Abstract
Nickel-catalyzed cross-coupling reactions of unactivated tertiary alkyl nucleophiles and aryl bromides have been developed using N-heterocyclic carbene ligands. These processes are reviewed alongside earlier attempts to employ unactivated tertiary alkyl nucleophiles in cross-coupling reactions. Potential mechanisms for the transformations, and future challenges in this field are discussed.
Keywords: nickel, cross-coupling, transition metals, homogeneous catalysis, Grignard reaction
The development of efficient metal-catalyzed carbon-carbon cross-coupling reactions has revolutionized the strategies by which we approach the construction of organic molecules.1 Although studies of these reactions have largely focused on the formation of C(sp2)-C(sp2) bonds, metal-catalyzed processes involving C(sp3) nucleophiles and electrophiles have also been developed.1b,2 As a result of the propensity of alkyl ligands to undergo β-hydride elimination, the use of C(sp3) nucleophiles is generally more challenging than the use of C(sp2) nucleophiles. Over the past few years, different methods have been developed that permit the limited use of secondary nucleophiles in metal-catalyzed C–C bond-forming reactions.3 However, tertiary nucleophiles have remained incompatible with most cross-coupling reactions. Prior to the title work, no general Pd- or Ni-catalyzed cross-coupling protocol that tolerates the use of tertiary alkyl nucleophiles had been reported. The Cu-catalyzed method previously reported by Hintermann constituted the only successful, substantial effort towards the development of a method that permitted the use of tertiary alkyl nucleophiles in cross-coupling reactions.4,5 However, the method was limited to the cross-coupling of tertiary alkylmagnesium halides and certain polychlorinated aza-aryl electrophiles.
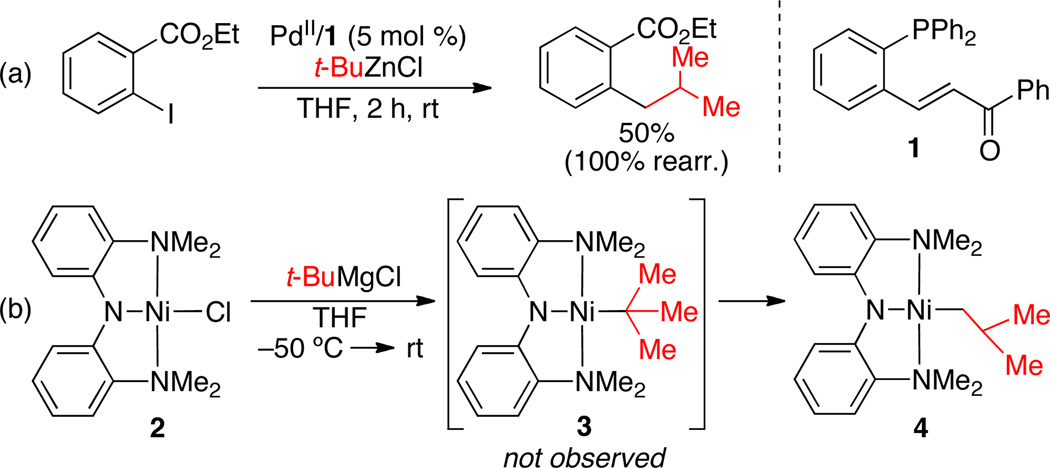
Few attempts to employ tertiary alkyl nucleophiles in Pd- and Ni-catalyzed processes have been reported. The Lei group has described its effort to employ t-BuZnCl in a Pd-catalyzed Negishi cross-coupling reaction with an aryl iodide (Scheme 2a) using hemi-labile bidentate ligand 1.6 This reaction resulted exclusively in the formation of the iso-butyl cross-coupling product that arises from the rearrangement of the tert-butyl nucleophile. The Hu group recently attempted to employ t-BuMgCl in a stoichiometric reaction with Ni(II)Cl pincer complex 2 (Scheme 2b).7,8 Compound 3, which should arise from the direct reaction of 2 and t-BuMgCl was not observed. Instead, only i-BuNi(II) pincer complex 4 was observed. These reactions highlight the rearrangement of the nucleophile as a major obstacle in the development of an efficient method for Pd- or Ni-catalyzed cross-coupling reactions employing tertiary alkyl nucleophiles.
Scheme 2.
(a) Lei’s use of t-BuZnCl in a Pd-catalyzed cross-coupling reaction with an aryl iodide. (b) Hu’s stoichiometric treatment of a Ni pincer complex with t-BuMgCl.
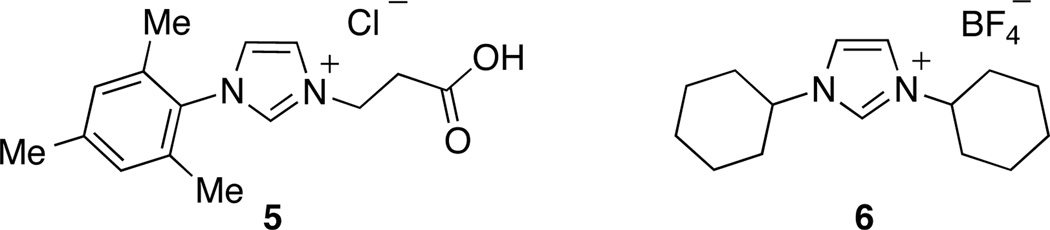
Recently, our research group,9a as well as the Glorius group,9b independently reported the ability of N-heterocyclic carbene (NHC) ligands (5 and 6) to support the Ni-catalyzed cross-coupling of aryl electrophiles and tertiary alkylmagnesium nucleophiles. For the first time, a general catalytic system was developed that supported the transmetallation of a sterically-hindered, tertiary nucleophile, while also supporting oxidative addition of an aryl halide and the rapid reductive elimination of the cross-coupling product. Facile reductive elimination is essential to circumvent the β-hydride elimination/reinsertion sequence (vide infra) that results in the formation of undesired isomers of the cross-coupling product.
The Ni-catalyzed process reported by the Glorius group for the cross-coupling of t-BuMgCl and aryl bromides employed NHC ligand 5.9b It was proposed that the presence of the remote carboxylate group of 5 helps to retard competitive β-hydride elimination by occupying an additional coordination site on the Ni center. Isolated yields as high as 85% were obtained in these reactions; the formation of up to 15% of inseparable isomers was also observed.
In our work, NHC ligand 6 was used as the supporting ligand for the Ni-catalyzed cross-coupling of t-BuMgCl and aryl bromides.9a In general, good yields and minimal isomerization were observed when 6 was employed (Table 2). The outcome of the reaction showed little dependence on the electronic properties of the aryl bromide. An ortho-substituent (entry 10) was surprisingly well tolerated under these conditions. However, the use of heterocyclic substrates resulted in incomplete conversion. Tertiary alkylmagnesium halides other than t-BuMgCl were also investigated using this reaction protocol (Table 3). While a tertiary alkylmagnesium halide nucleophile containing one α-branch was employed successfully, increased branching coincided with higher levels of isomerization. It was also observed that a hydrated nickel source was required for optimal catalytic efficiency. A thorough analysis of the influence of NiCl2 hydration on yield revealed 1.2–1.8 equivalents of H2O to be conducive to highest yields. While partially hydrated NiCl2 salts were readily prepared from NiCl2•(H2O)6, the use of commercially available NiBr2•H2O provided an additional option for the nickel source and resulted in only slightly diminished yields.
Table 2.
Representative products formed using Biscoe’s method for the Ni-catalyzed cross-coupling of t-BuMgCl and ArBra
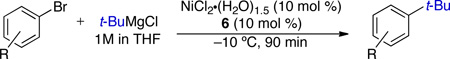 | |||||
|---|---|---|---|---|---|
| entry | product | yield | entry | product | yield |
| 1 |  |
81% (>50:1) | 7 |  |
79% (47:1) |
| 2 | 75% (34:1) | 8 | 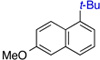 |
86% (>50:1) | |
| 3 | 84% (>50:1) | 9 | 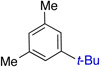 |
85% (32:1) | |
| 4 | 84% (45:1) | 10 | 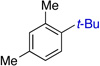 |
55% (10:1) | |
| 5 | 70% (>50:1) | 11 | 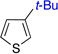 |
47% (20:1) | |
| 6 | 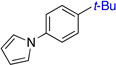 |
75% (>50:1) | 12 | 52% (13:1) | |
Average isolated yields of two runs. Ratio of retention product to isomerization product in parentheses.
Table 3.
Use of α-branched tertiary alkylmagnesium nucleophiles in Ni-catalyzed cross-coupling reactions with arylbromidesa
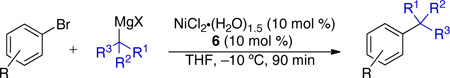 | ||
|---|---|---|
| entry | product | yield |
| 1 | 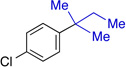 |
73% (>50:1) |
| 2 | 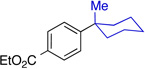 |
76% (12:1) |
| 3 | 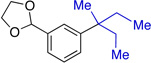 |
51%b (5:1) |
Average isolated yields of two runs. Ratio (determined by 1H spectroscopy) of retention product to isomerization product in parentheses.
Reaction was warmed to rt and stirred overnight.
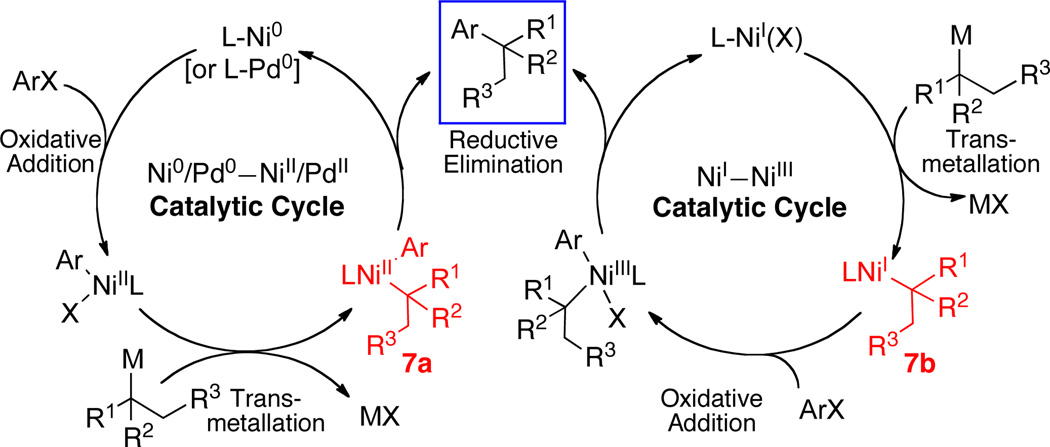
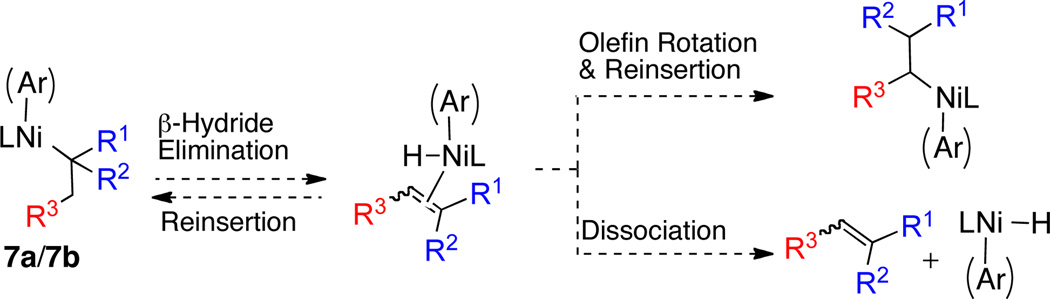
Scheme 3 depicts two plausible catalytic cycles for Ni- and Pd-catalyzed cross-coupling reactions employing tertiary alkyl nucleophiles. While it is likely that such a Pd-catalyzed process would proceed via the Pd(0)–Pd(II) catalytic cycle that is well-established for cross-coupling reactions,1 the Ni-catalyzed cycle is not so clearly defined. Since multiple stable oxidation states are potentially accessible to organonickel complexes, a Ni(0)–Ni(II) catalytic cycle or a Ni(I)–Ni(III) cycle can be envisioned in these systems.10 A Ni(0)–Ni(II) cycle would be expected to mirror the analogous Pd(0)–Pd(II) cycle where oxidative addition occurs on the low valent metal, followed by transmetallation and ensuing reductive elimination. On the other hand, transmetallation would likely precede oxidative addition in the Ni(I)–Ni(III) cycle (Scheme 3).11 In either catalytic cycle, the products that arise from transmetallation (7a and 7b) may isomerize via a rapid β-hydride elimination/re-insertion sequence (Scheme 4), thus generating inseparable by-products. It is important that the supporting ligand retard such isomerization in order to establish an efficient catalytic cycle.
Scheme 3.
Possible catalytic cycles for Ni- and Pd-catalyzed cross-coupling reactions of alkyl nucleophiles and aryl halides
Scheme 4.
Side-products resulting from the competing β-hydride elimination pathway
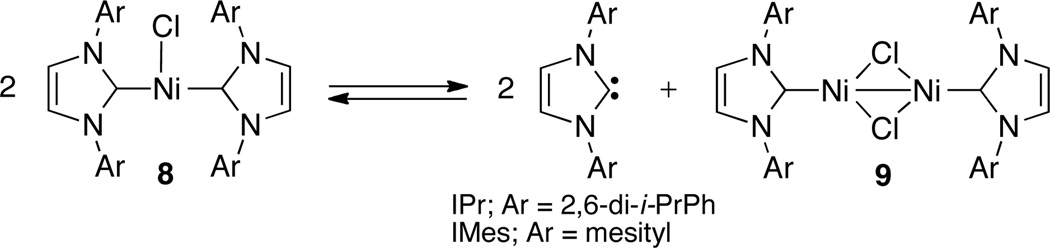
While we cannot definitively exclude the possibility of a Ni(0)–Ni(II) catalytic cycle, several existing studies lead us to support the Ni(I)–Ni(III) pathway for this process.12,13 Multiple groups have investigated the stoichiometric formation of NHC-ligated Ni(I) complexes from Ni(0) and Ni(II) precursors.12 When using NHC ligands bearing bulky aryl substituents, the formation of nickel (I) halide complexes (8) that are bis-ligated, three-coordinate, monovalent, and T-shaped has been reported.12b,c In the specific case where IPr is the supporting ligand, complex 8 exists in equilibrium with a mono-ligated μ-chloronickel dimer (9) that can also be isolated (Scheme 5).12b When IMes is the supporting ligand, complex 8 has been shown to constitute a kinetically competent precatalyst for Kumada and Suzuki cross-coupling reactions.12c It is possible that complex 8 is an active intermediate of the catalytic cycle, while complex 9 exists as an off-cycle resting state.12a–c
Scheme 5.
Equilibrium between tricoordinate (NHC)2NiCl and its μ-chloronickel dimer
Although analogous Ni(I) complexes bearing NHC ligands 5 and 6 have not been successfully isolated, tricoordinate complexes similar to 8 may play an important role in Ni-catalyzed cross-coupling reactions between tertiary alkylmagnesium halides and aryl bromides. Indeed, more detailed structural information regarding the intermediate complexes of the catalytic cycle would be helpful in explaining why Ni/NHC complexes can uniquely support such reactions involving tertiary alkyl nucleophiles. Any additional structural information would also aid in the rational development of improvements and extensions of this method.
At present, several challenges still limit the exciting potential of this chemistry. The method employing ligand 6 is realistically limited to tertiary alkylmagnesium halide nucleophiles bearing at least two α-methyl units (i.e. a maximum of one α-branch). Although the desired cross-coupling product can be obtained when two α-branches are present on the nucleophile, approximately 15% of the total isolated product is the inseparable rearranged species. This system needs to be improved such that the use of bulkier, more substituted nucleophiles no longer coincides with the increased formation of isomerized products. Such a process would enhance the scope of the reaction and open the door for the potential use of optically active tertiary nucleophiles in the cross-coupling reactions.
In order to maximize functional group tolerance and to enable the direct use of optically active tertiary nucleophiles in this method, new extensions must be developed that permit the use of less nucleophilic, more configurationally-stable tertiary alkyl organometallic nucleophiles. The use of Grignard reagents places an obviously large limitation on the functional groups that are compatible with this reaction. Additionally, enantioenriched Grignard reagents undergo rapid racemization under non-cryonic conditions and cannot be prepared in a straightforward manner.14 The development of cross-coupling methods that tolerate the use of tertiary alkylzinc, tertiary alkyltin, or tertiary alkylboron reagents would significantly expand the substrate scope of this reaction and would increase the likelihood that a general, stereoretentive cross-coupling procedure will be possible.15
This account has detailed the development of the first transition metal-catalyzed cross-coupling reactions of tertiary alkyl nucleophiles and aryl halides. Catalytic systems comprised of nickel and NHC ligands can successfully effect this transformation, often without the concurrent formation of isomeric by-products. These processes constitute a new example of reactivity that is accessible in catalytic systems that exploit the putative Ni(I)–Ni(III) redox system. We expect that this work has laid the groundwork for the development of alternate, more versatile catalytic systems for the metal-catalyzed cross-coupling of tertiary alkyl nucleophiles and aryl electrophiles.
Figure 1.
NHC ligands employed by the Glorius group (5) and by the Biscoe group (6) in Ni-catalyzed cross-coupling reactions of t-BuMgCl and arylbromides
Scheme 1.
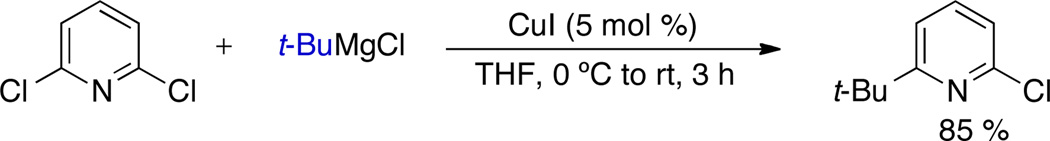
Hintermann’s method for the Cu-catalyzed cross-coupling of tertiary alkylmagnesium reagents and polychlorinated aza-aryl electrophiles
Table 1.
Representative products formed using Glorius’ method for the Ni-catalyzed cross-coupling of t-BuMgCl and ArBra
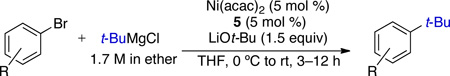 | |||||
|---|---|---|---|---|---|
| entry | product | yield | entry | product | yield |
| 1 | 73% (5:1) | 5 | 80%b | ||
| 2 | 68%b | 6 | 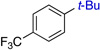 |
73% (4:1) | |
| 3 | 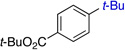 |
85% (6:1) | 7 | 75%b | |
| 4 | 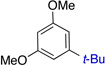 |
75% (4:1) | 8 | 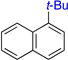 |
80%b |
Average isolated yields of two runs. Ratio of retention product to isomerization product in parentheses.
Ratio of retention product to isomerization product not given.
Acknowledgement
We thank The City College of New York (CCNY) and PSC-CUNY for financial support. We additionally acknowledge the donors of the American Chemical Society Petroleum Research Fund (50307-DNI1) for partial support of this research.
Biography

Amruta Joshi-Pangu (left) was raised in Kolhapur, India. In 2007, she received her B.Tech. degree in pharmaceuticals and fine chemicals from ICT, Mumbai. She is currently a Ph.D. student in chemistry at The City College of New York. Mark Biscoe (right) was raised in Bristol, CT. In 1999, he received a B.A. degree in chemistry from Wesleyan University where he conducted research in the laboratory of Albert J. Fry. In 2005, Mark received his Ph.D. from Columbia University under the guidance of Ronald Breslow. After three years as an NIH postdoctoral fellow in the laboratory of Stephen Buchwald at MIT, Mark joined the faculty at The City College of New York in 2009 as an Assistant Professor of Chemistry.
References and Notes
- 1.de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Weinheim: Wiley-VCH; 2004. [Google Scholar]
- 2.a) Chemler SR, Trauner D, Danishefsky SJ. Angew. Chem. Int. Ed. 2001;40:4544. doi: 10.1002/1521-3773(20011217)40:24<4544::aid-anie4544>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]; b) Netherton MR, Fu GC. In: Topics in Organometallic Chemistry: Palladium in Organic Synthesis. Tsuji J, editor. New York: Springer; 2005. pp. 85–108. [Google Scholar]; c) Rudolph A, Lautens M. Angew. Chem. Int. Ed. 2009;48:2656. doi: 10.1002/anie.200803611. [DOI] [PubMed] [Google Scholar]; d) Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111 doi: 10.1021/cr100327p. 1417 and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For recent examples of secondary nucleophiles in Pd catalysis: Campos KR, Klapars A, Walsman JH, Dormer PG, Chen C. J. Am. Chem. Soc. 2006;128:3538. doi: 10.1021/ja0605265. Dreher SD, Dormer PG, Sandrock DL, Molander GA. J. Am. Chem. Soc. 2008;130:9257. doi: 10.1021/ja8031423. Han C, Buchwald SL. J. Am. Chem. Soc. 2009;131:7532. doi: 10.1021/ja902046m. Thaler T, Haag B, Gavryushin A, Schober K, Hartman E, Gschwing RM, Zipse H, Mayer P, Knochel P. Nat. Chem. 2010;2:125. doi: 10.1038/nchem.505. Nakao Y, Takeda M, Matsumoto T, Hiyama T. Angew. Chem. Int. Ed. 2010;45:4447. doi: 10.1002/anie.201000816. Sandrock DL, Jean-Gerard L, Chen C-Y, Dreher SD, Molander GA. J. Am. Chem. Soc. 2010;132:17108. doi: 10.1021/ja108949w. Thaler T, Guo L-N, Mayer P, Knochel P. Angew. Chem. Int. Ed. 2011;50:2147. doi: 10.1002/anie.201006879. Calimsiz S, Organ MG. Chem. Commun. 2011;47:5181. doi: 10.1039/c0cc04835f. For recent examples of secondary nucleophiles in Ni catalysis: Melzig L, Gavryushin A, Knochel P. Org. Lett. 2007;9:5529. doi: 10.1021/ol702499h. Smith SW, Fu GC. Angew. Chem. Int. Ed. 2008;47:9334. doi: 10.1002/anie.200802784. Phapale VB, Guisan-Ceinos M, Bunuel E, Cardenas DJ. Chem. Eur. J. 2009;15:12681. doi: 10.1002/chem.200901913. Joshi-Pangu A, Ganesh M, Biscoe MR. Org. Lett. 2011;13:1218. doi: 10.1021/ol200098d.
- 4.Hintermann L, Xiao L, Labonne A. Angew. Chem. Int. Ed. 2008;47:8246. doi: 10.1002/anie.200803312. [DOI] [PubMed] [Google Scholar]
- 5.For a Cu-catalyzed cross-coupling reaction involving t-BuMgCl and primary alkyl halides see: Terao J, Todo H, Begum SA, Kuniyasu H, Kambe N. Angew. Chem. Int. Ed. 2007;46:2086. doi: 10.1002/anie.200603451.
- 6.Luo X, Zhang H, Duan H, Liu Q, Zhu L, Zhang T, Lei A. Org. Lett. 2007;9:4571. doi: 10.1021/ol701995t. [DOI] [PubMed] [Google Scholar]
- 7.Breitenfeld J, Vechorkin O, Corminboeuf C, Scopelliti R, Hu X. Organometallics. 2010;29:3686. [Google Scholar]
- 8.Examples of the Ni-catalyzed cross-coupling of trans-β-bromostyrene and t-BuMgCl have been previously reported in: Hayashi T, Konishi M, Yokota K, Kumada M. Chem. Lett. 1980:767. Nugent WA. Org. Lett. 2002;4:2133. doi: 10.1021/ol0259488.
- 9.a) Joshi-Pangu A, Wang C-Y, Biscoe MR. J. Am. Chem. Soc. 2011;133:8478. doi: 10.1021/ja202769t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lohre C, Droge T, Wang C, Glorius F. Chem. Eur. J. 2011;17:6052. doi: 10.1002/chem.201100909. [DOI] [PubMed] [Google Scholar]
- 10.Tamaru, editor. Modern Organonickel Chemistry. Weinheim: Wiley-VCH; 2005. [Google Scholar]
- 11.In Cu(I)-catalyzed C-heteroatom cross-coupling reactions, it is likewise proposed that transmetallation procedes oxidative addition. See: Jones GO, Liu P, Houk KN, Buchwald SL. J. Am. Chem. Soc. 2010;132:6205. doi: 10.1021/ja100739h. Yu H-Z, Jiang Y-Y, Fu Y, Liu L. J. Am. Chem. Soc. 2010;132:18078. doi: 10.1021/ja104264v.
- 12.a) Dible BR, Sigman MS, Arif AM. Inorg. Chem. 2005;44:3774. doi: 10.1021/ic050445u. [DOI] [PubMed] [Google Scholar]; b) Miyazaki S, Koga Y, Matsumoto T, Matsubara K. Chem. Commun. 2010;46:1932. doi: 10.1039/b924716e. [DOI] [PubMed] [Google Scholar]; c) Zhang K, Conda-Sheridan M, Cooke SR, Louie J. Organometallics. 2011;30:2546. doi: 10.1021/om200090d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nagao S, Matsumoto T, Koga Y, Matsubara K. Chem. Lett. 2011;40:1036. [Google Scholar]
- 13.Previous mechanistic studies of Ni-catalyzed processes involving tridentate nitrogen ligands also support a Ni(I)–Ni(III) catalytic cycle. See: Lin X, Phillips DL. J. Org. Chem. 2008;73:3680. doi: 10.1021/jo702497p. Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J. Am. Chem. Soc. 2006;128:13175. doi: 10.1021/ja063334i. Anderson TJ, Jones GD, Vicic DA. J. Am. Chem. Soc. 2004;126:8100. doi: 10.1021/ja0483903. ; Phapale VB, Guisan-Ceinos M, Bunuel E, Cardenas DJ. Chem. Eur. J. 2009;15:12681. doi: 10.1002/chem.200901913. [DOI] [PubMed] [Google Scholar]
- 14.a) Holzer B, Hoffmann RW. Chem. Commun. 2003:732. doi: 10.1039/b300033h. [DOI] [PubMed] [Google Scholar]; b) Hoffmann RW, Holzer B. Chem. Commun. 2001:491. [Google Scholar]; c) Hoffmann RW, Holzer B, Knopff O, Harms K. Angew. Chem. Int. Ed. 2000;39:3072. doi: 10.1002/1521-3773(20000901)39:17<3072::aid-anie3072>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Boudier A, Bromm LO, Lotz M, Knochel P. Angew. Chem. Int. Ed. 2000;39:4414. [PubMed] [Google Scholar]