Abstract
BACKGROUND AND OBJECTIVE:
Clinic-based programs for childhood obesity are not available to a large proportion of the population. The purpose of this study was to evaluate the efficacy of a guided self-help treatment of pediatric obesity (GSH-PO) compared with a delayed treatment control and to evaluate the impact of GSH-PO 6-months posttreatment.
METHODS:
Fifty overweight or obese 8- to 12-year-old children and their parents were randomly assigned to immediate treatment or to delayed treatment. The GSH-PO includes 12 visits over 5 months and addresses key components included in more intensive clinic-based programs. Children and parents in the immediate treatment arm were assessed at time 1 (T1), participated in GSH-PO between T1 and T2, and completed their 6-month posttreatment assessment at T3. Children and parents in the delayed treatment arm were assessed at T1, participated in GSH-PO between T2 and T3, and completed their 6-month posttreatment assessment at T4. The main outcome measures were BMI, BMI z score, and percentage overweight (%OW).
RESULTS:
Children in the immediate treatment GSH-PO arm decreased their BMI significantly more than did the delayed treatment arm (BMI group × time = −1.39; P < .001). Similar results were found for BMI z score and %OW. At the 6-month posttreatment assessment, changes resulting from GSH-PO were maintained for BMI z score and %OW but not BMI (BMI time effect = −0.06, not significant; BMI z score time effect = −0.10, P < .001; %OW time effect = −4.86, P < .05).
CONCLUSIONS:
The GSH-PO showed initial efficacy in decreasing BMI for children in this study. Additional efficacy and translational studies are needed to additionally evaluate GSH-PO.
Keywords: childhood, obesity, guided self-help, treatment
What’s Known on This Subject:
Clinic-based weight control programs for pediatric obesity are time and personnel intensive and not accessible to a large proportion of the population.
What This Study Adds:
This is the first study to reveal the efficacy of a low-intensity, 5-month, guided self-help treatment of childhood obesity with effects on the target child’s weight immediately posttreatment and 6 months later.
Recent data suggest that 31% of children in the United States are overweight or obese,1 affecting 4 to 5 million children. Family-based behavioral treatment of childhood obesity that combines nutrition and exercise education with behavior therapy techniques has been shown to be effective,2 and one-third of children treated are no longer overweight in adulthood.3,4 However, there are a number of barriers that limit accessibility to these treatments for overweight and obese children, including time needed to counsel families, reimbursement, and the ability of primary care practitioners to deliver behavioral treatment.5 Very few providers have training in the theory and techniques of behavior therapy needed to provide these treatments.6 Moreover, childhood obesity is considered an epidemic, and it is unlikely that there will ever be sufficient trained resources to address the large number of cases that exist. To disseminate these treatments to a greater proportion of the population, alternative models of delivery must be developed.
Guided self-help treatment includes offering structure along with a self-help program to enhance adherence and implementation of the program. The guidance offered is not therapy in its purest sense, but includes clarifying materials, answering questions, helping families remain on task, and modifying the program to fit the individual family’s needs. This form of guidance makes the didactic material more readily accessible to users with diverse levels of literacy or educational backgrounds. Ultimately, a guided self-help program could be adeptly administered by primary care practitioners or other health care providers with minimal training.
Thus, the purpose of this study was to assess the extent to which a guided self-help intervention, compared with a delayed treatment control, resulted in a decrease in BMI in children. We also evaluated whether the intervention promoted improvements in eating behavior and physical activity among children and parents and a decrease in BMI in the parents. In addition, we evaluated whether the guided self-help treatment of pediatric obesity (GSH-PO) intervention led to maintenance of intervention outcomes at 6-months posttreatment.
Methods
Children aged 8 to 12 years who were overweight or obese (BMI percentile: 85th to 98th) and their parents were recruited through a number of methods, including newspaper advertisements, list serves, pediatrician referrals, and direct mailing. We limited the upper end of BMI for children to the 98th percentile because those with higher BMIs are significantly more likely to present with obesity-related health comorbidities7 and require more intensive treatment. Because these children are not yet significantly obese, they are more likely to respond to a minimal intervention such as GSH-PO and prevent additional development of obesity. At least 1 parent or guardian participated with the child. Families were excluded if either the child or parent was currently involved in any other psychological or weight-loss treatment, was using medications that affected appetite or weight, had a psychiatric condition, or did not speak English. Parents provided written informed consent, and children completed an assent.
If a family responded to an advertisement, and had a child that could qualify, they were invited for an assessment at which their height and weight would be measured and where they would complete surveys. Fifty parent-child pairs were randomly assigned by using computer-generated random numbers to a GSH-PO or a delayed treatment control. Parents and children assigned to the immediate intervention started GSH-PO after the baseline assessment [time (T) 1]. Immediate intervention families also attended posttreatment (T2) and 6-month posttreatment (T3) assessments. Delayed treatment families attended a baseline assessment (T1) and waited 5 months before attending another assessment (T2) to start treatment. After treatment, the delayed treatment families attended a posttreatment assessment (T3) and a 6-month posttreatment assessment (T4).
Treatment sessions were conducted at the University of California, San Diego, and the study was approved by the University of California, San Diego, Institutional Review Board. All treatment sessions were led by graduate students in clinical psychology. All interventionists attended a 4-hour training regarding behavioral intervention for the study and were supervised by the first author on a weekly basis during treatment.
GSH-PO
The GSH-PO intervention included 12 visits over 5 months. The treatment frequency and time for visits (every other week for 20 minutes) was deliberately chosen to develop a method that could be used in primary care clinics. Families received a parent manual, a child manual, and an activities manual. The activities manual was designed to provide activities and games that parents and children could do together to enhance program learning at home. The parent, child, and activity manuals focused on the same topics each week, with the exception of parenting skills, and the information in the child manuals was provided in age-appropriate language. The manuals were designed by the project team to address the major components of family-based behavioral treatment programs for childhood obesity in a self-help manner. Topics for the manuals included the causes of childhood obesity, the traffic light eating plan, stimulus control, increasing physical activity, decreasing sedentary activity, increasing lifestyle activity, motivation, teasing, body image, cognitive skills, social support, planning for high-risk situations, and relapse prevention.8 Families were told to read the assigned chapter in the parent and child manuals between visits, apply the skills, and complete any activities from the activities manual that were of interest to their family.
Meetings with the interventionists were a maximum of 20 minutes in length, with the exception of session 2, which was designed to be 1 hour to allow for time to discuss the dietary recommendations. Visits were focused on monitoring weight of parent and child, reflecting on child and parent behaviors that led to any weight changes (to improve self-regulation), answering any questions regarding program material, and problem solving any barriers to implementing program recommendations. Parents and children were given self-monitoring booklets and were told to write down their food intake and physical activity daily in as much detail as possible. Interventionists also collected self-monitoring books from parents and children and praised them for any self-monitoring or other program efforts.
Delayed Treatment Control
The delayed treatment group did not have any contact with the project team during the 5-month delay, and they received the GSH-PO intervention starting at T2.
Outcome Measures
All measurements were completed by all participants at T1, T2, and T3. Measurements were completed at T4 by the delayed treatment group only. Demographic characteristics were reported by the parents at T1.
Child Outcomes
Child BMI
The primary outcome measures in this trial were child body size measures, including child BMI, BMI z score, and percentage overweight (%OW). Child height was measured by using a portable Schorr height board (Schorr Inc, Olney, MD) in duplicate. Body weight was measured in duplicate on a Tanita digital scale (model WB-110A; Tanita, Arlington, IL), and the average of the 2 values was used in analyses. Height and weight were converted to BMI (kg/m2) and translated to BMI-for-age percentile score by using the Centers for Disease Control and Prevention growth charts.9 Child %OW was calculated by using the following formula: %OW = 100*(BMI − M)/M, where M is the median BMI for gender and age according to the Centers for Disease Control and Prevention growth chart data files.
Dietary Intake
Dietary intake of the child was assessed with three 24-hour dietary recalls with the child (and with the parent present) at each assessment point on 3 nonconsecutive days by telephone. Validation studies have provided support for the use of this method of dietary assessment for children.10–12 All of the interviews used the Nutrition Data Systems for Research nutrient calculation software and food content database (http://www.ncc.umn.edu/products/ndsr.html).
Physical Activity
Physical activity of the child was assessed by using GT1M Actigraph accelerometers (Actigraph, Inc, Pensacola, FL). Actigraph technology has been shown to be valid for quantifying activity levels in laboratory and field settings.13 Light and moderate-to-vigorous activity intensities were determined from the Freedson age-adjusted equation.14,15 Sedentary time was determined as accelerometer counts between 0 and 100. Activity categories were summed to calculated minutes of valid days (>10 hours), and each category was reported as a percentage of total Actigraph wear-time to adjust for differences in the amount of time the children wore the accelerometers.
Parent Outcomes
Parent BMI
Parent height and weight were measured in the same manner as for the child and translated to BMI (kg/m2).
Parent Dietary Intake.
The Dietary History Questionnaire (DHQ) is a cognitively based food-frequency questionnaire (FFQ) developed by the National Cancer Institute to assess dietary intake and nutrient consumption (http://www.riskfactor.cancer.gov/DHQ). Validation studies have revealed the DHQ to be an improvement over the Block FFQ and the Willett FFQ.16,17 The DHQ has been widely used in normal-weight, overweight, and obese populations to assess dietary intake18–20 and change in dietary intakes during intervention trials.21
Parent Physical Activity
The Global Physical Activity Questionnaire22 is a 16-item comprehensive assessment of health-related physical activity and sedentary behavior in adults and captures a range of daily physical activity habits: occupational, active transportation, leisure, and sedentary behavior. The Global Physical Activity Questionnaire has been validated against objective and self-report measures of activity.22
Acceptability and Liking Survey
After treatment, children and parents completed a brief survey about their acceptability and liking of the GSH-PO program.
Statistical Analysis
Initial tests of treatment group differences in child and parent baseline demographic characteristics were conducted with χ2 tests for categorical variables and t tests for continuous variables. Maximum likelihood repeated-measures models tested between-group differences over time. Analyses were conducted by using all available data assuming data were missing at random. Models were specified with a between-subject factor of treatment group (0.5 = GSH-PO immediate treatment; −0.5 = delayed treatment), a within-subject factor of time (0 = time 1 baseline, 1 = time 2 post–immediate treatment, 2 = 6 months post–immediate treatment) and the treatment × time interaction. In addition, the 2 treatment groups were combined in repeated-measures models to test for sustained treatment effects from baseline to 6 months posttreatment.
All analyses were conducted by using SPSS 19 (SPSS Inc, Chicago, IL). P values were not adjusted for multiple tests. All reported P values are for 2-sided tests with effects considered to be statistically significant at P < .05.
Results
Recruitment and Completion
Table 1 shows sample demographic characteristics by group. No statistical differences were found between groups on any of the child or parent demographic characteristics. At baseline, 64.0% of the immediate treatment group were obese and 68.0% of those in delayed treatment group were obese [χ2(1) = 0.089, P = .765]. At baseline, 5 of the GSH-PO immediate treatment parents were overweight and 8 were obese. In the delayed treatment group, 9 of the parents were overweight and 7 parents were obese.
TABLE 1.
Sample Demographic Characteristics by Study Group
| Immediate (n = 25) | Delayed (n = 25) | |
|---|---|---|
| Child | ||
| Gender, % (n) | ||
| Girls | 60.0 (15) | 64.0 (16) |
| Boys | 40.0 (10) | 36.0 (9) |
| Age, mean ± SD, y | 10.3 ± 1.3 | 10.5 ± 1.4 |
| Ethnicity, % (n) | ||
| Asian | 4.0 (1) | 12.0 (3) |
| African American | 8.0 (2) | 4.0 (1) |
| Native Hawaiian/Pacific Islander | 4.0 (1) | 0.0 |
| Hispanic | 12.0 (3) | 16.0 (4) |
| White | 72.0 (18) | 68.0 (17) |
| Parent | ||
| Gender, % (n) | ||
| Female | 84.0 (21) | 82.6 (19) |
| Male | 16.0 (4) | 17.4 (4) |
| Age, mean ± SD, y | 42.9 ± 5.7 | 43.2 ± 4.8 |
| Ethnicity, % (n) | ||
| Asian | 8.0 (2) | 17.4 (4) |
| African American | 12.0 (3) | 0 |
| Hispanic | 4.0 (1) | 8.7 (2) |
| White | 76 (19) | 73.9 (17) |
| Marital status, % (n) | ||
| Married | 88.0 (22) | 78.3 (18) |
| Never married or divorced | 12.0 (3) | 21.7 (5) |
| Income, % (n) | ||
| <$20,000–$60,000 | 8.0 (2) | 21.7 (5) |
| >$60,000 | 84.0 (21) | 69.6 (16) |
| Don’t know | 8.0 (2) | 8.7 (2) |
| Education, % (n) | ||
| Less than college degree | 40.0 (10) | 26.0 (6) |
| College degree | 36.0 (9) | 17.4 (4) |
| Master’s or professional degree | 24.0 (6) | 56.5 (13) |
| BMI, mean ± SD | 27.5 ± 6.1 | 27.9 ± 6.1 |
| Parents’ weight, % (n) | ||
| Normal weight | 48.0 (12) | 36.0 (9) |
| Overweight | 20.0 (5) | 36.0 (9) |
| Obese | 32.0 (8) | 28.0 (7) |
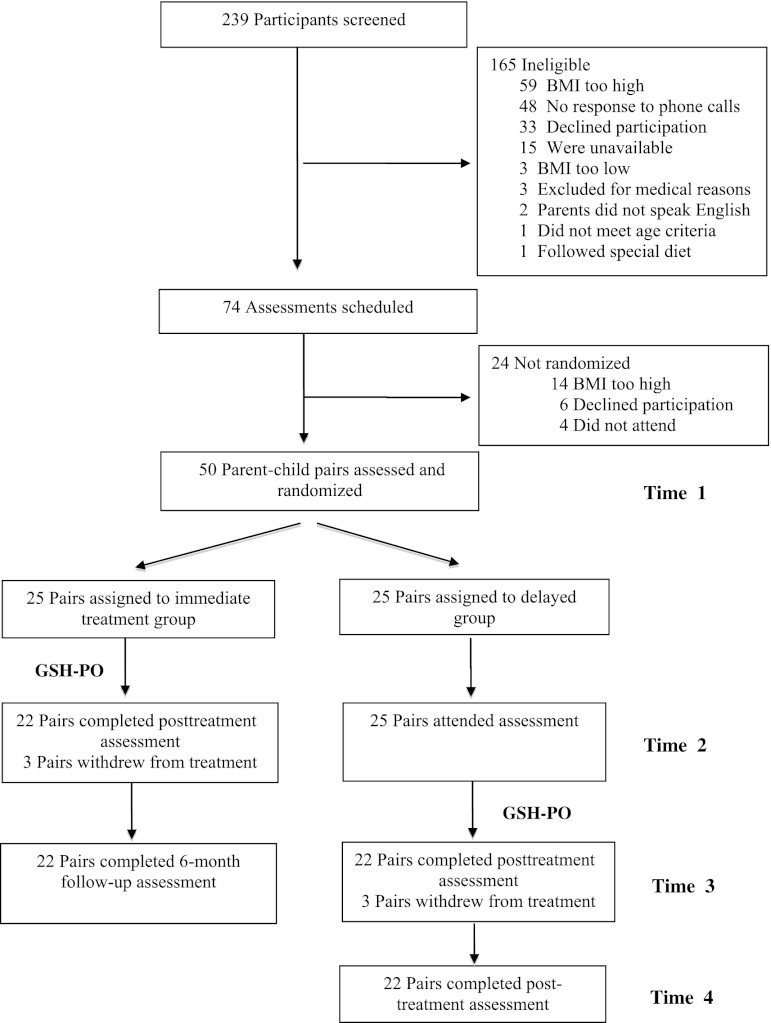
Figure 1 shows the study recruitment and completion rate of parent-child dyads in this study. Seventy-four families were scheduled for an assessment, and 50 were enrolled in the trial and were randomly assigned to the immediate treatment or delayed treatment arm. No parent-child pairs were lost in the delayed treatment arm from T1 to T2. Twenty-five parent-child pairs started treatment in both immediate (T1) and delayed treatment (T2) time points, and 22 families finished treatment in both arms (12% dropout total). No parent-child pairs were lost between posttreatment and 6-month follow-up time points. Table 2 shows the observed body size (means and SD) at each assessment point.
FIGURE 1.
Study enrollment and retention.
TABLE 2.
Observed Weight Data at Each Assessment Point
| T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
| Child BMI | ||||
| Immediate | 24.07 ± 1.92 | 23.34 ± 2.11 | 24.03 ± 2.64 | — |
| Delayed | 24.40 ± 2.55 | 25.17 ± 2.79 | 24.48 ± 3.01 | 25.01 ± 3.23 |
| Child BMI z score | ||||
| Immediate | 1.71 ± 0.25 | 1.49 ± 0.32 | 1.50 ± 0.37 | — |
| Delayed | 1.71 ± 0.28 | 1.74 ± 0.30 | 1.56 ± 0.34 | 1.55 ± 0.39 |
| Child, %OW | ||||
| Immediate | 39.35 ± 9.27 | 32.75 ± 10.65 | 34.05 ± 12.71 | — |
| Delayed | 40.21 ± 11.09 | 42.37 ± 12.37 | 36.04 ± 12.47 | 38.97 ± 12.60 |
| Parent BMI | ||||
| Immediate | 27.53 ± 6.11 | 26.79 ± 6.43 | 27.15 ± 6.46 | — |
| Delayed | 27.90 ± 6.05 | 28.01 ± 6.22 | 27.46 ± 5.91 | 28.31 ± 5.93 |
Data are means ± SDs. Sample sizes for immediate group are as follows: T1, n = 25; T2, n = 22; T3, n = 22. Sample sizes for delayed group are as follows: T1, n = 25; T2, n = 25; T3, n = 22; T4, n = 22.
Comparison of Immediate Treatment and Delayed Treatment Control Groups (T1 to T2)
Table 2 shows that from T1 to T2 child outcomes decreased in the immediate treatment group but remained the same or increased in the delayed treatment control group. Table 3 shows parameter estimates for the repeated-measures models. The intercept is the initial status of the sample at T1. The group parameter is the difference between groups at T1. As expected, no differences were found because of random assignment to groups. The time parameter is change from T1 to T2 experienced by both groups. The group × time interaction is the differential change from T1 to T2 in the immediate treatment group compared with the delayed treatment group, which represents the intervention treatment effect. For child BMI, the group × time interaction indicated a statistically significant treatment effect of a BMI point change of −1.39 (95% CI: −1.91 to −0.87) in the immediate treatment group compared with the delayed treatment control group from T1 to T2 (P < .001). A similar differential change between groups was found for child BMI z score (−0.24) and child %OW (−8.31) (P < 0.001). In a mixed-model including only those parents with a BMI >25 (n = 29), the group × time interaction was −0.72 (SE = 0.40; P = .08), suggesting a change in parent BMI that did not reach statistical significance.
TABLE 3.
Repeated-measures Model Parameter Estimates for Child and Parent Outcomes From T1 to T2
| Outcome | Intercept | Group | Time | Group × Time |
|---|---|---|---|---|
| Child | ||||
| BMI | 24.23 (0.31)** | −0.32 (0.62) | 0.06 (0.13) | −1.39 (0.26)** |
| BMI z score | 1.71 (0.04)** | 0.01 (0.07) | −0.09 (0.02)** | −0.24 (0.04)** |
| %OW | 39.78 (1.42)** | −0.86 (2.83) | −1.99 (0.75) | −8.31 (0.75)** |
| Parent | ||||
| BMIa | 31.34 (1.01)** | 1.15 (2.03) | −0.20 (0.20) | −0.73 (0.40) |
Data are estimates (SE). Sample sizes for parents are as follows: T1, n = 13; T2, n = 11. Sample sizes for delayed group are as follows: T1, n = 16; T2, n = 16. **P < .001.
Includes only overweight and obese parents.
Comparison of Maintenance of GSH-PO for the Combined Immediate and Delayed Treatment Groups
We combined the immediate and delayed treatment groups by matching baseline, posttreatment, and 6-month follow-up points (ie, T1, T2, and T3 for the immediate treatment group; T2, T3, and T4 for the delayed treatment group). Table 2 shows the pattern of means for child and parent body size outcomes from baseline to the 6-month follow-up. Outcomes generally decreased from pre- to postintervention and then increased from post- to 6-month follow-up. Table 4 shows the parameter estimates for the repeated-measures models. For child outcomes, change in BMI was not statistically significant. However, the age- and gender-adjusted measures of BMI z score (−0.10) and %OW (−4.86) were significant, indicating that although children did gain back some of their weight postintervention, their normed weight change was still statistically different from baseline. No difference was found for change in parent BMI (−0.17; SE = 0.22, P = .446) from baseline to the 6-month follow-up.
TABLE 4.
Repeated-measures Model Parameter Estimates for Child and Parent Outcomes From Baseline to 6 Months After Treatment
| Outcome | Intercept | Time |
|---|---|---|
| Child | ||
| BMI | 24.61 (0.34)** | -0.06 (0.12) |
| BMI z score | 1.72 (0.04)** | −0.10 (0.02)** |
| %OW | 39.35 (1.82)** | −4.86 (1.90)* |
| Parent | ||
| BMI | 31.37 (1.03)** | −0.17 (0.22) |
Baseline to 6-months after treatment was T1 to T3 for immediate treatment; and T2 to T4 for delayed treatment. *P < .05, **P < .001.
Child and Parent Physical Activity and Dietary Outcomes From T1 to T2
No between-group differences were found from T1 to T2 for child accelerometer-measured sedentary, light, or moderate-to-vigorous physical activity. No differences were found between groups for child total energy, percentage of energy from fat, or log-transformed servings for fruits and vegetables per 1000 kcal. No difference was found for parent total metabolic equivalent task minutes per week of moderate-to-vigorous physical activity. No between-group differences were found for parent energy intake and percentage of energy from fat.
Acceptability and Liking of GSH-PO Intervention
All families (immediate and delayed treatment) completed the acceptability and liking survey posttreatment. One hundred percent of parents said that they liked the program, 74% liked the program “a lot” or “loved it.” Ninety-three percent of the children liked the program, 55% liked it “a lot” or “loved it.” Eighty-three percent of parents would recommend the program to other families, and 77% of the children thought that other children their age would like the program.
In reference to changing their lifestyle, 95% of the parents found the program helpful in changing the lifestyle of the child and family, 85% found the traffic light program helpful, and 71% of parents thought that the program helped their child to have more control over eating.
Ninety-five percent of the parents reported that the interventionist feedback to questions was helpful (“somewhat” or “very”), and 90% thought that the dietary advice and 90% thought the physical activity advice was helpful (“somewhat” or “very”). Ninety-three percent of parents rated the weekly weighing helpful (“somewhat” or “very”), and 90% found the positive parenting advice helpful (“somewhat” or “very”). Finally, 95% of parents thought that being accountable for their child’s behavior was helpful (“somewhat” or “very”).
Discussion
To our knowledge, this is the first evaluation of a guided self-help treatment of overweight or obese children. The GSH-PO intervention showed a significant decrease in child BMI, BMI z score, and %OW immediately after completing the 5-month treatment. In addition, the intervention resulted in decreases in child BMI z score and %OW that were maintained 6 months after the intervention.
It is interesting that there were no differences in the other child or parent measures in this study. Recent reviews report biases in self-reported measures of diet in children,23 but there should be less bias in the accelerometer measurements. Studies in adults show that changes in diet are necessary for weight loss, whereas physical activity is more important for weight maintenance.24,25 We believe that the changes in body size in the children were most likely related to changes in their diets, but these were likely to be inaccurately reported, a well-recognized limitation of self-reported dietary data.
In terms of parent outcomes, there were no statistically significant changes in parent BMI, diet, or physical activity in this study. We allowed parent weight status to vary in our recruitment for this study, resulting in a sample of normal-weight and overweight parents. However, we evaluated parent weight loss in only the overweight parents and found decrease of nearly .75 points in BMI for the immediate treatment compared with delayed treatment group but did not have adequate statistical power to detect this between-group difference. Parent diet and physical activity were measured by self-report instruments and are also subject to bias.
The GSH-PO intervention has a number of strengths. It is a low-intensity intervention, which could be easily translated to primary care providers or other health care providers. The GSH-PO intervention provides ∼4.5 hours of direct contact over 5 months, as opposed to the 30 hours of clinic family-based treatment programs. It has the potential to be more cost-effective and represents a potential advancement in the efficiency of current standards of care for overweight and obese children. In addition, it can be administered to families on an individual family basis, which allows for flexibility in scheduling.
GSH-PO emphasizes “self-help” and places less significance on the role of the interventionist by shifting the emphasis to the individual family as the primary agent of change. This shift in emphasis has the potential to provide greater self-efficacy to children and parents. In addition to providing the families with specific information and tools for behavior change, this treatment model may also provide greater self-sufficiency and self-efficacy to the parents and children that may make the treatment effects more durable over time.
Primary care providers are often the gateway to psychological treatment because families typically do not seek specialty psychological services as their initial source of care. Because the GSH-PO is developed for dissemination in primary care settings, it has the potential to intervene with patients earlier in the disease process as well as reach a larger proportion of the population, including those who might not normally seek more intensive interventions. In addition, GSH-PO may be more easily incorporated into checkups and visits so that it can become a part of routine health care. Because primary care providers initially detect and screen for obesity, providing these skills in primary care offices could reduce any stigma associated with a weight-loss program.
As in all studies, there are limitations that need to be considered. This study included a 6-month follow-up and a modest sample size, and larger studies are needed to provide additional efficacy data and to translate these methods to health care clinics. Our participants were also treatment-seeking and severely overweight children were excluded, limiting generalizability. In addition, we did not include a control group that could be followed over the length of the entire study, which limits our conclusions on the program’s efficacy to the randomly assigned groups at the T1 and T2 only.
Conclusions
This study revealed that the 5-month GSH-PO intervention results in decreases in BMI, BMI z score, and %OW in the target child and maintenance of these losses at 6 months posttreatment. In addition, the intervention was well received by families, provides treatment in less time that traditional family-based treatment, and has the potential to be provided by health care providers in the future. The GSH-PO has the potential to become the initial standard of care for overweight and obese children.
Acknowledgments
We acknowledge Hanaah Fannin, who was instrumental in the data collection in this project. We also acknowledge all of the children and parents who participated in this study.
Glossary
- DHQ
Dietary History Questionnaire
- FFQ
food-frequency questionnaire
- GSH-PO
guided self-help treatment of pediatric obesity
- MAR
missing at random
- T
time
- %OW
percentage overweight
Footnotes
Dr Boutelle conceived of the project, designed the study, collected the data, supervised the interventionists, contributed to data analysis and interpretation, and drafted the manuscript; Dr Norman collaborated on project conception and design of the study, analyzed the data and contributed to the interpretation, and revised the manuscript for intellectual content, specifically focusing on methods and physical activity; Dr Rock collaborated on project conception and the design of the study, contributed to data analysis and interpretation, and collaborated in writing and revising the manuscript, specifically focusing on nutritional assessment and interpretation; Dr Rhee collaborated on the design of the study, assisted with data collection, contributed to data analysis and interpretation, collaborated on supervising the interventionists, and collaborated in writing and revising the manuscript, specifically focusing on medical and parenting issues; and Dr Crow conceived of the project (with Dr Boutelle), collaborated on the design of the study, contributed to data analysis and interpretation, collaborated on supervising the interventionists, and collaborated in writing and revising the manuscript, specifically focusing on the provision of the guided self-help intervention. All of the authors contributed to the manuscript and publically take responsibility for its content.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01145833).
FINANCIAL DISCLOSURE: Dr Crowe has research grants from Shire, Alkermes, and Novartis; the other authors indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (NIH) DK080266.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847–2850 [DOI] [PubMed] [Google Scholar]
- 2.Epstein LH. Family-based behavioural intervention for obese children. Int J Obes Relat Metab Disord. 1996;20(suppl 1):S14–S21 [PubMed] [Google Scholar]
- 3.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol. 1994;13(5):373–383 [DOI] [PubMed] [Google Scholar]
- 4.Epstein LH, McCurley J, Wing RR, Valoski A. Five-year follow-up of family-based behavioral treatments for childhood obesity. J Consult Clin Psychol. 1990;58(5):661–664 [DOI] [PubMed] [Google Scholar]
- 5.Dietz WH, Nelson A. Barriers to the treatment of childhood obesity: a call to action. J Pediatr. 1999;134(5):535–536 [DOI] [PubMed] [Google Scholar]
- 6.Task Force on Promotion and Dissemination of Psychological Procedures DoC, Psychology . Training in and dissemination of empirically-validated psychological treatments: report and recommendations. Clin Psychol. 1995;48:3–23 [Google Scholar]
- 7.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–2374 [DOI] [PubMed] [Google Scholar]
- 8.Epstein LH, Myers MD, Raynor HA, Saelens BE. Treatment of pediatric obesity. Pediatrics. 1998;101(3 pt 2):554–570 [PubMed] [Google Scholar]
- 9.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27 [PubMed] [Google Scholar]
- 10.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc. 1996;96(11):1140–1144 [DOI] [PubMed] [Google Scholar]
- 11.Lytle LA, Nichaman MZ, Obarzanek E, et al. The CATCH Collaborative Group . Validation of 24-hour recalls assisted by food records in third-grade children. J Am Diet Assoc. 1993;93(12):1431–1436 [DOI] [PubMed] [Google Scholar]
- 12.Lytle LA, Murray DM, Perry CL, Eldridge AL. Validating fourth-grade students’ self-report of dietary intake: results from the 5 A Day Power Plus program. J Am Diet Assoc. 1998;98(5):570–572 [DOI] [PubMed] [Google Scholar]
- 13.Nichols JF, Morgan CG, Chabot LE, Sallis JF, Calfas KJ. Assessment of physical activity with the Computer Science and Applications, Inc., accelerometer: laboratory versus field validation. Res Q Exerc Sport. 2000;71(1):36–43 [DOI] [PubMed] [Google Scholar]
- 14.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781 [DOI] [PubMed] [Google Scholar]
- 15.Trost SG, Pate RR, Sallis JF, et al. Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc. 2002;34(2):350–355 [DOI] [PubMed] [Google Scholar]
- 16.Thompson FE, Subar AF, Brown CC, et al. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc. 2002;102(2):212–225 [DOI] [PubMed] [Google Scholar]
- 17.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099 [DOI] [PubMed] [Google Scholar]
- 18.Thompson FE, Subar AF, Smith AF, et al. Fruit and vegetable assessment: performance of 2 new short instruments and a food frequency questionnaire. J Am Diet Assoc. 2002;102(12):1764–1772 [DOI] [PubMed] [Google Scholar]
- 19.Lee RE, Mama SK, Medina AV, et al. Multiple measures of physical activity, dietary habits and weight status in African American and Hispanic or Latina women. J Community Health. 2011;36(6):1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams CD, Satia JA, Adair LS, et al. Dietary patterns, food groups, and rectal cancer risk in whites and African-Americans. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1552–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherwood NE, Crain AL, Martinson BC, et al. Keep it off: a phone-based intervention for long-term weight-loss maintenance. Contemp Clin Trials. 2011;32(4):551–560 [DOI] [PMC free article] [PubMed]
- 22.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009;6(6):790–804 [DOI] [PubMed] [Google Scholar]
- 23.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc. 2010;110(10):1501–1510 [DOI] [PubMed] [Google Scholar]
- 24.Wing RR, Papandonatos G, Fava JL, et al. Maintaining large weight losses: the role of behavioral and psychological factors. J Consult Clin Psychol. 2008;76(6):1015–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller WC, Koceja DM, Hamilton EJ. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int J Obes Relat Metab Disord.1997;21(10):941–947 [DOI] [PubMed] [Google Scholar]