Abstract
Chronic arsenic ingestion predisposes to vascular disease, but underlying mechanisms are poorly understood. In the present study we have analyzed the effects of short-term arsenite exposure on vascular function and endothelium-dependent relaxation.
Endothelium-dependent relaxations, nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF)-type, were studied in rabbit iliac artery and aortic rings using the G protein-coupled receptor agonist acetylcholine (ACh) and by cyclopiazonic acid (CPA), which promotes store-operated Ca2+ entry by inhibiting the endothelial SERCA pump. Production of reactive oxygen species (ROS) in the endothelium of rabbit aortic valve leaflets and endothelium-denuded RIA and aortic rings was assessed by imaging of dihydroethidium.
In the iliac artery, exposure to 100 μM arsenite for 30 min potentiated EDHF-type relaxations evoked by both CPA and ACh. Potentiation was prevented by catalase, the catalase/superoxide dismutase mimetic manganese porphyrin and the NADPH oxidase inhibitor apocynin. By contrast in aortic rings, that exhibited negligible EDHF-type responses, endothelium-dependent NO-mediated relaxations evoked by CPA and ACh were unaffected by arsenite. Arsenite induced apocynin-sensitive increases in ROS production in the aortic valve endothelium, but not in the media and adventitia of the iliac artery and aorta.
Our results suggest that arsenite can potentiate EDHF-type relaxations via a mechanism that is dependent on hydrogen peroxide, thus demonstrating that dismutation of the superoxide anion generated by NADPH oxidase can potentially offset loss of NO bioavailability under conditions of reduced eNOS activity. By contrast, selective increases in endothelial ROS production following exposure to arsenite failed to modify relaxations mediated by endogenous NO.
Abbreviations: ACh, acetylcholine; CPA, cyclopiazonic acid; DHE, dihydroethidium; ER, endoplasmic reticulum; KCa, calcium-activated K+ channels; NO, nitric oxide; H2O2, hydrogen peroxide; L-NAME, NG-nitro-L-arginine methyl ester; O2•−, superoxide anion; PE, phenylephrine; RIA, rabbit iliac artery; ROS, reactive oxygen species; SOD, superoxide dismutase
Keywords: Nitric oxide, EDHF, Arsenite, Endothelium, Iliac artery
1. Introduction
In the cardiovascular system, exposure to arsenic accelerates the development of atherosclerosis and predisposes to hypertension and peripheral microvascular abnormalities such as Blackfoot Disease (Balakumar and Kaur, 2009; Prozialeck et al., 2008; States et al., 2009). Underlying mechanisms have been suggested to involve increased oxidant stress, because exposure of endothelial cells to arsenite at concentrations within the range found in contaminated drinking water (0.3–15 μM) causes excess production of the superoxide anion (O2•−) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Barchowsky et al., 1999; Smith et al., 2001; Qian et al., 2005; Straub et al., 2008). O2•− may contribute to vascular dysfunction through a rapid interaction with, and inactivation of, the potent vascular relaxing factor endothelium-derived nitric oxide (NO) (Lassègue and Griendling, 2010). Dismutation of O2•− by superoxide dismutase (SOD) also generates hydrogen peroxide (H2O2), and the production of both reactive oxygen species (ROS) increases within minutes of exposing endothelial cells to low concentrations of arsenite (5 μM) (Barchowsky et al., 1999; Smith et al., 2001). Notably, endothelium-derived H2O2 is now thought to participate in the physiological response to endothelium-dependent agonists and fluid shear stress (Matoba et al., 2002; Liu et al., 2011), and can compensate for the loss of NO bioavailability observed in experimental models of hypertension and diabetes and in patients with arterial disease (Karasu, 2000; Landmesser et al., 2003; Phillips et al., 2007; Larsen et al., 2009). One possible mode of action may be an ability of H2O2 to relax subjacent smooth muscle cells by acting as a freely diffusible endothelium-derived hyperpolarizing factor (EDHF) (Matoba et al., 2002; Liu et al., 2011). However, H2O2 may also promote depletion of the endothelial endoplasmic reticulum (ER) Ca2+ store and amplify increases in cytosolic Ca2+ evoked by pharmacological stimulation of the endothelium (Hu et al., 2000; Edwards et al., 2008). This synergy can enhance the opening of calcium-activated K+ channels (KCa) thereby allowing H2O2 to potentiate “EDHF-type” relaxations that are mediated by the spread of endothelial hyperpolarization into the arterial media via myoendothelial and homocellular smooth muscle gap junctions (Edwards et al., 2008; Garry et al., 2009). Recently it has been reported that EDHF-type responses to the endocannabinoid-like molecule N-oleoylethanolamine are modulated by H2O2 (Wheal et al., 2012).
The aim of the current study was to investigate how inorganic AsIII, which is intrinsically more toxic than inorganic AsV (Vahter, 2002), affects EDHF-type and NO-mediated relaxations via the generation of O2•− and H2O2. Endothelium-dependent relaxations of rabbit iliac artery (RIA) and aortic rings were elicited by the G protein-coupled receptor agonist acetylcholine (ACh) and by cyclopiazonic acid (CPA), which promotes store-operated Ca2+ entry by depleting ER Ca2+ by inhibiting the endothelial SERCA pump (Fernandez-Rodriguez et al., 2009). In the RIA such relaxations consist of dual NO-mediated and EDHF-type gap junction-dependent components (Griffith et al., 2004, 2005; Chaytor et al., 2005), whereas in the aorta the EDHF-type component is negligible, so that the two mechanisms of relaxation can be dissociated (Ruiz et al., 1997; Fernandez-Rodriguez et al., 2009). The effects of arsenite were compared in the presence and absence of endogenous NO production, and the functional role of H2O2 investigated with catalase and a manganese-based SOD/catalase mimetic (Day et al., 1997). The role of NADPH oxidase was investigated with apocynin, which blocks the assembly of specific forms of this enzyme, and prevents the generation of O2•− and H2O2 in cultured endothelial cells treated with arsenite (Barchowsky et al., 1999; Touyz, 2008). Dihydroethidium (DHE) was used to assess ROS production in the different layers of the arterial wall (Zielonka and Kalyanaraman, 2010).
2. Methods
2.1. Mechanical responses
Iliac arteries, aortae and aortic valve leaflets (RAV) were obtained from male NZW rabbits (2–2.5 kg) killed by injection of sodium pentobarbital (150 mg/kg; i.v.) via the marginal ear vein and in accordance with local University guidelines. Rings of iliac artery or aorta 2–3 mm wide were mounted in a myograph (model 610M, Danish Myotechnology, Aarhus, Denmark) containing oxygenated (95% O2; 5% CO2) Holman's buffer (composition in mM: NaCl 120, KCl 5, NaH2PO4 1.3, NaHCO3 25, CaC12 2.5, glucose 11, and sucrose 10) at 37 °C and maintained at a resting tension of 1 mN over a 60 min equilibration period, with frequent readjustments in baseline tension to correct for stress relaxation. To evaluate EDHF-type responses, preparations were incubated for 30 min with the eNOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 300 μM) and the cyclooxygenase inhibitor indomethacin (10 μM) to inhibit prostanoid formation. Sodium arsenite (30 or 100 μM) was then added for 30 or 90 min prior to constriction with phenylephrine (PE, 1 μM). Once constrictor responses had reached a stable plateau, relaxation was studied by constructing cumulative concentration–response curves to CPA or ACh in the continued presence of arsenite. These curves were generally completed within ∼60 min so that total cumulative exposure to arsenite was 90 min and 150 min in the two protocols. Preliminary experiments demonstrated that lower concentrations of arsenite (10 μM) did not affect relaxation under these experimental conditions. To evaluate the role of O2•− and H2O2, catalase (2000 units/ml, from bovine liver), manganese(III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP, 100 μM) or the NADPH oxidase inhibitor apocynin (1-(4-hydroxy-3-methoxyphenyl)ethanone, 100 μM) were co-administered with L-NAME and indomethacin.
2.2. Detection of superoxide/hydrogen peroxide
RAV leaflets, and endothelium-denuded rings of iliac artery and aorta were incubated with arsenite (100 μM), apocynin (100 μM) or both for 60 min in oxygenated Holman's buffer containing L-NAME (300 μM) and indomethacin (10 μM) at 37 °C. To assess the production of reactive oxygen species (ROS) dihydroethidium (DHE, 5 μM) was then added for a further 30 min, following which the preparations were washed and fixed in 4% paraformaldehyde and images collected with a Leica SP5 confocal microscope (excitation 514 nm, emission 560–630 nm). This protocol was designed to match the total exposure of rings preincubated with 100 μM arsenite for 30 min in mechanical experiments in which it took a further ∼60 min to construct full concentration–relaxation curves. It should be noted that oxidation of DHE can generate two products, ethidium and 2-hydroxyethidium, which possess overlapping emission spectra and whose fluorescence is enhanced by binding to DNA (Zielonka and Kalyanaraman, 2010). Although H2O2 does not oxidize DHE directly and the formation of 2-hydroxyethidium is specific for O2•−, H2O2 may promote the formation of ethidium in the presence of peroxidase activity or haem proteins so that increased fluorescence in DHE-loaded vascular smooth muscle/endothelial cells may reflect production of both O2•− and H2O2 (Fernandes et al., 2007; Ray et al., 2011). The RAV was used to circumvent the complicating effects of signals transmitted from subjacent smooth muscle to the endothelium. All imaging data presented were acquired in the presence of L-NAME in order to avoid potentially confounding effects of NO which has been reported to promote the formation of ethidium in the presence of molecular oxygen (Zielonka and Kalyanaraman, 2010).
2.3. Statistics
The maximal percentage reversal of PE-induced tone (Rmax) by CPA or ACh and concentrations giving 50% reversal of this constrictor response (IC50 for CPA) or 50% of maximal relaxation (EC50 for ACh) were determined for each experiment. The use of IC50 rather than EC50 was necessary to allow for small initial constrictor responses to CPA that were observed in many experiments and can be attributed to an effect of CPA on smooth muscle Ca2+ stores (Chaytor et al., 2005). All metrics are given as mean ± SEM and compared using paired or unpaired Student's t-tests or one-way ANOVA followed by a Bonferroni's post-test as appropriate. Significance was accepted at P < 0.05; n denotes the number of animals studied in each experimental group.
2.4. Reagents
Pharmacological agents were purchased from Sigma–Aldrich (UK), except CPA (Ascent Scientific) and MnTMPyP (Calbiochem), and were dissolved in Holman's buffer, except apocynin and indomethacin (absolute ethanol), and CPA and DHE (DMSO).
3. Results
3.1. Effects of arsenite on responses to CPA and ACh in the RIA
3.1.1. EDHF-type relaxations
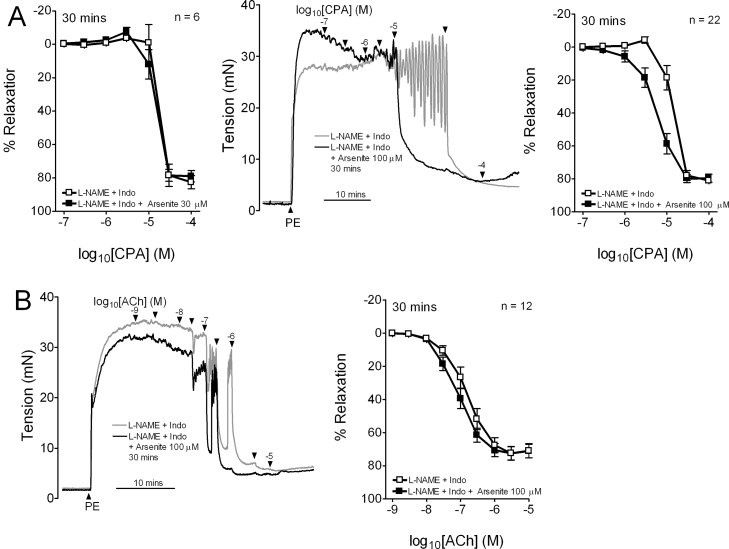
Responses evoked by CPA in the presence of L-NAME/indomethacin were unaffected by exposure to 30 μM arsenite for 30 min, whereas exposure to 100 μM arsenite for 30 min caused a leftward shift in the concentration–relaxation curve, such that pIC50 increased from ∼4.8 to ∼5.2 without change in Rmax (Fig. 1A; Table 1). EDHF-type relaxations evoked by ACh were similarly potentiated by exposure to 100 μM arsenite for 30 min, exhibiting a significant increase in pEC50 from ∼6.8 to ∼7.0 without change in Rmax (Fig. 1B; Table 1).
Fig. 1.
Effects of arsenite on EDHF-type relaxations in endothelium-intact iliac artery rings. (A) Concentration–response curves and original recordings showing that 100 μM arsenite, but not 30 μM, potentiated CPA-evoked EDHF-type relaxations after 30 min. (B) Exposure to 100 μM arsenite for 30 min also potentiated EDHF-type relaxations evoked by ACh. Rings were constricted by 1 μM phenylephrine (PE). n denotes the number of animals studied.
Table 1.
Effects of exposure to arsenite for 30 min on endothelium-dependent relaxations to CPA and ACh in the iliac artery.
| CPA | n | pIC50 | Rmax, % |
|---|---|---|---|
| Control | 9 | 4.99 ± 0.07 | 94.4 ± 2.8 |
| Arsenite 100 μM | 4.98 ± 0.10 | 91.6 ± 3.3 | |
| L-NAME + indo | 6 | 4.76 ± 0.07 | 82.7 ± 3.9 |
| L-NAME + indo + arsenite 30 μM | 4.85 ± 0.05 | 79.8 ± 3.3 | |
| L-NAME + indo | 22 | 4.85 ± 0.05 | 82.1 ± 1.8 |
| L-NAME + indo + arsenite 100 μM | 5.16 ± 0.08*** | 83.1 ± 1.7 |
| ACh | n | pEC50 | Rmax, % |
|---|---|---|---|
| Control | 12 | 7.32 ± 0.10 | 91.3 ± 2.2 |
| Arsenite 100 μM | 7.52 ± 0.10 | 91.1 ± 2.3 | |
| L-NAME + indo | 12 | 6.83 ± 0.09 | 72.7 ± 4.1 |
| L-NAME + indo + arsenite 100 μM | 7.08 ± 0.09*** | 72.4 ± 4.0 |
Experiments were performed in the absence (control) and presence of L-NAME (300 μM) and indomethacin (10 μM). Potency (negative log IC50 or log EC50) and maximal responses (Rmax) are given as mean ± SEM. n denotes the number of animals studied.
P < 0.001 compared with time-matched preparations not exposed to arsenite.
3.1.2. EDHF-type/NO-mediated relaxations
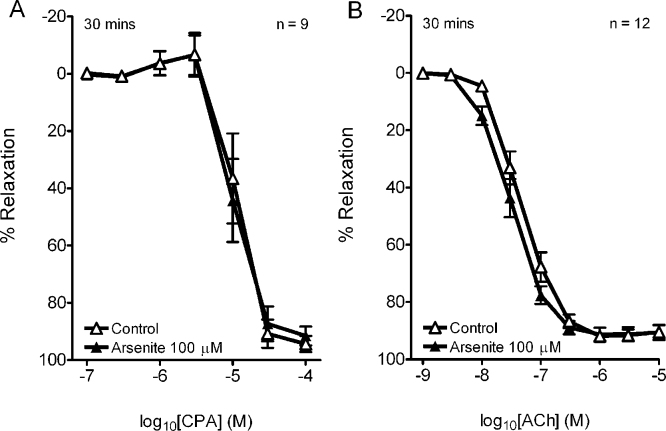
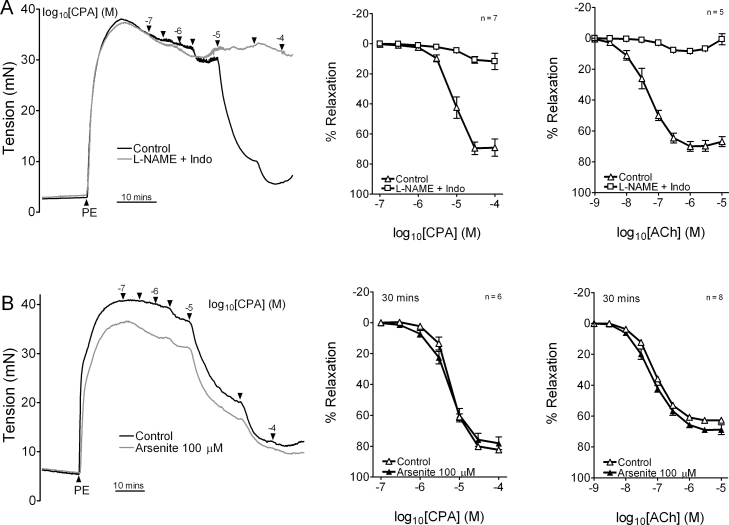
In control rings with intact endothelium incubated in the absence of L-NAME/indomethacin, the additional contribution of NO to CPA- and ACh-evoked relaxations was evidenced by pIC50 values of ∼5.0 and ∼7.3, and increases in Rmax to ∼90% from ∼80% and ∼70% compared to the corresponding EDHF-type concentration–relaxation curves (Table 1). Responses to CPA and ACh were unaffected by incubation with 100 μM arsenite for 30 min (Fig. 2A and B; Table 1).
Fig. 2.
Concentration–response curves showing that exposure to 100 μM arsenite for 30 min did not affect endothelium-dependent relaxations evoked by CPA or ACh in the iliac artery in the absence of L-NAME/indomethacin. n denotes the number of animals studied.
3.1.3. Contraction
In control rings incubated in the absence of L-NAME/indomethacin, the magnitude of the constrictor response to 1 μM PE was unaffected by exposure to 30 μM arsenite for 30 min, but was reduced by ∼15% following exposure to 100 μM arsenite for 30 min (from 30.1 ± 1.7 mN to 26.7 ± 1.8 mN, pooled data from all experiments n = 21, P < 0.01). Incubation with L-NAME/indomethacin increased PE-induced constriction by ∼15% and this increment in tone was reversed by exposure to 100 μM arsenite for 30 min (from 35.3 ± 1.2 mN to 30.0 ± 1.1 mN, pooled data from all experiments n = 73, P < 0.01), such that constriction then matched the level observed in the absence of L-NAME/indomethacin. No attempt was made to correct for these small overlapping effects on pre-relaxation tone.
3.2. Effects of arsenite on NO-mediated aortic relaxation
Maximal relaxations evoked by CPA and ACh in aortic rings with intact endothelium were equivalent to ∼70% of PE-induced tone and were mediated by NO because no significant EDHF-type component was evident in the presence of L-NAME/indomethacin (Fig. 3A). Rmax and pIC50/pEC50 values for concentration–relaxation curves constructed for CPA and ACh were unaffected by incubation with 100 μM arsenite for 30 min (Table 2). As in the RIA, this incubation protocol reduced PE-induced constriction by ∼15% (from 26.9 ± 1.6 mN to 22.9 ± 1.3 mN, pooled data from all experiments n = 14, P < 0.01).
Fig. 3.
Effects of arsenite on relaxation in aortic rings with intact endothelium. (A) EDHF-type relaxations were <5% of induced tone so that endothelium-dependent relaxation in this vessel can be attributed to NO. (B) Concentration–relaxation curves for CPA and ACh constructed in the absence of L-NAME/indomethacin were unaffected by exposure to 100 μM arsenite for 30 min. n denotes the number of animals studied.
Table 2.
Effects of exposure to 100 μM arsenite for 30 min on endothelium-dependent relaxations to CPA and ACh in the aorta.
| CPA | n | pIC50 | Rmax, % |
|---|---|---|---|
| Control | 6 | 4.73 ± 0.24 | 82.4 ± 1.7 |
| Arsenite | 5.00 ± 0.12 | 78.1 ± 4.1 |
| ACh | n | pEC50 | Rmax, % |
|---|---|---|---|
| Control | 8 | 7.09 ± 0.03 | 63.0 ± 1.2 |
| Arsenite | 7.19 ± 0.07 | 69.5 ± 2.5 |
Potency (negative log IC50 or log EC50) and maximal responses (Rmax) are given as mean ± SEM. n denotes the number of animals studied.
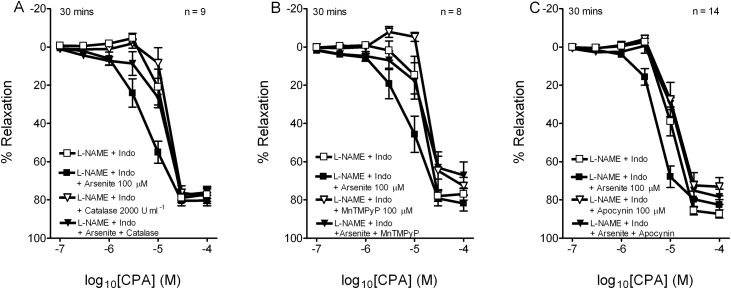
3.3. Effects of catalase, manganese porphyrin and apocynin on EDHF-type responses to CPA in the RIA
To investigate the mechanisms responsible for the selective potentiation of relaxations elicited in the presence of L-NAME/indomethacin, rings were preincubated with 2000 units/ml catalase, 100 μM MnTMPyP or 100 μM apocynin (Fig. 4). None of these agents alone significantly affected pIC50 and Rmax values for relaxation in the absence of arsenite, whereas the enhancement of relaxation observed following exposure to 100 μM arsenite for 30 min was fully prevented in each case (Table 3).
Fig. 4.
Inhibitory effects of (A) 2000 units/ml catalase, (B) 100 μM MnTMPyP, and (C) 100 μM apocynin on the potentiation of CPA-evoked EDHF-type relaxations by exposure to 100 μM arsenite for 30 min. n denotes the number of animals studied.
Table 3.
Effects of catalase, MnTMPyP and apocynin on the potentiation of CPA-evoked EDHF-type relaxations in the iliac artery by 100 μM arsenite for 30 min.
| CPA | n | pIC50 | Rmax, % |
|---|---|---|---|
| L-NAME + indo | 9 | 4.92 ± 0.05 | 79.5 ± 2.3 |
| + Arsenite 100 μM | 5.21 ± 0.07*** | 81.0 ± 2.5 | |
| + Catalase 2000 U ml−1 | 4.79 ± 0.05 | 82.2 ± 2.8 | |
| + Arsenite + catalase | 4.80 ± 0.04 | 77.9 ± 3.0 | |
| L-NAME + indo | 8 | 4.68 ± 0.07 | 78.9 ± 2.8 |
| + Arsenite 100 μM | 5.05 ± 0.05* | 84.1 ± 3.1 | |
| + MnTMPyP 100 μM | 4.59 ± 0.07 | 73.3 ± 4.5 | |
| + Arsenite + MnTMPyP | 4.69 ± 0.10 | 70.7 ± 5.6 | |
| L-NAME + indo | 14 | 4.99 ± 0.07 | 86.2 ± 1.9 |
| + Arsenite 100 μM | 5.31 ± 0.05** | 83.1 ± 2.1 | |
| + Apocynin 100 μM | 4.89 ± 0.07 | 78.7 ± 3.7 | |
| + Arsenite + apocynin | 5.04 ± 0.06 | 82.9 ± 3.1 | |
All experiments were performed in the presence of L-NAME (300 μM) and indomethacin (10 μM). Potency (negative log IC50) and maximal responses (Rmax) are given as means ± SEM. n denotes the number of animals studied.
P < 0.05 compared with time-matched controls.
P < 0.01 compared with time-matched controls.
P < 0.001 compared with time-matched controls.
3.4. Fluorescence imaging of ROS production
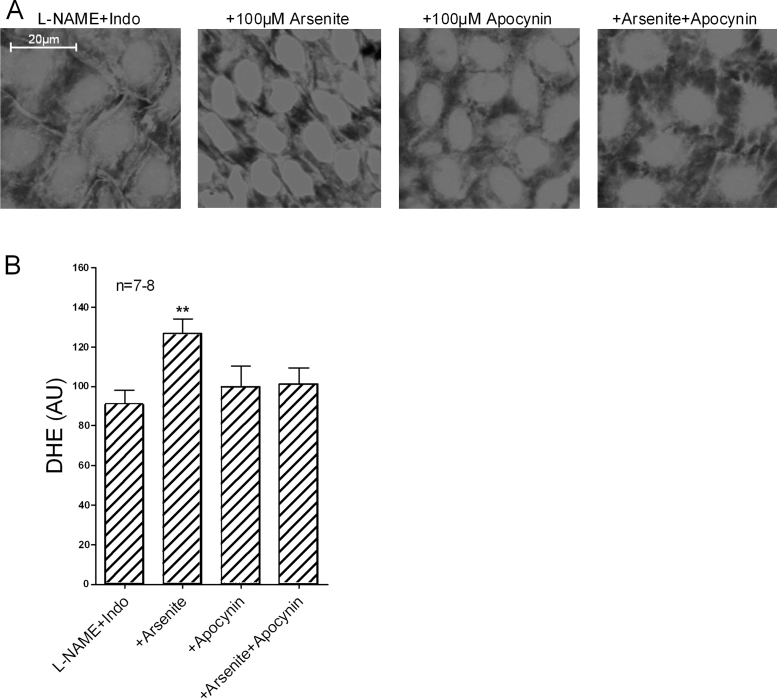
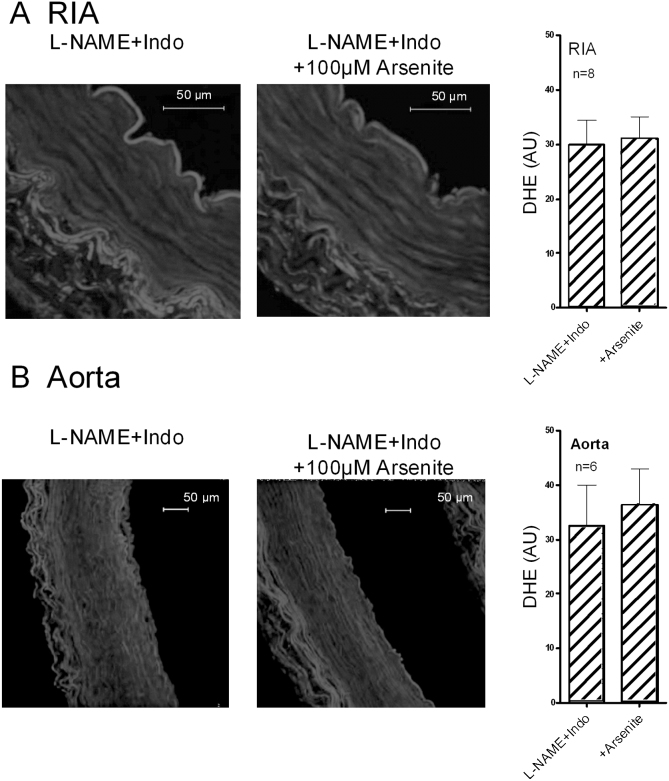
Exposure to 100 μM arsenite for 90 min significantly enhanced endothelial nuclear fluorescence in RAV leaflets loaded with DHE in the presence of L-NAME/indomethacin, an effect that was fully prevented by preincubation with 100 μM apocynin (Fig. 5). Exposure to 100 μM arsenite for 90 min did not increase fluorescence in either the media or adventitia of endothelium-denuded RIA and aortic rings loaded with DHE (Fig. 6).
Fig. 5.
Endothelial ROS production in rabbit isolated aortic valve leaflets loaded with DHE in the presence of L-NAME/indomethacin. (A) Nuclear fluorescence increased following exposure to 100 μM arsenite for 90 min, but was unaffected by apocynin or arsenite in combination with apocynin. (B) Bar graphs confirming statistical significance. n denotes the number of animals studied.
Fig. 6.
Fluorescence studies of endothelium-denuded iliac artery and aortic rings loaded with DHE in the presence of L-NAME/indomethacin. (A) and (B) Images and bar graphs showing that neither artery type exhibited evidence of excess ROS production in the media of the vessel wall following exposure to 100 μM arsenite for 90 min. The adventitia of both vessels displayed autofluorescence, but its appearances were unaltered by exposure to arsenite. Scalebars identify the vessel lumen. n denotes the number of animals studied.
3.5. Effects of longer term exposure to arsenite
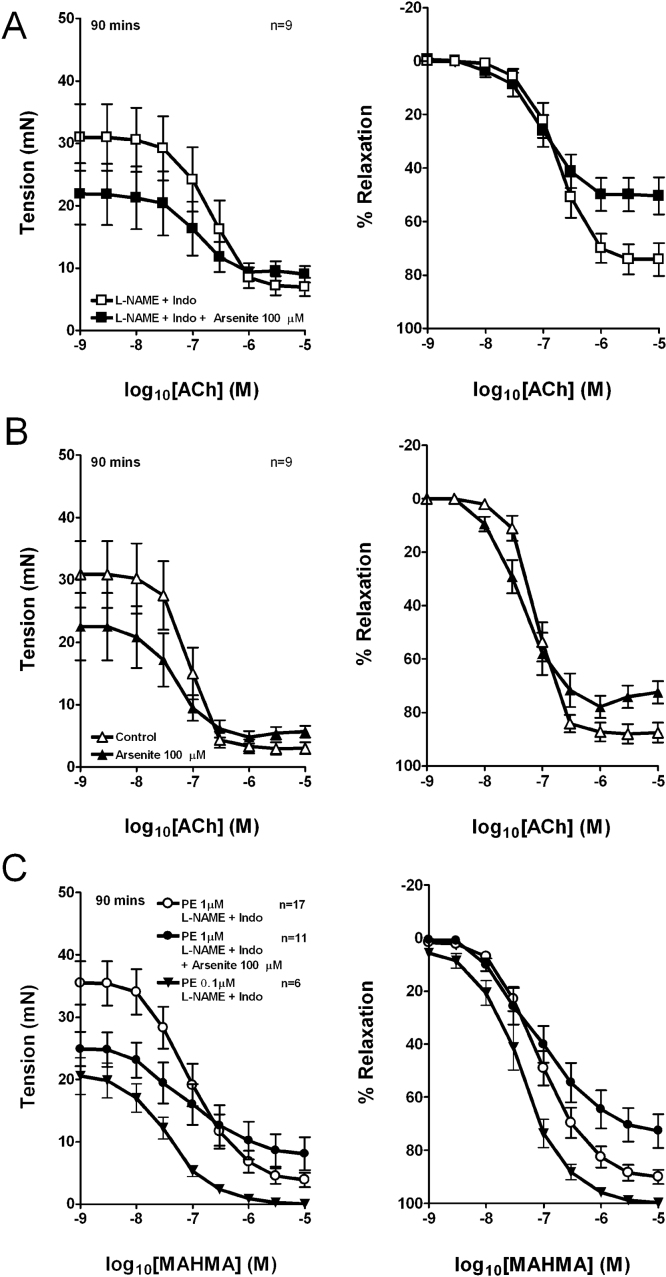
Exposure to 100 μM arsenite for 90 min caused a ∼30% reduction in force development in RIA rings constricted by 1 μM PE from 33.9 ± 2.9 mN to 23.5 ± 2.6 mN (n = 26 and 20) in the presence of L-NAME/indomethacin and from 30.9 ± 5.4 mN to 22.4 ± 5.3 mN (n = 9 and 9) in control rings (pooled data from Fig. 7; P < 0.01 in each case). This large depressor effect affected the analysis of endothelium-dependent relaxation, because absolute tension ultimately converged to a similar plateau in the presence and absence of arsenite at the highest concentrations of ACh. Consequently, normalization to initial pre-relaxation tone led to an apparent decrease in Rmax on a % basis (Fig. 7A and B), whereas pEC50 values derived from the concentration–relaxation curves were unaffected by arsenite (Table 4).
Fig. 7.
Effects of 90 min exposure to 100 μM arsenite on relaxation in endothelium-intact iliac artery rings. Data are presented as absolute reversal (left panels) and % reversal (right panels) of PE-induced constriction. (A) EDHF-type responses to ACh. (B) Responses to ACh with L-NAME/indomethacin omitted from the buffer. (C) Responses to MAHMA NONOate in the presence of L-NAME/indomethacin.
Table 4.
Effects of exposure to 100 μM arsenite for 90 min on endothelium-dependent relaxations to ACh and direct relaxation to MAHMA NONOate in the RIA.
| ACh | n | pEC50 |
|---|---|---|
| Control | 7 | 7.11 ± 0.06 |
| Arsenite | 7 | 7.37 ± 0.14 |
| L-NAME + indo | 9 | 6.74 ± 0.09 |
| L-NAME + indo + arsenite | 9 | 7.03 ± 0.13 |
| MAHMA NONOate | n | pEC50 |
|---|---|---|
| 1 μM PE, L-NAME + indo | 17 | 7.01 ± 0.11 |
| 1 μM PE, L-NAME + indo + arsenite | 11 | 7.02 ± 0.15 |
| 0.1 μM PE, L-NAME + indo | 6 | 7.39 ± 0.11 |
Potency (negative log EC50) is given as mean ± SEM. n denotes the number of animals studied.
Similar experiments demonstrated that exposure to 100 μM arsenite for 90 min also impaired smooth muscle relaxations to the exogenous NO donor MAHMA NONOate in endothelium-intact RIA rings incubated with L-NAME/indomethacin. The use of an exogenous NO donor excludes any potential effect of arsenite on the NO synthase pathway. Again this incubation protocol did not statistically affect pEC50 values derived from concentration–relaxation curves, whereas Rmax was reduced (Fig. 7C; Table 4).
Experiments were also performed in which the relaxant effects of arsenite on pre-relaxation tone were mimicked by reducing the concentration of PE used to induce constriction to 0.1 μM (Fig. 7C). Rmax and pEC50 values for MAHMA NONOate were then larger than in experiments conducted in the presence of 1 μM PE or 1 μM PE plus 100 μM arsenite, as complete relaxation was obtained in the presence of the lower concentration of 0.1 μM PE (Fig. 7; Table 4).
4. Discussion
The present study has provided new insights into the mechanisms through which short-term exposure to inorganic arsenic can modulate endothelial, and therefore vascular, function. Arsenite was shown to potentiate EDHF-type relaxations by stimulating endothelial NADPH oxidase activity and thereby promoting the formation of H2O2 from O2•−, whereas relaxations mediated by endothelium-derived NO were unaffected.
4.1. Potentiating effects of arsenite on the EDHF phenomenon
Arsenite amplified EDHF-type relaxations evoked by the SERCA inhibitor CPA in RIA rings in a concentration-dependent manner, with potentiation being apparent following exposure to 100 μM, but not 30 μM, arsenite for 30 min. This potentiation was prevented by catalase and the catalase/SOD mimetic MnTMPyP, thus confirming a central role for endogenously generated H2O2. Enhanced relaxation was also prevented by apocynin, and this NADPH oxidase inhibitor abolished arsenite-induced increases in fluorescence in RAV leaflets loaded with the ROS sensitive probe DHE. Arsenite similarly enhanced EDHF-type relaxations to ACh, although this effect was less prominent than with CPA, consistent with previous observations that exogenous H2O2 amplifies EDHF-type relaxations to ACh at a higher threshold compared with CPA (Garry et al., 2009). Taken together, these findings indicate that excess O2•− generated by the activation of endothelial NADPH oxidase by arsenite can serve as a source of H2O2 that modulates the EDHF phenomenon. Previous analysis has demonstrated that exogenous H2O2 synergistically enhances depletion of the ER Ca2+ store by CPA and amplifies electrotonically conducted relaxations by promoting endothelial KCa channel opening (Edwards et al., 2008; Garry et al., 2009). The present study extends these observations by demonstrating that endogenously generated H2O2 can enhance the biological role of the EDHF phenomenon under conditions of increased oxidative stress.
The classical phagocytic NADPH oxidase comprises a membrane-bound flavocytochrome b558 component constructed from a catalytic Nox subunit (designated as Nox2 or gp91phox) and a p22phox regulatory subunit. p22phox co-activation requires translocation of additional protein subunits (p47phox, p67phox, p40phox and the small GTPase Rac1) to the cell membrane where they associate with the b558 heterodimer in a cascade that can be interrupted by apocynin at the level of p47phox (Ray and Shah, 2005; Touyz, 2008; Lassègue and Griendling, 2010). Exposure to arsenite increases the overall Nox catalytic activity of membrane fractions from cultured intact endothelial cells by twofold within 1 h, whereas treatment of isolated endothelial membranes is without effect (Smith et al., 2001). More specifically, the ability of arsenite to stimulate endothelial O2•− production has an obligatory requirement for gp91phox, p47phox and p67phox and Rac1, consistent with activation of the Nox2-based oxidase (Smith et al., 2001; Qian et al., 2005; Straub et al., 2008). It should be noted that the Nox2-based oxidase can also be detected in a perinuclear distribution where it is associated with the endothelial cytoskeleton and might contribute to intracellular O2•− production directly (Ray and Shah, 2005). Other Nox homologues are also expressed in the arterial wall, with the dominant subtypes found in large arteries being Nox2 and Nox4 in the endothelium and Nox1 and Nox4 in smooth muscle (Brandes and Schroder, 2008; Lassègue and Griendling, 2010). In contrast to Nox2, the Nox4 homologue is constitutively active, localizes to the endoplasmic/sarcoplasmic reticulum, generates H2O2 in preference to O2•−, and is insensitive to apocynin because catalytic activity depends on Nox4/p22phox without the requirement for p47phox and other proteins that characterizes the phagocytic complex (Brandes and Schroder, 2008; Chen et al., 2008; Dikalov et al., 2008; Ray et al., 2011). The present findings therefore imply that the Nox4-based oxidase does not contribute to the potentiating effects of arsenite, as EDHF-type relaxations were fully blocked by apocynin. While it has been suggested that apocynin might act as an antioxidant rather than an inhibitor of NADPH oxidase, the antioxidant effects were detected only at 1 mM and were absent at the 100 μM apocynin concentration employed in the present study (Heumuller et al., 2008).
4.2. Differential effects of arsenite on EDHF-type and NO-mediated relaxations
Activation of endothelial NADPH oxidase should in theory impair NO-mediated arterial relaxations as a consequence of the reaction between O2•− and NO (Griffith et al., 1987), whose existence following exposure to arsenite has been inferred from evidence of tissue protein nitrosation, presumably by peroxynitrite, in endothelial cells (Straub et al., 2008). However, we found that arsenite did not affect aortic relaxations evoked by CPA and ACh, even though such responses were mediated exclusively by NO, and arsenite was confirmed to stimulate ROS production in the RAV endothelium. Furthermore, while arsenite potentiated EDHF-type relaxations, no evidence of potentiation was evident in the absence of L-NAME/indomethacin. Taken together, these observations suggest (i) that the flux of NO generated by CPA or ACh substantially exceeds the rate of formation of O2•− induced by arsenite in rabbit endothelial cells, and (ii) that NO may limit the availability of O2•− for dismutation to H2O2, thereby compromising the ability of arsenite to potentiate any co-existent EDHF-type component of relaxation. Notably, we also demonstrated that arsenite did not enhance ROS generation in the media of the RIA or aorta, and this is likely to explain its inability to impair NO-mediated relaxation, despite increased ROS production by the endothelium. In this regard it should be noted that selective increases in endothelial O2•− production also fail to impair NO-mediated aortic relaxations to ACh or nitroprusside in transgenic mice with targeted endothelial overexpression of Nox2 (Bendall et al., 2007), and that overexpression of Nox4 in the endothelium, to increase intracellular production of H2O2 (but not O2•−) may enhance EDHF-type relaxations in transgenic mice without altering NO bioavailability (Ray et al., 2011). By contrast, the well-documented ability of angiotensin II to depress NO-mediated endothelium-dependent relaxation is accompanied by a global increase in ROS production across the vessel wall secondary to dual activation of the Nox1-based smooth muscle oxidase and the Nox2-based endothelial oxidase (Lassègue et al., 2001; Touyz et al., 2002; Lassègue and Griendling, 2010). Angiotensin II may also stimulate ROS generation by vascular adventitial cells (Pagano et al., 1997), whereas no evidence for excess arsenite-induced adventitial DHE fluorescence was apparent in the present study.
4.3. Relationship with previous studies
Previous reports have provided evidence that chronic in vivo exposure to inorganic arsenic can impair subsequent ex vivo endothelium-dependent relaxations to ACh in the rabbit and the rat aorta (Pi et al., 2003; Verma et al., 2009). While these studies hypothesized that impaired NO-mediated relaxations reflected overproduction of O2•−, the measurements made were indirect (plasma [H2O2], nitrite and cGMP levels), and assessment of ROS production in the vessel wall was not attempted. Lee et al. (2003) also observed apparent reductions in endothelium-dependent relaxations to ACh in rat aortic rings exposed to 50 μM arsenite for 14 h, but attributed these to impaired cGMP-mediated mechanisms of relaxation and impaired conversion of L-arginine to L-citrulline by eNOS, rather than increased ROS production. In view of these conflicting observations, we evaluated the effects of more prolonged 90 min incubation with arsenite on both EDHF-type and NO-mediated relaxation evoked by ACh in RIA rings. Notably, this protocol reduced the contractile response to 1 μM PE by ∼30%, both in the presence or absence of L-NAME/indomethacin, without greatly affecting the residual level of tone observed at the point of maximal ACh-induced relaxation, so that standard analysis led to an apparent decrease in Rmax, calculated on a % basis relative to the initial level of pre-relaxation tone. However, pEC50 values for the corresponding concentration–relaxation curves were not affected by arsenite, and were essentially unchanged compared to those obtained after exposure to 100 μM arsenite for 30 min. We observed a similar phenomenon in experiments where direct smooth muscle relaxation was elicited with MAHMA NONOate after constriction by 1 μM PE and arsenite again reduced Rmax but not pEC50 values. By contrast, when tone was induced by 0.1 μM PE, to match the depressed constriction observed with 1 μM PE in the presence of arsenite, the reversal of tone by MAHMA NONOate was essentially complete. Taken together, such observations suggest that apparent reductions in Rmax in the presence of arsenic primarily reflect a generalized impairment of smooth muscle function, rather than specific effects against EDHF-type and NO-mediated relaxations.
5. Conclusion
The present study has identified complex effects of short-term exposure to inorganic arsenic on EDHF-type and NO-mediated arterial relaxations. An ability of arsenite to increase endothelial production of H2O2 secondary to activation of NADPH oxidase can potentiate EDHF-type responses, whereas selective increases in endothelial O2•− production appeared insufficient to impair smooth muscle relaxations to endothelium-derived NO. Further studies are required to correlate in vitro observations with the long term vascular effects of arsenic ingestion in vivo at levels found in contaminated drinking water, and to clarify conflicting reports that in vivo conversion to more toxic metabolites such as monomethylarsonous acid that may result in direct inhibition of eNOS (Vahter, 2002; Lee et al., 2003). Possible iatrogenic effects of trivalent arsenic on vascular function also remain to be investigated given that arsenic trioxide is now widely used in the treatment of haematological conditions such as acute promyelocytic leukaemia (Jing et al., 1999).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
The work was supported by the British Heart Foundation (Grant No. PG/08/072/25474) and the School of Medicine, Cardiff University
References
- Balakumar P., Kaur J. Arsenic exposure and cardiovascular disorders: an overview. Cardiovasc. Toxicol. 2009;9:169–176. doi: 10.1007/s12012-009-9050-6. [DOI] [PubMed] [Google Scholar]
- Barchowsky A., Klei L.R., Dudek E.J., Swartz H.M., James P.E. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free. Radic. Biol. Med. 1999;27:1405–1412. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- Bendall J.K., Rinze R., Adlam D., Tatham A.L., de Bono J., Wilson N., Volpi E., Channon K.M. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin I: studies in endothelial-targeted Nox2 transgenic mice. Circ. Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- Brandes R.P., Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends. Cardiovasc. Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Chaytor A.T., Bakker L.M., Edwards D.H., Griffith T.M. Connexin-mimetic peptides dissociate electrotonic EDHF-type signalling via myoendothelial and smooth muscle gap junctions in the rabbit iliac artery. Br. J. Pharmacol. 2005;144:108–114. doi: 10.1038/sj.bjp.0706046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Kirber M.T., Xiao H., Yang Y., Keaney J.F., Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell. Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B.J., Fridovich I., Crapo J.D. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- Dikalov S.I., Dikalova A.E., Bikineyeva A.T., Schmidt H.H., Harrison D.G., Griendling K.K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D.H., Li Y., Griffith T.M. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler. Thromb. Vasc. Biol. 2008;28:1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- Fernandes D.C., Wosniak J., Jr., Pescatore L.A., Bertoline M.A., Liberman M., Laurindo F.R., Santos C.X. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am. J. Physiol. Cell Physiol. 2007;292:C413–C422. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- Fernandez-Rodriguez S., Edwards D.H., Newton B., Griffith T.M. Attenuated store-operated Ca2+ entry underpins the dual inhibition of nitric oxide and EDHF-type relaxations by iodinated contrast media. Cardiovasc. Res. 2009;84:470–478. doi: 10.1093/cvr/cvp239. [DOI] [PubMed] [Google Scholar]
- Garry A., Edwards D.H., Fallis I.F., Jenkins R.L., Griffith T.M. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc. Res. 2009;84:218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T.M., Chaytor A.T., Bakker L.M., Edwards D.H. 5-Methyltetrahydrofolate and tetrahydrobiopterin can modulate electrotonically mediated endothelium-dependent vascular relaxation. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7008–7013. doi: 10.1073/pnas.0408919102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T.M., Chaytor A.T., Edwards D.H. The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol. Res. 2004;49:551–564. doi: 10.1016/j.phrs.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Griffith T.M., Edwards D.H., Henderson A.H. Unstimulated release of endothelium derived relaxing factor is independent of mitochondrial ATP generation. Cardiovasc. Res. 1987;2:565–568. doi: 10.1093/cvr/21.8.565. [DOI] [PubMed] [Google Scholar]
- Hu Q., Zheng G., Zweier J.L., Deshpande S., Irani K., Ziegelstein R.C. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J. Biol. Chem. 2000;275:15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- Heumuller S., Wind S., Barbosa-Sicard E., Schmidt H.H.H.W., Busse R., Schroder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Jing Y., Dai J., Chalmers-Redman R.M., Tatton W.G., Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- Karasu C. Time course of changes in endothelium-dependent and -independent relaxation of chronically diabetic aorta: role of reactive oxygen species. Eur. J. Pharmacol. 2000;392:163–173. doi: 10.1016/s0014-2999(00)00140-0. [DOI] [PubMed] [Google Scholar]
- Landmesser U., Dikalov S., Price S.R., McCann L., Fukai T., Holland S.M., Mitch W.E., Harrison D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B.T., Bubolz A.H., Mendoza S.A., Pritchard K.A., Jr., Gutterman D.D. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2009;29:739–745. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassègue B., Griendling K.K. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassègue B., Sorescu D., Szöcs K., Yin Q., Akers M., Zhang Y., Grant S.L., Lambeth J.D., Griendling K.K. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Lee M.Y., Jung B.I., Chung S.M., Bae O.N., Lee J.Y., Park J.D., Yang J.S., Lee H., Chung J.H. Arsenic-induced dysfunction in relaxation of blood vessels. Environ. Health Perspect. 2003;111:513–517. doi: 10.1289/ehp.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bubolz A.H., Mendoza S., Zhang D.X., Gutterman D.D. H2O2 is the transferable factor mediating flow-induced dilation in human coronary arterioles. Circ. Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T., Shimokawa H., Kubota H., Morikawa K., Fujiki T., Kunihiro I., Mukai Y., Hirakawa Y., Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- Pagano P.J., Clark J.K., Cifuentes-Pagano M.E., Clark S.M., Callis G.M., Quinn M.T. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S.A., Hatoum O.A., Gutterman D.D. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am. J. Physiol. 2007;292:H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- Pi J., Horiguchi S., Sun Y., Nikaido M., Shimojo N., Hayashi T., Yamauchi H., Itoh K., Yamamoto M., Sun G., Waalkes M.P., Kumagai Y. A potential mechanism for the impairment of nitric oxide formation caused by prolonged oral exposure to arsenate in rabbits. Free Radic. Biol. Med. 2003;35:102–113. doi: 10.1016/s0891-5849(03)00269-7. [DOI] [PubMed] [Google Scholar]
- Prozialeck W.C., Edwards J.R., Nebert D.W., Woods J.M., Barchowsky A., Atchison W.D. The vascular system as a target of metal toxicity. Toxicol. Sci. 2008;102:207–218. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Liu K.J., Chen Y., Flynn D.C., Castranova V., Shi X. Cdc42 regulates arsenic-induced NADPH oxidase activation and cell migration through actin filament reorganization. J. Biol. Chem. 2005;280:3875–3884. doi: 10.1074/jbc.M403788200. [DOI] [PubMed] [Google Scholar]
- Ray R., Murdoch C.E., Wang M., Santos C.X., Zhang M., Alom-Ruiz S., Anilkumar N., Ouattara A., Cave A.C., Walker S.J., Grieve D.J., Charles R.L., Eaton P., Brewer A.C., Shah A.M. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- Ray R., Shah A.M. NADPH oxidase and endothelial cell function. Clin. Sci. (Lond.) 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- Ruiz E., Lorente R., Tejerina T. Effects of calcium dobesilate on the synthesis of endothelium-dependent relaxing factors in rabbit isolated aorta. Br. J. Pharmacol. 1997;121:711–716. doi: 10.1038/sj.bjp.0701184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K.R., Klei L.R., Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L442–L449. doi: 10.1152/ajplung.2001.280.3.L442. [DOI] [PubMed] [Google Scholar]
- States J.C., Srivastava S., Chen Y., Barchowsky A. Arsenic and cardiovascular disease. Toxicol. Sci. 2009;107:312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub A.C., Clark K.A., Ross M.A., Chandra A.G., Li S., Gao X., Pagano P.J., Stolz D.B., Barchowsky A. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J. Clin. Invest. 2008;118:3980–3989. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz R.M. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 2008;51:172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- Touyz R.M., Chen X., Tabet F., Yao G., He G., Quinn M.T., Pagano P.J., Schiffrin E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ. Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Verma S., Reddy K., Balakumar P. The defensive effect of benfotiamine in sodium arsenite-induced experimental vascular endothelial dysfunction. Biol. Trace. Elem. Res. 2009;137:96–109. doi: 10.1007/s12011-009-8567-7. [DOI] [PubMed] [Google Scholar]
- Wheal A.J., Alexander S.P., Randall M.D. Hydrogen peroxide as a mediator of vasorelaxation evoked by N-oleoylethanolamine and anandamide in rat small mesenteric arteries. Eur. J. Pharmacol. 2012;674:384–390. doi: 10.1016/j.ejphar.2011.11.033. [DOI] [PubMed] [Google Scholar]
- Zielonka J., Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free. Radic. Biol. Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]