Abstract
Background
The link between daily changes in ambient fine particulate matter air pollution (PM2.5) and cardiovascular morbidity and mortality is well established. Whether PM2.5 at levels below current US National Ambient Air Quality Standards also increases the risk of ischemic stroke remains uncertain.
Methods
We reviewed the medical records of 1705 Boston-area patients hospitalized with neurologist-confirmed ischemic stroke and abstracted data on the time of symptom onset and clinical characteristics. PM2.5 concentrations were measured at a central monitoring station. We used the time-stratified case-crossover study design to assess the association between the risk of ischemic stroke onset and PM2.5 levels in the hours and days preceding each event. We examined whether the association with PM2.5 differed by ischemic stroke etiology and patient characteristics.
Results
The estimated odds ratio of ischemic stroke onset was 1.34 (95% confidence interval (CI): 1.13, 1.58; p<0.001) following a 24-hour period classified as “moderate” (PM2.5 15–40 μg/m3) by the US Environmental Protection Agency’s (EPA) Air Quality Index compared to a 24-hour period classified as “good” (≤15 μg/m3). Considering PM2.5 as a continuous variable, the estimated odds ratio of ischemic stroke onset was 1.11 (95% CI: 1.03, 1.20; p=0.006) per interquartile range increase in PM2.5 (6.4 μg/m3). The increase in risk was greatest within 12–14 hours of exposure to PM2.5 and was most strongly associated with markers of traffic-related pollution.
Conclusion
These results suggest that exposure to PM2.5 levels considered generally safe by the US EPA increase the risk of ischemic stroke onset within hours of exposure.
Keywords: Stroke, risk factors, air pollution, epidemiology
Daily changes in ambient fine particulate matter (PM2.5) have been associated with higher risk of acute cardiovascular events, excess hospitalizations and deaths.1 These cardiovascular effects of PM2.5 appear to be mediated through a combination of autonomic, hemostatic, inflammatory, and vascular endothelial disturbances with consequent changes in cardiac and vascular function.2–7 Based on the current evidence, the US Environmental Protection Agency (EPA) regulates mean daily and annual PM2.5 levels. Whether the current regulatory standards are sufficient to protect public health remains controversial.8
Although a number of studies have examined the association between other air pollutants and the risk of stroke,9–12 relatively fewer studies have evaluated the effects of PM2.5 on stroke risk and the results remain equivocal. Specifically, some,13–15 but not all,16–18 studies suggest that PM2.5 may increase the incidence of the combined endpoint of acute cerebrovascular diseases, an etiologically diverse group with multiple underlying pathophysiologic mechanisms. Prior work has suggested that ambient air pollution is more strongly associated with ischemic stroke than intracranial hemorrhage.19 However, few studies have specifically evaluated the link between PM2.5 and ischemic stroke risk,14, 20–22 and only one of these studies assessed whether the associations differ by ischemic stroke etiology.22
We therefore evaluated the association between daily and hourly changes in PM2.5 and the risk of ischemic stroke onset among patients residing in the greater Boston area and admitted between 1999 and 2008 to the Beth Israel Deaconess Medical Center (BIDMC). Of note, during the study period PM2.5 levels in the Boston area did not exceed current EPA standards.
Methods
This study was approved by the Committee on Clinical Investigations at BIDMC. We identified 1763 consecutive patients ≥21 years old admitted to the BIDMC between April 1, 1999, and October 31, 2008, with neurologist-confirmed ischemic stroke, excluding patients with in-hospital strokes or transient ischemic attacks. BIDMC is a 650-bed teaching hospital of Harvard Medical School and designated as a Primary Stroke Service Hospital by the state. The Stroke Service consists of 5 Vascular Neurologists who see ~550 stroke patients annually. As in previous studies,23 we excluded patients residing >40 km from the Harvard ambient monitoring station to reduce exposure misclassification. Patients potentially eligible for this study were identified by reviewing daily emergency department admission logs, stroke service admission logs, stroke service consult logs, and hospital electronic discharge records. For patients meeting eligibility criteria, we abstracted data on demographics, presenting symptoms, medical history (including history of prior stroke) and imaging results from each patient’s medical record. We classified stroke etiology as due to: 1) large artery atherosclerosis, 2) small vessel occlusion, 3) cardioembolism, 4) other determined etiology or undetermined etiology according to the approach developed for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST).24 Time of stroke symptom onset or time last seen normal as documented by the attending stroke neurologist at the time of hospital presentation was classified as “exact”, “estimated” or “unknown”.22 Given the documented morning peak in ischemic stroke incidence,25 we assumed stroke onset occurred at 9am for 221 (13%) patients where the date of stroke symptom onset was documented but not the time. We excluded 58 (3%) patients for whom neither the date nor time of stroke onset were documented, leaving 1705 patients available for analysis.
PM2.5 and black carbon concentrations were measured continuously and sulfate particles (SO42−) were measured daily (9am–9am) at the Harvard ambient monitoring station, as previously described.23 Hourly measures of nitrogen dioxide (NO2), carbon monoxide (CO), and ozone (O3) were obtained from local monitoring sites operated by the Massachusetts Department of Environmental Protection and averaged. We obtained hourly meteorological data from the National Weather Service station at Boston’s Logan Airport and calculated apparent temperature, an index of thermal comfort, as previously described.26 In a secondary analysis, we estimated exposure to black carbon at each subject’s home as a marker of traffic pollution using a validated temporal-spatial model.27 Briefly, black carbon predictions are based on over 6,000 black carbon measurements at 82 locations in the greater Boston area, meteorological and other characteristics of a given day, and measures of land use (e.g. traffic and population density) at a given location.
We used the time-stratified case-crossover study design28 to assess the association between the risk of ischemic stroke onset and PM2.5 concentrations in the hours and days preceding each event. In this design, each subject’s exposure prior to a case-defining event (case period) is compared with his or her own exposure experience during one or more control periods when the subject did not become a case (control period). Control periods were chosen such that exposures during the case period were compared to exposures occurring on other days of the same month falling on the same day of the week and time of day as the case period. The use of control periods from both before and after the index event is appropriate in this setting because individual events do not affect the distribution of future exposure in the overall study population.29 This approach effectively controls for seasonality, time-trends, and chronic and slowly-varying potential confounders because the exposure information for cases and controls within the same stratum come from the same calendar month.30
We performed conditional logistic regression, stratifying on each hospitalization, to obtain estimates of odds ratios associated with PM2.5 and corresponding 95 percent confidence intervals (CI). In all analyses, we controlled for ambient temperature and dew point temperature using natural cubic splines (3 degrees of freedom each) and barometric pressure modeled as a linear continuous variable. We first considered 2 categories of PM2.5 levels defined a priori by the EPA’s Air Quality Index as either “good” (≤15 μg/m3) or “moderate” (15–40 μg/m3), excluding 11 (0.3%) days where PM2.5 levels exceeded 40 μg/m3. Next we considered 5 a priori categories of PM2.5 levels (breakpoints at 5, 10, 15, and 20 μg/m3). Finally, we considered PM2.5 as a continuous variable.
In all analyses, PM2.5 exposure was assessed relative to the time of stroke symptom onset. We separately evaluated the association between the risk of ischemic stroke onset and PM2.5 levels averaged 0 to <24, 24 to <48, 48 to <72, and 72 to <96 hours prior to stroke symptom onset. Results were subsequently confirmed using unconstrained distributed lag models such that pollutant levels at each time point were considered jointly in a single model. To explore associations with shorter exposures in more detail, we additionally evaluated the association between risk of stroke onset and PM2.5 levels averaged 0 to <2, 2 to <4, 4 to <6, … 36 to <38 hours prior to stroke symptom onset. We repeated these analyses for black carbon, NO2, CO, O3 and SO4.
We evaluated whether the associations with PM2.5 differed by ischemic stroke etiology or according to the presence of major stroke risk factors including diabetes mellitus, atrial fibrillation, hypertension, and past history of stroke or transient ischemic attack using fully stratified models. We used the Chi-squared test for homogeneity31 to evaluate whether associations differ significantly across subgroups. A two-sided p value of <0.05 was considered statistically significant. Analyses were performed using SAS V9.2 (SAS Institute Inc., Cary, NC) and the R statistical package (R v 2.8.1).
Results
The 1705 patients were predominantly white women with a mean age of 73.1 (SD: 14.5) years (Table 1). Small vessel strokes (26%) were the most common determined stroke etiology, followed by strokes due to cardioembolism (25%) and large artery atherosclerosis (20%). The median delay time from stroke symptom onset to hospital presentation was 10 hours (25th percentile: 3 hours, 75th percentile: 26 hours). In-hospital mortality was 5.8%. The majority (87%) of patients resided <20 km from the Boston/Harvard ambient monitoring site (eTable 1).
Table 1.
Characteristics of 1705 patients hospitalized with acute ischemic stroke and residing in the Boston metropolitan area, 1999–2008.
| n (%) or mean ± SD | |
|---|---|
| Age, y (mean ± SD) | 73.1 ± 14.5 |
| Female, n (%) | 931 (54.6) |
| White, n (%) | 1165 (68.3) |
| Past Medical History, n (%) | |
| Stroke or TIA | 482 (28.3) |
| Atrial Fibrillation | 424 (24.9) |
| Hypertension | 1216 (71.3) |
| Coronary Artery Disease | 432 (25.3) |
| Heart Failure | 221 (13.0) |
| Diabetes Mellitus | 495 (29.0) |
| Chronic Obstructive Pulmonary Disease | 105 (6.2) |
| Smoking History, n (%) | |
| Current | 236 (13.8) |
| Former | 457 (26.8) |
| Presumed Stroke Etiology, n (%) | |
| Large Artery Atherosclerosis | 339 (19.9) |
| Small Vessel Occlusion | 450 (26.4) |
| Cardioembolism | 427 (25.0) |
| Other or Undetermined | 489 (28.7) |
During the study period 2888 (83%) days were classified as “good” according to the EPA’s Air Quality Index for PM2.5 (with the accompanying statement: “air pollution poses little or no risk”), and 572 (16%) days were classified as “moderate” (“air quality is acceptable; however, there may be a moderate health concern for a very small number of people”).
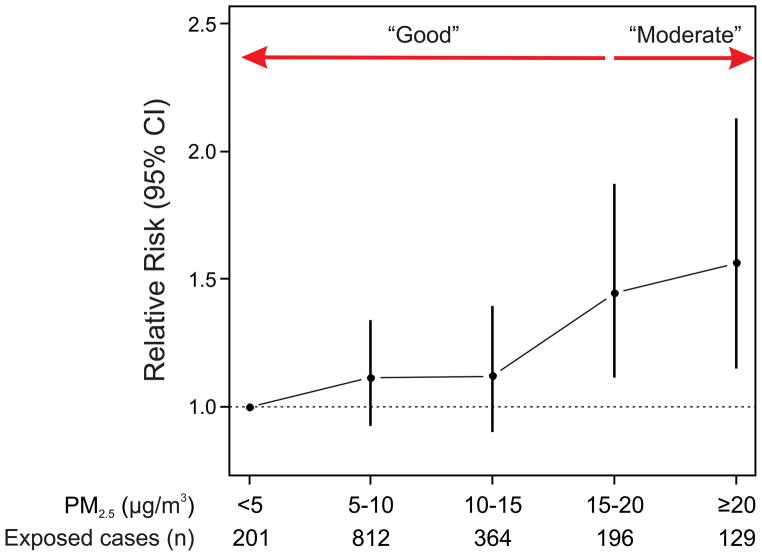
The odds ratio of ischemic stroke onset was 1.34 (95% CI: 1.13, 1.58; p<0.001) following a 24-hour period classified by the EPA as “moderate” compared to a period classified as “good”. The association between PM2.5 and risk of ischemic stroke onset was approximately linear (Fig. 1). Considering PM2.5 as a continuous variable, the odds ratio of stroke onset was 1.11 (95% CI: 1.03, 1.20; p=0.006) comparing the 75th to 25th percentile of PM2.5 (6.4 μg/m3) over the previous 24 hours. PM2.5 levels preceding stroke onset by more than 24 hours were not associated with higher risk.
Figure 1.
Odds ratio of ischemic stroke onset for categories of mean PM2.5 levels in the 24 hrs preceding stroke onset.
The results were not materially different when we excluded from analyses patients living >20 km from the monitoring site; those where we imputed time of symptom onset; those presenting more than 48 hours after symptom onset; or after statistical adjustment for apparent temperature or ozone (eTable 2). The results from an unconstrained distributed lag model were also similar.
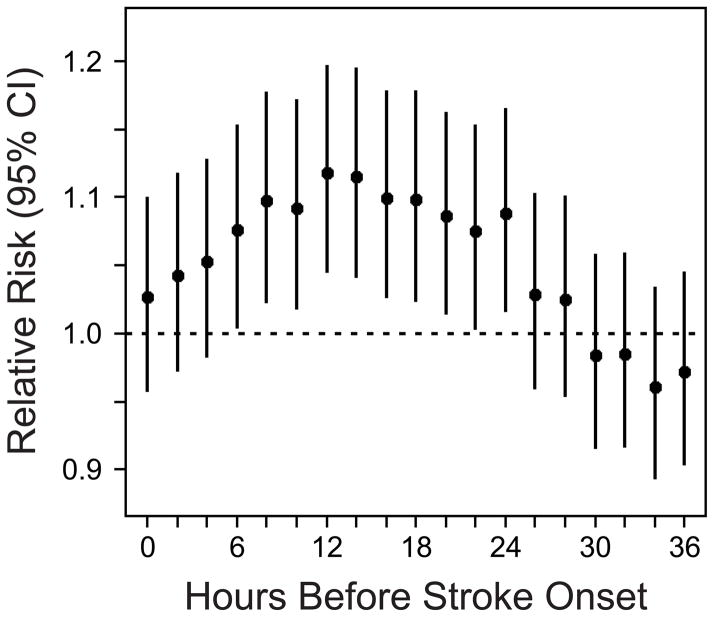
Figure 2 shows that the odds ratio of stroke onset was elevated immediately, peaked in association with mean PM2.5 levels 12 to <14 hours earlier (odds ratio comparing the 75th to 25th percentile of PM2.5: 1.10, 95% CI: 1.04, 1.17; p=0.001), and decreased thereafter.
Figure 2.
Odds ratio of ischemic stroke onset per interquartile range increase in PM2.5 (6.4 μg/m3) in the hours preceding stroke onset.
We examined whether the association between PM2.5 and stroke onset varied across subgroups of patients with specific clinical characteristics (Table 3). PM2.5 was associated with stroke onset among patients with ischemic stroke classified as due to large artery atherosclerosis and small vessel disease, but not cardioembolism. There was no evidence suggesting that the association varied according to the presence of comorbid diabetes mellitus, atrial fibrillation or hypertension, past history of stroke or transient ischemic attack, or age.
Table 3.
Odds ratio of ischemic stroke onset per interquartile range increase in PM2.5 levels 0–24 hours prior to stroke symptom onset among subgroups of patients.
| Subgroup | OR (95% CI) | Ph | |
|---|---|---|---|
| Presumed Stroke Etiology | Large Artery | 1.24 (1.04,1.48) | 0.19 |
| Small Vessel | 1.19 (1.02,1.37) | ||
| Cardioembolic | 1.09 (0.93,1.27) | ||
| Other/Undetermined | 0.99 (0.85,1.15) | ||
| History of Diabetes | Yes | 1.10 (1.00,1.21) | 0.67 |
| No | 1.14 (0.99,1.31) | ||
| History of Atrial Fibrillation | Yes | 1.11 (1.02,1.22) | 0.92 |
| No | 1.13 (0.96,1.32) | ||
| History of Hypertension | Yes | 1.10 (0.96,1.27) | 0.86 |
| No | 1.12 (1.02,1.23) | ||
| History of Stroke | Yes | 1.12 (1.02,1.23) | 0.80 |
| No | 1.09 (0.95,1.26) | ||
| Age | ≥75 years | 1.17 (1.05, 1.31) | 0.23 |
| <75 years | 1.06 (0.96, 1.19) | ||
We considered the association between the risk of stroke onset and other pollutants (Table 2). Results for black carbon (measured at either the Harvard ambient monitoring site or estimated at each patient’s home address) and NO2 were similar to those for PM2.5, while results for CO, O3, and SO42− were not statistically significant.
Table 2.
Odds ratio of ischemic stroke onset comparing the 75th to 25th percentile (interquartile range) of each pollutant in the 24 hours preceding stroke onset.
| Pollutant | Interquartile Range | Odds Ratio (95% CI) | p |
|---|---|---|---|
| PM2.5† | 6.4 μg/m3 | 1.11 (1.03, 1.20) | 0.006 |
| Black Carbon† | 0.5 μg/m3 | 1.10 (1.02, 1.19) | 0.017 |
| Estimated Residential Black Carbon‡ | 0.6 μg/m3 | 1.08 (1.01, 1.16) | 0.018 |
| NO2 | 8.1 ppb | 1.12 (1.03, 1.22) | 0.009 |
| CO | 0.3 ppm | 1.07 (0.96, 1.19) | 0.24 |
| O3 | 15.2 ppb | 0.97 (0.87, 1.09) | 0.65 |
| SO42−† | 2.1 μg/m3 | 1.06 (0.99, 1.13) | 0.12 |
Measured at the Boston/Harvard Ambient Monitoring Station;
Mean daily black carbon levels estimated at each patient’s address using a validated spatial-temporal land use regression model.
Discussion
In this Boston-area study conducted while the region was in attainment of EPA regulatory standards, we found that ischemic stroke risk was 34% (95% CI: 13, 58%; p<0.001) higher on days with “moderate” PM2.5 levels as compared to days with “good” levels according to the EPA’s air quality index. The relationship between higher PM2.5 and increased risk of stroke onset was linear, strongest within 12 hours of PM2.5 exposure, and observed among patients presenting with strokes classified as due to large artery atherosclerosis or small vessel occlusion, but not cardioembolism. Stroke risk was more strongly associated with black carbon and NO2, markers of traffic pollution, than with components linked to non-traffic sources.
From a public health perspective, it is noteworthy that we observed an association between PM2.5 levels and ischemic stroke onset in an area in attainment of the US National Ambient Air Quality Standards. Although the observed relative risk is modest, the number of strokes attributable to PM2.5 may be high given the high incidence of ischemic stroke and the fact that nearly everyone is exposed to ambient fine particulate matter. If the association observed in this study is causal and assuming a linear dose-response function, a 2 μg/m3 (approximately 20%) reduction in mean PM2.5 levels during this time period may have averted approximately 6,100 of the 184,000 stroke hospitalizations observed in the US Northeast region in 2007 alone.32
An analysis of Medicare beneficiaries in 204 US counties found a 0.4% (95% CI: 0.0, 0.9%) higher risk of admission for the combined endpoint of cerebrovascular disease per 10 μg/m3 increase in same day PM2.5.13 Smaller studies in Taiwan14 and Southern California15 have reported excess relative risks of approximately 2% per 10 μg/m3 increase in PM2.5, while others have found no evidence of an association.16–18 Of four prior studies that have specifically evaluated the association between PM2.5 and the risk of ischemic stroke, two Canadian studies found no association,20, 22 while studies from Taiwan14 and Nueces County, Texas21 found a 3% (95% CI: −1 to 7%) and 6% (95% CI: −1 to 13%) excess risk per 10 μg/m3 increase in PM2.5, respectively.
The estimate from the current study scaled to a 10 μg/m3 increase in PM2.5 would be 18% (95% CI: 5, 34%), substantially larger than previous reports. We believe that this difference is attributable, at least in part, to the use in the current study of data on the timing of ischemic stroke onset. Most prior studies have assessed exposure to PM2.5 based on the calendar day of hospital admission; an exposure assessment strategy which can bias health effects estimates towards the null by as much as 60%.33 Our previous study in a quality of care registry in Ontario, Canada22 may have been null partly due to misclassification of stroke onset time for patients presenting beyond the limited time window where they may benefit from thrombolytic therapy. Differences in outcome assessment methods, population and pollutant characteristics, and other aspects of the exposure assessment strategy very likely also contribute to heterogeneity across studies. For example, health effects of PM2.5 in North America are known to vary geographically depending on the local pollutant sources and components,34 as well as community characteristics.35
In the current study we were able to estimate the time course of the association between PM2.5 and stroke onset in greater detail than has been previously possible. We observed an immediate increase in the odds ratio for stroke onset that peaked 12 hours after PM2.5 exposure, and decreased thereafter (Fig. 2). Experimental studies in humans and animals have shown that exposure to concentrated ambient PM2.5 can induce increases in blood pressure and heart rate and reductions in heart rate variability within this time frame,7, 36 suggesting that altered hemodynamics could play an important role. Other potential mechanisms include alterations in hemostatic factors, systemic inflammation, endothelial cell injury, and vascular dysfunction.4–6 Although these physiologic intermediates have typically been investigated in association with PM2.5 exposures lasting a day or longer, there is some evidence suggesting that these effects may also follow exposures shorter than 24 hours.7, 37–39
The observation that PM2.5 was more strongly associated with stroke onset in patients with strokes due to large artery atherosclerosis is consistent with a mechanism involving altered hemodynamics and/or vascular dysfunction that results in disruption of a vulnerable atherosclerotic plaque with subsequent thrombosis and/or downstream embolism, as well as with results from a prior study.22 This result is also consistent with a Boston-area study showing a higher risk of acute myocardial infarction within 2 hours of exposure to elevated levels of PM2.5.40 The mechanisms underlying the observed association between PM2.5 and small vessel stroke are less clear since the pathology of these strokes remains poorly understood. However, evidence suggests that endothelial dysfunction and injury, potentially triggered by PM2.5 or its components, may contribute to the distinct non-atherosclerotic arteriopathy which likely underlies many small vessel strokes.41 We did not find evidence to suggest that the presence of comorbid diabetes, hypertension, atrial fibrillation or a past history of stroke increased patients’ vulnerability to PM2.5-related stroke.
Identifying the components or sources of PM2.5 responsible for the observed associations is of public health and regulatory interest. We found that the risk of stroke onset was most strongly associated with PM2.5, but also significantly associated with black carbon and NO2, markers of traffic pollution. This finding is in agreement with past studies suggesting that traffic pollution may trigger ischemic strokes.20, 42–43 We did not find any association between risk of stroke onset and ozone, a secondary pollutant formed from the reaction of oxides of nitrogen and volatile organic compounds in the presence of sunlight. This is in agreement with most,14, 16–17, 20 but not all,44 prior studies. We also did not find any association between risk of stroke onset and SO42−, consistent with prior studies using administrative data.16, 45 In the Northeast, SO42− generally represents regional pollution from coal-fired power plants.46
Our study has some limitations. First, the use of air quality measures from a single monitoring site is expected to lead to exposure misclassification, increasing the width of our confidence intervals but not otherwise biasing our results.47 However, PM2.5 levels measured at this monitoring site have been shown to be strong proxies for personal exposure to particles of ambient origin in communities surrounding Boston.48 In support of this, our results were not materially different when we restricted the analyses to patients living <20 km from the monitoring site and the results were similar when we evaluated the association between black carbon estimated at patients’ homes and ischemic stroke onset. Second, we did not study the association between PM2.5 and stroke resulting in death prior to coming to medical attention. Third, in 13% of patients we were able to estimate the day but not the time of stroke symptom onset, likely resulting in some exposure misclassification and biasing our results towards the null hypothesis of no association. Consistent with this notion, the association between PM2.5 and ischemic stroke was more pronounced after exclusion of these patients from analysis. Fourth, this study involved patients from a single tertiary care center in Boston. Since the effects of PM2.5 likely vary depending on population characteristics, pollution sources, and particle constituents, our results are not necessarily generalizable to other populations or geographic locations.
As in previous studies, important strengths of this study include detailed data on the time of stroke symptom onset22 and patient clinical characteristics.22, 43 In particular, our use of data on the time of stroke symptom onset provides novel insights into the mechanisms by which PM2.5 may increase the risk of ischemic stroke onset.
In conclusion, these results suggest that PM2.5 increases the risk of ischemic stroke at levels below those currently considered safe under US regulations. These associations can be observed within hours of exposure and are most strongly associated with pollution from local or transported traffic emissions. If pollution levels decline with regulation, data on timing of stroke onset, patient clinical characteristics, and stroke etiology will be essential for proper evaluation of the clinical benefits of pollution control on stroke risk.
Supplementary Material
Acknowledgments
Funding/Support and Role of Sponsor
The project described was supported by grants ES015774, ES009825, ES017125 and ES000002 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and grants R832416 and RD83479801 from the US Environmental Protection Agency (US EPA). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, or US EPA. The funding agencies were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
The authors are grateful to Dr. Elissa Wilker for helpful suggestions regarding the manuscript.
Footnotes
Author Contributions: Drs. Wellenius and Mittleman had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wellenius, Coull, Schwartz, Mittleman
Acquisition of data: Wellenius, Burger, Schlaug, Suh, Koutrakis, Mittleman
Analysis and interpretation of data: Wellenius, Coull, Schwartz, Koutrakis, Gold, Mittleman
Drafting of manuscript: Wellenius, Mittleman
Critical revision of manuscript: Wellenius, Burger, Coull, Schwartz, Suh, Koutrakis, Schlaug, Gold, Mittleman
Statistical analysis: Wellenius, Coull, Schwartz, Mittleman
Obtaining funding: Wellenius, Koutrakis, Gold, Mittleman
Administrative, technical, or material support: Suh, Schlaug, Burger
Disclosures: One or more of the authors have previously received or currently receive funding from the following sources: Health Effects Institute, Boston, MA; Electric Power Research Institute, Palo Alto, CA; US Environmental Protection Agency; and NIH.
References
- 1.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, Frohlich M, Doring A, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22(14):1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112(3):339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 6.Ruckerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173(4):432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa D. Air quality in a changing climate. Environ Health Perspect. 2011;119(4):A154–155. doi: 10.1289/ehp.1103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateen FJ, Brook RD. Air pollution as an emerging global risk factor for stroke. JAMA. 2011;305(12):1240–1241. doi: 10.1001/jama.2011.352. [DOI] [PubMed] [Google Scholar]
- 10.Vidale S, Bonanomi A, Guidotti M, Arnaboldi M, Sterzi R. Air pollution positively correlates with daily stroke admission and in hospital mortality: a study in the urban area of Como, Italy. Neurol Sci. 2010;31(2):179–182. doi: 10.1007/s10072-009-0206-8. [DOI] [PubMed] [Google Scholar]
- 11.Tsai SS, Goggins WB, Chiu HF, Yang CY. Evidence for an association between air pollution and daily stroke admissions in Kaohsiung, Taiwan. Stroke. 2003;34(11):2612–2616. doi: 10.1161/01.STR.0000095564.33543.64. [DOI] [PubMed] [Google Scholar]
- 12.Oudin A, Stromberg U, Jakobsson K, Stroh E, Bjork J. Estimation of short-term effects of air pollution on stroke hospital admissions in southern Sweden. Neuroepidemiology. 2010;34(3):131–142. doi: 10.1159/000274807. [DOI] [PubMed] [Google Scholar]
- 13.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CC, Chuang KJ, Chien LC, Chen WJ, Chang WT. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur Heart J. 2006;27(10):1238–1244. doi: 10.1093/eurheartj/ehi835. [DOI] [PubMed] [Google Scholar]
- 15.Delfino RJ, Brummel S, Wu J, et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup Environ Med. 2009;66(3):189–197. doi: 10.1136/oem.2008.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the west midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulphate. Occup Environ Med. 2001;58(8):504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett AG, Williams GM, Schwartz J, et al. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. 2006;114(7):1018–1023. doi: 10.1289/ehp.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halonen JI, Lanki T, Yli-Tuomi T, Tiittanen P, Kulmala M, Pekkanen J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology. 2009;20(1):143–153. doi: 10.1097/EDE.0b013e31818c7237. [DOI] [PubMed] [Google Scholar]
- 19.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36(12):2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- 20.Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21(9):689–700. doi: 10.1007/s10654-006-9050-9. [DOI] [PubMed] [Google Scholar]
- 21.Lisabeth LD, Escobar JD, Dvonch JT, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64(1):53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine Particulate Air Pollution (PM2.5) and the Risk of Acute Ischemic Stroke. Epidemiology. 2011;22(3):422–431. doi: 10.1097/EDE.0b013e3182126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environ Health Perspect. 2005;113(6):670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 26.Kalkstein L, Valimon K. An evaluation of summer discomfort in the United States using a relative climatological index. Bulletin Am Meterological Soc. 1986;67:842–848. [Google Scholar]
- 27.Gryparis A, Coull BA, Schwartz J, Suh HH. Semiparametric latent variable regression models for spatiotemporal modelling of mobile source particles in the greater Boston area. Appl Statist. 2007;56(2):183–209. [Google Scholar]
- 28.Lumley T, Levy D. Bias in the case-crossover design: implications for studies of air pollution. Environmetrics. 2000;11:689–704. [Google Scholar]
- 29.Maclure M, Mittleman MA. Case-crossover designs compared with dynamic follow-up designs. Epidemiology. 2008;19(2):176–178. doi: 10.1097/EDE.0b013e318162afb9. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J, Zanobetti A, Bateson TF. Revised Analyses of Time-Series Studies of Air Pollution and Health. Special Report. Boston: Health Effects Institute; 2003. Morbidity and mortality among elderly residents in cities with daily PM measurements; pp. 25–58. [Google Scholar]
- 31.Rothman KJ, Greenland S. Modern epidemiology. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 32.National Center for Health Statistics. [Accessed April 21, 2011.];Health Data Interactive. 2007 www.cdc.gov/nchs/hdi.htm.
- 33.Lokken PR, Wellenius GA, Coull BA, et al. Air Pollution and Risk of Stroke: Underestimation of Effect Due to Misclassification of Time of Event Onset. Epidemiology. 2008 doi: 10.1097/ede.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital Admissions and Chemical Composition of Fine Particle Air Pollution. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell ML, Ebisu K, Peng RD, Dominici F. Air Conditioning Use Lowers Particulate Matter’s Adverse Health Effects: Results from National United States Studies on Hospital Admissions and Mortality. Epidemiology. 2009 [Google Scholar]
- 36.Bartoli CR, Wellenius GA, Diaz EA, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117(3):361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105(13):1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 38.Dales R, Liu L, Szyszkowicz M, et al. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health. 2007;81(2):159–164. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 39.Riediker M, Cascio WE, Griggs TR, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 40.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 41.Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005;76(5):617–619. doi: 10.1136/jnnp.2004.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maynard D, Coull BA, Gryparis A, Schwartz J. Mortality risk associated with short-term exposure to traffic particles and sulfates. Environ Health Perspect. 2007;115(5):751–755. doi: 10.1289/ehp.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersen ZJ, Olsen TS, Andersen KK, Loft S, Ketzel M, Raaschou-Nielsen O. Association between short-term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. Eur Heart J. 2010;31(16):2034–2040. doi: 10.1093/eurheartj/ehq188. [DOI] [PubMed] [Google Scholar]
- 44.Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med. 2007;64(7):439–445. doi: 10.1136/oem.2006.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lippmann M, Ito K, Nadas A, Burnett RT. Association of particulate matter components with daily mortality and morbidity in urban populations. Res Rep Health Eff Inst. 2000;(95):5–72. discussion 73–82. [PubMed] [Google Scholar]
- 46.Bell ML, Belanger K, Ebisu K, et al. Prenatal exposure to fine particulate matter and birth weight: variations by particulate constituents and sources. Epidemiology. 2010;21(6):884–891. doi: 10.1097/EDE.0b013e3181f2f405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown KW, Sarnat JA, Suh HH, Coull BA, Koutrakis P. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407(12):3754–3765. doi: 10.1016/j.scitotenv.2009.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.