Abstract
OBJECTIVES:
Short sleep has been associated with adolescent obesity. Most studies used a cross-sectional design and modeled BMI categories. We sought to determine if sleep duration was associated with BMI distribution changes from age 14 to 18.
METHODS:
Adolescents were recruited from suburban high schools in Philadelphia when entering ninth grade (n = 1390) and were followed-up every 6 months through 12th grade. Height and weight were self-reported, and BMIs were calculated (kg/m2). Hours of sleep were self-reported. Quantile regression was used to model the 10th, 25th, 50th, 75th, and 90th BMI percentiles as dependent variables; study wave and sleep were the main predictors.
RESULTS:
BMI increased from age 14 to 18, with the largest increase observed at the 90th BMI percentile. Each additional hour of sleep was associated with decreases in BMI at the 10th (–0.04; 95% confidence interval [CI]: –0.11, 0.03), 25th (–0.12; 95% CI: –0.20, –0.04), 50th (–0.15; 95% CI: –0.24, –0.06), 75th (–0.25; 95% CI: –0.38, –0.12), and 90th (–0.27; 95% CI: -0.45, -0.09) BMI percentiles. The strength of the association was stronger at the upper tail of the BMI distribution. Increasing sleep from 7.5 to 10.0 hours per day at age 18 predicted a reduction in the proportion of adolescents >25 kg/m2 by 4%.
CONCLUSIONS:
More sleep was associated with nonuniform changes in BMI distribution from age 14 to 18. Increasing sleep among adolescents, especially those in the upper half of the BMI distribution, may help prevent overweight and obesity.
Keywords: adolescence, longitudinal study, obesity, sleep
What’s Known on This Subject:
Short sleep may be an adolescent obesity risk factor, but most evidence is from cross-sectional studies. Three longitudinal studies have investigated the association between sleep duration and adolescent obesity, finding mixed results.
What This Study Adds:
Shorter sleep was associated with increases in BMI from age 14 to 18, especially at the upper tail of the BMI distribution. Increasing daily sleep to 10 hours per day could help to prevent adolescent obesity.
The untoward psychosocial and physical consequences of adolescent obesity have been well documented.1,2 Unfortunately, the prevalence of adolescent obesity has more than tripled over the past 4 decades (5.2% to 18.4%).3,4 The rise in obesity has been paralleled by decreases in the amount of time that adolescents have spent sleeping,5 leading to the hypothesis that short sleep duration has contributed to the rise in adolescent obesity.
Cross-sectional studies support an association between less sleep and adolescent obesity,6–8 but longitudinal studies are needed to establish temporal precedence. Three longitudinal studies have investigated the relationship between sleep duration and adolescent BMI.9–11 Two of those studies found evidence that less sleep led to an increased likelihood of being classified as obese at follow-up,10,11 whereas 1 study found no association.9 These studies only measured BMI at 2 time points and categorized participants into nonobese and obese groups based on BMI. Such categorization of a continuous variable reduces statistical power and considers those in close proximity to a category cutoff, but on opposite sides, as being very different, as opposed to being very similar.12
Longitudinal studies that incorporate >2 study waves and consider changes in the entire BMI distribution will further our understanding of the relationship between sleep duration and the development of adolescent overweight and obesity. We used quantile regression because it allows for the investigation of predictors (eg, sleep) across the distribution of an outcome variable (eg, BMI).13 The purpose of our study was to determine if sleep duration was associated with changes across the BMI distribution, over 8 study waves, from age 14 to 18.
Methods
Participants
The participants were recruited from 4 suburban high schools in Philadelphia, when they were entering ninth grade. We identified 1517 adolescents, but 30 were ineligible to participate due to having a special classroom placement or not being a native English speaker. The 1487 eligible adolescents were invited to participate, and of those, 1478 (99%) provided parental consent and were enrolled into the study. The baseline survey was completed by 1429 (97%) of the participants enrolled; reasons for not completing the survey included being absent from school (n = 30) and withdrawing from the study (n = 19). The University of Pennsylvania Institutional Review Board granted ethical approval for the study.
BMI
The participants self-reported their height and weight, from which BMI was calculated (kg/m2). The height and weight data were screened for high and low values and were compared with Center for Disease Control and Prevention growth chart data.14 There is high correlation between adolescent BMI calculated from self-report and measured height and weight.15–17 Also, similar measures of association were observed between obesity risk factors and BMI when calculated from self-reported and measured height and weight.18
Sleep
Typical duration of sleep on a school night (Sunday to Thursday) and on a weekend night (Fri and Sat) were self-reported by the participants, to the nearest 15 minutes. Past studies have shown that adolescents are able to recall their typical duration of sleep.19 The sleep times reported were screened for low and high values, and if a participant reported sleep times that were 3 standard deviations above or below the age and gender-specific mean hours of sleep, then their sleep data were coded as missing. The average time spent sleeping per day was calculated for each participant [(school night × 5) + (weekend night × 2)/7].8
Covariates
It has been shown in previous studies that BMI and sleep duration differ by gender,3,20 race,3,20 and socioeconomic status.20,21 Therefore, these demographic variables were included in the current study as covariates. Maternal education was used as a marker of socioeconomic status. Physical activity has been associated with lower BMI in adolescents,22 and it has been suggested that more physical activity is associated with more sleep.23 For these reasons, self-reported time spent in moderate-to-vigorous physical activity (MVPA) was included as a covariate. It has been observed that more time spent watching television and playing video games is associated with higher BMI in adolescents,24 and data suggest that more screen time is associated with less sleep.23 For these reasons, self-reported screen time (television/video and video games) was included as a covariate.
Statistical Analysis
The means and standard deviations are presented for the continuous variables, and the frequencies and percentages are provided for the categorical variables. Longitudinal quantile regression was used to address the aim of our study.25 Quantile regression is related to ordinary least squares regression but can model any point in the distribution of a continuous outcome variable, not just the mean.13,25 Furthermore, the dependent variable is modeled as a continuous variable, and categorization does not take place. The coefficients from quantile regression are interpreted in the exact same way as those from ordinary least squares regression. In the current study, the coefficients represent the change in BMI for every unit change in the independent variable; study wave and sleep were modeled as the main independent variables. Study wave was coded 0, 1, 2, 3, 4, 5, 6, and 7 to represent each 6-month follow-up, and sleep was modeled as a continuous variable (hours per day). The coefficients at the 10th, 25th, 50th, 75th, and 90th BMI percentiles are presented. Model 1 included study wave and gender to describe the change in the BMI distribution from age 14 to 18. In model 2, sleep was added as an independent variable to determine if sleep was associated with changes in the BMI distribution from age 14 to 18. Race and maternal education were then added as independent variables (model 3), self-reported MVPA was then added as an independent variable (model 4), and finally screen time was added as an independent variable (model 5). The purpose of models 3, 4, and 5 was to determine if any association between sleep and change in BMI remained after their inclusion in the model. The data were arranged in the long format; to account for the repeated measures on the participants, a first-order autoregressive correlation structure was modeled, and 95% confidence intervals (CIs) were estimated from 500 bootstrap samples.25 All analyses were conducted by using Stata 12.1 (StataCorp LP, College Station, TX).
Results
Of those enrolled 1336 provided valid BMI data at baseline (93%), and 1089 (82%) provided BMI data at the eighth study wave (Table 1). Our sample had equal proportions of male and female participants, most of the sample was white (>74%), and the majority of the participants had mothers who attained a high school education or greater (>70%; Table 1). The average hours of sleep was ∼8 hours per day for both male and female participants at baseline, and this decreased to an average of 7.5 hours per day at the eighth study wave (Table 1).
TABLE 1.
Demographics, Sleep Duration, and Anthropometric Data by Study Wave
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | Wave 5 | Wave 6 | Wave 7 | Wave 8 | |
|---|---|---|---|---|---|---|---|---|
| Sample size | 1336 | 1261 | 1067 | 1076 | 1019 | 1045 | 971 | 1089 |
| Age, mean (SD), y | 14.3 (0.56) | 14.8 (0.59) | 15.3 (0.53) | 15.7 (0.55) | 16.3 (0.55) | 16.8 (0.55) | 17.3 (0.54) | 17.7 (0.56) |
| Gender, n (%) | ||||||||
| Female | 666 (50.0) | 631 (50.1) | 544 (51.1) | 542 (50.4) | 520 (51.1) | 531 (50.9) | 446 (46.0) | 562 (51.7) |
| Male | 667 (50.0) | 629 (49.9) | 521 (48.9) | 533 (49.6) | 498 (48.9) | 513 (49.1) | 523 (54.0) | 525 (48.3) |
| Race, n (%) | ||||||||
| Black | 187 (14.0) | 169 (13.4) | 138 (12.9) | 138 (12.8) | 123 (12.1) | 131 (12.5) | 128 (13.2) | 135 (12.4) |
| White | 996 (74.6) | 958 (76.0) | 805 (75.5) | 812 (75.5) | 765 (75.1) | 789 (75.5) | 737 (75.9) | 823 (75.6) |
| Other | 153 (11.5) | 134 (10.6) | 124 (11.6) | 126 (11.7) | 131 (12.9) | 125 (12.0) | 106 (10.9) | 131 (12.0) |
| Maternal education, n (%) | ||||||||
| High school education or greater | 936 (70.8) | 880 (70.6) | 765 (72.6) | 765 (72.0) | 719 (71.2) | 730 (70.6) | 686 (71.5) | 764 (70.9) |
| Less than high school education | 387 (29.3) | 367 (29.4) | 289 (27.4) | 298 (28.0) | 288 (28.6) | 304 (29.4) | 273 (28.5) | 314 (29.1) |
| Sleep, mean (SD), hr/d | ||||||||
| Male | 8.13 (1.69) | 8.01 (1.75) | 7.93 (1.75) | 7.94 (1.62) | 7.93 (1.70) | 7.82 (1.58) | 7.72 (1.51) | 7.54 (1.61) |
| Female | 8.16 (1.68) | 7.98 (1.75) | 7.95 (1.65) | 7.82 (1.64) | 7.69 (1.64) | 7.67 (1.59) | 7.65 (1.55) | 7.52 (1.59) |
| Height, mean (SD), m | ||||||||
| Male | 1.69 (0.09) | 1.72 (0.08) | 1.73 (0.08) | 1.74 (0.08) | 1.75 (0.08) | 1.76 (0.08) | 1.76 (0.08) | 1.76 (0.08) |
| Female | 1.61 (0.07) | 1.62 (0.07) | 1.62 (0.07) | 1.63 (0.07) | 1.63 (0.08) | 1.63 (0.07) | 1.64 (0.07) | 1.63 (0.07) |
| Wt, mean (SD), kg | ||||||||
| Male | 63.9 (13.9) | 67.0 (14.3) | 68.7 (13.7) | 69.9 (13.8) | 71.9 (14.5) | 73.4 (14.5) | 74.7 (14.6) | 75.3 (14.5) |
| Female | 56.6 (10.4) | 58.2 (10.9) | 58.2 (10.2) | 58.8 (10.3) | 60.0 (11.1) | 60.8 (10.9) | 64.4 (10.6) | 61.4 (11.0) |
| BMI, mean (SD) | ||||||||
| Male | 22.2 (4.04) | 22.6 (4.11) | 22.9 (4.02) | 23.0 (4.08) | 23.3 (4.22) | 23.6 (4.20) | 24.0 (4.39) | 24.1 (4.28) |
| Female | 21.7 (3.67) | 22.1 (3.85) | 22.0 (3.44) | 22.1 (3.47) | 22.5 (3.66) | 22.8 (3.70) | 23.9 (3.87) | 23.0 (3.73) |
The average BMI at baseline was 22.2 kg/m2 for the boys and 21.7 kg/m2 for the girls (Table 1). BMI increased on average to 24.1 kg/m2 for the boys and 23.0 kg/m2 for the girls at the eighth study wave (Table 1). Changes across the BMI distribution are described in Table 2 (model 1). At the 50th BMI percentile, BMI increased at a rate of 0.24 kg/m2 every 6 months. In comparison, increases in BMI were lower at the 10th BMI percentile (0.22 kg/m2 every 6 months) and greater at the 90th BMI percentile (0.35 kg/m2 every 6 months).
TABLE 2.
Changes in the BMI Distribution From Age 14 to 18 and the Influence of Sleep
| BMI percentile | |||||
|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | |
| Model 1a | |||||
| Intercept | 18.1 (17.8, 18.4) | 19.6 (19.4, 19.8) | 21.5 (21.2, 21.8) | 24.2 (23.7, 24.7) | 27.8 (27.1, 28.6) |
| Wave | 0.22 (0.19, 0.26) | 0.22 (0.19, 0.25) | 0.24 (0.21, 0.28) | 0.24 (0.17, 0.30) | 0.35 (0.25, 0.44) |
| Model 2a | |||||
| Intercept | 18.7 (18.1, 19.4) | 20.6 (20.0, 21.3) | 22.8 (22.0, 23.6) | 26.4 (25.3, 27.5) | 30.3 (28.5, 32.1) |
| Wave | 0.21 (0.18, 0.25) | 0.21 (0.18, 0.24) | 0.23 (0.20, 0.27) | 0.21 (0.15, 0.27) | 0.28 (0.19, 0.38) |
| Sleep | −0.07 (–0.14, 0.01) | −0.13 (–0.21, –0.05) | −0.17 (–0.26, –0.07) | −0.26 (–0.39, –0.14) | −0.28 (–0.48, –0.08) |
| Model 3b | |||||
| Intercept | 18.6 (18.0, 19.3) | 20.6 (19.9, 21.3) | 22.5 (21.7, 23.3) | 25.9 (24.7, 27.0) | 29.9 (28.0, 31.7) |
| Wave | 0.21 (0.18, 0.25) | 0.21 (0.18, 0.23) | 0.24 (0.20, 0.27) | 0.21 (0.16, 0.27) | 0.28 (0.18, 0.38) |
| Sleep | −0.06 (–0.13, 0.01) | −0.12 (–0.21, –0.04) | −0.15 (–0.24, –0.06) | −0.25 (–0.38, –0.12) | −0.24 (–0.44, –0.05) |
| Model 4c | |||||
| Intercept | 18.3 (17.6, 19.1) | 20.5 (19.8, 21.2) | 22.3 (21.4, 23.2) | 25.8 (24.7, 27.0) | 30.4 (28.5, 32.2) |
| Wave | 0.23 (0.19, 0.27) | 0.22 (0.19, 0.25) | 0.24 (0.21, 0.28) | 0.22 (0.17, 0.28) | 0.27 (0.17, 0.36) |
| Sleep | −0.05 (–0.13, 0.03) | −0.13 (–0.21, –0.05) | −0.15 (–0.25, –0.06) | −0.23 (–0.34, –0.11) | −0.23 (–0.41, –0.05) |
| Model 5d | |||||
| Intercept | 18.1 (17.4, 18.9) | 20.3 (19.5, 21.0) | 21.8 (20.9, 22.6) | 24.9 (23.6, 26.3) | 28.8 (27.0, 30.5) |
| Wave | 0.24 (0.20, 0.27) | 0.22 (0.19, 0.25) | 0.25 (0.22, 0.28) | 0.24 (0.18, 0.30) | 0.30 (0.20, 0.40) |
| Sleep | −0.04 (–0.11, 0.03) | −0.12 (–0.20, –0.04) | −0.15 (–0.24, –0.06) | −0.25 (–0.38, –0.12) | −0.27 (–0.45, –0.09) |
Data presented are coefficients and 95% CIs in parentheses. Wave is coded 0, 1, 2, 3, 4, 5, 6, and 7 to represent each 6-month follow-up, and thus the wave coefficients are interpreted as change in BMI per 6 months. Sleep is modeled in hours per day, and thus the sleep coefficients are interpreted as the change in BMI for each additional hour spent sleeping.
Adjusted for gender.
Adjusted for gender, race, and maternal education.
Adjusted for gender, race, maternal education, and MVPA.
Adjusted for gender, race, maternal education, MVPA, and screen time.
Each additional hour of sleep was associated with a reduction in BMI, at all BMI percentiles (Table 2, model 2). The strength of the association was weaker at the lower tail of the BMI distribution, compared with the upper tail of the BMI distribution (Table 2, model 2). For example, each additional hour of sleep was associated with a 0.07 kg/m2 reduction in BMI at the 10th BMI percentile, compared with a 0.17 kg/m2 reduction in BMI at the 50th BMI percentile, and a 0.28 kg/m2 reduction in BMI at the 90th BMI percentile (Table 2, model 2). The associations at all BMI percentiles remained similar after adjusting for race and maternal education (Table 2, model 3), self-reported MVPA (Table 2, model 4), and screen time (Table 2, model 5).
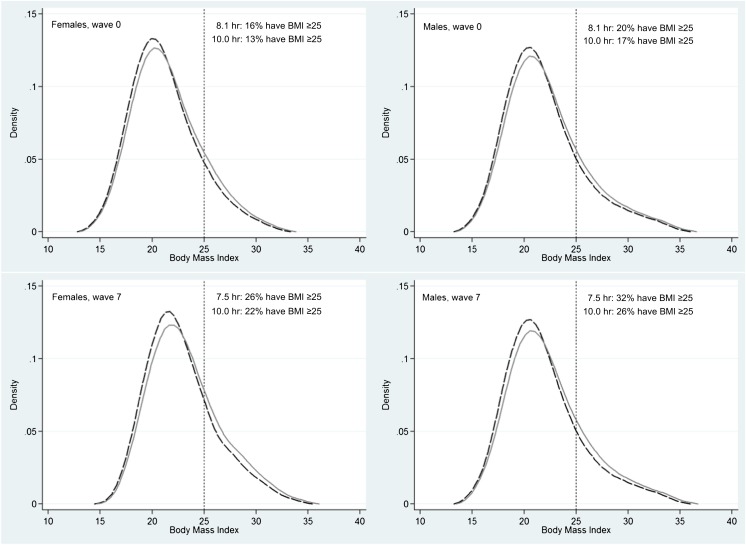
To aid the interpretation of the coefficients presented in Table 2, BMI distributions at wave 0 and wave 7 are illustrated in Fig 1. The dashed line represents the predicted BMI distribution if all adolescents accumulated 8.1 hours of sleep at age 14 and 7.5 hours of sleep per day at age 18. The solid line represents the BMI distribution if all adolescents accumulated 10.0 hours of sleep per day at each age. The stronger negative association between sleep duration and BMI at the upper tail of the distribution can be observed, which translates to a leftward shift at the upper tail of the BMI distribution with more sleep (Fig 1).
FIGURE 1.
Predicted BMI distributions at wave 0 and wave 7 by gender. The dashed line represents the BMI distribution if all adolescents slept for 10 hours per day, and the solid line represents the BMI distribution if all adolescents slept for 8.1 hours per day at age 14 and 7.5 hours per day at age 18 (age-specific average hours of sleep, Table 1).
Discussion
The current study was designed to investigate the effect of sleep duration on changes in BMI from mid to late adolescence. Importantly, we observed a large sample of adolescents over 8 study waves and considered the entire BMI distribution. We found that less sleep was associated with greater increases in BMI from age 14 to 18, but the association was not uniform across the BMI distribution. Less sleep was more strongly associated with increases in BMI at the upper tail, compared with the lower tail, of the distribution. This provides strong evidence that sleep duration is an important adolescent obesity risk factor and that it is especially important to ensure that adolescents in the upper half of the BMI distribution at age 14 accumulate sufficient hours of sleep and also maintain sufficient hours of sleep throughout adolescence. It is recommended that adolescents sleep for 8.5 to 10.5 hours per day.5 On the basis of our results, sleeping for 10 hours per day versus <8 hours per day could reduce the proportion of adolescents with a BMI ≥25 by 3% at age 14 and by 4% to 6% at age 18. The prevalence of adolescent obesity increased by 7.5% from 1991 to 20083,4; at the population level, a 4% reduction in the proportion of adolescents with a BMI ≥25 translates to ∼500 000 fewer 14- to 18-year-olds who are overweight.26 Therefore, increasing the average time spent sleeping each night by 2 hours across mid to late adolescence could have a significant impact on obesity prevention.
Two smaller longitudinal studies reported associations between less sleep at baseline and an increased likelihood of being classified as obese at follow-up.10,11 Seegers et al observed 1916 preadolescents from age 10 to 13 and found that those in the low sleep trajectory group were 3 times more likely to be obese, compared with those in the high sleep trajectory group.10 Similarly, Silva et al recruited 304 children (aged 6–12) who were followed-up 5 years later (aged 10 to 18), and observed that sleeping for <7.5 hours per day at baseline, compared with ≥9.0 hours per day at baseline, was associated with a threefold increased likelihood of being obese at follow-up.11 Our findings are consistent with these findings and extend the research by following adolescents over a longer time period and studying the entire BMI distribution. However, 1 study reported a null association between sleep duration and BMI defined obesity at 2-year follow-up in a sample of 13 568 adolescents.9 It would be of interest to study the BMI distribution in that sample of adolescents to determine if the association between sleep duration and changes in BMI were uniform across the BMI distribution.
It is important to note that the associations we observed between sleep duration and BMI remained after adjusting for screen time and physical activity. This implies that more sleep could contribute to the prevention of adolescent obesity, even if the screen time and physical activity guidelines are met.27,28 It is a limitation that we were not able to include any measure of dietary intake in our study, and we cannot exclude the possibility that our findings are explained, in part, by increases in caloric intake with less sleep.29 However, the association between less sleep and adolescent obesity reported by Silva et al was adjusted for caloric intake,11 which implies that more sleep could contribute to the prevention of adolescent obesity, even for those who consume an excess of calories. It has been proposed that less sleep increases adolescent BMI by decreasing physical activity, as a consequence of fatigue and changes in hormones that regulate energy expenditure,30,31 and increasing energy intake, as a consequence of more eating opportunities and changes in hormones that regulate energy intake.30,31 It is therefore surprising that adjusting for the energy balance variables did not attenuate the associations observed between sleep duration and changes in adolescent BMI. Future studies could adjust for objectively measured sleep duration, physical activity, and sedentary behavior, as well as for caloric intake, to investigate this mechanism in more detail.
Alternatively, the association we observed could be explained by short sleep disrupting circadian rhythms.32,33 Adolescents with short sleep may be more likely to be awake at night and be exposed to light during the dark cycle. This could affect the central clock, which in turn could affect the peripheral clock in adipose tissue, leading to abnormal timing of adipocyte differentiation and the release of adipokines.32,33 In support, a cross-sectional study observed that adolescents going to bed late tended to have higher BMIs, independent of sleep duration, compared with adolescents who go to bed early.34 To test if this circadian rhythm hypothesis explains the relationship between sleep duration and adolescent weight, future studies could specifically control for evening electronic screen exposure, and evening food intake; and investigate if variants in clock genes (eg, CLOCK and BAML1) modify the association between sleep duration and adolescent BMI.
Educating adolescents on the benefits of sleep and informing adolescents of sleep hygiene practices has shown little impact on adolescent sleep duration. A review of school-based sleep education programs found no evidence that such education led to increases in sleep duration among adolescents.35 Alternatively, schools could help to increase adolescent sleep duration by delaying the start to the school day. Owen et al reported a 45-minute per day increase in sleep by delaying the start of the school day from 8:00 to 8:30.36 Outside of school, observational data suggest that modifying the bedroom environment, such as removing electronic media from the bedroom, could be important for increasing adolescent sleep duration,37 although no studies appear to have experimentally tested whether these changes lead to increases in sleep among adolescents. This is an area of research that needs to be further developed to help determine the best approaches for increasing sleep duration among adolescents.
Our longitudinal study has several strengths. We followed adolescents over 8 study waves, whereas previous longitudinal studies included only 2 study waves.9–11 Our analytical method allowed for the investigation of sleep duration at the tails of the BMI distribution, and we were able to determine how sleep duration affected the shape of the BMI distribution. Our study also has limitations. Sleep duration was self-reported, and we were not able to measure sleep quality. Our participants self-reported their height and weight, and replication of our findings using BMI calculated from objectively measured height and weight is needed. If these self-report data can be corroborated with objective monitoring data, this would help to establish sleep duration as an important adolescent obesity risk factor. We adjusted for key covariates, but residual confounding may remain due to missing covariates, such as caloric intake. Our participants were sampled from a single region in the United States, and attempts to replicate our findings in other populations of adolescents are needed.
In summary, we conducted a longitudinal quantile regression analysis to assess the association between sleep duration and BMI. Using this novel method, we found that less sleep was associated with greater increases in adolescent BMI from age 14 to 18, with the association strongest at the upper tail of the BMI distribution. This is an important finding and suggests that increasing sleep duration, especially for those in the upper half of the BMI distribution, could help to reduce the prevalence of adolescent obesity.
Glossary
- CI
confidence interval
- MVPA
moderate-to-vigorous physical activity
Footnotes
Dr Mitchell conceived and designed the study, analyzed the data, drafted the manuscript, and approved the final manuscript as submitted; Dr Rodriguez manages and maintains the data set that was used in the current study, revised the manuscript, interpreted the data, and approved the final manuscript as submitted; Dr Schmitz reviewed and revised the manuscript, interpreted the data, and approved the final manuscript as submitted; and Dr Audrain-McGovern acquired the data, reviewed and revised the manuscript, interpreted the data, and approved the final submitted manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Cancer Institute (grant RO1 CA126958 to Dr Audrain-McGovern). Funded by the National Institutes of Health (NIH).
References
- 1.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond). 2009;33(suppl 1):S60–S65 [DOI] [PubMed] [Google Scholar]
- 2.Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168(1):30–37 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149(10):1085–1091 [DOI] [PubMed] [Google Scholar]
- 5.Matricciani LA, Olds TS, Blunden S, Rigney G, Williams MT. Never enough sleep: a brief history of sleep recommendations for children. Pediatrics. 2012;129(3):548–556 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring). 2008;16(2):265–274 [DOI] [PubMed] [Google Scholar]
- 7.Knutson KL, Lauderdale DS. Sleep duration and overweight in adolescents: self-reported sleep hours versus time diaries. Pediatrics. 2007;119(5):e1056–e1062 [DOI] [PubMed] [Google Scholar]
- 8.Garaulet M, Ortega FB, Ruiz JR, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study. Int J Obes (Lond). 2011;35(10):1308–1317 [DOI] [PubMed] [Google Scholar]
- 9.Calamaro CJ, Park S, Mason TB, et al. Shortened sleep duration does not predict obesity in adolescents. J Sleep Res. 2010;19(4):559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seegers V, Petit D, Falissard B, et al. Short sleep duration and body mass index: a prospective longitudinal study in preadolescence. Am J Epidemiol. 2011;173(6):621–629 [DOI] [PubMed] [Google Scholar]
- 11.Silva GE, Goodwin JL, Parthasarathy S, et al. Longitudinal association between short sleep, body weight, and emotional and learning problems in Hispanic and Caucasian children. Sleep. 2011;34(9):1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao L, Naimen DQ. Quantile Regression. Thousand Oaks, CA: Sage; 2007 [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002; (246):1–190 [PubMed] [Google Scholar]
- 15.Elgar FJ, Roberts C, Tudor-Smith C, Moore L. Validity of self-reported height and weight and predictors of bias in adolescents. J Adolesc Health. 2005;37(5):371–375 [DOI] [PubMed] [Google Scholar]
- 16.Tokmakidis SP, Christodoulos AD, Mantzouranis NI. Validity of self-reported anthropometric values used to assess body mass index and estimate obesity in Greek school children. J Adolesc Health. 2007;40(4):305–310 [DOI] [PubMed] [Google Scholar]
- 17.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106(1 pt 1):52–58 [DOI] [PubMed] [Google Scholar]
- 18.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring). 2007;15(1):188–196 [DOI] [PubMed] [Google Scholar]
- 19.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26(2):213–216 [DOI] [PubMed] [Google Scholar]
- 20.Blair PS, Humphreys JS, Gringras P, et al. Childhood sleep duration and associated demographic characteristics in an English cohort. Sleep. 2012;35(3):353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatakis E, Wardie J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes. 2010;34(1):41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimm SY, Glynn NW, Obarzanek E, et al. Relation between the changes in physical activity and body-mass index during adolescence: a multicentre longitudinal study. Lancet. 2005;366(9482):301–307 [DOI] [PubMed] [Google Scholar]
- 23.Foti KE, Eaton DK, Lowry R, McKnight-Ely LR. Sufficient sleep, physical activity, and sedentary behaviors. Am J Prev Med. 2011;41(6):596–602 [DOI] [PubMed] [Google Scholar]
- 24.Chinapaw MJ, Proper KI, Brug J, van Mechelen W, Singh AS. Relationship between young peoples’ sedentary behaviour and biomedical health indicators: a systematic review of prospective studies. Obes Rev. 2011;12(7):e621–e632 [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Pere A, Koenker R, He X. Quantile regression methods for reference growth charts. Stat Med. 2006;25(8):1369–1382 [DOI] [PubMed] [Google Scholar]
- 26.Howden LM, Meyer JA. Age and sex composition: 2010. May 2011. Available at: www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed July 31, 2012
- 27.American Academy of Pediatrics, Committee on Public Education . American Academy of Pediatrics: Children, adolescents, and television. Pediatrics. 2001;107(2):423–426 [DOI] [PubMed] [Google Scholar]
- 28.Physical Activity Guidelines Advisory Committee Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S. Department of Health and Human Services; 2008 [Google Scholar]
- 29.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33(9):1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91(11):881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston JD, Frost G, Otway DT. Adipose tissue, adipocytes and the circadian timing system. Obes Rev. 2009;10(suppl 2):52–60 [DOI] [PubMed] [Google Scholar]
- 34.Olds TS, Maher CA, Matricciani L. Sleep duration or bedtime? Exploring the relationship between sleep habits and weight status and activity patterns. Sleep. 2011;34(10):1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blunden SL, Chapman J, Rigney GA. Are sleep education programs successful? The case for improved and consistent research efforts. Sleep Med Rev. 2011;16(4):355–370. [DOI] [PubMed] [Google Scholar]
- 36.Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Arch Pediatr Adolesc Med. 2010;164(7):608–614 [DOI] [PubMed] [Google Scholar]
- 37.Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010;11(8):735–742 [DOI] [PubMed] [Google Scholar]