Abstract
Secondhand smoke exposure (SHSe) is a known cause of many adverse health effects in adults and children. Increasingly, SHSe assessment is an element of tobacco control research and implementation worldwide. In spite of decades of development of approaches to assess SHSe, there are still unresolved methodological issues; therefore, a multidisciplinary expert meeting was held to catalogue the approaches to assess SHSe and with the goal of providing a set of uniform methods for future use by investigators and thereby facilitate comparisons of findings across studies. The meeting, held at Johns Hopkins, in Baltimore, Maryland, USA, was supported by the Flight Attendant Medical Research Institute (FAMRI). A series of articles were developed to summarise what is known about self-reported, environmental and biological SHSe measurements. Non-smokers inhale toxicants in SHS, which are mainly products of combustion of organic materials and are not specific to tobacco smoke exposure. Biomarkers specific to SHSe are nicotine and its metabolites (eg, cotinine), and metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cotinine is the preferred blood, saliva and urine biomarker for SHSe. Cotinine and nicotine can also be measured in hair and toenails. NNAL (4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol), a metabolite of NNK, can be determined in the urine of SHS-exposed non-smokers. The selection of a particular biomarker of SHSe and the analytic biological medium depends on the scientific or public health question of interest, study design and setting, subjects, and funding. This manuscript summarises the scientific evidence on the use of biomarkers to measure SHSe, analytical methods, biological matrices and their interpretation.
Keywords: Secondhand smoke exposure, questionnaires, biological markers, environmental exposure, validation, surveillance and monitoring, prevalence, human rights, secondhand smoke, smoking caused disease, environmental tobacco smoke, harm reduction, product analysis, carcinogens, global health, litigation, carcinogens
Introduction
This article presents the scientific evidence on the use of biomarkers of secondhand smoke exposure (SHSe), analytical methods, biological matrices and their interpretation. A biomarker for SHSe should be: (a) unique to SHSe; (b) easily detectable using analytic methods reproducible across laboratories; (c) reflect known toxic exposures or high correlation with such exposures; and (d) exhibit changed levels with a corresponding change in disease risk.1 Most toxicants in tobacco smoke result from combustion of organic materials and are not specific to SHSe.1 Metabolites of nicotine (cotinine, trans-3′-hydroxycotinine and their glucuronides, and nicotine glucuronide) and NNK (NNAL (4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol) and its glucuronides) can be measured in SHS-exposed individuals, with high sensitivity in various biological matrices (table 1).2 3
Table 1.
Biomarkers of secondhand smoke exposure (SHSe), characteristics and cut-off points for distinguishing smokers from non-smokers
| Biomarker | Half-life | Invasiveness | Cut-off point | Pros | Cons |
| Cotinine | Reflects recent SHSe | ||||
| Urine | 16 h (average) | Non-invasive | 50 ng/ml for higher SHSe | Higher concentrations than other matrices (higher sensitivity) | Need of facilities with privacy during collection Difficulty for population-based or children studies Need for creatinine clearance adjustment Collect data on renal disease and some prescription drugs |
| Blood | 16 h (average) | Invasive |
|
No adjustment required for hydration | Pregnant women have increased clearance rate Difficulty for infants and young children Lower sensitivity |
| Saliva | 16 h (average) | Non-invasive | 14 ng/ml for higher SHSe | Good for multiple measurements over a limited period of time | Potential issues with age, gender, race, oral pH, type of diet, dehydration, or drug treatment Lower sensitivity |
| Nicotine/cotinine | |||||
| Hair | 1 cm of hair proximal to the scalp is approximately equal to the last month's exposure | Non-invasive |
|
Easy to collect, ship and store (room temperature ≤5 years) Less affected by daily variability (fluctuating exposure, varying metabolism and nicotine elimination) Represents longer exposure |
Scarcity of hair in infants and adults Chemical hair treatments can reduce concentrations by 9% to 30% Age, gender and race may play roles in determining hair nicotine concentrations |
| Toenails | 1 mm is approximately equal to last month's exposure | Non-invasive | Not available | Easy to collect, ship and store (room temperature ≤20 years) Overcomes day-to-day exposure variability Represents longer exposure |
Need for further research and population concentrations |
| NNAL* | |||||
| Urine | Up to 3 weeks | Non-invasive | Not available | Related to a lung carcinogen Represents longer exposure than cotinine (urine/blood/saliva) |
Analytical expertise Costly equipment NNAL is carcinogenic and mutagenic, special lab handling Further research needed |
NNAL (4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol).
Selection of biomarkers of SHSe
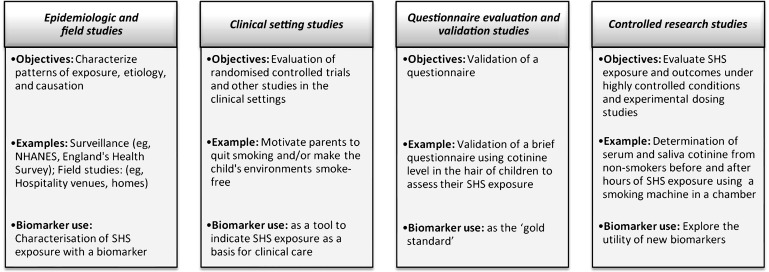
Selection of a SHSe biomarker depends on the scientific or public health question of interest, study design and setting, subjects, funding and laboratory access (figure 1). Novel biomarkers under development for use in highly controlled settings, such as chamber studies of exposures to volunteers, are not discussed here.
Figure 1.
Types of study designs and biomarker use. NHANES, National Health and Nutrition Examination Survey; SHS, secondhand smoke.
Nicotine and metabolites
Nicotine is present in substantial concentrations in virtually all tobacco products and in insignificant amounts in some foods.4 5 Nicotine is extensively metabolised, primarily in the liver, and its major proximate metabolite is cotinine: on average, 75% of nicotine is converted to cotinine, primarily by the liver enzyme cytochrome P450 2A6.6 Cotinine's half-life (t1/2), the time in which its concentration halves, is longer (average: 16 h) than nicotine's (2 h). Cotinine concentrations are more stable throughout the day, making it the preferred blood, saliva and urine biomarker for SHSe (table 1). Blood's cotinine concentrations and saliva are highly correlated. Urine cotinine concentrations average fourfold to sixfold higher than those in blood or saliva, making urine a more sensitive matrix to detect low-concentration exposure.7 Six metabolites (nicotine, cotinine and trans-3′-hydroxycotinine (3-HC) and their respective glucuronide conjugates) account for about 85% to 90% of a nicotine dose, and the sum of these metabolites in urine provides an approximate estimate of daily nicotine intake.
Considerable between-individual variability exists in the rate and pattern of nicotine metabolism, possibly affecting cotinine concentration resulting from a given nicotine exposure. Factors influencing nicotine metabolism can include genetic variation, race, gender, oral contraceptive use or other oestrogen-containing hormones, kidney failure and drugs, including anticonvulsants and rifampin.6 Cotinine concentrations in biofluids and nicotine in hair are generally higher in infants and children, compared to SHS-exposed adults; this is probably due to greater inhaled nicotine doses (closer proximity to smokers and higher minute ventilation per body mass) and slower cotinine metabolism.8
NNK and metabolites
NNK is a nitrosamine and potent carcinogen formed primarily during tobacco curing, when nicotine or pseudo-oxynicotine reacts with nitrite in tobacco.9 NNK is metabolised in the body to NNAL and NNAL-glucuronides, commonly measured together, total NNAL (tNNAL). tNNAL remains in the body longer than cotinine (t1/2=10 days to 3 weeks) (table 1). NNAL is a potent lung carcinogen, with activity similar to NNK, and tNNAL detection in the urine of SHS-exposed non-smokers forms a biochemical link between exposure and lung cancer. In rats, NNK and NNAL are known to induce tumours of the pancreas, and NNK causes nasal mucosa and liver tumours, although at higher doses than for lung tumours.9
Concentrations of urine and plasma cotinine are highly correlated with urine tNNAL,10 11 although no data link these biomarkers to other SHS chemicals. The dynamic nature of SHS is critical when interpreting biomarkers for particular exposure patterns, including brief high-intensity versus sustained low-concentration exposures. Volatile compounds including nicotine leave the smoke and adsorb to room surfaces (eg, walls, floors, furniture) quickly, while other volatile compounds may persist in the air.12
Analytical methods for biomarkers of SHSe
Choosing a laboratory for analysis should occur early in a study to assure that the collection protocol is suited to the assay. Analysis actually begins with sampling, and the collection protocol may have significant implications for subsequent assays. Analytical methods include radioimmunoassay,13 14 ELISA,14 15 gas chromatography (GC)-nitrogen-phosphorous detection (NPD),16 GC-thermal energy analysis (GC-TEA),17 GC-mass spectrometry (GC-MS),18 liquid chromatography (LC)-electrochemical detection (ECD),19 LC-tandem mass spectrometry (LC-MS/MS) and GC-MS/MS20 (table 2).
Table 2.
Analytical methods for measurement of biomarkers of secondhand smoke exposure (SHSe)
| Method | Sensitivity | Specificity | Cost | Comments |
| Cotinine | ||||
| Radioimmunoassay (RIA)13 14 | 0.10–2.00 ng/ml | Variable (poorest in urine) | Low | Quick and relatively low cost analysis of large number of samples |
| ELISA14 15 | 0.10–0.20 ng/ml | Good | Low | Quick and relatively low cost analysis of large number of samples |
| Gas chromatography-nitrogen-phosphorous detection (GC-NPD)16 | 0.10–0.20 ng/ml | Good | Moderate | Lacks sensitivity for very low level of SHSe |
| Gas chromatography-mass spectrometry (GC-MS)18 | 0.10–0.20 ng/ml | Excellent | High | |
| Liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry (LC-APCI MS/MS)21 | <0.05 ng/ml | Excellent | Extremely high | Current state-of-the-art method for urine cotinine. It can perform 100 samples/day |
| High performance liquid chromatography (HPLC)22 23 | 0.13–1.00 ng/ml | Good | Moderate | |
| Nicotine | ||||
| GC-MS23 | 0.02–0.25 ng/ml | Excellent | Moderate-high | It has been used for nicotine determination in hair and toenails |
| HPLC19 23 | 0.10–0.32 ng/ml | Good | Low | It has been used for nicotine determination in hair and with electrochemical detection for toenails |
| NNAL (4-[methylnitrosamino]-1-[3-pyridyl]-1-butanol) | ||||
| Liquid chromatography-tandem mass spectrometry (LC-MS/MS)20 | 0.25 pg/ml | Excellent | Extremely high | NNAL assay of choice. |
| Gas chromatography-thermal energy analysis (GC-TEA)17 | 0.15 pmol/ml | Excellent | Moderate | Difficult implementation |
Modified from Benowitz 1996.4
Urine nicotine and metabolites
Urine cotinine is a widely used biomarker of SHSe, with the sum of free cotinine and cotinine glucuronide (conjugated) resulting in higher concentrations in some studies.10 24–27 For comparison purposes, researchers must consider whether free cotinine or total (free plus conjugated) cotinine was measured. Free cotinine is preferable to use as it correlates better with plasma cotinine than total cotinine.7 Current state-of-the-art methods are GC-MS/MS and LC-MS/MS, with limits of detection (LOD) of 0.05 ng/ml,20 21 a sensitivity concentration needed in countries with low SHSe due to clean air regulations and lower air nicotine concentrations.
The advantages of determining SHSe with urine are that cotinine concentrations and other metabolites are higher than in other biological fluids; it represents relatively acute exposure; and collection is non-invasive. Disadvantages include the facilities for privacy during collection, difficulty collecting in population-based or children studies, variability in cotinine conversion factor and needing to adjust for creatinine clearance (data on renal diseases and prescription drugs that interfere with metabolism and renal secretion).
Blood nicotine and metabolites
Nicotine, cotinine and 3-HC can all be measured in blood, but cotinine is generally the preferred biomarker4 because of its longer half-life.28 With regular, sustained exposures, the longer cotinine t1/2 results in higher, more uniform steady-state concentrations than nicotine. However, the cotinine t1/2 is <1 day, indicating only recent SHSe. Cotinine t1/2 may be longer in young children than in older children and adults,29 and slightly shorter in women than in men.30 This reduction is especially notable in pregnant women due to increased clearance rates,31 and is critical when evaluating relative concentrations and applying cotinine values to identify pregnant smokers and non-smokers.
Cotinine is commonly measured in serum or plasma, with comparable results from either matrix; although uncommon, whole blood may also be used. Current analytic methods require no more than 1 ml of serum, which is readily obtainable from adults, although infants and young children may present challenges. GC-MS/MS and LC-MS/MS are preferred analytical methods for these matrices.
A potential disadvantage of serum assays versus urine is lower sensitivity, as urine cotinine concentrations are typically fourfold to sixfold higher than in serum. If extremely sensitive analytic methods are used, however, this is not a concern. As serum does not require adjustment for hydration differences among individuals, it provides a more uniform matrix measurement than urine. Blood collection is invasive, however, and salivary cotinine may be a necessary alternative. An advantage for US studies is that using serum cotinine facilitates direct comparison with representative data for US non-smokers from the National Health and Nutrition Examination Survey (NHANES).32 33
Saliva cotinine and metabolites
Cotinine molecules are small and relatively water soluble with minimal protein binding in the blood, with concentration in saliva that parallels serum, but approximately 15% to 40% higher.34 Thus, the analytical sensitivity in this matrix is approximately equal to serum, and most methods can process either serum or saliva. The primary advantage of salivary cotinine measurements is that is a relatively non-invasive matrix when collecting a blood sample is not feasible, or when requiring multiple measurements over a limited period. Collection procedures are relatively simple and well tolerated. Contamination, though unusual, is clearly a greater risk for saliva samples than for serum samples. Issues of age, gender, race, oral pH, type of diet, dehydration, or drug treatment and their effect on salivary cotinine concentrations should be addressed for variability.
Hair nicotine and metabolites
Hair nicotine has been used and validated as a biomarker of SHSe among children and adults.35–38 Nicotine is incorporated into hair if it is present in the circulation, and environmental contamination of nicotine is minimal after washing samples.35 37 The hair growth rate is 1 cm/month, 1 cm of hair proximal to the scalp represents the last month's exposure.39 Hair can characterise exposure and time; theoretically, 10-cm of hair represents 10 months of past exposure, a longer period than covered by other biomarkers. The average hair nicotine dose (cumulative exposure/duration of exposure) is therefore less affected by daily variability from fluctuating exposure, varying metabolism and elimination of nicotine.40
The sensitivity of hair nicotine methods varies (LODs range 0.02–0.2 ng nicotine/mg hair).19 41 42 LC-ultraviolet (UV),42 or GC-MS41 43 can be used for hair nicotine determination. Hair nicotine concentrations are highly reproducible over 1 year, proving the method's sensitivity for detecting individual changes in smoking habits and SHSe.43
Advantages of the hair matrix include collection ease, storage at room temperature without degradation for up to 5 years, and shipping without special handling.35 44 Scarcity of hair can preclude using this approach for infants or some adults. Chemical hair treatments, however, can reduce hair nicotine concentration by 9% to 30% and relevant information should be collected with hair samples for any necessary adjustments.45 46 Similar to most biomarkers, gender and race may play roles in determining hair nicotine concentrations. Hair colour could likely influence nicotine concentrations, since nicotine is bound to melanin and the type and amount of melanin in hair varies with hair colour. However, Zahlsen et al 47 found that nicotine uptake did not differ due to hair colour or thickness, or person's age or gender. Among children, younger children have higher hair nicotine concentrations than older children, with the same SHSe.45 48
Toenail nicotine and metabolites
Toenail nicotine concentration shows promise for SHSe assessment, as it reflects relatively long exposure periods: depending on the length of the clipping, the concentration can represent up to several months of past exposure (toenails grow at a rate of approximately 1 mm/month).49 In one study, toenail nicotine concentrations strongly predicted self-reported exposure, even after 20 years' storage at room temperature.50 Similar to hair samples, toenails can be collected easily and shipped without temperature restrictions.
Toenails are less directly exposed than hair to environmental nicotine, with concentrations only reflecting nicotine taken up from blood circulation by nails during growth. Slow toenail growth rates overcome day-to-day exposure variability and provide potentially more stable estimates of average exposure, which is critical to assessing long-term exposures.50 51 In one study, toenail nicotine concentrations were significantly correlated with reported tobacco smoking (r=0.63).52 They are also predictive of high and low SHSe among non-smokers50; in newborns, fingernails and toenails have been used to determine nicotine exposure in utero.53
Population data on toenail biomarker concentrations are not available, and determining normative population concentrations is critical to relating these concentrations to disease risk and tobacco exposure. Given the low nicotine concentration per mg of toenail, collecting clippings from all 10 toenails is recommended. Age is inversely related to toenail nicotine concentrations in women, even after controlling for cigarette consumption and SHSe frequency,54 while nail fungus infection and nail polish do not appear to influence concentrations or exposure. No data exist on toenail nicotine concentrations relating to gender or race. Laboratory methods to analyse nicotine in toenail samples include high-performance liquid chromatography-electrochemical detection (HPLC-ECD) (LOD of 0.1 ng/mg toenail),19 or GC-MS (LOD range 0.025–0.01 ng/mg toenail).55 56
NNK metabolites in urine
NNK is a tobacco-specific lung carcinogen shown to induce adenocarcinoma of the lung in rats, mice, or hamsters.9 Its metabolites, tNNAL can be measured in the urine of SHS-exposed non-smokers. NNK itself is not found in human urine because of its extensive metabolism to NNAL and other metabolites.9 57 As NNAL represents only 15% of NNK dose intake, it is prone to interindividual and intraindividual variability due to metabolic variability of the other 85% of metabolites. tNNAL has been quantified in smokers' blood,58 but its measurement in SHS-exposed non-smokers' blood has not been reported and may be too low for current detection methods. Highly sensitive, validated analytical methods available to quantify tNNAL in urine are GC-TEA, GC-MS/MS and LC-MS/MS. LC-MS/MS is currently the assay of choice for NNAL as concentrations can be determined in first morning urine, spot urine and 24 h urine samples.
The major advantages of the tNNAL biomarker are its specificity to tobacco smoke and its direct relationship to a lung carcinogen. NNK is found only in tobacco products—never in the general environment, unless SHS is present. Thus, NNAL detection in urine signifies exposure to, and uptake of, the lung carcinogen NNK. The tNNAL biomarker is well established and it is not detected in the urine of non-exposed individuals. Multiple studies have demonstrated uptake of NNK by SHS-exposed non-smokers, as well as transplacental exposure from smoking mothers by analysing amniotic fluid or first morning urine.59 60 NNAL has a longer half-life than cotinine, thus representing longer exposure. However, NNAL's t1/2 of up to 3 weeks is much less than nicotine's half-life in hair or toenail matrices.
The disadvantages of tNNK are the needs for expertise in analytical chemistry and costly equipment. NNAL is carcinogenic and mutagenic and must be handled with extreme caution in the laboratory. To date, the highest tNNAL concentration in the urine have been observed in infants and children with SHSe (80–90 fmol/ml urine), compared to concentrations of 20–50 fmol/ml urine in non-smoking adults.10 24 It is not clear whether these are differences in metabolism between children and adults, or in exposure or other factors. One study comparing tNNAL concentrations in teen versus adult smokers did not find significant differences, suggesting that metabolic differences are unimportant, but further study is needed.26 Studies of smokers do not indicate gender differences in tNNAL concentrations; this is also true for exposed non-smokers. There may be racial differences in tNNAL concentrations in smokers' urine, as some studies suggest higher concentrations in African Americans compared to Caucasians.61 62 Whether these observations extend to non-smokers is unknown.
Utility of NNK metabolites versus nicotine metabolites
A moderately strong correlation is evident between NNK metabolites and nicotine metabolites in the urine of SHS-exposed non-smokers, with similar results in smokers. In a study of 74 children, as tNNAL increased from 0.05 to 0.35 pmol/ml urine, total cotinine (the sum of cotinine and its glucuronide) increased from 15 to 50 ng/ml urine,25 leaving little doubt that cotinine at these concentrations in non-smokers' urine implies the presence of tNNAL. Since it is easier and less expensive to measure nicotine than tNNAL, one could argue that the latter is not necessary. tNNAL in urine, however, may have greater public health impact and better predictive utility for the adverse health effects of SHSe, compared to detection of nicotine metabolites. This specificity reflects the pulmonary carcinogen NNAL and its parent NNK, although nicotine may be related to carcinogenesis, atherosclerosis, platelet adhesion and coronary heart disease (CHD) vasoconstriction, and has addictive properties and high-dose toxicity. Detection of an actual carcinogen, tNNAL, in non-smokers' urine, signals a hazard, and using this biomarker to discourage smoking (eg, feedback for parents of exposed children) has been proposed. Its detection in the urine of SHS-exposed non-smokers repeatedly attracts media attention, leading to further support for tobacco-free legislation and tobacco control.
Factors affecting concentrations of biomarkers
Age
Differences in drug elimination between children and adults are well documented, particularly during neonatal and early infant periods, when hepatic, renal, cardiac and lung functions are immature.63 64 Nicotine and cotinine pharmacokinetics in neonates exposed in utero to nicotine had significantly longer nicotine t1/2 in the infants’ serum than that reported for adults (neonates t1/2 nicotine=11.2 h (95% CI 8.0 to 18.9 h); adults t1/2 nicotine=approximately 2 h) with no differences for cotinine,29 suggesting that differences in the nicotine t1/2 but not in the cotinine t1/2 between neonates and adults may relate to differences in nicotine metabolism.65 Several studies report cotinine elimination in children's urine, and all show younger children having slower elimination and/or higher concentrations than older children or adults66–68; also observed with hair (nicotine and cotinine).45 48 69
Disease state
Disease states may affect metabolite concentrations in children and adults. Children with asthma show higher cotinine concentrations in hair and urine compared to children without asthma.70 71 However, it is difficult to determine if the differences are due to metabolism or exposure as the studies only used parental report to measure environmental exposure.
Other sources of exposure to nicotine
Other sources of nicotine exposure in non-smokers include breastfed infants with smoking mothers, even if the infant does not have SHSe. Children breast fed by mothers who smoke outside the house can also have higher urine cotinine concentrations than bottle-fed children with direct SHSe.72 One study reported Alaska Native children, aged 4 years and older, chewing tobacco.73 Finally, through hand-to-mouth activity, children may ingest floor dust containing nicotine or touch fabrics that have been exposed to SHS, such as smokers' clothing.74
Interpreting biomarker concentrations
Results of quantitative biomarkers' concentrations of SHSe need careful interpretation, which could consider incorporating cut-off points to separate smokers from SHS-exposed non-smokers; using threshold concentrations for significant consequences of SHSe; and assessing the severity of SHSe in non-smokers.
Separating smokers and non-smokers
As cotinine is specific for nicotine exposure, any detectable concentration indicates exposure to tobacco, tobacco smoke, or medicinal nicotine. NNK is also specific for tobacco and is not present in medications or food; any NNAL concentration indicates exposure to tobacco or tobacco smoke.
Daily smokers typically have plasma or serum cotinine concentrations of 100 ng/ml or higher, or urine tNNAL concentrations of 1000 fmol/ml or higher,57 while light or non-daily smokers can have cotinine concentrations below 10 ng/ml. Heavy SHSe may result in plasma cotinine concentrations up to 25 ng/ml.75 Thus, overlap may occur between cotinine concentrations of non-smokers who experience heavy SHSe and light/occasional smokers. The optimal cut-off point should minimise false classification of SHS-exposed non-smokers versus smokers, and will depend on the extent of non-smokers' SHSe and the smoking behaviour of the population's smokers.
The most widely used serum cotinine cut-off point (14 ng/ml) to distinguish smokers from non-smokers is based on work from the early 1980s in England.76 More recently, a cut-off point of 12 ng/ml was determined using UK data (1996–2004) from a representative sample,77 which suggests that SHSe in England did not declined dramatically over 20 years. SHSe in the US is much lower today, however, compared to 1980s England. An optimal cut-off point of 3 ng/ml was determined using NHANES data (1999–2004).78 Due to differences in smoking behaviours and perhaps in cotinine metabolism, the optimal US cut-off point, for adults, varies by race/ethnicity (non-Hispanic whites: 5 ng/ml, non-Hispanic blacks: 6 ng/ml, Mexican–Americans: 1 ng/ml).78 The low cut-off in Mexican–Americans reflects lower SHSe and higher prevalence of light/occasional smoking.
Generalisability from national sampling to particular subpopulations requires careful consideration, particularly if the target population's exposure profile is unusual. Thus, researchers should consider the target population when selecting the optimal cut-off point to separate smokers from non-smokers (eg, casino workers with high SHSe (optimal cut-off point: >3 ng/ml). Hair cotinine cut-off point values in women, pregnant women and children (0.8, 0.2 and 0.2 ng/mg, respectively) have been determined using data from the USA, Canada and France.79 Lack of representative population data for other biomarkers in the different matrices makes prediction of optimal cut-off values difficult for these markers.
Assessing risk from SHSe
For researchers interpreting a particular nicotine, cotinine, or NNAL concentration as a biomarker of risk from exposure, an appropriate comparison group is required. Three possible approaches are available: (1) to consider that any concentration resulting from a specific biomarker of SHSe such as nicotine, cotinine, or NNAL, indicates an increased risk28; (2) to classify exposure with respect to tertile or quartile of a general population of exposed individuals, if available (eg, US NHANES (serum),80 England's Health Survey (saliva),77); and (3) to classify exposure severity based on known environmental exposure (eg, those heavily exposed in bars or casinos) or on association with disease (see Relationship of Biomarkers to Disease Risk).
Interpreting total NNAL concentrations in urine
Average tNNAL concentrations in the urine of SHS-exposed non-smokers range from 18 to 90 fmol/ml urine, while for smokers this figure is 1000 fmol/ml urine or higher.3 57 Exceptions occur, however, and there may be some overlap in smokers and non-smokers' NNAL concentrations. No cut point differentiating smokers from non-smokers has been determined.
Based on a 50 fmol/ml urine concentration in SHS-exposed non-smokers, tNNAL excretion is estimated at 75 pmol/day.81 Since tNNAL represents 15% of the NNK dose, NNK exposure in SHS-exposed non-smokers is estimated at 500 pmol/day, or a dose of about 1.1 mg (0.01 mg/kg) in 30 years of SHSe. The lowest total dose of NNK shown to induce lung tumours in rats is 1.8 mg/kg,9 or 200 times higher than the dose of a SHS-exposed non-smoker.
Another method is to compare tNNAL concentrations in SHS-exposed non-smokers to tNNAL concentrations in smokers. In one study, tNNAL concentrations in the urine of SHS-exposed women were 5.6% of that in the urine of their partners who smoked.82 Epidemiological studies estimate that the excess risk for lung cancer in SHS-exposed women is about 20% higher than that for unexposed women83, or 1% to 2% of the excess risk for lung cancer in smokers (1400% to 1900%) compared with non-smokers, a figure consistent with the 5.6% relative NNAL concentrations.82
Relationship of biomarkers to disease risk
Two criteria for valid biomarkers of risk are that they predict disease risk and that a change in biomarker concentration corresponds to a change in disease risk. Such research is problematic because most SHSe-related diseases take years to develop, and established biomarkers, such as cotinine, measure short-term exposure. If, however, measuring a biomarker at a particular time reflects chronic SHSe over a longer period, then a quantitative relationship between biomarker concentrations and disease may exist.
SHSe is a known cause of CHD.28 Several studies among adults have found a positive relationship with cotinine levels and CHD prevalence (170%; p<0.05),84 and CHD development (50%; p<0.05).85 Toenail nicotine concentrations were associated with CHD events among SHS-exposed nurses,54 although this association was not statistically significant, possible because of drastic SHSe reduction in US hospitals between 1982 and 1998.
SHSe causes respiratory disease among children.28 Higher saliva cotinine concentrations were associated with doubling the ‘tendency for colds to go to the chest’ and reduction of lung function markers in children.86 Among adults with asthma or chronic obstructive pulmonary disease (COPD), higher urine cotinine was associated with greater COPD severity and lower physical health status and disease-specific quality of life.87 COPD outcomes, however, were not associated with self-reported SHSe or personal badge nicotine concentrations. Urine NNAL concentrations is a better predictor of SHSe effects among COPD subjects.88 The risk of asthma-related hospital admissions in adults increased with higher hair concentrations of nicotine, but not cotinine.89 Significant decreases in lung function and increases in inflammatory markers were observed after acute SHSe (1 h), particularly in men.90 In addition, although the effects on lung function appear to disappear within 60 min; inflammatory cytokines remain elevated for at least 3 h after SHSe.
SHSe reduces birth weight28 and cotinine levels of SHS-exposed pregnant women have been statistically significantly associated with reductions in mean birth weight (range: 73 g to 200 g).28 91–93 Non-significant associations have found with hair nicotine levels.94 95
Conclusions
This article summarises the current scientific evidence on the use of biomarkers to measure SHSe, analytical methods, biological matrices and their interpretation. Cotinine is the biomarker of choice for measuring SHSe in urine, blood and saliva. Nicotine can be measured in hair and toenails. NNK is a tobacco-specific lung carcinogen. NNAL, a NNK metabolite, represents only 15% of the NNK dose intake but can be detected in the urine of SHS-exposed non-smokers. Use of each biomarker has its advantages and disadvantages, making selection dependent on the study's objectives, subjects, design and setting, funding, issues of privacy, invasiveness and subject's age. The length of SHSe may result in selecting hair or toenails over biofluids. The information provided here may assist investigators in selecting the optimal biomarker when designing their study.
Acknowledgments
The authors would like to thank Nicole Ammerman and Charlotte Gerczak for their technical and editing assistance, respectively. The authors would also like to thank Drs Benjamin Apelberg, Dana Best, Michael Cummings, Geoffrey Fong, Lara Gundel, Katherine Hammond, Melbourne Hovell, Andrew Hyland, Jonathan Klein, Neil Klepeis, Robert McMillen, James Repace, and Jonathan Winickoff for their participation in the expert meeting.
Footnotes
Contributors: EA-T organised and participated in the expert meeting, drafted and revised the paper. She is guarantor. WKA-D, DLA, NB, JTB, SK and SH participated in the expert meeting, drafted and revised the paper. JMS initiated and organised the expert meeting and revised the draft paper.
Funding: This work was supported by the Flight Attendant Medical Research Institute's grant for the Centers of Excellence at Johns Hopkins; the University of California, San Francisco Bland Lane; and the American Academy of Pediatrics Julius B Richmond. The funding organisation had no role in the preparation of the manuscripts.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. U.S. National Research Council Committee on Passive Smoking Assessing Exposures to Environmental Tobacco Smoke in the External Environment. Environmental Tobacco Smoke: Measuring Exposures and Assessing Health Effects. Washington, D.C.: U.S. National Research Council, 1986:69–100 [PubMed] [Google Scholar]
- 2. Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009;192:29–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control 2004;13(Suppl 1):i48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 1996;18:188–204 [DOI] [PubMed] [Google Scholar]
- 5. Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric Food Chem 1999;47:3113–20 [DOI] [PubMed] [Google Scholar]
- 6. Hukkanen J, Jacob P, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev 2005;57:79–115 [DOI] [PubMed] [Google Scholar]
- 7. Benowitz NL, Dains KM, Dempsey D, et al. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res 2009;11:954–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matt GE, Quintana PJ, Liles S, et al. Evaluation of urinary trans-3'-hydroxycotinine as a biomarker of children's environmental tobacco smoke exposure. Biomarkers 2006;11:507–23 [DOI] [PubMed] [Google Scholar]
- 9. Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 1998;11:559–603 [DOI] [PubMed] [Google Scholar]
- 10. Stepanov I, Hecht SS, Duca G, et al. Uptake of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by Moldovan children. Cancer Epidemiol Biomarkers Prev 2006;15:7–11 [DOI] [PubMed] [Google Scholar]
- 11. Stepanov I, Hecht SS, Lindgren B, et al. Relationship of human toenail nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol to levels of these biomarkers in plasma and urine. Cancer Epidemiol Biomarkers Prev 2007;16:1382–6 [DOI] [PubMed] [Google Scholar]
- 12. Singer BC, Hodgson AT, Guevarra KS, et al. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environ Sci Technol 2002;36:846–53 [DOI] [PubMed] [Google Scholar]
- 13. Knight GJ, Wylie P, Holman MS, et al. Improved 125I radioimmunoassay for cotinine by selective removal of bridge antibodies. Clin Chem 1985;31:118–21 [PubMed] [Google Scholar]
- 14. Benkirane S, Nicolas A, Galteau MM, et al. Highly sensitive immuno-assays for the determination of cotinine in serum and saliva. Comparison between RIA and an avidin-biotin ELISA. Eur J Clin Chem Clin Biochem 1991;29:405–10 [DOI] [PubMed] [Google Scholar]
- 15. Bjercke RJ, Cook G, Rychlik N, et al. Stereospecific monoclonal antibodies to nicotine and cotinine and their use in enzyme-linked immunosorbent assays. J Immunol Methods 1986;90:203–13 [DOI] [PubMed] [Google Scholar]
- 16. Feyerabend C, Russell MA. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol 1990;42:450–2 [DOI] [PubMed] [Google Scholar]
- 17. Church TR, Anderson KE, Le C, et al. Temporal stability of urinary and plasma biomarkers of tobacco smoke exposure among cigarette smokers. Biomarkers 2010;15:345–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacob P, III, Yu L, Wilson M, et al. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom 1991;20:247–52 [DOI] [PubMed] [Google Scholar]
- 19. Mahoney GN, Al Delaimy W. Measurement of nicotine in hair by reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 2001;753:179–87 [DOI] [PubMed] [Google Scholar]
- 20. Jacob P, III, Havel C, Lee DH, et al. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem 2008;80:8115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 1997;43:2281–91 [PubMed] [Google Scholar]
- 22. Hariharan M, VanNoord T. Liquid-chromatographic determination of nicotine and cotinine in urine from passive smokers: comparison with gas chromatography with a nitrogen-specific detector. Clin Chem 1991;37:1276–80 [PubMed] [Google Scholar]
- 23. Massadeh AM, Gharaibeh AA, Omari KW. A single-step extraction method for the determination of nicotine and cotinine in Jordanian smokers' blood and urine samples by RP-HPLC and GC-MS. J Chromatogr Sci 2009;47:170–7 [DOI] [PubMed] [Google Scholar]
- 24. Hecht SS, Carmella SG, Le KA, et al. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides in the urine of infants exposed to environmental tobacco smoke. Cancer Epidemiol Biomarkers Prev 2006;15:988–92 [DOI] [PubMed] [Google Scholar]
- 25. Hecht SS, Ye M, Carmella SG, et al. Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiol Biomarkers Prev 2001;10:1109–16 [PubMed] [Google Scholar]
- 26. Hertsgaard LA, Hanson K, Hecht SS, et al. Exposure to a tobacco-specific lung carcinogen in adolescent versus adult smokers. Cancer Epidemiol Biomarkers Prev 2008;17:3337–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spector LG, Hecht SS, Ognjanovic S, et al. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev 2007;16:1902–5 [DOI] [PubMed] [Google Scholar]
- 28. United States Department of Health and Human Services The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, D.C.: U.S. Govt. Printing Office, 2006 [Google Scholar]
- 29. Dempsey D, Jacob P, III, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther 2000;67:458–65 [DOI] [PubMed] [Google Scholar]
- 30. Benowitz NL, Lessov-Schlaggar CN, Swan GE, et al. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther 2006;79:480–8 [DOI] [PubMed] [Google Scholar]
- 31. Dempsey D, Jacob P, III, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther 2002;301:594–8 [DOI] [PubMed] [Google Scholar]
- 32. Pirkle JL, Flegal KM, Bernert JT, et al. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA 1996;275:1233–40 [PubMed] [Google Scholar]
- 33. Pirkle JL, Bernert JT, Caudill SP, et al. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988-2002. Environ Health Perspect 2006;114:853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benowitz NL, Kuyt F, Jacob P, III, et al. Cotinine disposition and effects. Clin Pharmacol Ther 1983;34:604–11 [DOI] [PubMed] [Google Scholar]
- 35. Al-Delaimy WK. Hair as a biomarker for exposure to tobacco smoke. Tob Control 2002;11:176–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Delaimy WK, Crane J, Woodward A. Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health 2002;56:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al-Delaimy WK, Crane J, Woodward A. Questionnaire and hair measurement of exposure to tobacco smoke. J Expo Anal Environ Epidemiol 2000;10:378–84 [DOI] [PubMed] [Google Scholar]
- 38. Woodruff SI, Conway TL, Edwards CC, et al. Acceptability and validity of hair collection from Latino children to assess exposure to environmental tobacco smoke. Nicotine Tob Res 2003;22:375–85 [DOI] [PubMed] [Google Scholar]
- 39. Uematsu T, Mizuno A, Nagashima S, et al. The axial distribution of nicotine content along hair shaft as an indicator of changes in smoking behaviour: evaluation in a smoking-cessation programme with or without the aid of nicotine chewing gum. Br J Clin Pharmacol 1995;39:665–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stockwell HG, Goldman AL, Lyman GH, et al. Environmental tobacco smoke and lung cancer risk in nonsmoking women. J Natl Cancer Inst 1992;84:1417–22 [DOI] [PubMed] [Google Scholar]
- 41. Kim SR, Wipfli H, Avila-Tang E, et al. Method validation for measurement of hair nicotine level in nonsmokers. Biomed Chromatogr 2009;23:273–9 [DOI] [PubMed] [Google Scholar]
- 42. Pichini S, Altieri I, Pellegrini M, et al. Hair analysis for nicotine and cotinine: evaluation of extraction procedures, hair treatments, and development of reference material. Forensic Sci Int 1997;84:243–52 [DOI] [PubMed] [Google Scholar]
- 43. Zahlsen K, Nilsen OG. Nicotine in hair of smokers and non-smokers: sampling procedure and gas chromatographic/mass spectrometric analysis. Pharmacol Toxicol 1994;75:143–9 [DOI] [PubMed] [Google Scholar]
- 44. Nafstad P, Botten G, Hagen JA, et al. Comparison of three methods for estimating environmental tobacco smoke exposure among children aged between 12 and 36 months. Int J Epidemiol 1995;24:88–94 [DOI] [PubMed] [Google Scholar]
- 45. Kim S, Wipfli H, Navas-Acien A, et al. Determinants of hair nicotine concentrations in nonsmoking women and children: a multicountry study of secondhand smoke exposure in homes. Cancer Epidemiol Biomark Prev 2009;18:3407–14 [DOI] [PubMed] [Google Scholar]
- 46. Jurado C, Kintz P, Menendez M, et al. Influence of the cosmetic treatment of hair on drug testing. Int J Leg Med 1997;110:159–63 [DOI] [PubMed] [Google Scholar]
- 47. Zahlsen K, Nilsen T, Nilsen OG. Interindividual differences in hair uptake of air nicotine and significance of cigarette counting for estimation of environmental tobacco smoke exposure. Pharmacol Toxicol 1996;79:183–90 [DOI] [PubMed] [Google Scholar]
- 48. Groner JA, Huang H, Nicholson L, et al. Secondhand smoke exposure and endothelial progenitor cell depletion in children. Circulation 2008;117:e249 [Google Scholar]
- 49. Tosi A, Piraccini B. Biology of nail. In: Freedberg I, Az E, Wolff K, eds. Fitzpatrick's Dermatology in General Medicine. New York: Mc-Graw-Hill, 1999:239–44 [Google Scholar]
- 50. Al-Delaimy WK, Mahoney GN, Speizer FE, et al. Toenail nicotine levels as a biomarker of tobacco smoke exposure. Cancer Epidemiol Biomark Prev 2002;11:1400–4 [PubMed] [Google Scholar]
- 51. Hulka BS, Margolin BH. Methodological issues in epidemiologic studies using biologic markers. Am J Epidemiol 1992;135:200–9 [DOI] [PubMed] [Google Scholar]
- 52. Al-Delaimy WK, Willett WC. Measurement of tobacco smoke exposure: comparison of toenail nicotine biomarkers and self-reports. Cancer Epidemiol Biomarkers Prev 2008;17:1255–61 [DOI] [PubMed] [Google Scholar]
- 53. Mari F, Politi L, Bertol E. Nails of newborns in monitoring drug exposure during pregnancy. Forensic Sci Int 2008;179:176–80 [DOI] [PubMed] [Google Scholar]
- 54. Al-Delaimy WK, Stampfer MJ, Manson JE, et al. Toenail nicotine levels as predictors of coronary heart disease among women. Am J Epidemiol 2008;167:1342–8 [DOI] [PubMed] [Google Scholar]
- 55. Hahn EJ, Rayens MK, Butler KM, et al. Smoke-free laws and adult smoking prevalence. Prev Med 2008;47:206–9 [DOI] [PubMed] [Google Scholar]
- 56. Stepanov I, Feuer R, Jensen J, et al. Mass spectrometric quantitation of nicotine, cotinine, and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human toenails. Cancer Epidemiol Biomarkers Prev 2006;15:2378–83 [DOI] [PubMed] [Google Scholar]
- 57. Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis 2002;23:907–22 [DOI] [PubMed] [Google Scholar]
- 58. Carmella SG, Yoder A, Hecht SS. Combined analysis of r-1, t-2,3, c-4-tetrahydroxy-1,2,3,4-tetrahydrophenanthrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in smokers' plasma. Cancer Epidemiol Biomarkers Prev 2006;15:1490–4 [DOI] [PubMed] [Google Scholar]
- 59. Lackmann GM, Salzberger U, Tollner U, et al. Metabolites of a tobacco-specific carcinogen in urine from newborns. J Natl Cancer Inst 1999;91:459–65 [DOI] [PubMed] [Google Scholar]
- 60. Milunsky A, Carmella SG, Ye M, et al. A tobacco-specific carcinogen in the fetus. Prenat Diagn 2000;20:307–10 [DOI] [PubMed] [Google Scholar]
- 61. Muscat JE, Djordjevic MV, Colosimo S, et al. Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Cancer 2005;103:1420–6 [DOI] [PubMed] [Google Scholar]
- 62. Richie JP, Carmella SG, Muscat JE, et al. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev 1997;6:783–90 [PubMed] [Google Scholar]
- 63. Leeder JS, Kearns GL. Pharmacogenetics in pediatrics. Implications for practice. Pediatr Clin North Am 1997;44:55–77 [DOI] [PubMed] [Google Scholar]
- 64. Ginsberg G, Hattis D, Sonawane B, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 2002;66:185–200 [DOI] [PubMed] [Google Scholar]
- 65. Kuehl GE, Murphy SE. N-glucuronidation of nicotine and cotinine by human liver microsomes and heterologously expressed UDP-glucuronosyltransferases. Drug Metab Dispos 2003;31:1361–8 [DOI] [PubMed] [Google Scholar]
- 66. Etzel RA, Greenberg RA, Haley NJ, et al. Urine cotinine excretion in neonates exposed to tobacco smoke products in utero. J Pediatr 1985;107:146–8 [DOI] [PubMed] [Google Scholar]
- 67. Gomella T, Cunningham M. Neonatology. 5th edn New York: McGraw-Hill Medical, 2004 [Google Scholar]
- 68. Leong JW, Dore ND, Shelley K, et al. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther 1998;11:287–90 [DOI] [PubMed] [Google Scholar]
- 69. Groner JA, Hoshaw-Woodard S, Koren G, et al. Screening for children's exposure to environmental tobacco smoke in a pediatric primary care setting. Arch Pediatr Adolesc Med 2005;159:450–5 [DOI] [PubMed] [Google Scholar]
- 70. Willers S, Svenonius E, Skarping G. Passive smoking and childhood asthma. Urinary cotinine levels in children with asthma and in referents. Allergy 1991;46:330–4 [DOI] [PubMed] [Google Scholar]
- 71. Knight JM, Eliopoulos C, Klein J, et al. Pharmacokinetic predisposition to nicotine from environmental tobacco smoke: a risk factor for pediatric asthma. J Asthma 1998;35:113–17 [DOI] [PubMed] [Google Scholar]
- 72. Chilmonczyk BA, Knight GJ, Palomaki GE, et al. Environmental tobacco smoke exposure during infancy. Am J Public Health 1990;80:1205–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Etzel R, Jones D, Schlife C, et al. Passive smoking and tobacco chewing among Alaska children: measuring saliva cotinine. J Smoking Relat Dis 1992;3:161–5 [Google Scholar]
- 74. Matt GE, Quintana PJE, Destaillats H, et al. Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 2011;119:1218–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med 1990;19:190–7 [DOI] [PubMed] [Google Scholar]
- 76. Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, et al. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 1987;77:1435–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jarvis MJ, Fidler J, Mindell J, et al. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction 2008;103:1553–61 [DOI] [PubMed] [Google Scholar]
- 78. Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–48 [DOI] [PubMed] [Google Scholar]
- 79. Florescu A, Ferrence R, Einarson TR, et al. Reference values for hair cotinine as a biomarker of active and passive smoking in women of reproductive age, pregnant women, children, and neonates: systematic review and meta-analysis. Ther Drug Monit 2007;29:437–46 [DOI] [PubMed] [Google Scholar]
- 80. Centers for Disease Control and Prevention (CDC) Disparities in secondhand smoke exposure–United States, 1988-1994 and 1999-2004. MMWR Morb Mortal Wkly Rep 2008;57:744–7 [PubMed] [Google Scholar]
- 81. Hecht SS. A biomarker of exposure to environmental tobacco smoke (ETS) and Ernst Wynder's opinion about ETS and lung cancer. Prev Med 2006;43:256–60 [DOI] [PubMed] [Google Scholar]
- 82. Anderson KE, Carmella SG, Ye M, et al. Metabolites of a tobacco-specific lung carcinogen in nonsmoking women exposed to environmental tobacco smoke. J Natl Cancer Inst 2001;93:378–81 [DOI] [PubMed] [Google Scholar]
- 83. International Agency for Research on Cancer Tobacco Smoke and Involuntary Smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, FR: IARC, 2004:1191–413 [PMC free article] [PubMed] [Google Scholar]
- 84. Tunstall-Pedoe H, Brown CA, Woodward M, et al. Passive smoking by self report and serum cotinine and the prevalence of respiratory and coronary heart disease in the Scottish heart health study. J Epidemiol Community Health 1995;49:139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Whincup PH, Gilg JA, Emberson JR, et al. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ 2004;329:200–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Strachan DP. Impedance tympanometry and the home environment in seven-year-old children. J Laryngol Otol 1990;104:4–8 [DOI] [PubMed] [Google Scholar]
- 87. Eisner MD, Balmes J, Yelin EH, et al. Directly measured secondhand smoke exposure and COPD health outcomes. BMC Pulm Med 2006;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eisner MD, Jacob P, III, Benowitz NL, et al. Longer term exposure to secondhand smoke and health outcomes in COPD: impact of urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Nicotine Tob Res 2009;11:945–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eisner MD, Klein J, Hammond SK, et al. Directly measured second hand smoke exposure and asthma health outcomes. Thorax 2005;60:814–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Flouris AD, Metsios GS, Carrillo AE, et al. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am J Respir Crit Care Med 2009;179:1029–33 [DOI] [PubMed] [Google Scholar]
- 91. Schwartz-Bickenbach D, Schulte-Hobein B, Abt S, et al. Smoking and passive smoking during pregnancy and early infancy: effects on birth weight, lactation period, and cotinine concentrations in mother's milk and infant's urine. Toxicol Lett 1987;35:73–81 [DOI] [PubMed] [Google Scholar]
- 92. Martinez FD, Wright AL, Taussig LM. The effect of paternal smoking on the birthweight of newborns whose mothers did not smoke. Group Health Medical Associates. Am J Public Health 1994;84:1489–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jedrychowski W, Flak E. [Impact of active and passive smoking during pregnancy on birth weight of the newborn]. Pol Merkuriusz Lek 1996;1:379–82 [PubMed] [Google Scholar]
- 94. Nafstad P, Fugelseth D, Qvigstad E, et al. Nicotine concentration in the hair of nonsmoking mothers and size of offspring. Am J Public Health 1998;88:120–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jaakkola JJ, Jaakkola N, Zahlsen K. Fetal growth and length of gestation in relation to prenatal exposure to environmental tobacco smoke assessed by hair nicotine concentration. Environ Health Perspect 2001;109:557–61 [DOI] [PMC free article] [PubMed] [Google Scholar]