Highlights
-
•
Human adipose tissue (AT) expresses the bile acid receptor TGR5.
-
•
Human AT TGR5 expression is linked to obesity.
-
•
Resting metabolic rate and AT TGR5 expression is positively correlated.
-
•
TGR5 expression is not higher in brown compared to white human AT.
Keywords: TGR5, Human adipose tissue, Gene expression, Obesity, Resting metabolic rate
Abstract
Bile acids have emerged as a new class of signaling molecules that play a role in metabolism. Studies in mice have shown that the bile acid receptor TGR5 mediates several of these effects but the metabolic function of TGR5 in humans is less well established. Here we show that human adipose tissue TGR5 expression is positively correlated to obesity and reduced during diet-induced weight loss. Adipose tissue TGR5 expression was also positively correlated to resting metabolic rate. Our study indicates that human adipose tissue contributes to the TGR5 mediated metabolic effects of bile acids and plays a role in energy expenditure.
1. Introduction
Bile acids are synthesized in the liver and play a central role in dietary lipid emulsification in the intestine. The majority of the secreted bile acids is taken up by the distal parts of the small intestine and return to the liver and the gall bladder via the enterohepatic circulation. Beyond their well established function in dietary lipid emulsification, bile acids are signaling molecules with metabolic effects mediated by specific receptors. The farnesoid X receptor (FXR) is the most studied receptor for bile acids. FXR is a nuclear receptor that regulates bile acid synthesis as well as lipid and glucose metabolism [1]. The G protein-coupled receptor TGR5 (also denoted G protein-coupled bile acid receptor 1 or GBPAR1) has also been found to be a functional bile acid receptor [2]. In 2006, Watanabe et al. showed that bile acid administration augmented energy expenditure in mice [3], and that these effects were mediated via the TGR5 and its effects on the thyroid hormone activating enzyme iodothyronine deiodinase type II (DIO2) in brown adipose tissue (BAT). In addition, they showed that bile acid treatment of human skeletal myocytes increased both DIO2 activity and oxygen consumption, indicating that similar mechanisms are relevant also in humans. More recently, TGR5 activation has been shown to induce glucagon-like peptide-1 (GLP-1) release in mice, indicating a direct role for TGR5 in glucose homeostasis [4].
Despite these advances in understanding of TGR5, much less in known about TGR5 function in humans and its expression in other metabolically relevant organs such as adipose tissue. The aim of this study was therefore to investigate the potential role of adipose tissue TGR5 in human metabolism.
2. Materials and methods
The regional ethics committee in Gothenburg approved these studies.
2.1. Sib Pair study
The swedish obese subjects (SOS) Sib Pair study consists of 154 nuclear families with BMI discordant sibling pairs (BMI difference ⩾10 kg/m2), resulting in a study population consisting of 732 subjects [5]. The subjects were extensively phenotyped [5], including anthropometric measurements and determination of resting metabolic rate (RMR) in a ventilated hood [6]. Subcutaneous adipose tissue needle biopsies were obtained and used for gene expression analysis. For the current study, complete data from 353 siblings and 86 parents were available for the analysis.
2.2. Very low calorie diet (VLCD) study
The very low calorie diet (VLCD) study was performed to investigate gene expression changes in adipose tissue of obese subjects during weight loss induced by caloric restriction. Twenty-eight obese subjects (20 women and 8 men, age 39.7 ± 12.7 years, BMI 36.3 ± 3.7 kg/m2) were treated with VLCD (450 kcal/day) for 12 weeks [7,8]. Subcutaneous adipose tissue needle biopsies were obtained at the start of the VLCD treatment (day 0) and three times during the VLCD treatment (weeks 2, 6, and 12). After 12 weeks of VLCD treatment, the mean weight loss was 19%.
2.3. Perithyroid and perirenal adipose tissue studies
Perthyroid adipose tissue surgical samples were obtained from 24 patients undergoing surgery in the thyroid region for malignancies or endocrine disorders. Clinical characteristics of the patients have been described previously [9]. Perithyroid adipose tissue biopsies containing BAT was identified by expression analysis of uncoupling protein 1 (UCP1) and histological analysis. Samples with high UCP1 expression was classified as BAT positive (BAT+, n = 9). Paired subcutaneous white adipose tissue biopsies from the surgical incision area were also obtained from the same nine patients (2 men and 7 women, age 47 ± 21 years, BMI 23 ± 2 kg/m2).
Biopsies of perirenal adipose tissue were obtained from 55 healthy kidney donors. The perirenal adipose tissue samples were screened for UCP1 expression and samples with high UCP1 expression (samples from 4 men and 6 women) were classified as BAT positive (BAT+) samples. These samples constituted the BAT+ group. A control group (n = 10) with low perirenal adipose tissue UCP1 expression (classified as BAT− samples) was matched to the BAT+ group based on sex, age and BMI. The subjects in the BAT+ group had an average age of 42 ± 14 years and a BMI of 26 ± 2. The subjects in the BAT− group had an average age of 44 ± 9 years and a BMI of 26 ± 3.
2.4. Gene expression analysis
Total RNA was isolated from adipose tissue using the RNeasy lipid tissue midi kit (Qiagen, Chatsworth, CA) or the phenol–chloroform extraction method of Chomczynski and Sacchi [10].
Gene expression in adipose tissue from the Sib Pair study and in the perithyroid adipose tissue was analyzed study using Human Genome U133 plus 2.0 arrays (Affymetrix, Santa Clara, CA). Gene expression in the perirenal adipose tissue samples were analyzed by (Affymetrix Gene 1.0 ST arrays at the Uppsala Array Platform, Uppsala, Sweden). All arrays were analyzed according to the manufacturer’s instructions. Expression data were analyzed using the RMA algorithm (Affymetrix). TGR5 expression was assessed using probe sets 1552501_a_at and 8048249 for Human Genome U133 plus 2.0 and Expression assay Gene 1.0 ST arrays, respectively.
Adipose tissue total RNA from the VLCD study was reversed transcribed using the High Capacity cDNA RT kit (Life Technologies, Paisley, UK) according to the manufacturer’s protocol. Reagents for real-time PCR analysis of TGR5 (Hs00544894_m1) and low-density lipoprotein (LDL) receptor-related protein 10 (LPR10) (Hs00204094_m1) were purchased from Life Technologies and used according to the manufacturer’s instructions. cDNA was used for real-time PCR in the Applied Biosystems PRISM 7900HT Sequence Detection System (Life Technologies) using default cycle parameters. A standard curve was plotted for each primer-probe set with a serial dilution of cDNA synthesized from pooled RNA. All samples and standards were analyzed in triplicate and LRP10 gene expression was used as a reference gene [11].
2.5. Gene ontology (GO) enrichment analysis
GO enrichment analysis was performed using the DAVID web–accessible program [12,13] (http://david.abcc.ncifcrf.gov). Transcripts significantly correlated with TGR5 expression were identified in the Sib Pair study using Spearman correlation. Accounting for multiple testing, a threshold of p < 9.1 × 10−7 was used as a cut off for declaring statistical significance (Bonferroni correction). This yielded a total of 1755 negative and 1767 positivity correlated transcripts which were included separately in the GO enrichment analysis. The analysis was limited to the GOTERM_BP_FAT, GEOTERM_CC_FAT and GEOTERM_MF_FAT options and Human Genome U133 Plus 2.0 was used as a background.
2.6. Statistical analysis
Statistical analyses were performed using the SAS software package (v. 9.1.3, SAS Institute Inc., Cary, NC) or and PASW Statistics (Chicago, IL, USA). Quantitative data were transformed towards normal distribution using Box–Cox power transformations. Outliers beyond three standard deviations from the trait mean were excluded. Correlations between clinical traits and TGR5 expression were analyzed using the MIXED procedure in SAS. In the linear mixed models, we used a “sandwich estimator” of the covariance matrix to adjust for non-independence among family members. This asymptotically yields the same parameter as ordinary least squares or regression methods while standard errors and, consequently, hypothesis tests are adjusted for the family relatedness. Comparisons in gene expression between BAT and WAT were performed using Student’s t-test (paired or unpaired as appropriate). Gene expression during weight loss was analyzed using a one-way repeated measures analysis of variance (ANOVA) with Greenhouse–Geisser correction. Comparisons between time points were assessed by Bonferroni corrected post-hoc tests.
3. Results
3.1. TGR5 adipose tissue expression
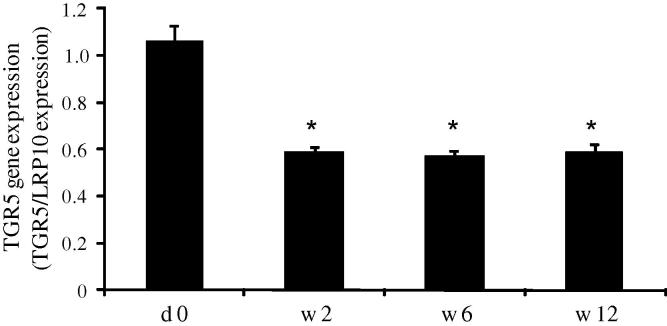
Initially, we investigated the link between TGR5 adipose tissue expression and obesity. Among the Sib Pair families, TGR5 expression was positively correlated with BMI (r = 0.40, p < 0.0001), independent of age, sex, family membership and generation. In line with this finding, obese subjects treated with VLCD displayed a drastic reduction of adipose tissue TGR5 expression determined by a repeated measures ANOVA (F(1.4, 38) = 38, p < 0.0005; Fig. 1). Compared to day 0, adipose tissue TGR5 expression was approximately half as low during the entire dieting period and post-hoc tests using the Bonferroni correction showed that TGR5 levels were reduced compared with day 0 at all subsequent time points measured (weeks 2, 6 and 12, p < 0.0005 for each test; Fig. 1).
Fig. 1.
Adipose tissue TGR5 expression during weight loss. Twenty-eight obese patients (20 women and 8 men) were treated with a very low caloric diet for 12 weeks. Subcutaneous adipose tissue biopsies were obtained by needle aspiration before the diet (day 0, d0) and after 2, 6 and 12 weeks (w) of diet. TGR5 gene expression was analyzed by real-time PCR and normalized to the reference gene LRP10. Data is presented as mean ± SEM. ∗∗p < 0.0005 compared with d0.
3.2. Gene ontology enrichment analysis
To gain insights into functions in adipose tissue that may be related to TGR5 expression, Gene ontology enrichment analysis was performed using transcripts positively or negatively correlated to TGR5 expression in subcutaneous adipose tissue in the Sib Pair offspring study. Transcripts negative correlated to TGR5 expression displayed highly significant (adjusted p-values down to 3 × 10−20) enrichment of several GO terms relating to mitochondria and ribosome (Table 1). Enrichment analysis of transcripts positively correlated to TGR5 expression resulted in identification of GO terms primarily related to endoplasmatic reticulum/Golgi and cytoskeleton function but with less striking p-values (Table 1).
Table 1.
Gene ontology enrichment analysis of transcripts correlated to TGR5 expression in adipose tissue in the Sib Pair study.
| GO term | Category | Counta | Adj p-valueb |
|---|---|---|---|
| Transcripts negatively correlated to TGR5 expression | |||
| Mitochondrion | CC | 160 | 3.0E−20 |
| Mitochondrial part | CC | 100 | 8.2E−16 |
| Intracellular organelle lumen | CC | 203 | 4.4E−13 |
| Organelle lumen | CC | 205 | 7.5E−13 |
| Membrane-enclosed lumen | CC | 208 | 9.0E−13 |
| Translation | BP | 67 | 1.6E−11 |
| Ribosome | CC | 46 | 7.1E−11 |
| Mitochondrial envelope | CC | 68 | 6.1E−10 |
| Organelle inner membrane | CC | 58 | 6.3E−10 |
| Mitochondrial membrane | CC | 64 | 1.9E−09 |
| Mitochondrial lumen | CC | 46 | 2.2E−09 |
| Mitochondrial matrix | CC | 46 | 2.2E−09 |
| Ribosomal subunit | CC | 32 | 3.5E−09 |
| Ribonucleoprotein complex | CC | 75 | 4.8E−09 |
| Mitochondrial inner membrane | CC | 53 | 6.1E−09 |
| Transcripts positively correlated to TGR5 expression | |||
| Endoplasmic reticulum | CC | 135 | 5.7E−08 |
| Actin binding | MF | 54 | 0.000023 |
| Cytoskeletal protein binding | MF | 74 | 0.000031 |
| GOLGI apparatus | CC | 115 | 0.000033 |
| Lysosome | CC | 41 | 0.00006 |
| Lytic vacuole | CC | 41 | 0.00006 |
| Endoplasmic reticulum part | CC | 54 | 0.00067 |
| Endoplasmic reticulum lumen | CC | 21 | 0.00078 |
| Vacuole | CC | 42 | 0.0014 |
| Actin cytoskeleton organization | BP | 39 | 0.0019 |
| Cortical cytoskeleton | CC | 15 | 0.0019 |
| Actin filament-based process | BP | 41 | 0.0023 |
| Cell cortex | CC | 28 | 0.0023 |
| Proteinaceous extracellular matrix | CC | 48 | 0.0023 |
| Extracellular matrix part | CC | 24 | 0.0027 |
CC = cellular component; BP = biological process; MF = molecular function.
Number of correlated transcripts included in the specific GO term.
The table displays the top 15 enriched GO terms based on the adjusted p-value (Benjamini).
3.3. TGR5 adipose tissue expression and energy expenditure
Since mitochondria play an important role in energy expenditure and nutrient combustion, we next investigated the relation between adipose tissue TGR5 expression and resting metabolic rate (RMR) in the Sib Pair study. Unexpectedly, given the negative correlation to mitochondrial genes, TGR5 expression was positively correlated with RMR (r = 0.25, p < 0.0001). In a model including age, polynomials of age, sex, lean body mass, generation and family membership, the statistical significance of this correlation persisted.
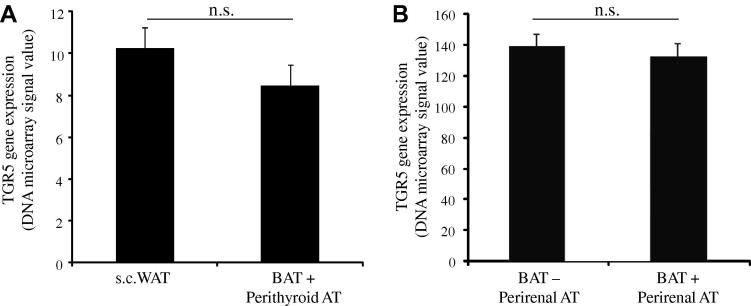
Brown adipocytes have, in comparison to white adipocytes, substantially more mitochondria. In mice TGR5 has been shown to be expressed in brown adipose tissue (BAT) and treatment of brown adipocytes with bile acids has been shown to increase oxygen expenditure via TGR5-mediarted activation of DIO2 in BAT [3]. The expression of TGR5 was therefore investigated in two sets of human adipose tissue samples (the perithyriod and the perirenal adipose tissue studies) containing islets of BAT (BAT+) and compared to expression in white adipose tissue. However, no significant difference in TGR5 expression was observed between the BAT containing samples and white adipose tissue (Fig. 2A and B).
Fig. 2.
TGR5 gene expression in human brown and white adipose tissue. (A) TGR5 expression in BAT containing (BAT+) perithyroid adipose tissue and paired subcutaneous white adipose tissue (s.c. WAT, n = 9). (B) TGR5 expression in perirenal adipose tissue containing BAT (BAT+, n = 10) or only containing white adipose tissue (BAT−, n = 10). Data is presented as mean microarray signal ± SEM. n.s. = not significant. Note: the different signal values are due to different microarray types.
4. Discussion
In this study, we have characterized the gene expression of TGR5 in human adipose tissue. Our main findings are that TGR5 adipose tissue expression is reduced during weight loss, and that it is positively correlated with obesity as well as with resting metabolic rate.
Our results clearly show that TGR5 expression in adipose tissue is regulated in a manner that supports a functional metabolic role for TGR5 in adipose tissue. However, our results are not easily reconciled with the results from the studies of mice treated with bile acids [3]. Whereas mice treated with bile acids lose weight, high human adipose tissue TGR5 expression is associated with obesity. In addition, the mice studies also show that the mitochondria rich BAT is a major target organ for the effects of bile acids whereas we show a negative correlation of TGR5 to the expression of mitochondrial genes in white adipose tissue. In the light of this, it is not unthinkable that the up regulation of TGR5 in human adipose tissue can be viewed as compensatory mechanism to prevent adipose tissue growth. However, one has to consider that regulation studies in one single organ may not capture the more complex regulation of the entire body and that receptor gene or protein expression cannot directly be translated to the amount of receptor signaling.
Expression analysis in human brown fat depots is challenging because, in contrast to mouse, human BAT represents a much smaller fraction of adipose tissue and is usually not located in distinct depots but rather as islets within the white adipose tissue [9,14]. Only the expression of a very limited number of genes has been investigated in human BAT [9,15,16]. Our analysis of TGR5 expression in human BAT-containing adipose tissue samples provide no evidence that TGR5 is expressed at higher levels in human BAT compared to WAT. This together with the limited amount of BAT in humans speaks against that the observed association between TGR5 adipose tissue expression and RMR is related to human BAT. A more likely scenario is that TGR5 expression in adipose tissue is co regulated with the TGR5 expression in other metabolically more active organs such as skeletal muscle or that it is related to adipose tissue thermogenesis not involving BAT. The correlation between adipose tissue TGR5 expression and RMR is well in line with the down-regulation of TGR5 during the diet intervention, a situation where RMR is reduced [17]. Interestingly, the drastic reduction of TGR5 expression seen already after 2 weeks preceded the maximal weight loss which occurred at week 12 [8]. This indicates that TGR5 expression in adipose tissue is regulated by energy intake or metabolic rate rather than the level of obesity. One study has shown that human plasma levels of bile acids are not associated with energy metabolism [18], indicating that if these systems play an important role in human energy expenditure future research should probably be directed at the receptor level.
Obesity represents an independent cardiovascular risk factor [19]. In addition to its metabolic role, TGR5 has recently been directly implicated in the development of atherosclerosis. Activation of TGR5 in mice was associated with attenuation of atherosclerosis by means of decreased pro-inflammatory cytokine production and reduced oxidized LDL uptake in macrophages [20]. These findings are of particular interest to our study since adipose tissue is a major source of circulating pro-inflammatory cytokines. However, the relative contribution of the adipose tissue TGR5 expression by adipocytes and macrophages is currently unknown.
In conclusion, we have shown that adipose tissue expression of human TGR5 is reduced during weight loss, and positively correlates to obesity as well as to resting metabolic rate, indicating a functional metabolic role for TGR5 in adipose tissue. However, more studies are needed to specifically define this role.
Acknowledgments
This study was supported by grants from the Wellcome Trust (GR079534), the Swedish Foundation for Strategic Research, Sahlgrenska Center for Cardiovascular and Metabolic Research, The Swedish Childhood Cancer Foundation and the Swedish federal government under the LUA/ALF agreement. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data or in the decision to submit the article for publication. The authors would like to acknowledge Margareta Jernås for expert technical assistance in the microarray analysis and Bengt Nilsson and Maria Svensson for the collection of samples in the perithyroid and perirenal adipose tissue studies, respectively. We would also like to acknowledge the help from Uppsala Array Platform.
References
- 1.Claudel T., Staels B., Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler. Thromb. Vasc. Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Itadani H., Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem. Biophys. Res. Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H., Messaddeq N., Harney J.W., Ezaki O., Kodama T., Schoonjans K., Bianco A.C., Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 4.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., Pellicciari R., Auwerx J., Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsson L.M., Jacobson P., Walley A., Froguel P., Sjöström L., Svensson P.A., Sjöholm K. ALK7 expression is specific for adipose tissue, reduced in obesity and correlates to factors implicated in metabolic disease. Biochem. Biophys. Res. Commun. 2009;382:309–314. doi: 10.1016/j.bbrc.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nookaew I., Svensson P.A., Jacobson P., Jernås M., Taube M., Larsson I., Andersson-Assarsson J.C., Sjöström L., Froguel P., Walley A., Nielsen J., Carlsson L.M. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J. Clin. Endocrinol. Metab. 2012;98:E370–E378. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saiki A., Olsson M., Jernås M., Gummesson A., McTernan P.G., Andersson J., Jacobson P., Sjöholm K., Olsson B., Yamamura S., Walley A., Froguel P., Carlsson B., Sjöström L., Svensson P.A., Carlsson L.M. Tenomodulin is highly expressed in adipose tissue, increased in obesity, and down-regulated during diet-induced weight loss. J. Clin. Endocrinol. Metab. 2009;94:3987–3994. doi: 10.1210/jc.2009-0292. [DOI] [PubMed] [Google Scholar]
- 8.Magnusson B., Gummesson A., Glad C.A., Goedecke J.H., Jernås M., Lystig T.C., Carlsson B., Fagerberg B., Carlsson L.M., Svensson P.A. Cell death-inducing DFF45-like effector C is reduced by caloric restriction and regulates adipocyte lipid metabolism. Metabolism. 2008;57:1307–1313. doi: 10.1016/j.metabol.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Svensson P.A., Jernås M., Sjöholm K., Hoffmann J.M., Nilsson B.E., Hansson M., Carlsson L.M. Gene expression in human brown adipose tissue. Int. J. Mol. Med. 2011;27:227–232. doi: 10.3892/ijmm.2010.566. [DOI] [PubMed] [Google Scholar]
- 10.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 11.Gabrielsson B.G., Olofsson L.E., Sjogren A., Jernås M., Elander A., Lönn M., Rudemo M., Carlsson L.M. Evaluation of reference genes for studies of gene expression in human adipose tissue. Obes. Res. 2005;13:649–652. doi: 10.1038/oby.2005.72. [DOI] [PubMed] [Google Scholar]
- 12.Dennis G., Jr., Sherman B.T., Hosack D.A., Yang J., Gao W., Lane H.C., Lempicki R.A. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 13.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 14.Zingaretti M.C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 15.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.J., Enerback S., Nuutila P. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H., Khandekar M., Virtanen K.A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W.D., Hoeks J., Enerback S., Schrauwen P., Spiegelman B.M. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Gaal L.F., Vansant G.A., De Leeuw I.H. Factors determining energy expenditure during very-low-calorie diets. Am. J. Clin. Nutr. 1992;56:224S–229S. doi: 10.1093/ajcn/56.1.224S. [DOI] [PubMed] [Google Scholar]
- 18.Brufau G., Bahr M.J., Staels B., Claudel T., Ockenga J., Boker K.H., Murphy E.J., Prado K., Stellaard F., Manns M.P., Kuipers F., Tietge U.J. Plasma bile acids are not associated with energy metabolism in humans. Nutr. Metab. (Lond.) 2010;7:73. doi: 10.1186/1743-7075-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubert H.B., Feinleib M., McNamara P.M., Castelli W.P. Obesity as an independent risk factor for cardiovascular disease: a 26 year follow-up of participants in the Framingham heart study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 20.Pols T.W., Nomura M., Harach T., Lo Sasso G., Oosterveer M.H., Thomas C., Rizzo G., Gioiello A., Adorini L., Pellicciari R., Auwerx J., Schoonjans K. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]