Abstract
The potentiating action of the flavonolignan, (-)-hydnocarpin, in combination with vincristine was evaluated in the 697 acute lymphoblastic leukemia cell line and a P-gp-expressing variant, 697-R. Vincristine at 3 nM caused nearly complete growth inhibition in 697 cells, versus a 17% growth inhibition in 697-R cells. When combined with (-)-hydnocarpin at concentrations of 10 and 5 µM, vincristine-mediated growth inhibition in the 697-R cells increased significantly over the sum of the individual agents to 72% (p ≤ 0.0001), and 41% (p = 0.0256), respectively. Vincristine at 1.5 nM (66% growth inhibition) and 0.75 nM (39% growth inhibition) combined with (-)-hydnocarpin at 10 µM (42% growth inhibition) in the 697 cells, caused a significant increase in growth inhibition to 83% (p = 0.03) and to 61% (p < 0.0001), respectively, when compared to vincristine treatment as a single agent. To investigate the mechanism for the vincristine re-sensitization caused by (-)-hydnocarpin, the P-gp inhibitory effect of (-)-hydnocarpin was evaluated.
Keywords: P-glycoprotein, multidrug resistance, (-)-hydnocarpin, vincristine
INTRODUCTION
Tumors may become resistant to chemotherapeutic agents over time, a phenomenon known as multidrug resistance (MDR). Once MDR appears, malignant tumors are unresponsive to antineoplastic agents even with increased doses (Ozben, 2006). MDR due to elevated expression of the transmembrane protein, P-glycoprotein (P-gp), encoded by the ABCB1 gene, renders many established oncology therapies inactive and hence remains a serious clinical problem (Longley, 2005).
Natural compounds derived from plants are being sought as potential P-gp modulating agents. Macrocyclic lathyrane diterpenes (e.g., latilagascenes A-F), isolated from Euphorbia species (Duarte et al., 2006; Duarte et al., 2007), and alkaloids (e.g., pervilleines A-F and tetrandrine), isolated from Erythroxylum pervillei and Stephania tetrandra, respectively, showed MDR reversal activity in vitro, in vivo, and in clinical trials (Mi et al., 2001; Chin et al., 2006; Sun et al., 2009).
Flavonoid derivatives such as amorphigenin and rotenone, and the flavonoids, chrysin, epigallocatechin, and formononetin have been shown to inhibit P-gp function effectively in MDR1 gene-transfected mouse lymphoma cells (L1210). Rotenone was also found to be an effective P-gp inhibitor using Colo320 P-gp-expressing human colon cancer cells (Molnár et al., 2010). Another example of interest in the flavonoids as potential MDR inhibitors is the report of an amine-linked flavonoid dimer that can resensitize a P-gp-expressing estrogen independent human breast cancer cell line (MDA435/LCC6MDR) to daunorubicin, doxorubicin, mitoxantrone, paclitaxel, vinblastine, and vincristine with EC50 values of 90 to 179 nM and a high therapeutic index of 574.3 (Chan et al., 2012). In vivo P-gp modulating activity of flavonoids such as biochanin A, morin, naringin, and quercetin has also been reported previously. Moreover, the increased bioavailability of anticancer drugs by several of these flavonoids is due to a dual inhibition of P-gp and the metabolizing enzyme, CYP3A (Alvarez et al., 2010).
(-)-Hydnocarpin has previously been isolated from the plants Brucea javanica, Hydnocarpus wightiana, Verbascum sinaiticum, and certain Berberis species (Sharma and Hall, 1991; Afifi et al., 1993; Guz and Stermitz, 2000; Stermitz et al., 2000; Stermitz et al., 2001; Reddy et al., 2005; Pan et al., 2009). Hydnocarpin cytotoxicity has been reported in numerous cancer cell lines (ED50 <10 µM) (Sharma and Hall, 1991; Afifi et al., 1993). In earlier work, (-)-hydnocarpin was found to be inactive (ED50 >10 µM) against the MCF-7 human breast cancer cell line, but when combined with bruceantin and bruceine A, it caused a 7- to 10-fold increase in the cytotoxic potency of the latter compounds, respectively (Pan et al., 2009). Hydnocarpin was also shown to possess free-radical scavenging activity and to inhibit the bacterial Staphylococcus aureus NorA MDR pump in vitro (Guz and Stermitz, 2000; Stermitz et al., 2000; Stermitz et al., 2001; Reddy et al., 2005).
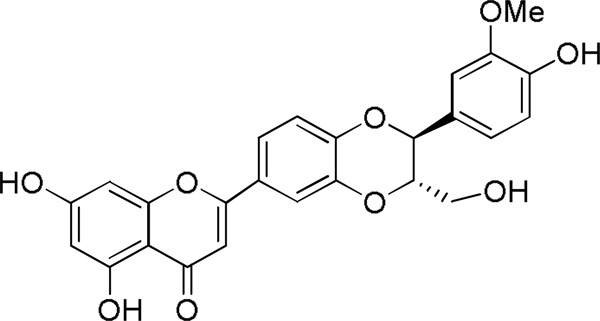
Chemosensitization involves the co-administration of MDR modulators (e.g., a P-gp inhibitor) with an anticancer drug to block P-gp function and enhance the intracellular accumulation of the drug (Krishna and Mayer, 2000). Several MDR modulators have been demonstrated to restore chemotherapeutic sensitivity in cancer cells and tissues in vitro and in vivo, but few MDR inhibitors have reached clinical trials and none are in clinical use as of today (Crowley et al., 2010; Nobili et al., 2011). In an attempt to address this problem, several natural products were screened in the present study as possible MDR inhibitors. These studies showed that the flavonolignan, (-)-hydnocarpin (Fig. 1), potentiates vincristine cytotoxicity in 697 cells and can re-sensitize resistant 697-R cells (Gupta et al., 2011) to vincristine treatment.
Figure 1. Structure of (-)-hydnocarpin.
MATERIALS AND METHODS
Test compounds
(-)-Hydnocarpin was previously isolated from Brucea javanica collected in Vietnam (Pan et al., 2009). Silvestrol was previously isolated from Aglaia foveolata (Pan et al., 2010). Vincristine sulfate and rhodamine 123 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Verapamil was obtained from Enzo Life Sciences (Plymouth Meeting, PA, USA).
Cell lines
The 697 pre-B acute lymphoblastic leukemia cell line was obtained from DSMZ (Braunschweig, Germany). The 697-R derivative was generated by stepwise increase in exposure to the P-gp substrate silvestrol. This resistant cell line was shown to exhibit increased P-gp expression as well as function (Gupta et al., 2011). The 697 cell line and the 697-R derivative were cultured in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (Sigma). The 697-R cells were cultured with bi-weekly addition of 80 nM silvestrol to maintain the MDR phenotype. Cell viability was monitored by trypan blue dye exclusion. All cells were confirmed to be at least 90% viable before in vitro testing. In addition, cells were divided the day prior to testing to ensure a logarithmic cell growth condition at the time of assay.
Cytotoxicity assays
The CellTiter 96® Aqueous Cell Proliferation (MTS) assay (Promega, Madison, WI, USA), which monitors mitochondrial activity as a surrogate for cell growth, was used to evaluate the re-sensitization activity (Fig. 2), and combination treatment of (-)-hydnocarpin and vincristine (Fig. 3) in 697 and 697-R cells. The density of 697 and 697-R cells was adjusted to 200,000 cells/mL. Sample aliquots of 100 µL were placed in a 96-well microplate in quadruplicate and incubated for 48 h in a humidified 5% CO2 atmosphere at 37°C. After the incubation period, 20 µL of MTS/PMS solution was added to each well and incubated for 5 h in a 5% CO2, 37°C atmosphere. Absorbance was read in a microplate reader at 490 nm.
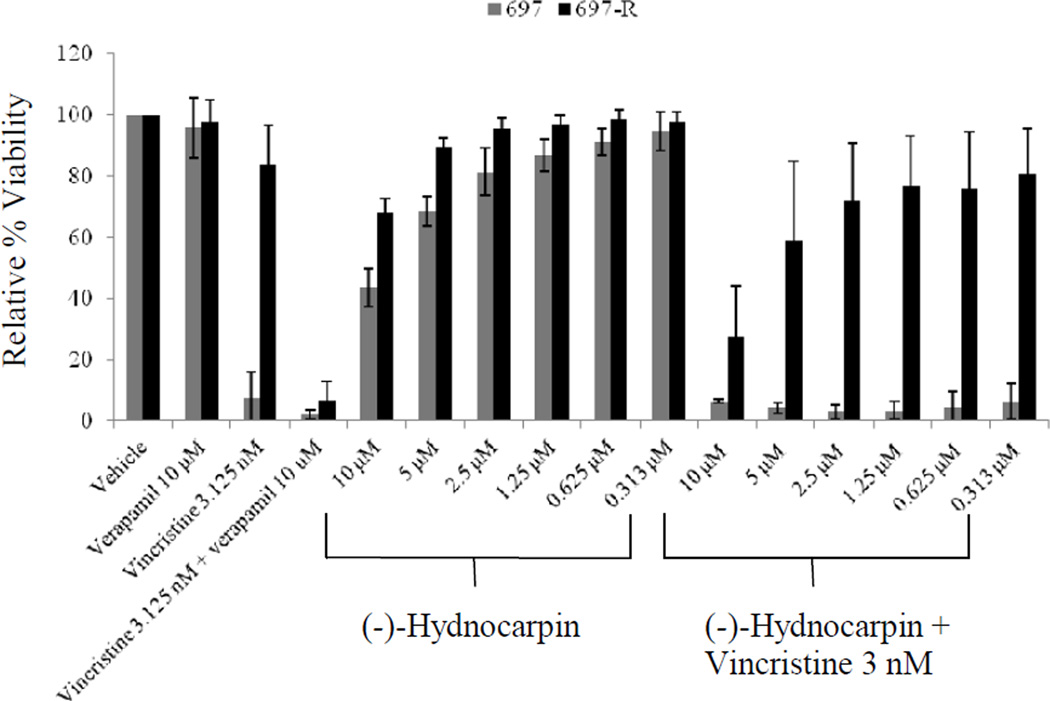
Figure 2. Re-sensitization test using (-)-hydnocarpin.
(-)-Hydnocarpin alone or in combination with 3 nM vincristine was incubated with 697 and 697-R cells for 48 h (n = 4). Viability was determined by the MTS assay and results were calculated relative to untreated cells. Error bars represent the standard deviation of four independent experiments.
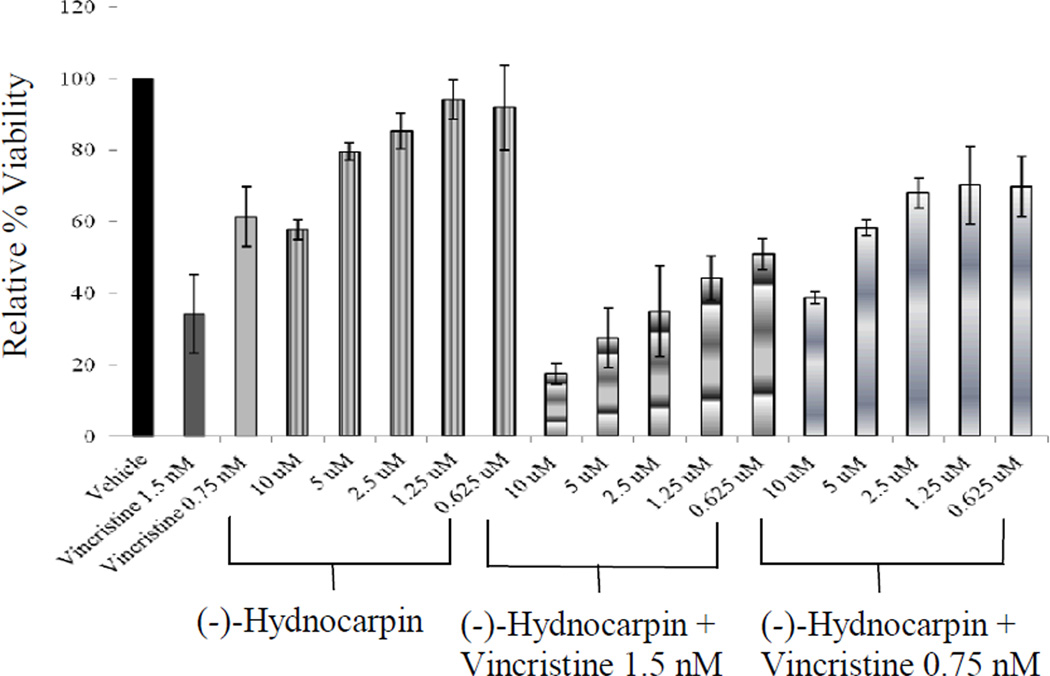
Figure 3. Combination treatment of vincristine and (-)-hydnocarpin.
(-)-Hydnocarpin alone or in combination with 1.5 or 0.75 nM vincristine was incubated with 697 cells for 48 h (n = 3). Viability was determined by the MTS assay and results were calculated relative to untreated cells. Error bars represent the standard deviation of three independent experiments.
Rhodamine 123 assay
To monitor P-gp function, efflux of the fluorescent P-gp substrate rhodamine 123 was assessed by flow cytometry (Fig. 4) as previously described (Gupta et al., 2011). A 2 h incubation was used for testing, as previous results showed that most of the rhodamine dye is effluxed out of 697-R cells at this time compared to the unstained control (Gupta et al., 2011).
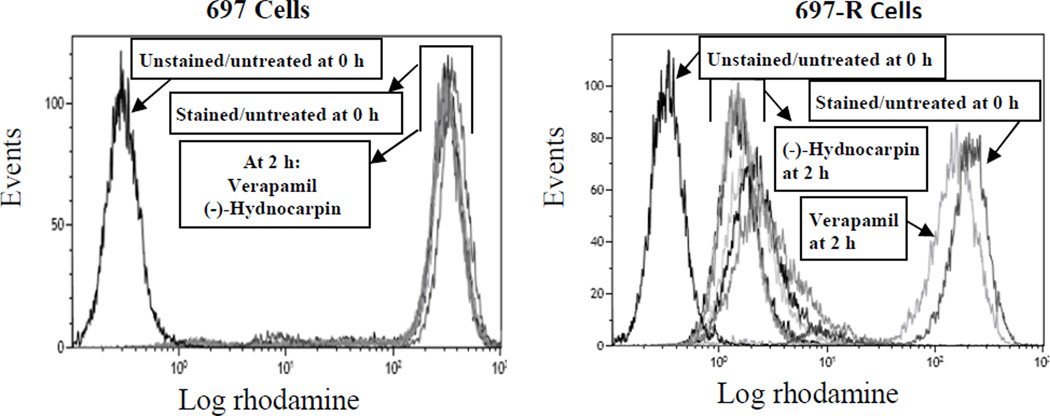
Figure 4. P-gp functional assay with (-)-hydnocarpin.
697 cells (no P-gp expression; left) and 697-R cells (P-gp expressing; right) were incubated with rhodamine 123 for 30 min and washed. Unstained cells and untreated cells (negative controls) were immediately read by flow cytometry. Verapamil at 10 µM was used as a positive control for P-gp inhibition. (-)-Hydnocarpin at 10, 5, 2.5, 1.25, and 0.625 µM was incubated with the cells for 2 h and washed, then fluorescence was read immediately after by flow cytometry. Values are reported as mean fluorescence intensity (MFI). Data shown are representative of three independent experiments.
Statistical analysis
For the cytotoxicity assays, the absorbance results were normalized to the absorbance of vehicle treated cells. The average of four independent experiments was used to construct a graph and the standard deviation was calculated. The IC50 value of (-)-hydnocarpin in the 697 cells for the re-sensitization test was calculated with the average of the four experiments and using the TableCurve 2Dv.4 software. For Figure 2, a linear mixed effects model with an interaction term between (-)-hydnocarpin and vincristine was used to assess the effects of combination treatments on cytotoxicity. From the model, the estimated combination effect of (-)-hydnocarpin and vincristine was compared to the sum of the individual effects of (-)-hydnocarpin and vincristine at each dose of (-)-hydnocarpin separately, with 95% confidence intervals (CI). Holm’s method was used for the six different dose comparisons with an overall Type I error rate of α = 0.05. Similarly, for Figure 3, a linear mixed effects model was used to compare the cytotoxicity of the combination treatment with the effect of vincristine alone at each dose combination, separately, with 95% CI. Holm’s method was used for the ten separate comparisons, with an overall Type I error rate of α = 0.05. All analyses were performed using SAS/STAT software v.9.2 (SAS Institute, Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
The possible re-sensitization activity of (-)-hydnocarpin when combined with vincristine, a potent anticancer drug and a well-known P-gp substrate (Fig. 2), was tested against both 697 cells and P-gp-expressing 697-R cells. Vincristine at 3 nM caused an almost complete growth inhibition for 697 cells, but only 17% growth inhibition against 697-R cells. Verapamil, an established P-gp inhibitor (Ramalhete et al., 2009) used as a control, showed no toxicity to either cell line at concentrations up to 10 µM. (-)-Hydnocarpin exhibited an IC50 (concentration resulting in 50% growth inhibition) of 8.7 µM for 697 cells and an IC50 of more than 10 µM against 697-R cells. The combination of (-)-hydnocarpin at 10 and 5 µM with vincristine using 697-R cells caused significant increases in growth inhibition to 72% (p ≤ 0.0001) and 41% (p = 0.0256), respectively.
The potential increase in vincristine cytotoxicity when combined with (-)-hydnocarpin (Fig. 3) was also evaluated using the parental 697 cells. (-)-Hydnocarpin at 10 µM caused a 42% growth inhibition, vincristine at 1.5 and 0.75 nM produced 66% and 39% growth inhibition, respectively, and the combination of (-)-hydnocarpin at 10 µM and vincristine at 1.5 and 0.75 nM resulted in 83% and 61% growth inhibition, respectively. The effect of the combination of (-)-hydnocarpin at 10 µM and vincristine at 1.5 and 0.75 nM was significantly greater than the effect of vincristine alone (p = 0.03 and < 0.0001, respectively).
To test the hypothesis that the observed increased in cytotoxicity of the combination treatment was due to inhibition of the P-gp pump by (-)-hydnocarpin, efflux of a specific fluorescent P-gp substrate, rhodamine 123 (Neyfakh, 1988), was assessed by flow cytometry using 697-R cells that express high levels of P-gp (Fig. 4). In these experiments, (-)-hydnocarpin at 10 µM did not prevent rhodamine efflux, indicating that this agent does not directly inhibit P-gp function.
The potentiating effect of (-)-hydnocarpin with vincristine in a multidrug resistant cell line and the possible use of (-)-hydnocarpin as a chemosensitizer is reported herein for the first time. (-)-Hydnocarpin can sensitize resistant cells to vincristine treatment as observed from the significant increase in vincristine cytotoxicity when combined with (-)-hydnocarpin. (-)-Hydnocarpin may serve as an MDR reversal agent, although the mechanism of action for the observed increase in vincristine cytotoxicity when combined with (-)-hydnocarpin in the 697-R cells does not appear to be through P-gp inhibition, and thus remains to be determined. The use of (-)-hydnocarpin as a lead compound to synthesize analogs and perform structure-activity relationship studies offers considerable potential for the development of a non-P-gp dependent MDR modulator with increased potency.
Acknowledgement
Partial support from grant P01 CA125066 from the National Cancer Institute, NIH, Bethesda, MD, is acknowledged. L.B.P. was supported from NIH grant T32 GM008512 as part of the Chemistry-Biology Interface Program (CBIP), The Ohio State University, Columbus, OH, USA, 2009-2011.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
REFERENCES
- Afifi MSA, Ahmed MM, Pezzuto JM, Kinghorn AD. Cytotoxic flavonolignans and flavones from Verbascum sinaiticum leaves. Phytochemistry. 1993;34:839–841. [Google Scholar]
- Alvarez AI, Real R, Pérez M, Mendoza G, Prieto JG, Merino G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J Pharm Sci. 2010;99:598–617. doi: 10.1002/jps.21851. [DOI] [PubMed] [Google Scholar]
- Chan K-F, Wong ILK, Kan JWY, Yan CSW, Chow LMC, Chan TH. Amine linked flavonoid dimers as modulators for P-glycoprotein-based multidrug resistance: structure-activity relationship and mechanism of modulation. J Med Chem. 2012;55:1999–2014. doi: 10.1021/jm201121b. [DOI] [PubMed] [Google Scholar]
- Chin Y-W, Jones WP, Waybright TJ, McCloud TG, Rasoanaivo P, Cragg GM, Cassady JM, Kinghorn AD. Tropane aromatic ester alkaloids from a large-scale recollection of Erythroxylum pervilleii stem bark obtained in Madagascar. J Nat Prod. 2006;69:414–417. doi: 10.1021/np050366v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley E, McDevitt CA, Callaghan R. Generating inhibitors of P-glycoprotein: where to, now? Methods Mol Biol. 2010;596:405–432. doi: 10.1007/978-1-60761-416-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte N, Gyemant N, Abreu PM, Molnar J, Ferreira M-JU. New macrocyclic lathyrane diterpenes, from Euphorbia lagascae, as inhibitors of multidrug resistance of tumour cells. Planta Med. 2006;72:162–168. doi: 10.1055/s-2005-873196. [DOI] [PubMed] [Google Scholar]
- Duarte N, Varga A, Cherepnev G, Radics R, Molnar J, Ferreira M-JU. Apoptosis induction and modulation of P-glycoprotein mediated multidrug resistance by new macrocyclic lathyrane-type diterpenoids. Bioorg Med Chem. 2007;15:546–554. doi: 10.1016/j.bmc.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Gupta SV, Sass EJ, Davis ME, Edwards RB, Lozanski G, Heerema NA, Lehman A, Zhang X, Jarjoura D, Byrd JC, Pan L, Chan KK, Kinghorn AD, Phelps MA, Grever MR, Lucas DM. Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J. 2011;13:357–364. doi: 10.1208/s12248-011-9276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guz NR, Stermitz FR. Synthesis and structures of regioisomeric hydnocarpin-type flavonolignans. J Nat Prod. 2000;63:1140–1145. doi: 10.1021/np000166d. [DOI] [PubMed] [Google Scholar]
- Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- Mi Q, Cui B, Silva GL, Lantvit D, Lim E, Chai H, You M, Hollingshead MG, Mayo JG, Kinghorn AD, Pezzuto JM. Pervilleine A, a novel tropane alkaloid that reverses the multidrug-resistance phenotype. Cancer Res. 2001;61:4030–4037. [PubMed] [Google Scholar]
- Molnár J, Engi H, Hohmann J, Molnár P, Deli J, Wesolowska O, Michalak K, Wang Q. Reversal of multidrug resistance by natural substances from plants. Curr Top Med Chem. 2010;10:1757–1768. doi: 10.2174/156802610792928103. [DOI] [PubMed] [Google Scholar]
- Neyfakh AA. Use of fluorescent dyes as molecular probes for the study of multidrug resistance. Exp Cell Res. 1988;174:168–176. doi: 10.1016/0014-4827(88)90152-8. [DOI] [PubMed] [Google Scholar]
- Nobili S, Landini I, Mazzei T, Mini E. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med Res Rev. 2011 doi: 10.1002/med.20239. [DOI] [PubMed] [Google Scholar]
- Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006;580:2903–2909. doi: 10.1016/j.febslet.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Pan L, Chin Y-W, Chai H-B, Ninh TN, Soejarto DD, Kinghorn AD. Bioactivity-guided isolation of cytotoxic constituents of Brucea javanica collected in Vietnam. Bioorg Med Chem. 2009;17:2219–2224. doi: 10.1016/j.bmc.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Kardono LBS, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale recollection of Aglaia foveolata. J Nat Prod. 2010;73:1873–1878. doi: 10.1021/np100503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalhete C, Molnar J, Mulhovo S, Rosario VE, Ferreira M-JU. New potent P-glycoprotein modulators with the cucurbitane scaffold and their synergistic interaction with doxorubicin on resistant cancer cells. Bioorg Med Chem. 2009;17:6942–6951. doi: 10.1016/j.bmc.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Reddy SV, Tiwari AK, Kumar US, Rao RJ, Rao JM. Free radical scavenging, enzyme inhibitory constituents from antidiabetic ayurvedic medicinal plant Hydnocarpus wightiana Blume. Phytother Res. 2005;19:277–281. doi: 10.1002/ptr.1491. [DOI] [PubMed] [Google Scholar]
- Sharma DK, Hall IH. Hypolipidemic, anti-inflammatory, and antineoplastic activity and cytotoxicity of flavonolignans isolated from Hydnocarpus wightiana seeds. J Nat Prod. 1991;54:1298–1302. doi: 10.1021/np50077a010. [DOI] [PubMed] [Google Scholar]
- Stermitz FR, Beeson TD, Mueller PJ, Hsiang JF, Lewis K. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem Syst Ecol. 2001;29:793–798. doi: 10.1016/s0305-1978(01)00025-4. [DOI] [PubMed] [Google Scholar]
- Stermitz FR, Lorenz P, Tawara JN, Zenewicz LA, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5'-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci USA. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]