Abstract
Oxidative stress is implicated as an important molecular mechanism underlying fibrosis in a variety of organs, including the lungs. However, the causal role of reactive oxygen species (ROS) released from environmental exposures and inflammatory / interstitial cells in mediating fibrosis as well as how best to target an imbalance in ROS production in patients with fibrosis are not firmly established. We focus on the role of ROS in pulmonary fibrosis and, where possible, highlight overlapping molecular pathways in other organs. The key origins of oxidative stress in pulmonary fibrosis (e.g. environmental toxins, mitochondria / NADPH oxidase of inflammatory and lung target cells, and depletion of antioxidant defenses) are reviewed. The role of alveolar epithelial cell (AEC) apoptosis by mitochondria- and p53-regulated death pathways are examined. We emphasize an emerging role for the endoplasmic reticulum (ER) in pulmonary fibrosis. After briefly summarizing how ROS trigger a DNA damage response, we concentrate on recent studies implicating a role for mitochondrial DNA (mtDNA) damage and repair mechanisms focusing on 8-oxoguanine DNA glycosylase (Ogg1) as well as crosstalk between ROS production, mtDNA damage, p53, Ogg1, and mitochondrial aconitase (ACO2). Finally, the association between ROS and TGF-β1-induced fibrosis is discussed. Novel insights into the molecular basis of ROS-induced pulmonary diseases and, in particular, lung epithelial cell death may promote the development of unique therapeutic targets for managing pulmonary fibrosis as well as fibrosis in other organs and tumors, and in aging; diseases for which effective management is lacking.
Keywords: Reactive oxygen species, Epithelium, Mitochondria, NADPH Oxidase, Apoptosis
1. Introduction
Fibrosis is characterized by exuberant extracellular matrix (ECM) protein deposition in the basement membrane and interstitial tissue in the setting of an injured overlying epithelium and expansion of activated mesenchymal cells (myofibroblasts). Fibrosis is pathologically evident in numerous diseases involving nearly every organ. Accumulating evidence over the past several decades have identified many of the important pathogenic mechanisms that promote fibrosis, yet the precise molecular mechanisms involved and the crosstalk between implicated pathways are not fully understood. Oxidative stress is one important molecular mechanism underlying fibrosis in a variety of organs, including the lungs. However, the causal role of reactive oxygen species (ROS) released from environmental exposures and inflammatory / interstitial cells in mediating fibrosis as well as how best to target an imbalance in ROS production in patients with fibrosis are not firmly established.
The term “oxidative stress” encompasses all the molecular, cellular and tissue abnormalities resulting from excess ROS production and/or depleted antioxidant defenses [1–3]. As compared to other organs, the lungs are particularly vulnerable to oxidative stress because they are exposed to the highest levels of oxygen; oxygen pressure of inhaled air is 150 mmHg and that of alveolar air is 100 mmHg while venous blood oxygen pressures returning from various other organs ranges from a high of ~45 mm Hg to a low of ~1 mmHg [2]. As emphasized by others, the traditional concept of fibrosis resulting from an imbalance in ROS production and antioxidant defenses is overly simplistic given the diverse pathways that are independently regulated and the ineffectual results from numerous antioxidant trials [1–3].
The purpose of this review is to highlight our current understanding of the causal role of oxidative stress in promoting fibrosis. Given the enormity of the topic and space constraints, we have chosen to restrict our focus to the role of ROS in mediating pulmonary fibrosis. In particular, we emphasize the role of ROS in the pathobiology of asbestosis, which is pulmonary fibrosis resulting from asbestos exposure. Asbestosis shares radiographic and pathologic features with idiopathic pulmonary fibrosis (IPF), a much more common disease that has a worse prognosis, and lacks effective treatment and ideal animal models. We underscore some of the important overlapping oxidative stress-induced molecular pathways resulting in fibrosis in other organs (e.g. liver, heart, etc.) that have recently been reviewed in detail elsewhere [4–6]. We first describe the principal origins of oxidative stress that can lead to pulmonary fibrosis including environmental toxins (e.g. tobacco, occupational exposure such as asbestos or silica, radiation, and drugs, such as bleomycin), mitochondria and NADPH oxidase (NOX), especially NADPH oxidase-4 (NOX4) in inflammatory and lung target cells. Oxidative stress also results from inadequate or deficient antioxidant defenses. Reactive nitrogen species, which are beyond the scope of this review, also contribute to the free radical burden of fibrotic lungs (see for review: [7]). We explore the evidence for alveolar epithelial cell (AEC) apoptosis being important in promoting asbestosis and IPF with an emphasis on ROS derived from the mitochondria- and p53-regulated death pathways as well as recent evidence for an activated endoplasmic reticulum (ER) stress response in patients with IPF. After briefly summarizing how ROS trigger a DNA damage response, we discuss the role for mitochondrial DNA (mtDNA) damage and repair mechanisms focusing on 8-oxoguanine DNA glycosylase (Ogg1) as well as crosstalk between ROS production, mtDNA damage, p53, Ogg1, and mitochondrial aconitase (ACO2), which is a mitochondrial redox-sensor molecule involved in mtDNA maintenance. Finally, the association between oxidative stress and transforming growth factor β (TGF-β)-induced fibrosis is discussed. We examine recent investigations highlighting important crosstalk between ROS, apoptosis, and inflammation. A general hypothetical model depicting the major ROS-driven pathways described in this review is illustrated in Figure 1. Collectively, these studies are revealing new insights into the molecular basis of ROS-induced pulmonary fibrosis and that may prove useful in the development of novel anti-fibrotic treatment strategies.
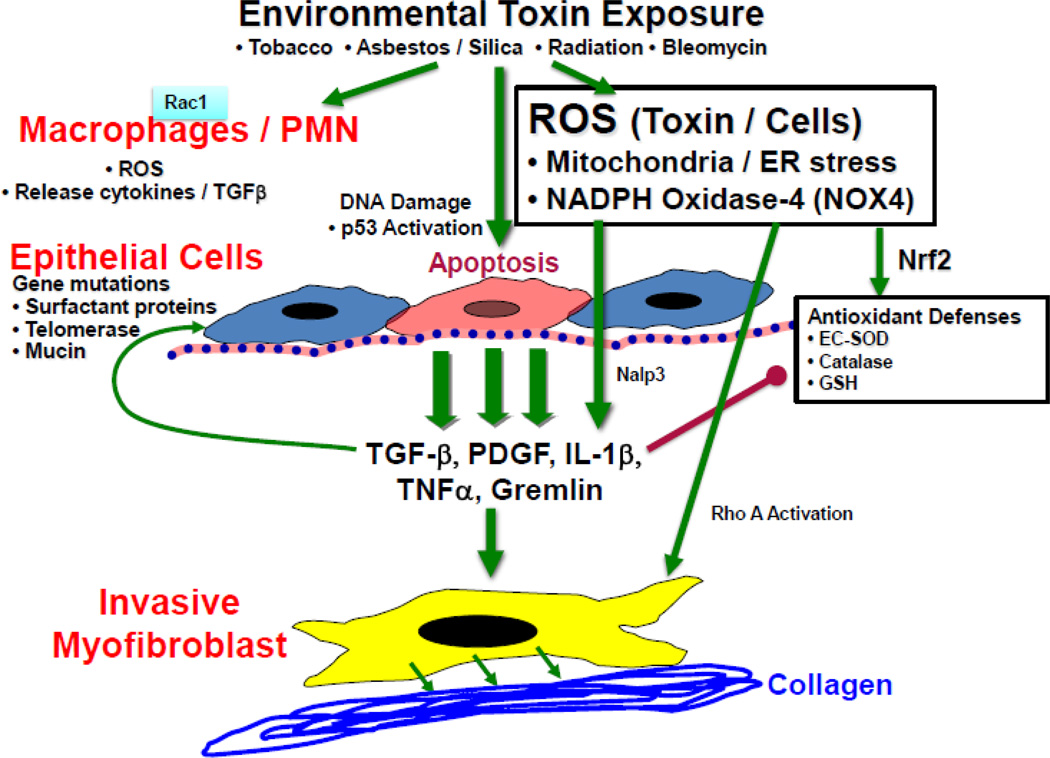
Figure 1. Hypothetical model of environmental stress/oxidant induced pulmonary fibrosis.
Environmental toxins/oxidants may induce the generation of ROS via induction of ER stress, NOX4 and uncoupling of mitochondrial electron transport or Rac 1 mediated activation of macrophages and/or PMNs. In conjunction with DNA damage and p53 activation, ROS promote apoptosis of airway epithelial cells, and elicit the production of cytokines and growth factors that may be important for invasive myofibroblastic differentiation and collagen deposition and may alter antioxidant defenses. The susceptibility of epithelial cells to apoptosis may be affected by mutations in surfactant, telomerase and mucin genes.
2. Origins of Oxidative Stress in Pulmonary Fibrosis
Biological systems are continually exposed to both extrinsic sources of reactive oxidants (e.g. tobacco, asbestos/silica, radiation, bleomycin and other drugs, etc.) and those that arise endogenously in inflammatory cells as well as epithelial, mesenchymal and endothelial cells within tissues [8]. Several enzymatic systems contribute to ROS production including NOXs, xanthine oxidase, nitric oxide synthase (NOS), and the mitochondrial electron transport chain. [8, 9]. They are also produced from the metabolism of a wide spectrum of drugs and xenobiotics [8]. ROS, including superoxide (O2-) and hydrogen peroxide (H2O2), play a central role in host defense by killing microbes in phagocytic cells. Although excess amounts of ROS are toxic and can promote fibrosis, lower levels of ROS are ‘physiologic’ by functioning as signaling molecules that mediate various cellular responses including proliferation, migration, differentiation, and gene expression [7–9]. The highly reactive HO• can be generated from O2- and H2O2 in the presence of small amounts of redox-activate ferrous iron complexed with toxins such as asbestos fiber from outside of cell, via the Fenton-catalyzed Haber-Weiss reaction shown in equation 1 [9, 10]. Alkoxyl radicals are also formed by iron catalysis of organic hydroperoxides as shown in equation 2.
| (1) |
| (2) |
Redox cycling is a common mechanism by which quinones and related species are reduced by a flavoenzyme (e.g. cytochrome P450 reductase) to a free radical that then reacts with oxygen to generate O2- [9]. Some fibrogenic agents implicated in redox cycling include the herbicide paraquat, the anticancer drug doxorubicin and the diabetogenic compound alloxan [8]. Free radicals also arise through the oxidation of phenols, aromatic amines and hydrazines by heme proteins, and some compounds undergo autoxidation catalyzed by transition metal ions [9, 11]. Because these ROS-producing reactions are associated with fibrosis, they have been the focus of investigative interest [10]. Finally, as reviewed in this section, oxidative stress can also be caused by depletion of antioxidant defense mechanisms, resulting in increased levels of ROS. In general, low levels of ROS production can result in cell proliferation and activation of antioxidant defenses, but higher ROS levels trigger DNA damage, p53 activation, cell cycle blockade, and cell death via apoptosis and/or necrosis; all of these may be important in a culminating fibrotic response.
At least 4 lines of evidence convincingly show that ROS generated internally or induced by environmental exposure play a vital role in the development of pulmonary fibrotic diseases as reviewed elsewhere [1, 7, 12–14]: (1) Oxidized proteins and lipid products like 8-isoprostrane and carbonylated proteins have been identified in exhaled air, bronchoalveolar lavage fluid, and lung tissue from patients with fibrotic lung diseases. (2) Bleomycin-induced pulmonary fibrosis, the most commonly utilized experimental model, is associated with marked increases in the levels of ROS, oxidized proteins, DNA and lipids. (3) Increased oxidative DNA damage is detected in patients with IPF as well as workers with silicosis and asbestosis and animal models with silica or asbestos induced lung fibrosis. (4) Antioxidants and iron chelators can attenuate bleomycin- and asbestos-induced pulmonary fibrosis in rodent models.
2.1. Mitochondria-derived ROS
ROS generated from the mitochondria of key target cells appear important in mediating pulmonary fibrosis. Mitochondrial dysfunction results in the generation of ROS (e.g. H2O2 and O2-) as the electron transport chain is uncoupled from proton pumping and ROS are released into the cytosol [15]. Carter and associates [16–19] have established a key role for H2O2 production by the mitochondria of alveolar macrophages (AM) in causing asbestosis. They reported that AM exposed to asbestos fibers produce H2O2, which is blocked by catalase or mitigation of AM mitochondrial stress. Further, Rac1, a Rho GTP binding protein family member, increases AM mitochondrial H2O2 production while asbestos-induced AM H2O2 production is reduced by knockdown of the iron-sulfur protein of complex III in the mitochondrial electron transport chain, a major site or ROS production. Increased levels of Rac1 are localized in the mitochondria of AM of asbestosis patients, and asbestos exposed mice with a conditional deletion of Rac1 have less oxidative stress and pulmonary fibrosis [16–19] (Figure 2). Taken together, these studies demonstrate that asbestos triggers AM H2O2 production by transferring electrons from complex III to Rac1, which then drive down-stream signaling pathways, inflammation and cellular injury that result in asbestosis in mice. Carter et.al have proposed that Rac1 is a novel biomarker for pulmonary fibrosis. An important role for H2O2 mediating pulmonary fibrosis is also supported by the protective effects of catalase in a rodent model of asbestosis [20]. As reviewed elsewhere, exogenous toxins, such as asbestos fibers can activate mitochondrial ROS production in other important lung target cells implicated in pulmonary fibrosis, including epithelial cells [14, 21]. However, additional studies are warranted to better understand the relevance of these findings in pulmonary fibrosis and to determine which cell should be primarily targeted.
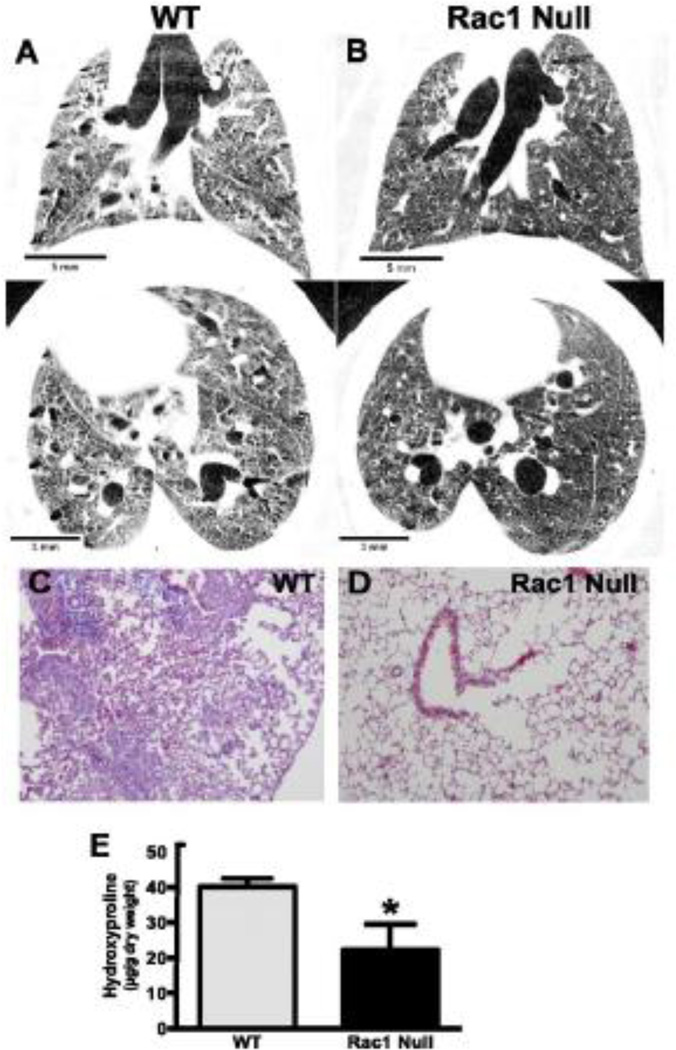
Figure 2. Asbestos-induced pulmonary fibrosis is blocked in Rac-1 null mice.
Wild-type (WT) and Rac1 null mice were intratracheally exposed to chrysotile asbestos (100 µg). Micro-CT scan images were obtained on live mice (in vivo) 21 days after exposure. Images are representative of three WT (A) and three Rac1 null (B) mice. Twenty one days after exposure, the mice were euthanized and the lungs were removed and processed for collagen deposition using Masson's trichrome staining. Micrographs are representative of 10 WT (C) and 10 Rac1 null (D) mice. (E), mice were euthanized 21 days after exposure, and lungs were removed and homogenized for hydroxyproline assay. WT (n = 4) and Rac1 null (n = 4); *p < 0.0208. Experimental data from Osborn-Heaford et.al (reference 16) was used with permission.
2.2. NAD(P)H Oxidase
NOX family of oxidoreductases catalyze one or two electron reductions to form O2- and H2O2, which contribute to cellular signaling, killing of invading microbes, and damage of surrounding host tissues when released primarily by activated inflammatory cells but also by non-inflammatory cells (See for reviews: [22, 23]). Recent studies have established the central role of NOX, especially isoforms NOX1, NOX2 and NOX4, in the pathogenesis of pulmonary fibrosis [22, 23]. NOX4 is induced by TGF-β1, which subsequently promotes key events in the development of fibrotic lung disease such as myofibroblast differentiation and impaired re-epithelialization discussed later (see Section 5). Thannikal and colleagues [24] demonstrated that NOX4-dependent generation of H2O2 is required for TGF-β1-induced myofibroblast differentiation and ECM production, and that genetic knockdown or pharmacologic inhibition of NOX4 prevents bleomycin-induced fibrosis in mice. Moreover, NOX-4 is upregulated in lungs of mice treated with bleomycin and humans with IPF [24]. TGF-β1 mediated myofibroblast differentiation is associated with the enzymatic production of extracellular H2O2 that can promote AEC cell death, a key event initiating and perpetuating pulmonary fibrosis as reviewed in section 3.1 [25, 26]. NOX4 is strongly expressed in the hyperplastic alveolar epithelium of IPF patients, particularly in alveolar type 2 (AT2) cells [25]. Interestingly, NOX4-deficient mice are completely protected against bleomycin-induced AT2 cell apoptosis and pulmonary fibrosis, but not against bleomycin-induced pulmonary inflammation [25]. NOX-4 deficient epithelial cells and/or AEC treated with NOX-4 chemical inhibitors (fluvene-5- inhibits NOX-4/2- and GK13601-inhibits NOX-1/4) decrease TGF-β1-mediated ROS generation and AEC apoptosis [25]. Collectively, these findings firmly implicate that ROS produced by NOX4 are crucial for mediating AEC apoptosis during the development of pulmonary fibrosis. In an experimental model of diabetic lung fibrosis, NOX activation in fibroblasts by angiotensin II also appears important [27]. A role of NOX2 in pulmonary fibrosis is supported by the finding that mice genetically deficient in NOX2 are protected from bleomycin- or carbon nanotube-induced lung fibrosis [22, 28].
NOXs are also important in mediating fibrosis in other organs. For example, a mutated SOD, which enhances O2- production, increases NOX1 in hepatic stellate cells during liver fibrosis [29]. Hepatocyte apoptosis and liver fibrosis are attenuated by GKT137831, an inhibitor of NOX4/NOX1, in vivo [29, 30]. In the heart, NOX4 is also implicated in promoting myofibroblastic differentiation of cardiac fibroblasts and is a major source of mitochondrial oxidative stress in cardiac myocytes in the failing heart [31, 32]. Taken together, these data firmly support a causal role of NOXs in tissue fibrogenesis and indicates that targeting NOXs could afford a novel approach to treatment of fibrogenic diseases. Future studies are necessary to further characterize the primary source of NOXs and role of NOX family members in the setting of ROS-induced organ fibrosis. It will also be of interest to determine the translational significance of targeting NOXs in humans with various forms of fibrosis.
Oxidative stress by NOXs and mitochondria-derived ROS can also trigger inflammatory signaling via the Nalp3 inflammosome sensing that may be important in driving silicosis, asbestosis and perhaps other forms of fibrosis [33–36]. The inflammosome, which is primarily expressed within macrophages and neutrophils, is a protein complex that serves as a host defense cellular danger sensor by activating IL-1β-driven innate pathway. Studies using pharmacologic ROS inhibitors, iron chelated asbestos, and targeted murine knockouts, demonstrate that ROS generated by NOXs during phagocytosis and/or by mitochondrial oxidative phosphorylation, are essential for activation of Nalp3 inflammosomes [33–36]. Thus, environmental toxins such as silica and asbestos, induce oxidative stress from NOXs and/or the mitochondria of inflammatory cells thereby activating multiple signaling pathways, including Nalp3, which may represent another novel therapeutic target for the remediation of pulmonary fibrosis.
2.3. Depletion of Antioxidant Defense Mechanisms
All organs, including the lungs, express a wide variety of antioxidants to protect against oxidative stress. Depletion of antioxidant defenses can result in oxidative stress that augments fibrosis. Herein, we focus on some of the primary lung antioxidant defenses implicated in animal models of pulmonary fibrosis and humans with IPF, including catalase, glutathione (GSH), and superoxide dismutase (SOD) as well as nuclear factor erythroid 2-related factor 2 (Nrf2), the primary regulator of antioxidant enzymes.
2.3.1 Catalase
a scavenger of H2O2 expressed in lung AEC and inflammatory cells, can inhibit H2O2-mediated activation of fibroblasts in IPF lungs [7]. Continuous intravenous infusion of polyethylene glycol catalase blocks asbestosis in a rat model [20]. More recently, intratracheal catalase administration to asbestos-treated mice was shown to prevent pulmonary fibrosis by inhibiting H2O2 production through Rac 1-activated inflammatory cells [18].
2.3.2. Glutathione
a low molecular weight antioxidant found in normal lungs, is reduced in the epithelial lining fluid and fibrotic foci in IPF lungs [7, 37]. The anti-fibrotic effects of N-acetyl cysteine (NAC), a GSH precursor, are observed in rodent models of fibrosis in which NAC increases lung GSH levels and attenuates bleomycin-induced fibrosis [38, 39]. Administration of aerosolized NAC to patients with IPF augments their antioxidant/oxidant balance [40]. One phase III clinical trial suggested a possible benefit of NAC therapy [41]. However, the results of an ongoing NIH-sponsored, multi-center, Phase III trial (PANTHER) should definitively address whether NAC is efficacious in IPF.
2.3.3. Superoxide dismutase
SOD decomposes superoxide radicals to H2O2. All three isoforms of mammalian SOD (intracellular copper-zinc SOD; mitochondrial manganese SOD; and extracellular SOD [EC-SOD]) exist in lung cells but cell-specific localization and expression varies (see for review: [2, 7]). EC-SOD is highly expressed in the lung and is implicated in the pathogenesis of ROS-induced pulmonary diseases, including pulmonary fibrosis [2, 7]. EC-SOD is protective in several murine models of pulmonary fibrosis (e.g. bleomycin, asbestos and radiation) whereas EC-SOD knockout mice have significantly more fibrosis following exposure to environmental toxins [2, 7]. Although beyond the scope of this review, accumulating evidence demonstrates that EC-SOD exerts its anti-fibrotic effects, in part by preventing oxidative degradation of the ECM and release of ECM degradation products that can augment fibrosis by effects on lung epithelial, mesenchymal and inflammatory cells [2, 7]. The role of the other two SOD isoforms in the pathogenesis of pulmonary fibrosis is less well understood. Mitochondial Cu, Zn, SOD−/− mice have less oxidative stress and do not develop asbestosis [19].
2.3.4. Nuclear factor-erythroid 2-related factor 2
Nrf2 is a transcription factor considered the “master regulator” of the antioxidant response (see for review: [42]). When Nrf2 is released from its negative regulator Kelch-like erythroid cell-derived protein CNC homology – associated protein 1 in the cytosol, Nrf2 traffics to the nucleus and activates the antioxidant response element (ARE) that controls hundreds of genes, including NAD(P)H quinone-oxidoreductase 1, antioxidant-related genes involved in glutathione biosynthesis, Phase II detoxifying “stress response” genes, and genes limiting the inflammatory and fibrotic responses. [42] Several recent studies underscore the importance of Nrf2 in regulating pulmonary fibrosis. First, Nrf2 knockout mice are more sensitive to bleomycin- and paraquat-induced pulmonary fibrosis as compared to wild-type animals [43, 44]. Second, in a study of primary lung fibroblasts cultured from healthy or IPF patients, decreased Nrf2 expression was associated with a myofibroblast phenotype (increased α-smooth muscle actin [α-SMA], and type 1 collagen expression) while Nrf2 activation increased antoxidant defenses and myofibroblastic dedifferentiation in IPF fibroblasts [45]. Moreover, a Nrf2 activator, sulfaphane, increased Nrf2 expression in cultured fibroblasts from normal and IPF patients; effects that were associated with increased expression of antioxidants, decreased ROS levels, and myofibroblastic dedifferentiation. Sulfaphane inhibited TGF-β1- mediated profibrotic effects in both normal and IPF fibroblasts but not in Nrf-2 knockdown fibroblasts [45]. Resveratrol, a phytoalexin polyphenol produced by several plants, attenuates bleomycin- and paraquat-induced pulmonary fibrosis in part by augmenting Nrf2 levels and downstream antioxidant enzymes [44]. Collectively, these results in animal models suggest that Nrf2 may be a novel target for IPF therapy as well as other oxidative stress-induced fibrogenic diseases
3. The Role of ROS and AEC Apoptosis in Pulmonary Fibrosis
3.1. AEC apoptosis is important to the pathophysiology of pulmonary fibrosis
Cells undergo apoptosis by two mechanisms, the extrinsic (death receptor) and intrinsic (mitochondria–regulated) pathways. Several lines of evidence convincingly show that lung epithelial cell death / apoptosis is one of the critical pathophysiologic events limiting normal lung repair and thereby facilitating pulmonary fibrosis. These studies have been extensively reviewed in detail elsewhere and will only be briefly summarized here (see for review: [1, 14, 21, 46, 47]). First, patients with IPF and animal models of pulmonary fibrosis show significant lung epithelial cell injury and apoptosis. In contrast to catastrophic lytic cell death that can trigger an inflammatory response, apoptosis is a regulated, ATP-dependent process of cell death that results in the elimination of cells with extensive DNA damage without eliciting an inflammatory response. Second, fibrogenic environmental toxins, including silica and asbestos, can induce both lytic and apoptotic AEC death in part by generating ROS derived from the mitochondria or NOXs. Third, DNA strand break formation, a potent trigger of apoptosis, occurs in the AEC of human patients with IPF. p53, a critical DNA damage response molecule reviewed below (see Section 4), is activated in patients with IPF and rodent models of asbestosis. Fourth, several murine models have established that AEC apoptosis is sufficient for inducing pulmonary fibrosis and that blocking AEC-targeted apoptosis prevents fibrosis [48, 49]. Janssen-Heininger and colleagues [47] have established that redox-based alterations in the FAS death receptor by protein S-glutathionylation are required for mediating extrinsic apoptosis in lung epithelial cells and pulmonary fibrosis. Fas is S-glutathionylated independently of NOX-induced ROS after caspase-8 degradation of glutathione reductase 1, thereby increasing caspase-3 activation and apoptosis. Glutathione reductase 1 overexpression attenuates S-glutathionylation and attenuates Fas ligand induced apoptosis and pulmonary fibrosis. In the ER of lung epithelial cells the Fasligand induces Fas glutathionylation with ERp57 (protein disulfide isomerase 3) and glutathione S-transferase π-1, which subsequently increases caspase-3 and -8 activity, AEC apoptosis, and bleomycin-induced lung fibrosis [50]. Notably, RNAi mediated knockdown of ERp57 and glutathione S-transferase π-1 attenuates the development of bleomycin-induced pulmonary fibrosis. Finally, preventing αvβ6 integrin release from damaged lung epithelial cells, blocks activation of latent TGF-β and pulmonary fibrosis following radiation or bleomycin exposure [51]. Thus, these studies establish a crucial role of AEC apoptosis in the pathophysiology of pulmonary fibrosis following exposure to oxidative stress. Future work is required to further characterize the precise molecular mechanisms involved and to determine the translational significance of identified targets in animals exposed to fibrogenic agents and humans with IPF.
3.2. Genetic predisposition to pulmonary fibrosis – Role of oxidative stress and the lung epithelium
IPF appears to have a genetic predisposition in nearly 5% of patients (see for review: [46]). Numerous mutations are associated with the development of pulmonary fibrosis, some expressed only in genes of epithelial cells, and others more ubiquitously expressed. Mutations in surfactant protein C and A2 genes, which are expressed only in the alveolar epithelium, are present in unique cohorts of familial pulmonary fibrosis patients [46]. Mutated surfactant proteins can induce an ER stress in AEC and this is important in mediating pulmonary fibrosis (discussed below). The most common gene mutations identified in familial pulmonary fibrosis are in the telomerase genes (TERT and TERC), and unlike surfactant proteins, are expressed outside the lung, especially in stem cells and progenitor cells (see for review: [52]). Notably, AEC from 60 of 62 patients with IPF had shortened telomeres that were < 50% of predicted [53]. Since shortened telemeres are directly associated with aging-related diseases in part due to oxidative stress, shortened AEC telomeres in IPF may in part account for their susciptibility to fibrosis following injury [3, 52, 53]. There is also some evidence that mitochondrial ROS production is a crucial determinant of telomerase-dependent senescence [54]. However, additional genetic and/or environmental factors appear important since AEC telomerase shortening alone does not promote bleomycin-induced fibrosis in mice [55]. Polymorphisms in the MUC5B gene promoter are evident in 38% of patients with sporadic IPF and 34% of patients with familiar pulmonary fibrosis [56]. Further studies are necessary to determine how alterations in MUC5B, which is expressed in the bronchial epithelium but not in AT2 cells, augments pulmonary fibrosis. It will also be of interest determining how oxidative stress adversely impacts these various mutant genes and their protein products in the development of pulmonary fibrosis in animal models as well as in humans.
3.3. Mitochondria-regulated AEC death pathways in pulmonary fibrosis
Diverse fibrotic stimuli (e.g. ROS, DNA damage, asbestos, etc.) activate the intrinsic death pathway by increasing the permeability of the outer mitochondrial membrane, reducing the mitochondrial membrane potential (Δψm), and releasing numerous apoptotic proteins, including cytochrome c (see for reviews: [14, 21, 46, 57–59]). The Bcl-2 family of –BH3 domain proteins are grouped into a four-class hierarchy dependent on structure and function (either pro- or anti-apoptotic). Pro-apoptotic proteins, such as Bax and Bak, can induce outer mitochondrial membrane permeabilization, which is the point of no return in the intrinsic apoptotic pathway causing the release of apoptogenic molecules from the mitochondria and caspase activation. Proapoptotic Bid, which is activated by the death receptor pathway and triggers intrinsic apoptosis by blocking anti-apoptotic molecules enabling Bax/Bak-mediated apoptosis, is necessary for mediating bleomycin-induced fibrosis in mice [49]. It will be interest knowing whether AEC or other cell types are primarily responsible for mediating the effects of Bcl-2 family members in pulmonary fibrosis.
Oxidative stress resulting from exposure to asbestos fibers activates the mitochondrial death pathway in AEC that may be important in the development of pulmonary fibrosis. Since this area has been extensively reviewed elsewhere (see for reviews: [1, 14, 21, 46, 58, 59]), herein we highlight the evidence with asbestos. Asbestos fibers cause mitochondrial dysfunction as evidenced by decreased Δψm, the release of cytochrome c from the mitochondria into the cytosol, and caspase-9 and 3 (but not caspase-8) activation [60]. These effects are blocked by phytic acid (an iron chelator), benzoic acid (a free-radical scavenger), and overexpression of Bcl-XL [60]. A crucial role for mitochondrial ROS in mediating AEC apoptosis is suggested by two lines of evidence: (1) asbestos-induced AEC intrinsic apoptosis and p53 activation are blocked in cells lacking mitochondrial DNA and mitochondrial ROS production (2) asbestos induces mitochondrial ROS production using a highly sensitive rho-GFP probe targeted to the mitochondria [60–63]. An important role for mitochondrial ROS and p53 in mediating intrinsic AEC following asbestos exposure is suggested by the protective effects of pifthrin-alpha, an inhibitor of p53-dependent transcription [63]. Activated protein kinase delta (PKCδ) migrates to the mitochondria of lung epithelial cells in vitro and in vivo following asbestos exposure and is necessary for inducing asbestos-induced intrinsic apoptosis and fibrosis via Bim activation [64, 65]. Taken together, mitochondrial ROS production and PKCδ activation following asbestos exposure appear important for inducing p53 activation and intrinsic lung epithelial cell apoptosis. However, the translational significance of these findings for pulmonary fibrosis awaits further study.
3.4. AEC ER Stress in fibrotic lung injury
Several groups have documented that the alveolar epithelium in patients with IPF have evidence of ER stress response in cells undergoing apoptosis [66, 67]. However, the pathophysiologic significance of these findings and the role of oxidative stress are uncertain. The ER primarily regulates protein folding and transport as well as Ca2+ homeostasis (see for reviews: [58, 68]). The ER can modulate intrinsic apoptosis, but the detailed mechanism underlying crosstalk between the ER and mitochondria and how this regulates mitochondrial metabolism, mitochondrial Ca2+ levels, and cell survival/death signaling are not fully understood. Close tethering of the ER to the mitochondria appears crucial for transferring ER-derived Ca2+ that is important for triggering intrinsic apoptosis [58, 69]. Mitofusin 2, one of the several proteins localized at mitochondrial associated membranes, physically attaches the mitochondria to the ER and is crucial for mitochondrial Ca2+ uptake from the ER [58, 69]. Exogenous and endogenous stressors, including mitochondrial ROS production, augment unfolded protein accumulation in the ER, leading to ER stress and activation of three unfolded protein response (UPR) signaling pathways including: (1) inositol requiring protein 1 (IRE1-α)/ X box binding protein 1 (Xbp-1), (2) activated transcription factor 6, and (3) protein kinase RNA-like endoplasmic reticulum kinase/ eukaryotic initiation factor-2a. (see for review: [58, 68]). The UPR is vitally important for coordinating numerous adaptive cellular responses that enhance cell survival in the setting of oxidative stress and other noxious stimuli, but apoptosis / autophagy (cell death with extensive cytoplasmic vacuolization) occur in the setting of a sustained ER stress response [57, 58, 68].
The Bcl-2 family members function at both the mitochondria and the ER, although the precise molecular mechanisms regulating crosstalk between these two organelles and the role of oxidative stress are not fully established [58, 59, 68, 69]. Transient ER Ca2+ release favors pro-survival signaling within cells while sustained ER Ca2+ release generally results in intrinsic apoptosis [58, 59, 69]. Sarcoplasmic ER Ca2+ATPase (SERCA) overexpression in Bax/Bak double knockout cells, which are resistant to mitochondria-regulated apoptosis, restores ER Ca2+ levels and intrinsic apoptotic response following exposure to oxidative stress [69]. The antiapoptotic effects of Bcl-2 are linked to Ca2+ mobilization from the ER to the mitochondrion and blocks homodimerization of pro-apoptotic Bax that displaces SERCA from the mitochondrial associated membranes [58, 59, 69]. Oxidative stress-induced NF-kB transcriptionally regulates the expression of sigma-1 receptors located in the mitochondrial associated membranes, and downstream of Bcl-2 expression [70]. ER Ca2+ release to the mitochondria is necessary, but not sufficient for induction of intrinsic apoptosis by coordination of ER stress survival signals through IRE1-α/ TNF associated factor-2, resulting in increased apoptosis signal regulating kinase 1 and JNK [71].
Accumulating evidence implicate an important role for AEC ER stress response in patients with IPF and possibly in mediating asbestos pulmonary toxicity. First, as noted above, AEC in patients with IPF that are undergoing apoptosis co-localize with markers of ER-UPR stress [66–68]. Second, ER stress augments epithelial-mesenchymal transition (EMT) and the formation of myofibroblasts, one of the primary effector cells in IPF [72]. Third, AEC ER stress and EMT can result from over-expression of a mutant, and accumulation of misfolded forms of surfactant protein C in the ER and are associated with familial pulmonary fibrosis [72, 73]. Notably, transgenic mice conditionally expressing mutated SP-CL188Q in their AT2 cells and them exposed to ER stress (e.g. intratracheal tunicamycin) do not develop pulmonary fibrosis unless a profibrotic stimuli (e.g. bleomycin) is added [74]. Thus, AEC ER stress appears to render the cells susceptible to oxidative stress-induced apoptosis following exposure to environmental toxins or drugs, and thereby promotes pulmonary fibrosis. As reviewed elsewhere [14], preliminary studies from our group show that asbestos-exposed AEC cells release AEC ER Ca2+ and activate the UPR signaling proteins (IRE1-α and Xbp-1). Further, the ER chemical chaperone 4-phenyl butyric acid (4-PBA) abolishes asbestos-induced increases in IRE1-α and Xbp-1, but does not prevent ER Ca2+ release or apoptosis. A recent study demonstrates that ROS are responsible for mediating TGF-β-induced UPR and that 4-PBA suppresses both TGF-β-induced UPR and myofibroblast differentiation [75]. Aging, which is a major cofactor in the development of IPF, is associated with increased oxidative stress, a decline in ER protein folding, reduced ability of the UPR to maintain cellular homeostasis during ER stress, and increased AEC apoptosis following injury (see for review: [1, 3, 57, 68]). Unlike young mice, older mice challenged with murine gamma herpesvirus 68 (MHV68) develop lung fibrosis that is associated with increased AER ER stress and apoptosis [76]. Taken together, these data show that AEC ER stress occurs in IPF as well as following exposure to various toxins that can promote fibrotic remodeling. Figure 3 illustrates a hypothetical model by which crosstalk between the ER and the mitochondria prevent AEC apoptosis that can lead to pulmonary fibrosis. It will be of considerable interest to better understand the molecular mechanisms by which oxidative stress following exposure to environmental toxins, causes an ER-UPR and apoptosis and also to determine whether modulation of this pathway affords a unique therapeutic target in the management of pulmonary fibrosis as well as other fibrotic diseases.
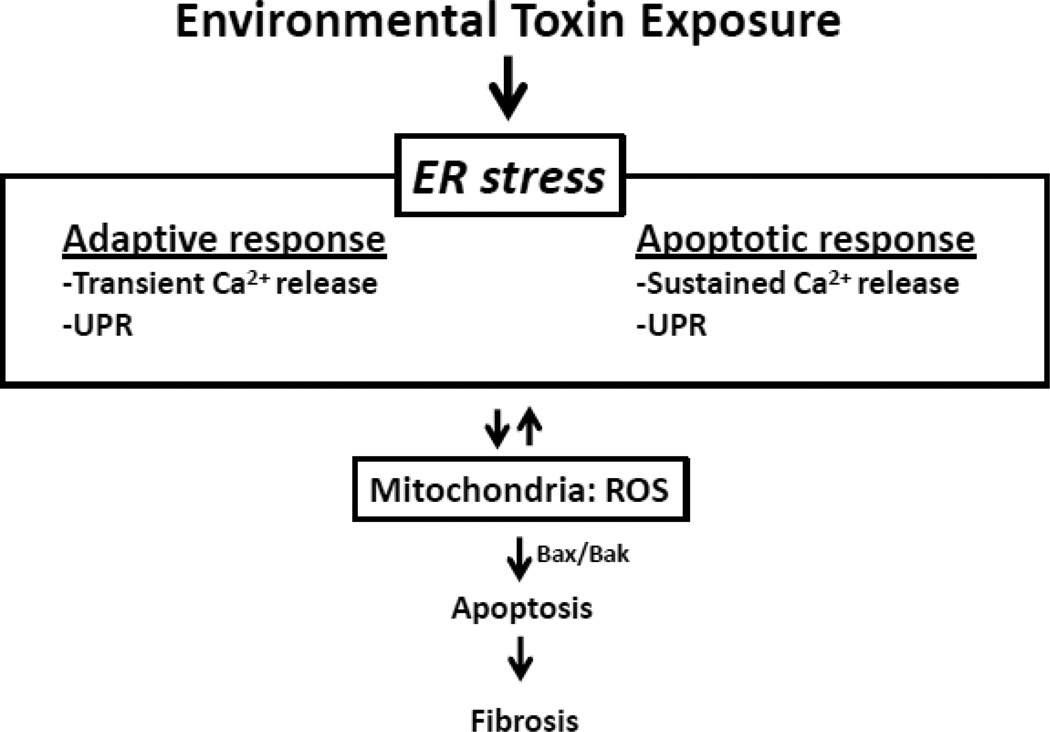
Figure 3. Hypothetical model of AEC ER-mitochondria crosstalk-induced apoptosis and fibrosis.
Exposure of AEC cells to environmental toxins induces ER that can lead to the release of ROS from the mitochondria that promotes apoptosis and pulmonary fibrosis (see text for details).
4. Oxidative stress and DNA damage response in Pulmonary Fibrosis
4.1. Oxidative stress induces a p53-dependent DNA damage response
Oxidative stress activates p53, which is considered the genome gatekeeper because p53 integrates key signals regulating cellular responses such as cell-cycle arrest, differentiation, apoptosis, senescence, and antiangiogenesis (see for review: [21, 77–81]). p53 is a transcriptional activator modulating downstream target gene expression involved in DNA-damage responses and tumor suppression that are important in preventing the accumulation of gene mutations [82]. As such, p53-dependent DNA damage response following oxidative stress serves as a barrier to tissue injury/fibrosis and tumor progression by augmenting DNA repair and/or promoting cell death of cells with DNA damage that overwhelms the repair mechanisms [78]. The key role of p53 is highlighted by the fact that over half of all human cancers have p53 mutations and also by the increased cancers seen in p53 null mice [83]. p53 is also redox sensitive and its transcriptional function is integrally linked to oxidative stress by orchestrating downstream cellular effects including the induction of apoptotic cell death [21, 81]. Stabilization of p53 can also promote further ROS generation via effects on the mitochondria [21, 81]. By regulating thousands of genes, either directly or indirectly, p53 modulates numerous vital cellular roles, including mtDNA maintenance (discussed below) [84, 85].
Accumulating evidence implicate p53 in the pathophysiology of pulmonary fibrosis, including that caused by asbestos [21, 63, 86–89]. Asbestos increases p53 and p21 expression in lung epithelial and mesothelial cells that result in cell-cycle arrest and apoptosis [63, 90–92]. p53 levels are elevated in lung cancers of patients with asbestosis [93], and p53 point mutations are present in the lung epithelium of smokers and asbestos-exposed patients [94]. Crocidolite asbestos augments p53 gene mutations in BALB/c-3 T3 cells [95]. In lung-specific dominant-negative p53 mice, chrysotile asbestos induces TGF-β and other proinfammatory/fibrotic signaling and increases adenocarcinoma formation [96]. Finally, gene-expression microarray studies in lung epithelial and mesothelial cells demonstrate that p53 activation has a very prominent role along with nearly 2,500 other genes of over 54,000 genes profiled [97, 98]. Collectively, ROS-induced p53 is cricial in regulating the lung cellular DNA-damage response, as occurs with fibrogenic agents such as asbestos and tobacco smoke.
ROS-induced DNA damage can trigger p53-dependent apoptosis that may be important in driving a fibrogenic response. Further, p53 can promote additional ROS generation, often via effects on the mitochondria [77, 85]. p53-regulated apoptosis can occur via activating the intrinsic and extrinsic death pathways either directly or indirectly via crosstalk with other ROS-activated signaling, such as MAPK [63, 99, 100]. Prompt repair of DNA lesions in most instances will restore the DNA. However, abnormal DNA repair may occur resulting in gene mutations, chromosomal aberrations and ultimately cell transformation. Damaged DNA can trigger apoptosis and contribute to the fibrogenic and malignant potential of oxidative stress [21, 101, 102]. A variety of DNA lesions, such as single or double strand breaks, intra- and inter-strand cross-linking and base damage produced by oxidant-induced ROS induce a DNA damage response [77, 103]. Under normal conditions, p53 expression is maintained at low levels through constitutive degradation by mouse double-minute 2 protein (MDM2), an E3 ubiquitin ligase, whereas upon DNA damage, p53 is released from MDM2-mediated degradation and is rapidly stabilized [104–106]. Further, deubiquitinating enzymes including herpesvirus-associated ubiquitin-specific protease (HAUSP) and ubiquitin specific peptidase (Usp) 10 have been shown to stabilize p53 protein by antagonizing MDM2-dependent ubiquitination in response to DNA damage [104, 107]. Transcriptional activity of p53 is also regulated by interacting partners such as transcriptional coactivators or corepressors [108]. The functional consequences of DNA damage-dependent p53 stabilization and transcriptional activation are induction of downstream target genes, such as p21, Bax, Noxa and BBC3 (encoding PUMA), that are involved in mediating p53’s cellular functions noted above [98, 105]. p53 also modulates apoptosis, at least partially, via transcription-independent mechanisms through associations with several members of the Bcl2 family of proteins in the cytoplasm or at the mitochondria [80, 101]. As illustrated in Figure 4, p53-Bcl2 and/or p53-BclXL complexes can promote Bax- and/or Bak-mediated intrinsic apoptosis; supporting a dual role of p53 in gene transactivation as well as in mitochondria-regulated apoptosis [80, 109]. To maintain the appropriate cellular redox state, ROS levels are tightly controlled; a task which is performed by two p53-regulated interconnected systems: thioredoxin and glutathione [79, 110]. The anti-apoptotic funtion of p53 is attributed to the effects on genes regulating the thioredoxin and glutathione systems including glutathione peroxidase, manganese SOD, aldehyde dehydrogenase 4, p53-induced glycolysis, apoptosis regulator, PA26 and Hi95 [79].
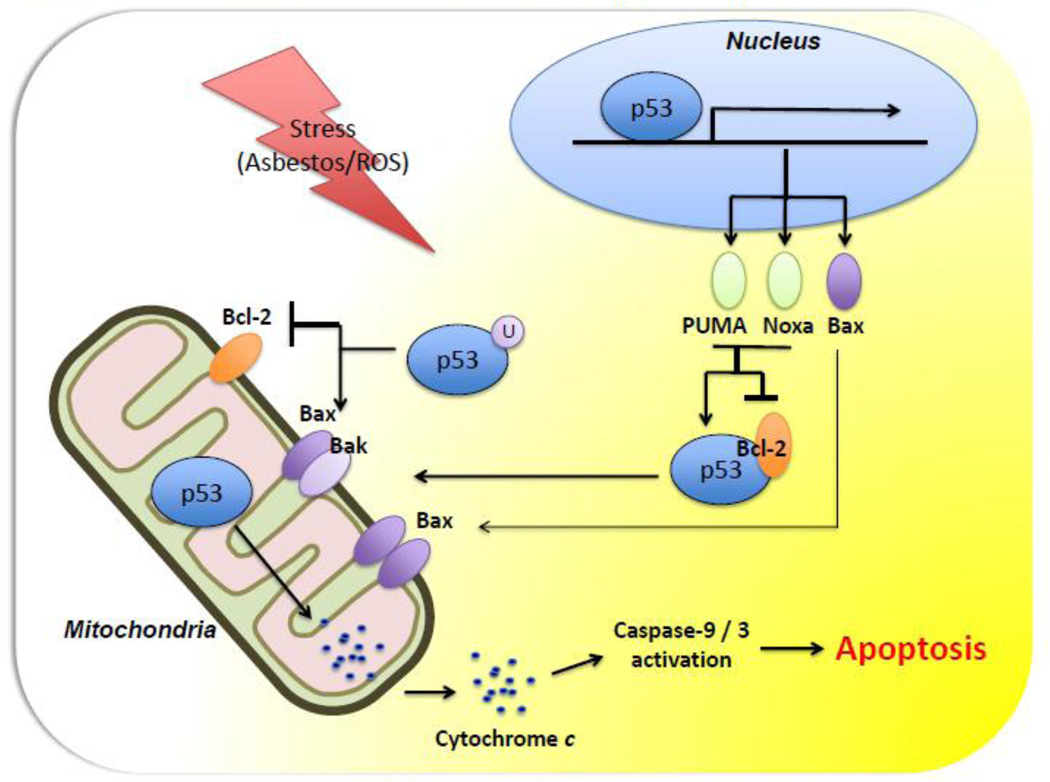
Figure 4. Hypothetical model of p53-dependent cytosolic and mitochondrial apoptotic pathways.
In response to stressors such as oxidative stress, nuclear-localized p53 induces the transcription of target genes that mediate p53’s function (see text for details).
4.2. The role of p53 during DNA repair triggered by oxidative stress
Damaged DNA following oxidative stress and other genotoxic exposures can be corrected by p53-related repair systems; a clearly protective role for p53 [79]. The observation that p53 translocation to the mitochondria does not necessarily lead to apoptosis and may enhance mtDNA repair also suggests an important role for p53 in maintaining normal cellular homeostasis in the setting of oxidative stress [84, 85, 111]. Further, there appear to be distinct genes activated by p53 for mediating the DNA damage response and tumor suppression following exposure to genotoxic stress as occurs with ROS [82]. p53 transactivation-dependent and -independent functions act as coordinators of the DNA repair process and involve most of the cellular DNA repair systems, such as mismatch repair, non-homologous end-joining, homologous recombination, nucleotide excision repair, and base excision repair (BER) [112]. Deficiency of components of the BER induced by oxidative stress, such as apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) or DNA polymerase β, cause a p53-dependent pre-and postnatal lethality, respectively, and lead to hypersensitivity toward certain types of DNA-damaging agents [79]. APE/Ref-1 or 8-oxoguanine-DNA glycosylase (OGG1), another important BER enzyme, are up-regulated in various cancers and promote apoptosis resistance [112, 113]. OGG1 has glycosylase activity that removes abnormal bases in DNA, especially 8-hydroxyguanine (8-oxoG), and generates an apurinic or apyrimidinic (AP) site recognized by the multifunctional enzyme APE/Ref-1 [112]. Oxidative stress causes multiple types of DNA base damage, however 8-oxoG has been most widely studied since 8-oxoG pairs with adenine rather than cytosine during DNA replication potentially resulting in a G:C to T:A transversion that is a pre-mutagenic lesion implicated in cancer and aging [21, 114]. Notably, p53 activation by oxidative stress in vitro enhances the excision of 8-oxoG nucleotides from the DNA by enhancing the sequential activities of OGG1 and APE/Ref-1 [81]. The transactivation-independent functions of p53 during DNA repair and recombination are also involved during p53-induced apoptosis [113]. Although much has been uncovered recently in how p53 regulates DNA repair in the setting of oxidative stress, the precise molecular pathways and their in vivo relevance in the setting of pulmonary fibrosis are not firmly established.
Mitochondrial ROS production following oxidative stress causes mtDNA damage that must be efficiently repaired or else mtDNA damage will trigger cell death or possibly mutagenic abnormalities contributing to the fibrogenic and malignant potential of ROS [21, 114, 115]. The mtDNA is particularly vulnerable to oxidative attack given its location at the inner mitochondrial membrane in close proximity to the mitochondrial electron transport chain and given that ~1–5% of total molecular oxygen used by the mitochondria for energy production results in the generation of ROS [21, 114–117]. Malignant transformation involves periods of increased mitochondrial ROS production when select cells survive oxidative stress-induced cell death [115, 116, 118]. As recently reviewed [21, 114, 115, 119], several lines of evidence suggest that mtDNA oxidative injury triggers apoptosis that is important in driving ROS-induced fibrogenesis and, perhaps, inflammation-associated cancers including: (1) cell death is better associated with mtDNA oxidative lesions than with nuclear DNA damage, (2) mtDNA damage results in ATP depletion and mitochondrial dysfunction, (3) augmenting mtDNA repair can block apoptosis, and (4) defective mtDNA repair promotes apoptosis. Because mtDNA oxidative damage is primarily repaired by BER, defects in mitochondrial BER enzymes, all of which are encoded in the nucleus and imported into the mitochondria, can promote mtDNA damage and instability [114, 120]. We focus on mitochondrial Ogg1 (mt-Ogg1) since it is the best-characterized mitochondrial BER enzyme involved in preventing the deleterious effects of oxidative stress in a wide variety of cells.
4.3. The role of hOgg1 during mitochondrial DNA repair caused by oxidative stress
The first step of the BER involves recognition and removal of the aberrant 8-oxoG base pair catalyzed by DNA glycosylases, such as Ogg1. DNA glycosylases recognize the modified or inappropriate base and cleave the N-glycosidic bond, creating an abasic site [114]. Notably, in vitro fluorometric techniques document that oxidative stress preferentially activates mt-Ogg1, rather than nuclear Ogg1 [121]. Further, Ogg1-deficient mice have increased 8-oxoG levels in mtDNA and nuclear DNA suggesting the importance of Ogg1 for most of the BER activity following exposure to oxidative stress [122]. An emerging role for mt-Ogg1 in regulating apoptosis is suggested by the findings that mt-OGG1 overexpression reduces mtDNA damage and intrinsic apoptosis caused by ROS-exposed vascular endothelial and asbestos-exposed cells [61, 123–129]. Crocidolite asbestos fibers, which are among the most potent amphiboles for inducing fibrosis and malignancies, cause preferential mtDNA damage, rather than nuclear DNA damage, in lung mesothelial cells that results in apoptosis [129]. The human OGG1 gene produces two major-alternatively spliced OGG1 mRNAs in humans: α-Ogg1, and β-Ogg1 [130]. α-Ogg1 is mainly located in the nucleus and present to a lesser extent in the mitochondria whereas β-Ogg1 is localized exclusively in mitochondria [130, 131]. A curious unexplained observation is that β-Ogg1 levels in the mitochondria are 20-fold higher than α-Ogg1 levels, yet only the α-Ogg1 isoform has 8-oxoG DNA repair glycosylase activity [132]. We reasoned that there must be a role for Ogg1 that is independent of DNA repair [61]. Support for this possibility was our observation that overexpression of mitochondrial α-Ogg1 mutants lacking 8-oxoG DNA repair activity were as effective as wild type mt-Ogg1 in preventing oxidant (asbestos and H2O2)-induced caspase-9 activation and intrinsic apoptosis in AEC cells yet did not affect the levels of mitochondrial ROS production [61]. It is unclear whether β-Ogg1 acts similarly in preventing oxidant-induced mitochondrial dysfunction and apoptosis. Interestingly, overexpression of mitochondrial α-Ogg1 preserved mitochondrial aconitase (ACO2) protein levels and activity in the setting of oxidative stress suggesting a novel role for Ogg1 that is considered in more detail below [61].
ACO2, which is an essential mitochondrial enzyme for energy metabolism and involved in the interconversion of citrate to isocitrate in the tricarboxylic acid (TCA) cycle, contains four iron–sulphur moieties which render ACO2 as a source for ROS and iron in vitro and in vivo [133–136]. ACO2 is uniquely sensitive to O2˙− mediated oxidative inactivation because of the presence of a single unligated iron atom, such that oxidation of the [4Fe-4S]2+ cluster renders it unstable and promotes removal of the labile iron atom, consequently forming H2O2 by the reduction of O2˙ [135]. Inactivation of ACO2 reduces mitochondrial manganese SOD levels [137], decreases the life span in Drosophila [134], and may have a pathophysiologic role in neurodegenerative diseases such as progressive supranuclear palsy [138], Friedreich’s ataxia [139], and Huntington’s disease [140]. In yeast, ACO2 preserves mtDNA independently of ACO2’s catalytic activity, which was the first suggestion of a novel dual function for ACO2 in mtDNA maintenance as well as a mitochondrial TCA enzyme [141]. We reported a novel role for mt-hOgg1 in chaperoning ACO2 in the setting of oxidative stress that prevents AEC apoptosis [61]. Notably, several groups have reported that suppression of Ogg1 expression increases oxidant-induced apoptosis [142–144]. Collectively, these findings suggest that intrinsic AEC apoptosis following exposure to oxidative stress is regulated by a novel interaction between mt-Ogg1 and ACO2. However, the role of Ogg1 and ACO2 in mediating pulmonary fibrosis following AEC apoptosis requires further study.
The precise molecular mechanisms by which mt-hOgg1 and ACO2 interact to prevent oxidant-induced AEC apoptosis are unclear, but there are at least two possibilities, which are not mutually exclusive. First, mt-hOgg1 may chaperone ACO2 and thereby block ACO2 oxidative modification that results in its degradation by mitochondrial Lon protease [145]. An example of this mechanism was uncovered with Friedreich’s Ataxia in which ACO2 coprecipitates with a mutant frataxin incapable of preventing oxidative inactivation of ACO2 [146]. Frataxin, which is an iron chaperone protein that blocks ACO2 oxidative inactivation and/or augments ACO2 reactivation, may function similarly to mt-hOgg1. Second, overexpression of mt-hOgg1 or ACO2 may preserve the mtDNA levels necessary to prevent activation of p53 and intrinsic apoptosis. Overexpression of mt-Ogg1 or ACO2 may attenuate the accumaulation of mtDNA lesions in the setting of oxidative stress that can result in intrinsic apoptosis via p53-dependent DNA damage signaling. Support for this latter possibility includes accumulating evidence showing a potentially crucial association between p53, Ogg1 and ACO2. p53 regulates the transcription of the OGG1 gene in colon and renal epithelial cells [142]. Further, p53 deficient cells have reduced expression and activity of OGG1 due partly because of reduced p53 binding to the putative cis elements within the OGG1 promoter; a defect that can be overcome by p53 overexpression. p53 may also modulate ACO2 since thymoquinone, a p53-dependent antineoplastic drug, decreases ACO2 activity in liver mitochondria [147]. Both endogenous p53 induction by camptothecin and exogenous transient p53 overexpression decrease ACO2 gene expression in prostate carcinoma cells [148]. Camptothecin does not affect ACO2 reporter activity in p53-null PC-3 cells suggesting that the decrease in ACO2 gene expression by camptothecin occurs via p53 activation. These findings may account for the observation that Ogg1 decreases oxidative stress-induced fibroblast apopotosis through p53-mediated signaling [142]. Additional investigations are warranted to further explore these various possibilities and to determine the molecular mechanism by which mt-hOgg1 interacts with aconitase to affect p53 signaling. The in vivo relevance of these findings to oxidative stress-induced fibrosis also requires study.
5. Oxidative Stress and TGF-β-induced Fibrosis
The interactive effects between oxidative stress and TGF-β are important for promoting fibrosis (see for review: [12]). TGF-β, which is the most potent profibrogenic cytokine evident in nearly all fibrotic diseases, is a multi-function protein that exists in different isoforms (TGF-β1, 2, and 3) and plays a vital role in ECM deposition by regulating crucial cellular activities such as cell proliferation, differentiation, adhesion, migration, apoptosis, and EMT. TGF-β stimulates ROS production leading to oxidative stress while oxidative stress can activate latent TGF-β setting up a vicious profibrogenic circle. Considerable evidence firmly implicate that TGF-β is required for the development of fibrosis including: (1) There is an increase in TGF-β mRNA and/or protein expression in fibrotic diseases of almost all organ systems in humans and in experimental animal models of fibrosis. (2) Transgenic mice that overexpress activated TGF-β globally have fibrosis in multiple organs and tissue. (3) Increased constitutively active TGF-β in lungs and peritoneum leads to lung and peritoneal fibrosis, respectively. (4) Fibrosis is attenuated in experimental animal models by blocking activated TGF-β in various organ systems by administration of either TGF-β binding proteins, anti-TGF-β antibody, or TGF-β type 1 receptor, as well as by the overexpression of dominant-negative TGF-β type 2 receptor [12]. Herein, we focus on some of the recently identified important molecular pathways by which TGF-β and oxidative stress collude to drive pulmonary fibrosis.
5.1 ROS activate latent TGF-β and induces TGF-β gene expression
All three isoforms of TGF-β are secreted in an inactive, latent form attached to a latency association protein (LAP). ROS increase TGF-β -induced fibrosis in part by activating latent TGF-β by two proposed mechanisms: (1) directly through oxidation of the LAP thereby releasing TGF-β and (2) indirectly by ROS-induced activation of matrix metalloproteinases (MMPs), which then cleave LAP and release active TGF-β [12]. ROS generated by asbestos fibers can directly activate latent TGF-β [149]. Using a site mutagenesis approach, a redox switch located on methionine 252 of the LAP has been identified as a possible extracellular ROS-sensor, since mutant LAP were incapable of ROS-induced TGF-β activation [150].
In addition to activating latent TGF-β, ROS can augment gene expression and secretion of TGF-β in many cell types including AEC and macrophages (see for review: [12]). Notably, free radical scavenging agents, iron chelators, as well as NOX2 null mice all substantially reduce ROS-induced TGF-β gene expression in several fibrogenic model systems [12]. Also, H2O2 promotes EMT in lung epithelial cells via a TGF-β1-dependent mechanism [151]. The fibrotic response caused by adenoviral vectors encoding constitutively activated TGF-β1 are blocked by concomitant treatment with adenoviruses encoding EC-SOD [152]. Collectively, these studies show the closely linked interactive effects between ROS and TGF-β activation in promoting pulmonary fibrosis.
5.2 TGF-β increases ROS production
Although ROS promote activation of TGF-β gene expression and release from LAP, TGF-β can also increase ROS production. TGF-β-induced generation of oxidative stress occurs in part by decreasing antioxidant defenses as well as by increasing ROS production. TGF-β decreases the concentration of important antioxidants in the lungs and hepatocytes, such as catalase, GSH and SOD, all of which have the capacity to inhibit fibrogenic responses due to oxidative stress as discussed in Section 2.3 [12, 153]. Intra-nasal instillation of adenoviral vectors encoding for constitutively activated TGF-β1 in murine lungs suppresses enzymes involved in GSH synthesis before the development of pulmonary fibrosis and that this occurs by inducing Activating Transcription Factor 3 (ATF3), a transcriptional repressor involved in regulation of glutamate-cysteine ligase [13]. These changes in GSH synthesis result in oxidative stress, lung epithelial cell apoptosis and pulmonary fibrosis [13].
There are several mechanisms by which TGF-β can augment the production of ROS that include the following: (1) activating oxidases in the cell membrane that promote H2O2 release to the extracellular space in human lung fibroblasts and bovine pulmonary artery endothelial cells. (2) inducing mitochondrial ROS production in part by decreasing complex IV activity in lung epithelial cells and hepatocytes. (3) activating Nox4 to generate ROS in various types of cells in vitro and in vivo [12, 154–156]. Taken together, these studies establish that TGF-β can augment oxidative stress, important in the pathogenesis of pulmonary fibrosis.
5.3 Possible mechanisms by which ROS mediates TGF-β-induced fibrogenesis
The detailed molecular mechanisms by which TGF-β promotes fibrosis is beyond the scope of this report but has recently been reviewed [12]. Herein we highlight some of the newer findings centered on the four possible mechanisms by which ROS mediate TGF-β-induced fibrogenesis including: (1) activation of fibroblast proliferation and collagen production, (2) induction of EMT, (3) promotion of epithelial cell apoptosis, and (4) activation of ECM gene expression and suppression of ECM protein degradation. Using primary human cardiac fibroblasts, TGF-β increases NOX4 and α-SMA, a myofibroblast marker, while knockdown of NOX4 by siRNA reduces production of ROS and expression of α-SMA mRNA suggesting that ROS mediate cardiac fibroblast differentiation to myofibroblasts [31]. Further, these investigators showed that ROS activate Smad2/3 and that activated Smad2/3 alone can cause cardiac fibroblast differentiation to myofibroblasts. As noted above (Section 2.2), TGF-β induced NOX4 expression is crucial for mediating ROS production that drives myofibroblast activation in the lungs of patients with IPF and mice with bleomycin-induced fibrosis as well as increase in AEC apoptosis [33, 157]. TGF-β-induced NOX4 expression and oxidative stress also appear important in mediating hepatocyte apoptosis and liver fibrosis [158, 159]. ROS can induce EMT; a process that results in a loss of epithelial markers and acquisition of mesenchymal phenotype as seen in many fibrotic diseases, including IPF [160–162]. TGF-β is a key mediator of EMT in vitro [163, 164] and in vivo [165, 166]. A role for ROS is suggested by the observation that antioxidants block TGF-β-induced EMT in lung and renal epithelial cells [151, 167]. A recent study demonstrated that TGF-β coordinately regulates EMT by interactions with β-catenin through its interaction with cAMP-response element-binding protein (CREB)-binding protein (CBP) in AECs [168]. Using the bleomycin mouse model of pulmonary fibrosis, these investigators demonstrate that ICG-001, a small molecule specific inhibitor of β-catenin /CBP interaction, attenuates pulmonary fibrosis and α-SMA and collagen induction in AEC. Given the robust β-catenin signaling in pulmonary fibrosis as well as emerging evidence of crosstalk between ROS levels and β-catenin signaling pathway, this suggests a novel mechanistic link that may be relevant to our understanding of pulmonary fibrosis [57, 168–170].
6. Conclusions
Table 1 summarizes some of the important molecular mechanisms underlying ROS-induced pulmonary fibrosis that we reviewed as well as some of the crucial areas that require further investigation. Patients with pulmonary fibrosis have increased oxidative stress as measued by various techniques as well as reductions in crucial anti-oxidant defenses. With the possible exception of NAC, none of the strategies aimed at limiting oxidative stress to date have proven efficacious in the management of pulmonary fibrosis. A simple imbalance between ROS levels and anti-oxidant defenses is unlikely to fully account for the multiple, independently regulated pathways involving various cells and organelles [1–3]. Targeted anti-oxants to the mitochondria or limiting NOX4 activation warrant further study. Emerging evidence demonstrates that mitochondria- and p53-regulated death pathways as well as ER stress mediate AEC apoptosis, which is an important early event in patients with IPF and asbestosis, all of which are modulated by the levels of ROS. There may also be an important interactive effect among mtOgg1, ACO2, and p53 in mtDNA repair and oxidant-induced intrinsic apoptosis. It seems likely that many of the interactive effects among mtOgg1, ACO2, p53, and intrinsic apoptosis described herein with asbestos fibers will have important broader implications regarding pulmonary fibrosis and, perhaps, mutagenesis. Future investigations exploring the role of these various interactive effects in transgenic animal models and in humans are warranted. Furthermore, the role of mt-Ogg1 and ACO2 in preventing oxidant-induced mtDNA damage, p53 activation, and intrinsic apoptosis requires additional study. ROS activate latent TGF-β, TGF-β gene expression and fibroblast collagen production by a RhoA-dependent mechanism. Finally, and perhaps most importantly, the ROS / pulmonary fibrosis paradigm is shedding light into the molecular basis underlying fibrosis in other organs involving the liver, heart and kidney as well as our understanding of tumorigenesis, for which effective treatment regimens are urgently required.
Table 1.
Some of the important molecular mechanisms underlying ROS-induced pulmonary fibrosis and areas requiring further investigation
| What we know | What we need to know |
|---|---|
| There is increased oxidative stress in the lungs of patients with pulmonary fibrosis. |
|
| There is decreased antioxidant defenses in the lungs of patients with pulmonary fibrosis. |
|
| Apoptosis of AECs occurs in patients with IPF and asbestosis. | Is AEC apoptosis essential for mediating pulmonary fibrosis? |
| Pulmonary fibrosis is associated with activation of an ER stress response that may be due partly to ROS production. | What is the causal role of ROS in activating the ER stress response in AEC as well as the in vivo relevance of ER stress in the context of treating pulmonary fibrosis? |
| ROS cause AEC apoptosis in vitro while AEC apoptosis occurs in patients with IPF and asbestosis. | Is AEC apoptosis essential for mediating pulmonary fibrosis? |
| Asbestos induces AM Rac1-dependent mitochondrial ROS production that is important in murine asbestosis. | What role does AM Rac1 play in humans with asbestosis, and is it a useful biomarker of pulmonary fibrosis? |
| p53-dependent transcription mediates ROS/asbestos-induced intrinsic AEC apoptosis in vitro and is evident in gene-profiling studies of normal and malignant cells. | Is p53-depenedent transcription necessary for pulmonary fibrosis following ROS exposure in animal models or humans? |
| Mitochondrial hOgg1 and ACO2 prevent oxidant-induced AEC mitochondrial dysfunction and intrinsic apoptosis in vitro. | What is the in vivo relevance of these findings in the context of asbestosis and other forms of pulmonary fibrosis? |
| ROS activate latent TGF-β, induce TGF-β gene expression, and activate fibroblast collagen production | What is the in vivo relevance of these findings in the context of treating pulmonary fibrosis and which cell(s) should be targeted? |
| ROS activates Nalp3 inflammasomes. | Is Nalp3 activation induced by ROS necessary and sufficient for inducing pulmonary fibrosis, and if so, which cell is responsible? |
Abbreviations: ACO2, mitochondrial aconitase; AEC, alveolar epithelial cell; AM, alveolar macrophage; BALF, bronchoalveolar lavage fluid; ER, endoplasmic reticulum; hOgg1, human 8-oxoguanine-DNA glycosylase 1; IPF, idiopathic pulmonary fibrosis; ROS, reactive oxygen
Highlights.
Oxidative stress is an important mechanism underlying fibrosis
ROS derived from the mitochondria and NOX4 can promote fibrosis
AEC apoptosis in lung fibrosis is coupled with mitochondria, ER, and p53 activation
ROS-induced AEC apoptosis is blocked in cells overexpressing Ogg1 or ACO2
The interactive effects between oxidative stress and TGF-β augment fibrosis
Acknowledgements
The authors acknowledge the important work of many investigators in the field, only some of whom were mentioned here because of space constraints. We have cited several recent reviews that include a more thorough listing of all the relevant literature. Our work was supported by a Merit Review grant from the Department of Veteran Affairs (to DWK) and NIH-RO1ES020357 (to DWK).
Glossary
Acronyms
- AEC
alveolar epithelial cell
- AT2
alveolar epithelial type II
- AM
alveolar macrophage
- ARE
antioxidant response element
- BALF
bronchoalveolar lavage fluid
- JNK
c-Jun N-terminal kinase
- EGFR
epidermal growth factor receptor
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- ERK
extracellular-signal-regulated kinase
- H2O2
hydrogen peroxide
- IPF
idiopathic pulmonary fibrosis
- IRE1α
inositol requiring kinase 1 alpha
- IL-1β
interleukin-1 beta
- ACO2
mitochondrial aconitase
- mtDNA
mitochondrial DNA
- mt-hOgg1
mitochondrial human 8-oxoguanine-DNA glycosylase 1
- Δψm
mitochondrial membrane potential
- MDM2
mouse double-minute 2 protein
- NAC
n-acetyl cysteine
- NOX
NADPH oxidase
- NOX4
DPH oxidase-4
- NOS
nitric oxide synthase
- Nrf2
nuclear factor erythroid 2-related factor 2
- PKCδ
protein kinase C delta
- ROS
reactive oxygen species
- SERCA
sarcoplasmic ER Ca2+ ATPase
- αSMA
α smooth muscle actin
- O2-
superoxide
- SOD
superoxide dismutase
- SP-C
surfactant protein C
- TGF-β
transforming growth factor beta
- TNFα
tumor necrosis factor-alpha
- UPR
unfolded protein response
- XBP1
x-box binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faner R, Rojas M, Macnee W, Agusti A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012;186:306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 2.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am. J. Respir. Crit. Care. Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 3.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Valle V, Chavez-Tapia NC, Uribe M, Mendez-Sanchez N. Role of Oxidative Stress and Molecular Changes in Liver Fibrosis: A Review. Curr. Med. Chem. 2012 doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- 5.Friedrichs K, Baldus S, Klinke A. Fibrosis in Atrial Fibrillation - Role of Reactive Species and MPO. Front. Physiol. 2012;3:214. doi: 10.3389/fphys.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrielli A, Svegliati S, Moroncini G, Amico D. New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol. J. 2012;6:87–95. doi: 10.2174/1874312901206010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Rad. Biol. Med. 2010;49:707–717. doi: 10.1016/j.freeradbiomed.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 8.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 9.Kamp DW, Graceffa P, Pryor WA, Weitzman SA. The role of free radicals in asbestos-induced diseases. Free Rad. Biol. Med. 1992;12:293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- 10.Turci F, Tomatis M, Lesci IG, Roveri N, Fubini B. The iron-related molecular toxicity mechanism of synthetic asbestos nanofibres: a model study for high-aspect-ratio nanoparticles. Chemistry. 2011;17:350–358. doi: 10.1002/chem.201001893. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien PJ. Radical formation during the peroxidase catalyzed metabolism of carcinogens and xenobiotics: the reactivity of these radicals with GSH, DNA, and unsaturated lipid. Free Rad. Biol. Med. 1988;4:169–183. doi: 10.1016/0891-5849(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 12.Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Rad. Biol. Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, Kavanagh TJ, Matthews QL, Postlethwait EM. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Rad. Biol. Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G, Cheresh P, Kamp DW. Molecular Basis of Asbestos-Induced Lung Disease. Annu. Rev. Pathol.: Mech. Dis. 2013;8:161–187. doi: 10.1146/annurev-pathol-020712-163942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep. 2012;13:322–330. doi: 10.1038/embor.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborn-Heaford HL, Ryan AJ, Murthy S, Racila AM, He C, Sieren JC, Spitz DR, Carter AB. Mitochondrial Rac1 import and electron transfer from cytochrome c is required for pulmonary fibrosis. J. Biol. Chem. 2012;287:3301–3312. doi: 10.1074/jbc.M111.308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy S, Ryan A, He C, Mallampalli RK, Carter AB. Rac1-mediated mitochondrial H2O2 generation regulates MMP-9 gene expression in macrophages via inhibition of SP-1 and AP-1. J. Biol. Chem. 2010;285:25062–25073. doi: 10.1074/jbc.M109.099655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy S, Adamcakova-Dodd A, Perry SS, Tephly LA, Keller RM, Metwali N, Meyerholz DK, Wang Y, Glogauer M, Thorne PS, Carter AB. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L846–L855. doi: 10.1152/ajplung.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He C, Murthy S, McCormick ML, Spitz DR, Ryan AJ, Carter AB. Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J. Biol. Chem. 2011;286:15597–15607. doi: 10.1074/jbc.M110.187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossman BT, Marsh JP, Sesko A, Hill S, Shatos MA, Doherty J, Petruska J, Adler KB, Hemenway D, Mickey R, et al. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycolconjugated catalase in a rapid inhalation model of asbestosis. Am. Rev. Respir. Dis. 1990;141:1266–1271. doi: 10.1164/ajrccm/141.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- 21.Huang SX, Jaurand MC, Kamp DW, Whysner J, Hei TK. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J. Toxicol. Environ. Health B. Crit. Rev. 2011;14:179–245. doi: 10.1080/10937404.2011.556051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crestani B, Besnard V, Boczkowski J. Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int. J. Bioichem. Cell Biol. 2011;43:1086–1089. doi: 10.1016/j.biocel.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Hecker L, Cheng J, Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell. Molec. Life Sci. 2012;69:2365–2371. doi: 10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Tan Y, Zhao F, Ma Z, Wang Y, Zheng S, Epstein PN, Yu J, Yin X, Zheng Y, Li X, Miao L, Cai L. Angiotensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of NADPH oxidase-mediated nitrosative damage. Am. J. Physiol, Endocrinol. Metab. 2011;301:E132–E144. doi: 10.1152/ajpendo.00629.2010. [DOI] [PubMed] [Google Scholar]
- 28.Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois JM, Bertrand CP, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir. Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P. ABD, Nicotinamide adenine dinucleotide phosphate oxidase (nox) in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012 doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, Torok NJ. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic. Biol. Med. 2012;53:289–296. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 32.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Nat. Acad. Sci. U.S.A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Nat. Acad. Sci. U.S.A. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossman BT, Lippmann M, Hesterberg TW, Kelsey KT, Barchowsky A, Bonner JC. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B. Crit. Rev. 2011;14:76–121. doi: 10.1080/10937404.2011.556047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 37.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 38.Giri SN, Hyde DM, Schiedt MJ. Effects of repeated administration of N-acetyl-L-cysteine on sulfhydryl levels of different tissues and bleomycin-induced lung fibrosis in hamsters. J. Lab. Clin. Med. 1988;111:715–724. [PubMed] [Google Scholar]
- 39.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am. J. Respir. Crit. Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 40.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–216. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 41.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 42.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, Yamamoto M, Hizawa N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X, Wang L, Szklarz G, Bi Y, Ma Q. Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J. Pharmacol. Exp. Ther. 2012;342:81–90. doi: 10.1124/jpet.112.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Romer S, Boutten A, Bonay M. Nuclear Factor Erythroid 2-Related Factor 2 Nuclear Translocation Induces Myofibroblastic Dedifferentiation in Idiopathic Pulmonary Fibrosis. Antioxid. Redox Signal. 2012 doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 46.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J. Clin. Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anathy V, Roberson EC, Guala AS, Godburn KE, Budd RC, Janssen-Heininger YM. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid. Redox Signal. 2012;16:496–505. doi: 10.1089/ars.2011.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, Homer RJ, Elias JA. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budinger GR, Mutlu GM, Eisenbart J, Fuller AC, Bellmeyer AA, Baker CM, Wilson M, Ridge K, Barrett TA, Lee VY, Chandel NS. Proapoptotic Bid is required for pulmonary fibrosis. Proc. Nat. Acad. Sci. U.S.A. 2006;103:4604–4609. doi: 10.1073/pnas.0507604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, Janssen-Heininger YM. Oxidative processing of latent fas in the endoplasmic reticulum controls the strength of apoptosis. Mol. Cell Biol. 2012;32:3464–3478. doi: 10.1128/MCB.00125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K, Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O'Hara C, Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 2008;177:56–65. doi: 10.1164/rccm.200706-805OC. [DOI] [PubMed] [Google Scholar]
- 52.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutation Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, Phillips JA, 3rd, Lansdorp PM, Loyd JE, Armanios MY. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Nat. Acad. Sci. U.S.A. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birket MJ, Harold G, Schaeuble K, Birch-Machin MA, Kirkwood TB, von Zglinicki T. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Degryse AL, Xu XC, Newman JL, Mitchell DB, Tanjore H, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Phillips JA, 3rd, Cogan JD, Blackwell TS, Lawson WE. Telomerase deficiency does not alter bleomycin-induced fibrosis in mice. Exp. Lung Res. 2012;38:124–134. doi: 10.3109/01902148.2012.658148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 60.Panduri V, Weitzman SA, Chandel N, Kamp DW. The mitochondria-regulated death pathway mediates asbestos-induced alveolar epithelial cell apoptosis. Am. J. Resp. Cell Molec. Biol. 2003;28:241–248. doi: 10.1165/rcmb.4903. [DOI] [PubMed] [Google Scholar]
- 61.Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GR, Schumacker PT, Weitzman SA, Kamp DW. Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Radic. Biol. Med. 2009;47:750–759. doi: 10.1016/j.freeradbiomed.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Panduri V, Weitzman SA, Chandel NS, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2004;286:L1220–L1227. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- 63.Panduri V, Surapureddi S, Soberanes S, Weitzman SA, Chandel N, Kamp DW. P53 mediates amosite asbestos-induced alveolar epithelial cell mitochondria-regulated apoptosis. Am. J. Respir. Cell Mol. Biol. 2006;34:443–452. doi: 10.1165/rcmb.2005-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lounsbury KM, Stern M, Taatjes D, Jaken S, Mossman BT. Increased localization and substrate activation of protein kinase C delta in lung epithelial cells following exposure to asbestos. Am. J. Pathol. 2002;160:1991–2000. doi: 10.1016/s0002-9440(10)61149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buder-Hoffmann SA, Shukla A, Barrett TF, MacPherson MB, Lounsbury KM, Mossman BT. A protein kinase Cdelta-dependent protein kinase D pathway modulates ERK1/2 and JNK1/2 phosphorylation and Bim-associated apoptosis by asbestos. Am. J. Pathol. 2009;174:449–459. doi: 10.2353/ajpath.2009.080180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 67.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L721–L729. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29:2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meunier J, Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J. Pharmacol. Exp. Ther. 2010;332:388–397. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, Kim KJ, duBois RM, Crandall ED, Beers MF, Borok Z. Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol. 2011;45:498–509. doi: 10.1165/rcmb.2010-0347OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by Surfactant Protein C BRICHOS mutants. Int. J. Biochem. Cell Biol. 2012;44:101–112. doi: 10.1016/j.biocel.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, Morrisey EE, Beers MF, Yull FE, Blackwell TS. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc. Nat. Acad. Sci. U.S.A. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baek HA, Kim do S, Park HS, Jang KY, Kang MJ, Lee DG, Moon WS, Chae HJ, Chung MJ. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012;46:731–739. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- 76.Torres-Gonzalez E, Bueno M, Tanaka A, Krug LT, Cheng DS, Polosukhin VV, Sorescu D, Lawson WE, Blackwell TS, Rojas M, Mora AL. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am. J. Respir. Cell Mol. Biol. 2012;46:748–756. doi: 10.1165/rcmb.2011-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu G, Beri R, Mueller A, Kamp DW. Molecular mechanisms of asbestos-induced lung epithelial cell apoptosis. Chem. Biol. Int. 2010;188:309–318. doi: 10.1016/j.cbi.2010.03.047. [DOI] [PubMed] [Google Scholar]
- 78.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol. Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 79.Janicke RU, Sohn D, Schulze-Osthoff K. The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ. 2008;15:959–976. doi: 10.1038/cdd.2008.33. [DOI] [PubMed] [Google Scholar]
- 80.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 81.Achanta G, Huang P. Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species-generating agents. Cancer Res. 2004;64:6233–6239. doi: 10.1158/0008-5472.CAN-04-0494. [DOI] [PubMed] [Google Scholar]
- 82.Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Kenzelmann Broz D, Basak S, Park EJ, McLaughlin ME, Karnezis AN, Attardi LD. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell. 2011;145:571–583. doi: 10.1016/j.cell.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kenzelmann Broz D, Attardi LD. In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis. 2010;31:1311–1318. doi: 10.1093/carcin/bgp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakhanashvili M, Grinberg S, Bonda E, Simon AJ, Moshitch-Moshkovitz S, Rahav G. p53 in mitochondria enhances the accuracy of DNA synthesis. Cell Death Differ. 2008;15:1865–1874. doi: 10.1038/cdd.2008.122. [DOI] [PubMed] [Google Scholar]
- 85.Lebedeva MA, Eaton JS, Shadel GS. Loss of p53 causes mitochondrial DNA depletion and altered mitochondrial reactive oxygen species homeostasis. Biochim. Biophys. Acta. 2009;1787:328–334. doi: 10.1016/j.bbabio.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson A, Mendoza T, Hoyle GW, Brody AR, Fermin C, Morris GF. Enhancement of fibrogenesis by the p53 tumor suppressor protein in asbestos-exposed rodents. Chest. 2001;120:33S–34S. doi: 10.1378/chest.120.1_suppl.s33. [DOI] [PubMed] [Google Scholar]
- 87.Burmeister B, Schwerdtle T, Poser I, Hoffmann E, Hartwig A, Muller WU, Rettenmeier AW, Seemayer NH, Dopp E. Effects of asbestos on initiation of DNA damage, induction of DNA-strand breaks, P53-expression and apoptosis in primary, SV40-transformed and malignant human mesothelial cells. Mutation Res. 2004;558:81–92. doi: 10.1016/j.mrgentox.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–274. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 89.Mishra A, Liu JY, Brody AR, Morris GF. Inhaled asbestos fibers induce p53 expression in the rat lung. Am. J. Respir. Cell Mol. Biol. 1997;16:479–485. doi: 10.1165/ajrcmb.16.4.9115760. [DOI] [PubMed] [Google Scholar]
- 90.Johnson NF, Jaramillo RJ. p53, Cip1, and Gadd153 expression following treatment of A549 cells with natural and man-made vitreous fibers. Environ Health Perspect. 1997;105(Suppl 5):1143–1145. doi: 10.1289/ehp.97105s51143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matsuoka M, Igisu H, Morimoto Y. Phosphorylation of p53 protein in A549 human pulmonary epithelial cells exposed to asbestos fibers. Environ Health Perspect. 2003;111:509–512. doi: 10.1289/ehp.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kopnin PB, Kravchenko IV, Furalyov VA, Pylev LN, Kopnin BP. Cell type-specific effects of asbestos on intracellular ROS levels, DNA oxidation and G1 cell cycle checkpoint. Oncogene. 2004;23:8834–8840. doi: 10.1038/sj.onc.1208108. [DOI] [PubMed] [Google Scholar]
- 93.Nuorva K, Makitaro R, Huhti E, Kamel D, Vahakangas K, Bloigu R, Soini Y, Paakko P. p53 protein accumulation in lung carcinomas of patients exposed to asbestos and tobacco smoke. Am. J. Respir. Crit. Care Med. 1994;150:528–533. doi: 10.1164/ajrccm.150.2.8049841. [DOI] [PubMed] [Google Scholar]
- 94.Husgafvel-Pursiainen K, Kannio A, Oksa P, Suitiala T, Koskinen H, Partanen R, Hemminki K, Smith S, Rosenstock-Leibu R, Brandt-Rauf PW. Mutations, tissue accumulations, and serum levels of p53 in patients with occupational cancers from asbestos and silica exposure. Environ. Mol. Mutagen. 1997;30:224–230. [PubMed] [Google Scholar]
- 95.Lin F, Liu Y, Keshava N, Li S. Crocidolite induces cell transformation and p53 gene mutation in BALB/c-3T3 cells. Teratog. Carcinog. Mutagen. 2000;20:273–281. doi: 10.1002/1520-6866(2000)20:5<273::aid-tcm3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 96.Yee H, Yie TA, Goldberg J, Wong KM, Rom WN. Immunohistochemical study of fibrosis and adenocarcinoma in dominant-negative p53 transgenic mice exposed to chrysotile asbestos and benzo(a)pyrene. J. Environ. Pathol. Toxicol. Oncol. 2008;27:267–276. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i4.30. [DOI] [PubMed] [Google Scholar]
- 97.Nymark P, Lindholm PM, Korpela MV, Lahti L, Ruosaari S, Kaski S, Hollmen J, Anttila S, Kinnula VL, Knuutila S. Gene expression profiles in asbestos-exposed epithelial and mesothelial lung cell lines. BMC Genomics. 2007;8:62. doi: 10.1186/1471-2164-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hevel JM, Olson-Buelow LC, Ganesan B, Stevens JR, Hardman JP, Aust AE. Novel functional view of the crocidolite asbestos-treated A549 human lung epithelial transcriptome reveals an intricate network of pathways with opposing functions. BMC Genomics. 2008;9:376. doi: 10.1186/1471-2164-9-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 100.Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol. Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 101.Michalak E, Villunger A, Erlacher M, Strasser A. Death squads enlisted by the tumour suppressor p53. Biochem. Biophys. Res. Commun. 2005;331:786–798. doi: 10.1016/j.bbrc.2005.03.183. [DOI] [PubMed] [Google Scholar]
- 102.Shukla A, Gulumian M, Hei TK, Kamp D, Rahman Q, Mossman BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003;34:1117–1129. doi: 10.1016/s0891-5849(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 103.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 104.Brooks CL, Gu W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell. 2006;21:307–315. doi: 10.1016/j.molcel.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat. Rev. Molec. Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 106.Aylon Y, Oren M. Living with p53, dying of p53. Cell. 2007;130:597–600. doi: 10.1016/j.cell.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 107.Olsson A, Manzl C, Strasser A, Villunger A. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 2007;14:1561–1575. doi: 10.1038/sj.cdd.4402196. [DOI] [PubMed] [Google Scholar]
- 108.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomita Y, Marchenko N, Erster S, Nemajerova A, Dehner A, Klein C, Pan H, Kessler H, Pancoska P, Moll UM. WT p53, but not tumor-derived mutants, bind to Bcl2 via the DNA binding domain and induce mitochondrial permeabilization. J. Biol. Chem. 2006;281:8600–8606. doi: 10.1074/jbc.M507611200. [DOI] [PubMed] [Google Scholar]
- 110.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid. Redox. Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 111.Essmann F, Pohlmann S, Gillissen B, Daniel PT, Schulze-Osthoff K, Janicke RU. Irradiation-induced translocation of p53 to mitochondria in the absence of apoptosis. J. Biol. Chem. 2005;280:37169–37177. doi: 10.1074/jbc.M502052200. [DOI] [PubMed] [Google Scholar]
- 112.Sengupta S, Harris CC. p53: traffic cop at the crossroads of DNA repair and recombination. Nat. Rev. Mol. Cell Biol. 2005;6:44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 113.Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006;12:440–450. doi: 10.1016/j.molmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Gredilla R, Bohr VA, Stevnsner T. Mitochondrial DNA repair and association with aging--an update. Exp. Gerontol. 2010;45:478–488. doi: 10.1016/j.exger.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R. The causes of cancer revisited: "mitochondrial malignancy" and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Mol. Aspects Med. 2010;31:145–170. doi: 10.1016/j.mam.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 116.Gredilla R, Garm C, Holm R, Bohr VA, Stevnsner T. Differential age-related changes in mitochondrial DNA repair activities in mouse brain regions. Neurobiol. Aging. 2010;31:993–1002. doi: 10.1016/j.neurobiolaging.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 118.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Nat. Acad. of Sci. U.S.A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25:400–410. [PubMed] [Google Scholar]
- 120.Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic. Biol. Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 121.Mirbahai L, Kershaw RM, Green RM, Hayden RE, Meldrum RA, Hodges NJ. Use of a molecular beacon to track the activity of base excision repair protein OGG1 in live cells. DNA Repair. 2010;9:144–152. doi: 10.1016/j.dnarep.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 122.Bohr VA, Stevnsner T, de Souza-Pinto NC. Mitochondrial DNA repair of oxidative damage in mammalian cells. Gene. 2002;286:127–134. doi: 10.1016/s0378-1119(01)00813-7. [DOI] [PubMed] [Google Scholar]
- 123.Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J. Biol. Chem. 2000;275:37518–37523. doi: 10.1074/jbc.M000831200. [DOI] [PubMed] [Google Scholar]
- 124.Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi AB, Gillespie MN. Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am. J. Physiol. Lung. Cell. Mol. Physiology. 2005;288:L530–L535. doi: 10.1152/ajplung.00255.2004. [DOI] [PubMed] [Google Scholar]
- 125.Rachek LI, Grishko VI, Ledoux SP, Wilson GL. Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic. Biol. Med. 2006;40:754–762. doi: 10.1016/j.freeradbiomed.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 126.Harrison JF, Rinne ML, Kelley MR, Druzhyna NM, Wilson GL, Ledoux SP. Altering DNA base excision repair: use of nuclear and mitochondrial-targeted N-methylpurine DNA glycosylase to sensitize astroglia to chemotherapeutic agents. Glia. 2007;55:1416–1425. doi: 10.1002/glia.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruchko MV, Gorodnya OM, Zuleta A, Pastukh VM, Gillespie MN. The DNA glycosylase Ogg1 defends against oxidant-induced mtDNA damage and apoptosis in pulmonary artery endothelial cells. Free Radic. Biol. Med. 2011;50:1107–1113. doi: 10.1016/j.freeradbiomed.2010.10.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN. Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am. J. Physiol. Lung Cell. Molec. Physiol. 2011;301:L892–L898. doi: 10.1152/ajplung.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shukla A, Jung M, Stern M, Fukagawa NK, Taatjes DJ, Sawyer D, Van Houten B, Mossman BT. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am. J. Physiol. Lung Cell. Molec. Physiol. 2003;285:L1018–L1025. doi: 10.1152/ajplung.00038.2003. [DOI] [PubMed] [Google Scholar]
- 130.Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, Nakabeppu Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Molec. Biol. Cell. 1999;10:1637–1652. doi: 10.1091/mbc.10.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takao M, Aburatani H, Kobayashi K, Yasui A. Mitochondrial targeting of human DNA glycosylases for repair of oxidative DNA damage. Nucleic Acids Res. 1998;26:2917–2922. doi: 10.1093/nar/26.12.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hashiguchi K, Stuart JA, de Souza-Pinto NC, Bohr VA. The C-terminal alphaO helix of human Ogg1 is essential for 8-oxoguanine DNA glycosylase activity: the mitochondrial beta-Ogg1 lacks this domain and does not have glycosylase activity. Nucleic Acids Res. 2004;32:5596–5608. doi: 10.1093/nar/gkh863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc. Nat. Acad. Sci. U.S.A. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc. Nat. Acad. Sci. U.S.A. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cantu D, Fulton RE, Drechsel DA, Patel M. Mitochondrial aconitase knockdown attenuates paraquat-induced dopaminergic cell death via decreased cellular metabolism and release of iron and H(2)O(2) J. Neurochem. 2011;118:79–92. doi: 10.1111/j.1471-4159.2011.07290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Molecular cancer. 2006;5:14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- 138.Park LC, Albers DS, Xu H, Lindsay JG, Beal MF, Gibson GE. Mitochondrial impairment in the cerebellum of the patients with progressive supranuclear palsy. J. Neurosci. Res. 2001;66:1028–1034. doi: 10.1002/jnr.10062. [DOI] [PubMed] [Google Scholar]
- 139.Bradley JL, Blake JC, Chamberlain S, Thomas PK, Cooper JM, Schapira AH. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum. Mol. Genet. 2000;9:275–282. doi: 10.1093/hmg/9.2.275. [DOI] [PubMed] [Google Scholar]
- 140.Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington's disease brain. Ann. Neurol. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 141.Chen XJ, Wang X, Kaufman BA, Butow RA. Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science. 2005;307:714–717. doi: 10.1126/science.1106391. [DOI] [PubMed] [Google Scholar]
- 142.Youn CK, Song PI, Kim MH, Kim JS, Hyun JW, Choi SJ, Yoon SP, Chung MH, Chang IY, You HJ. Human 8-oxoguanine DNA glycosylase suppresses the oxidative stress induced apoptosis through a p53-mediated signaling pathway in human fibroblasts. Mol. Cancer. Res. 2007;5:1083–1098. doi: 10.1158/1541-7786.MCR-06-0432. [DOI] [PubMed] [Google Scholar]
- 143.Bacsi A, Chodaczek G, Hazra TK, Konkel D, Boldogh I. Increased ROS generation in subsets of OGG1 knockout fibroblast cells. Mech. Ageing Dev. 2007;128:637–649. doi: 10.1016/j.mad.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xie Y, Yang H, Miller JH, Shih DM, Hicks GG, Xie J, Shiu RP. Cells deficient in oxidative DNA damage repair genes Myh and Ogg1 are sensitive to oxidants with increased G2/M arrest and multinucleation. Carcinogenesis. 2008;29:722–728. doi: 10.1093/carcin/bgn033. [DOI] [PubMed] [Google Scholar]
- 145.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 146.Bulteau AL, O'Neill HA, Kennedy MC, Ikeda-Saito M, Isaya G, Szweda LI. Frataxin acts as an iron chaperone protein to modulate mitochondrial aconitase activity. Science. 2004;305:242–245. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- 147.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, Schneider-Stock R, Gali-Muhtasib H. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol. Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 148.Tsui KH, Feng TH, Lin YF, Chang PL, Juang HH. p53 downregulates the gene expression of mitochondrial aconitase in human prostate carcinoma cells. Prostate. 2011;71:62–70. doi: 10.1002/pros.21222. [DOI] [PubMed] [Google Scholar]
- 149.Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab. Invest. 2004;84:1013–1023. doi: 10.1038/labinvest.3700109. [DOI] [PubMed] [Google Scholar]
- 150.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, Barcellos-Hoff MH. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat. Res. 2006;166:839–848. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 151.Gorowiec MR, Borthwick LA, Parker SM, Kirby JA, Saretzki GC, Fisher AJ. Free radical generation induces epithelial-to-mesenchymal transition in lung epithelium via a TGF-beta1-dependent mechanism. Free Radic. Biol. Med. 2012;52:1024–1032. doi: 10.1016/j.freeradbiomed.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 152.Cui Y, Robertson J, Maharaj S, Waldhauser L, Niu J, Wang J, Farkas L, Kolb M, Gauldie J. Oxidative stress contributes to the induction and persistence of TGF-beta1 induced pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2011;43:1122–1133. doi: 10.1016/j.biocel.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Kayanoki Y, Fujii J, Suzuki K, Kawata S, Matsuzawa Y, Taniguchi N. Suppression of antioxidative enzyme expression by transforming growth factor-beta 1 in rat hepatocytes. J. Biol. Chem. 1994;269:15488–15492. [PubMed] [Google Scholar]
- 154.Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGF- beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–1903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 155.Herrera B, Alvarez AM, Sanchez A, Fernandez M, Roncero C, Benito M, Fabregat I. Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor (beta) in fetal hepatocytes. FASEB J. 2001;15:741–751. doi: 10.1096/fj.00-0267com. [DOI] [PubMed] [Google Scholar]
- 156.Albright CD, Salganik RI, Craciunescu CN, Mar MH, Zeisel SH. Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-beta1 in immortalized rat hepatocytes. J. Cell. Biochem. 2003;89:254–261. doi: 10.1002/jcb.10498. [DOI] [PubMed] [Google Scholar]
- 157.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Molec. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 158.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, Fabregat I. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J. Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 159.Sancho P, Bertran E, Caja L, Carmona-Cuenca I, Murillo MM, Fabregat I. The inhibition of the epidermal growth factor (EGF) pathway enhances TGF-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim. Biophys. Acta. 2009;1793:253–263. doi: 10.1016/j.bbamcr.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 160.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am. J. Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am. J. Physiol. Ren. Physiol. 2007;293:F723–F731. doi: 10.1152/ajprenal.00480.2006. [DOI] [PubMed] [Google Scholar]
- 163.Yao HW, Xie QM, Chen JQ, Deng YM, Tang HF. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76:29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 164.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir. Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Margetts PJ, Bonniaud P, Liu L, Hoff CM, Holmes CJ, West-Mays JA, Kelly MM. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 2005;16:425–436. doi: 10.1681/ASN.2004060436. [DOI] [PubMed] [Google Scholar]
- 166.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Nat. Acad. Sci. U.S.A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, Lee HB. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 168.Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS, Liebler JM, Minoo P, Crandall ED, Borok Z. Interactions between beta-catenin and transforming growth factor-beta signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP) J. Biol. Chem. 2012;287:7026–7038. doi: 10.1074/jbc.M111.276311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang DY, Wang HJ, Tan YZ. Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PloS One. 2011;6:e21397. doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wen JW, Hwang JT, Kelly GM. Reactive oxygen species and Wnt signalling crosstalk patterns mouse extraembryonic endoderm. Cell. Signal. 2012;24:2337–2348. doi: 10.1016/j.cellsig.2012.07.024. [DOI] [PubMed] [Google Scholar]