Abstract
Human γδ T cells display potent cytotoxicity against various tumor cells pretreated with zoledronic acid (Zol). Zol has shown benefits when added to adjuvant endocrine therapy for patients with early-stage breast cancer or to standard chemotherapy for patients with multiple myeloma. Although γδ T cells may contribute to this additive effect, the responsiveness of γδ T cells from early-stage breast cancer patients has not been fully investigated. In this study, we determined the number, frequency, and responsiveness of Vγ2Vδ2 T cells from early- and late-stage breast cancer patients and examined the effect of IL-18 on their ex vivo expansion. The responsiveness of Vγ2Vδ2 T cells from patients with low frequencies of Vγ2Vδ2 T cells was significantly diminished. IL-18, however, enhanced ex vivo proliferative responses of Vγ2Vδ2 T cells and helper NK cells from patients with either low or high frequencies of Vγ2Vδ2 T cells. Treatment of breast cancer patients with Zol alone decreased the number of Vγ2Vδ2 T cells and reduced their ex vivo responsiveness. These results demonstrate that Zol can elicit immunological responses by γδ T cells from early-stage breast cancer patients, but that frequent in vivo treatment reduces Vγ2Vδ2 T cell numbers and their responsiveness to stimulation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-012-1368-4) contains supplementary material, which is available to authorized users.
Keywords: γδ T cells, Helper NK cells, Breast cancer, Phosphoantigen, N-BP, IL-18
Introduction
Vγ2Vδ2 T cells (also termed Vγ9Vδ2 T cells) recognize phosphoantigens derived from isoprenoid pathways, including isopentenyl diphosphate (IPP) and (E)-4-hydroxy-3-methylbut-2-enyl diphosphate (HMBPP), in an MHC class I- and MHC class II-independent manner [1–9]. Vγ2Vδ2 T cells target and lyse tumor cells that have been treated with nitrogen-containing bisphosphonates (N-BPs), such as pamidronate, zoledronic acid (Zol) and risedronate, in a γδ TCR-dependent manner [10–12]. N-BPs have been used as therapeutic agents for osteoporosis and hypercalcemia of malignancy. The primary target for N-BPs is farnesyl diphosphate synthase (FPPS) whose inhibition leads to the accumulation of its upstream metabolite, IPP [13, 14]. Although the precise molecular mechanism underlying Vγ2Vδ2 TCR recognition of phosphoantigens presented by tumor cells remains unclear, stimulation of γδ T cells has received much attention as a potential therapeutic intervention for infections and malignancies [15, 16].
Zoledronic acid has recently been tested in clinical trials as an additive therapeutic for early-stage breast cancer and multiple myeloma. In early-stage breast cancer, the addition of Zol to standard therapy improved disease-free survival in estrogen receptor–positive patients in a low-estrogen environment [17–19]. Zol also had treatment benefits in patients with newly diagnosed multiple myeloma—improving overall survival independently of the prevention of skeletal-related events [20]. Taken together, these studies clearly demonstrate that Zol has a beneficial effect on cancer patients. However, the mechanism by which Zol mediates these effects is still unclear since the effects appear to extend beyond the prevention of skeletal-related events [20, 21]. This has lead to the proposal that Vγ2Vδ2 T cells contribute to the observed beneficial effect of Zol in early-stage breast cancer patients [22]. Moreover, adoptive Vγ2Vδ2 T cell therapy has shown some promise in the treatment of breast cancer with a complete remission achieved in one patient with concurrent hormonal therapy that had previously failed hormonal, radiation, and chemotherapy treatments [23]. To understand the role of Vγ2Vδ2 T cells in early-stage breast cancer patients treated with Zol and to develop γδ T cell treatments for these patients, we have investigated the characteristics of Vγ2Vδ2 T cells from these patients and have determined the ability of Vγ2Vδ2 T cells to expand ex vivo and the effect of IL-18 on this expansion.
Materials and methods
Patients
Breast cancer patients (n = 80) were enrolled after institutional review board approval and with written informed consent. Patient characteristics are summarized in Online Resource 1. Early-stage (stage 0, I, or II) breast cancer patients constituted 62/80 (77.5 %) of the patients, whereas late-stage (stage III, IV) patients constituted 18/80 (22.5 %).
Reagents
Zol was purchased from Novartis Pharmaceuticals Corp. (Basel, Switzerland) and converted to its sodium salt using a Na+ form of Dowex 50 W × 8 (Muromachi Kogyo Kaisha Ltd., Chuo-ku, Tokyo, Japan). Recombinant human IL-18 and IL-2 were kindly provided by GlaxoSmithKline plc (Research Triangle Park, NC) and Shionogi Pharmaceutical Co., Ltd. (Chuo-ku, Osaka, Japan), respectively.
Stimulation of Vγ2Vδ2 T cells
PBMC were purified as described in Online Resource 2. The cells were stimulated with Zol/IL-2 or Zol/IL-2/IL-18 for 2–10 days as described in Online Resource 3.
Flow cytometry and mAbs
Flow cytometric analysis was performed using a FACSCalibur cytometer (Becton–Dickinson, Franklin Lakes, NJ). The following Abs were used: fluorescein isothiocyanate (FITC)-conjugated anti-Vδ1 and anti-Vδ2 mAbs (Beckman Coulter Inc., Fullerton, CA), phycoerythrin (PE)-conjugated anti-CD3 mAb (BD Biosciences, San Diego, CA), and isotype control Abs (Biolegend, San Diego, CA), and FcR blocking reagent (human, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The gating strategy is depicted in Online Resource 2.
Cytokine production
PBMC were stimulated with 5 μM of Zol and 100 U/ml of IL-2 in the presence or absence of 100 ng/ml of IL-18. After incubation at 37 °C with 5 % CO2 for 2 days, the culture supernatants were frozen at −80 °C overnight. The samples were then thawed and IFN-γ and TNF-α cytokine levels determined by ELISA (Peprotech, Rocky Hill, NJ).
Effect of repeated in vivo injections of Zol
A breast cancer patient (stage II) was given 4 mg of Zol twice, 1 month apart. Peripheral blood samples were obtained just before the first injection, 1 month after the first injection, and 1 month after the second injection. PBMC were purified and cultured for 14 days as described in Online Resource 3. The cells before and after culture were stained and analyzed using a FACSCalibur flow cytometer.
Statistical analysis
Correlations between Vδ2+ T cell frequencies on day 0 and cell numbers or Vδ2+ T cell frequencies on day 10, or between the Vδ2+ T cell frequencies on day 0 and cytokine production on day 2 were examined using Spearman’s rank correlation coefficient (ρ). Paired t test was used to assess the effect of IL-18 on the expansion of Vδ2+ T cells and helper NK cells and cytokine productions. A p value of less than 0.05 was considered statistically significant.
Results
The frequency of Vδ2+ T cells in breast cancer patients is inversely correlated with age and disease stage
To characterize γδ T cells in breast cancer patients, we first determined the frequencies of Vδ1+ and Vδ2+ γδ T cells among CD3+ T cells in peripheral blood mononuclear cells (PBMC) from 80 patients with breast cancer composed of 62 early-stage (stage 0–II) patients and 18 late-stage (stage III, IV) patients. Overall, the frequency of Vδ2+ T cells (5.2 ± 6.8 %) was significantly higher than that of Vδ1+ T cells (1.5 ± 1.7 %) in both early- and late-stage patients (Fig. 1 and Online Resource 2). Whereas 57.5 % of patients had Vδ1+ T cell frequencies of less than 1 %, only 21.2 % showed Vδ2+ T cell frequencies of less than 1 % (Fig. 1). As summarized in Online Resource 1 and Table 1, the frequency of Vδ2+ T cells inversely correlated with the age (p = 0.044) and disease stage (p = 0.001). When stratified by disease stages, there was no significant difference in Vδ1+ T cell frequencies between early- and late-stage patients (early: 1.4 ± 1.4 %; late: 1.7 ± 2.5 %). In contrast, higher Vδ2+ T cell frequencies were observed in early-stage patients compared with late-stage patients (early: 5.9 ± 7.4 %; late: 2.6 ± 2.8 %). Although the proportion of patients whose Vδ2+ T cell frequencies were less than 1 % was essentially the same between early- and late-stage patients (early: 21.0 %; late: 22.2 %), a markedly higher proportion of early-stage patients had Vδ2+ T cell frequencies of greater than 5.2 % compared with late-stage patients (early: 35.5 %; late: 5.6 %). This difference in Vδ2+ T cell frequency between early- and late-stage patients suggests that Vγ2Vδ2 T cells might be immunologically perturbed based on the severity of disease. Besides age and cancer stage, there was no correlation with any other patient background characteristic, with the expression of either the human epidermal growth factor receptor 2 (HER2) or the estrogen receptor (ER), or with the cancer subtype.
Fig. 1.
Frequencies of peripheral blood Vδ1+ and Vδ2+ T cells in early- and late-stage breast cancer patients. a Dot plots of PBMC from breast cancer patients stained for expression of CD3 and Vδ1 or Vδ2. PBMC derived from 80 patients were analyzed by two-color flow cytometric analysis. Values shown are the percent of CD3+ T cells. Dot plots of three representative patients are shown (BC01: stage II; BC02: stage II; BC03: stage I). b Frequency of Vδ1+ and Vδ2+ T cells in early- (stage 0–II) and late-stage (stage III, IV) breast cancer patients. The percentages of Vδ1+ and Vδ2+ T cells were calculated based on the dot plots and are depicted in the scatter plots
Table 1.
Relationship between patients’ characteristics and Vγ2/CD3 ratio
| N | 80 | <5.2 % | ≥5.2 % | Total | p |
|---|---|---|---|---|---|
| Age | <60 | 23 | 15 | 38 | 0.044 |
| ≥60 | 34 | 8 | 42 | ||
| Stage | 0, I, II | 40 | 22 | 62 | 0.001 |
| III, IV | 17 | 1 | 18 | ||
| Grade | 1, 2 | 36 | 18 | 54 | 0.194 |
| 3 | 14 | 5 | 19 | ||
| Unknown | 7 | ||||
| Node | + | 20 | 5 | 25 | 0.113 |
| − | 29 | 18 | 47 | ||
| Unknown | 8 | ||||
| ER | Positive | 39 | 15 | 54 | 0.384 |
| Negative | 13 | 8 | 21 | ||
| Unknown | 5 | ||||
| HER2 | Positive | 11 | 3 | 14 | 0.406 |
| Negative | 41 | 20 | 61 | ||
| Unknown | 5 | ||||
| Ki67 | <14 % | 20 | 14 | 34 | 0.097 |
| ≥14 % | 30 | 9 | 39 | ||
| Unknown | 7 | ||||
| Subtype | Luminal A | 17 | 11 | 28 | 0.494* |
| Luminal B | 12 | 4 | 16 | ||
| Luminal–Her2 | 8 | 0 | 8 | ||
| HER2 | 3 | 3 | 6 | ||
| Triple negative | 10 | 5 | 15 | ||
| Unknown | 7 |
* Luminal A plus B versus other subtypes. Correlations between the Vδ2/CD3 ratio and clinicopathological parameters were assessed using χ 2 test. A p value of less than 0.05 was considered to indicate statistically significant
Ex vivo expansion of Vγ2Vδ2 T cells from early-stage breast cancer patients stimulated by Zol/IL-2
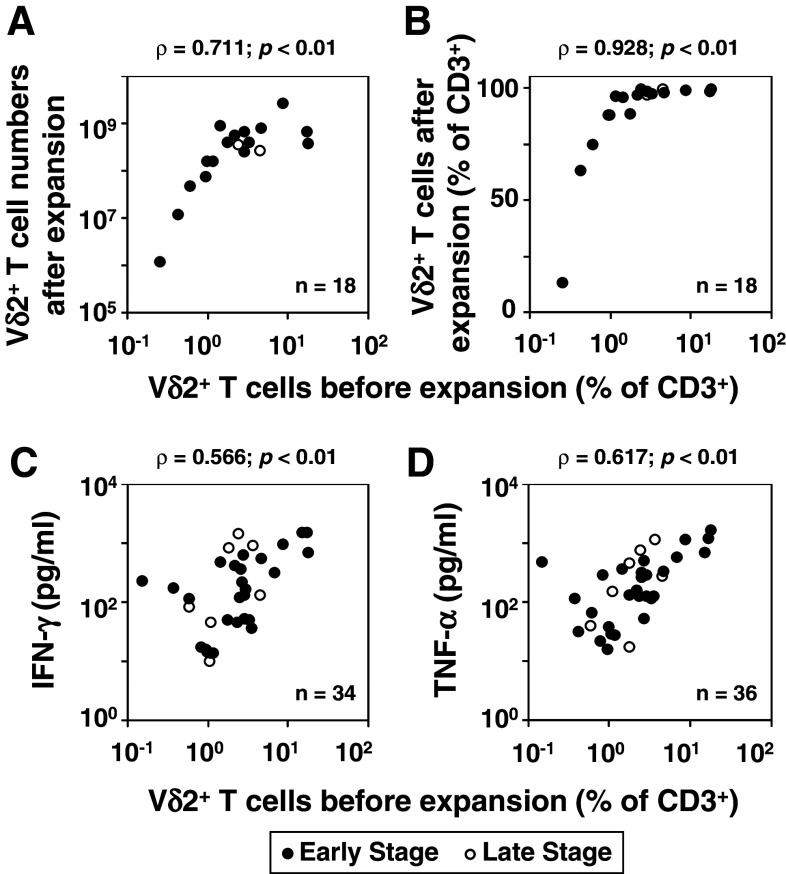
Adoptive immunotherapy with Vγ2Vδ2 T cells requires their ex vivo expansion. To assess the feasibility of adoptive immunotherapy with Vγ2Vδ2 T cells in early-stage breast cancer, PBMC from patients were stimulated with Zol in the presence of IL-2 and the expansion of Vδ2+ T cells determined by flow cytometry 10 days later. Dot plots of four representative breast cancer patients are shown (Fig. 2 and Online Resource 2). The proliferation of Vδ2+ T cells to Zol/IL-2 correlated with the initial Vδ2+ T cell frequency. Patients with 1 % or greater Vγ2Vδ2 T cells at day 0 expanded to near 100 % of the T cells by day 10. Both the number (ρ = 0.711; p < 0.01, Fig. 3a, closed circles) and the frequency (ρ = 0.928; p < 0.01, Fig. 3b, closed circles) of Vγ2Vδ2 T cells strongly correlated with the initial Vδ2+ T cell frequency. Patients with initial Vδ2+ T cell frequencies of 1 % or less expanded poorly.
Fig. 2.
Zol/IL-2-induced expansion of Vγ2Vδ2 T cells from PBMC of breast cancer patients. PBMC derived from 18 breast cancer patients were analyzed for expression of CD3 and Vδ2. The cells were cultured in the presence of Zol and IL-2 for 10 days and analyzed by two-color flow cytometry. Dot plots of four representative patients (BC04: stage II; BC05: stage II; BC06: stage I; BC07: stage II) are shown. Values shown are the percent of CD3+ T cells
Fig. 3.
Correlation between the initial frequency of Vδ2+ T cells and the responsiveness of Vδ2+ T cells to Zol/IL-2 stimulation. a Correlation between the initial frequencies of Vδ2+ T cells and their numbers after Zol/IL-2 stimulation. b Correlation between the initial proportions of Vδ2+ T cells and their proportions after Zol/IL-2 stimulation. c, d Correlation between the initial frequency of Vδ2+ T cells and their production of IFN-γ (c) and TNF-α (d) after Zol/IL-2 stimulation. PBMC from 18 patients with breast cancer were stimulated with Zol/IL-2 for 10 days, the number of Vδ2+ T cells determined by means of dye exclusion and flow cytometry, and the data shown as a scatter plot. For production of IFN-γ and TNF-α, PMBC from 34 (c) or 36 (d) patients were stimulated with Zol/IL-2 for 2 days, the culture supernatants harvested, and IFN-γ or TNF-α levels determined by ELISA. The cytokine levels are plotted against the initial frequency of Vδ2+ T cells. Data obtained from early- and late-stage breast cancer patients were plotted as closed and open circles, respectively
We also analyzed the correlation between the initial Vδ2+ T cell frequency and the expansion rate of Vδ2+ T cells. When stratified by the initial Vδ2+ T cell frequency, the average expansion rates ± SD were 722 ± 284-fold, 1,610 ± 588-fold, and 3,447 ± 1,823-fold for patients whose initial frequency was <0.5, 0.5–1, and 1–10 %, respectively. This demonstrated that the responsiveness of Vδ2+ T cells from patients with low initial frequency was functionally impaired. In addition, the Vδ2+ T cells/CD3 ratios after expansion by Zol for 10 days were 38.2 ± 35.3, 83.7 ± 7.5, and 96.9 ± 3.1 %, respectively. Whereas the functional impairment was prominent when the initial frequency was less than 0.5 %, the relatively large SD, 35.3 %, indicated variability in responsiveness among patients with low initial frequency of Vδ2+ T cells.
Similarly, the production of IFN-γ (ρ = 0.566; p < 0.01) and TNF-α (ρ = 0.617; p < 0.01) also correlated with the initial frequency of Vδ2+ T cells (Figs. 3c, d, respectively). The correlation between the initial Vδ2+ T cell frequency and the responsiveness of Vδ2+ T cells to Zol/IL-2 stimulation was also observed in late-stage breast cancer patients (Fig. 3; open circles).
Effect of IL-18 on the ex vivo expansion of Vγ2Vδ2 T cells from early-stage breast cancer patients
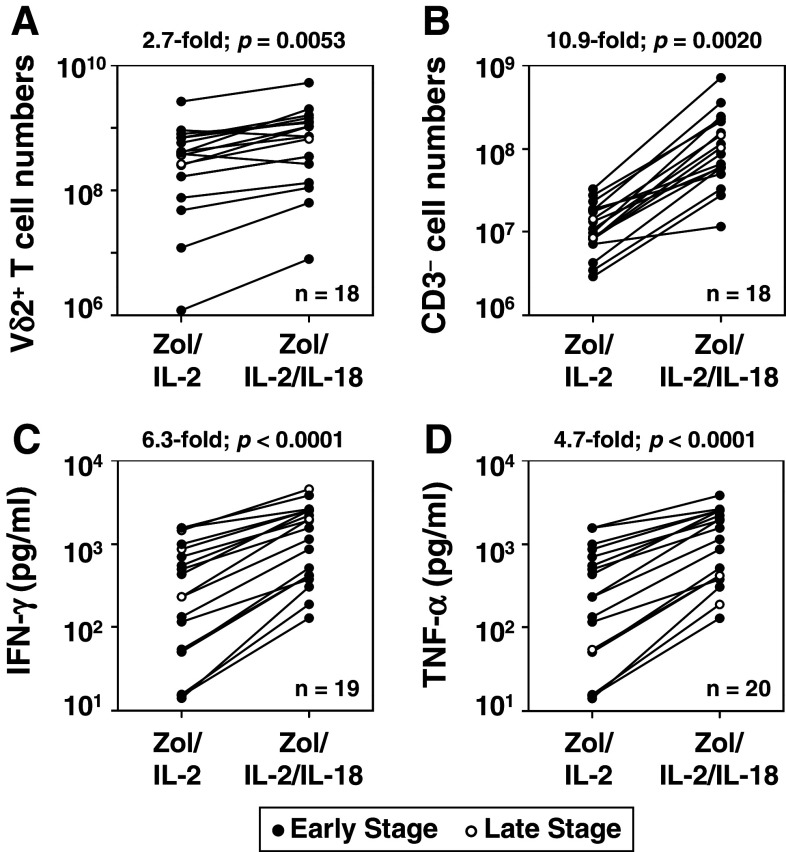
Significant numbers of breast cancer patients had initial Vδ2+ T cell frequency of 1 % or less (Fig. 1b). Based on our findings, for some patients, Zol treatment might not stimulate in vivo Vγ2Vδ2 T cell expansions and ex vivo expansion of Vγ2Vδ2 T cells for adoptive immunotherapy would be poor. We previously reported that IL-18 promoted expansion of γδ T cells from healthy individuals [24]. Therefore, the effect of IL-18 on the expansion of Vγ2Vδ2 T cells from breast cancer patients was investigated. Addition of IL-18 to Zol/IL-2 treatment significantly increased the expansion of Vδ2+ T cells by 2.7-fold (p = 0.0053) (Fig. 4a). For example, for three representative breast cancer patients, the addition of IL-18 increased the total number of Vδ2+ T cells 2.4–4.1-fold (BC08: 1.1 × 108 cells to 3.5 × 108 cells, BC09: 5.8 × 108 cells to 14.2 × 108 cells, and BC10: 2.5 × 108 cells to 10.4 × 108 cells) (Online Resource 3). The proportion and the total number of CD3− cells were also significantly up-regulated by the addition of IL-18. For the three representative breast cancer patients (Online Resource 3), IL-18 addition increased CD3− cells 7.7–10.3-fold (BC08: 11.6–38.8 %; 2.3 × 107 cells to 22.9 × 107 cells, BC09: 1.8–7.1 %; 1.1 × 108 cells to 11.3 × 108 cells, BC10: 1.4–3.0 %; 2.7 × 108 cells to 20.8 × 108 cells). Thus, CD3− cells increased by an average of 10.9-fold (p = 0.0020) (Fig. 4b). The addition of IL-18 to PBMC stimulated with Zol/IL-2 also increased the production of INF-γ by 6.3-fold (p < 0.0001) and TNF-α by 4.7-fold (p < 0.0001) (Fig. 4c, d, respectively). This enhancement by IL-18 of proliferative responses and cytokine secretion was observed in all patients regardless of their initial Vδ2+ T cell frequency, demonstrating that Vγ2Vδ2 T cells could be expanded by Zol/IL-2/IL-18 even in cancer patients with lower Vδ2+ T cell frequencies.
Fig. 4.
IL-18 augments expansion of Vδ2+ T cells and CD3− “helper” NK cells and the effector functions of Vδ2+ T cells. a, b Effect of IL-18 on the expansion of Vδ2+ T cells (a) and CD3− “helper” NK cells (b). PBMC derived from 18 breast cancer patients were stimulated with Zol/IL-2 or Zol/IL-2/IL-18 for 10 days, and the numbers of Vδ2+ T cells and CD3− “helper” NK cells determined by vital dye exclusion and flow cytometry. Each line connects the cell numbers after expansion by Zol/IL-2 with the cell numbers after expansion by Zol/IL-2/IL-18 from the same patient. c, d Effect of IL-18 on the production of IFN-γ (c) and TNF-α (d) by PBMC after stimulation with Zol/IL-2 or Zol/IL2/IL-18. PBMC from 19 (c) or 20 (d) breast cancer patients were stimulated with Zol/IL-2 or Zol/IL-2/IL-18 for 2 days, the culture supernatants harvested, and IFN-γ and TNF-α levels determined by ELISA. Each line connects cytokines produced in response to Zol/IL-2 or Zol/IL-2/IL-18 by PBMC from the same patient. Data obtained from early- and late-stage breast cancer patients were plotted as closed and open circles, respectively
Expansion of “helper” NK cells from early-stage breast cancer patients after Zol/IL-2/IL-18 stimulation and functional analyses of expanded Vδ2+ T cells
After stimulation of PBMC with Zol/IL-2 in the presence or absence of IL-18 for 10 days, the majority of the cells expressed Vδ2 TCR, and the remaining cells consisted of CD3− cells and a few CD3+ αβ T cells (Online Resource 3, right panels). Because NK cells with “helper” properties have been reported to develop under the influence of IL-2 and IL-18 [25], we examined cell surface markers expressed by CD3− cells. Consistent with the identification of the CD3− cells as NK cells, almost all of these cells expressed NK surface markers including CD56, NKG2D, and CD16, but not monocytic markers such as CD14 (Online Resource 4). We also examined expression of APC-related molecules, such as CD86, CD80, HLA-DR, and HLA-DQ—all were strongly expressed, whereas the expression of NKp44, a positive NK receptor, was down-regulated, suggesting that “helper” NK cells were expanded along with Vδ2+ T cells.
Frequent Zol administration as used in the AZURE trial leads to decreasing blood Vγ2Vδ2 T cell levels and decreased ability for their ex vivo expansion
Some recent clinical trials on early breast cancer patients demonstrated additional clinical benefits when Zol was added to standard cancer therapy [26]. Whereas two trials (the Austrian Breast and Colorectal Cancer Study Group trial-12 (ABCSG-12) [17, 18] and the Zometa-Ferma Adjuvant Synergy Trials (ZO-FAST) [19]) showed clinical benefits for most patients, another (the Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) trial [27]) showed benefits only with postmenopausal women. Besides the increased frequency of chemotherapy [27], one other important difference between the trials was that the AZURE trial gave Zol every 3 weeks initially for 6 doses and quite frequently thereafter, whereas the ABCSG-12 and ZO-FAST trials gave Zol only every 6 months. Therefore, we examined the effect of in vivo Zol administration on Vδ2+ T cells in breast cancer patients when given at 4 week intervals. The Vδ2+ T cell frequency in the peripheral blood of one stage II patient decreased after repeated Zol injection spaced at 1 month interval from 0.79 % prior to therapy to 0.55 % after the first dose, and 0.46 % after the second dose (Fig. 5). The efficiency of ex vivo Vδ2+ T cell expansion also progressively decreased, with Vδ2/CD3 ratios of 94.2, 88.7, and 64.0 %, and numbers of Vδ2+ cells of 4.00 × 108, 2.68 × 108, 0.08 × 108, respectively. Similar decreases in peripheral blood Vγ2Vδ2 T cells were noted in four other patients withVδ2+ T cell frequencies decreasing from 3.7 to 3.0 % (stage IV), 1.8–1.4 % (stage IV), 0.6–0.3 % (stage IV), and 0.6–0.3 % (stage I). Overall, Vδ2+ T cell frequency in the blood decreased an average of 34.4 % for the five patients 1 month after one dose of Zol. These pilot studies suggest that frequent Zol treatments of breast cancer patients leads to decreasing blood Vγ2Vδ2 T cell levels and decreased ability for ex vivo expansion.
Fig. 5.
Effect of repeated in vivo injections of Zol on the Vγ2Vδ2 T cells of an early-stage breast cancer patient. a Dot plots showing ex vivo expansion of Vγ2Vδ2 T cells by Zol/IL-2/IL-18 before or after one or two Zol injections. b Effect of Zol administration to a breast cancer patient on the frequency of blood Vδ2+ T cells. The initial Vδ2+ T cell frequency before Zol injection (0), 1 month after the first injection (1), or 1 month after the second injection (2). c Ex vivo expansion of Vδ2+ T cells by Zol/IL-2/IL-18 from PBMC derived from a patient before receiving Zol or after receiving Zol once or twice. The effect of repetitive Zol injection on Vδ2+ T cells as % of CD3 (left panel) or as cell numbers (right panel) before treatment (closed circles), 1 month after the first injection (closed triangles), or 1 month after the second injection (closed squares)
Discussion
Recent clinical trials demonstrate that Zol provides clinical benefits in the treatment of patients with early-stage breast cancer and multiple myeloma, although the contribution of γδ T cells to the beneficial effects is unclear [26]. In the present study, we have examined the number, frequency, and responsiveness of peripheral blood γδ T cells from patients with breast cancer. The average Vδ2+ T cell frequency was significantly higher in early-stage patients compared with late-stage patients (5.9 vs 2.6 %) and slightly higher than healthy donors (4.3 ± 2.2 %). In fact, many breast cancer patients had elevated Vδ2+ T cell frequencies with 35.5 % of patients having greater than 5.2 % Vδ2+ T cells (22/62) and 19.4 % (12/62) greater than 10 %. When stratified by patients’ background, the frequency of Vδ2+ T cells correlated negatively with age and disease stages. These findings further support the hypothesis that Vγ2Vδ2 T cells are immunologically perturbed by the development of mammary carcinomas.
The expansion of Vδ2+ T cells was impaired in some breast cancer patients, consistent with a recent report [23]. To study this hyporesponsiveness, we stimulated PBMC from donors with varying frequencies of Vδ2+ TCR with Zol/IL-2 and compared the relationship between the responsiveness of the Vδ2+ T cells with the initial Vδ2+ T cell frequencies. The frequency of Vδ2+ T cells before expansion strongly correlated with both the number and the percentage of Vδ2+ T cells after expansion. This correlation was most evident when the initial frequency of Vδ2+ T cells was less than 1 %. Because the proportion of patients with a Vδ2+ T cell frequency of less than 1 % is significantly higher in breast cancer patients than in healthy individuals, it is thus more difficult to expand Vδ2+ T cells from patients. IFN-γ and TNF-α cytokine production also correlated with the initial frequency of Vδ2+ T cells although somewhat less strongly. This may be because the inhibition of FPPS by Zol also affects other cells in PBMC, such as monocytes and NK cells, and can cause apoptosis that may stimulate cytokine secretion by innate NK cells [28].
We previously reported that IL-18 enhanced proliferative responses of Vδ2+ T cells from healthy donors in response to phosphoantigens [24]. In this study, we examined whether IL-18 could compensate for the low Vδ2+ T cell frequencies observed in many breast cancer patients. IL-18 increased the numbers of Vδ2+ T cells after expansion by Zol/IL-2 and augmented cytokine secretion regardless of the initial frequency of Vδ2+ T cells, thus demonstrating that IL-18 would be useful in expanding Vδ2+ T cells from breast cancer patients with low numbers of blood Vδ2+ T cells.
In healthy individuals, CD11cintCD56+ cells are concomitantly increased in cultures of PBMC stimulated with Zol/IL-2/IL-18 and are likely involved in the enhancement of Vδ2+ T cell proliferation [24]. In fact, the depletion of CD56+ cells from PBMC resulted in only poor proliferation of Vδ2+ T cells [24]. The present study demonstrated that in breast cancer patients this subset can expand to a greater degree than Vδ2+ T cells. These cells exhibited a “helper” APC phenotype because they expressed the APC-related molecules, HLA-DQ, HLA-DR, CD86, and CD80. This is consistent with previous reports that NK cells may acquire a “helper” phenotype upon exposure to IL-18 [25, 29]. We therefore have termed this subset “helper” NK cells. Although “helper” NK cells appear to preferentially promote expansion of Vδ2+ T cells under the condition in this study, the precise mechanism underlying the helper function remains to be determined. Because large aggregates of cells are observed in cultures in the presence of Zol/IL-2/IL-18 [24] and because the disruption of adhesion molecule-mediated cell-to-cell contact by anti-LFA-1 mAb greatly reduced the proliferative responses of Vδ2+ T cells [9], promotion of cell-to-cell contact by IL-18 may play a pivotal role in the marked expansion observed. Interestingly, the CD11cintCD56+ cells tend to lose the expression of CD11c as culture proceeds, and the resulting CD11c−/lowCD56+ cells no longer support the proliferation of Vδ2+ T cells [24]. These findings suggest that IL-18/IL-2 might primarily promote the differentiation and expansion of “helper” NK cells, which in turn support the expansion of Vδ2+ T cells through the adhesion molecule-mediated interaction. Studies to identify the precursor of this NK subset and its differentiation pathway are ongoing.
While the effect of IL-18 on Vδ2+ T cells is not as marked as that on “helper” NK cells, Vδ2+ T cells also tend to develop a stronger “helper” APC phenotype upon stimulation with phosphoantigens in the presence of IL-18 given that there is further up-regulation of the expression of APC-related molecules. Because both cell subsets can be considered to be types of innate immune cells and play a crucial role in provoking adaptive immune responses, it is reasonable to assume that “helper” NK cells and Vδ2+ T cells present classical peptide antigens to adaptive αβ T cells, through which effective, highly specific immune reactions take place to eliminate infected cells and malignant tumors [30]. Whereas we focused on the effect of “helper” NK cells on Vδ2+ T cells in this study, a recent report also suggested that Vδ2+ T cells induced robust NK-cell-mediated cytotoxicity through CD137 engagement, indicating a cross-talk between NK cells and Vδ2+ T cells [31].
Endogenous production of IL-18 also played a role in Zol/IL-2 responses. Both cell aggregation and cytokine production were impaired by blocking IL-18 signaling, clearly supporting a crucial role of IL-18 in the expansion of Vδ2+ T cells [24]. Inhibition of mevalonate pathway by Zol inhibition of FPPS results in the depletion of downstream isoprenoid metabolites, including geranylgeraniol, that in turn is likely to lead to the activation of caspase-1 [32]. Because IL-18 is processed by caspase-1 to become a biologically active species, the exposure of cells to Zol may enhance the production of active IL-18. Although it is difficult to define the physiological function of IL-18, this study shows that the cytokine may play an important role in cytotoxic activity elicited by Vδ2+ T cells.
The presence of IL-2 and other T cell growth factors, such as IL-15, is essential for the long-term proliferative response of Vδ2+ T cells, as opposed to short-term responses where Vδ2+ T cells themselves transiently produce IL-2 [33]. We therefore examined the in vivo effect of Zol on peripheral blood Vδ2+ T cells in breast cancer patients who underwent standard Zol therapy monthly without exogenous IL-2. The number of peripheral blood Vδ2+ T cells decreased after repetitive Zol administration as did their ex vivo expansion, clearly demonstrating that Vδ2+ T cells are negatively affected by frequent Zol treatment [34–36]. A previous preclinical study showed that the infusion of low-dose IL-2 together with Zol successfully expanded Vγ2Vδ2 T cells in patients with prostate cancer and breast cancer [37, 38]. Thus, IL-2 or another T cell growth factor is necessary when Zol infusion is used to augment Vδ2+ T cells for cancer immunotherapy. Otherwise, Zol infusion will lead to the exhaustion of Vδ2+ T cells, and this subset will dwindle to a marginal level where the efficient expansion of Vδ2+ T cells is unfeasible. Consistent with these findings, it has been well documented that patients suffer from fever and flu-like symptoms due to the activation of Vδ2+ T cells after the initial infusion of N-BP, but that the subsequent infusion of N-BP 1–2 months later does not cause such symptoms [39].
This loss of Vγ2Vδ2 T cell function may partially explain the differences observed between the various clinical trials of Zol in early-stage breast cancer patients. Whereas the AZURE trial gave Zol every 3–4 weeks for 6 doses, every 3 months for 8 doses, and then every 6 months [27], the ABCSG-12 [18] and ZO-FAST [19] trials gave Zol much less frequently—only every 6 months. This long interval between Zol infusion in the ABCSG-12 and ZO-FAST trials may allow partial recovery of Vγ2Vδ2 T cell immune function. Consistent with this possibility, we found that administration of Zol twice in 2 months in rhesus monkeys caused decreased Vγ2Vδ2 T cell responsiveness, but that partial recovery of Vγ2Vδ2 T cell responsiveness was observed in 3 out of 4 monkeys after a rest period of 9 months (C. Jin and C. T. Morita, personal observation). Thus, less frequent Zol infusion may actually be better than intensive Zol therapy at promoting the immunotherapeutic effects of Vγ2Vδ2 T cells in early-stage breast cancer patients. Less-intensive therapy would also serve to decrease the frequency of osteonecrosis of the jaw, a major side effect of intensive Zol therapy.
Besides direct in vivo administration of Zol, adoptive immunotherapy with Vγ2Vδ2 T cells expanded ex vivo with either Zol or 2-methyl-3-butenyl diphosphate has shown promise in clinical trials. Complete remissions have been observed after adoptive immunotherapy with Vγ2Vδ2 T cells in an early-stage breast cancer patient also treated with hormonal suppression therapy [23] and a renal cancer patient with metastatic disease [40]. Our results clearly demonstrate that adoptive immunotherapy with Vγ2Vδ2 T cells would be feasible for the majority of both early- and late-stage breast cancer patients given that most patients had significant ex vivo expansion of Vγ2Vδ2 T cells. Moreover, for the ~20 % of patients that have Vγ2Vδ2 T cells less than 1 % which is correlated with poor ex vivo expansion, IL-18 can be shown to augment Vγ2Vδ2 T cell expansion which may allow immunotherapy even in these patients. The Vγ2Vδ2 T cells generated by ex vivo expansion have been shown to traffic to tumors in patients as early as 1 h after administration [23].
In summary, this study provides support for an immunotherapeutic role for Vγ2Vδ2 T cells in early-stage breast cancer patients. Many breast cancer patients had elevated Vδ2+ T cell frequencies and the majority exhibited large ex vivo expansions of Vγ2Vδ2 T cells that could be augmented further by IL-18. However, the frequent in vivo administration of Zol was detrimental to Vγ2Vδ2 T cells strongly suggesting that less frequent treatment may be preferable to preserve Vγ2Vδ2 T cell function and to avoid Zol side effects. This study shows that adoptive immunotherapy with Vγ2Vδ2 T cells is feasible for the majority of early- and late-stage breast cancer patients. Successful treatment of a breast cancer patient with Vγ2Vδ2 T cells provides proof-of-concept that Vγ2Vδ2 T cells can be used for immunotherapy. The challenge will be to optimize Vγ2Vδ2 T cell immunotherapy and to select the patients most likely to respond.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Ms. Chiyomi Inoue for excellent technical assistance and to GlaxoSmithKline plc (Research Triangle Park, NC) and Shionogi Pharmaceutical Co., Ltd. (Chuo-ku, Osaka, Japan) for providing recombinant human IL-18 and IL-2, respectively. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (MEXT) (to Y. T.), by “Coordination, Support, and Training Program for Translational Research” from MEXT (to Y. T., N. M., T. S., and M. T.), by “Special Coordination Funds for Promoting Science and Technology” from MEXT and Astellas Pharma Inc. through the “Formation of Center for Innovation by Fusion of Advanced Technologies” program (to Y. T.), by “Platform for Drug Discovery, Informatics, and Structural Life Science” from MEXT (to Y. T.), and by grants from the National Institute of Arthritis and Musculoskeltal and Skin Disease, National Institutes of Health (AR045504), National Cancer Institute, National Institutes of Health (CA113874), and the Department of Veterans Affairs (BX000972) (to C. T. M.).
Conflict of interest
C. T. M. is a co-inventor of US Patent 8,012,466 on the development of live bacterial vaccines for activating γδ T cells. The other authors declare that they have no financial or commercial conflict of interest.
Footnotes
Tomoharu Sugie and Kaoru Murata-Hirai contributed equally to this work.
References
- 1.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonneville M, O’Brien RL, Born WK. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kabelitz D, Wesch D, He W. Perspectives of γδ T cells in tumor immunology. Cancer Res. 2007;67:5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 5.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. doi: 10.1016/S0014-5793(01)03191-X. [DOI] [PubMed] [Google Scholar]
- 8.Rohdich F, Hecht S, Gartner K, Adam P, Krieger C, Amslinger S, Arigoni D, Bacher A, Eisenreich W. Studies on the nonmevalonate terpene biosynthetic pathway: metabolic role of IspH (LytB) protein. Proc Natl Acad Sci USA. 2002;99:1158–1163. doi: 10.1073/pnas.032658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 10.Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 11.Kato Y, Tanaka Y, Miyagawa F, Yamashita S, Minato N. Targeting of tumor cells for human γδ T cells by nonpeptide antigens. J Immunol. 2001;167:5092–5098. doi: 10.4049/jimmunol.167.9.5092. [DOI] [PubMed] [Google Scholar]
- 12.Bukowski JF, Morita CT, Tanaka Y, Bloom BR, Brenner MB, Band H. Vγ2Vδ2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154:998–1006. [PubMed] [Google Scholar]
- 13.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Sarikonda G, Puan KJ, Tanaka Y, Feng J, Giner JL, Cao R, Monkkonen J, Oldfield E, Morita CT. Indirect stimulation of human Vγ2Vδ2 T cells through alterations in isoprenoid metabolism. J Immunol. 2011;187:5099–5113. doi: 10.4049/jimmunol.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu W, Zheng J, Liu Y, Sia SF, Liu M, Qin G, Ng IH, Xiang Z, Lam KT, Peiris JS, Lau YL. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice. J Exp Med. 2011;208:1511–1522. doi: 10.1084/jem.20110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, Foglietta M, Palumbo A, Bosia A, Coscia M, Boccadoro M, Massaia M. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vγ9Vδ2 T cells, alphabeta CD8+ T cells, regulatory T cells, and dendritic cells. J Immunol. 2011;187:1578–1590. doi: 10.4049/jimmunol.1002514. [DOI] [PubMed] [Google Scholar]
- 17.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 18.Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber EP, Fesl C, Greil R. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 19.Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, von Minckwitz G, Miller J, Schenk N, Coleman R. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21:2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 20.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, Ashcroft AJ, Ross F, Byrne J, Roddie H, Rudin C, Cook G, Jackson GH, Child JA. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aft R. Bisphosphonates in breast cancer: antitumor effects. Clin Adv Hematol Oncol. 2011;9:292–299. [PubMed] [Google Scholar]
- 22.Kunzmann V, Wilhelm M. Adjuvant zoledronic acid for breast cancer: mechanism of action? Lancet Oncol. 2011;12:991–992. doi: 10.1016/S1470-2045(11)70252-2. [DOI] [PubMed] [Google Scholar]
- 23.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous γδ T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuda J, Li W, Yamanishi H, Yamamoto H, Okuda A, Kubo S, Ma Z, Terada N, Tanaka Y, Okamura H. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human γδ T cells. J Immunol. 2011;186:2003–2012. doi: 10.4049/jimmunol.1001919. [DOI] [PubMed] [Google Scholar]
- 25.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton E, Clay TM, Blackwell KL. New perspectives on zoledronic acid in breast cancer: potential augmentation of anticancer immune response. Cancer Invest. 2011;29:533–541. doi: 10.3109/07357907.2011.605413. [DOI] [PubMed] [Google Scholar]
- 27.Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, Gil M, Houston SJ, Grieve RJ, Barrett-Lee PJ, Ritchie D, Pugh J, Gaunt C, Rea U, Peterson J, Davies C, Hiley V, Gregory W, Bell R. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365:1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 28.Nussbaumer O, Gruenbacher G, Gander H, Thurnher M. DC-like cell-dependent activation of human natural killer cells by the bisphosphonate zoledronic acid is regulated by γδ T lymphocytes. Blood. 2011;118:2743–2751. doi: 10.1182/blood-2011-01-328526. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Martinez A, Iyengar R, Gan K, Chotsampancharoen T, Rooney B, Holladay M, Ramirez M, Leung W. Blood dendritic cells suppress NK cell function and increase the risk of leukemia relapse after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2011;17:598–607. doi: 10.1016/j.bbmt.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moser B, Eberl M. γδ T cells: novel initiators of adaptive immunity. Immunol Rev. 2007;215:89–102. doi: 10.1111/j.1600-065X.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- 31.Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Human gd T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–1733. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montero MT, Matilla J, Gomez-Mampaso E, Lasuncion MA. Geranylgeraniol regulates negatively caspase-1 autoprocessing: implication in the Th1 response against Mycobacterium tuberculosis . J Immunol. 2004;173:4936–4944. doi: 10.4049/jimmunol.173.8.4936. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki M, Tanaka Y, Kobayashi H, Murata-Hirai K, Miyabe H, Sugie T, Toi M, Minato N. Expression and function of PD-1 in human γδ T cells that recognize phosphoantigens. Eur J Immunol. 2011;41:345–355. doi: 10.1002/eji.201040959. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi H, Tanaka Y, Yagi J, Minato N, Tanabe K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, Liu G, Eickhoff JC, McNeel DG, Malkovsky M. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–1460. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, Valerio MR, Meraviglia S, Di Sano C, Sireci G, Salerno A. Induction of γδ T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 37.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D’Asaro M, Orlando V, Scarpa F, Roberts A, Caccamo N, Stassi G, Dieli F, Hayday AC. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siris ES. Extensive personal experience: Paget’s disease of bone. J Clin Endocrinol Metab. 1995;80:335–338. doi: 10.1210/jcem.80.2.7852484. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi H, Tanaka Y, Shimmura H, Minato N, Tanabe K. Complete remission of lung metastasis following adoptive immunotherapy using activated autologous γδ T-cells in a patient with renal cell carcinoma. Anticancer Res. 2010;30:575–579. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.