Abstract
Aims and setting
Descriptions of behavioural epidemics have received little attention compared with infectious disease epidemics in Eastern Europe. Here we report a study aimed at estimating trends in the prevalence of injection drug use between 2005 and 2009 in Estonia.
Design and methods
The number of injection drug users (IDUs) aged 15–44 each year between 2005 and 2009 was estimated using capture-recapture methodology based on 4 data sources (2 treatment data bases: drug abuse and non-fatal overdose treatment; criminal justice (drug related offences) and mortality (injection drug use related deaths) data). Poisson log-linear regression models were applied to the matched data, with interactions between data sources fitted to replicate the dependencies between the data sources. Linear regression was used to estimate average change over time.
Findings
there were 24305, 12292, 238, 545 records and 8100, 1655, 155, 545 individual IDUs identified in the four capture sources (Police, drug treatment, overdose, and death registry, accordingly) over the period 2005 – 2009. The estimated prevalence of IDUs among the population aged 15–44 declined from 2.7% (1.8–7.9%) in 2005 to 2.0% (1.4–5.0%) in 2008, and 0.9% (0.7–1.7%) in 2009. Regression analysis indicated an average reduction of over 1700 injectors per year.
Conclusion
While the capture-recapture method has known limitations, the results are consistent with other data from Estonia. Identifying the drivers of change in the prevalence of injection drug use warrants further research.
Keywords: injection drug user, IDU, prevalence, Eastern Europe, HIV
INTRODUCTION
There is evidence that both risk behaviours (high risk sexual behaviour, illicit drug use) and related infectious diseases (sexually transmitted and blood borne infections) are constantly evolving in the interplay between social-environmental factors, pathogens and their hosts (Bello et al., 2011). In an epidemic outbreak of an infectious disease the prevalence in a particular population does not grow indefinitely, but saturates at some level. Following the initial spread there is generally a fall in the incidence followed by a reduction in prevalence. The same is likely to apply to behavioral epidemics. There are similarities between the spread of drug use, in particular the spread of the use addictive drugs (such as heroin), and that of infectious diseases. The use of drugs is communicated, not as an organic agent, but as a kind of “innovative” social practice or custom, and not to everyone but only to those who, for whatever reason, are not immune (i.e. susceptible individuals) (Rossi, 2002). Behavioural epidemics have received little attention compared with infectious disease epidemics in Eastern Europe.
Estonia is a small country in the northern-eastern part of Europe with a population of about 1,340,000 (Statistics Estonia, 2012). According to a global review of injection drug use and HIV epidemiology, Estonia has one of the highest prevalences of injection drug users (IDUs) among people aged 15–64 years (1.51% in 2007) coupled with a high HIV prevalence among IDUs (Uusküla et al., 2008; Mathers et al., 2008). We have previously estimated the prevalence of IDU among ages 15–44 in Estonia to be 2.4% in 2004 (Uusküla et al., 2007). Capture-recapture (CRC) is an indirect method that estimates population size from the degree of overlap between two or more separate samples from a population (Hook & Regal, 1995). The method calculates the extent to which the same individuals appear in datasets from different sources, and extrapolates from this to estimate the number of individuals who do not appear in any of the sources. Several assumptions are made when using CRC: the population is closed; overlaps between datasets can accurately be identified; the samples are independent or multiple sources are used to account for dependencies; all members of the population have an equal probability of occurring in any of the sources; representative samples of the population can be obtained (Millar et al., 2008). As injection drug use encompasses criminal and health problems, CRC studies should obtain data from both criminal justice and healthcare sources to target the population (Hickman et al., 2001).
We have used CRC methodology to estimate the number of IDUs in the 15–44 year age range in Estonia for each year between 2005 and 2009and to examine the trend in prevalence of injection drug users during this period.
METHODS
DATA SOURCES AND DEFINITIONS
Our target population was men and women aged 15–44 who were residents in Estonia and injecting drugs at some time between 2005 and 2009. Series of cross-sectional studies conducted in several location and across the period of 2004 to 2011 have documented that overwhelming majority (~ 95%) of IDUs in Estonia are of age 15–44 (Talu et al., 2009; Uusküla et al., 2012). We used four data sources: 1) police data on drug-related offences, 2) data on drug treatment from the Estonian Health Insurance Fund (EHIF), 3) data on drug overdoses from EHIF, and 4) data about injection drug use related deaths from the Estonian Causes of Death Registry (ECDR). Each data source is described below and the main characteristics are summarized in Table 1.
TABLE 1.
Data sources, descriptions of abstractions and definitions
| Police: POLIS database | Estonian Health Insurance Fund | Estonian Causes of Death Registry | |
|---|---|---|---|
| Coverage | National | National | National |
| Information recorded |
|
||
| Definition of IDU | Cases of drug-related offences (i.e. unlawful acquisition or storage of small quantities of narcotic drugs or psychotropic substances, or use of narcotic drugs or psychotropic substances without doctor's prescription) (drug-related misdemeanours: Article §151 of the Act on Narcotic Drugs and Psychotropic Substances and Precursors) | In/out-patient treatment (billing) episodes/records for health conditions coded according to ICD-10 as Drug treatment F11.0-F11.9 Overdose (life threatening non-fatal) T40 (T40.0, T40.1, T40.2) |
Deaths coded (ICD-10) in relation to the use of narcotics T40.2, T40.3, T40.4 or T43.6 (if heroin, morphine, methadone, or 3-methyl/fentanyl were listed as putative drugs detected) Deaths from the year following the reference year were used (i.e. for the IDU prevalence estimation in 2007 deaths from 2008 were used) |
To differentiate injection drug users from non injection drug users among case files we limited the data abstraction to health events (overdose, drug treatment, death) related to the opiates use. This evidence is supported by the data from the Estonian drug treatment database. According to the data from 2010 only 5.6% of persons receiving drug treatment and admitting opiates use (heroin, fentanyl, poppy liquid, other opiates) have never injected drugs (personal communication Dr. Kaire Vals, administrator, Estonian drug treatment database). Further, attribution of non-fatal overdoses and deaths related to the opiates use being associated with injection drug use is confirmed in the discussions with the health care/prevention services providers and Estonian Forensic Science Institute researchers (Denissov, 2012; personal communication Dr. Marika Väli, Deputy Director of Estonian Forensic Science Institute). For Police data – preparatory key informants (drug users, police officials) interviews documented that 90 % of the drug users arrested/detained at the Ida-Virumaa county and close to 80% in Tallinn area (Harjumaa county) are injection drug users (personal communication, Ave Talu, Estonian Drug Monitoring Center).
Police: drug-related offences
Data on drug-related offences detected and registered by the police was abstracted from the POLIS database which is a national database comprising data on all registered crimes. According to police information, the POLIS database is relatively complete due to the mandatory character of reporting, technical control and regular auditing. However, POLIS is an administrative database and does not record details on routes of drug administration (i.e. injected or other). We defined IDUs as people registered in the database for the unlawful acquisition, storage, or use of small quantities of narcotic drugs or psychotropic substances without a doctor's prescription.
Estonian Health Insurance Fund (EHIF): drug treatment and overdoses
Healthcare in Estonia is funded through a compulsory scheme under which employers are obliged to fund health insurance for their employees. In addition, certain groups (i.e. pregnant women, persons under 19 years of age, persons receiving an Estonian state pension) do not pay but are covered by the insurance. As of 31 December 2010, EHIF had 1,256,240 members representing 93.7% of the Estonian population (Estonian Health Insurance Fund, 2010). Emergency care (i.e in the case of drug overdose) is free and covered for everyone in need irrespective of the health insurance status. It is important to note, however, that studies conducted in Estonia among IDUs found less than 50% health insurance coverage in this group (Vorobjov et al., 2012). The EHIF database was used to construct a data source of people who received treatment related to the use of opioids (International Classification of Diseases, tenth edition (ICD-10) codes F11.0-F11.9) and a separate data source of people who had an opioid overdose requiring emergency care (ICD-10 T40.0, T40.1, T40.2) from 2005 to 2009.
Estonian Causes of Death Registry (ECDR) (E): Injection drug use related deaths
ECDR is developed in collaboration between Statistics Estonia and the National Institute for Health Development, containing individual data from 1983 and onwards. Our study considered all deaths deemed after autopsy to be due to an acute reaction to an opiate and confirmed by the pathologist's report on the basis of organic samples (blood and/or tissue), including ICD-10 codes T40.2, T40.3, T40.4, and T43.6 if heroin, morphine, methadone, or fentanyl/3-methyl-fentanyl had been detected.
MATCHING
In all data sets an identification (ID) variable consisting of initials of first name and surname, full date of birth (day/month/year) and gender was constructed. Additionally, from the police and EHIF database an encoded identification code (which does not permit personal identification or linking to other registries) was obtained. This code could not be used for matching purposes, but allowed us to identify different records belonging to the same individuals within the respective data sets, and thus assess the quality of the constructed ID variable. The persons’ identity remained unknown to us at all times.
The data sets from police and EHIF were originally composed of episodes and not individuals. Unique individuals were identified by the ID variable and one record per person was kept for each year. Records belonging to individuals under 15 or over 44 were removed. The ID variable was then used for cross-matching between the data sources. Individuals cannot appear in any other data source after death and this therefore violates the requirement for defining a closed population. Therefore, when estimating the number of IDUs in a given year, deaths from the following year were used (e.g. for the 2007 estimation, we used deaths from 2008).
ANALYSES
We evaluated the dependence among sources using the Poisson log-linear model for complete data. Data from each year of observation were arranged in the 24 incomplete multiway contingency table with one missing cell corresponding to absence in all sources. Log-linear models were fitted to this contingency table to estimate the number of missing cases, taking into account the pattern of association between sources. The table has 15 observations (corresponding to the presence in one, two, three or four sources). Any model may contain up to 14 terms: four independence terms (each corresponding to the main effect of a source), six first-order interaction terms (each corresponding to the interaction effect between two sources) and four second-order interaction terms (corresponding to the interaction effect between three of the considered sources). Interaction terms represent different aspects of the dynamics of the population or of its response to the sampling procedure (Bishop et al., 1975). This means that if four independence terms and a first order interaction term are considered in a model, the corresponding estimate of the number of missing cases is adjusted for dependence between the two sources. For the data from each year, we fitted a model with four independence terms (each corresponding to the main effect of a source) and all six first order interaction terms (each corresponding to the interaction effect between two sources).The interaction terms were then removed one by one and the model with the smallest variance (based on the AKAIKE'S (AIC) standard information criteria) was chosen to cover the missing cell (i.e. the number of IDUs not captured by any data source), thus yielding an estimate of the total population size with 95% CI (data on Bayesian information criteria (BIC) and deviance are also presented in the Table 4).
TABLE 4.
Total population estimates from the capture-recapture analysis
| CRC estimate | df | Dev | AIC | BIC | |
|---|---|---|---|---|---|
| 2005 | |||||
| Main effects | 7034 (3522, 12087) | 6 | 18.7 | 7.6 | 4.3 |
| All pairwise interactions | 25135 (19405, 112042) | 1 | 0.90 | 6.9 | −1.5 |
| Best estimate by AIC | 15675 (10230, 46018)A1 | 3 | 3.17 | 6.7 | −4.0 |
| 2006 | |||||
| Main effects | 6429 (3650, 10241) | 6 | 12.2 | 7.3 | −2.2 |
| All pairwise interactions | 10323 (7763, 31288) | 1 | 0.54 | 7.1 | −1.9 |
| Best estimate by AIC | 7989 (4697, 15031)A2 | 3 | 1.94 | 6.9 | −5.3 |
| 2007 | |||||
| Main effects | 7073 (3958, 11219) | 8 | 24.4 | 7.6 | 3.9 |
| All pairwise interactions | 15126 (10431, 44020) | 2 | 1.45 | 6.7 | −3.7 |
| Best estimate by AIC | 7182 (3978, 11462)A3 | 6 | 9.30 | 6.7 | −6.1 |
| 2008 | |||||
| Main effects | 7343 (4084, 11618) | 6 | 26.2 | 8.8 | 11.9 |
| All pairwise interactions | 11493 (7843, 28518) | 1 | 0.76 | 7.4 | −1.6 |
| Best estimate by AIC | 11493 (7843, 28518)A4 | 1 | 0.76 | 7.4 | −1.6 |
| 2009 | |||||
| Main effects | 9862 (3865, 18157) | 5 | 48.0 | 11.0 | 36.5 |
| All pairwise interactions | 7795 (6720, 30911) | 0 | 0.00 | 7.2 | 0.0 |
| Best estimate by AIC | 5362 (3906, 9837)A5 | 3 | 2.83 | 6.9 | −4.1 |
Interaction terms included in the models
Police*Treatment, Police*Deaths, Treatment*Overdose
Police*Treatment, Treatment*Overdose, Treatment*Deaths
Treatment*Overdose, Overdose*Deaths
Police*Treatment, Police*Overdose, Police*Deaths, Treatment*Overdose, Treatment*Deaths
Police*Treatment, Police*Deaths
The prevalence rate (hereafter: prevalence) of injection drug users in the population was then calculated using the official population aged 15 to 44 as the denominator (Statistics Estonia, 2012). The trend in prevalence of injection drug users over the period of observation was estimated with linear regression analysis.
RESULTS
The four capture sources are described in Table 2. Gender was not recorded in 0.3% of records in the POLIS database,and these were therefore excluded from the analyses. In the other data sets, full identification was possible for all records. Comparison of the ID variable with the databases’ own ID code revealed that 19 persons in the police and 4 persons in the EHIF data set had identical characteristics to another person but the database’s inner ID indicated that they were different persons. When this occurred, one of the two was randomly deleted from the data set. There were no cases of the same person (according to the inner/within data source identification code) getting different ID variables in any of the sources.
TABLE 2.
Total number of records, and the number of individual IDU cases defined in the capture sources used and selected characteristics of IDU cases (aged 15–44) in the capture sources for the period of 2005–2009
| Data source | Police | EHIF: drug treatment |
EHIF: overdose |
Mortality Database |
|---|---|---|---|---|
|
Total number of records identified as IDUs (for definition see Table 1) |
24305 | 12292 | 238 | 560 |
| % of records where full identification was possible (initials, gender, day/month/year of birth) |
99.7% | 100% | 100% | 100% |
| Number of individual IDU cases | 8331 | 1811 | 188 | 560 |
| Number of individuals defined as IDUs aged 15–44 (n, % of all) |
8100 (97.2%) |
1655 (91.4%) |
155 (82.4%) |
545 (97.3%) |
|
Number of individual IDUs aged 15–44 years identified |
||||
| 2005 | 2484 | 407 | 40 | 81 |
| 2006 | 2808 | 524 | 29 | 108 |
| 2007 | 3018 | 661 | 55 | 103 |
| 2008 | 3090 | 722 | 43 | 152 |
| 2009 | 2100 | 758 | 49 | 101 |
|
Age: mean (SD) (in the age group of 15– 44 years) |
||||
| 2005 | 24.1 (5.6) | 24.8 (5.2) | 25.3 (7.0) | 26.1 (4.9) |
| 2006 | 24.3 (5.7) | 25.8 (5.3) | 24.4 (5.7) | 26.8 (4.9) |
| 2007 | 24.9 (5.8) | 26.7 (5.0) | 24.7 (5.9) | 28.1 (5.7) |
| 2008 | 25.0 (5.8) | 27.5 (5.2) | 24.9 (5.1) | 28.5 (4.7) |
| 2009 | 25.4 (5.8) | 28.7 (5.2) | 28.5 (5.2) | 29.1 (5.2) |
| p-value * | <0.01 | <0.01 | 0.02 | <0.01 |
|
Proportion of residents in Tallinn area (Harjumaa county) (%) (in the age group of 15–44 years) |
||||
| 2005 | 72.3 | 50.9 | 55.0 | 80.3 |
| 2006 | 68.0 | 51.5 | 55.2 | 65.7 |
| 2007 | 63.6 | 49.0 | 38.2 | 83.5 |
| 2008 | 57.3 | 46.0 | 60.5 | 73.0 |
| 2009 | 57.7 | 42.4 | 55.1 | 73.3 |
| p-value * | <0.01 | <0.01 | 0.99 | 0.27 |
P-value for trend
The numbers of estimated IDU cases from 2005 to 2009 by data source is presented in Table 2. Over this period, the number of people receiving drug treatment increased (from 407 in 2005 to 758 in 2009). There was a sizeable decline in the number of people in the police database at the end of the observation period (from 3090 in 2008 to 2100 in 2009). Over the study period, the mean age increased with each year in all data sources, except for overdoses (Table 2). The proportion of females was highest among the drug treatment and overdose data sets, and lowest in the police data set. The proportion of females varied during the years, without any clear trend.
The number of individual IDU cases and the overlaps between the data sources are presented in Table 3. Each year, some individuals occurred in up to three capture sources but no individuals occurred in all four sources. The number of overlaps was fairly stable. CRC estimates of the total population are listed in Table 4. For each year, the results from the independence model (main effects only), the model with all first order interactions, and the best model by AIC are presented. Given that including interaction terms into the model in most of the cases/years inflated the prevalence estimates suggests positive dependence between the data sources.
TABLE 3.
Numbers of individual IDU cases identified in each of the capture sources (Police, Health Insurance Fund: drug treatment, Health Insurance Fund: overdoses, Mortality database) and overlaps among the capture sources
| Year | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|
| In 1 capture source (n, %) | 2637 (93.5%) | 2889 (91.0%) | 3138 (90.2%) | 3234 (89.6%) | 2614 (93.1%) |
| In 2 capture sources (n, %) | 177 (6.3%) | 275 (8.7%) | 324 (9.3%) | 355 (9.8%) | 191 (6.8%) |
| In 3 capture sources (n, %) | 7 (0.2%) | 10 (0.3%) | 17 (0.5%) | 21 (0.6%) | 4 (0.1%) |
| Total number of captured individuals | 2821 | 3174 | 3479 | 3610 | 2809 |
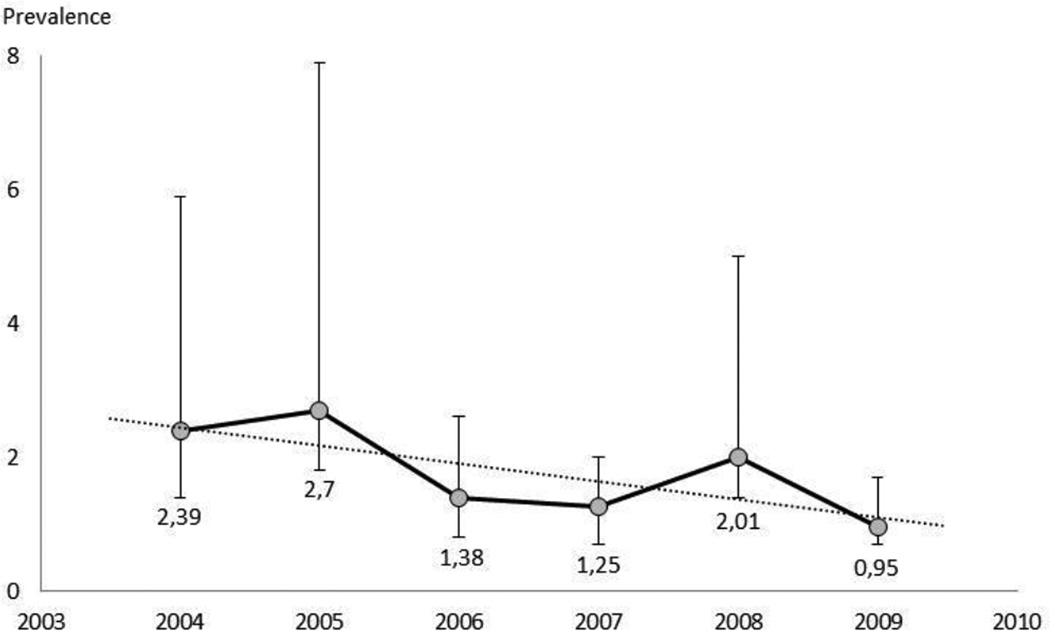
The estimated size of the total IDU population according to the best model decreased from 15675 (95% CI 10230 to 46018) in 2005 to 5362 (3906 to 9837) in 2009. The corresponding estimates of IDU prevalence are presented in Figure 1. According to our estimates, the IDU population decreased by 56% over the study period (from 14262 in 2004 to 6266 in 2009; p=0.095). Regression analysis indicated a yearly decrease in the number of IDUs (n=1599) and IDU prevalence (0.27%).
Figure 1. Estimated injection drug use prevalence (%) in 15–44 year age group in Estonia.
Prevalence estimate for 2004 from Uusküla et al 2007 [42]
DISCUSSION
This is the first study from Eastern Europe designed to describe the course of the injection drug users epidemic. There was a reduction in the estimated number of injection drug users over time. An estimated number of the injection drug users in Estonia were 15675, 11493 and 5362 for the years 2005, 2008 and 2009, accordingly. These estimates translate as a prevalence of IDUs among the population aged 15–44 in Estonia 2.7% (1.8–7.9%) in 2005 to 2.0% (1.4–5.0%) in 2008, and 0.9% (0.7–1.7%) in 2009.
It is not possible to compare these estimates with those of other studies directly. Still, these findings agree with a model estimating the numbers of new cases of HIV among IDUs in the Estonian capital, Tallinn. According to the current analysis and previous research (Uusküla et al., 2007) about two thirds of IDUs in Estonia reside in the Tallinn area. The HIV incidence model (Pinkerton, 2010) was based on the exchange of over 700,000 syringes per year in Tallinn (Uusküla et al., 2011), and estimated an incidence of 4% to 5% per year in a population of 6000 injectors with 50% HIV prevalence, leading to 120 to 150 new infections per year. If the injecting population in Tallinn area were 10,000 (no decline since 2004; (Uusküla et al., 2007), then we would expect 200 to 250 new infections per year. The actual number of newly reported HIV infections in Tallinn is approximately 180, over half of whom are likely to be IDUs (Rüütel et al., 2011). Thus, the modeling is consistent with a considerable (approximately 50%) reduction in the IDU population in Tallinn rather than a stable population of 10,000 injectors in the city (Des Jarlais et al. in press Advances in Preventive Medicine 2012).
Further, the reduction in the numbers of IDUs is consistent with a series of cross-sectional studies in Estonia over the study period that used respondent-driven sampling and which showed a significant reduction in the number of new injectors (the proportion of those starting injecting up to 3 years before the interview has decreased from 21% of the sample in 2005 to 8% in 2011) (Uusküla et al., 2011).
It is important to note that from 2005 to 2010, an increase in drug-related deaths: from 4.2 to 7.5/100 000 (Tuusov et al., 2012; National Institute for Health Development, 2012), and AIDS-related deaths: from 2.5 to 3.1/100 000 (Statistics Estonia, 2012) has been documented. We found a slight increase in the estimated numbers of IDUs in the overdose and mortality data sources. These observations are consistent with the increasing age of IDUs, and high prevalence of HIV (~50%) among IDUs in Estonia (Uusküla et al., 2008; Vorobjov et al., 2011; Uusküla et al., 2011). Further, injecting specific drugs - (synthetic, designer-drugs) fentanyles – has been attributed to the increased number on overdoses and overdose deaths among IDUs in Estonia (Tuusov et al., 2012; Talu et al., 2009; Wilson, 2012). In 2009, 87% and in 2011, 80% of illicit drug related deaths have been related to the use of fentanyles (Denissov, 2012). Because fentanyl is extremely potent by weight, small variations in the illicit production or processing of the drug may lead to large variations in the effective doses, increasing the likelihood of overdoses. The data on overdoses indicate that community-based distribution of naloxone may be effective in reducing fatal overdoses (MMWR, 2012).
As with all CRC analyses, the main source of inaccuracy lies in the uncertainty of the estimates. The number of IDUs identified in all four datasets was relatively small and wide confidence intervals were observed around estimates means that results obtained with a regression of central estimates should be interpreted with caution (Hickmann et al., 1999). Our data do not support attribution of the observed decline in the estimated prevalence of IDUs only to the changes in the numbers of IDUs captured in the different sites or to increases in the overlaps. There have been no significant changes in “capture procedures” (service provision or administrative changes) during the study period in the EHIF or ECDR. However, we are aware of some changes in police priorities which could affect data capture, namely, an increased focus on criminal gangs involved in organized crime and drug supply and reduced attention on individual drug users and small-scale suppliers (potentially leading to under-registration of drug-related misdemeanours) (National instituute for Health Development, 2012).
The illegal nature of drug use may also reduce the accuracy of the CRC method in this setting (Domingo-Salvany et al., 1998). The first consideration is case definition, as most sources tend to provide cases whose conditions are more severe than those of the average drug user. However, since this aspect is constant over time it should not affect temporal trends, therefore the observed decrease in IDU prevalence over the study period is likely to be valid. The police database may include drug dealers who are not users/injectors themselves. However, it is reasonable to assume that an overwhelming majority of those convicted of the acquisition or storage of small quantities of narcotic drugs are drug users. The IDU status in the EHIF and Death registry capture sources were defined using ICD-10 classification codes for opiate use, in an attempt to identify injection drug users as specifically as possible (Papoz et al., 1996). According to published data, opioids (home-made opioids, heroin or fentanyl/analogues) are the main group of illicit drug used (Vorobjov et al., 2012) and administration other than by injection is rare. Further, the proportion of IDUs injecting only stimulants (rather than opioids) is less than 8% according to a series of cross-sectional studies conducted in Tallinn from 2005–9 that used respondent-driven sampling (Uusküla et al., 2008; Vorobjov et al., 2012; Uusküla A unpublished data), and deaths related to stimulant use have contributed less than 7% of the total of drug-related deaths in Estonia (Tuusov et al., 2012). Another requirement for CRC studies is a closed population. However, drug addiction may be considered a chronic condition which many persons may temporarily cease using drugs only to relapse later, and some of whom may die from the condition or other causes (Domingo-Salvany et al., 1995). To minimize these problems, we used a relatively short (1 year) reference period for yearly prevalence estimations.
Bias can also arise from inability to match data between sources due to poor data quality. Still, police, EHIF and ECDR records are reliable sources with high quality identifier information as we confirmed by comparing the constructed ID variable with the databases’ inner ID code. False negative and false positive matching resulting in over- or underestimates are thus not probable. The appropriateness of using data from these sources is further supported by the small number of records that had to be excluded due to missing information (0.3% of the police records).
Another consideration, independence between samples, may be intrinsic to the "captures" in the strict sense or may reflect a failure to meet the requirements for homogeneity and a closed population. In the current study, the use of four samples – police, two EHIF and the Death registry as capture sources – reduces the importance of the independence assumption. To account for any dependencies between the data sources, the models were explored with 2-factor interactions as suggested by Cormack 2007 (Cormack, 1999).
The assumption that each member of the population should have an equal, non-zero probability of being “captured” might be violated. Several possible entry points provide preferential access to one subset of IDUs compared to another. A significant proportion of IDUs (> 50%) (Uusküla et al., 2008; Vorobjov et al, 2012) have virtually no chance to enter the EHIF drug treatment dataset, as they are not covered by health insurance. However, the chance of IDUs ending up in the EHIF ‘overdose’ dataset should be equally distributed, as emergency care in Estonia is freely provided for everyone in need.
Reliable identification of individuals is essential to accurately quantify the recapture cases. The quality and reliability of the data sources used was discussed above. We assume that the six identifiers (gender, day/month/year of birth, and initials) we used were sufficient to provide a true match, although it is possible that such a strict matching procedure could miss true matches if there were slight discrepancies or errors in the identifiers.
Further, the assumption of homogeneity is important as behaviours may be related to available resources and hence affect capture probabilities. Although our study covered a whole country, both available resources (health care) and response policy (Police) are distributed uniformly throughout the country. In addition, results from other studies of drug users (Domingo-Salvany et al., 1995; Frischer et al., 1991) have suggested that heterogeneity does not seriously affect the results. We could not explore heterogeneity by gender or age group as the numbers in each stratum were too small.
It has been recommended that a wide variety of sources be sought in an attempt to cover as many different areas as possible (Hook & Regal, 1995). This aspect is important to consider when using the capture-recapture method to estimate populations that may escape routine sources of information. Mortality data have been used in CRC studies (Morrison & Stone, 2000), including in those estimating the size of an IDU population (Domingo-Salvany et al., 1998).
Last but not least, variability in year to year estimates is a potential limitation but we do not believe that there were meaningful variations in the data used, with the possible exception of changes in police policy for the last year.
There is a tendency for epidemics of certain diseases to synchronize across a population, sometimes producing strong oscillations in the number of affected individuals over time. Such effects are well known for diseases including measles (Grenfell et al., 2001; He & Stone, 2003) and syphilis (Grassly et al., 2005). The timing of syphilis cycles fits well with our understanding of the immune response associated with the disease (Easley et al., 2010). A cyclical course of drug use epidemics has been described as well (Kozel ja Adams, 1986; Johnston et al., 2004). The United States has experienced several cycles of heroin epidemics: the first began after World War II, with the highest incidence occurring in the late 1940s and early 1950s, and the second began in the late 1960s, with the highest incidence occurring between 1971 and 1977 (Hughes & Rieche, 1995) and another occurred in the 1990s (Schulden et al., 2009). In Europe, heroin use was first described as an “epidemic” in the late 1960s and early 1970s, and (injecting) drug use levels increased dramatically during the 1980s and 1990s (Kozel & Adams, 1986; DuPont & Greene, 1973; European Monitoring Center for Drugs and Drug Addiction, 2010). In Eastern Europe injection drug use epidemics emerged in the 1990s and have led to the highest IDU prevalences reported across the globe (Mathers et al., 2008). Behrens et al hypothesize (Behrens et al., 2002) that cycles of drug use occur when the current generation of youth no longer remembers the adverse experiences of their forebears. This could explain the explosive growth of the IDU epidemics in Eastern Europe given that drug use was introduced to a naïve / susceptible population with no preexisting ‘immunity’ (memory of the adverse experiences of drug use).
We do not yet have a good explanation for the decline in the estimated numbers of persons who inject drugs in Estonia. However, the social/economic/political situation in which the injecting drug users epidemic first arose (the dissolution of the Soviet Union) has clearly improved. There may also have been a “community learning” effect, in which younger persons observed the harmful consequences of injecting drugs (including high rates of HIV infection) which then made younger persons much less likely to begin injecting.
CONCLUSIONS
The costs that illicit drugs impose on society are high and illicit drug use is notoriously difficult to control (Ryan, 1998; Room & Reuter, 2012). Research into the course of drug use epidemics and their drivers are important contributors to the knowledge needed to develop effective control interventions and policies.
This study, estimating IDU prevalence using CRC methodology documented a decrease in IDU prevalence over a 5-year period in Estonia. While acknowledging the limitations of the methodology used, we still believe that a real decrease in the prevalence of injection drug users has occurred. No interventions targeting IDU initiation curtailment or IDU cessation (i.e. opioid substitution therapy) were implemented at the public health scale in Estonia over the study period (Rüütel et al., 2011). Identifying the drivers of the observed decrease is an important and interesting question that warrants further research. Identification of these drivers could lead to programs to further decrease initiation into illicit drug injection.
ACKNOWLEDGEMENTS
The study was supported by the grant SF0180060s09 from the Estonian Ministry of Education and Research; by the R01 AI 083035 grant from the NIH (USA); and the EMCDDA grant No GA.12.RTX.007.1.0.
We thank Senior Inspector Ain Borodin and Chief Superintendent of the Police Work Department, Analysis and Planning Division Marilis Sepp; Ms Triin Tõrvand from the Estonian Health Insurance Fund; and the Head of the Estonian Causes of Death Registry Gleb Denissov for their valuable contributions.
Appendix 1
Overlaps matrix for data from four data sources (Police, drug treatment, overdose and death registry) used
| Police | Drug treatment |
Overdose | Deaths | 2005 | 2006 | 2007 | 2008 | 2009 |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | |||||
| 0 | 0 | 0 | 1 | 49 | 65 | 57 | 72 | 98 |
| 0 | 0 | 1 | 0 | 29 | 13 | 25 | 15 | 24 |
| 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| 0 | 1 | 0 | 0 | 254 | 280 | 368 | 419 | 581 |
| 0 | 1 | 0 | 1 | 2 | 5 | 3 | 13 | 0 |
| 0 | 1 | 1 | 0 | 3 | 3 | 6 | 1 | 6 |
| 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| 1 | 0 | 0 | 0 | 2305 | 2531 | 2688 | 2728 | 1911 |
| 1 | 0 | 0 | 1 | 27 | 31 | 32 | 52 | 2 |
| 1 | 0 | 1 | 0 | 4 | 10 | 15 | 21 | 16 |
| 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 0 | 141 | 226 | 267 | 268 | 167 |
| 1 | 1 | 0 | 1 | 3 | 7 | 9 | 15 | 1 |
| 1 | 1 | 1 | 0 | 4 | 3 | 7 | 6 | 3 |
| 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Total | 2821 | 3174 | 3479 | 3610 | 2809 | |||
| Present in one source (capture) | 2637 | 2889 | 3138 | 3234 | 2614 | |||
| Present in two sources (captures) | 177 | 275 | 324 | 355 | 191 | |||
| Present in three sources (captures) | 7 | 10 | 17 | 21 | 4 | |||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
There is no conflict of interest
REFERENCES
- 1.Bello G, Simwaka B, Ndhlovu T, Salaniponi F, Hallett TB. Evidence for changes in behaviour leading to reductions in HIV prevalence in urban Malawi. Sex Transm Infect. 2011;87(4):296–300. doi: 10.1136/sti.2010.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens DA, Caulkins JP, Tragler G, Feichtinger G. Why present-oriented societies undergo cycles of drug epidemics. Journal of Economic Dynamics and Control. 2002;26:919–936. [Google Scholar]
- 3.Bishop YMM, Fienberg SE, Holland PW. Discrete multivariate analysis: theory and practice. Cambridge, MA: MIT Press; 1975. pp. 229–256. [Google Scholar]
- 4.Bobkov A, Kazennova E, Selimova L, Bobkova M, Khanina T. A sudden epidemic of HIV type 1 among injecting drug users in the former Soviet Union: identification of subtype A, subtype B, and novel gagA/envB recombinants. AIDS Res Hum Retroviruses. 1998;14(8):669–676. doi: 10.1089/aid.1998.14.669. [DOI] [PubMed] [Google Scholar]
- 5.Borisenko KK, Tichonova LI, Renton AM. Syphilis and other sexually transmitted infections in the Russian Federation. Int J STD AIDS. 1999;10(10):665–668. doi: 10.1258/0956462991913240. [DOI] [PubMed] [Google Scholar]
- 6.Cormack RM. Problems with using capture-recapture in epidemiology: an example of a measles epidemic. J Clin Epidemiol. 1999;52(10):909–914. doi: 10.1016/s0895-4356(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 7.Denissov G. DRD Expert Meeting 12–13 Nov. Lisbon, Portugal: European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); 2012. Recent developments concerning the DRD Key Indicator in Estonia. [Google Scholar]
- 8.Domingo-Salvany A, Hartnoll RL, Maguire A, Suelves JM, Antó JM. Use of capture-recapture to estimate the prevalence of opiate addiction in Barcelona, Spain, 1989. Am J Epidemiol. 1995;141:567–574. doi: 10.1093/oxfordjournals.aje.a117472. [DOI] [PubMed] [Google Scholar]
- 9.Domingo-Salvany A, Hartnoll RL, Maguire A, Brugal MT, Albertín P, Caylà JA. Analytical considerations in the use of capture-recapture to estimate prevalence: case studies of the estimation of opiate use in the metropolitan area of Barcelona, Spain. Am J Epidemiol. 1998;148(8):732–740. doi: 10.1093/oxfordjournals.aje.a009694. [DOI] [PubMed] [Google Scholar]
- 10.DuPont RL, Greene MH. The dynamics of a heroin addiction epidemic. Science. 1973;181:716–722. doi: 10.1126/science.181.4101.716. [DOI] [PubMed] [Google Scholar]
- 11.Easley D, Kleinberg J. Networks, Crowds, and Markets: Reasoning about a Highly Connected World. Cambridge University Press; 2010. Chapter 21. [Google Scholar]
- 12.Estonian health insurance fund (EHIF) The insured by 31.12.2010 (Kindlustatute jaotus seisuga 31.12.2010) Retrieved 03rd Feb 2011 from http://www.haigekassa.ee/haigekassa/statistika/el.
- 13.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Trends in injecting drug use in Europe. Selected issue 2010. Retrieved 23rd May 2012 from http://www.emcdda.europa.eu/attachements.cfm/att_108590_EN_EMCDDA_SI10_injecting.pdf.
- 14.Frischer M, Bloor M, Finlay A, Goldberg D, Green S, Haw S. A new method of estimating prevalence of injecting drug use in an urban population: results from a Scottish city. Int J Epidemiol. 1991;20:997–1000. doi: 10.1093/ije/20.4.997. [DOI] [PubMed] [Google Scholar]
- 15.Grassly NC, Fraser C, Garnett GP. Host immunity and synchronized epidemics of syphilis across the United States. Nature. 2005;433:417–421. doi: 10.1038/nature03072. [DOI] [PubMed] [Google Scholar]
- 16.Grenfell BT, Bjornstad ON, Kappey J. Travelling waves and spatial hierarchies in measles epidemics. Nature. 2001;414:716–723. doi: 10.1038/414716a. [DOI] [PubMed] [Google Scholar]
- 17.He D, Stone L. Spatio-temporal synchronization of recurrent epidemics. Proc. Royal Soc. London. 2003;270:1519–1526. doi: 10.1098/rspb.2003.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickman M, Sutcliffe H, Sondhi A, Stimson GV. Surveillance of problem drug use in the UK: a review of a Regional Drug Misuse Database. J Public Health Med. 1999;21(3):271–277. doi: 10.1093/pubmed/21.3.271. [DOI] [PubMed] [Google Scholar]
- 19.Hickman M, Seaman S, de Angelis D. Estimating the relative incidence of heroin use: application of a method for adjusting observed reports of first visits to specialized drug treatment agencies. Am J Epidemiol. 2001;153(7):632–641. doi: 10.1093/aje/153.7.632. [DOI] [PubMed] [Google Scholar]
- 20.Hilliard N, Jenkins R, Pashayan N, Powles J. Informal knowledge transfer in the period before formal health education programmes: case studies of mass media coverage of HIV and SIDS in England and Wales. BMC Public Health. 2007;7:293. doi: 10.1186/1471-2458-7-293. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook EB, Regal RR. Capture-recapture methods in epidemiology: methods and limitations. Epidemiol Rev. 1995;17(2):243–264. doi: 10.1093/oxfordjournals.epirev.a036192. [DOI] [PubMed] [Google Scholar]
- 22.Hughes PH, Rieche O. Heroin epidemics revisited. Epidemiol Rev. 1995;17(1):66–73. doi: 10.1093/oxfordjournals.epirev.a036186. [DOI] [PubMed] [Google Scholar]
- 23.Ingram M. Russia fears rapid increase in HIV infection. BMJ. 1997;314(7097):1783. doi: 10.1136/bmj.314.7097.1781e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National Survey Results on Drug Use, 1975–2003. NIH Publication 04–5507. Bethesda, MD: National Institute on Drug Abuse (vol I); 2004. [Google Scholar]
- 25.Kozel HJ, Adams EH. Epidemiology of drug abuse: an overview. Science. 1986;234:970–974. doi: 10.1126/science.3490691. [DOI] [PubMed] [Google Scholar]
- 26.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 27.Millar T, Domingo-Salvany A, Eastwood C, Hay G. Glossary of terms relating to capture-recapture methods. J Epidemiol Community Health. 2008;62:677–681. doi: 10.1136/jech.2007.070433. [DOI] [PubMed] [Google Scholar]
- 28.MMWR. Community-based opioid overdose prevention programs providing naloxone – United States, 2010. Morb Mortal Wkly Rep. 2012;61(6):101–105. [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison A, Stone DH. Capture-recapture: a useful methodological tool for counting traffic related injuries? Inj Prev. 2000;6(4):299–304. doi: 10.1136/ip.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute for Health Development (NIHD) 2011 NATIONAL REPORT to the EMCDDA (using 2010’s data), by the Reitox National Focal Point ESTONIA. New developments, trends and in-depth information on selected issues. 2012 Retrieved 24 May 2012 from http://www.tai.ee/et/terviseandmed/uuringud/download/168.
- 31.Papoz L, Balkau B, Lellouch J. Case counting in epidemiology: limitations of methods based on multiple data sources. Int J Epidemiol. 1996;25(3):474–478. doi: 10.1093/ije/25.3.474. [DOI] [PubMed] [Google Scholar]
- 32.Pinkerton SD. Is Vancouver Canada's supervised injection facility cost-saving? Addiction. 2010;105(8):1429–1436. doi: 10.1111/j.1360-0443.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 33.Room R, Reuter P. How well do international drug conventions protect public health? Lancet. 2012;379(9810):84–91. doi: 10.1016/S0140-6736(11)61423-2. [DOI] [PubMed] [Google Scholar]
- 34.Rossi C. The role of dynamic modelling in drug abuse epidemiology. Bulletin on Narcotics. 2002;LIV:33–44. [Google Scholar]
- 35.Rüütel K, Trummal A, Salekesin M, Pervilhac C. Estonia: Analysis of Strategic Information: a case study. World Health Organization; 2011. Retrieved 23rd May 2012 from http://www.euro.who.int/__data/assets/pdf_file/0020/155630/e96096.pdf. [Google Scholar]
- 36.Ryan KF. Clinging to failure: the rise and continued life of US drug policy. Law and Society Review. 1998;32(1):221–242. [Google Scholar]
- 37.Schulden JD, Thomas Y, Compton WM. Substance Abuse in the United States: Findings From Recent Epidemiologic Studies. Curr Psychiatry Rep. 2009;11(5):353–359. doi: 10.1007/s11920-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Statistics Estonia. Retrieved 23rd May 2012 from www.stat.ee.
- 39.Tichonova L, Borisenko K, Ward H, Meheus A, Gromyko A, Renton A. Epidemics of syphilis in the Russian Federation: trends, origins, and priorities for control. Lancet. 1997;350(9072):210–213. doi: 10.1016/S0140-6736(97)01382-2. [DOI] [PubMed] [Google Scholar]
- 40.Tuusov J, Vals K, Tõnisson M, Riikoja A, Denissov G, Väli M. Fatal poisoning in Estonia 2000–2009. Trends in illegal drug-related deaths. Journal of Forensic and Legal Medicine. 2012;1:6. doi: 10.1016/j.jflm.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Uusküla A, Silm H, Vessin T. Sexually transmitted diseases in Estonia: past and present. Int J STD AIDS. 1997;8(7):446–450. doi: 10.1258/0956462971920505. [DOI] [PubMed] [Google Scholar]
- 42.Uusküla A, Rajaleid K, Talu A, Abel K, Rüütel K, Hay G. Estimating injection drug use prevalence using state wide administrative data sources: Estonia, 2004. Addiction research & theory. 2007;4:411–424. [Google Scholar]
- 43.Uusküla A, Kals M, Rajaleid K, Abel K, Talu A, Rüütel K. High-prevalence and high-estimated incidence of HIV infection among new injecting drug users in Estonia: need for large scale prevention programs. J Public Health (Oxf) 2008;30(2):119–125. doi: 10.1093/pubmed/fdn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uusküla A, Des Jarlais DC, Kals M, Rüütel K, Abel-Ollo K, Talu A. Expanded syringe exchange programs and reduced HIV infection among new injection drug users in Tallinn, Estonia. BMC Public Health. 2011;11:517. doi: 10.1186/1471-2458-11-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uusküla A, Abel-Ollo K, Markina A, McNutt LA, Heimer R. Condom use and partnership intimacy among drug injectors and their sexual partners in Estonia. Sex Transm Infect. 2012;88(1):58–62. doi: 10.1136/sextrans-2011-050195. [DOI] [PubMed] [Google Scholar]
- 46.Vorobjov S, Uusküla A, Des Jarlais DC, Abel-Ollo K, Talu A, Rüütel K. Multiple routes of drug administration and HIV risk among injecting drug users. J Subst Abuse Treat. 2012;42(4):413–420. doi: 10.1016/j.jsat.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson S. Synthetic drug fentanyl causes overdose boom in Estonia. BBC News Europe: 30. March 2012. 2012 ( http://www.bbc.co.uk/news/world-europe-17524945)