin B1 by estradiol and polyamines in MCF-7 breast cancer cells. Cancer Res. 1994;54:1077–84. [PubMed] [Google Scholar]
- 15.Kramer DL, Vujcic S, Diegelman P, Alderfer J, Miller JT, Black JD, Bergeron RJ, Porter CW. Polyamine analogue induction of the p53-p21WAF1/CIP1-Rb pathway and G1 arrest in human melanoma cells. Cancer Res. 1999;59:1278–86. [PubMed] [Google Scholar]
- 16.McCloskey DE, Casero RA, Woster PM, Davidson NE. Induction of programmed cell death in human breast cancer cells by an unsymmetrically alkylated polyamine analogue. Cancer Res. 1995;55:3233–6. [PubMed] [Google Scholar]
- 17.Vujcic S, Halmekyto M, Diegelman P, Gan G, Kramer DL, Janne J, Porter CW. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J Biol Chem. 275:38319–28. doi: 10.1074/jbc.M003270200. 20000. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Hager ER, Phillips DL, Dunn VR, Hacker A, Frydman B, Kink JA, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Davidson NE. A novel polyamine analog inhibits growth and induces apoptosis in human breast cancer cells. Clin Cancer Res. 2003;9:2769–77. [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Keen J, Hager ER, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Casero RA, Davidson NE. Regulation of polyamine analogue cytotoxicity by c-Jun in human cancer MDA-MB-435 Cells. Mol Cancer Res. 2004;2:81–8. [PubMed] [Google Scholar]
- 20.Saab MH, West EE, Bieszk NC, Preuss C, Mank AR, Casero RA, Jr, Woster PM. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine/spermine-N1-acetyltransferase (SSAT) and as potential anti-tumor agents. J Med Chem. 1993;36:2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 21.Rubin E, Mittnacht S, Villa-Moruzzi E, Ludlow JW. Site-specific and temporally-regulated retinoblastoma protein dephosphorylation by protein phosphatase type 1. Oncogene. 2001;20:3776–85. doi: 10.1038/sj.onc.1204518. [DOI] [PubMed] [Google Scholar]
- 22.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;19:296, 550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Fan W. IkB Kinase Activation is involved in the regulation of paclitaxel-induced apoptosis in human tumor cell Lines. Mol Pharmacol. 2002;61:105–13. doi: 10.1124/mol.61.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Swanton C. Cell-cycle targeted therapies. Lancet Oncol. 2004;5:27–36. doi: 10.1016/s1470-2045(03)01321-4. [DOI] [PubMed] [Google Scholar]
- 25.Seidenfeld J, Gray JW, Marton LJ. Depletion of 9L rat brain tumor cell polyamine content by treatment with D,L-alpha-difluoromethylornithine inhibits proliferation and the G1 to S transition. Exp Cell Res. 1981;131:209–16. doi: 10.1016/0014-4827(81)90420-1. [DOI] [PubMed] [Google Scholar]
- 26.Heby O, Sarna GP, Marton LJ, Omine M, Perry S, Russell DH. Polyamine content of AKR leukemic cells in relation to the cell cycle. Cancer Res. 1973;33:2959–64. [PubMed] [Google Scholar]
- 27.Kramer DL, Fogel-Petrovic M, Diegelman P, Cooley JM, Bernacki RJ, McManis JS, Bergeron RJ, Porter CW. Effects of novel spermine analogues on cell cycle progression and apoptosis in MALME-3M human melanoma cells. Cancer Res. 1997;57:5521–7. [PubMed] [Google Scholar]
- 28.Webb HK, Wu Z, Sirisoma N, Ha HC, Casero RA, Jr, Woster P. 1-(N-alkylamino)-11-(N-ethylamino)-4,8-diazaundecanes: simple synthetic polyamine analogues that differentially alter tubulin polymerization. J Med Chem. 1999;42:1415–21. doi: 10.1021/jm980603+. [DOI] [PubMed] [Google Scholar]
- 29.Bettuzzi S, Astancolle S, Guidetti G, Moretti M, Tiozzo R, Corti A. Clusterin (SGP-2) gene expression is cell cycle dependent in normal human dermal fibroblasts. FEBS Lett. 1999;448:297–300. doi: 10.1016/s0014-5793(99)00375-0. [DOI] [PubMed] [Google Scholar]
- 30.Ray RM, Zimmerman BJ, McCormack SA, Patel TB, Johnson LR. Polyamine depletion arrests cell cycle and induces inhibitors p21(Waf1/Cip1), p27(Kip1), and p53 in IEC-6 cells. Am J Physiol. 1999;276:C684–91. doi: 10.1152/ajpcell.1999.276.3.C684. [DOI] [PubMed] [Google Scholar]
- 31.Marty C, Mori G, Sabini L, Rivarola V. Effects of alpha-difluoromethylornithine on the cyclin A expression in Hep-2 cells. Biocell. 2000;24:49–52. [PubMed] [Google Scholar]
- 32.Kramer DL, Chang BD, Chen Y, Diegelman P, Alm K, Black AR, Roninson IB, Porter CW. Polyamine depletion in human melanoma cells leads to G1 arrest associated with induction of p21WAF1/CIP1/SDI1, changes in the expression of p21-regulated genes, and a senescence-like phenotype. Cancer Res. 2001;61:7754–62. [PubMed] [Google Scholar]
- 33.Mitchell JL, Leyser A, Holtorff MS, Bates JS, Frydman B, Valasinas AL, Reddy VK, Marton LJ. Antizyme induction by polyamine analogues as a factor of cell growth inhibition. Biochem J. 2002;366:663–71. doi: 10.1042/BJ20011612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–11. [PubMed] [Google Scholar]
- 35.Vousden KH. p53: death star. Cell. 2000;103:691–4. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]