Abstract
Background and purpose
Wear particles from metal–on–metal arthroplasties are under suspicion for adverse effects both locally and systemically, and the DePuy ASR Hip Resurfacing System (RHA) has above–average failure rates. We compared lymphocyte counts in RHA and total hip arthroplasty (THA) and investigated whether cobalt and chromium ions affected the lymphocyte counts.
Method
In a randomized controlled trial, we followed 19 RHA patients and 19 THA patients. Lymphocyte subsets and chromium and cobalt ion concentrations were measured at baseline, at 8 weeks, at 6 months, and at 1 and 2 years.
Results
The T–lymphocyte counts for both implant types declined over the 2–year period. This decline was statistically significant for CD3+CD8+ in the THA group, with a regression coefficient of –0.04 × 109cells/year (95% CI: –0.08 to –0.01). Regression analysis indicated a depressive effect of cobalt ions in particular on T–cells with 2–year whole–blood cobalt regression coefficients for CD3+ of –0.10 (95% CI: –0.16 to –0.04) × 109 cells/parts per billion (ppb), for CD3+CD4+ of –0.06 (–0.09 to –0.03) × 109 cells/ppb, and for CD3+CD8+ of –0.02 (–0.03 to –0.00) × 109 cells/ppb.
Interpretation
Circulating T–lymphocyte levels may decline after surgery, regardless of implant type. Metal ions—particularly cobalt—may have a general depressive effect on T– and B–lymphocyte levels.
Registered with ClinicalTrials.gov under # NCT01113762
The increasing use of resurfacing metal–on–metal (MoM) articulations in young people has raised concerns about the possible adverse effects of the cobalt and chromium ions released in the body. A strong link between high wear rates, raised systemic ion levels, and painful hips or pseudotumors, (Pandit et al. 2008, Hart et al. 2009a, Kwon et al. 2010) puts wear products under suspicion of inducing a local toxic and immunological reaction, but elevated blood ion concentrations have also been associated with reduced systemic CD3+ and CD8+ T–cell count (Hart et al. 2006, 2009b). These studies are, however, biased from the lack of a baseline value, and there is currently no clinical evidence of a detrimental effect of the proposed reduced lymphocyte count.
The DePuy ASR Hip Resurfacing System was recalled from the market in 2010 due to higher than average failure rates (Porter et al. 2010). It has been suggested that the ASR has increased risk of edge load wear, especially in the small implants—particularly if the components are suboptimally placed, which might give rise to accelerated particle production and elevated ion concentrations ( De Haan et al. 2008, Langton et al. 2010).
We compared cobalt and chromium concentrations and the absolute levels of lymphocytes and the subgroups CD3+, CD3+CD4+, and CD3+CD8+ T–cells, CD16+CD56+ NK–cells, and CD3–CD19+ B–cells between patients implanted with the ASR RHA or a ceramic–on–polyethylene THA. We related the absolute levels of these lymphocyte subgroups to the metal ion concentrations.
Patients and methods
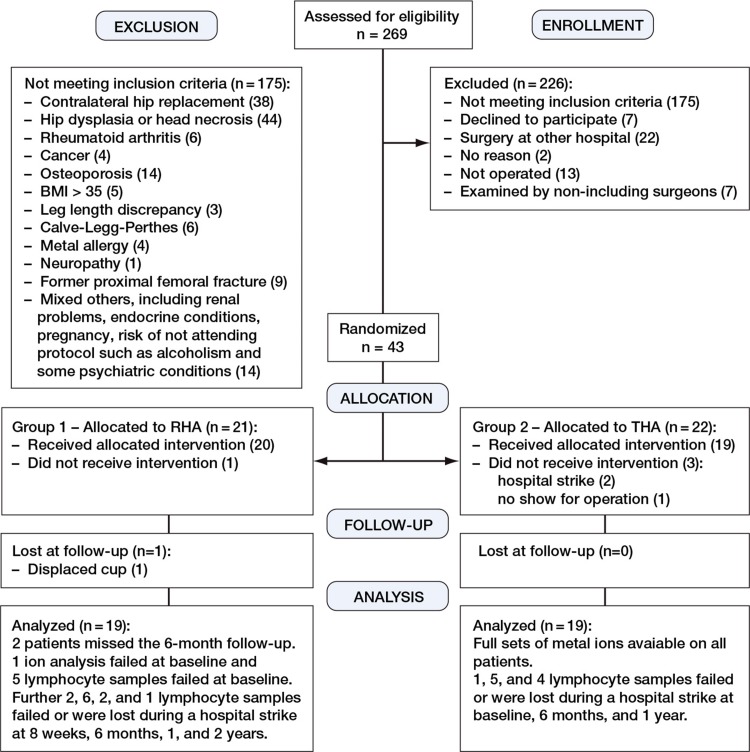
The cobalt ion levels, chromium ion levels, and lymphocyte counts in the present study represent secondary outcomes from a larger random controlled trial (RCT) using range of motion as primary outcome (Penny et al. in press). The patients included for the study of ion levels were randomized to RHA (n = 19) or standard ceramic–on–polyethylene THA (n = 19) after having given written and verbal informed consent (Figure 1). This was done using sealed envelopes that had been filled by a nurse in blocks of 10. At inclusion, the patient picked an envelope and the intervention was known to the patient before surgery.
Figure 1.
CONSORT Flow chart showing the inclusion and analysis process of the RCT.
The inclusion criteria were primary osteoarthritis or secondary osteoarthritis due to mild dysplasia and age between 40 and 65 years (Table 1). 65% of the patients who were assessed for participation in this study were excluded. This large proportion was because of exclusion of patients with contralateral hip implants (as metal ion analysis was undertaken) and exclusion of patients with moderate or severe hip dysplasia (as such patients were not eligible for RHA at our institution). Our study therefore involved a selected subgroup of osteoarthritic patients. The patients were operated from April 2007 to March 2009 at Odense University Hospital. Tobacco use was recorded at baseline; no patients gave up smoking, and exact exposure at follow–up visits was not recorded. The RHA group had 1 smoker (30 cigarettes a day) and the THA group had 2 (14 and 20 cigarettes a day). None of the patients used oral steroids or other immunosuppressive drugs, but otherwise their medication, including vitamins, was not scrutinized.
Table 1.
Baseline demographic data expressed as median (range)
| RHA | THA | |
|---|---|---|
| n | 19 | 19 |
| Females | 8 | 3 |
| Age, years | 57 (46–64) | 55 (44–64) |
| BMI, kg/m2 | 28 (19–37) | 28 (23–36) |
The RHA group received an Articular Surface Replacement (ASR; DePuy, Leeds, UK) made from a high–carbon cobalt–chromium–molybdenium alloy. The 28–mm ceramic–on–polyethylene THAs were implanted with a titanium Mallory–head acetabular shell, a 28–mm Biolox Delta modular ceramic head, an Arcom Ringlock polyethylene liner, and a titanium Bimetric stem (Biomet, Bridge End, UK).
All ASR patients underwent hip resurfacing using a standard posterior approach, performed by 2 consultant surgeons who had previously done more than 30 ASRs. The distal gluteal insertion was detached along with the external rotators. The cup was placed cementless in press–fit and the femoral component was cemented with SmartSet GHV Bone Cement (DePuy, Leeds, UK).
The THAs were inserted without cement via a posterolateral approach of short incision length and without distal muscle release. All patients received identical doses of tranexamic acid and cefuroxime preoperatively as well as 2 postoperative doses of dicloxacillin and low–molecular–weight heparin until they were well mobilized. Apart from free range of motion in the RHA group, they followed the same postoperative rehabilitation program including full weight bearing.
The patients were evaluated clinically with the UCLA activity score (Amstutz et al. 1984), range of motion (ROM), and pain measured on a WOMAC VAS scale (Bellamy et al. 1988) preoperatively (baseline) and at 8 weeks, at 6 months, and after 1 and 2 years. Lymphocyte and metal ion levels were also measured at each visit. 2 THA patients had a contralateral THA after 15 and 20 months, but no RHAs were bilateral within the study period.
Blood analysis
The blood was sampled between the hours of 9 a.m. and 1 p.m. Both whole blood and serum samples were analyzed. Whole blood was chosen for a complete characterization of the actual blood ion levels, and serum was included to enable comparison with a wide variety of studies (MacDonald et al. 2004). The serum results are summarized in Figure 3 and Table 5 (see supplementary data).
Whole blood was sampled in trace element 6/7–mL Plus K2 EDTA tubes (368381) (BectonDickinson, NJ), and sampling and handling were done as described previously (Penny and Overgaard 2010), discarding the first sample. The whole blood was transferred to 3.8–mL acid–washed Nunc tubes (Thermo Fisher Scientific, Denmark), it was stored at –80°C, and it was analyzed for cobalt and chromium content on an ICP–SFMS Finnigan ELEMENT mass spectrometer (Finnigan MAT, Bremen, Germany) in an independent ISO 17025/ISO 9001:2000–accredited laboratory (ALS Scandinavia’s laboratories, Luleå, Sweden).
Lymphocyte analysis
Initially, a 7–mLVacuette (456057) (BectonDickinson, NJ) CPDA tube was used for the subpopulation analysis and a 4–mL Venosafe EDTA tube (Terumo) was used for the total lymphocyte count. From the start of 2010, the EDTA tubes were used for both samples.
Subsets were determined according to their immuno phenotype (CD3+, CD3+CD4+, CD3+CD8+, CD3–CD19+, and CD16+CD56+). Until the beginning of 2010, the samples were analyzed on a Becton Dickinson FacsCalibur flow cytometer with three color combinations of monoclonal antibodies (MAbs) obtained from DakoCytomation. From 2010, the laboratory switched to using a Becton Dickinson FacsCanto II flow cytometer. Absolute counts of lymphocyte subpopulations were determined by a single–platform, lyse–no–wash procedure using the BD Multitest 6–Color TBNK reagent (TruCount tubes; BD Biosciences). Before switching systems, it was validated that there was no significant difference between the two methods (data not shown).
Statistics
The primary endpoint of the RCT was range of motion. The sample size of the RCT was based on range of motion, where we aimed to include 20 patients in each group.
With baseline sample and 4 follow–up samples, a correlation of 0.88 (data from own study) and a type–2 error of 0.05, the study should be large enough to reproduce previous CD3+CD8+ differences of 0.152 109/L (Hart et al. 2009b) with a power of 90%.
As the analyses represent a post hoc calculation our results are given with 95% CIs. Data are presented as median (range), apart from the functional parameters at 2 years, which are presented as means.
Ion concentrations below the detection limit were assigned a value of half the detection limit and reported as parts per billion (ppb) (equivalent to µg/L). The differences between ion concentrations were compared using regression analysis adjusted for sex but not smoking, as only 3 patients smoked. The development of lymphocyte (and subgroup) levels over time was analyzed with a repeated–measures ANCOVA adjusted for baseline values and sex, but not smoking.
Regression analysis on the delta lymphocyte values adjusted for sex and baseline values was performed, but with the proviso that metal ion levels and lymphocyte levels are measured on the same day. The half–life of mature NK–cells in the peripheral blood system appears to be about 7–10 days; it is 5–6 weeks for B–cells and approximately 3 months for T–cells (Fulcher and Basten 1997, Hellerstein et al. 1999, Yokoyama et al. 2004). If we assume the metal ions affects the lymphocytes, the lymphocyte counts actually reflect the levels of metal ions weeks to months prior to testing, so for these analyses we assume that the ion levels were similar in that period.
Values of p below 0.05 indicated a significant difference. All analyses were carried out using STATA software version 11.2.
Ethics
The study was approved by the regional ethics board of Region Southern Denmark (VF–20050133) and the procedures followed were in accordance with the Helsinki Declaration.
Results
Cobalt and chromium ion concentrations
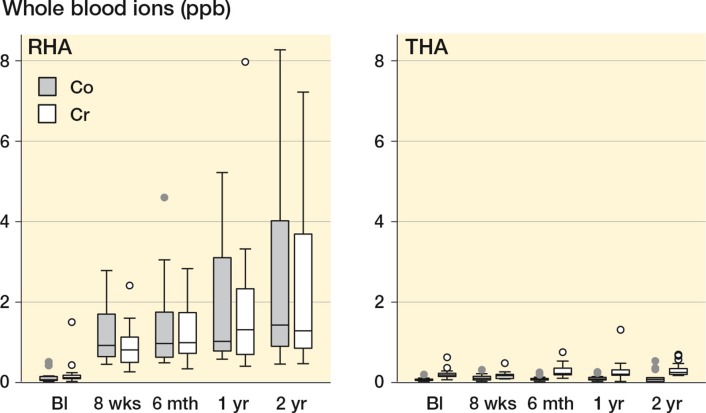
At 2 years, the median whole–blood cobalt and chromium levels in RHA patients reached 1.43 (0.46–8.27) ppb and 1.29 (0.47–7.22) ppb (Figure 2), and at all follow–up times all metal ion levels were higher in patients with an RHA than in those with a THA (p ≤ 0.001). By the first year, the median RHA whole–blood ion concentrations had reached a steady state. In some individuals, the cobalt and chromium ion concentrations increased markedly after the first year, but the effect on the median was not statistically significant (p = 0.1 for both ions). 2 RHA patients had whole–blood levels above 7 ppb and their functional status at 2 years is summarized in Table 2. The numbers in each group were too small for statistical analysis, but the patients with raised metal ion levels did not appear to suffer clinically as a result of their raised ion levels.
Figure 2.
Box plot depicting median, lower and upper quartiles (box). Whiskers mark the adjacent values and dots represent the outliers.
Table 2.
Mean values (SD) of functional parameters at 2 years. None were considered failures. The numbers in the group > 7 ppb were too low for meaningful testing of statistical significance
| Groups | n | UCLA | ROM, ° | Increase in ROM from baseline, ° | HHS | Pain (100 is max) |
|---|---|---|---|---|---|---|
| RHA > 7 ppb | 2 | 6.5 (2.1) | 225 (28) | 80 (23) | 95 (3) | 0.2 (0.3) |
| RHA < 7 ppb | 17 | 7.4 (1.8) | 220 (39) | 58 (44) | 92 (10) | 7.5 (14) |
| THA | 19 | 7.2 (2.1) | 226 (33) | 59 (37) | 91 (14) | 11.3 (22) |
Lymphocyte counts
Only 1 RHA patient had CD3+CD8+ levels below a normal 95% CI range (Bisset et al. 2004), with a decline from 0.18 to 0.12 × 109cells/L at 2 years (Table 3). The patient was male, with a highly functional hip, whole–blood cobalt of 1.5, and whole–blood chromium of 1.6 ppb. 3 additional THA patients had low levels at some stage and they were all well.
Table 3.
Details of lymphocyte counts at each follow–up time, reported as median values 95%CI and range. Development of lymphocyte and subgroup counts are reported separately for RHA and THA together with the mean 95% CI difference between the groups. The linear regression analysis was adjusted for gender and baseline values, and repeated measures were accounted for by the Cluster option in STATA. The lymphocyte change/year is reported as mean 95% CI, and in the analysis between THA and RHA a negative mean change/year indicates that the decline in lymphocytes was greater for RHA
| Baseline | 8 weeks | 6 month | 1 year | 2 years | Mean change/year | p–value | ||
|---|---|---|---|---|---|---|---|---|
| RHA | ||||||||
| Total lymphocytes | median | 1.65 | 1.54 | 1.64 | 1.54 | 1.66 | –0.05 | 0.5 |
| (×109/L) | 95%CI | 1.31–2.01 | 1.21–1.76 | 1.17–1.81 | 1.38–1.74 | 1.28–1.87 | –0.19 to 0.09 | |
| range | 0.84–2.34 | 0.91–2.77 | 0.76–2.21 | 0.98–3.14 | 0.73–3.01 | |||
| CD3+ (×109/L) | 1.13 | 1.25 | 1.10 | 1.15 | 1.13 | –0.06 | 0.3 | |
| 0.95–1.55 | 0.88–1.35 | 0.85–1.43 | 1.01–1.27 | 0.99–1.35 | –0.18 to 0.07 | |||
| 0.66–1.85 | 0.63–2.08 | 0.57–1.58 | 0.71–2.32 | 0.53–2.37 | ||||
| CD3+CD4+ (×109/L) | 0.74 | 0.73 | 0.66 | 0.73 | 0.76 | –0.05 | 0.3 | |
| 0.59–1.18 | 0.64–1.02 | 0.59–0.94 | 0.67–0.92 | 0.65–0.86 | –0.13 to 0.04 | |||
| 0.47–1.30 | 0.41–1.57 | 0.40–1.18 | 0.50–1.83 | 0.38–1.80 | ||||
| CD3+CD8+ (×109/L) | 0.32 | 0.31 | 0.33 | 0.30 | 0.29 | –0.03 | 0.1 | |
| 0.22–0.41 | 0.23–0.49 | 0.21–0.43 | 0.25–0.40 | 0.20–0.41 | –0.08 to 0.01 | |||
| 0.18–0.70 | 0.14–0.73 | 0.15–0.65 | 0.16–0.63 | 0.12–0.61 | ||||
| CD3–CD19+ (×109/L) | 0.18 | 0.15 | 0.18 | 0.19 | 0.19 | 0.00 | 0.8 | |
| 0.12–0.27 | 0.13–0.22 | 0.15–0.24 | 0.15–0.23 | 0.14–0.27 | –0.02 to 0.02 | |||
| 0.08–0.41 | 0.09–0.31 | 0.12–0.40 | 0.14–0.39 | 0.10–0.40 | ||||
| CD16+CD56+ (×109/L) | 0.19 | 0.17 | 0.20 | 0.19 | 0.17 | 0.01 | 0.4 | |
| 0.08–0.29 | 0.15–0.24 | 0.06–0.26 | 0.11–0.28 | 0.13–0.28 | –0.01 to 0.02 | |||
| 0.05–0.45 | 0.05–0.56 | 0.03–0.59 | 0.05–0.38 | 0.06–0.56 | ||||
| THA | ||||||||
| Total lymphocytes | median | 1.66 | 1.70 | 1.74 | 1.67 | 1.55 | –0.07 | 0.2 |
| (×109/L) | 95%CI | 1.27–2.31 | 1.29–1.96 | 1.07–2.23 | 1.29–1.89 | 1.29–1.90 | –0.18 to 0.04 | |
| range | 0.76–2.52 | 0.68–3.13 | 0.90–3.94 | 0.86–2.57 | 0.53–2.66 | |||
| CD3+ (×109/L) | 1.39 | 1.24 | 1.33 | 1.22 | 1.15 | –0.06 | 0.1 | |
| 0.82–1.59 | 0.97–1.56 | 0.84–1.65 | 0.98–1.51 | 1.06–1.42 | –0.15 to 0.02 | |||
| 0.53–2.16 | 0.43–2.35 | 0.65–3.31 | 0.52–2.16 | 0.42–2.29 | ||||
| CD3+CD4+ (×109/L) | 0.86 | 0.75 | 0.88 | 0.73 | 0.75 | –0.01 | 0.8 | |
| 0.59–0.96 | 0.64–0.98 | 0.66–1.04 | 0.63–0.96 | 0.69–0.97 | –0.09 to 0.07 | |||
| 0.39–1.47 | 0.31–1.87 | 0.40–2.36 | 0.37–1.43 | 0.32–1.61 | ||||
| CD3+CD8+ (×109/L) | 0.41 | 0.37 | 0.45 | 0.37 | 0.35 | –0.04 | 0.02 | |
| 0.26–0.66 | 0.25–0.61 | 0.21–0.66 | 0.24–0.55 | 0.20–0.53 | –0.08 to –0.01 | |||
| 0.11–1.27 | 0.09–0.89 | 0.13–1.50 | 0.11–1.16 | 0.08–0.97 | ||||
| CD3–CD19+ (×109/L) | 0.18 | 0.13 | 0.13 | 0.14 | 0.15 | 0.00 | 0.8 | |
| 0.12–0.22 | 0.10–0.20 | 0.08–0.24 | 0.10–0.28 | 0.10–0.21 | –0.02 to 0.02 | |||
| 0.04–0.56 | 0.03–0.38 | 0.04–0.61 | 0.03–0.44 | 0.03–0.55 | ||||
| CD16+CD56+ (×109/L) | 0.19 | 0.17 | 0.15 | 0.16 | 0.17 | –0.01 | 0.5 | |
| 0.13–0.26 | 0.13–0.25 | 0.11–0.20 | 0.11–0.22 | 0.13–0.22 | –0.03 to 0.01 | |||
| 0.09–0.53 | 0.09–0.52 | 0.08–0.27 | 0.06–0.33 | 0.06–0.34 | ||||
| Difference between THA and RHA | ||||||||
| Total lymphocytes | mean diff. | 0.05 | 0.05 | 0.26 | 0.02 | –0.04 | –0.01 | 0.9 |
| (×109/L) | 95%CI | –0.34–0.44 | –0.35–0.44 | –0.26–0.78 | –0.32–0.36 | –0.39–0.31 | –0.17 to 0.16 | |
| CD3+ (×109/L) | 0.07 | 0.06 | 0.31 | 0.03 | 0.01 | 0.01 | 0.9 | |
| –0.26–0.40 | –0.26–0.37 | –0.14–0.76 | –0.25–0.32 | –0.28–0.30 | –0.13 to 0.15 | |||
| CD3+CD4+ (×109/L) | –0.03 | –0.02 | 0.18 | –0.02 | 0.01 | 0.05 | 0.4 | |
| –0.25–0.20 | –0.24–0.21 | –0.12–0.48 | –0.23–0.18 | –0.21–0.23 | –0.06 to 0.15 | |||
| CD3+CD8+ ×109/L | 0.12 | 0.07 | 0.18 | 0.08 | 0.06 | –0.01 | 0.7 | |
| –0.06–0.30 | –0.07–0.21 | –0.06–0.42 | –0.08–0.23 | –0.07–0.20 | –0.06 to 0.04 | |||
| CD19+ (×109/L) | –0.01 | –0.02 | –0.01 | –0.02 | –0.03 | 0.00 | 0.8 | |
| –0.09–0.07 | –0.08–0.05 | –0.12–0.09 | –0.09–0.05 | –0.10–0.05 | –0.02 to 0.03 | |||
| CD16+CD56+ (×109/L) | 0.01 | –0.01 | –0.03 | –0.04 | –0.04 | –0.02 | 0.2 | |
| –0.08–0.10 | –0.09–0.07 | –0.13–0.06 | –0.11–0.03 | –0.11–0.04 | –0.04 to 0.01 |
T–cells: CD3+, CD3+CD4+, and CD3+CD8+. Defend the host cells from infection by recognizing foreign antigens.
B–cells: CD19+. Present antigens and make antibodies against antigens.
NK–cells: CD16+CD56+. Defend host cells from infection by recognizing non–self organisms in an unspecific way.
The T–lymphocyte counts tended to decline over the 2–year period, as tested by the trend over time repeated–measures ANCOVA analysis. This decline was statistically significant for CD3+CD8+ in the THA group, but there was no difference between RHA and THA regarding total lymphocyte counts or counts of any of the lymphocyte subgroups during the 2–year study period.
Relationship between cobalt and chromium ion concentrations and lymphocyte counts
The regression analysis (Table 4) showed a depressing effect of metal ions on all lymphocyte subgroups except for NK–cells, which showed a modest and not statistically significant effect of being stimulated. Statistical significance for a depressive effect of metal ions was primarily seen for cobalt in the T-lymphocytes, but the statistical significance was not consistent at all follow-up times.
Table 4.
Regression analysis of RHA and THA combined between ion level and change in lymphocytes, with regression coefficients measured as ×109 cells/L/ppb. Dependent variable: change in T–cell levels from baseline to the time given; independent variable: metal ion concentration at the time given. ANCOVA adjusted for baseline values of T–cells and for gender
| 8 weeks |
6 months |
1 year |
2 years |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| coeff. | 95% CI | coeff. | 95% CI | coeff. | 95% CI | coeff. | 95% CI | |||
| Total | Co | –0.00 | (–0.24 to 0.23) | –0.32 a | (–0.61 to –0.03) | –0.02 | (–0.16 to 0.12) | –0.07 a | (–0.14 to –0.01) | |
| lymphocytes | Cr | –0.05 | (–0.32 to 0.22) | –0.28 | (–0.60 to 0.04) | –0.04 | (–0.10 to 0.01) | –0.06 | (–0.12 to 0.00) | |
| CD3+ | Co | –0.08 | (–0.34 to 0.18) | –0.30 a | (–0.57 to –0.03) | –0.05 | (–0.16 to 0.07) | –0.10 b | (–0.16 to –0.04) | |
| Cr | –0.12 | (–0.46 to 0.23) | –0.23 | (–0.55 to 0.09) | –0.06 | (–0.13 to 0.00) | –0.07 | (–0.15 to 0.01) | ||
| CD3+CD4+ | Co | 0.02 | (–0.13 to 0.17) | –0.19 a | (–0.37 to –0.01) | –0.02 | (–0.10 to 0.07) | –0.06 c | (–0.09 to –0.03) | |
| Cr | 0.00 | (–0.18 to 0.18) | –0.17 | (–0.38 to 0.05) | –0.03 a | (–0.05 to –0.00) | –0.05 a | (–0.08 to –0.01) | ||
| CD3+CD8+ | Co | –0.02 | (–0.07 to 0.03) | –0.09 a | (–0.16 to –0.03) | –0.01 | (–0.05 to 0.02) | –0.02 a | (–0.03 to –0.00) | |
| Cr | –0.03 | (–0.09 to 0.03) | –0.10 b | (–0.16 to –0.04) | –0.00 | (–0.02 to 0.02) | –0.01 | (–0.03 to 0.01) | ||
| CD3–CD19+ | Co | 0.01 | (–0.03 to 0.04) | –0.04 | (–0.09 to 0.01) | –0.00 | (–0.02 to 0.02) | –0.01 | (–0.03 to 0.01) | |
| Cr | 0.00 | (–0.04 to 0.05) | –0.04 | (–0.09 to 0.01) | –0.00 | (–0.01 to 0.00) | –0.01 | (–0.03 to 0.00) | ||
| CD16+CD56+ | Co | 0.00 | (–0.04 to 0.04) | –0.02 | (–0.05 to 0.02) | 0.02 | (–0.00 to 0.04) | 0.01 | (–0.01 to 0.03) | |
| Cr | –0.00 | (–0.04 to 0.03) | –0.02 | (–0.05 to 0.01) | 0.01 a | (0.00 to 0.02) | 0.00 | (–0.01 to 0.02) | ||
a p < 0.05,
b p < 0.01,
c p < 0.001.
T–cells: CD3+, CD3+CD4+, and CD3+CD8+. Defend the host cells from infection by recognizing foreign antigens.
B–cells: CD19+. Present antigens and make antibodies against antigens.
NK–cells: CD16+CD56+. Defend host cells from infection by recognizing non–self organisms in an unspecific way.
Discussion
The present study is the first to present prospective lymphocyte data in a MoM RHA population. The major findings were indications of a suppressive effect of cobalt ions on T–lymphocytes and a general decline in T–lymphocyte levels over time for both RHA and THA.
The RHA implant that we used (ASR) was later withdrawn from the market due to excessive failure rates, but despite the fact that it included a rather large proportion of females and of heads below 51 mm, the median ion concentrations measured in the present study were comparable to those in other prospective RHA studies involving implants known to have either a high degree or a suboptimal degree of survival (Allan et al. 2007, Heisel et al. 2008, Daniel et al. 2009, Isaac et al. 2009, deSouza et al. 2010, Garbuz et al. 2010, Beaule et al. 2011, Pattyn et al. 2011). We therefore believe that our results are not specific for the ASR implant, but that they can be generally applied to metal ion and lymphocyte levels.
The absolute circulating CD3+CD8+ levels of 0.29 × 109 cells/L for the RHA group at 2 years were slightly below that of a normal population but were similar to those reported by Hart (Hart et al. 2009b); both sets of results were lower than those of Granchi (Granchi et al. 2003). But where other studies (Savarino et al. 1999, Granchi et al. 2003, Hart et al. 2009b) have found CD3+CD8+ values at the higher end of the normal reference range of values (Bisset et al. 2004, Jentsch–Ullrich et al. 2005) for non–MoM implants, the value of 0.35 × 109 cells/L in our study was identical to that in a normal population. It included older MoM patients than in earlier studies, which may help to explain the slightly lower CD3+CD8+levels in our RHA group, but paradoxically our THA patients were younger than those in the other studies and they still had lower CD3+CD8+ levels. Different laboratories may use different equipment or protocols, and since race, age, sex, smoking, age, and time of day may affect lymphocyte levels, it is difficult to compare lymphocyte counts from different studies.
Baseline values permitted a trend–over–time analysis, which did not indicate that the course of total lymphocyte levels and subgroup levels differed between the 2 study groups. Despite the general lack of statistical significance, we observed a slight decline in total lymphocyte counts and in CD3+, CD3+CD4+, and CD3+CD8+ counts (significant for THA) postoperatively in both groups, whereas B–cells did not appear to be affected. We saw no signs of clinical adverse effects such as increased rates of infection, but in general a depression in lymphocyte counts would be considered to be negative as the decline in CD3+CD4+ and CD3+CD8+ would impair the release of cytokines and growth factors that regulate other immune cells and impair the ability of the host to fight virus–infected cells and certain cancer cells (Ducloux et al. 2010, Martorelli et al. 2010). A generally declining effect over time could either be an age–related effect—an improvement from an increased level from chronic inflammation that disappears when the arthritic hip is replaced—or a depression due to a foreign object regardless of alloy. Without samples from the hip or the lymphatic system, we do not know if this could mean a redirection of T–lymphocytes elsewhere.
The regression analysis between metal ions and lymphocyte counts suggested the possibility of a general depressive effect of metal ions, and cobalt in particular, on T–lymphocytes. Weakening the link, however, was the 1–year regression results where the relationship to metal ion concentrations was almost non–existent. Given the turnover rate of lymphocytes and lymphocyte subgroups, a depressing effect should have been noted. Support for the theory comes from the study by Hart et al. (2009b), which not only found increased CD3+CD8+ counts in MoM patients but added support for a general depressive effect—as these authors also found inverse correlations between cobalt or chromium concentrations and absolute counts of T– and B–lymphocytes. Furthermore, they also found cobalt to be slightly more predictive than chromium, a finding that is repeated in the present results. Partial support for a depressive effect of chromium can be found in a study of revision MoP THAs where there was a negative correlation between chromium levels and all the lymphocyte parameters—statistically significantly so for total lymphocytes and CD3+CD4+ lymphocytes (Savarino et al. 1999). However, in contrast to our results, rising cobalt concentrations were associated with an increase in total lymphocyte counts and to a fall in the NK cell count. The connection between metal ion concentrations and lymphocyte counts has also been debated by other authors. Hailer et al. (2011) conducted an RCT (without baseline lymphocyte values), and despite the lack of significance, the results pointed in the opposite direction except for CD16+CD56+ and CD3+CD4+. Granchi et al. (2003) found lower lymphocyte levels on all counts except for B–lymphocytes, when MoM was compared to ceramic–on–ceramic (CoC) but not to metal–on–polyethylene (MoP). None of the observed differences were statistically significant and there were no associations between ion concentration and lymphocyte counts. Finally, a study of revision THA including a few MoM failed to relate either the amount or the type of metal in periprosthetic tissues or in the bone marrow to the proportion of lymphocytes in the peripheral blood (Case et al. 2000). It can be argued that some of the studies were small, had low metal ion concentrations, and lacked baseline levels, so the evidence for MoM implants affecting the immune system is still conflicting; however, there is the some agreement regarding an association between reduced CD3+CD4+ levels and raised cobalt levels.
This is interesting, as several in vitro studies have found cytotoxic effects of cobalt ions on a variety of cells (Gill et al. 2012). A study using lymphocytes (CD3+) (Akbar et al. 2011) found reduced proliferation at 10 µM Co(2+) and increased apoptosis at 100 µM Co(2+), suggesting an inhibitory but not lethal effect of cobalt at levels found clinically. The 2–year regression coefficient in the present study suggests increases from 0.01 × 109 cells/ppb (CD16+CD56+) to depression of –0.10 × 109 cells/ppb (CD3+) or roughly 10% per ppb with wide CIs. For at high–wear–rate patient, one would expect a serious detrimental effect on the immune system, but there is currently no evidence in support of increased viral infections. As for DNA aberrations in different cell cultures, cobalt seems less harmful than chromium (Gill et al. 2012), which accumulates intracellularly. Specific studies on lymphocyte cell lines (CD19+) {El–Yamani, 2011 719 /id} indicate impairment of DNA repair mechanisms, and studies on circulating lymphocytes and revision implants (Doherty et al. 2001) have suggested more aneuploidy in titanium implants and more translocations in chromium–cobalt implants. That study was limited by comparison with a younger population without implants and by having low levels of circulating metal ions, but MoM implants revised to MoP have shown less leukocyte DNA damage in the revised group (Dunstan et al. 2008). A prospective study of a primary MoM population (Ladon et al. 2004) supported the idea of an adverse effect of metal implants, with an increase in lymphocyte translocations over time, but it did not show any correlation with circulating cobalt and chromium ion levels.
The greatest fear with DNA damage is the risk of cancer, but presently the overall cancer incidence rate for MoM articulations is slightly lower than for the general population or for a THA population (Visuri et al. 2006, Smith et al. 2012, Makela et al. 2012). Skin cancers such as melanomas have increased in lymphocyte–depressed patients, and one study has found a small increase in basal cell cancers (Makela et al. 2012). However, in a larger study the hypothesized depressive effect of metal ions on lymphocytes was not reflected by increased skin cancer rates in the early years following MoM implantation (Smith et al. 2012)
Limitations of the study
This was a small–scale study but it was strengthened by the randomized design, with several follow–up measurements that compensated for the lower numbers. The limitation with a smaller scale was, however, evident during randomization where—by chance—the study had more women in the RHA group. A few studies have found higher CD3+CD4+ levels and statistically insignificantly raised CD3+CD8+ levels in females (Bisset et al. 2004, Jentsch–Ullrich et al. 2005). If the skewed distribution had had an effect on our results, the predominance of female RHA patients would have caused higher baseline values. As we in fact observed the opposite, we do not believe that the male–female distribution affected our conclusions, and adjusting the analysis for baseline values and using delta values further strengthened the study.
We did not measure activated lymphocytes or perform any functional tests. The study only addressed the absolute numbers and we cannot exclude the possibility that metal ions may have an adverse effect on lymphocyte function.
In addition, we assume that the metal ion levels reflected the total wear volume ( De Smet et al. 2008), but a great limitation of that study and the present study was the lack of knowledge of local concentrations around the hip joint, the bone marrow, and the lymph nodes and also the type of metal debris present. Some of the above studies have indicated that there is an adverse effect of MoM implants, and larger studies including lymphocyte function, hip joint and perhaps bone marrow aspirate could help clarify the matter, but such specimens may be difficult to obtain from patients with well–functioning hips.
Lymphocyte counts are not standard tests for MoM implants, but recent calls for regular metal ion testing of MoM hips in combination with data from national hip registries and the possibility of combining the patients with national morbidity and cancer registries could in time help answer the question of whether the changes we observed have clinical relevance.
Supplementary data
Figure 3 and Table 5 are available at our website (www.actaorthop.org), identification number 5347.
Acknowledgments
SO, OO, JEV, and JØP designed the study. JØP and CN obtained and analyzed the data. JØP, CN, SO, OO, and JEV wrote the initial draft. JØP and CN ensured the accuracy of the data and of the analyses.
We thank project nurse Annie Gam–Pedersen for practical/logistical work and Lars Korsholm for statistical help in choosing the relevant models for analysis and for writing the statistical programs.
Søren Overgaard has received institutional support from both the Danish ASR/DePuy distributor and from Biomet Denmark. The Danish Ministry of Health and Prevention (grant id: 2006–1022–59) provided general funding for the study.
References
- Akbar M, Brewer JM, Grant MH. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J Immunotoxicol. 2011;(8):140–9. doi: 10.3109/1547691X.2011.553845. [DOI] [PubMed] [Google Scholar]
- Allan DG, Trammell R, Dyrstad B, Barnhart B, Milbrandt JC. Serum cobalt and chromium elevations following hip resurfacing with the Cormet 2000 device. J Surg Orthop Adv. 2007;16:12–8. [PubMed] [Google Scholar]
- Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg (Am) 1984;66:228–41. [PubMed] [Google Scholar]
- Beaule PE, Kim PR, Hamdi A, Fazekas A. A prospective metal ion study of large–head metal–on–metal bearing: a matched–pair analysis of hip resurfacing versus total hip replacement. Orthop Clin North Am. 2011;42:251–7. doi: 10.1016/j.ocl.2011.01.005. ix. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol. 2004;72:203–12. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- Case CP, Langkamer VG, Lock RJ, Perry MJ, Palmer MR, Kemp AJ. Changes in the proportions of peripheral blood lymphocytes in patients with worn implants. J Bone Joint Surg (Br) 2000;82:748–54. doi: 10.1302/0301-620x.82b5.9946. [DOI] [PubMed] [Google Scholar]
- Daniel J, Ziaee H, Pynsent PB, McMinn DJ. The validity of serum levels as a surrogate measure of systemic exposure to metal ions in hip replacement. J Bone Joint Surg (Br) 2007;89:736–41. doi: 10.1302/0301-620X.89B6.18141. [DOI] [PubMed] [Google Scholar]
- Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six–year results of a prospective study of metal ion levels in young patients with metal–on–metal hip resurfacings. J Bone Joint Surg (Br) 2009;91:176–9. doi: 10.1302/0301-620X.91B2.21654. [DOI] [PubMed] [Google Scholar]
- De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De SK. Correlation between inclination of the acetabular component and metal ion levels in metal–on–metal hip resurfacing replacement. J Bone Joint Surg (Br) 2008;90:1291–7. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- De Smet K, De HR, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C, Gill HS. Metal ion measurement as a diagnostic tool to identify problems with metal–on–metal hip resurfacing. J Bone Joint Surg (Am) (Suppl 4) 2008;90:202–8. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- deSouza R M, Parsons N R, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten–year period. J Bone Joint Surg (Br) 2010;(92):1642–7. doi: 10.1302/0301-620X.92B12.24654. [DOI] [PubMed] [Google Scholar]
- Doherty AT, Howell RT, Ellis LA, Bisbinas I, Learmonth ID, Newson R, Case CP. Increased chromosome translocations and aneuploidy in peripheral blood lymphocytes of patients having revision arthroplasty of the hip. J Bone Joint Surg (Br) 2001;83:1075–81. doi: 10.1302/0301-620x.83b7.10102. [DOI] [PubMed] [Google Scholar]
- Ducloux D, Courivaud C, Bamoulid J, Vivet B, Chabroux A, Deschamps M, Rebibou JM, Ferrand C, Chalopin JM, Tiberghien P, Saas P, Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol. 2010;21:868–75. doi: 10.1681/ASN.2009090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan E, Ladon D, Whittingham–Jones P, Carrington R, Briggs TW. Chromosomal aberrations in the peripheral blood of patients with metal–on–metal hip bearings. J Bone Joint Surg (Am) 2008;90:517–22. doi: 10.2106/JBJS.F.01435. [DOI] [PubMed] [Google Scholar]
- Fulcher DA, Basten A. B cell life span: a review. Immunol Cell Biol. 1997;75:446–55. doi: 10.1038/icb.1997.69. [DOI] [PubMed] [Google Scholar]
- Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award: Metal–on–Metal Hip Resurfacing versus Large–diameter Head Metal–on–Metal Total Hip Arthroplasty: A Randomized Clinical Trial. Clin Orthop. 2010;468(2):318–25. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol Med. 2012;18(3):45–55. doi: 10.1016/j.molmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Granchi D, Savarino L, Ciapetti G, Cenni E, Rotini R, Mieti M, Baldini N, Giunti A. Immunological changes in patients with primary osteoarthritis of the hip after total joint replacement. J Bone Joint Surg (Br) 2003;85:758–64. [PubMed] [Google Scholar]
- Hailer NP, Blaheta RA, Dahlstrand H, Stark A. Elevation of circulating HLA DR(+) CD8(+) T–cells and correlation with chromium and cobalt concentrations 6 years after metal–on–metal hip arthroplasty. Acta Orthop. 2011;82:6–12. doi: 10.3109/17453674.2010.548028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AJ, Hester T, Sinclair K, Powell JJ, Goodship AE, Pele L, Fersht NL, Skinner J. The association between metal ions from hip resurfacing and reduced T–cell counts. J Bone Joint Surg (Br) 2006;88:449–54. doi: 10.1302/0301-620X.88B4.17216. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal–on–metal hip resurfacing. J Bone Joint Surg (Br) 2009a;91:738–44. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Skinner JA, Winship P, Faria N, Kulinskaya E, Webster D, Muirhead–Allwood S, Aldam CH, Anwar H, Powell JJ. Circulating levels of cobalt and chromium from metal–on–metal hip replacement are associated with CD8+ T–cell lymphopenia. J Bone Joint Surg (Br) 2009b;91:835–42. doi: 10.1302/0301-620X.91B6.21844. [DOI] [PubMed] [Google Scholar]
- Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running–in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg (Am) (Suppl 3) 2008;90:125–33. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune JM. Directly measured kinetics of circulating T lymphocytes in normal and HIV–1–infected humans. Nat Med. 1999;5:83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- Isaac GH, Siebel T, Oakeshott RD, Lennan–Smith R, Cobb AG, Schmalzried TP, Vail TP. Changes in whole blood metal ion levels following resurfacing: serial measurements in a multi–centre study. Hip Int. 2009;19:330–7. doi: 10.1177/112070000901900406. [DOI] [PubMed] [Google Scholar]
- Jentsch–Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets’ reference ranges in an age– and gender–balanced population of 100 healthy adults––a monocentric German study. Clin Immunol. 2005;116:192–7. doi: 10.1016/j.clim.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, Murray DW, Gill HS. Lymphocyte proliferation responses in patients with pseudotumors following metal–on–metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444–50. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal–on–metal hip arthroplasty. J Arthroplasty. 2004;19:78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal–on–metal bearings in hip resurfacing and large–diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg (Br) 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal–on–metal hip arthroplasties. J Arthroplasty. 2004;19:12–6. doi: 10.1016/j.arth.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Makela KT, Visuri T, Pulkkinen P, Eskelinen A, Remes V, Virolainen P, Junnila M, Pukkala E. Risk of cancer with metal–on–metal hip replacements: population based study. BMJ. 2012;345:e4646. doi: 10.1136/bmj.e4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorelli D, Muraro E, Merlo A, Turrini R, Rosato A, Dolcetti R. Role of CD4+ cytotoxic T lymphocytes in the control of viral diseases and cancer. Int Rev Immunol. 2010;29:371–402. doi: 10.3109/08830185.2010.489658. [DOI] [PubMed] [Google Scholar]
- Pandit H, Glyn–Jones S, Lardy–Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal–on–metal hip resurfacings. J Bone Joint Surg (Br) 2008:847–51. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- Pattyn CA, Lauwagie SN, Verdonk RC. Whole blood metal ion concentrations in correlation with activity level in three different metal–on–metal bearings. J Arthroplasty. 2011;26:58–64. doi: 10.1016/j.arth.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Penny JO, Overgaard S. Serum chromium levels sampled with steel needle versus plastic IV cannula. Does method matter? J Biomed Mater Res B Appl Biomater. 2010;92:1–4. doi: 10.1002/jbm.b.31479. [DOI] [PubMed] [Google Scholar]
- Pemmy JO, Ovesen O, Varmarken JE, Overgaard S. Similar range of motion and function after resurfacing large–head or standard total hip arthroplasty. 2–year results from a randomized clinical trial. Acta Orthop. 2013;84:x–x. doi: 10.3109/17453674.2013.788435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M, Borroff M, Gregg P, Howard P, MacGregor A, Tucker K. National Joint Registry for England and Wales. 7th. annual report 2010. Surgical data to December 2009. 7. 2010. 12–5–2011. [Google Scholar]
- Savarino L, Granchi D, Ciapetti G, Stea S, Donati ME, Zinghi G, Fontanesi G, Rotini R, Montanaro L. Effects of metal ions on white blood cells of patients with failed total joint arthroplasties. J Biomed Mater Res. 1999;47:543–50. doi: 10.1002/(sici)1097-4636(19991215)47:4<543::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Dieppe P, Porter M, Blom AW. Risk of cancer in first seven years after metal–on–metal hip replacement compared with other bearings and general population: linkage study between the National Joint Registry of England and Wales and hospital episode statistics. BMJ. 2012;344:e2383. doi: 10.1136/bmj.e2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visuri TI, Pukkala E, Pulkkinen P, Paavolainen P. Cancer incidence and causes of death among total hip replacement patients: a review based on Nordic cohorts with a special emphasis on metal–on–metal bearings. Proc Inst Mech Eng [H ] 2006;220:399–407. doi: 10.1243/095441105X63282. [DOI] [PubMed] [Google Scholar]
- Walter LR, Marel E, Harbury R, Wearne J. Distribution of chromium and cobalt ions in various blood fractions after resurfacing hip arthroplasty. J Arthroplasty. 2008;23:814–21. doi: 10.1016/j.arth.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–29. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.